

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM223332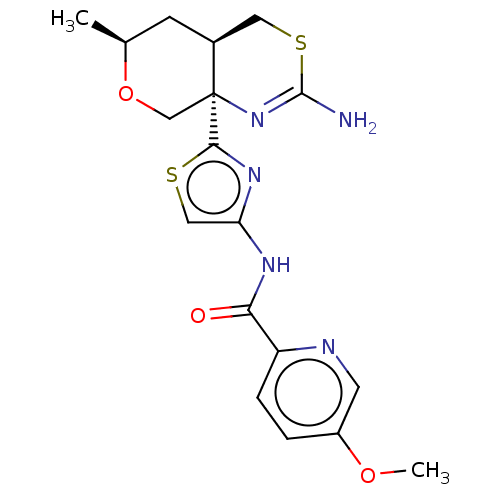 (US9315520, 19 | US9605007, Example 19 | US9744173,...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.0140 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of BACE1 in human H4 cells transfected with wild type APP assessed as reduction in soluble portion of APPbeta level in cells incubated for... | J Med Chem 61: 4476-4504 (2018) Article DOI: 10.1021/acs.jmedchem.8b00246 BindingDB Entry DOI: 10.7270/Q23J3GJ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50452875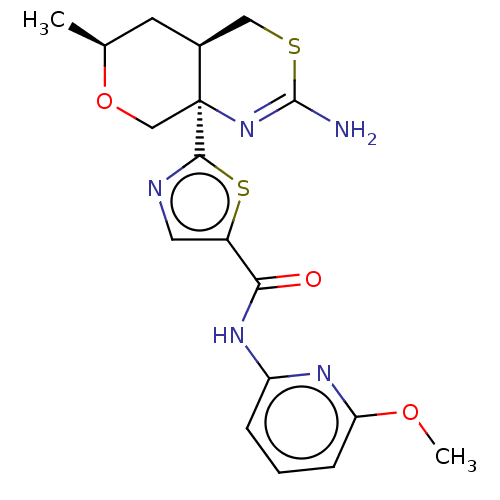 (CHEMBL4212046) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.0330 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of BACE1 in human H4 cells transfected with wild type APP assessed as reduction in soluble portion of APPbeta level in cells incubated for... | J Med Chem 61: 4476-4504 (2018) Article DOI: 10.1021/acs.jmedchem.8b00246 BindingDB Entry DOI: 10.7270/Q23J3GJ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50452884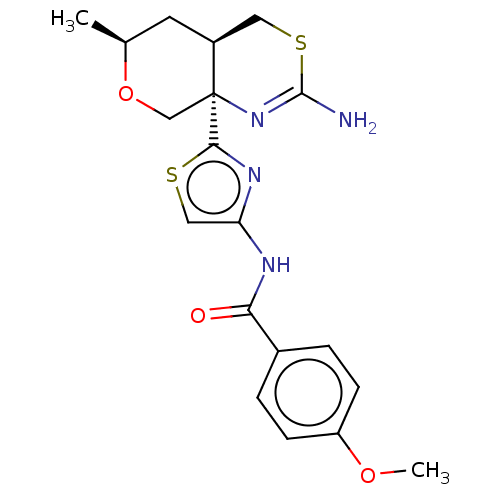 (CHEMBL4217023) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.0340 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of BACE1 in human H4 cells transfected with wild type APP assessed as reduction in soluble portion of APPbeta level in cells incubated for... | J Med Chem 61: 4476-4504 (2018) Article DOI: 10.1021/acs.jmedchem.8b00246 BindingDB Entry DOI: 10.7270/Q23J3GJ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50452883 (CHEMBL4203860) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of BACE1 in human H4 cells transfected with wild type APP assessed as reduction in soluble portion of APPbeta level in cells incubated for... | J Med Chem 61: 4476-4504 (2018) Article DOI: 10.1021/acs.jmedchem.8b00246 BindingDB Entry DOI: 10.7270/Q23J3GJ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4A [2-825] (Homo sapiens (Human)) | BDBM577094 ((7S)-10-(4-Chlorophenyl)-8-cyclopropyl-7-methyl-7,...) | PDB MMDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The PDE4A3, PDE4B1, PDE4C1 and PDE4D3 assays use the Scintillation Proximity Assay (SPA) technology to measure the inhibition of human recombinant PD... | Citation and Details BindingDB Entry DOI: 10.7270/Q2XW4P16 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4A [2-825] (Homo sapiens (Human)) | BDBM577172 (10-(4-Chloro-3-fluorophenyl)-8-cyclopropyl-3,4,7,8...) | PDB MMDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <0.310 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The PDE4A3, PDE4B1, PDE4C1 and PDE4D3 assays use the Scintillation Proximity Assay (SPA) technology to measure the inhibition of human recombinant PD... | Citation and Details BindingDB Entry DOI: 10.7270/Q2XW4P16 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 2 (Homo sapiens (Human)) | BDBM47353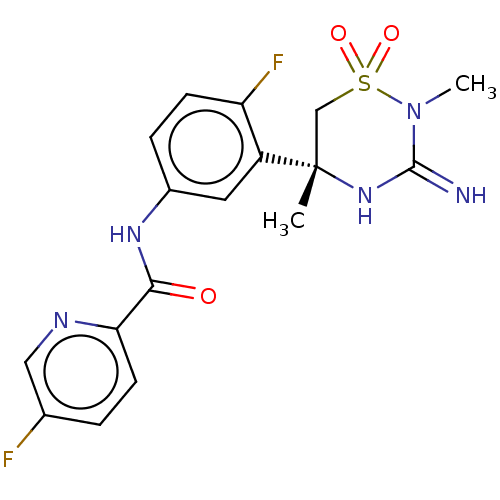 (BDBM143220 | US9029362, 173 | US9687494, 25) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.380 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human BACE2 | J Med Chem 61: 4476-4504 (2018) Article DOI: 10.1021/acs.jmedchem.8b00246 BindingDB Entry DOI: 10.7270/Q23J3GJ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4A [2-825] (Homo sapiens (Human)) | BDBM577135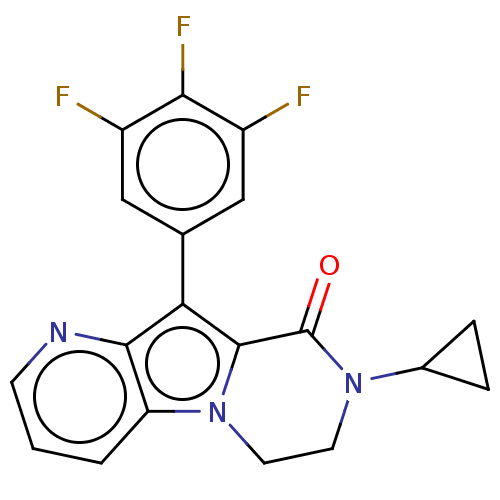 (8-Cyclopropyl-10-(3,4,5-trifluorophenyl)-7,8-dihyd...) | PDB MMDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <0.410 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The PDE4A3, PDE4B1, PDE4C1 and PDE4D3 assays use the Scintillation Proximity Assay (SPA) technology to measure the inhibition of human recombinant PD... | Citation and Details BindingDB Entry DOI: 10.7270/Q2XW4P16 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4B [122-736] (Homo sapiens (Human)) | BDBM577077 (10-(4-Chlorophenyl)-8-cyclopropyl-7,8-dihydropyrid...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <0.420 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q2XW4P16 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4A [2-825] (Homo sapiens (Human)) | BDBM577126 (10-(4-Chloro-3-fluorophenyl)-8-cyclopropyl-7,8-dih...) | PDB MMDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <0.450 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The PDE4A3, PDE4B1, PDE4C1 and PDE4D3 assays use the Scintillation Proximity Assay (SPA) technology to measure the inhibition of human recombinant PD... | Citation and Details BindingDB Entry DOI: 10.7270/Q2XW4P16 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4A (Homo sapiens (Human)) | BDBM285634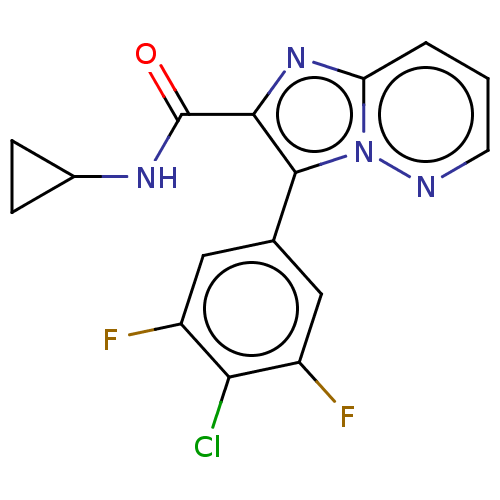 (3-(4-chloro-3,5-difluoro-phenyl)-N-cyclopropyl-imi...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <0.488 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description The PDE4A3, PDE4B1, PDE4C1 and PDE4D3 assays use the Scintillation Proximity Assay (SPA) technology to measure the inhibition of human recombinant PD... | US Patent US9598421 (2017) BindingDB Entry DOI: 10.7270/Q2K939KG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoform 4 of cAMP-specific 3',5'-cyclic phosphodiesterase 4A (RD1) (Homo sapiens (Human)) | BDBM285634 (3-(4-chloro-3,5-difluoro-phenyl)-N-cyclopropyl-imi...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <0.488 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description The PDE4A3, PDE4B1, PDE4C1 and PDE4D3 assays use the Scintillation Proximity Assay (SPA) technology to measure the inhibition of human recombinant PD... | US Patent US10077269 (2018) BindingDB Entry DOI: 10.7270/Q2P84DX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4A (Homo sapiens (Human)) | BDBM285634 (3-(4-chloro-3,5-difluoro-phenyl)-N-cyclopropyl-imi...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <0.488 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description Human PDE4A3 coding sequence (amino acids 2 to 825 from the sequence with accession number NP_001104779) was cloned into the baculovirus expression v... | US Patent US10669279 (2020) BindingDB Entry DOI: 10.7270/Q2765JC9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4B [122-736] (Homo sapiens (Human)) | BDBM577094 ((7S)-10-(4-Chlorophenyl)-8-cyclopropyl-7-methyl-7,...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <0.520 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q2XW4P16 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM143218 (US8940748, 34 | US9029362, 34 | US9687494, 34) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | <0.540 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of BACE1 in human H4 cells transfected with wild type APP assessed as reduction in soluble portion of APPbeta level in cells incubated for... | J Med Chem 61: 4476-4504 (2018) Article DOI: 10.1021/acs.jmedchem.8b00246 BindingDB Entry DOI: 10.7270/Q23J3GJ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4B [122-736] (Homo sapiens (Human)) | BDBM577172 (10-(4-Chloro-3-fluorophenyl)-8-cyclopropyl-3,4,7,8...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <0.550 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q2XW4P16 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4C [2-712] () | BDBM577094 ((7S)-10-(4-Chlorophenyl)-8-cyclopropyl-7-methyl-7,...) | PDB MMDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <0.570 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q2XW4P16 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50452874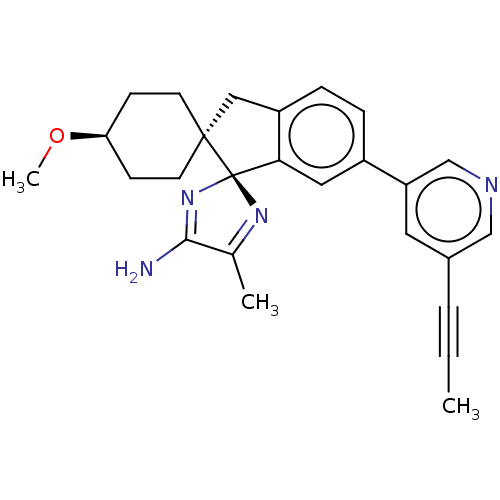 (CHEMBL4217620) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human BACE1 | J Med Chem 61: 4476-4504 (2018) Article DOI: 10.1021/acs.jmedchem.8b00246 BindingDB Entry DOI: 10.7270/Q23J3GJ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM312938 (N-{2-[(4aR,6S,8aR)-2-amino-6-methyl-4,4a,5,6-tetra...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.640 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of BACE1 in human H4 cells transfected with wild type APP assessed as reduction in soluble portion of APPbeta level in cells incubated for... | J Med Chem 61: 4476-4504 (2018) Article DOI: 10.1021/acs.jmedchem.8b00246 BindingDB Entry DOI: 10.7270/Q23J3GJ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4B [122-736] (Homo sapiens (Human)) | BDBM577128 (2-Chloro-5-(8-cyclopropyl-9-oxo-6,7,8,9-tetrahydro...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <0.640 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q2XW4P16 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4A (Homo sapiens (Human)) | BDBM285598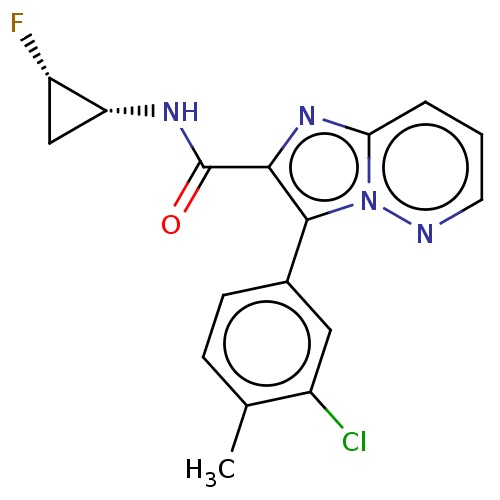 (3-(3-chloro-4-methylphenyl)-N-[(1R,2S)-2-fluorocyc...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <0.669 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description The PDE4A3, PDE4B1, PDE4C1 and PDE4D3 assays use the Scintillation Proximity Assay (SPA) technology to measure the inhibition of human recombinant PD... | US Patent US9598421 (2017) BindingDB Entry DOI: 10.7270/Q2K939KG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4A (Homo sapiens (Human)) | BDBM285598 (3-(3-chloro-4-methylphenyl)-N-[(1R,2S)-2-fluorocyc...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <0.669 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description Human PDE4A3 coding sequence (amino acids 2 to 825 from the sequence with accession number NP_001104779) was cloned into the baculovirus expression v... | US Patent US10669279 (2020) BindingDB Entry DOI: 10.7270/Q2765JC9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoform 4 of cAMP-specific 3',5'-cyclic phosphodiesterase 4A (RD1) (Homo sapiens (Human)) | BDBM285598 (3-(3-chloro-4-methylphenyl)-N-[(1R,2S)-2-fluorocyc...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <0.669 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description The PDE4A3, PDE4B1, PDE4C1 and PDE4D3 assays use the Scintillation Proximity Assay (SPA) technology to measure the inhibition of human recombinant PD... | US Patent US10077269 (2018) BindingDB Entry DOI: 10.7270/Q2P84DX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4B [122-736] (Homo sapiens (Human)) | BDBM577135 (8-Cyclopropyl-10-(3,4,5-trifluorophenyl)-7,8-dihyd...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <0.670 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q2XW4P16 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4A [2-825] (Homo sapiens (Human)) | BDBM577130 (10-(3-Chloro-4-fluorophenyl)-8-cyclopropyl-7,8-dih...) | PDB MMDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <0.670 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The PDE4A3, PDE4B1, PDE4C1 and PDE4D3 assays use the Scintillation Proximity Assay (SPA) technology to measure the inhibition of human recombinant PD... | Citation and Details BindingDB Entry DOI: 10.7270/Q2XW4P16 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4B [122-736] (Homo sapiens (Human)) | BDBM577126 (10-(4-Chloro-3-fluorophenyl)-8-cyclopropyl-7,8-dih...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q2XW4P16 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM312935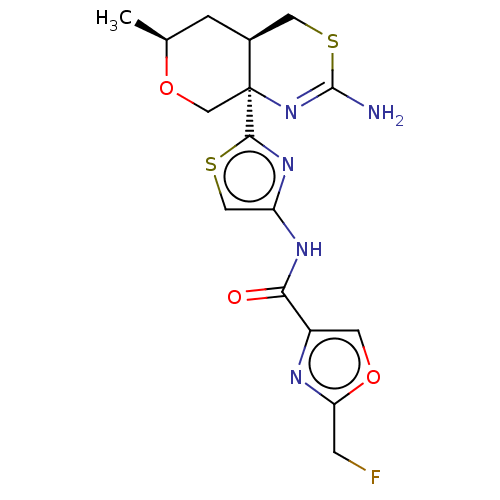 (N-{2-[(4aR,6S,8aR)-2-amino-6-methyl-4,4a,5,6-tetra...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description Beta-secretase (BACE) is one of the enzymes involved in the generation of the amyloid beta peptide found in the amyloid plaques of Alzheimer's Di... | US Patent US9605007 (2017) BindingDB Entry DOI: 10.7270/Q2JQ133S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4B [122-736] (Homo sapiens (Human)) | BDBM577143 (10-(3-Chlorophenyl)-8-cyclopropyl-7,8-dihydropyrid...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <0.720 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q2XW4P16 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4B [122-736] (Homo sapiens (Human)) | BDBM577079 (4-(8-Cyclopropyl-9-oxo-6,7,8,9-tetrahydropyrido[2&...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | US Patent | n/a | n/a | <0.810 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q2XW4P16 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4B [122-736] (Homo sapiens (Human)) | BDBM577173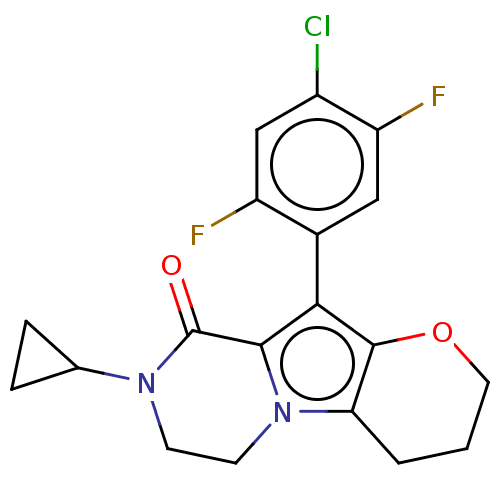 (10-(4-Chloro-2,5-difluorophenyl)-8-cyclopropyl-3,4...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.840 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q2XW4P16 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM143218 (US8940748, 34 | US9029362, 34 | US9687494, 34) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human BACE1 | J Med Chem 61: 4476-4504 (2018) Article DOI: 10.1021/acs.jmedchem.8b00246 BindingDB Entry DOI: 10.7270/Q23J3GJ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM312938 (N-{2-[(4aR,6S,8aR)-2-amino-6-methyl-4,4a,5,6-tetra...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description Beta-secretase (BACE) is one of the enzymes involved in the generation of the amyloid beta peptide found in the amyloid plaques of Alzheimer's Di... | US Patent US9605007 (2017) BindingDB Entry DOI: 10.7270/Q2JQ133S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 2 (Homo sapiens (Human)) | BDBM50452874 (CHEMBL4217620) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human BACE2 | J Med Chem 61: 4476-4504 (2018) Article DOI: 10.1021/acs.jmedchem.8b00246 BindingDB Entry DOI: 10.7270/Q23J3GJ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4A [2-825] (Homo sapiens (Human)) | BDBM577128 (2-Chloro-5-(8-cyclopropyl-9-oxo-6,7,8,9-tetrahydro...) | PDB MMDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.980 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The PDE4A3, PDE4B1, PDE4C1 and PDE4D3 assays use the Scintillation Proximity Assay (SPA) technology to measure the inhibition of human recombinant PD... | Citation and Details BindingDB Entry DOI: 10.7270/Q2XW4P16 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4A [2-825] (Homo sapiens (Human)) | BDBM577077 (10-(4-Chlorophenyl)-8-cyclopropyl-7,8-dihydropyrid...) | PDB MMDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <0.990 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The PDE4A3, PDE4B1, PDE4C1 and PDE4D3 assays use the Scintillation Proximity Assay (SPA) technology to measure the inhibition of human recombinant PD... | Citation and Details BindingDB Entry DOI: 10.7270/Q2XW4P16 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM223358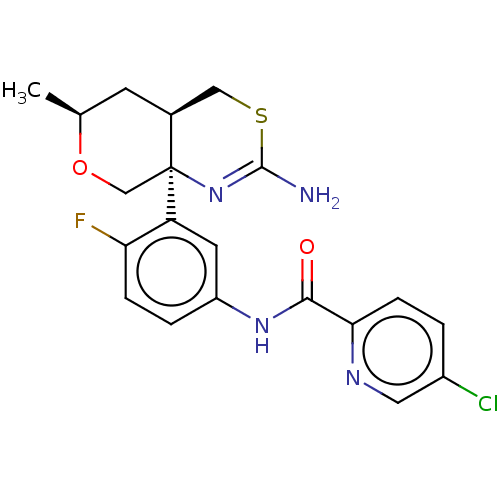 (US9315520, Comparator 7 | US9605007, Comparator 7 ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description The BACE1 and BACE2 binding assays measured beta-site amyloid precursor protein-cleaving enzyme (BACE) binding as a decrease in the counts of radioli... | US Patent US9605007 (2017) BindingDB Entry DOI: 10.7270/Q2JQ133S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoform 7 of cAMP-specific 3',5'-cyclic phosphodiesterase 4A (PDE4A8) (Homo sapiens (Human)) | BDBM399979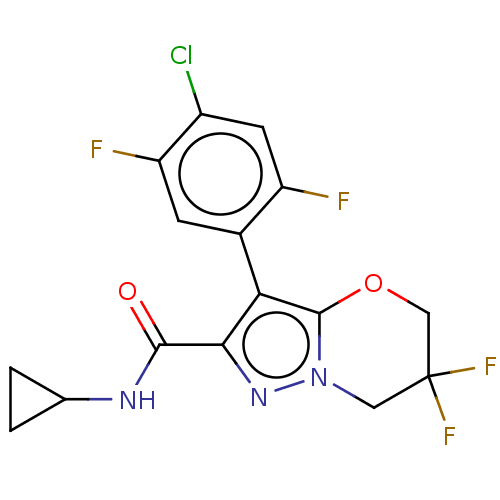 (US10323042, Example 58) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
CNR | Assay Description The PDE4A3, PDE4B1, PDE4C1 and PDE4D3 assays use the Scintillation Proximity Assay (SPA) technology to measure the inhibition of human recombinant PD... | J Med Chem 52: 5990-8 (2009) BindingDB Entry DOI: 10.7270/Q2NP26RH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4A [2-825] (Homo sapiens (Human)) | BDBM577173 (10-(4-Chloro-2,5-difluorophenyl)-8-cyclopropyl-3,4...) | PDB MMDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The PDE4A3, PDE4B1, PDE4C1 and PDE4D3 assays use the Scintillation Proximity Assay (SPA) technology to measure the inhibition of human recombinant PD... | Citation and Details BindingDB Entry DOI: 10.7270/Q2XW4P16 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM223358 (US9315520, Comparator 7 | US9605007, Comparator 7 ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description Beta-secretase (BACE) is one of the enzymes involved in the generation of the amyloid beta peptide found in the amyloid plaques of Alzheimer's Di... | US Patent US9744173 (2017) BindingDB Entry DOI: 10.7270/Q2K076CZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4A [2-825] (Homo sapiens (Human)) | BDBM457828 (US10738063, Example 58) | PDB MMDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description Human PDE4A3 coding sequence (amino acids 2 to 825 from the sequence with accession number NP_001104779) was cloned into the baculovirus expression v... | US Patent US10738063 (2020) BindingDB Entry DOI: 10.7270/Q20Z76BW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4B [122-736] (Homo sapiens (Human)) | BDBM577102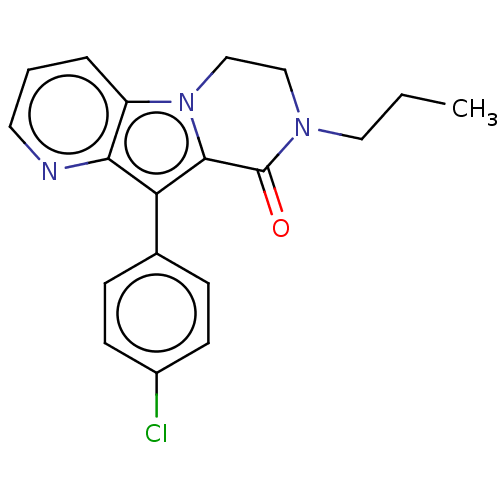 (10-(4-Chlorophenyl)-8-propyl-7,8-dihydropyrido[2',...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q2XW4P16 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoform D of Beta-secretase 1 (BACE-I-432) (Homo sapiens (Human)) | BDBM223358 (US9315520, Comparator 7 | US9605007, Comparator 7 ...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | 4.5 | 37 |
Pfizer Inc. US Patent | Assay Description The substrate is Biotin-GLTNIKTEEISEISY^EVEFR-C[Oregon Green]KK-OH. The BACE1 enzyme is affinity purified material from conditioned media of CHO-K1 c... | US Patent US9315520 (2016) BindingDB Entry DOI: 10.7270/Q20000Z5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4B [122-736] (Homo sapiens (Human)) | BDBM577123 (10-(4-Chloro-5-fluoro-2-methylphenyl)-8-cyclopropy...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q2XW4P16 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4A [2-825] (Homo sapiens (Human)) | BDBM577123 (10-(4-Chloro-5-fluoro-2-methylphenyl)-8-cyclopropy...) | PDB MMDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The PDE4A3, PDE4B1, PDE4C1 and PDE4D3 assays use the Scintillation Proximity Assay (SPA) technology to measure the inhibition of human recombinant PD... | Citation and Details BindingDB Entry DOI: 10.7270/Q2XW4P16 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4A [2-825] (Homo sapiens (Human)) | BDBM577143 (10-(3-Chlorophenyl)-8-cyclopropyl-7,8-dihydropyrid...) | PDB MMDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The PDE4A3, PDE4B1, PDE4C1 and PDE4D3 assays use the Scintillation Proximity Assay (SPA) technology to measure the inhibition of human recombinant PD... | Citation and Details BindingDB Entry DOI: 10.7270/Q2XW4P16 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4B [122-736] (Homo sapiens (Human)) | BDBM577130 (10-(3-Chloro-4-fluorophenyl)-8-cyclopropyl-7,8-dih...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q2XW4P16 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4B [122-736] (Homo sapiens (Human)) | BDBM577142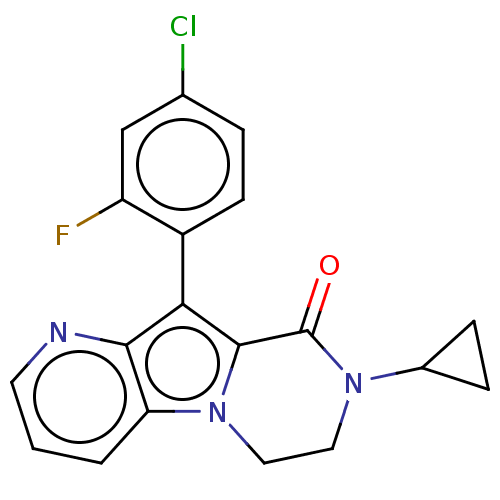 (10-(4-Chloro-2-fluorophenyl)-8-cyclopropyl-7,8-dih...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.14 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q2XW4P16 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4A [2-825] (Homo sapiens (Human)) | BDBM577091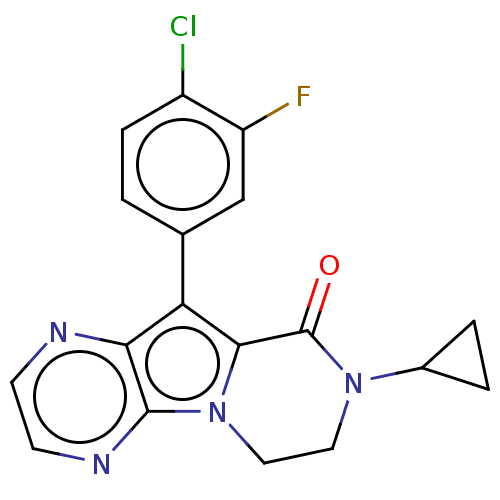 (10-(4-Chloro-3-fluorophenyl)-8-cyclopropyl-7,8-dih...) | PDB MMDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.19 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The PDE4A3, PDE4B1, PDE4C1 and PDE4D3 assays use the Scintillation Proximity Assay (SPA) technology to measure the inhibition of human recombinant PD... | Citation and Details BindingDB Entry DOI: 10.7270/Q2XW4P16 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4A [2-825] (Homo sapiens (Human)) | BDBM577171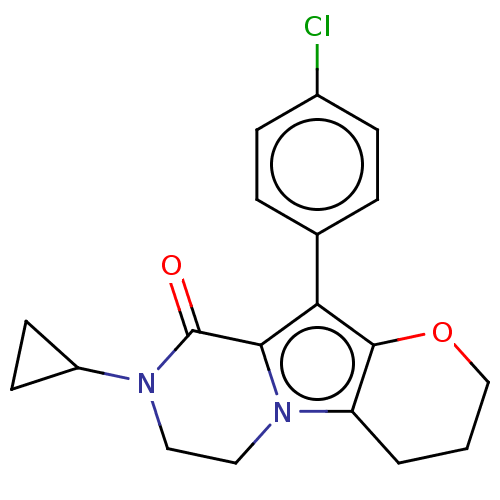 (10-(4-Chlorophenyl)-8-cyclopropyl-3,4,7,8-tetrahyd...) | PDB MMDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.26 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The PDE4A3, PDE4B1, PDE4C1 and PDE4D3 assays use the Scintillation Proximity Assay (SPA) technology to measure the inhibition of human recombinant PD... | Citation and Details BindingDB Entry DOI: 10.7270/Q2XW4P16 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4A [2-825] (Homo sapiens (Human)) | BDBM577136 (8-Cyclopropyl-10-(3-fluorophenyl)-7,8-dihydropyrid...) | PDB MMDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.26 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The PDE4A3, PDE4B1, PDE4C1 and PDE4D3 assays use the Scintillation Proximity Assay (SPA) technology to measure the inhibition of human recombinant PD... | Citation and Details BindingDB Entry DOI: 10.7270/Q2XW4P16 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 2631 total ) | Next | Last >> |