

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Genome polyprotein (Hepatitis C virus) | BDBM50142916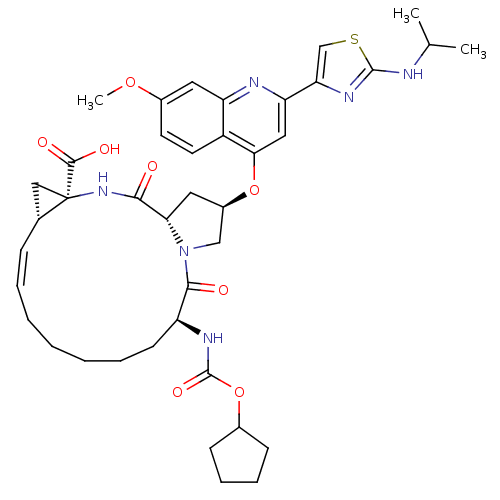 ((1S,4R,6S,14S,18R)-14-Cyclopentyloxycarbonylamino-...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
IDENIX Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of full length Hepatitis C virus genotype 1b Con1 NS3/4A by FRET assay | Bioorg Med Chem Lett 24: 4444-9 (2014) Article DOI: 10.1016/j.bmcl.2014.08.002 BindingDB Entry DOI: 10.7270/Q20C4XBK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM50023510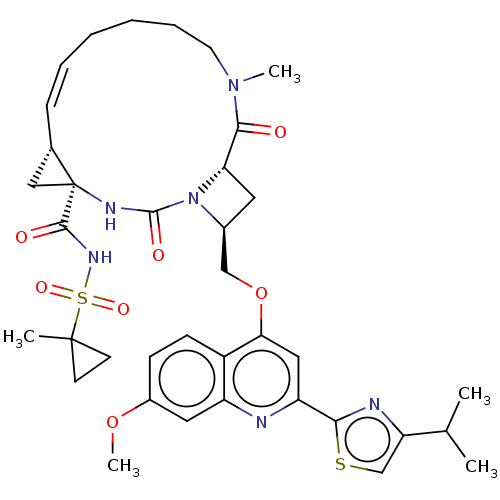 (CHEMBL3326826) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
IDENIX Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of full length Hepatitis C virus genotype 1b Con1 NS3/4A by FRET assay | Bioorg Med Chem Lett 24: 4444-9 (2014) Article DOI: 10.1016/j.bmcl.2014.08.002 BindingDB Entry DOI: 10.7270/Q20C4XBK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM50023579 (CHEMBL3326830) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
IDENIX Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of full length Hepatitis C virus genotype 1b Con1 NS3/4A by FRET assay | Bioorg Med Chem Lett 24: 4444-9 (2014) Article DOI: 10.1016/j.bmcl.2014.08.002 BindingDB Entry DOI: 10.7270/Q20C4XBK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM50023508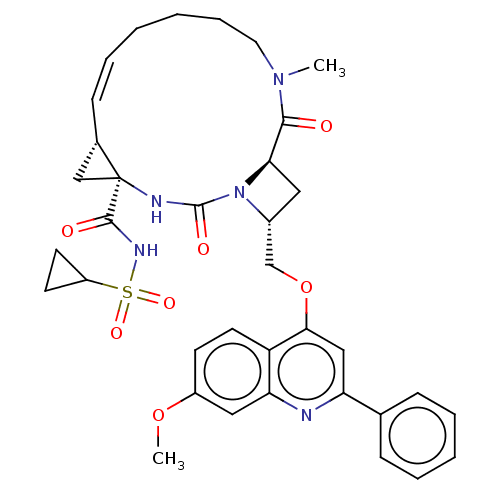 (CHEMBL3326539) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
IDENIX Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of full length Hepatitis C virus genotype 1b Con1 NS3/4A by FRET assay | Bioorg Med Chem Lett 24: 4444-9 (2014) Article DOI: 10.1016/j.bmcl.2014.08.002 BindingDB Entry DOI: 10.7270/Q20C4XBK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM50023578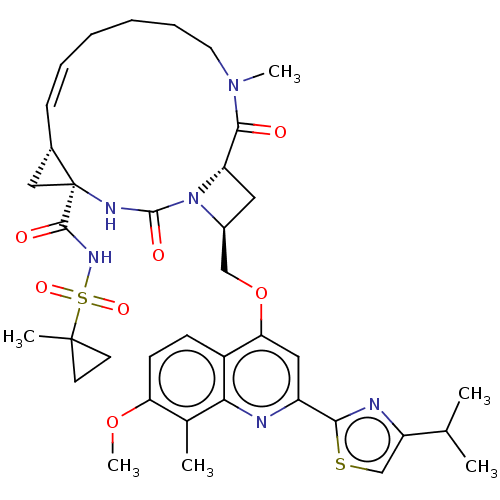 (CHEMBL3326829) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.60 | n/a | n/a | n/a | n/a | n/a | n/a |
IDENIX Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of full length Hepatitis C virus genotype 1b Con1 NS3/4A by FRET assay | Bioorg Med Chem Lett 24: 4444-9 (2014) Article DOI: 10.1016/j.bmcl.2014.08.002 BindingDB Entry DOI: 10.7270/Q20C4XBK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50138406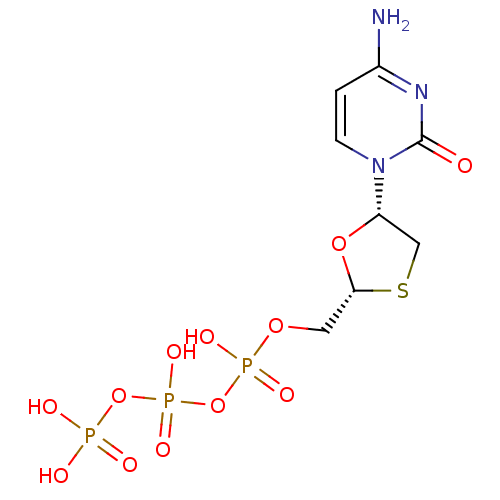 (3TC Triphosphate | CHEMBL1230 | LAMIVUDINE | Lamiv...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Idenix an MSD Company Curated by ChEMBL | Assay Description Inhibition of full-length wild-type HIV1 reverse transcriptase expressed in Escherichia coli BL21(DE3) pre-incubated for 30 mins before addition of t... | J Med Chem 61: 9218-9228 (2018) Article DOI: 10.1021/acs.jmedchem.8b00141 BindingDB Entry DOI: 10.7270/Q2NC63TK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM50023509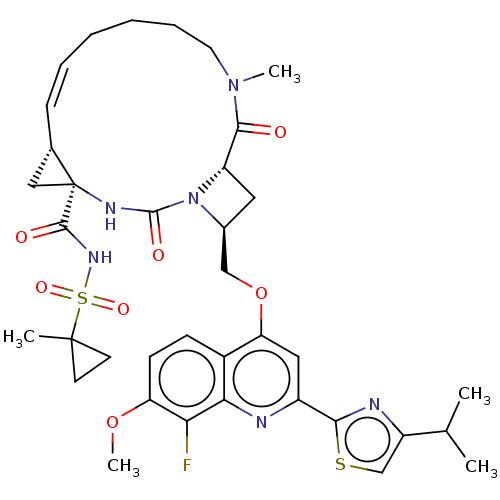 (CHEMBL3326540) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
IDENIX Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of full length Hepatitis C virus genotype 1b Con1 NS3/4A by FRET assay | Bioorg Med Chem Lett 24: 4444-9 (2014) Article DOI: 10.1016/j.bmcl.2014.08.002 BindingDB Entry DOI: 10.7270/Q20C4XBK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM50023507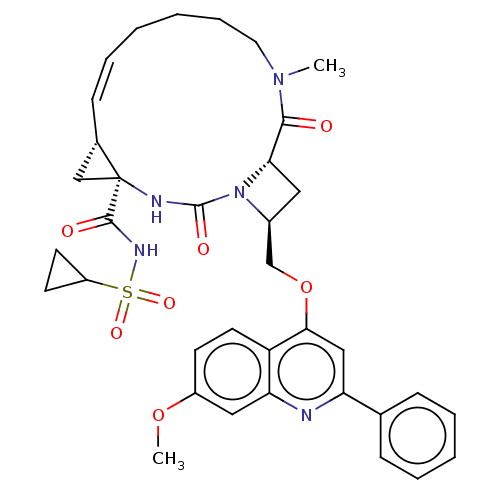 (CHEMBL3326538) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 45 | n/a | n/a | n/a | n/a | n/a | n/a |
IDENIX Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of full length Hepatitis C virus genotype 1b Con1 NS3/4A by FRET assay | Bioorg Med Chem Lett 24: 4444-9 (2014) Article DOI: 10.1016/j.bmcl.2014.08.002 BindingDB Entry DOI: 10.7270/Q20C4XBK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50459813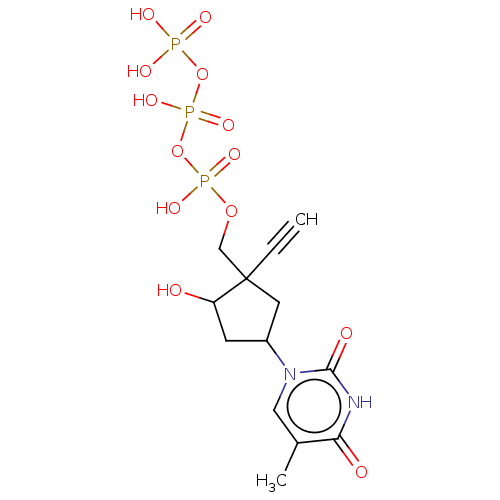 (CHEMBL4205384) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 114 | n/a | n/a | n/a | n/a | n/a | n/a |
Idenix an MSD Company Curated by ChEMBL | Assay Description Inhibition of full-length wild-type HIV1 reverse transcriptase expressed in Escherichia coli BL21(DE3) pre-incubated for 30 mins before addition of t... | J Med Chem 61: 9218-9228 (2018) Article DOI: 10.1021/acs.jmedchem.8b00141 BindingDB Entry DOI: 10.7270/Q2NC63TK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C Virus (Virus)) | BDBM426312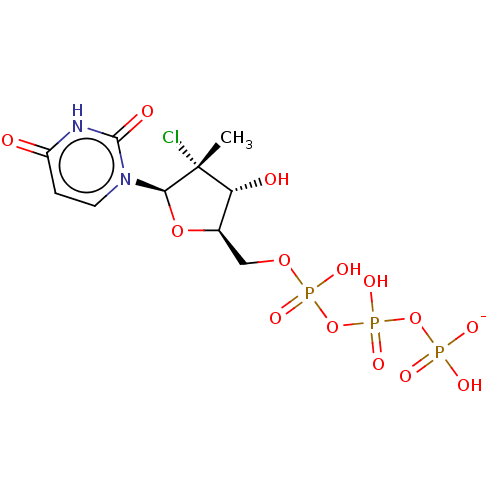 (US10513534, Compound 401) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | US Patent | n/a | n/a | <250 | n/a | n/a | n/a | n/a | n/a | n/a |
IDENIX PHARMACEUTICALS LLC; CENTRE NATIONAL DE LA RECHERCHE SCIENTIFIQUE; UNIVERSITE DE MONTPELLIER US Patent | Assay Description Test compounds in the form of nucleoside triphosphates were examined for inhibitory activity against purified HCV polymerase in a standard assay. Bac... | US Patent US10513534 (2019) BindingDB Entry DOI: 10.7270/Q2BC41XJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein [S283T] (Hepatitis C virus genotype 1b (isolate Japanese) (...) | BDBM347262 (US10202411, Compound 308) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | US Patent | n/a | n/a | <250 | n/a | n/a | n/a | n/a | n/a | n/a |
IDENIX PHARMACEUTICALS LLC US Patent | Assay Description Test compounds in the form of nucleoside triphosphates were examined for inhibitory activity against purified HCV polymerase in a standard assay. Bac... | US Patent US10202411 (2019) BindingDB Entry DOI: 10.7270/Q2RX9F67 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C Virus (Virus)) | BDBM347262 (US10202411, Compound 308) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | US Patent | n/a | n/a | <250 | n/a | n/a | n/a | n/a | n/a | n/a |
IDENIX PHARMACEUTICALS LLC US Patent | Assay Description Test compounds in the form of nucleoside triphosphates were examined for inhibitory activity against purified HCV polymerase in a standard assay. Bac... | US Patent US10202411 (2019) BindingDB Entry DOI: 10.7270/Q2RX9F67 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein [S283T] (Hepatitis C virus genotype 1b (isolate Japanese) (...) | BDBM347260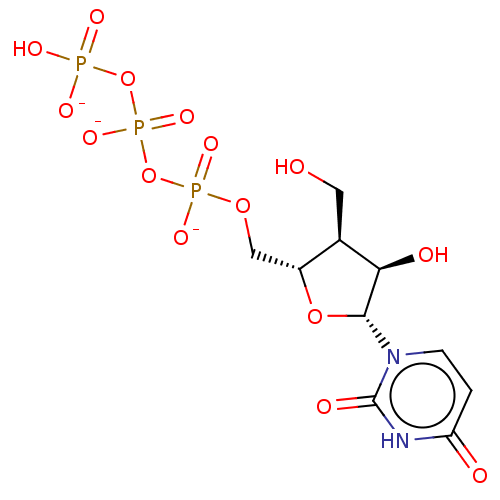 (US10202411, Compound 305) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | US Patent | n/a | n/a | <250 | n/a | n/a | n/a | n/a | n/a | n/a |
IDENIX PHARMACEUTICALS LLC US Patent | Assay Description Test compounds in the form of nucleoside triphosphates were examined for inhibitory activity against purified HCV polymerase in a standard assay. Bac... | US Patent US10202411 (2019) BindingDB Entry DOI: 10.7270/Q2RX9F67 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C Virus (Virus)) | BDBM347260 (US10202411, Compound 305) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | US Patent | n/a | n/a | <250 | n/a | n/a | n/a | n/a | n/a | n/a |
IDENIX PHARMACEUTICALS LLC US Patent | Assay Description Test compounds in the form of nucleoside triphosphates were examined for inhibitory activity against purified HCV polymerase in a standard assay. Bac... | US Patent US10202411 (2019) BindingDB Entry DOI: 10.7270/Q2RX9F67 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C Virus (Virus)) | BDBM347259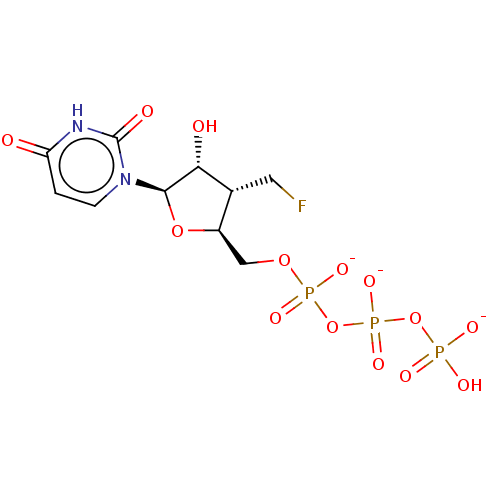 (US10202411, Compound 302) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | US Patent | n/a | n/a | <250 | n/a | n/a | n/a | n/a | n/a | n/a |
IDENIX PHARMACEUTICALS LLC US Patent | Assay Description Test compounds in the form of nucleoside triphosphates were examined for inhibitory activity against purified HCV polymerase in a standard assay. Bac... | US Patent US10202411 (2019) BindingDB Entry DOI: 10.7270/Q2RX9F67 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C Virus (Virus)) | BDBM426315 (US10513534, Compound 403) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | US Patent | n/a | n/a | <250 | n/a | n/a | n/a | n/a | n/a | n/a |
IDENIX PHARMACEUTICALS LLC; CENTRE NATIONAL DE LA RECHERCHE SCIENTIFIQUE; UNIVERSITE DE MONTPELLIER US Patent | US Patent US10513534 (2019) BindingDB Entry DOI: 10.7270/Q2BC41XJ | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C Virus (Virus)) | BDBM426317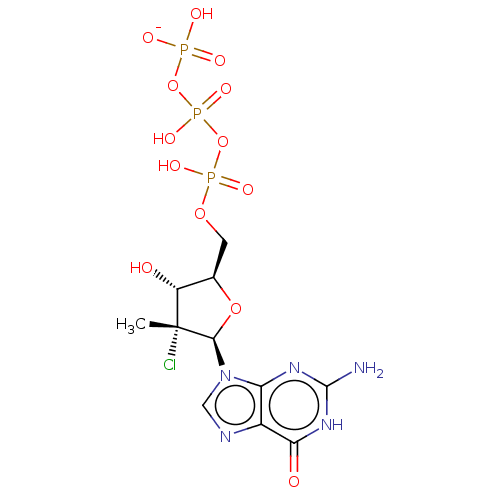 (US10513534, Compound 405) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | US Patent | n/a | n/a | <250 | n/a | n/a | n/a | n/a | n/a | n/a |
IDENIX PHARMACEUTICALS LLC; CENTRE NATIONAL DE LA RECHERCHE SCIENTIFIQUE; UNIVERSITE DE MONTPELLIER US Patent | US Patent US10513534 (2019) BindingDB Entry DOI: 10.7270/Q2BC41XJ | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C Virus (Virus)) | BDBM426318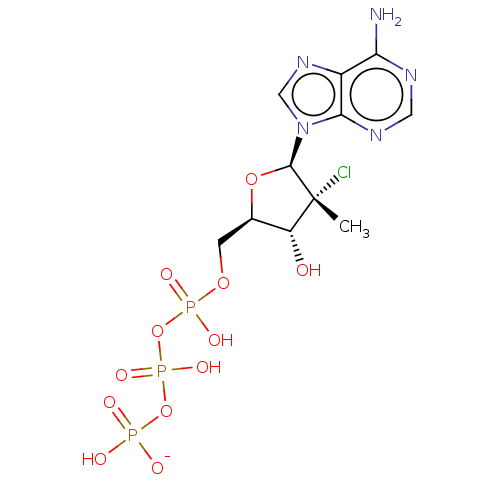 (US10513534, Compound 406) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | US Patent | n/a | n/a | <250 | n/a | n/a | n/a | n/a | n/a | n/a |
IDENIX PHARMACEUTICALS LLC; CENTRE NATIONAL DE LA RECHERCHE SCIENTIFIQUE; UNIVERSITE DE MONTPELLIER US Patent | Assay Description Test compounds in the form of nucleoside triphosphates were examined for inhibitory activity against purified HCV polymerase in a standard assay. Bac... | US Patent US10513534 (2019) BindingDB Entry DOI: 10.7270/Q2BC41XJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C Virus (Virus)) | BDBM426319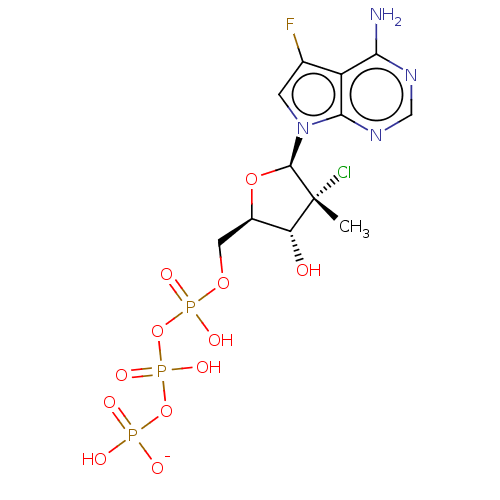 (US10513534, Compound 407) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | US Patent | n/a | n/a | <250 | n/a | n/a | n/a | n/a | n/a | n/a |
IDENIX PHARMACEUTICALS LLC; CENTRE NATIONAL DE LA RECHERCHE SCIENTIFIQUE; UNIVERSITE DE MONTPELLIER US Patent | US Patent US10513534 (2019) BindingDB Entry DOI: 10.7270/Q2BC41XJ | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein [S283T] (Hepatitis C virus genotype 1b (isolate Japanese) (...) | BDBM347259 (US10202411, Compound 302) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | US Patent | n/a | n/a | <250 | n/a | n/a | n/a | n/a | n/a | n/a |
IDENIX PHARMACEUTICALS LLC US Patent | Assay Description Test compounds in the form of nucleoside triphosphates were examined for inhibitory activity against purified HCV polymerase in a standard assay. Bac... | US Patent US10202411 (2019) BindingDB Entry DOI: 10.7270/Q2RX9F67 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein [S283T] (Hepatitis C virus genotype 1b (isolate Japanese) (...) | BDBM347266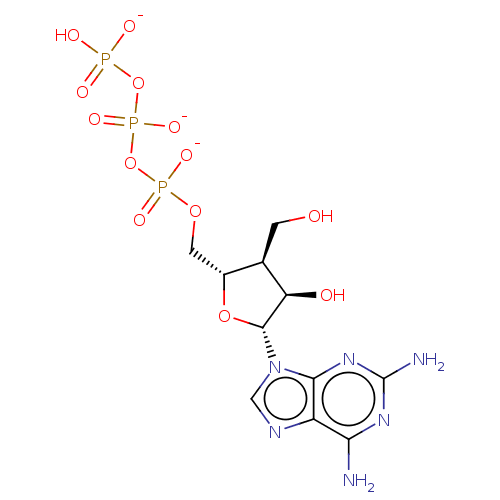 (US10202411, Compound 330) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | US Patent | n/a | n/a | <250 | n/a | n/a | n/a | n/a | n/a | n/a |
IDENIX PHARMACEUTICALS LLC US Patent | Assay Description Test compounds in the form of nucleoside triphosphates were examined for inhibitory activity against purified HCV polymerase in a standard assay. Bac... | US Patent US10202411 (2019) BindingDB Entry DOI: 10.7270/Q2RX9F67 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein [S283T] (Hepatitis C virus genotype 1b (isolate Japanese) (...) | BDBM347265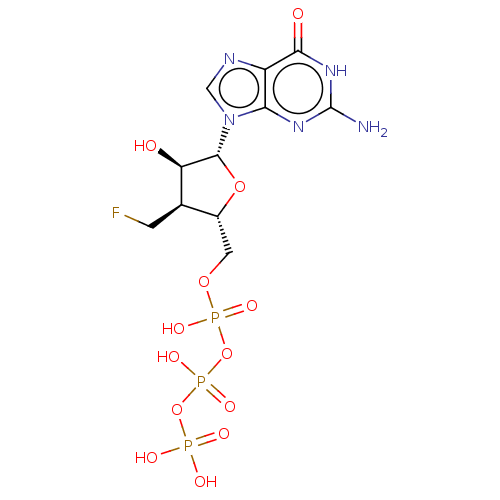 (US10202411, Compound 327) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | US Patent | n/a | n/a | <250 | n/a | n/a | n/a | n/a | n/a | n/a |
IDENIX PHARMACEUTICALS LLC US Patent | Assay Description Test compounds in the form of nucleoside triphosphates were examined for inhibitory activity against purified HCV polymerase in a standard assay. Bac... | US Patent US10202411 (2019) BindingDB Entry DOI: 10.7270/Q2RX9F67 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C Virus (Virus)) | BDBM347265 (US10202411, Compound 327) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | US Patent | n/a | n/a | <250 | n/a | n/a | n/a | n/a | n/a | n/a |
IDENIX PHARMACEUTICALS LLC US Patent | Assay Description Test compounds in the form of nucleoside triphosphates were examined for inhibitory activity against purified HCV polymerase in a standard assay. Bac... | US Patent US10202411 (2019) BindingDB Entry DOI: 10.7270/Q2RX9F67 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50459816 (CHEMBL518606) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 263 | n/a | n/a | n/a | n/a | n/a | n/a |
Idenix an MSD Company Curated by ChEMBL | Assay Description Inhibition of full-length wild-type HIV1 reverse transcriptase expressed in Escherichia coli BL21(DE3) pre-incubated for 30 mins before addition of t... | J Med Chem 61: 9218-9228 (2018) Article DOI: 10.1021/acs.jmedchem.8b00141 BindingDB Entry DOI: 10.7270/Q2NC63TK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM50023581 (CHEMBL3326537) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 278 | n/a | n/a | n/a | n/a | n/a | n/a |
IDENIX Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of full length Hepatitis C virus genotype 1b Con1 NS3/4A by FRET assay | Bioorg Med Chem Lett 24: 4444-9 (2014) Article DOI: 10.1016/j.bmcl.2014.08.002 BindingDB Entry DOI: 10.7270/Q20C4XBK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50459815 (CHEMBL4216055) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 324 | n/a | n/a | n/a | n/a | n/a | n/a |
Idenix an MSD Company Curated by ChEMBL | Assay Description Inhibition of full-length wild-type HIV1 reverse transcriptase expressed in Escherichia coli BL21(DE3) pre-incubated for 30 mins before addition of t... | J Med Chem 61: 9218-9228 (2018) Article DOI: 10.1021/acs.jmedchem.8b00141 BindingDB Entry DOI: 10.7270/Q2NC63TK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50459812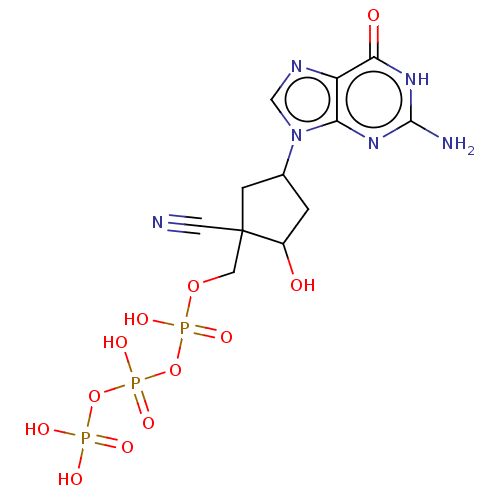 (CHEMBL4214561) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 444 | n/a | n/a | n/a | n/a | n/a | n/a |
Idenix an MSD Company Curated by ChEMBL | Assay Description Inhibition of full-length wild-type HIV1 reverse transcriptase expressed in Escherichia coli BL21(DE3) pre-incubated for 30 mins before addition of t... | J Med Chem 61: 9218-9228 (2018) Article DOI: 10.1021/acs.jmedchem.8b00141 BindingDB Entry DOI: 10.7270/Q2NC63TK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50459817 (CHEMBL4211088) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 523 | n/a | n/a | n/a | n/a | n/a | n/a |
Idenix an MSD Company Curated by ChEMBL | Assay Description Inhibition of full-length wild-type HIV1 reverse transcriptase expressed in Escherichia coli BL21(DE3) pre-incubated for 30 mins before addition of t... | J Med Chem 61: 9218-9228 (2018) Article DOI: 10.1021/acs.jmedchem.8b00141 BindingDB Entry DOI: 10.7270/Q2NC63TK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C Virus (Virus)) | BDBM347268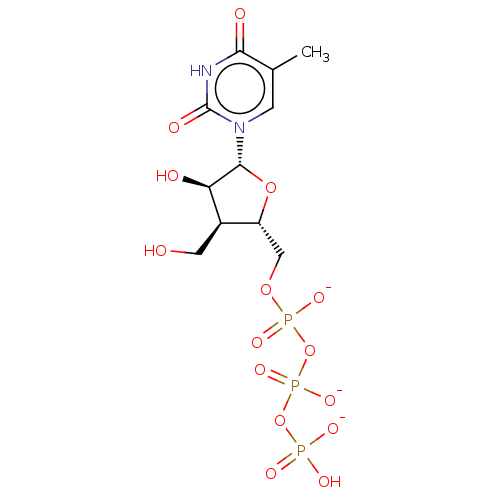 (US10202411, Compound 332) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | US Patent | n/a | n/a | 625 | n/a | n/a | n/a | n/a | n/a | n/a |
IDENIX PHARMACEUTICALS LLC US Patent | Assay Description Test compounds in the form of nucleoside triphosphates were examined for inhibitory activity against purified HCV polymerase in a standard assay. Bac... | US Patent US10202411 (2019) BindingDB Entry DOI: 10.7270/Q2RX9F67 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C Virus (Virus)) | BDBM347266 (US10202411, Compound 330) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | US Patent | n/a | n/a | 625 | n/a | n/a | n/a | n/a | n/a | n/a |
IDENIX PHARMACEUTICALS LLC US Patent | Assay Description Test compounds in the form of nucleoside triphosphates were examined for inhibitory activity against purified HCV polymerase in a standard assay. Bac... | US Patent US10202411 (2019) BindingDB Entry DOI: 10.7270/Q2RX9F67 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C Virus (Virus)) | BDBM347261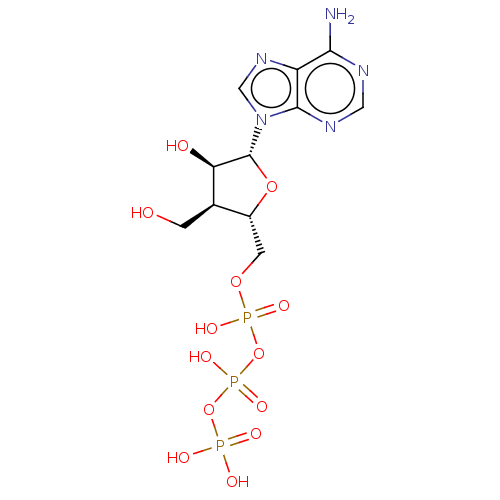 (US10202411, Compound 307) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | US Patent | n/a | n/a | 625 | n/a | n/a | n/a | n/a | n/a | n/a |
IDENIX PHARMACEUTICALS LLC US Patent | Assay Description Test compounds in the form of nucleoside triphosphates were examined for inhibitory activity against purified HCV polymerase in a standard assay. Bac... | US Patent US10202411 (2019) BindingDB Entry DOI: 10.7270/Q2RX9F67 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM50023577 (CHEMBL3326828) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | <1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
IDENIX Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of full length Hepatitis C virus genotype 1b Con1 NS3/4A by FRET assay | Bioorg Med Chem Lett 24: 4444-9 (2014) Article DOI: 10.1016/j.bmcl.2014.08.002 BindingDB Entry DOI: 10.7270/Q20C4XBK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM50023576 (CHEMBL3326827) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | <1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
IDENIX Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of full length Hepatitis C virus genotype 1b Con1 NS3/4A by FRET assay | Bioorg Med Chem Lett 24: 4444-9 (2014) Article DOI: 10.1016/j.bmcl.2014.08.002 BindingDB Entry DOI: 10.7270/Q20C4XBK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM50023580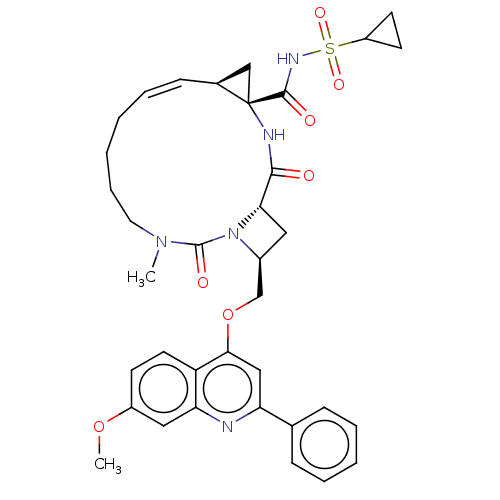 (CHEMBL3326536) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
IDENIX Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of full length Hepatitis C virus genotype 1b Con1 NS3/4A by FRET assay | Bioorg Med Chem Lett 24: 4444-9 (2014) Article DOI: 10.1016/j.bmcl.2014.08.002 BindingDB Entry DOI: 10.7270/Q20C4XBK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50459814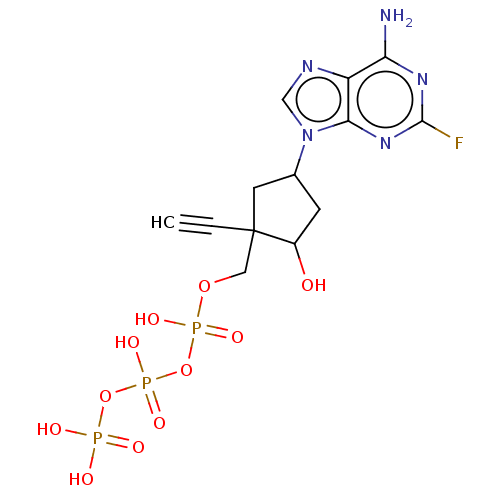 (CHEMBL4211602) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.46E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Idenix an MSD Company Curated by ChEMBL | Assay Description Inhibition of full-length wild-type HIV1 reverse transcriptase expressed in Escherichia coli BL21(DE3) pre-incubated for 30 mins before addition of t... | J Med Chem 61: 9218-9228 (2018) Article DOI: 10.1021/acs.jmedchem.8b00141 BindingDB Entry DOI: 10.7270/Q2NC63TK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C Virus (Virus)) | BDBM347264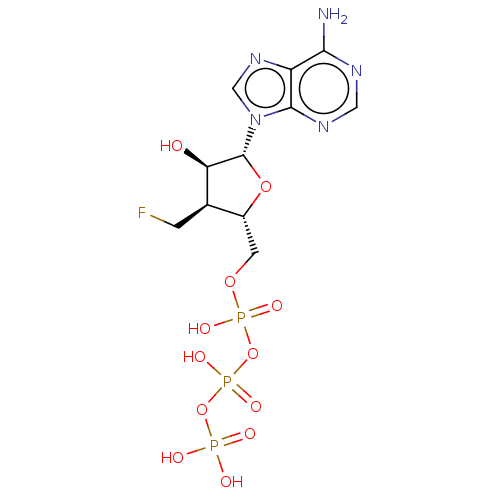 (US10202411, Compound 326) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | US Patent | n/a | n/a | 5.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
IDENIX PHARMACEUTICALS LLC US Patent | Assay Description Test compounds in the form of nucleoside triphosphates were examined for inhibitory activity against purified HCV polymerase in a standard assay. Bac... | US Patent US10202411 (2019) BindingDB Entry DOI: 10.7270/Q2RX9F67 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C Virus (Virus)) | BDBM426314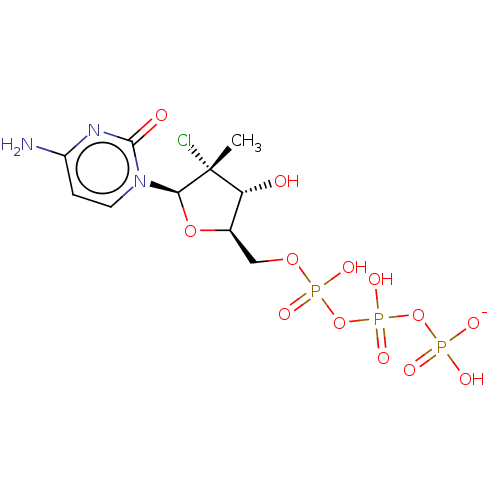 (US10513534, Compound 402) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | US Patent | n/a | n/a | 5.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
IDENIX PHARMACEUTICALS LLC; CENTRE NATIONAL DE LA RECHERCHE SCIENTIFIQUE; UNIVERSITE DE MONTPELLIER US Patent | Assay Description Test compounds in the form of nucleoside triphosphates were examined for inhibitory activity against purified HCV polymerase in a standard assay. Bac... | US Patent US10513534 (2019) BindingDB Entry DOI: 10.7270/Q2BC41XJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C Virus (Virus)) | BDBM347272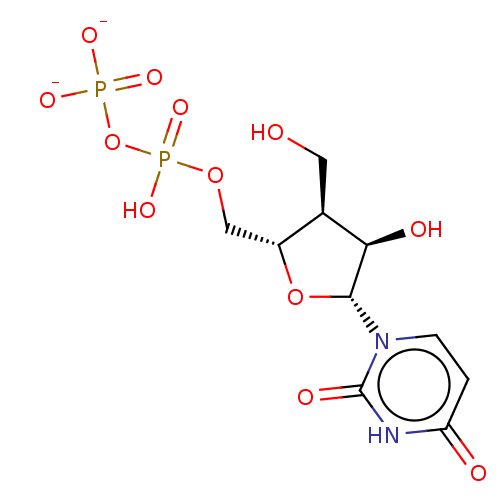 (US10202411, Compound 402) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | US Patent | n/a | n/a | 5.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
IDENIX PHARMACEUTICALS LLC US Patent | Assay Description Test compounds in the form of nucleoside triphosphates were examined for inhibitory activity against purified HCV polymerase in a standard assay. Bac... | US Patent US10202411 (2019) BindingDB Entry DOI: 10.7270/Q2RX9F67 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C Virus (Virus)) | BDBM347258 (US10202411, Compound 301) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | US Patent | n/a | n/a | 5.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
IDENIX PHARMACEUTICALS LLC US Patent | Assay Description Test compounds in the form of nucleoside triphosphates were examined for inhibitory activity against purified HCV polymerase in a standard assay. Bac... | US Patent US10202411 (2019) BindingDB Entry DOI: 10.7270/Q2RX9F67 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C Virus (Virus)) | BDBM347263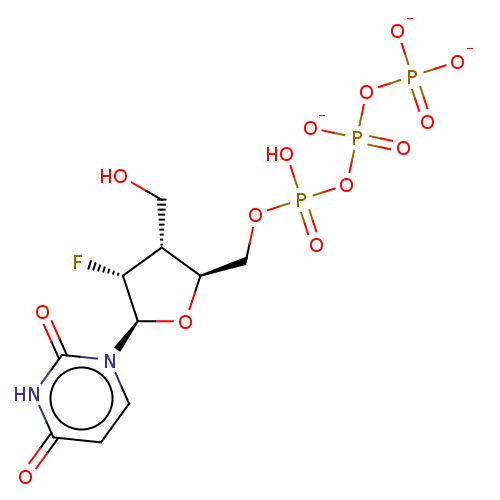 (US10202411, Compound 325) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | US Patent | n/a | n/a | 5.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
IDENIX PHARMACEUTICALS LLC US Patent | Assay Description Test compounds in the form of nucleoside triphosphates were examined for inhibitory activity against purified HCV polymerase in a standard assay. Bac... | US Patent US10202411 (2019) BindingDB Entry DOI: 10.7270/Q2RX9F67 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C Virus (Virus)) | BDBM426315 (US10513534, Compound 403) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | US Patent | n/a | n/a | 6.25E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
IDENIX PHARMACEUTICALS LLC; CENTRE NATIONAL DE LA RECHERCHE SCIENTIFIQUE; UNIVERSITE DE MONTPELLIER US Patent | Assay Description Test compounds in the form of nucleoside triphosphates were examined for inhibitory activity against purified HCV polymerase in a standard assay. Bac... | US Patent US10513534 (2019) BindingDB Entry DOI: 10.7270/Q2BC41XJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C Virus (Virus)) | BDBM426312 (US10513534, Compound 401) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | US Patent | n/a | n/a | 6.25E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
IDENIX PHARMACEUTICALS LLC; CENTRE NATIONAL DE LA RECHERCHE SCIENTIFIQUE; UNIVERSITE DE MONTPELLIER US Patent | US Patent US10513534 (2019) BindingDB Entry DOI: 10.7270/Q2BC41XJ | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C Virus (Virus)) | BDBM426322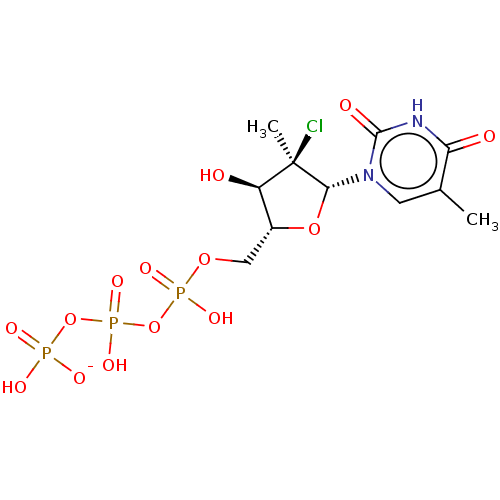 (US10513534, Compound 410) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | US Patent | n/a | n/a | 6.25E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
IDENIX PHARMACEUTICALS LLC; CENTRE NATIONAL DE LA RECHERCHE SCIENTIFIQUE; UNIVERSITE DE MONTPELLIER US Patent | Assay Description Test compounds in the form of nucleoside triphosphates were examined for inhibitory activity against purified HCV polymerase in a standard assay. Bac... | US Patent US10513534 (2019) BindingDB Entry DOI: 10.7270/Q2BC41XJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C Virus (Virus)) | BDBM426323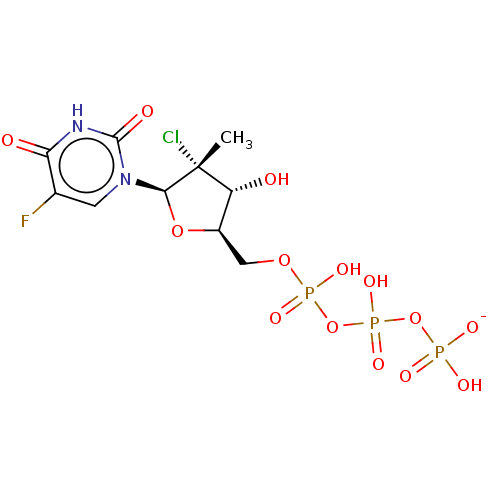 (US10513534, Compound 411) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | US Patent | n/a | n/a | 6.25E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
IDENIX PHARMACEUTICALS LLC; CENTRE NATIONAL DE LA RECHERCHE SCIENTIFIQUE; UNIVERSITE DE MONTPELLIER US Patent | Assay Description Test compounds in the form of nucleoside triphosphates were examined for inhibitory activity against purified HCV polymerase in a standard assay. Bac... | US Patent US10513534 (2019) BindingDB Entry DOI: 10.7270/Q2BC41XJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C Virus (Virus)) | BDBM426321 (US10513534, Compound 409) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | US Patent | n/a | n/a | 6.25E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
IDENIX PHARMACEUTICALS LLC; CENTRE NATIONAL DE LA RECHERCHE SCIENTIFIQUE; UNIVERSITE DE MONTPELLIER US Patent | Assay Description Test compounds in the form of nucleoside triphosphates were examined for inhibitory activity against purified HCV polymerase in a standard assay. Bac... | US Patent US10513534 (2019) BindingDB Entry DOI: 10.7270/Q2BC41XJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C Virus (Virus)) | BDBM426320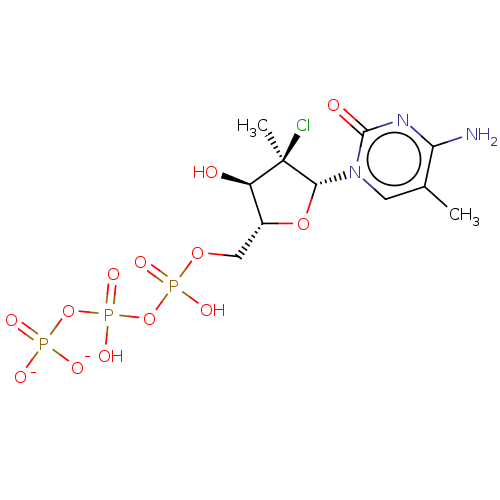 (US10513534, Compound 408) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | US Patent | n/a | n/a | 6.25E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
IDENIX PHARMACEUTICALS LLC; CENTRE NATIONAL DE LA RECHERCHE SCIENTIFIQUE; UNIVERSITE DE MONTPELLIER US Patent | Assay Description Test compounds in the form of nucleoside triphosphates were examined for inhibitory activity against purified HCV polymerase in a standard assay. Bac... | US Patent US10513534 (2019) BindingDB Entry DOI: 10.7270/Q2BC41XJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C Virus (Virus)) | BDBM426319 (US10513534, Compound 407) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | US Patent | n/a | n/a | 6.25E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
IDENIX PHARMACEUTICALS LLC; CENTRE NATIONAL DE LA RECHERCHE SCIENTIFIQUE; UNIVERSITE DE MONTPELLIER US Patent | Assay Description Test compounds in the form of nucleoside triphosphates were examined for inhibitory activity against purified HCV polymerase in a standard assay. Bac... | US Patent US10513534 (2019) BindingDB Entry DOI: 10.7270/Q2BC41XJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C Virus (Virus)) | BDBM426318 (US10513534, Compound 406) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | US Patent | n/a | n/a | 6.25E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
IDENIX PHARMACEUTICALS LLC; CENTRE NATIONAL DE LA RECHERCHE SCIENTIFIQUE; UNIVERSITE DE MONTPELLIER US Patent | US Patent US10513534 (2019) BindingDB Entry DOI: 10.7270/Q2BC41XJ | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C Virus (Virus)) | BDBM426317 (US10513534, Compound 405) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | US Patent | n/a | n/a | 6.25E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
IDENIX PHARMACEUTICALS LLC; CENTRE NATIONAL DE LA RECHERCHE SCIENTIFIQUE; UNIVERSITE DE MONTPELLIER US Patent | Assay Description Test compounds in the form of nucleoside triphosphates were examined for inhibitory activity against purified HCV polymerase in a standard assay. Bac... | US Patent US10513534 (2019) BindingDB Entry DOI: 10.7270/Q2BC41XJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C Virus (Virus)) | BDBM347270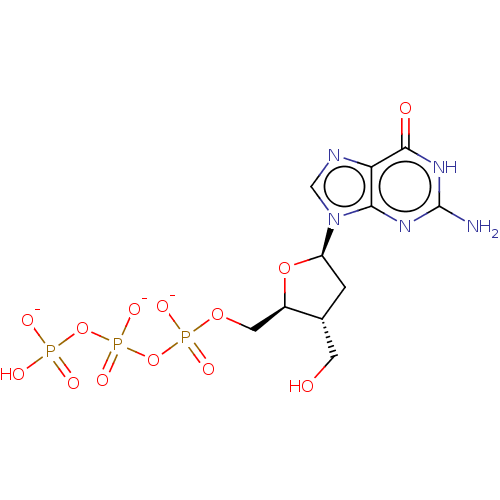 (US10202411, Compound 335) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | US Patent | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
IDENIX PHARMACEUTICALS LLC US Patent | Assay Description Test compounds in the form of nucleoside triphosphates were examined for inhibitory activity against purified HCV polymerase in a standard assay. Bac... | US Patent US10202411 (2019) BindingDB Entry DOI: 10.7270/Q2RX9F67 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 59 total ) | Next | Last >> |