

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Coagulation factor X (Homo sapiens (Human)) | BDBM50096105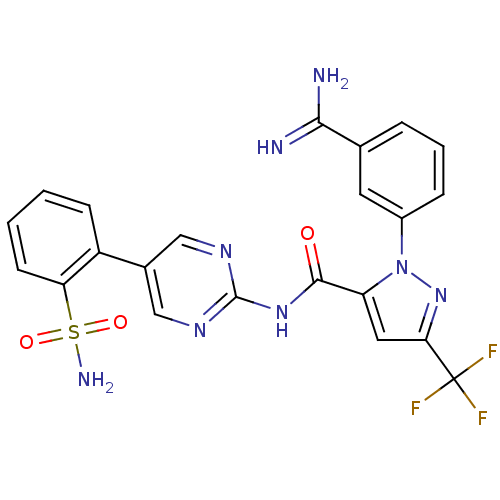 (2-(3-Carbamimidoyl-phenyl)-5-trifluoromethyl-2H-py...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.00100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company Curated by ChEMBL | Assay Description Binding affinity against human coagulation factor X | J Med Chem 44: 566-78 (2001) BindingDB Entry DOI: 10.7270/Q2RF5VQK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50096099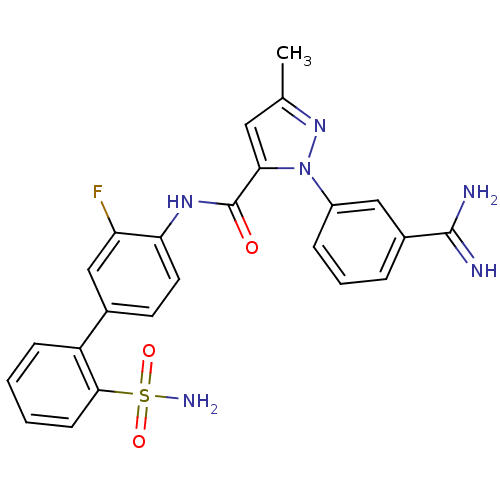 (2-(3-Carbamimidoyl-phenyl)-5-methyl-2H-pyrazole-3-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.00500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company Curated by ChEMBL | Assay Description Binding affinity against human coagulation factor X | J Med Chem 44: 566-78 (2001) BindingDB Entry DOI: 10.7270/Q2RF5VQK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50096101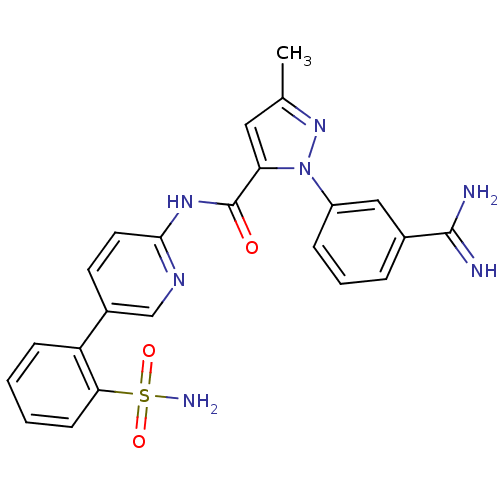 (2-(3-Carbamimidoyl-phenyl)-5-methyl-2H-pyrazole-3-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.00700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company Curated by ChEMBL | Assay Description Binding affinity against human coagulation factor X | J Med Chem 44: 566-78 (2001) BindingDB Entry DOI: 10.7270/Q2RF5VQK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50096110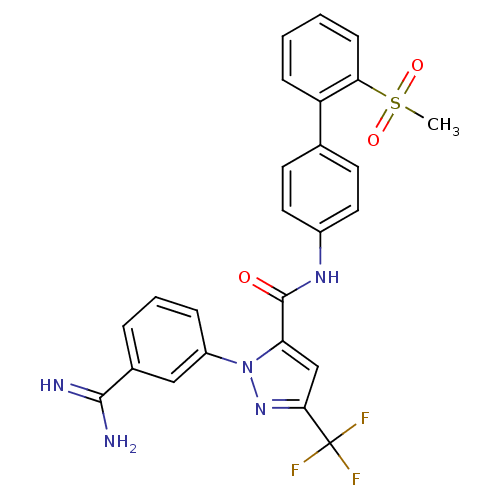 (2-(3-Carbamimidoyl-phenyl)-5-trifluoromethyl-2H-py...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.00800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company Curated by ChEMBL | Assay Description Binding affinity against human coagulation factor X | J Med Chem 44: 566-78 (2001) BindingDB Entry DOI: 10.7270/Q2RF5VQK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50096091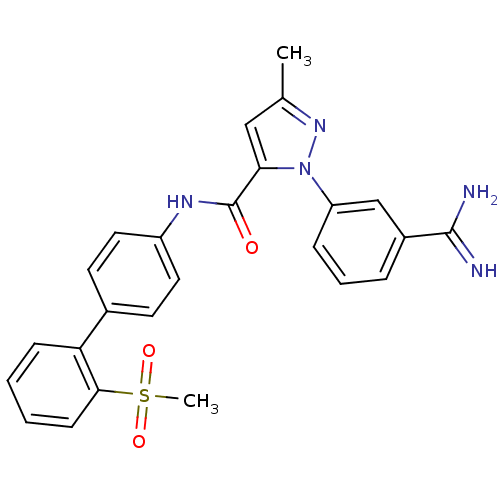 (2-(3-Carbamimidoyl-phenyl)-5-methyl-2H-pyrazole-3-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.00800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company Curated by ChEMBL | Assay Description Binding affinity against human coagulation factor X | J Med Chem 44: 566-78 (2001) BindingDB Entry DOI: 10.7270/Q2RF5VQK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50096108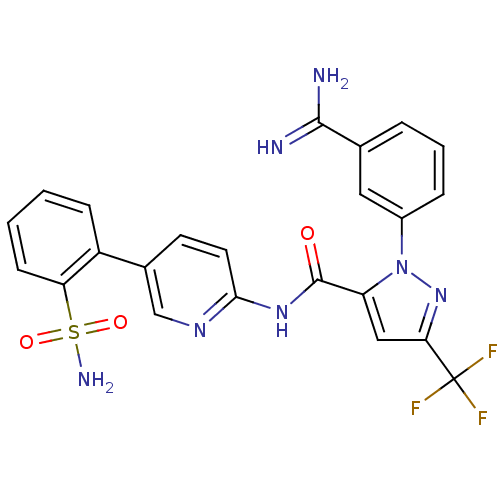 (2-(3-Carbamimidoyl-phenyl)-5-trifluoromethyl-2H-py...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.00900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company Curated by ChEMBL | Assay Description Binding affinity against human coagulation factor X | J Med Chem 44: 566-78 (2001) BindingDB Entry DOI: 10.7270/Q2RF5VQK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50096085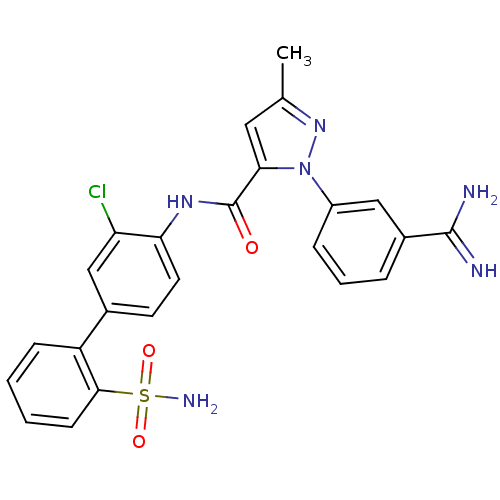 (2-(3-Carbamimidoyl-phenyl)-5-methyl-2H-pyrazole-3-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.00900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company Curated by ChEMBL | Assay Description Binding affinity against human coagulation factor X | J Med Chem 44: 566-78 (2001) BindingDB Entry DOI: 10.7270/Q2RF5VQK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50096098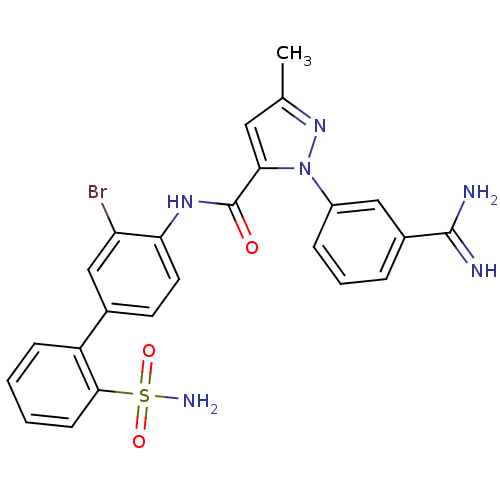 (2-(3-Carbamimidoyl-phenyl)-5-methyl-2H-pyrazole-3-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company Curated by ChEMBL | Assay Description Binding affinity against human coagulation factor X | J Med Chem 44: 566-78 (2001) BindingDB Entry DOI: 10.7270/Q2RF5VQK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM12751 (1-(3-carbamimidoylphenyl)-3-methyl-N-[4-(2-sulfamo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.0130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company Curated by ChEMBL | Assay Description Binding affinity against human coagulation factor X | J Med Chem 44: 566-78 (2001) BindingDB Entry DOI: 10.7270/Q2RF5VQK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50096111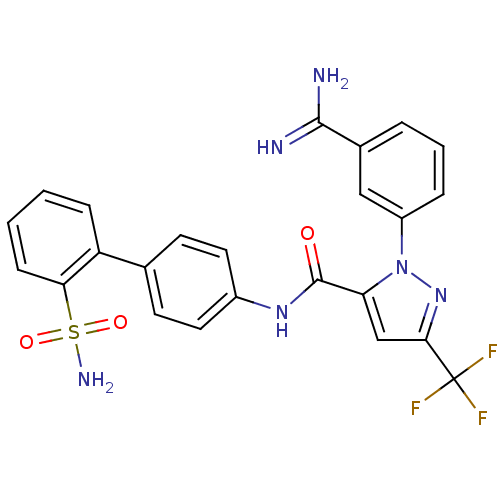 (2-(3-Carbamimidoyl-phenyl)-5-trifluoromethyl-2H-py...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company Curated by ChEMBL | Assay Description Binding affinity against human coagulation factor X | J Med Chem 44: 566-78 (2001) BindingDB Entry DOI: 10.7270/Q2RF5VQK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50096096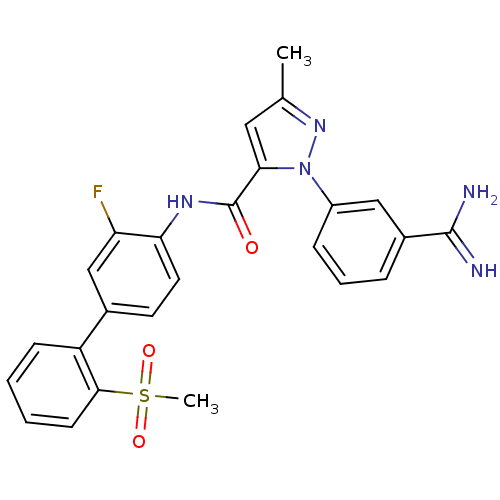 (2-(3-Carbamimidoyl-phenyl)-5-methyl-2H-pyrazole-3-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company Curated by ChEMBL | Assay Description Binding affinity against human coagulation factor X | J Med Chem 44: 566-78 (2001) BindingDB Entry DOI: 10.7270/Q2RF5VQK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM12751 (1-(3-carbamimidoylphenyl)-3-methyl-N-[4-(2-sulfamo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company Curated by ChEMBL | Assay Description In vitro activity against rabbit FXa. | J Med Chem 44: 566-78 (2001) BindingDB Entry DOI: 10.7270/Q2RF5VQK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM12693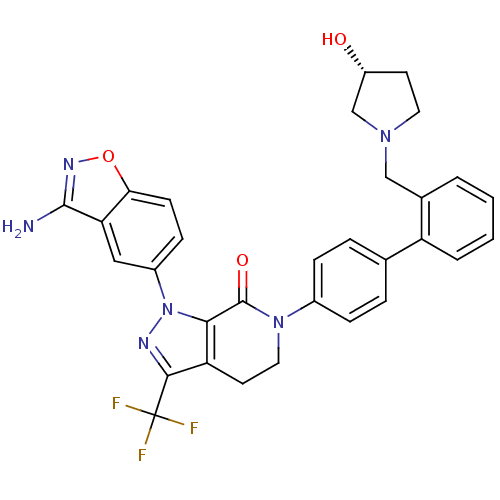 (1-(3-amino-1,2-benzoxazol-5-yl)-6-[4-(2-{[(3R)-3-h...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 0.0300 | -59.5 | n/a | n/a | n/a | n/a | n/a | 7.0 | 22 |
Bristol-Myers Squibb Pharmaceutical Research Institute | Assay Description Ki values were obtained from human purified enzyme. All assays were run in microtiter plates. Plates were read for 30 min at 405 nm. Rates were deter... | Bioorg Med Chem Lett 16: 4141-7 (2006) Article DOI: 10.1016/j.bmcl.2006.02.069 BindingDB Entry DOI: 10.7270/Q26T0JW1 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM12681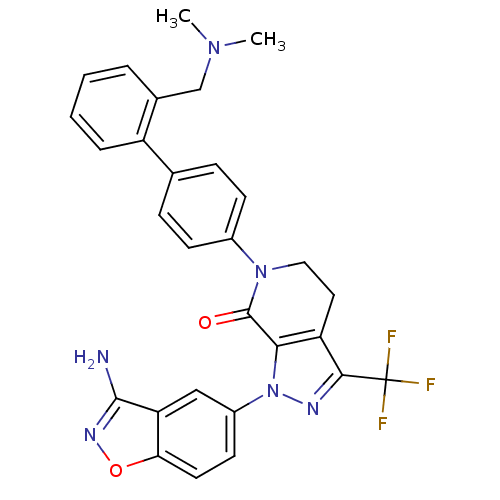 (1-(3-amino-1,2-benzoxazol-5-yl)-6-(4-{2-[(dimethyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0400 | -58.8 | n/a | n/a | n/a | n/a | n/a | 7.0 | 22 |
Bristol-Myers Squibb Pharmaceutical Research Institute | Assay Description Ki values were obtained from human purified enzyme. All assays were run in microtiter plates. Plates were read for 30 min at 405 nm. Rates were deter... | Bioorg Med Chem Lett 16: 4141-7 (2006) Article DOI: 10.1016/j.bmcl.2006.02.069 BindingDB Entry DOI: 10.7270/Q26T0JW1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50096094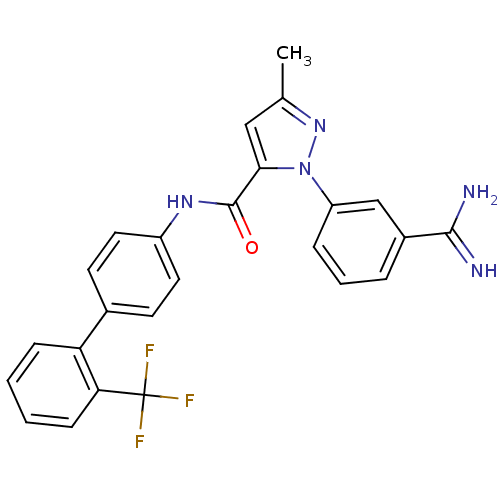 (2-(3-Carbamimidoyl-phenyl)-5-methyl-2H-pyrazole-3-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company Curated by ChEMBL | Assay Description Binding affinity against human coagulation factor X | J Med Chem 44: 566-78 (2001) BindingDB Entry DOI: 10.7270/Q2RF5VQK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50096112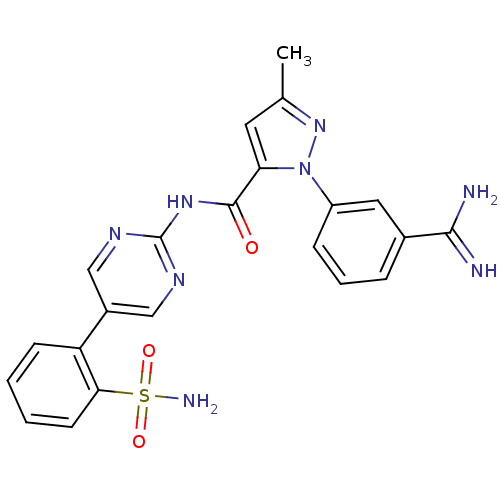 (2-(3-Carbamimidoyl-phenyl)-5-methyl-2H-pyrazole-3-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0410 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company Curated by ChEMBL | Assay Description Binding affinity against human coagulation factor X | J Med Chem 44: 566-78 (2001) BindingDB Entry DOI: 10.7270/Q2RF5VQK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50451005 (CHEMBL290376 | DuP-714) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB PubMed | 0.0420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company Curated by ChEMBL | Assay Description Inhibition of Coagulation factor II | Bioorg Med Chem Lett 9: 925-30 (1999) BindingDB Entry DOI: 10.7270/Q2KS6QQ6 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50096090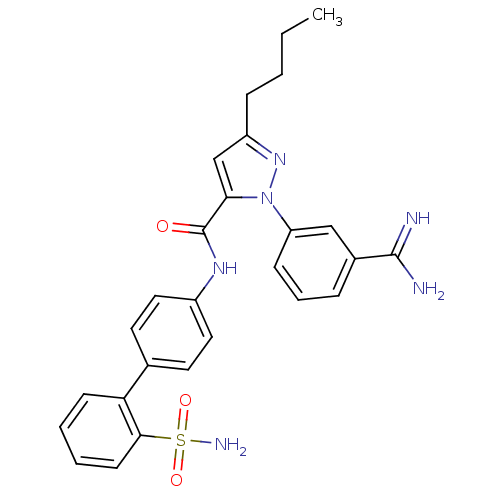 (5-Butyl-2-(3-carbamimidoyl-phenyl)-2H-pyrazole-3-c...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company Curated by ChEMBL | Assay Description Binding affinity against human coagulation factor X | J Med Chem 44: 566-78 (2001) BindingDB Entry DOI: 10.7270/Q2RF5VQK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM19023 (1-(4-methoxyphenyl)-7-oxo-6-[4-(2-oxopiperidin-1-y...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank MMDB PDB Article PubMed | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Inhibition of human factor 10a | Bioorg Med Chem Lett 20: 1373-7 (2010) Article DOI: 10.1016/j.bmcl.2010.01.011 BindingDB Entry DOI: 10.7270/Q2FQ9WQG | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM12690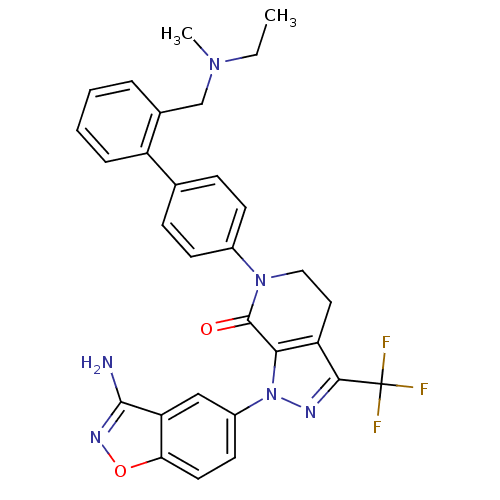 (1-(3-amino-1,2-benzoxazol-5-yl)-6-[4-(2-{[ethyl(me...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | <0.100 | <-56.5 | n/a | n/a | n/a | n/a | n/a | 7.0 | 22 |
Bristol-Myers Squibb Pharmaceutical Research Institute | Assay Description Ki values were obtained from human purified enzyme. All assays were run in microtiter plates. Plates were read for 30 min at 405 nm. Rates were deter... | Bioorg Med Chem Lett 16: 4141-7 (2006) Article DOI: 10.1016/j.bmcl.2006.02.069 BindingDB Entry DOI: 10.7270/Q26T0JW1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM12689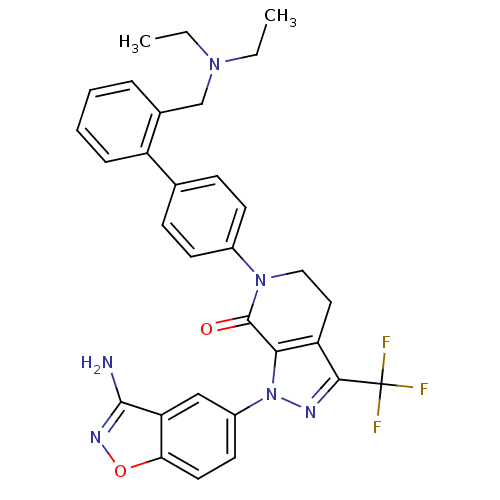 (1-(3-amino-1,2-benzoxazol-5-yl)-6-(4-{2-[(diethyla...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.110 | -56.3 | n/a | n/a | n/a | n/a | n/a | 7.0 | 22 |
Bristol-Myers Squibb Pharmaceutical Research Institute | Assay Description Ki values were obtained from human purified enzyme. All assays were run in microtiter plates. Plates were read for 30 min at 405 nm. Rates were deter... | Bioorg Med Chem Lett 16: 4141-7 (2006) Article DOI: 10.1016/j.bmcl.2006.02.069 BindingDB Entry DOI: 10.7270/Q26T0JW1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM12694 (1-(3-amino-1,2-benzoxazol-5-yl)-6-[4-(2-{[(3S)-3-h...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 0.120 | -56.1 | n/a | n/a | n/a | n/a | n/a | 7.0 | 22 |
Bristol-Myers Squibb Pharmaceutical Research Institute | Assay Description Ki values were obtained from human purified enzyme. All assays were run in microtiter plates. Plates were read for 30 min at 405 nm. Rates were deter... | Bioorg Med Chem Lett 16: 4141-7 (2006) Article DOI: 10.1016/j.bmcl.2006.02.069 BindingDB Entry DOI: 10.7270/Q26T0JW1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM12682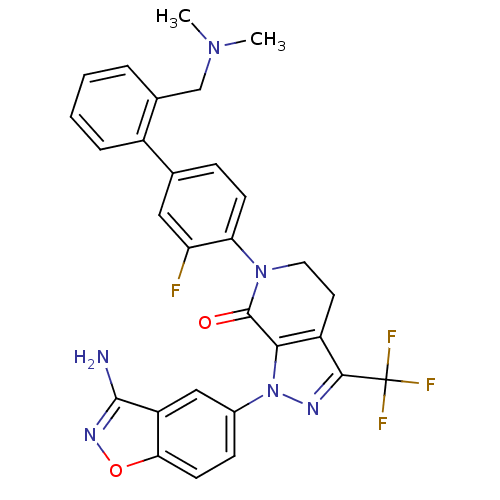 (1-(3-amino-1,2-benzoxazol-5-yl)-6-(4-{2-[(dimethyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.130 | -55.9 | n/a | n/a | n/a | n/a | n/a | 7.0 | 22 |
Bristol-Myers Squibb Pharmaceutical Research Institute | Assay Description Ki values were obtained from human purified enzyme. All assays were run in microtiter plates. Plates were read for 30 min at 405 nm. Rates were deter... | Bioorg Med Chem Lett 16: 4141-7 (2006) Article DOI: 10.1016/j.bmcl.2006.02.069 BindingDB Entry DOI: 10.7270/Q26T0JW1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM12692 (1-(3-amino-1,2-benzoxazol-5-yl)-6-{4-[2-(pyrrolidi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 0.150 | -55.5 | n/a | n/a | n/a | n/a | n/a | 7.0 | 22 |
Bristol-Myers Squibb Pharmaceutical Research Institute | Assay Description Ki values were obtained from human purified enzyme. All assays were run in microtiter plates. Plates were read for 30 min at 405 nm. Rates were deter... | Bioorg Med Chem Lett 16: 4141-7 (2006) Article DOI: 10.1016/j.bmcl.2006.02.069 BindingDB Entry DOI: 10.7270/Q26T0JW1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50087533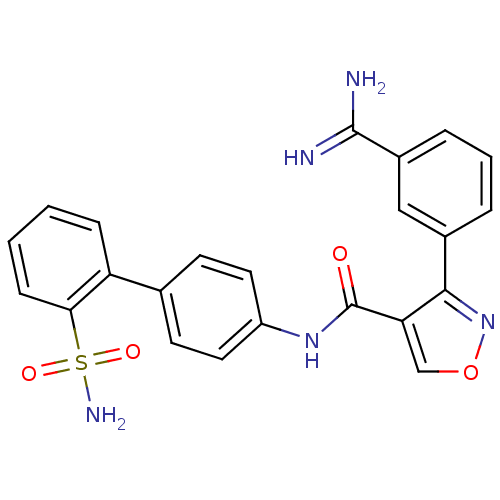 (3-(3-Carbamimidoyl-phenyl)-isoxazole-4-carboxylic ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | PubMed | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company Curated by ChEMBL | Assay Description Binding affinity against human coagulation factor X | J Med Chem 44: 566-78 (2001) BindingDB Entry DOI: 10.7270/Q2RF5VQK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM12657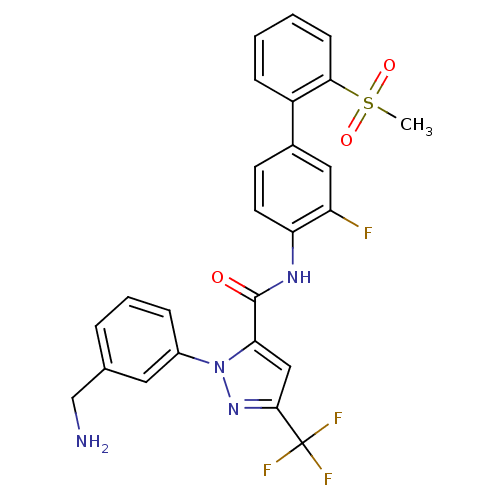 (1-[3-(Aminomethyl)phenyl]-N-[3-fluoro-2-(methylsul...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Inhibition of human factor 10a | Bioorg Med Chem Lett 20: 1373-7 (2010) Article DOI: 10.1016/j.bmcl.2010.01.011 BindingDB Entry DOI: 10.7270/Q2FQ9WQG | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM12700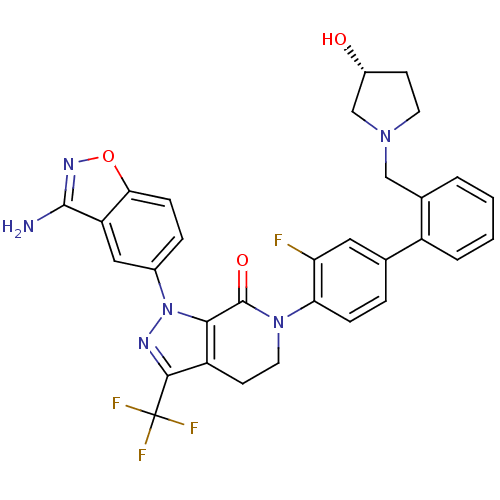 (1-(3-amino-1,2-benzoxazol-5-yl)-6-[2-fluoro-4-(2-{...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.160 | -55.3 | n/a | n/a | n/a | n/a | n/a | 7.0 | 22 |
Bristol-Myers Squibb Pharmaceutical Research Institute | Assay Description Ki values were obtained from human purified enzyme. All assays were run in microtiter plates. Plates were read for 30 min at 405 nm. Rates were deter... | Bioorg Med Chem Lett 16: 4141-7 (2006) Article DOI: 10.1016/j.bmcl.2006.02.069 BindingDB Entry DOI: 10.7270/Q26T0JW1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50096095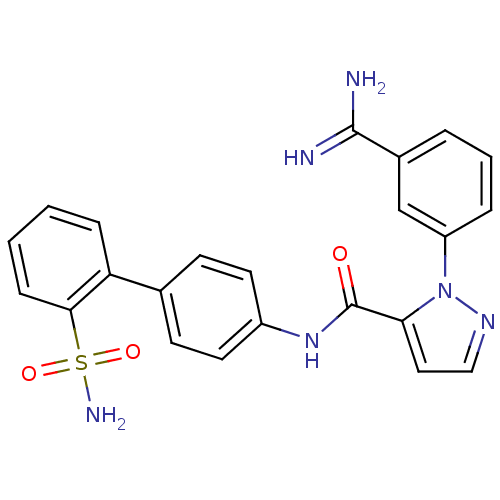 (2-(3-Carbamimidoyl-phenyl)-2H-pyrazole-3-carboxyli...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company Curated by ChEMBL | Assay Description Binding affinity against human coagulation factor X | J Med Chem 44: 566-78 (2001) BindingDB Entry DOI: 10.7270/Q2RF5VQK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM12676 (1-(3-Aminobenzisoxazol-5-yl)-3-trifluoromethyl-N-[...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Inhibition of human factor 10a | Bioorg Med Chem Lett 20: 1373-7 (2010) Article DOI: 10.1016/j.bmcl.2010.01.011 BindingDB Entry DOI: 10.7270/Q2FQ9WQG | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM12676 (1-(3-Aminobenzisoxazol-5-yl)-3-trifluoromethyl-N-[...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 0.190 | -54.9 | n/a | n/a | n/a | n/a | n/a | 7.0 | 22 |
Bristol-Myers Squibb Pharmaceutical Research Institute | Assay Description Ki values were obtained from human purified enzyme. All assays were run in microtiter plates. Plates were read for 30 min at 405 nm. Rates were deter... | Bioorg Med Chem Lett 16: 4141-7 (2006) Article DOI: 10.1016/j.bmcl.2006.02.069 BindingDB Entry DOI: 10.7270/Q26T0JW1 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM12695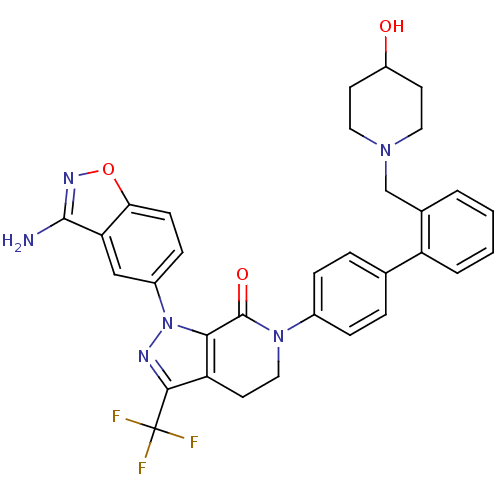 (1-(3-amino-1,2-benzoxazol-5-yl)-6-(4-{2-[(4-hydrox...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.220 | -54.6 | n/a | n/a | n/a | n/a | n/a | 7.0 | 22 |
Bristol-Myers Squibb Pharmaceutical Research Institute | Assay Description Ki values were obtained from human purified enzyme. All assays were run in microtiter plates. Plates were read for 30 min at 405 nm. Rates were deter... | Bioorg Med Chem Lett 16: 4141-7 (2006) Article DOI: 10.1016/j.bmcl.2006.02.069 BindingDB Entry DOI: 10.7270/Q26T0JW1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM12699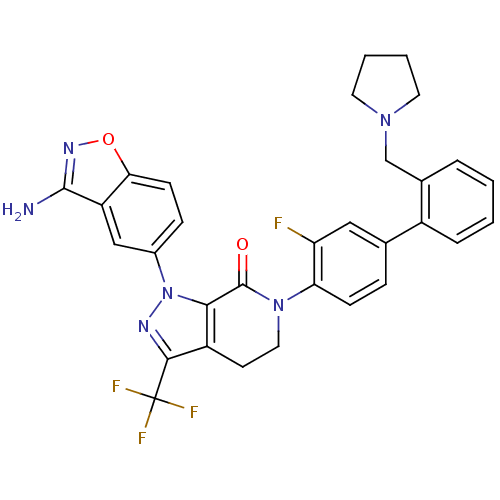 (1-(3-amino-1,2-benzoxazol-5-yl)-6-{2-fluoro-4-[2-(...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.240 | -54.4 | n/a | n/a | n/a | n/a | n/a | 7.0 | 22 |
Bristol-Myers Squibb Pharmaceutical Research Institute | Assay Description Ki values were obtained from human purified enzyme. All assays were run in microtiter plates. Plates were read for 30 min at 405 nm. Rates were deter... | Bioorg Med Chem Lett 16: 4141-7 (2006) Article DOI: 10.1016/j.bmcl.2006.02.069 BindingDB Entry DOI: 10.7270/Q26T0JW1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50307102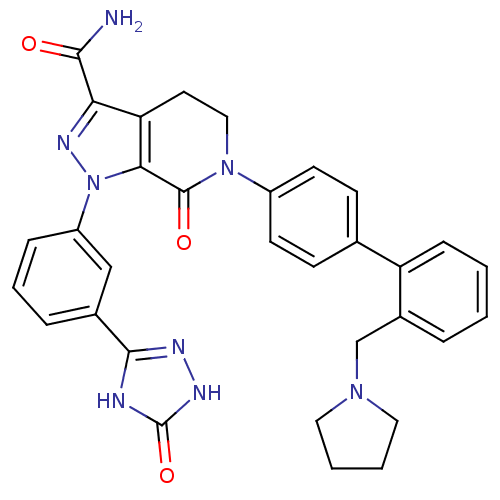 (7-oxo-1-(3-(5-oxo-4,5-dihydro-1H-1,2,4-triazol-3-y...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Inhibition of human factor 10a | Bioorg Med Chem Lett 20: 1373-7 (2010) Article DOI: 10.1016/j.bmcl.2010.01.011 BindingDB Entry DOI: 10.7270/Q2FQ9WQG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM12687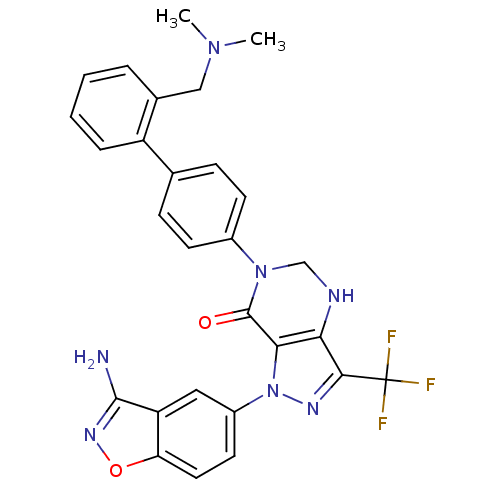 (1-(3-amino-1,2-benzoxazol-5-yl)-6-(4-{2-[(dimethyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 0.25 | -54.3 | n/a | n/a | n/a | n/a | n/a | 7.0 | 22 |
Bristol-Myers Squibb Pharmaceutical Research Institute | Assay Description Ki values were obtained from human purified enzyme. All assays were run in microtiter plates. Plates were read for 30 min at 405 nm. Rates were deter... | Bioorg Med Chem Lett 16: 4141-7 (2006) Article DOI: 10.1016/j.bmcl.2006.02.069 BindingDB Entry DOI: 10.7270/Q26T0JW1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM12688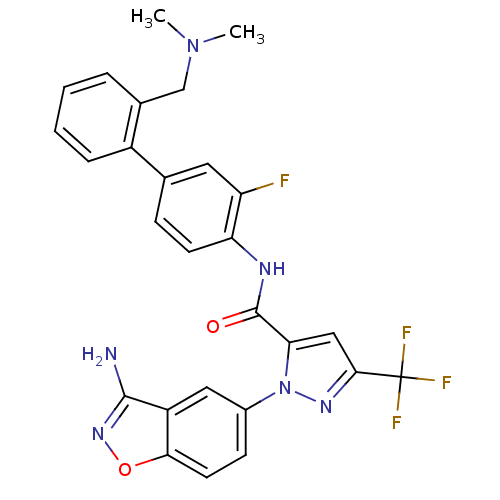 (1-(3-amino-1,2-benzoxazol-5-yl)-N-(4-{2-[(dimethyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.260 | -54.2 | n/a | n/a | n/a | n/a | n/a | 7.0 | 22 |
Bristol-Myers Squibb Pharmaceutical Research Institute | Assay Description Ki values were obtained from human purified enzyme. All assays were run in microtiter plates. Plates were read for 30 min at 405 nm. Rates were deter... | Bioorg Med Chem Lett 16: 4141-7 (2006) Article DOI: 10.1016/j.bmcl.2006.02.069 BindingDB Entry DOI: 10.7270/Q26T0JW1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM12703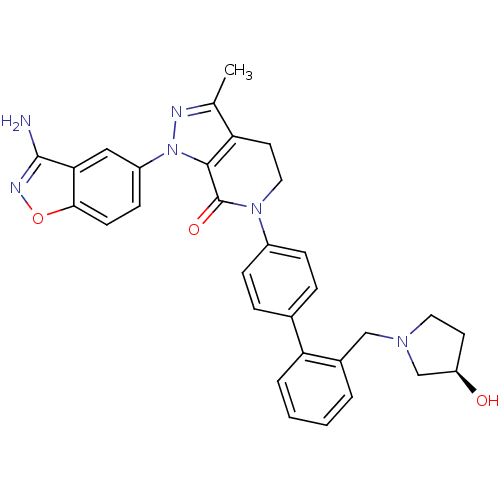 (1-(3-amino-1,2-benzoxazol-5-yl)-6-[4-(2-{[(3R)-3-h...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.270 | -54.1 | n/a | n/a | n/a | n/a | n/a | 7.0 | 22 |
Bristol-Myers Squibb Pharmaceutical Research Institute | Assay Description Ki values were obtained from human purified enzyme. All assays were run in microtiter plates. Plates were read for 30 min at 405 nm. Rates were deter... | Bioorg Med Chem Lett 16: 4141-7 (2006) Article DOI: 10.1016/j.bmcl.2006.02.069 BindingDB Entry DOI: 10.7270/Q26T0JW1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50096102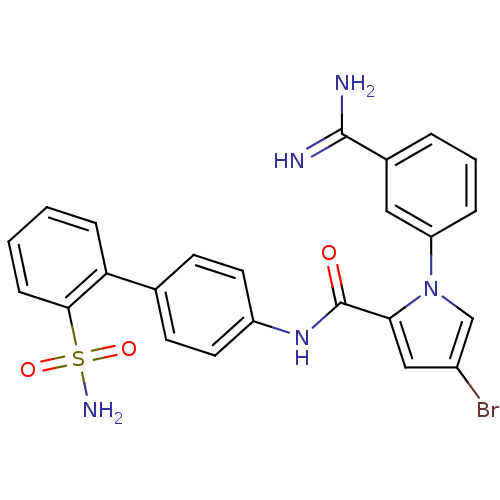 (4-Bromo-1-(3-carbamimidoyl-phenyl)-1H-pyrrole-2-ca...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company Curated by ChEMBL | Assay Description Binding affinity against human coagulation factor X | J Med Chem 44: 566-78 (2001) BindingDB Entry DOI: 10.7270/Q2RF5VQK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM12657 (1-[3-(Aminomethyl)phenyl]-N-[3-fluoro-2-(methylsul...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company Curated by ChEMBL | Assay Description In vitro activity against rabbit factor Xa | J Med Chem 44: 566-78 (2001) BindingDB Entry DOI: 10.7270/Q2RF5VQK | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM12702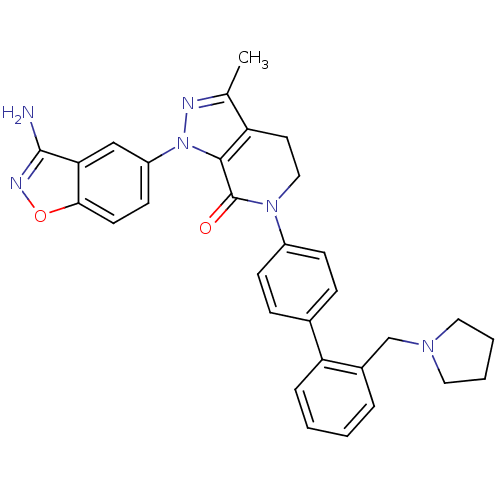 (1-(3-amino-1,2-benzoxazol-5-yl)-3-methyl-6-{4-[2-(...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 0.310 | -53.7 | n/a | n/a | n/a | n/a | n/a | 7.0 | 22 |
Bristol-Myers Squibb Pharmaceutical Research Institute | Assay Description Ki values were obtained from human purified enzyme. All assays were run in microtiter plates. Plates were read for 30 min at 405 nm. Rates were deter... | Bioorg Med Chem Lett 16: 4141-7 (2006) Article DOI: 10.1016/j.bmcl.2006.02.069 BindingDB Entry DOI: 10.7270/Q26T0JW1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM12691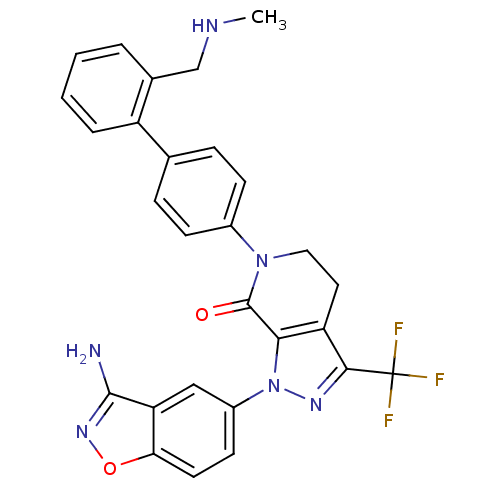 (1-(3-amino-1,2-benzoxazol-5-yl)-6-(4-{2-[(methylam...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.340 | -53.5 | n/a | n/a | n/a | n/a | n/a | 7.0 | 22 |
Bristol-Myers Squibb Pharmaceutical Research Institute | Assay Description Ki values were obtained from human purified enzyme. All assays were run in microtiter plates. Plates were read for 30 min at 405 nm. Rates were deter... | Bioorg Med Chem Lett 16: 4141-7 (2006) Article DOI: 10.1016/j.bmcl.2006.02.069 BindingDB Entry DOI: 10.7270/Q26T0JW1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM12704 (1-(3-amino-1,2-benzoxazol-5-yl)-6-[2-fluoro-4-(2-{...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.340 | -53.5 | n/a | n/a | n/a | n/a | n/a | 7.0 | 22 |
Bristol-Myers Squibb Pharmaceutical Research Institute | Assay Description Ki values were obtained from human purified enzyme. All assays were run in microtiter plates. Plates were read for 30 min at 405 nm. Rates were deter... | Bioorg Med Chem Lett 16: 4141-7 (2006) Article DOI: 10.1016/j.bmcl.2006.02.069 BindingDB Entry DOI: 10.7270/Q26T0JW1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM12696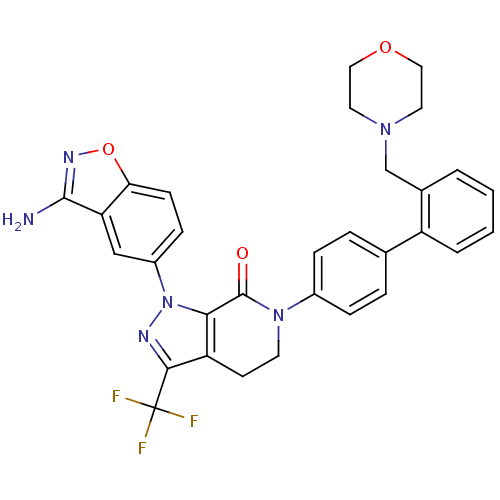 (1-(3-amino-1,2-benzoxazol-5-yl)-6-{4-[2-(morpholin...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 0.350 | -53.4 | n/a | n/a | n/a | n/a | n/a | 7.0 | 22 |
Bristol-Myers Squibb Pharmaceutical Research Institute | Assay Description Ki values were obtained from human purified enzyme. All assays were run in microtiter plates. Plates were read for 30 min at 405 nm. Rates were deter... | Bioorg Med Chem Lett 16: 4141-7 (2006) Article DOI: 10.1016/j.bmcl.2006.02.069 BindingDB Entry DOI: 10.7270/Q26T0JW1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50307100 (1-(3-(5-OXO-4,5-DIHYDRO-1H-1,2,4-TRIAZOL-3-YL)PHEN...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Inhibition of human factor 10a | Bioorg Med Chem Lett 20: 1373-7 (2010) Article DOI: 10.1016/j.bmcl.2010.01.011 BindingDB Entry DOI: 10.7270/Q2FQ9WQG | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50096088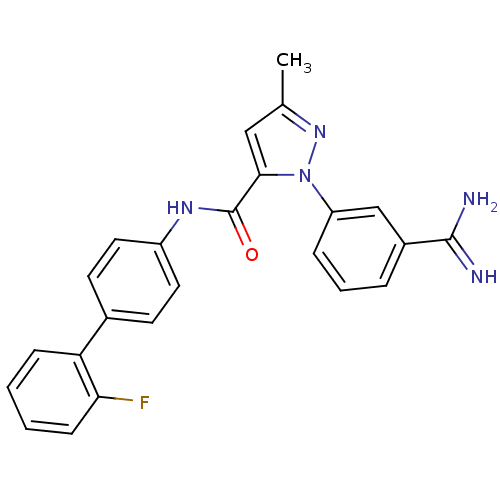 (2-(3-Carbamimidoyl-phenyl)-5-methyl-2H-pyrazole-3-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.460 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company Curated by ChEMBL | Assay Description Binding affinity against human coagulation factor X | J Med Chem 44: 566-78 (2001) BindingDB Entry DOI: 10.7270/Q2RF5VQK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50307093 (CHEMBL591955 | N-(3-fluoro-2'-(methylsulfonyl)biph...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Inhibition of human factor 10a | Bioorg Med Chem Lett 20: 1373-7 (2010) Article DOI: 10.1016/j.bmcl.2010.01.011 BindingDB Entry DOI: 10.7270/Q2FQ9WQG | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM12701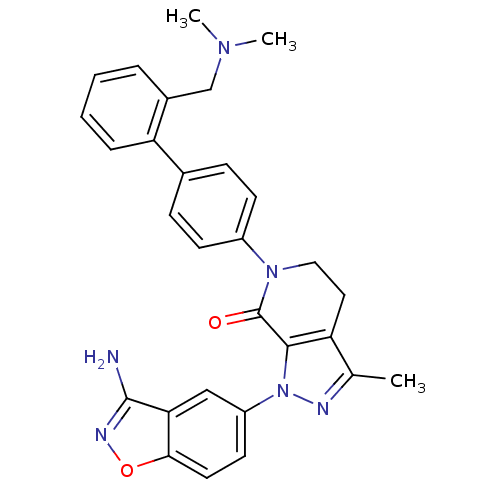 (1-(3-amino-1,2-benzoxazol-5-yl)-6-(4-{2-[(dimethyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 0.550 | -52.3 | n/a | n/a | n/a | n/a | n/a | 7.0 | 22 |
Bristol-Myers Squibb Pharmaceutical Research Institute | Assay Description Ki values were obtained from human purified enzyme. All assays were run in microtiter plates. Plates were read for 30 min at 405 nm. Rates were deter... | Bioorg Med Chem Lett 16: 4141-7 (2006) Article DOI: 10.1016/j.bmcl.2006.02.069 BindingDB Entry DOI: 10.7270/Q26T0JW1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM12686 (1-(3-amino-1,2-benzoxazol-5-yl)-7-(4-{2-[(dimethyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 0.600 | -52.1 | n/a | n/a | n/a | n/a | n/a | 7.0 | 22 |
Bristol-Myers Squibb Pharmaceutical Research Institute | Assay Description Ki values were obtained from human purified enzyme. All assays were run in microtiter plates. Plates were read for 30 min at 405 nm. Rates were deter... | Bioorg Med Chem Lett 16: 4141-7 (2006) Article DOI: 10.1016/j.bmcl.2006.02.069 BindingDB Entry DOI: 10.7270/Q26T0JW1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM12685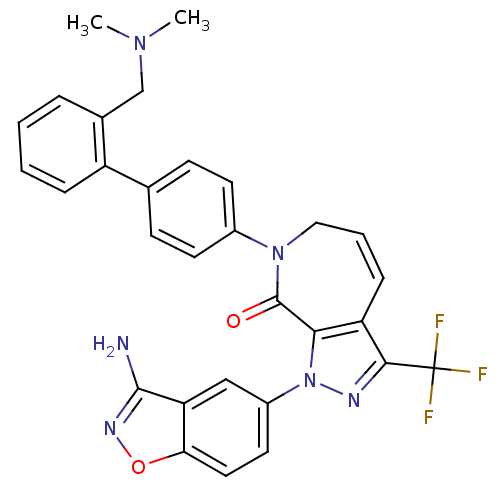 (1-(3-amino-1,2-benzoxazol-5-yl)-7-(4-{2-[(dimethyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 0.600 | -52.1 | n/a | n/a | n/a | n/a | n/a | 7.0 | 22 |
Bristol-Myers Squibb Pharmaceutical Research Institute | Assay Description Ki values were obtained from human purified enzyme. All assays were run in microtiter plates. Plates were read for 30 min at 405 nm. Rates were deter... | Bioorg Med Chem Lett 16: 4141-7 (2006) Article DOI: 10.1016/j.bmcl.2006.02.069 BindingDB Entry DOI: 10.7270/Q26T0JW1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM12697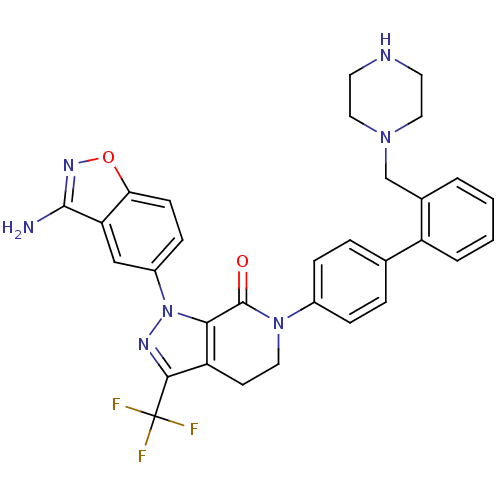 (1-(3-amino-1,2-benzoxazol-5-yl)-6-{4-[2-(piperazin...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.640 | -51.9 | n/a | n/a | n/a | n/a | n/a | 7.0 | 22 |
Bristol-Myers Squibb Pharmaceutical Research Institute | Assay Description Ki values were obtained from human purified enzyme. All assays were run in microtiter plates. Plates were read for 30 min at 405 nm. Rates were deter... | Bioorg Med Chem Lett 16: 4141-7 (2006) Article DOI: 10.1016/j.bmcl.2006.02.069 BindingDB Entry DOI: 10.7270/Q26T0JW1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM12698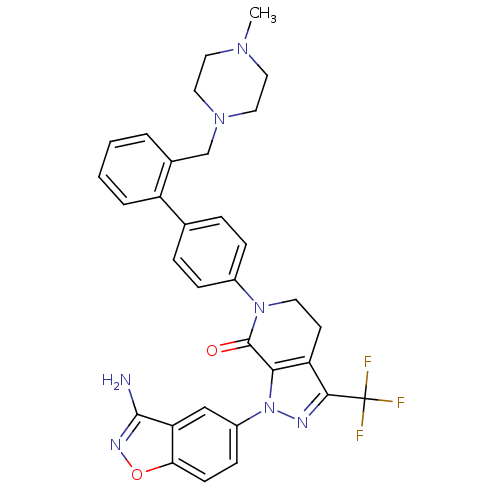 (1-(3-amino-1,2-benzoxazol-5-yl)-6-(4-{2-[(4-methyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.710 | -51.7 | n/a | n/a | n/a | n/a | n/a | 7.0 | 22 |
Bristol-Myers Squibb Pharmaceutical Research Institute | Assay Description Ki values were obtained from human purified enzyme. All assays were run in microtiter plates. Plates were read for 30 min at 405 nm. Rates were deter... | Bioorg Med Chem Lett 16: 4141-7 (2006) Article DOI: 10.1016/j.bmcl.2006.02.069 BindingDB Entry DOI: 10.7270/Q26T0JW1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 234 total ) | Next | Last >> |