

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Low molecular weight phosphotyrosine protein phosphatase (Homo sapiens (Human)) | BDBM50118216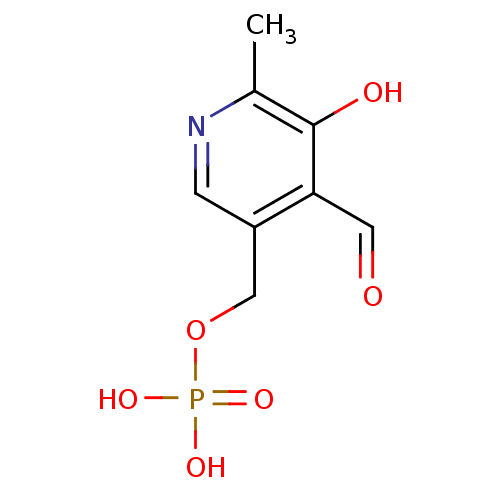 ((4-formyl-5-hydroxy-6-methylpyridin-3-yl)methyl di...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 1.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Saint John's University Curated by ChEMBL | Assay Description Competitive inhibition of LMW-PTP isoform A (unknown origin) | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2020.127342 BindingDB Entry DOI: 10.7270/Q2FJ2MBV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein phosphatase beta (Homo sapiens (Human)) | BDBM50441456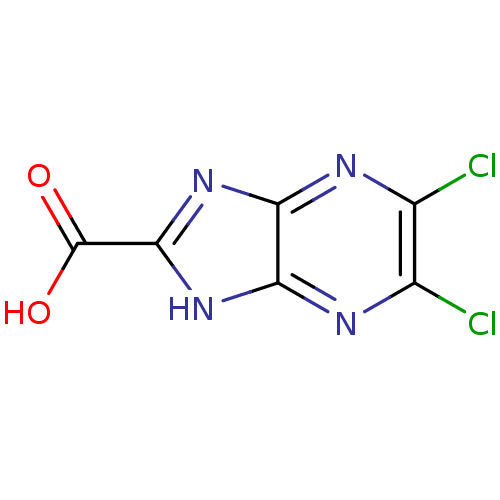 (CHEMBL1369374) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 2.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Saint John's University Curated by ChEMBL | Assay Description Noncompetitive inhibition of human GST-tagged LMW-PTP-B expressed in Escherichia coli JM 109 cells using p-nitrophenyl phosphate as substrate after 1... | Bioorg Med Chem Lett 23: 5912-4 (2013) Article DOI: 10.1016/j.bmcl.2013.08.079 BindingDB Entry DOI: 10.7270/Q2377B50 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein phosphatase beta (Homo sapiens (Human)) | BDBM50441457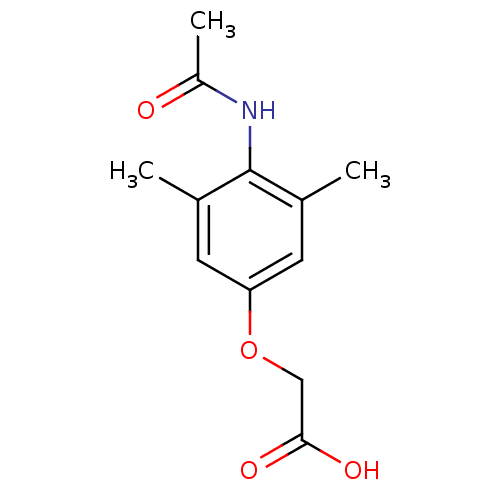 (CHEMBL1358072) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 7.32E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Saint John's University Curated by ChEMBL | Assay Description Noncompetitive inhibition of human GST-tagged LMW-PTP-B expressed in Escherichia coli JM 109 cells using p-nitrophenyl phosphate as substrate after 1... | Bioorg Med Chem Lett 23: 5912-4 (2013) Article DOI: 10.1016/j.bmcl.2013.08.079 BindingDB Entry DOI: 10.7270/Q2377B50 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM50022238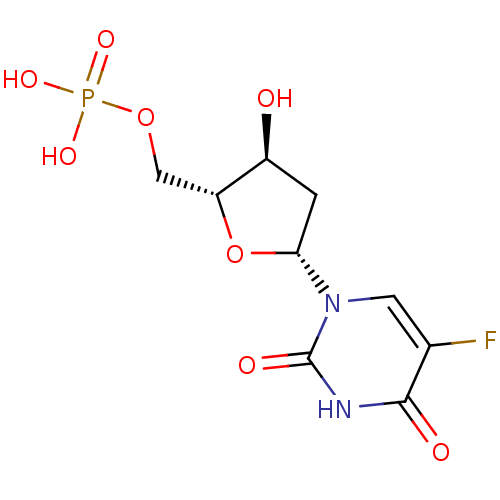 ((R)-5-Fluoro-1-((4S,5R)-4-hydroxy-5-methylphosphat...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Inhibition of cellular thymidylate synthase activity of mouse fibroblast (L929 TK-) in permeabilised cells. | J Med Chem 39: 4569-75 (1996) Article DOI: 10.1021/jm9603680 BindingDB Entry DOI: 10.7270/Q2445N57 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50002692 ((AZT) 1-(4-Azido-5-hydroxymethyl-tetrahydro-furan-...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | DrugBank Article | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Tested for the inhibition of the compound towards HIV-reverse transcriptase | Bioorg Med Chem Lett 5: 1819-1824 (1995) Article DOI: 10.1016/0960-894X(95)00302-A BindingDB Entry DOI: 10.7270/Q23T9H64 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM50022238 ((R)-5-Fluoro-1-((4S,5R)-4-hydroxy-5-methylphosphat...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Inhibition of cellular thymidylate synthase activity of murine leukemia cell line (L1210) in intact cells. | J Med Chem 39: 4569-75 (1996) Article DOI: 10.1021/jm9603680 BindingDB Entry DOI: 10.7270/Q2445N57 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM50022238 ((R)-5-Fluoro-1-((4S,5R)-4-hydroxy-5-methylphosphat...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 42 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Inhibition of cellular thymidylate synthase activity of murine leukemia cell line (L1210) in permeabilised cells. | J Med Chem 39: 4569-75 (1996) Article DOI: 10.1021/jm9603680 BindingDB Entry DOI: 10.7270/Q2445N57 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM50340678 (1-(beta-D-2-deoxy-erythro-pentofuranosyl)-5-fluoro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 43 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Inhibition of cellular thymidylate synthase activity of murine leukemia cell line (L1210) in intact cells. | J Med Chem 39: 4569-75 (1996) Article DOI: 10.1021/jm9603680 BindingDB Entry DOI: 10.7270/Q2445N57 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM50369176 (CHEMBL1791078) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 670 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Inhibition of cellular thymidylate synthase activity of mouse fibroblast (L929 TK-) in intact cells. | J Med Chem 39: 4569-75 (1996) Article DOI: 10.1021/jm9603680 BindingDB Entry DOI: 10.7270/Q2445N57 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Homo sapiens (Human)) | BDBM50369176 (CHEMBL1791078) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 890 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Inhibition of cellular thymidylate synthase activity of mouse fibroblast (L929 TK-) in permeabilised cells. | J Med Chem 39: 4569-75 (1996) Article DOI: 10.1021/jm9603680 BindingDB Entry DOI: 10.7270/Q2445N57 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM50054517 ((S)-2-{[5-(5-Fluoro-2,4-dioxo-3,4-dihydro-2H-pyrim...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Inhibition of cellular thymidylate synthase activity of mouse fibroblast (L929 TK-) in intact cells. | J Med Chem 39: 4569-75 (1996) Article DOI: 10.1021/jm9603680 BindingDB Entry DOI: 10.7270/Q2445N57 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM50369176 (CHEMBL1791078) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Inhibition of cellular thymidylate synthase activity of murine leukemia cell line (L1210) in permeabilised cells. | J Med Chem 39: 4569-75 (1996) Article DOI: 10.1021/jm9603680 BindingDB Entry DOI: 10.7270/Q2445N57 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM50054517 ((S)-2-{[5-(5-Fluoro-2,4-dioxo-3,4-dihydro-2H-pyrim...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Inhibition of cellular thymidylate synthase activity of murine leukemia cell line (L1210) in permeabilised cells. | J Med Chem 39: 4569-75 (1996) Article DOI: 10.1021/jm9603680 BindingDB Entry DOI: 10.7270/Q2445N57 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM50340678 (1-(beta-D-2-deoxy-erythro-pentofuranosyl)-5-fluoro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 7.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Inhibition of cellular thymidylate synthase activity of mouse fibroblast (L929 TK-) in intact cells. | J Med Chem 39: 4569-75 (1996) Article DOI: 10.1021/jm9603680 BindingDB Entry DOI: 10.7270/Q2445N57 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM50022238 ((R)-5-Fluoro-1-((4S,5R)-4-hydroxy-5-methylphosphat...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Inhibition of cellular thymidylate synthase activity of mouse fibroblast (L929 TK-) in intact cells. | J Med Chem 39: 4569-75 (1996) Article DOI: 10.1021/jm9603680 BindingDB Entry DOI: 10.7270/Q2445N57 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM50340678 (1-(beta-D-2-deoxy-erythro-pentofuranosyl)-5-fluoro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Inhibition of cellular thymidylate synthase activity of mouse fibroblast (L929 TK-) in permeabilised cells. | J Med Chem 39: 4569-75 (1996) Article DOI: 10.1021/jm9603680 BindingDB Entry DOI: 10.7270/Q2445N57 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM50369176 (CHEMBL1791078) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Inhibition of cellular thymidylate synthase activity of murine leukemia cell line (L1210) in intact cells. | J Med Chem 39: 4569-75 (1996) Article DOI: 10.1021/jm9603680 BindingDB Entry DOI: 10.7270/Q2445N57 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM50054517 ((S)-2-{[5-(5-Fluoro-2,4-dioxo-3,4-dihydro-2H-pyrim...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Inhibition of cellular thymidylate synthase activity of murine leukemia cell line (L1210) in intact cells. | J Med Chem 39: 4569-75 (1996) Article DOI: 10.1021/jm9603680 BindingDB Entry DOI: 10.7270/Q2445N57 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Homo sapiens (Human)) | BDBM50369176 (CHEMBL1791078) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Inhibition of cellular thymidylate synthase activity of mouse fibroblast (L929 TK-) in intact cells. | J Med Chem 39: 4569-75 (1996) Article DOI: 10.1021/jm9603680 BindingDB Entry DOI: 10.7270/Q2445N57 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM50340678 (1-(beta-D-2-deoxy-erythro-pentofuranosyl)-5-fluoro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Inhibition of cellular thymidylate synthase activity of murine leukemia cell line (L1210) in permeabilised cells. | J Med Chem 39: 4569-75 (1996) Article DOI: 10.1021/jm9603680 BindingDB Entry DOI: 10.7270/Q2445N57 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50060161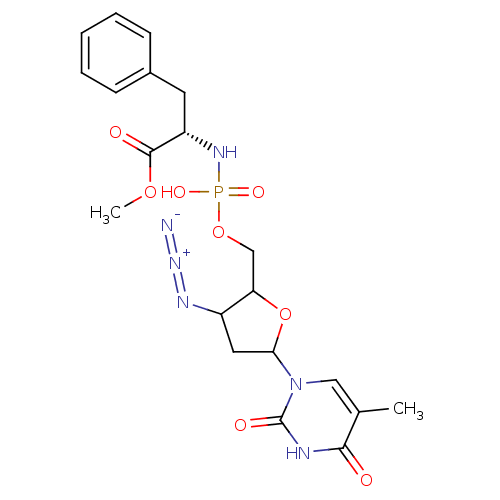 ((S)-2-{[3-Azido-5-(5-methyl-2,4-dioxo-3,4-dihydro-...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Tested for the inhibition of the compound towards HIV-reverse transcriptase | Bioorg Med Chem Lett 5: 1819-1824 (1995) Article DOI: 10.1016/0960-894X(95)00302-A BindingDB Entry DOI: 10.7270/Q23T9H64 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50060160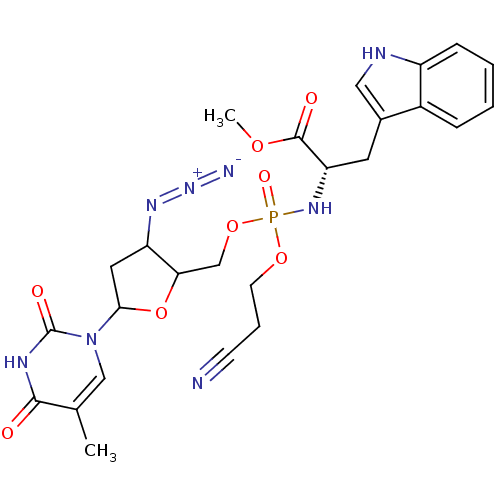 ((S)-2-[[3-Azido-5-(5-methyl-2,4-dioxo-3,4-dihydro-...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Tested for the inhibition of the compound towards HIV-reverse transcriptase | Bioorg Med Chem Lett 5: 1819-1824 (1995) Article DOI: 10.1016/0960-894X(95)00302-A BindingDB Entry DOI: 10.7270/Q23T9H64 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50060162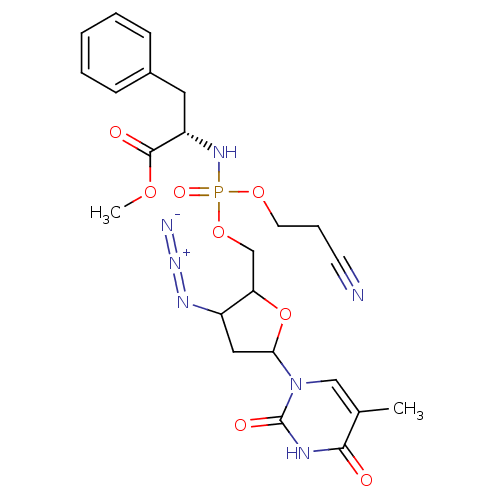 ((S)-2-[[3-Azido-5-(5-methyl-2,4-dioxo-3,4-dihydro-...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Tested for the inhibition of the compound towards HIV-reverse transcriptase | Bioorg Med Chem Lett 5: 1819-1824 (1995) Article DOI: 10.1016/0960-894X(95)00302-A BindingDB Entry DOI: 10.7270/Q23T9H64 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50213275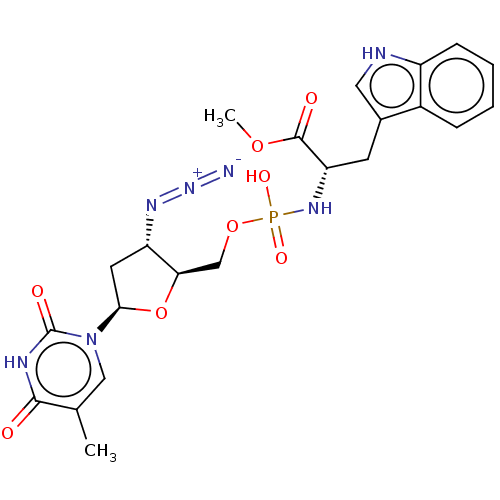 (CHEMBL2021382) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Tested for the inhibition of the compound towards HIV-reverse transcriptase | Bioorg Med Chem Lett 5: 1819-1824 (1995) Article DOI: 10.1016/0960-894X(95)00302-A BindingDB Entry DOI: 10.7270/Q23T9H64 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Homo sapiens (Human)) | BDBM50054517 ((S)-2-{[5-(5-Fluoro-2,4-dioxo-3,4-dihydro-2H-pyrim...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.90E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Inhibition of cellular thymidylate synthase activity of human leukemia cell line(CCRF-CEM) . | J Med Chem 39: 4569-75 (1996) Article DOI: 10.1021/jm9603680 BindingDB Entry DOI: 10.7270/Q2445N57 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Homo sapiens (Human)) | BDBM50054517 ((S)-2-{[5-(5-Fluoro-2,4-dioxo-3,4-dihydro-2H-pyrim...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.90E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Inhibition of cellular thymidylate synthase activity of human leukemia cell line(CCRF-CEM) . | J Med Chem 39: 4569-75 (1996) Article DOI: 10.1021/jm9603680 BindingDB Entry DOI: 10.7270/Q2445N57 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM50369176 (CHEMBL1791078) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Inhibition of cellular thymidylate synthase activity of mouse fibroblast (L929 TK-) in permeabilised cells | J Med Chem 39: 4569-75 (1996) Article DOI: 10.1021/jm9603680 BindingDB Entry DOI: 10.7270/Q2445N57 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM50054517 ((S)-2-{[5-(5-Fluoro-2,4-dioxo-3,4-dihydro-2H-pyrim...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Inhibition of cellular thymidylate synthase activity of mouse fibroblast (L929 TK-) in permeabilised cells | J Med Chem 39: 4569-75 (1996) Article DOI: 10.1021/jm9603680 BindingDB Entry DOI: 10.7270/Q2445N57 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||