 Found 828 hits with Last Name = 'chiarparin' and Initial = 'e'
Found 828 hits with Last Name = 'chiarparin' and Initial = 'e' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Orexin receptor type 2
(Rattus norvegicus (Rat)) | BDBM50417257

(SB-649868)Show SMILES Cc1nc(C(=O)N2CCCC[C@H]2CNC(=O)c2cccc3occc23)c(s1)-c1ccc(F)cc1 |r| Show InChI InChI=1S/C26H24FN3O3S/c1-16-29-23(24(34-16)17-8-10-18(27)11-9-17)26(32)30-13-3-2-5-19(30)15-28-25(31)21-6-4-7-22-20(21)12-14-33-22/h4,6-12,14,19H,2-3,5,13,15H2,1H3,(H,28,31)/t19-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0776 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicine Research Centre
Curated by ChEMBL
| Assay Description
Antagonist activity at recombinant rat OX2R expressed in CHO cells by FLIPR calcium based functional assay |
Bioorg Med Chem Lett 21: 5562-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.086
BindingDB Entry DOI: 10.7270/Q29K4CGM |
More data for this
Ligand-Target Pair | |
Orexin/Hypocretin receptor type 1
(Rattus norvegicus (Rat)) | BDBM50417257

(SB-649868)Show SMILES Cc1nc(C(=O)N2CCCC[C@H]2CNC(=O)c2cccc3occc23)c(s1)-c1ccc(F)cc1 |r| Show InChI InChI=1S/C26H24FN3O3S/c1-16-29-23(24(34-16)17-8-10-18(27)11-9-17)26(32)30-13-3-2-5-19(30)15-28-25(31)21-6-4-7-22-20(21)12-14-33-22/h4,6-12,14,19H,2-3,5,13,15H2,1H3,(H,28,31)/t19-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.117 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicine Research Centre
Curated by ChEMBL
| Assay Description
Antagonist activity at recombinant rat OX1R expressed in CHO cells by FLIPR calcium based functional assay |
Bioorg Med Chem Lett 21: 5562-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.086
BindingDB Entry DOI: 10.7270/Q29K4CGM |
More data for this
Ligand-Target Pair | |
Orexin/Hypocretin receptor type 1
(Homo sapiens (Human)) | BDBM50419142
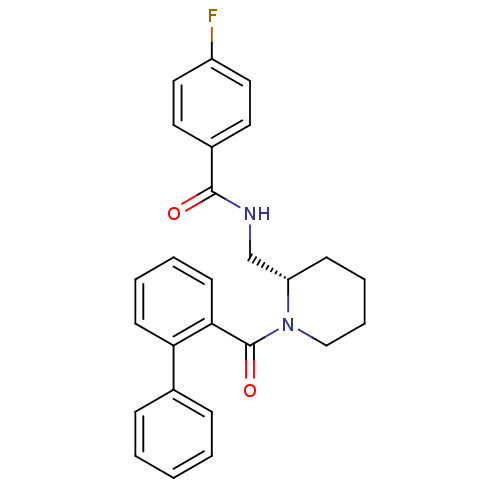
(CHEMBL1830963)Show SMILES Fc1ccc(cc1)C(=O)NC[C@@H]1CCCCN1C(=O)c1ccccc1-c1ccccc1 |r| Show InChI InChI=1S/C26H25FN2O2/c27-21-15-13-20(14-16-21)25(30)28-18-22-10-6-7-17-29(22)26(31)24-12-5-4-11-23(24)19-8-2-1-3-9-19/h1-5,8-9,11-16,22H,6-7,10,17-18H2,(H,28,30)/t22-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.126 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicine Research Centre
Curated by ChEMBL
| Assay Description
Antagonist activity at recombinant human OX1R expressed in CHO cells by FLIPR calcium based functional assay |
Bioorg Med Chem Lett 21: 5562-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.086
BindingDB Entry DOI: 10.7270/Q29K4CGM |
More data for this
Ligand-Target Pair | |
Orexin/Hypocretin receptor type 1
(Homo sapiens (Human)) | BDBM50419136
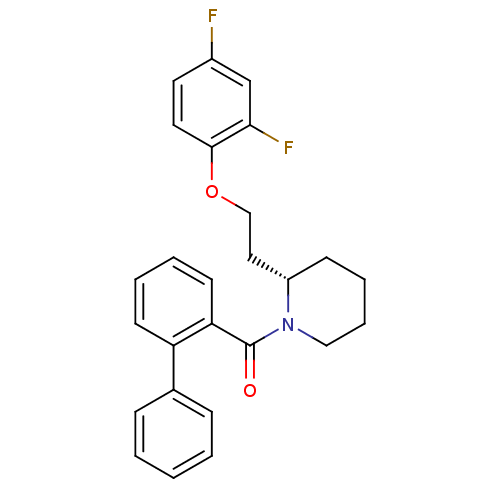
(CHEMBL1830961)Show SMILES Fc1ccc(OCC[C@@H]2CCCCN2C(=O)c2ccccc2-c2ccccc2)c(F)c1 |r| Show InChI InChI=1S/C26H25F2NO2/c27-20-13-14-25(24(28)18-20)31-17-15-21-10-6-7-16-29(21)26(30)23-12-5-4-11-22(23)19-8-2-1-3-9-19/h1-5,8-9,11-14,18,21H,6-7,10,15-17H2/t21-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.158 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicine Research Centre
Curated by ChEMBL
| Assay Description
Antagonist activity at recombinant human OX1R expressed in CHO cells by FLIPR calcium based functional assay |
Bioorg Med Chem Lett 21: 5562-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.086
BindingDB Entry DOI: 10.7270/Q29K4CGM |
More data for this
Ligand-Target Pair | |
Orexin receptor type 2
(Homo sapiens (Human)) | BDBM50419142

(CHEMBL1830963)Show SMILES Fc1ccc(cc1)C(=O)NC[C@@H]1CCCCN1C(=O)c1ccccc1-c1ccccc1 |r| Show InChI InChI=1S/C26H25FN2O2/c27-21-15-13-20(14-16-21)25(30)28-18-22-10-6-7-17-29(22)26(31)24-12-5-4-11-23(24)19-8-2-1-3-9-19/h1-5,8-9,11-16,22H,6-7,10,17-18H2,(H,28,30)/t22-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.251 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicine Research Centre
Curated by ChEMBL
| Assay Description
Antagonist activity at recombinant human OX2R expressed in CHO cells by FLIPR calcium based functional assay |
Bioorg Med Chem Lett 21: 5562-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.086
BindingDB Entry DOI: 10.7270/Q29K4CGM |
More data for this
Ligand-Target Pair | |
Orexin/Hypocretin receptor type 1
(Homo sapiens (Human)) | BDBM50417257

(SB-649868)Show SMILES Cc1nc(C(=O)N2CCCC[C@H]2CNC(=O)c2cccc3occc23)c(s1)-c1ccc(F)cc1 |r| Show InChI InChI=1S/C26H24FN3O3S/c1-16-29-23(24(34-16)17-8-10-18(27)11-9-17)26(32)30-13-3-2-5-19(30)15-28-25(31)21-6-4-7-22-20(21)12-14-33-22/h4,6-12,14,19H,2-3,5,13,15H2,1H3,(H,28,31)/t19-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.316 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicine Research Centre
Curated by ChEMBL
| Assay Description
Antagonist activity at recombinant human OX1R expressed in CHO cells by FLIPR calcium based functional assay |
Bioorg Med Chem Lett 21: 5562-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.086
BindingDB Entry DOI: 10.7270/Q29K4CGM |
More data for this
Ligand-Target Pair | |
Orexin receptor type 2
(Homo sapiens (Human)) | BDBM50417257

(SB-649868)Show SMILES Cc1nc(C(=O)N2CCCC[C@H]2CNC(=O)c2cccc3occc23)c(s1)-c1ccc(F)cc1 |r| Show InChI InChI=1S/C26H24FN3O3S/c1-16-29-23(24(34-16)17-8-10-18(27)11-9-17)26(32)30-13-3-2-5-19(30)15-28-25(31)21-6-4-7-22-20(21)12-14-33-22/h4,6-12,14,19H,2-3,5,13,15H2,1H3,(H,28,31)/t19-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.398 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicine Research Centre
Curated by ChEMBL
| Assay Description
Antagonist activity at recombinant human OX2R expressed in CHO cells by FLIPR calcium based functional assay |
Bioorg Med Chem Lett 21: 5562-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.086
BindingDB Entry DOI: 10.7270/Q29K4CGM |
More data for this
Ligand-Target Pair | |
Induced myeloid leukemia cell differentiation protein Mcl-1
(Homo sapiens (Human)) | BDBM50508937

(CHEMBL4448046)Show SMILES Cn1nc2CSCc3nn(C)c(Cl)c3-c3c(Cl)ccc4c(CCCOc5cc(SCc1c2)cc1ccccc51)c(C(O)=O)n(C)c34 Show InChI InChI=1S/C34H31Cl2N5O3S2/c1-39-31-25-10-11-26(35)29(31)30-27(38-41(3)33(30)36)18-45-16-20-14-21(40(2)37-20)17-46-22-13-19-7-4-5-8-23(19)28(15-22)44-12-6-9-24(25)32(39)34(42)43/h4-5,7-8,10-11,13-15H,6,9,12,16-18H2,1-3H3,(H,42,43) | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of Mcl1 (unknown origin) by TR-FRET assay |
J Med Chem 62: 9418-9437 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00716
BindingDB Entry DOI: 10.7270/Q2PV6PNH |
More data for this
Ligand-Target Pair | |
Orexin/Hypocretin receptor type 1
(Homo sapiens (Human)) | BDBM50417257

(SB-649868)Show SMILES Cc1nc(C(=O)N2CCCC[C@H]2CNC(=O)c2cccc3occc23)c(s1)-c1ccc(F)cc1 |r| Show InChI InChI=1S/C26H24FN3O3S/c1-16-29-23(24(34-16)17-8-10-18(27)11-9-17)26(32)30-13-3-2-5-19(30)15-28-25(31)21-6-4-7-22-20(21)12-14-33-22/h4,6-12,14,19H,2-3,5,13,15H2,1H3,(H,28,31)/t19-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.759 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicine Research Centre
Curated by ChEMBL
| Assay Description
Displacement of [3H]almorexant from recombinant human OX1R expressed in CHO cells |
Bioorg Med Chem Lett 21: 5562-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.086
BindingDB Entry DOI: 10.7270/Q29K4CGM |
More data for this
Ligand-Target Pair | |
Orexin receptor type 2
(Homo sapiens (Human)) | BDBM50419136

(CHEMBL1830961)Show SMILES Fc1ccc(OCC[C@@H]2CCCCN2C(=O)c2ccccc2-c2ccccc2)c(F)c1 |r| Show InChI InChI=1S/C26H25F2NO2/c27-20-13-14-25(24(28)18-20)31-17-15-21-10-6-7-16-29(21)26(30)23-12-5-4-11-22(23)19-8-2-1-3-9-19/h1-5,8-9,11-14,18,21H,6-7,10,15-17H2/t21-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.794 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicine Research Centre
Curated by ChEMBL
| Assay Description
Antagonist activity at recombinant human OX2R expressed in CHO cells by FLIPR calcium based functional assay |
Bioorg Med Chem Lett 21: 5562-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.086
BindingDB Entry DOI: 10.7270/Q29K4CGM |
More data for this
Ligand-Target Pair | |
Orexin/Hypocretin receptor type 1
(Homo sapiens (Human)) | BDBM50419131
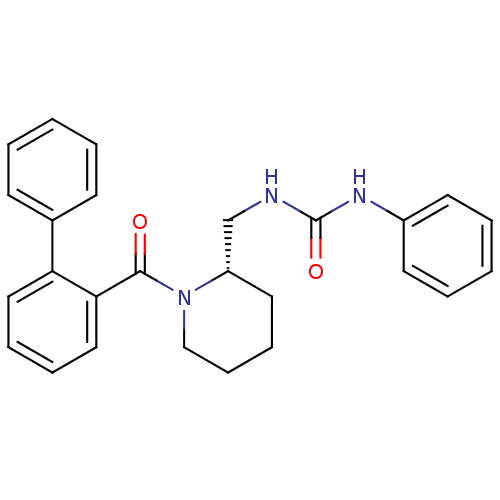
(CHEMBL1830968)Show SMILES O=C(NC[C@@H]1CCCCN1C(=O)c1ccccc1-c1ccccc1)Nc1ccccc1 |r| Show InChI InChI=1S/C26H27N3O2/c30-25(24-17-8-7-16-23(24)20-11-3-1-4-12-20)29-18-10-9-15-22(29)19-27-26(31)28-21-13-5-2-6-14-21/h1-8,11-14,16-17,22H,9-10,15,18-19H2,(H2,27,28,31)/t22-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicine Research Centre
Curated by ChEMBL
| Assay Description
Antagonist activity at recombinant human OX1R expressed in CHO cells by FLIPR calcium based functional assay |
Bioorg Med Chem Lett 21: 5562-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.086
BindingDB Entry DOI: 10.7270/Q29K4CGM |
More data for this
Ligand-Target Pair | |
Orexin receptor type 2
(Homo sapiens (Human)) | BDBM50417257

(SB-649868)Show SMILES Cc1nc(C(=O)N2CCCC[C@H]2CNC(=O)c2cccc3occc23)c(s1)-c1ccc(F)cc1 |r| Show InChI InChI=1S/C26H24FN3O3S/c1-16-29-23(24(34-16)17-8-10-18(27)11-9-17)26(32)30-13-3-2-5-19(30)15-28-25(31)21-6-4-7-22-20(21)12-14-33-22/h4,6-12,14,19H,2-3,5,13,15H2,1H3,(H,28,31)/t19-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.29 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicine Research Centre
Curated by ChEMBL
| Assay Description
Displacement of [3H]almorexant from recombinant human OX2R expressed in CHO cells |
Bioorg Med Chem Lett 21: 5562-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.086
BindingDB Entry DOI: 10.7270/Q29K4CGM |
More data for this
Ligand-Target Pair | |
Orexin receptor type 2
(Homo sapiens (Human)) | BDBM50419131

(CHEMBL1830968)Show SMILES O=C(NC[C@@H]1CCCCN1C(=O)c1ccccc1-c1ccccc1)Nc1ccccc1 |r| Show InChI InChI=1S/C26H27N3O2/c30-25(24-17-8-7-16-23(24)20-11-3-1-4-12-20)29-18-10-9-15-22(29)19-27-26(31)28-21-13-5-2-6-14-21/h1-8,11-14,16-17,22H,9-10,15,18-19H2,(H2,27,28,31)/t22-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 5.01 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicine Research Centre
Curated by ChEMBL
| Assay Description
Antagonist activity at recombinant human OX2R expressed in CHO cells by FLIPR calcium based functional assay |
Bioorg Med Chem Lett 21: 5562-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.086
BindingDB Entry DOI: 10.7270/Q29K4CGM |
More data for this
Ligand-Target Pair | |
Orexin/Hypocretin receptor type 1
(Homo sapiens (Human)) | BDBM50419137
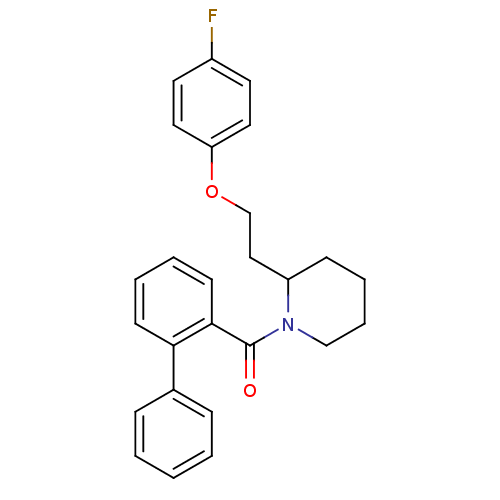
(CHEMBL1830959)Show SMILES Fc1ccc(OCCC2CCCCN2C(=O)c2ccccc2-c2ccccc2)cc1 Show InChI InChI=1S/C26H26FNO2/c27-21-13-15-23(16-14-21)30-19-17-22-10-6-7-18-28(22)26(29)25-12-5-4-11-24(25)20-8-2-1-3-9-20/h1-5,8-9,11-16,22H,6-7,10,17-19H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.01 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicine Research Centre
Curated by ChEMBL
| Assay Description
Antagonist activity at recombinant human OX1R expressed in CHO cells by FLIPR calcium based functional assay |
Bioorg Med Chem Lett 21: 5562-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.086
BindingDB Entry DOI: 10.7270/Q29K4CGM |
More data for this
Ligand-Target Pair | |
Orexin/Hypocretin receptor type 1
(Homo sapiens (Human)) | BDBM50419140
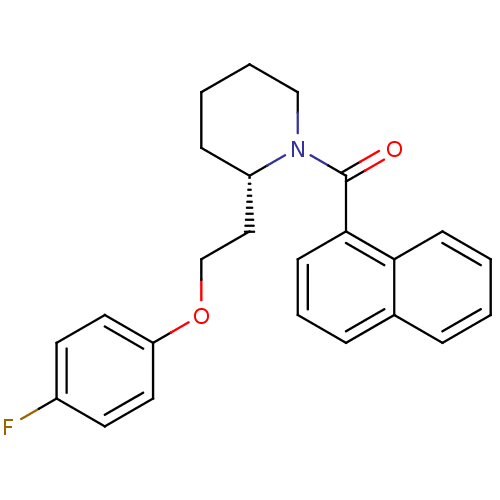
(CHEMBL1830960)Show SMILES Fc1ccc(OCC[C@@H]2CCCCN2C(=O)c2cccc3ccccc23)cc1 |r| Show InChI InChI=1S/C24H24FNO2/c25-19-11-13-21(14-12-19)28-17-15-20-8-3-4-16-26(20)24(27)23-10-5-7-18-6-1-2-9-22(18)23/h1-2,5-7,9-14,20H,3-4,8,15-17H2/t20-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 6.31 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicine Research Centre
Curated by ChEMBL
| Assay Description
Antagonist activity at recombinant human OX1R expressed in CHO cells by FLIPR calcium based functional assay |
Bioorg Med Chem Lett 21: 5562-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.086
BindingDB Entry DOI: 10.7270/Q29K4CGM |
More data for this
Ligand-Target Pair | |
Orexin/Hypocretin receptor type 1
(Homo sapiens (Human)) | BDBM50419135
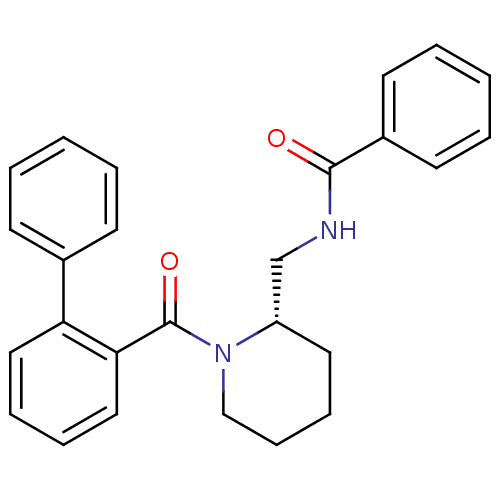
(CHEMBL1830962)Show SMILES O=C(NC[C@@H]1CCCCN1C(=O)c1ccccc1-c1ccccc1)c1ccccc1 |r| Show InChI InChI=1S/C26H26N2O2/c29-25(21-13-5-2-6-14-21)27-19-22-15-9-10-18-28(22)26(30)24-17-8-7-16-23(24)20-11-3-1-4-12-20/h1-8,11-14,16-17,22H,9-10,15,18-19H2,(H,27,29)/t22-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7.94 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicine Research Centre
Curated by ChEMBL
| Assay Description
Antagonist activity at recombinant human OX1R expressed in CHO cells by FLIPR calcium based functional assay |
Bioorg Med Chem Lett 21: 5562-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.086
BindingDB Entry DOI: 10.7270/Q29K4CGM |
More data for this
Ligand-Target Pair | |
Orexin/Hypocretin receptor type 1
(Homo sapiens (Human)) | BDBM50419132
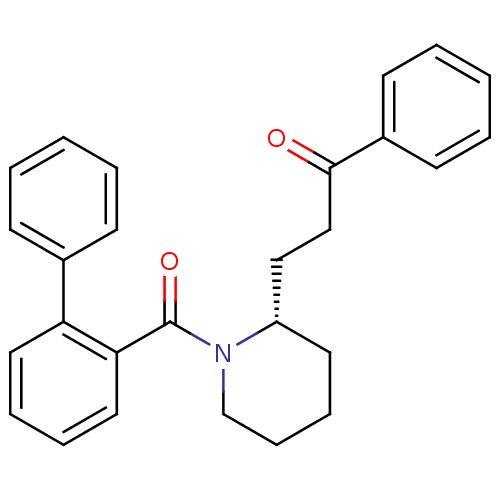
(CHEMBL1830967)Show SMILES O=C(CC[C@@H]1CCCCN1C(=O)c1ccccc1-c1ccccc1)c1ccccc1 |r| Show InChI InChI=1S/C27H27NO2/c29-26(22-13-5-2-6-14-22)19-18-23-15-9-10-20-28(23)27(30)25-17-8-7-16-24(25)21-11-3-1-4-12-21/h1-8,11-14,16-17,23H,9-10,15,18-20H2/t23-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicine Research Centre
Curated by ChEMBL
| Assay Description
Antagonist activity at recombinant human OX1R expressed in CHO cells by FLIPR calcium based functional assay |
Bioorg Med Chem Lett 21: 5562-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.086
BindingDB Entry DOI: 10.7270/Q29K4CGM |
More data for this
Ligand-Target Pair | |
Orexin receptor type 2
(Homo sapiens (Human)) | BDBM50419137

(CHEMBL1830959)Show SMILES Fc1ccc(OCCC2CCCCN2C(=O)c2ccccc2-c2ccccc2)cc1 Show InChI InChI=1S/C26H26FNO2/c27-21-13-15-23(16-14-21)30-19-17-22-10-6-7-18-28(22)26(29)25-12-5-4-11-24(25)20-8-2-1-3-9-20/h1-5,8-9,11-16,22H,6-7,10,17-19H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 19.9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicine Research Centre
Curated by ChEMBL
| Assay Description
Antagonist activity at recombinant human OX2R expressed in CHO cells by FLIPR calcium based functional assay |
Bioorg Med Chem Lett 21: 5562-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.086
BindingDB Entry DOI: 10.7270/Q29K4CGM |
More data for this
Ligand-Target Pair | |
Orexin receptor type 2
(Homo sapiens (Human)) | BDBM50419135

(CHEMBL1830962)Show SMILES O=C(NC[C@@H]1CCCCN1C(=O)c1ccccc1-c1ccccc1)c1ccccc1 |r| Show InChI InChI=1S/C26H26N2O2/c29-25(21-13-5-2-6-14-21)27-19-22-15-9-10-18-28(22)26(30)24-17-8-7-16-23(24)20-11-3-1-4-12-20/h1-8,11-14,16-17,22H,9-10,15,18-19H2,(H,27,29)/t22-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 19.9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicine Research Centre
Curated by ChEMBL
| Assay Description
Antagonist activity at recombinant human OX2R expressed in CHO cells by FLIPR calcium based functional assay |
Bioorg Med Chem Lett 21: 5562-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.086
BindingDB Entry DOI: 10.7270/Q29K4CGM |
More data for this
Ligand-Target Pair | |
Orexin receptor type 2
(Homo sapiens (Human)) | BDBM50419132

(CHEMBL1830967)Show SMILES O=C(CC[C@@H]1CCCCN1C(=O)c1ccccc1-c1ccccc1)c1ccccc1 |r| Show InChI InChI=1S/C27H27NO2/c29-26(22-13-5-2-6-14-22)19-18-23-15-9-10-20-28(23)27(30)25-17-8-7-16-24(25)21-11-3-1-4-12-21/h1-8,11-14,16-17,23H,9-10,15,18-20H2/t23-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 19.9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicine Research Centre
Curated by ChEMBL
| Assay Description
Antagonist activity at recombinant human OX2R expressed in CHO cells by FLIPR calcium based functional assay |
Bioorg Med Chem Lett 21: 5562-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.086
BindingDB Entry DOI: 10.7270/Q29K4CGM |
More data for this
Ligand-Target Pair | |
Orexin receptor type 2
(Homo sapiens (Human)) | BDBM50419139
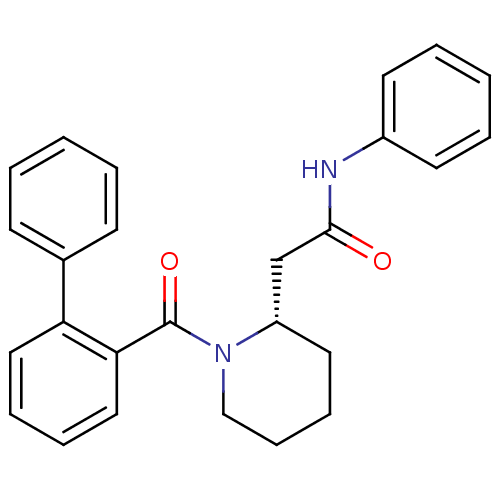
(CHEMBL1830966)Show SMILES O=C(C[C@@H]1CCCCN1C(=O)c1ccccc1-c1ccccc1)Nc1ccccc1 |r| Show InChI InChI=1S/C26H26N2O2/c29-25(27-21-13-5-2-6-14-21)19-22-15-9-10-18-28(22)26(30)24-17-8-7-16-23(24)20-11-3-1-4-12-20/h1-8,11-14,16-17,22H,9-10,15,18-19H2,(H,27,29)/t22-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 31.6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicine Research Centre
Curated by ChEMBL
| Assay Description
Antagonist activity at recombinant human OX2R expressed in CHO cells by FLIPR calcium based functional assay |
Bioorg Med Chem Lett 21: 5562-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.086
BindingDB Entry DOI: 10.7270/Q29K4CGM |
More data for this
Ligand-Target Pair | |
Orexin receptor type 2
(Homo sapiens (Human)) | BDBM50419134
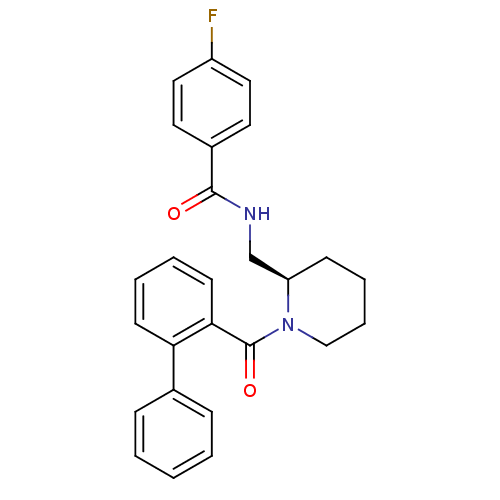
(CHEMBL1830964)Show SMILES Fc1ccc(cc1)C(=O)NC[C@H]1CCCCN1C(=O)c1ccccc1-c1ccccc1 |r| Show InChI InChI=1S/C26H25FN2O2/c27-21-15-13-20(14-16-21)25(30)28-18-22-10-6-7-17-29(22)26(31)24-12-5-4-11-23(24)19-8-2-1-3-9-19/h1-5,8-9,11-16,22H,6-7,10,17-18H2,(H,28,30)/t22-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 31.6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicine Research Centre
Curated by ChEMBL
| Assay Description
Antagonist activity at recombinant human OX2R expressed in CHO cells by FLIPR calcium based functional assay |
Bioorg Med Chem Lett 21: 5562-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.086
BindingDB Entry DOI: 10.7270/Q29K4CGM |
More data for this
Ligand-Target Pair | |
Orexin/Hypocretin receptor type 1
(Homo sapiens (Human)) | BDBM50419134

(CHEMBL1830964)Show SMILES Fc1ccc(cc1)C(=O)NC[C@H]1CCCCN1C(=O)c1ccccc1-c1ccccc1 |r| Show InChI InChI=1S/C26H25FN2O2/c27-21-15-13-20(14-16-21)25(30)28-18-22-10-6-7-17-29(22)26(31)24-12-5-4-11-23(24)19-8-2-1-3-9-19/h1-5,8-9,11-16,22H,6-7,10,17-18H2,(H,28,30)/t22-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 39.8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicine Research Centre
Curated by ChEMBL
| Assay Description
Antagonist activity at recombinant human OX1R expressed in CHO cells by FLIPR calcium based functional assay |
Bioorg Med Chem Lett 21: 5562-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.086
BindingDB Entry DOI: 10.7270/Q29K4CGM |
More data for this
Ligand-Target Pair | |
Induced myeloid leukemia cell differentiation protein Mcl-1
(Homo sapiens (Human)) | BDBM50508939

(CHEMBL4443085)Show SMILES Cc1cc(CSCc2nn(C)c(C)c2-c2cccc3c(CCCOc4cccc5ccccc45)c([nH]c23)C(O)=O)nn1C |(21.19,-26.33,;22.68,-25.93,;23.37,-24.56,;24.9,-24.79,;25.99,-23.71,;27.47,-24.11,;28.56,-23.02,;30.05,-23.42,;30.52,-24.88,;32.06,-24.88,;32.84,-26.21,;32.54,-23.42,;34.03,-23.02,;31.29,-22.51,;31.29,-20.97,;29.96,-20.21,;29.96,-18.67,;31.29,-17.89,;32.62,-18.66,;34.09,-18.19,;34.49,-16.7,;35.97,-16.3,;36.37,-14.82,;37.86,-14.42,;38.26,-12.93,;37.17,-11.83,;37.57,-10.35,;39.06,-9.95,;40.14,-11.03,;41.62,-10.63,;42.72,-11.71,;42.34,-13.2,;40.85,-13.61,;39.75,-12.52,;34.99,-19.43,;34.09,-20.68,;32.62,-20.2,;36.53,-19.43,;37.3,-20.76,;37.3,-18.1,;25.13,-26.32,;23.76,-27.02,;23.36,-28.5,)| Show InChI InChI=1S/C34H35N5O3S/c1-21-18-24(36-38(21)3)19-43-20-29-31(22(2)39(4)37-29)28-14-8-13-26-27(33(34(40)41)35-32(26)28)15-9-17-42-30-16-7-11-23-10-5-6-12-25(23)30/h5-8,10-14,16,18,35H,9,15,17,19-20H2,1-4H3,(H,40,41) | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 42 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of Mcl1 (unknown origin) by TR-FRET assay |
J Med Chem 62: 9418-9437 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00716
BindingDB Entry DOI: 10.7270/Q2PV6PNH |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Induced myeloid leukemia cell differentiation protein Mcl-1
(Homo sapiens (Human)) | BDBM50508950

(CHEMBL4472439)Show SMILES C[C@H]1CCN(C)C(=O)C[C@@H](Cc2ccc(Cl)c(Cl)c2)NC(=O)CN2c3ccccc3CC[C@H](NC(=O)C[C@H](Cc3ccc(Cl)cc3)N1)C2=O |r| Show InChI InChI=1S/C37H42Cl3N5O4/c1-23-15-16-44(2)36(48)21-29(18-25-9-13-30(39)31(40)19-25)42-35(47)22-45-33-6-4-3-5-26(33)10-14-32(37(45)49)43-34(46)20-28(41-23)17-24-7-11-27(38)12-8-24/h3-9,11-13,19,23,28-29,32,41H,10,14-18,20-22H2,1-2H3,(H,42,47)(H,43,46)/t23-,28-,29+,32-/m0/s1 | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 48 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of Mcl1 (unknown origin) by TR-FRET assay |
J Med Chem 62: 9418-9437 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00716
BindingDB Entry DOI: 10.7270/Q2PV6PNH |
More data for this
Ligand-Target Pair | |
Orexin/Hypocretin receptor type 1
(Homo sapiens (Human)) | BDBM50419139

(CHEMBL1830966)Show SMILES O=C(C[C@@H]1CCCCN1C(=O)c1ccccc1-c1ccccc1)Nc1ccccc1 |r| Show InChI InChI=1S/C26H26N2O2/c29-25(27-21-13-5-2-6-14-21)19-22-15-9-10-18-28(22)26(30)24-17-8-7-16-23(24)20-11-3-1-4-12-20/h1-8,11-14,16-17,22H,9-10,15,18-19H2,(H,27,29)/t22-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 50.1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicine Research Centre
Curated by ChEMBL
| Assay Description
Antagonist activity at recombinant human OX1R expressed in CHO cells by FLIPR calcium based functional assay |
Bioorg Med Chem Lett 21: 5562-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.086
BindingDB Entry DOI: 10.7270/Q29K4CGM |
More data for this
Ligand-Target Pair | |
Orexin receptor type 2
(Homo sapiens (Human)) | BDBM50419140

(CHEMBL1830960)Show SMILES Fc1ccc(OCC[C@@H]2CCCCN2C(=O)c2cccc3ccccc23)cc1 |r| Show InChI InChI=1S/C24H24FNO2/c25-19-11-13-21(14-12-19)28-17-15-20-8-3-4-16-26(20)24(27)23-10-5-7-18-6-1-2-9-22(18)23/h1-2,5-7,9-14,20H,3-4,8,15-17H2/t20-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 126 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicine Research Centre
Curated by ChEMBL
| Assay Description
Antagonist activity at recombinant human OX2R expressed in CHO cells by FLIPR calcium based functional assay |
Bioorg Med Chem Lett 21: 5562-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.086
BindingDB Entry DOI: 10.7270/Q29K4CGM |
More data for this
Ligand-Target Pair | |
Orexin/Hypocretin receptor type 1
(Homo sapiens (Human)) | BDBM50419130

(CHEMBL1830956)Show SMILES COc1cccc(c1)C(=O)N1CCCCC1CCOc1ccc(Cl)c(Cl)c1 Show InChI InChI=1S/C21H23Cl2NO3/c1-26-17-7-4-5-15(13-17)21(25)24-11-3-2-6-16(24)10-12-27-18-8-9-19(22)20(23)14-18/h4-5,7-9,13-14,16H,2-3,6,10-12H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 126 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicine Research Centre
Curated by ChEMBL
| Assay Description
Antagonist activity at recombinant human OX1R expressed in CHO cells by FLIPR calcium based functional assay |
Bioorg Med Chem Lett 21: 5562-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.086
BindingDB Entry DOI: 10.7270/Q29K4CGM |
More data for this
Ligand-Target Pair | |
Induced myeloid leukemia cell differentiation protein Mcl-1
(Homo sapiens (Human)) | BDBM50508947
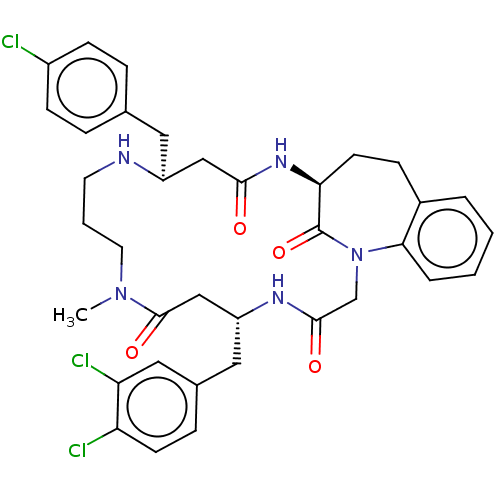
(CHEMBL4460550)Show SMILES CN1CCCN[C@@H](Cc2ccc(Cl)cc2)CC(=O)N[C@H]2CCc3ccccc3N(CC(=O)N[C@H](Cc3ccc(Cl)c(Cl)c3)CC1=O)C2=O |r| Show InChI InChI=1S/C36H40Cl3N5O4/c1-43-16-4-15-40-27(17-23-7-11-26(37)12-8-23)20-33(45)42-31-14-10-25-5-2-3-6-32(25)44(36(31)48)22-34(46)41-28(21-35(43)47)18-24-9-13-29(38)30(39)19-24/h2-3,5-9,11-13,19,27-28,31,40H,4,10,14-18,20-22H2,1H3,(H,41,46)(H,42,45)/t27-,28+,31-/m0/s1 | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 155 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of Mcl1 (unknown origin) by TR-FRET assay |
J Med Chem 62: 9418-9437 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00716
BindingDB Entry DOI: 10.7270/Q2PV6PNH |
More data for this
Ligand-Target Pair | |
Induced myeloid leukemia cell differentiation protein Mcl-1
(Homo sapiens (Human)) | BDBM50508938

(CHEMBL1984039)Show SMILES Cc1nn(C)c(C)c1-c1cccc2c(CCCOc3cccc4ccccc34)c([nH]c12)C(O)=O |(-6.44,-15.46,;-4.9,-15.46,;-3.99,-16.71,;-2.53,-16.23,;-1.28,-17.14,;-2.53,-14.69,;-1.28,-13.79,;-3.99,-14.22,;-4.47,-12.75,;-5.97,-12.43,;-6.45,-10.97,;-5.42,-9.83,;-3.91,-10.15,;-2.67,-9.24,;-2.67,-7.7,;-1.33,-6.93,;-1.33,-5.39,;,-4.62,;,-3.08,;-1.33,-2.31,;-1.33,-.77,;;1.33,-.77,;2.67,,;4,-.77,;4,-2.31,;2.67,-3.08,;1.33,-2.31,;-1.42,-10.15,;-1.9,-11.61,;-3.44,-11.61,;.04,-9.67,;1.19,-10.7,;.36,-8.16,)| Show InChI InChI=1S/C28H27N3O3/c1-17-25(18(2)31(3)30-17)23-13-7-12-21-22(27(28(32)33)29-26(21)23)14-8-16-34-24-15-6-10-19-9-4-5-11-20(19)24/h4-7,9-13,15,29H,8,14,16H2,1-3H3,(H,32,33) | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of Mcl1 (unknown origin) by TR-FRET assay |
J Med Chem 62: 9418-9437 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00716
BindingDB Entry DOI: 10.7270/Q2PV6PNH |
More data for this
Ligand-Target Pair | |
Orexin/Hypocretin receptor type 1
(Homo sapiens (Human)) | BDBM50419138
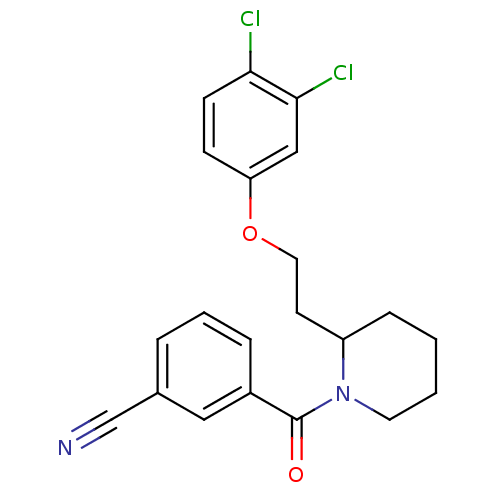
(CHEMBL1830957)Show SMILES Clc1ccc(OCCC2CCCCN2C(=O)c2cccc(c2)C#N)cc1Cl Show InChI InChI=1S/C21H20Cl2N2O2/c22-19-8-7-18(13-20(19)23)27-11-9-17-6-1-2-10-25(17)21(26)16-5-3-4-15(12-16)14-24/h3-5,7-8,12-13,17H,1-2,6,9-11H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 398 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicine Research Centre
Curated by ChEMBL
| Assay Description
Antagonist activity at recombinant human OX1R expressed in CHO cells by FLIPR calcium based functional assay |
Bioorg Med Chem Lett 21: 5562-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.086
BindingDB Entry DOI: 10.7270/Q29K4CGM |
More data for this
Ligand-Target Pair | |
Orexin receptor type 2
(Homo sapiens (Human)) | BDBM50419130

(CHEMBL1830956)Show SMILES COc1cccc(c1)C(=O)N1CCCCC1CCOc1ccc(Cl)c(Cl)c1 Show InChI InChI=1S/C21H23Cl2NO3/c1-26-17-7-4-5-15(13-17)21(25)24-11-3-2-6-16(24)10-12-27-18-8-9-19(22)20(23)14-18/h4-5,7-9,13-14,16H,2-3,6,10-12H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 501 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicine Research Centre
Curated by ChEMBL
| Assay Description
Antagonist activity at recombinant human OX2R expressed in CHO cells by FLIPR calcium based functional assay |
Bioorg Med Chem Lett 21: 5562-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.086
BindingDB Entry DOI: 10.7270/Q29K4CGM |
More data for this
Ligand-Target Pair | |
Orexin/Hypocretin receptor type 1
(Homo sapiens (Human)) | BDBM50419133
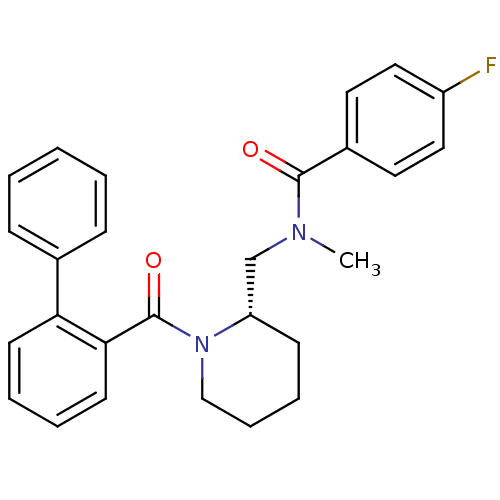
(CHEMBL1830965)Show SMILES CN(C[C@@H]1CCCCN1C(=O)c1ccccc1-c1ccccc1)C(=O)c1ccc(F)cc1 |r| Show InChI InChI=1S/C27H27FN2O2/c1-29(26(31)21-14-16-22(28)17-15-21)19-23-11-7-8-18-30(23)27(32)25-13-6-5-12-24(25)20-9-3-2-4-10-20/h2-6,9-10,12-17,23H,7-8,11,18-19H2,1H3/t23-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 501 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicine Research Centre
Curated by ChEMBL
| Assay Description
Antagonist activity at recombinant human OX1R expressed in CHO cells by FLIPR calcium based functional assay |
Bioorg Med Chem Lett 21: 5562-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.086
BindingDB Entry DOI: 10.7270/Q29K4CGM |
More data for this
Ligand-Target Pair | |
Orexin receptor type 2
(Homo sapiens (Human)) | BDBM50419138

(CHEMBL1830957)Show SMILES Clc1ccc(OCCC2CCCCN2C(=O)c2cccc(c2)C#N)cc1Cl Show InChI InChI=1S/C21H20Cl2N2O2/c22-19-8-7-18(13-20(19)23)27-11-9-17-6-1-2-10-25(17)21(26)16-5-3-4-15(12-16)14-24/h3-5,7-8,12-13,17H,1-2,6,9-11H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 631 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicine Research Centre
Curated by ChEMBL
| Assay Description
Antagonist activity at recombinant human OX2R expressed in CHO cells by FLIPR calcium based functional assay |
Bioorg Med Chem Lett 21: 5562-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.086
BindingDB Entry DOI: 10.7270/Q29K4CGM |
More data for this
Ligand-Target Pair | |
Induced myeloid leukemia cell differentiation protein Mcl-1
(Homo sapiens (Human)) | BDBM50508940
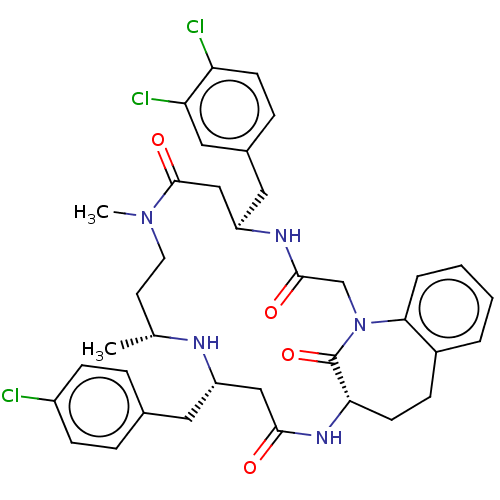
(CHEMBL4582512)Show SMILES C[C@@H]1CCN(C)C(=O)C[C@@H](Cc2ccc(Cl)c(Cl)c2)NC(=O)CN2c3ccccc3CC[C@H](NC(=O)C[C@H](Cc3ccc(Cl)cc3)N1)C2=O |r| Show InChI InChI=1S/C37H42Cl3N5O4/c1-23-15-16-44(2)36(48)21-29(18-25-9-13-30(39)31(40)19-25)42-35(47)22-45-33-6-4-3-5-26(33)10-14-32(37(45)49)43-34(46)20-28(41-23)17-24-7-11-27(38)12-8-24/h3-9,11-13,19,23,28-29,32,41H,10,14-18,20-22H2,1-2H3,(H,42,47)(H,43,46)/t23-,28+,29-,32+/m1/s1 | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 739 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of Mcl1 (unknown origin) by TR-FRET assay |
J Med Chem 62: 9418-9437 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00716
BindingDB Entry DOI: 10.7270/Q2PV6PNH |
More data for this
Ligand-Target Pair | |
Orexin receptor type 2
(Homo sapiens (Human)) | BDBM50419133

(CHEMBL1830965)Show SMILES CN(C[C@@H]1CCCCN1C(=O)c1ccccc1-c1ccccc1)C(=O)c1ccc(F)cc1 |r| Show InChI InChI=1S/C27H27FN2O2/c1-29(26(31)21-14-16-22(28)17-15-21)19-23-11-7-8-18-30(23)27(32)25-13-6-5-12-24(25)20-9-3-2-4-10-20/h2-6,9-10,12-17,23H,7-8,11,18-19H2,1H3/t23-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.26E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicine Research Centre
Curated by ChEMBL
| Assay Description
Antagonist activity at recombinant human OX2R expressed in CHO cells by FLIPR calcium based functional assay |
Bioorg Med Chem Lett 21: 5562-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.086
BindingDB Entry DOI: 10.7270/Q29K4CGM |
More data for this
Ligand-Target Pair | |
Induced myeloid leukemia cell differentiation protein Mcl-1
(Homo sapiens (Human)) | BDBM50508942
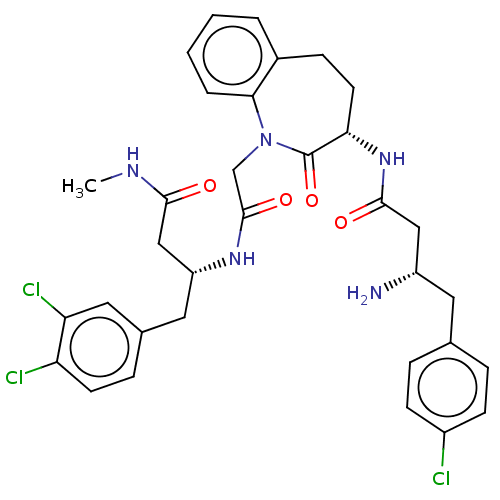
(CHEMBL4449849)Show SMILES CNC(=O)C[C@@H](Cc1ccc(Cl)c(Cl)c1)NC(=O)CN1c2ccccc2CC[C@H](NC(=O)C[C@@H](N)Cc2ccc(Cl)cc2)C1=O |r| Show InChI InChI=1S/C33H36Cl3N5O4/c1-38-30(42)18-25(15-21-8-12-26(35)27(36)16-21)39-32(44)19-41-29-5-3-2-4-22(29)9-13-28(33(41)45)40-31(43)17-24(37)14-20-6-10-23(34)11-7-20/h2-8,10-12,16,24-25,28H,9,13-15,17-19,37H2,1H3,(H,38,42)(H,39,44)(H,40,43)/t24-,25+,28-/m0/s1 | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.99E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of Mcl1 (unknown origin) by TR-FRET assay |
J Med Chem 62: 9418-9437 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00716
BindingDB Entry DOI: 10.7270/Q2PV6PNH |
More data for this
Ligand-Target Pair | |
Orexin/Hypocretin receptor type 1
(Homo sapiens (Human)) | BDBM50419141

(CHEMBL1830958)Show SMILES Clc1ccc(OCCC2CCCCN2C(=O)c2ccc(Cl)c(Cl)c2)cc1Cl Show InChI InChI=1S/C20H19Cl4NO2/c21-16-6-4-13(11-18(16)23)20(26)25-9-2-1-3-14(25)8-10-27-15-5-7-17(22)19(24)12-15/h4-7,11-12,14H,1-3,8-10H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.51E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicine Research Centre
Curated by ChEMBL
| Assay Description
Antagonist activity at recombinant human OX1R expressed in CHO cells by FLIPR calcium based functional assay |
Bioorg Med Chem Lett 21: 5562-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.086
BindingDB Entry DOI: 10.7270/Q29K4CGM |
More data for this
Ligand-Target Pair | |
Orexin receptor type 2
(Homo sapiens (Human)) | BDBM50419141

(CHEMBL1830958)Show SMILES Clc1ccc(OCCC2CCCCN2C(=O)c2ccc(Cl)c(Cl)c2)cc1Cl Show InChI InChI=1S/C20H19Cl4NO2/c21-16-6-4-13(11-18(16)23)20(26)25-9-2-1-3-14(25)8-10-27-15-5-7-17(22)19(24)12-15/h4-7,11-12,14H,1-3,8-10H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >3.16E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicine Research Centre
Curated by ChEMBL
| Assay Description
Antagonist activity at recombinant human OX2R expressed in CHO cells by FLIPR calcium based functional assay |
Bioorg Med Chem Lett 21: 5562-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.086
BindingDB Entry DOI: 10.7270/Q29K4CGM |
More data for this
Ligand-Target Pair | |
Induced myeloid leukemia cell differentiation protein Mcl-1
(Homo sapiens (Human)) | BDBM50508941

(CHEMBL4442625)Show SMILES CC1CCN[C@@H](Cc2ccc(Cl)cc2)CC(=O)N[C@H]2CCc3ccccc3N(CC(=O)N[C@H](Cc3ccc(Cl)c(Cl)c3)CC(=O)N1C)C2=O |r| Show InChI InChI=1S/C37H42Cl3N5O4/c1-23-15-16-41-28(17-24-7-11-27(38)12-8-24)20-34(46)43-32-14-10-26-5-3-4-6-33(26)45(37(32)49)22-35(47)42-29(21-36(48)44(23)2)18-25-9-13-30(39)31(40)19-25/h3-9,11-13,19,23,28-29,32,41H,10,14-18,20-22H2,1-2H3,(H,42,47)(H,43,46)/t23?,28-,29+,32-/m0/s1 | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 4.96E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of Mcl1 (unknown origin) by TR-FRET assay |
J Med Chem 62: 9418-9437 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00716
BindingDB Entry DOI: 10.7270/Q2PV6PNH |
More data for this
Ligand-Target Pair | |
Induced myeloid leukemia cell differentiation protein Mcl-1
(Homo sapiens (Human)) | BDBM50508941

(CHEMBL4442625)Show SMILES CC1CCN[C@@H](Cc2ccc(Cl)cc2)CC(=O)N[C@H]2CCc3ccccc3N(CC(=O)N[C@H](Cc3ccc(Cl)c(Cl)c3)CC(=O)N1C)C2=O |r| Show InChI InChI=1S/C37H42Cl3N5O4/c1-23-15-16-41-28(17-24-7-11-27(38)12-8-24)20-34(46)43-32-14-10-26-5-3-4-6-33(26)45(37(32)49)22-35(47)42-29(21-36(48)44(23)2)18-25-9-13-30(39)31(40)19-25/h3-9,11-13,19,23,28-29,32,41H,10,14-18,20-22H2,1-2H3,(H,42,47)(H,43,46)/t23?,28-,29+,32-/m0/s1 | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 4.96E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of Mcl1 (unknown origin) by TR-FRET assay |
J Med Chem 62: 9418-9437 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00716
BindingDB Entry DOI: 10.7270/Q2PV6PNH |
More data for this
Ligand-Target Pair | |
Induced myeloid leukemia cell differentiation protein Mcl-1
(Homo sapiens (Human)) | BDBM50508936
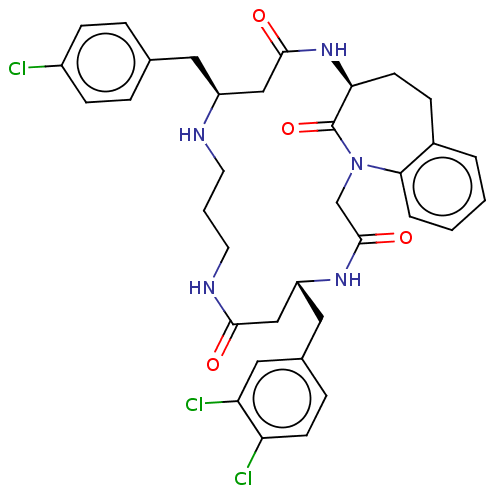
(CHEMBL4590345)Show SMILES Clc1ccc(C[C@H]2CC(=O)N[C@H]3CCc4ccccc4N(CC(=O)N[C@H](Cc4ccc(Cl)c(Cl)c4)CC(=O)NCCCN2)C3=O)cc1 |r| Show InChI InChI=1S/C35H38Cl3N5O4/c36-25-10-6-22(7-11-25)16-26-19-33(45)42-30-13-9-24-4-1-2-5-31(24)43(35(30)47)21-34(46)41-27(20-32(44)40-15-3-14-39-26)17-23-8-12-28(37)29(38)18-23/h1-2,4-8,10-12,18,26-27,30,39H,3,9,13-17,19-21H2,(H,40,44)(H,41,46)(H,42,45)/t26-,27+,30-/m0/s1 | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 5.21E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of Mcl1 (unknown origin) by TR-FRET assay |
J Med Chem 62: 9418-9437 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00716
BindingDB Entry DOI: 10.7270/Q2PV6PNH |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase XIAP
(Homo sapiens (Human)) | BDBM50239422
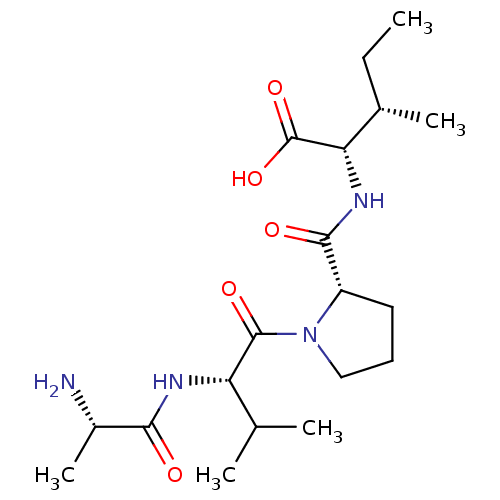
(CHEMBL234346)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@@H]1CCCN1C(=O)[C@@H](NC(=O)[C@H](C)N)C(C)C)C(O)=O |r| Show InChI InChI=1S/C19H34N4O5/c1-6-11(4)15(19(27)28)22-17(25)13-8-7-9-23(13)18(26)14(10(2)3)21-16(24)12(5)20/h10-15H,6-9,20H2,1-5H3,(H,21,24)(H,22,25)(H,27,28)/t11-,12-,13-,14-,15-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.0170 | n/a | n/a | n/a | n/a | n/a | n/a |
Astex Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of SMAC-derived peptide abuRPFK (5 and 6FAM)-amide interaction with XIAP BIR3 domain (unknown origin) by fluorescence polarization assay |
J Med Chem 60: 4611-4625 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01877
BindingDB Entry DOI: 10.7270/Q2KK9DX7 |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM300953
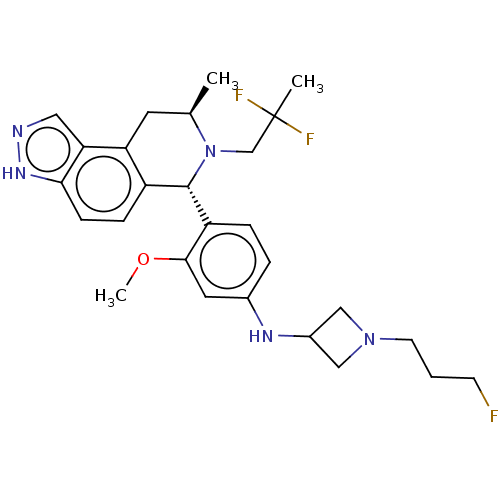
(N-(4-((6S,8R)-7-(2,2-difluoropropyl)-8-methyl-6,7,...)Show SMILES COc1cc(NC2CN(CCCF)C2)ccc1[C@H]1N(CC(C)(F)F)[C@H](C)Cc2c1ccc1[nH]ncc21 |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.0398 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Induction of ERalpha degradation in human MCF7 cells assessed as decrease in ER-alpha level incubated for 18 to 24 hrs by Alexa fluor 488/Hoechst sta... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01163
BindingDB Entry DOI: 10.7270/Q2KK9GDB |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM300962
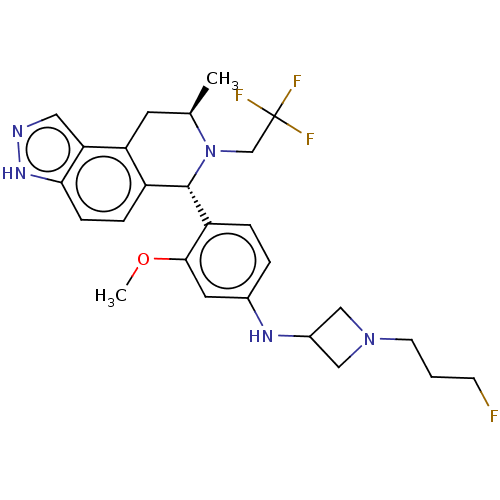
(1-(3-fluoropropyl)-N-(3-methoxy-4-((6S,8R)-8-methy...)Show SMILES COc1cc(NC2CN(CCCF)C2)ccc1[C@H]1N(CC(F)(F)F)[C@H](C)Cc2c1ccc1[nH]ncc21 |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.0631 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Induction of ERalpha degradation in human MCF7 cells assessed as decrease in ER-alpha level incubated for 18 to 24 hrs by Alexa fluor 488/Hoechst sta... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01163
BindingDB Entry DOI: 10.7270/Q2KK9GDB |
More data for this
Ligand-Target Pair | |
Baculoviral IAP repeat-containing protein 2
(Homo sapiens (Human)) | BDBM50450049

(CHEMBL4166057)Show SMILES C[C@@H]1CN(CC(=O)N2CC(C)(C)c3nc(CO)c(Cc4ccc(F)cc4)cc23)[C@@H](CN2[C@H](C)COC[C@H]2C)CN1 |r| Show InChI InChI=1S/C31H44FN5O3/c1-20-13-35(26(12-33-20)14-36-21(2)17-40-18-22(36)3)15-29(39)37-19-31(4,5)30-28(37)11-24(27(16-38)34-30)10-23-6-8-25(32)9-7-23/h6-9,11,20-22,26,33,38H,10,12-19H2,1-5H3/t20-,21-,22-,26-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Astex Pharmaceuticals
Curated by ChEMBL
| Assay Description
Induction of intracellular cIAP1 degradation in human MDA-MB-231 cells after 2 hrs |
J Med Chem 61: 7314-7329 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00900
BindingDB Entry DOI: 10.7270/Q2TT4THH |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM300955

(N-(4-((6S,8R)-7-(2,2-difluoroethyl)-8-methyl-6,7,8...)Show SMILES COc1cc(NC2CN(CCCF)C2)ccc1[C@H]1N(CC(F)F)[C@H](C)Cc2c1ccc1[nH]ncc21 |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Induction of ERalpha degradation in human MCF7 cells assessed as decrease in ER-alpha level incubated for 18 to 24 hrs by Alexa fluor 488/Hoechst sta... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01163
BindingDB Entry DOI: 10.7270/Q2KK9GDB |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM300969
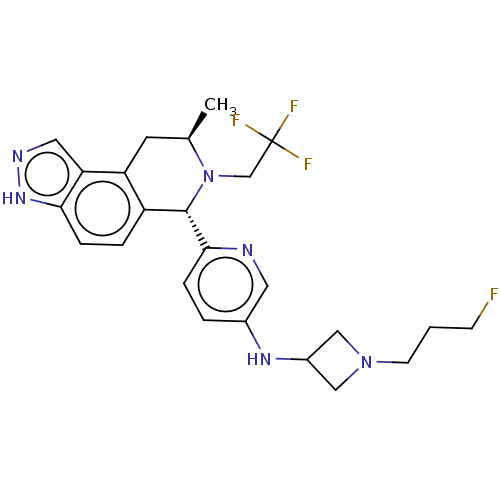
(BDBM300970 | N-(1-(3- fluoropropyl) azetidin-3-yl)...)Show SMILES C[C@@H]1Cc2c(ccc3[nH]ncc23)[C@H](N1CC(F)(F)F)c1ccc(NC2CN(CCCF)C2)cn1 |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.126 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Induction of ERalpha degradation in human MCF7 cells assessed as decrease in ERalpha expression in presence of cycloheximide by Western blot analysis |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01163
BindingDB Entry DOI: 10.7270/Q2KK9GDB |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM50548427

(CHEMBL4780809)Show SMILES C[C@@H]1Cc2c(ccc3[nH]ncc23)[C@H](N1CC(F)(F)F)c1ccc(N[C@H]2CCN(CCCF)C2)cn1 |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.126 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Induction of ERalpha degradation in human MCF7 cells assessed as decrease in ER-alpha level incubated for 18 to 24 hrs by Alexa fluor 488/Hoechst sta... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01163
BindingDB Entry DOI: 10.7270/Q2KK9GDB |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM299117

((6S,8R)-7-((l-fluorocyclopropyl)methyl)-6-(4-(2-(3...)Show SMILES COc1cc(OCCN2CC(CF)C2)ccc1[C@H]1N(CC2(F)CC2)[C@H](C)Cc2c1ccc1[nH]ncc21 |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.126 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Induction of ERalpha degradation in human MCF7 cells assessed as decrease in ER-alpha level incubated for 18 to 24 hrs by Alexa fluor 488/Hoechst sta... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01163
BindingDB Entry DOI: 10.7270/Q2KK9GDB |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data