 Found 124 hits with Last Name = 'salgado-polo' and Initial = 'f'
Found 124 hits with Last Name = 'salgado-polo' and Initial = 'f' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Ectonucleotide pyrophosphatase/phosphodiesterase family member 2
(Homo sapiens (Human)) | BDBM50501623

(CHEMBL4062929)Show SMILES [H][C@@]1(CC[C@@]2([H])[C@]3([H])[C@@H](O)C[C@]4([H])C[C@H](O)CC[C@]4(C)[C@@]3([H])CC[C@]12C)[C@H](C)CCC(=O)N[C@@H]1CCN(C1)C(=O)OCc1cc(Cl)cc(Cl)c1 |r| Show InChI InChI=1S/C36H52Cl2N2O5/c1-21(4-7-32(43)39-26-10-13-40(19-26)34(44)45-20-22-14-24(37)18-25(38)15-22)28-5-6-29-33-30(9-12-36(28,29)3)35(2)11-8-27(41)16-23(35)17-31(33)42/h14-15,18,21,23,26-31,33,41-42H,4-13,16-17,19-20H2,1-3H3,(H,39,43)/t21-,23+,26-,27-,28-,29+,30+,31+,33+,35+,36-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Netherlands Institute
Curated by ChEMBL
| Assay Description
Competitive inhibition of human ATX expressed in HEK293 Flp-In cells assessed as decrease in LPC hydrolysis measured every 30 secs for 90 mins by Lin... |
J Med Chem 60: 2006-2017 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01743
BindingDB Entry DOI: 10.7270/Q22F7RGR |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 2
(Homo sapiens (Human)) | BDBM50580260

(CHEMBL5093347)Show SMILES CCc1nc2ccc(cn2c1N(C)c1nc(c(s1)C#N)-c1ccc(F)cc1)N1CCN(CC(=O)N2CCC(CF)C2)CC1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of ATX lysoPLD (unknown origin) using 18:1 LPC as substrate assessed as reduction in choline release by HVA fluorescence based analysis |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00913
BindingDB Entry DOI: 10.7270/Q20P13WS |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 2
(Rattus norvegicus) | BDBM50605406

(CHEMBL5190935)Show SMILES [H][C@@]1(CC[C@@]2([H])C3[C@@H](O)C[C@]4([H])C[C@H](O)CC[C@]4(C)[C@@]3([H])CC[C@]12C)[C@H](C)CCC(=O)N1CCC(CC1)Oc1ccc(cc1)B(O)O |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00368
BindingDB Entry DOI: 10.7270/Q25H7MCC |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 2
(Homo sapiens (Human)) | BDBM50580262
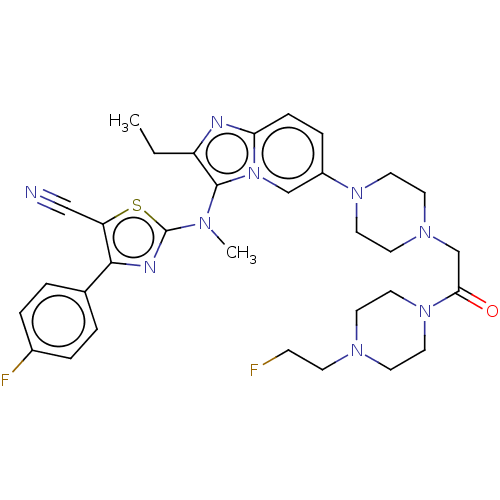
(CHEMBL5085520)Show SMILES CCc1nc2ccc(cn2c1N(C)c1nc(c(s1)C#N)-c1ccc(F)cc1)N1CCN(CC(=O)N2CCN(CCF)CC2)CC1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of ATX lysoPLD (unknown origin) using 18:1 LPC as substrate assessed as reduction in choline release by HVA fluorescence based analysis |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00913
BindingDB Entry DOI: 10.7270/Q20P13WS |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 2
(Homo sapiens (Human)) | BDBM50580256
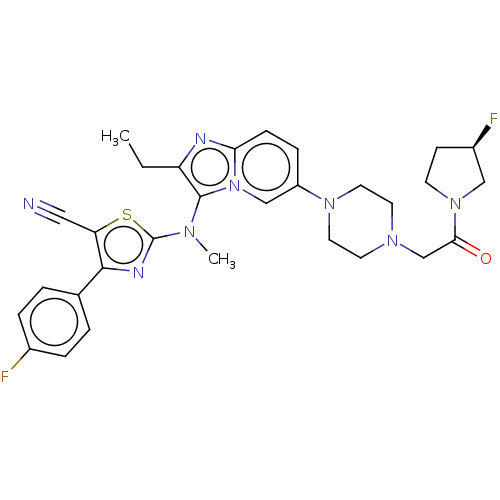
(CHEMBL5091502)Show SMILES CCc1nc2ccc(cn2c1N(C)c1nc(c(s1)C#N)-c1ccc(F)cc1)N1CCN(CC(=O)N2CC[C@@H](F)C2)CC1 |r| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of ATX lysoPLD (unknown origin) using 18:1 LPC as substrate assessed as reduction in choline release by HVA fluorescence based analysis |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00913
BindingDB Entry DOI: 10.7270/Q20P13WS |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 2
(Homo sapiens (Human)) | BDBM50580261
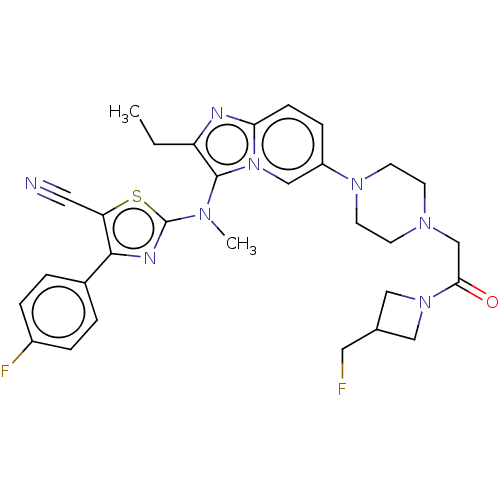
(CHEMBL5084424)Show SMILES CCc1nc2ccc(cn2c1N(C)c1nc(c(s1)C#N)-c1ccc(F)cc1)N1CCN(CC(=O)N2CC(CF)C2)CC1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of ATX lysoPLD (unknown origin) using 18:1 LPC as substrate assessed as reduction in choline release by HVA fluorescence based analysis |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00913
BindingDB Entry DOI: 10.7270/Q20P13WS |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 2
(Homo sapiens (Human)) | BDBM50580259

(CHEMBL5088520)Show SMILES CCc1nc2ccc(cn2c1N(C)c1nc(c(s1)C#N)-c1ccc(F)cc1)N1CCN(CC(=O)N2CCCC(F)C2)CC1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of ATX lysoPLD (unknown origin) using 18:1 LPC as substrate assessed as reduction in choline release by HVA fluorescence based analysis |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00913
BindingDB Entry DOI: 10.7270/Q20P13WS |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 2
(Homo sapiens (Human)) | BDBM50580255
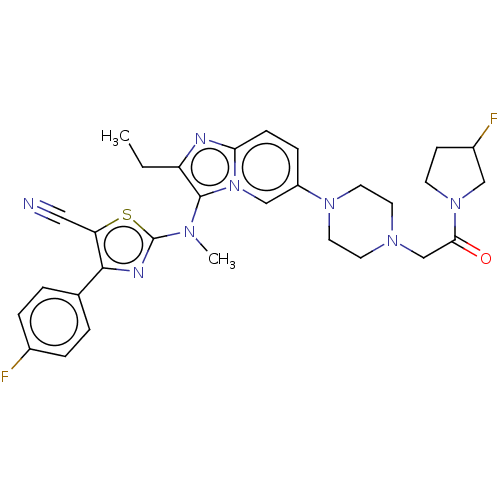
(CHEMBL5084058)Show SMILES CCc1nc2ccc(cn2c1N(C)c1nc(c(s1)C#N)-c1ccc(F)cc1)N1CCN(CC(=O)N2CCC(F)C2)CC1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of ATX lysoPLD (unknown origin) using 18:1 LPC as substrate assessed as reduction in choline release by HVA fluorescence based analysis |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00913
BindingDB Entry DOI: 10.7270/Q20P13WS |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 2
(Homo sapiens (Human)) | BDBM50580258
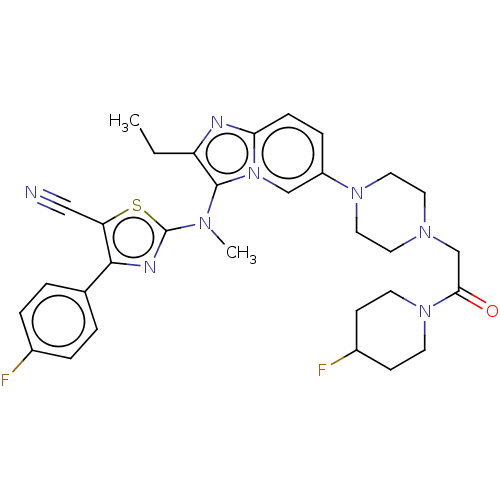
(CHEMBL5076928)Show SMILES CCc1nc2ccc(cn2c1N(C)c1nc(c(s1)C#N)-c1ccc(F)cc1)N1CCN(CC(=O)N2CCC(F)CC2)CC1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of ATX lysoPLD (unknown origin) using 18:1 LPC as substrate assessed as reduction in choline release by HVA fluorescence based analysis |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00913
BindingDB Entry DOI: 10.7270/Q20P13WS |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 2
(Homo sapiens (Human)) | BDBM50580263
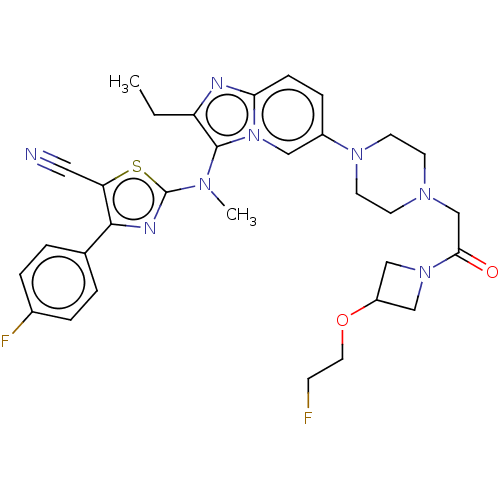
(CHEMBL5088371)Show SMILES CCc1nc2ccc(cn2c1N(C)c1nc(c(s1)C#N)-c1ccc(F)cc1)N1CCN(CC(=O)N2CC(C2)OCCF)CC1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of ATX lysoPLD (unknown origin) using 18:1 LPC as substrate assessed as reduction in choline release by HVA fluorescence based analysis |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00913
BindingDB Entry DOI: 10.7270/Q20P13WS |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 2
(Homo sapiens (Human)) | BDBM50580262

(CHEMBL5085520)Show SMILES CCc1nc2ccc(cn2c1N(C)c1nc(c(s1)C#N)-c1ccc(F)cc1)N1CCN(CC(=O)N2CCN(CCF)CC2)CC1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of ATX lysoPLD (unknown origin) using 18:1 LPC as substrate assessed as reduction in choline release by HVA fluorescence based analysis |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00913
BindingDB Entry DOI: 10.7270/Q20P13WS |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 2
(Rattus norvegicus) | BDBM50605405
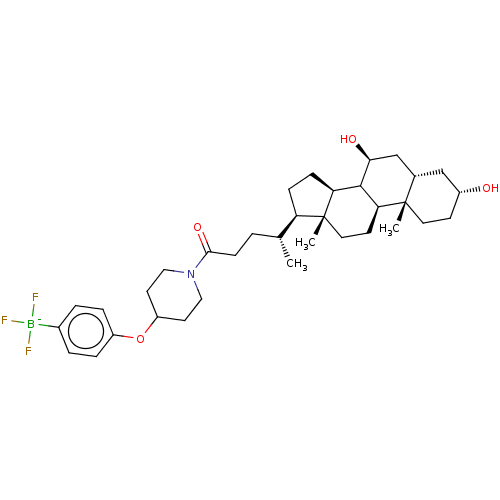
(CHEMBL5196449)Show SMILES [K;v0+].[H][C@@]1([#6]-[#6][C@@]2([H])[#6]3-[#6@@H](-[#8])-[#6][C@]4([H])[#6]-[#6@H](-[#8])-[#6]-[#6][C@]4([#6])[C@@]3([H])[#6]-[#6][C@]12[#6])[#6@H](-[#6])-[#6]-[#6]-[#6](=O)-[#7]-1-[#6]-[#6]-[#6](-[#6]-[#6]-1)-[#8]-c1ccc(cc1)[B-](F)(F)F |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 24 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00368
BindingDB Entry DOI: 10.7270/Q25H7MCC |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 2
(Homo sapiens (Human)) | BDBM192943
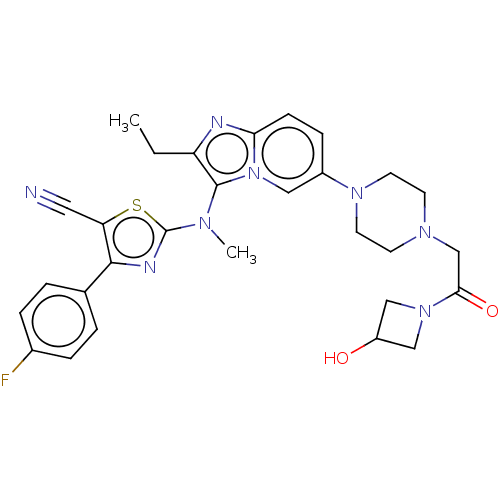
(US10526329, Compound 139 | US9670204, 138 2-((2-et...)Show SMILES CCc1nc2ccc(cn2c1N(C)c1nc(c(s1)C#N)-c1ccc(F)cc1)N1CCN(CC(=O)N2CC(O)C2)CC1 Show InChI InChI=1S/C29H31FN8O2S/c1-3-23-28(34(2)29-33-27(24(14-31)41-29)19-4-6-20(30)7-5-19)38-15-21(8-9-25(38)32-23)36-12-10-35(11-13-36)18-26(40)37-16-22(39)17-37/h4-9,15,22,39H,3,10-13,16-18H2,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of ATX lysoPLD (unknown origin) using 18:1 LPC as substrate assessed as reduction in choline release by HVA fluorescence based analysis |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00913
BindingDB Entry DOI: 10.7270/Q20P13WS |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 2
(Homo sapiens (Human)) | BDBM50580257
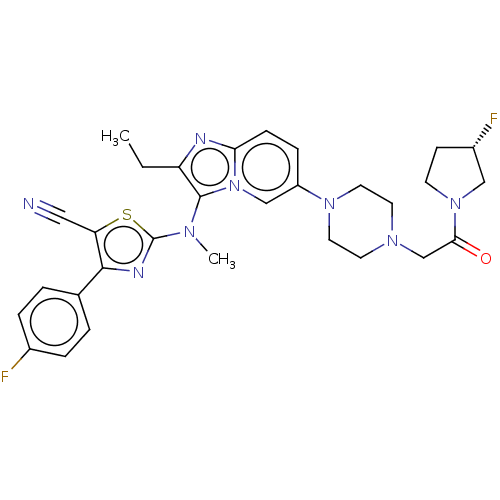
(CHEMBL5078471)Show SMILES CCc1nc2ccc(cn2c1N(C)c1nc(c(s1)C#N)-c1ccc(F)cc1)N1CCN(CC(=O)N2CC[C@H](F)C2)CC1 |r| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 32 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of ATX lysoPLD (unknown origin) using 18:1 LPC as substrate assessed as reduction in choline release by HVA fluorescence based analysis |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00913
BindingDB Entry DOI: 10.7270/Q20P13WS |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 2
(Homo sapiens (Human)) | BDBM50580254
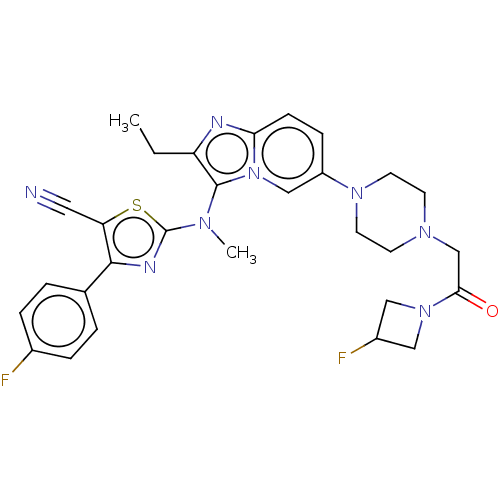
(CHEMBL5088215)Show SMILES CCc1nc2ccc(cn2c1N(C)c1nc(c(s1)C#N)-c1ccc(F)cc1)N1CCN(CC(=O)N2CC(F)C2)CC1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 41 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of ATX lysoPLD (unknown origin) using 18:1 LPC as substrate assessed as reduction in choline release by HVA fluorescence based analysis |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00913
BindingDB Entry DOI: 10.7270/Q20P13WS |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 2
(Homo sapiens (Human)) | BDBM50233915
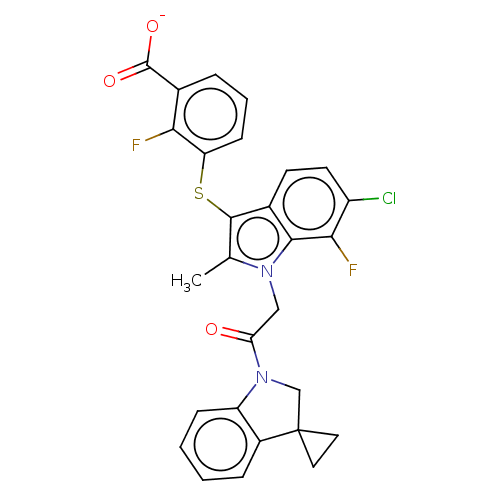
(CHEMBL4102874)Show SMILES [Na+].Cc1c(Sc2cccc(C([O-])=O)c2F)c2ccc(Cl)c(F)c2n1CC(=O)N1CC2(CC2)c2ccccc12 Show InChI InChI=1S/C28H21ClF2N2O3S/c1-15-26(37-21-8-4-5-16(23(21)30)27(35)36)17-9-10-19(29)24(31)25(17)32(15)13-22(34)33-14-28(11-12-28)18-6-2-3-7-20(18)33/h2-10H,11-14H2,1H3,(H,35,36)/p-1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Strathclyde
Curated by ChEMBL
| Assay Description
Inhibition of ATX (unknown origin) using lysophosphatidyl choline as substrate |
J Med Chem 60: 722-748 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01597
BindingDB Entry DOI: 10.7270/Q2MW2KCW |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 2
(Homo sapiens (Human)) | BDBM50187693

(CHEMBL3186509)Show SMILES Clc1cc(Cl)cc(COC(=O)N2CCN(CCC(=O)c3ccc4[nH]c(=O)oc4c3)CC2)c1 Show InChI InChI=1S/C22H21Cl2N3O5/c23-16-9-14(10-17(24)12-16)13-31-22(30)27-7-5-26(6-8-27)4-3-19(28)15-1-2-18-20(11-15)32-21(29)25-18/h1-2,9-12H,3-8,13H2,(H,25,29) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Strathclyde
Curated by ChEMBL
| Assay Description
Inhibition of human ATX expressed in HEK293 cells using FS-3 as substrate preincubated for 15 mins followed by substrate addition measured after 30 m... |
J Med Chem 60: 722-748 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01597
BindingDB Entry DOI: 10.7270/Q2MW2KCW |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 2
(Homo sapiens (Human)) | BDBM50187693

(CHEMBL3186509)Show SMILES Clc1cc(Cl)cc(COC(=O)N2CCN(CCC(=O)c3ccc4[nH]c(=O)oc4c3)CC2)c1 Show InChI InChI=1S/C22H21Cl2N3O5/c23-16-9-14(10-17(24)12-16)13-31-22(30)27-7-5-26(6-8-27)4-3-19(28)15-1-2-18-20(11-15)32-21(29)25-18/h1-2,9-12H,3-8,13H2,(H,25,29) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Netherlands Institute
Curated by ChEMBL
| Assay Description
Inhibition of human ATX expressed in HEK293 cells using FS-3 as substrate after 15 mins |
J Med Chem 60: 2006-2017 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01743
BindingDB Entry DOI: 10.7270/Q22F7RGR |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 2
(Homo sapiens (Human)) | BDBM50187692
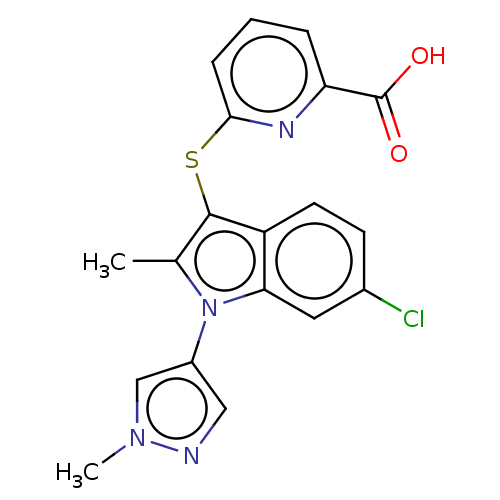
(CHEMBL3827757)Show SMILES Cc1c(Sc2cccc(n2)C(O)=O)c2ccc(Cl)cc2n1-c1cnn(C)c1 Show InChI InChI=1S/C19H15ClN4O2S/c1-11-18(27-17-5-3-4-15(22-17)19(25)26)14-7-6-12(20)8-16(14)24(11)13-9-21-23(2)10-13/h3-10H,1-2H3,(H,25,26) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Strathclyde
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human ATX using bisP-nitrophenyl phosphate as substrate measured after 30 mins |
J Med Chem 60: 722-748 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01597
BindingDB Entry DOI: 10.7270/Q2MW2KCW |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 2
(Homo sapiens (Human)) | BDBM50233913
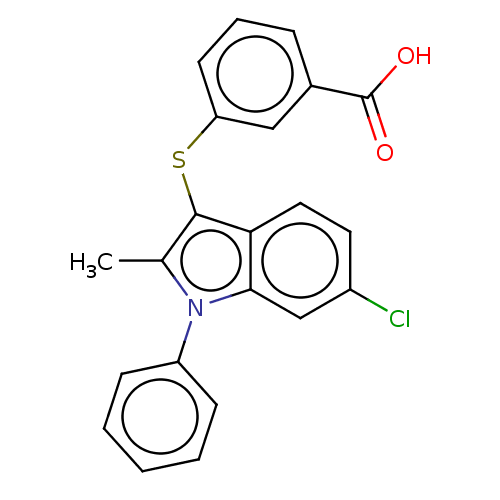
(CHEMBL4073215)Show SMILES Cc1c(Sc2cccc(c2)C(O)=O)c2ccc(Cl)cc2n1-c1ccccc1 Show InChI InChI=1S/C22H16ClNO2S/c1-14-21(27-18-9-5-6-15(12-18)22(25)26)19-11-10-16(23)13-20(19)24(14)17-7-3-2-4-8-17/h2-13H,1H3,(H,25,26) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Strathclyde
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human ATX using bisP-nitrophenyl phosphate as substrate measured after 30 mins |
J Med Chem 60: 722-748 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01597
BindingDB Entry DOI: 10.7270/Q2MW2KCW |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 2
(Homo sapiens (Human)) | BDBM50233919
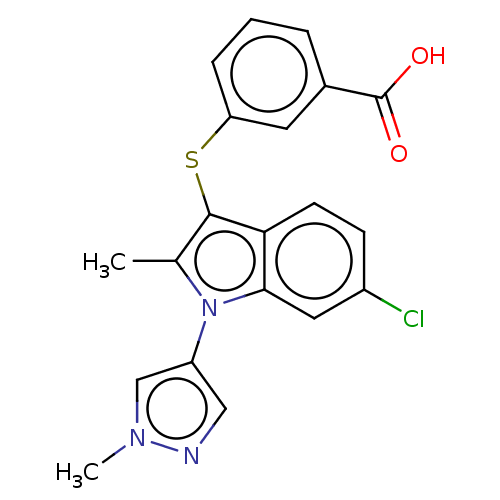
(CHEMBL4093897)Show SMILES Cc1c(Sc2cccc(c2)C(O)=O)c2ccc(Cl)cc2n1-c1cnn(C)c1 Show InChI InChI=1S/C20H16ClN3O2S/c1-12-19(27-16-5-3-4-13(8-16)20(25)26)17-7-6-14(21)9-18(17)24(12)15-10-22-23(2)11-15/h3-11H,1-2H3,(H,25,26) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Strathclyde
Curated by ChEMBL
| Assay Description
Inhibition of human ATX expressed in HEK293 Flp-In cells using LPC as substrate measured every 30 sec for 90 mins by fluorescence assay |
J Med Chem 60: 722-748 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01597
BindingDB Entry DOI: 10.7270/Q2MW2KCW |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 2
(Homo sapiens (Human)) | BDBM50501623

(CHEMBL4062929)Show SMILES [H][C@@]1(CC[C@@]2([H])[C@]3([H])[C@@H](O)C[C@]4([H])C[C@H](O)CC[C@]4(C)[C@@]3([H])CC[C@]12C)[C@H](C)CCC(=O)N[C@@H]1CCN(C1)C(=O)OCc1cc(Cl)cc(Cl)c1 |r| Show InChI InChI=1S/C36H52Cl2N2O5/c1-21(4-7-32(43)39-26-10-13-40(19-26)34(44)45-20-22-14-24(37)18-25(38)15-22)28-5-6-29-33-30(9-12-36(28,29)3)35(2)11-8-27(41)16-23(35)17-31(33)42/h14-15,18,21,23,26-31,33,41-42H,4-13,16-17,19-20H2,1-3H3,(H,39,43)/t21-,23+,26-,27-,28-,29+,30+,31+,33+,35+,36-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
The Netherlands Institute
Curated by ChEMBL
| Assay Description
Inhibition of human ATX expressed in HEK293 Flp-In cells assessed as decrease in choline release from LPC measured every 30 secs for 90 mins by HVA b... |
J Med Chem 60: 2006-2017 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01743
BindingDB Entry DOI: 10.7270/Q22F7RGR |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 2
(Rattus norvegicus) | BDBM50501623

(CHEMBL4062929)Show SMILES [H][C@@]1(CC[C@@]2([H])[C@]3([H])[C@@H](O)C[C@]4([H])C[C@H](O)CC[C@]4(C)[C@@]3([H])CC[C@]12C)[C@H](C)CCC(=O)N[C@@H]1CCN(C1)C(=O)OCc1cc(Cl)cc(Cl)c1 |r| Show InChI InChI=1S/C36H52Cl2N2O5/c1-21(4-7-32(43)39-26-10-13-40(19-26)34(44)45-20-22-14-24(37)18-25(38)15-22)28-5-6-29-33-30(9-12-36(28,29)3)35(2)11-8-27(41)16-23(35)17-31(33)42/h14-15,18,21,23,26-31,33,41-42H,4-13,16-17,19-20H2,1-3H3,(H,39,43)/t21-,23+,26-,27-,28-,29+,30+,31+,33+,35+,36-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00368
BindingDB Entry DOI: 10.7270/Q25H7MCC |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 2
(Homo sapiens (Human)) | BDBM50187692

(CHEMBL3827757)Show SMILES Cc1c(Sc2cccc(n2)C(O)=O)c2ccc(Cl)cc2n1-c1cnn(C)c1 Show InChI InChI=1S/C19H15ClN4O2S/c1-11-18(27-17-5-3-4-15(22-17)19(25)26)14-7-6-12(20)8-16(14)24(11)13-9-21-23(2)10-13/h3-10H,1-2H3,(H,25,26) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Strathclyde
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human ATX using bisP-nitrophenyl phosphate as substrate measured after 30 mins |
J Med Chem 60: 722-748 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01597
BindingDB Entry DOI: 10.7270/Q2MW2KCW |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 2
(Homo sapiens (Human)) | BDBM50187696
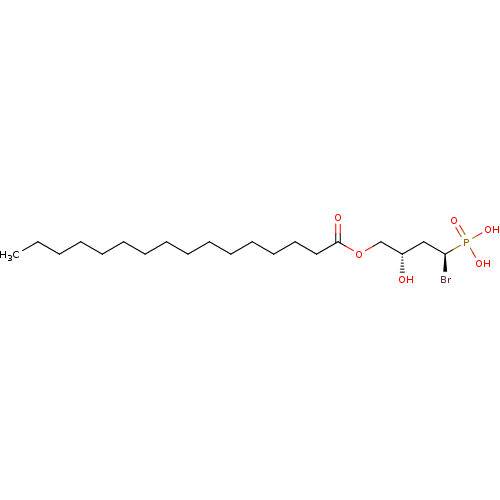
(CHEMBL3621356)Show SMILES CCCCCCCCCCCCCCCC(=O)OC[C@@H](O)C[C@H](Br)P(O)(O)=O |r| Show InChI InChI=1S/C20H40BrO6P/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-15-20(23)27-17-18(22)16-19(21)28(24,25)26/h18-19,22H,2-17H2,1H3,(H2,24,25,26)/t18-,19+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Strathclyde
Curated by ChEMBL
| Assay Description
Inhibition of recombinant HA-tagged ATX (unknown origin) using FS-3 as substrate measured after 2 hrs by fluorescence assay |
J Med Chem 60: 722-748 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01597
BindingDB Entry DOI: 10.7270/Q2MW2KCW |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 2
(Homo sapiens (Human)) | BDBM50233930
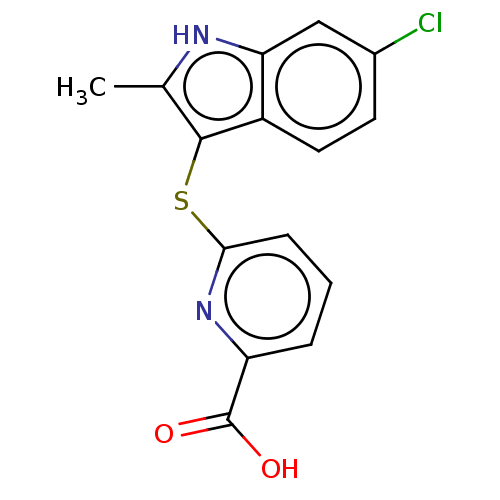
(CHEMBL4073328)Show InChI InChI=1S/C15H11ClN2O2S/c1-8-14(10-6-5-9(16)7-12(10)17-8)21-13-4-2-3-11(18-13)15(19)20/h2-7,17H,1H3,(H,19,20) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Strathclyde
Curated by ChEMBL
| Assay Description
Inhibition of human ATX expressed in HEK293 Flp-In cells using LPC as substrate measured every 30 sec for 90 mins by fluorescence assay |
J Med Chem 60: 722-748 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01597
BindingDB Entry DOI: 10.7270/Q2MW2KCW |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 2
(Homo sapiens (Human)) | BDBM50233921
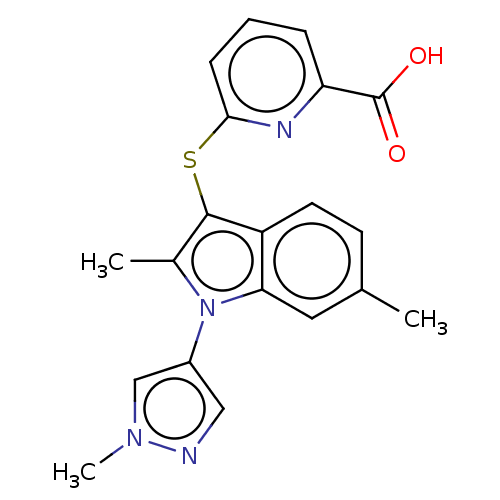
(CHEMBL4086837)Show SMILES Cc1c(Sc2cccc(n2)C(O)=O)c2ccc(C)cc2n1-c1cnn(C)c1 Show InChI InChI=1S/C20H18N4O2S/c1-12-7-8-15-17(9-12)24(14-10-21-23(3)11-14)13(2)19(15)27-18-6-4-5-16(22-18)20(25)26/h4-11H,1-3H3,(H,25,26) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Strathclyde
Curated by ChEMBL
| Assay Description
Inhibition of human ATX expressed in HEK293 Flp-In cells using LPC as substrate measured every 30 sec for 90 mins by fluorescence assay |
J Med Chem 60: 722-748 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01597
BindingDB Entry DOI: 10.7270/Q2MW2KCW |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 2
(Rattus norvegicus) | BDBM50605406

(CHEMBL5190935)Show SMILES [H][C@@]1(CC[C@@]2([H])C3[C@@H](O)C[C@]4([H])C[C@H](O)CC[C@]4(C)[C@@]3([H])CC[C@]12C)[C@H](C)CCC(=O)N1CCC(CC1)Oc1ccc(cc1)B(O)O |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00368
BindingDB Entry DOI: 10.7270/Q25H7MCC |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 2
(Rattus norvegicus) | BDBM50605406

(CHEMBL5190935)Show SMILES [H][C@@]1(CC[C@@]2([H])C3[C@@H](O)C[C@]4([H])C[C@H](O)CC[C@]4(C)[C@@]3([H])CC[C@]12C)[C@H](C)CCC(=O)N1CCC(CC1)Oc1ccc(cc1)B(O)O |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00368
BindingDB Entry DOI: 10.7270/Q25H7MCC |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 2
(Homo sapiens (Human)) | BDBM50233924
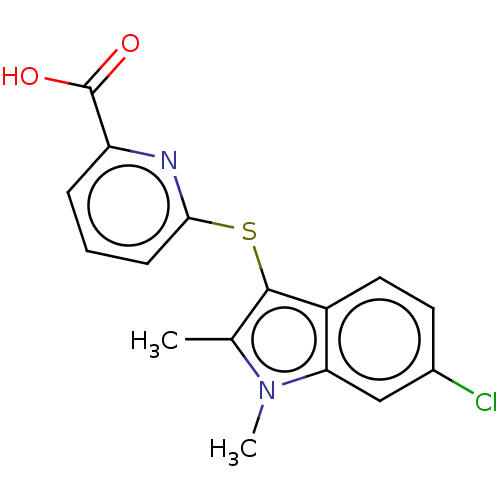
(CHEMBL4090230)Show InChI InChI=1S/C16H13ClN2O2S/c1-9-15(11-7-6-10(17)8-13(11)19(9)2)22-14-5-3-4-12(18-14)16(20)21/h3-8H,1-2H3,(H,20,21) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Strathclyde
Curated by ChEMBL
| Assay Description
Inhibition of human ATX expressed in HEK293 Flp-In cells using LPC as substrate measured every 30 sec for 90 mins by fluorescence assay |
J Med Chem 60: 722-748 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01597
BindingDB Entry DOI: 10.7270/Q2MW2KCW |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 2
(Rattus norvegicus) | BDBM50605405

(CHEMBL5196449)Show SMILES [K;v0+].[H][C@@]1([#6]-[#6][C@@]2([H])[#6]3-[#6@@H](-[#8])-[#6][C@]4([H])[#6]-[#6@H](-[#8])-[#6]-[#6][C@]4([#6])[C@@]3([H])[#6]-[#6][C@]12[#6])[#6@H](-[#6])-[#6]-[#6]-[#6](=O)-[#7]-1-[#6]-[#6]-[#6](-[#6]-[#6]-1)-[#8]-c1ccc(cc1)[B-](F)(F)F |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00368
BindingDB Entry DOI: 10.7270/Q25H7MCC |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 2
(Homo sapiens (Human)) | BDBM50233909
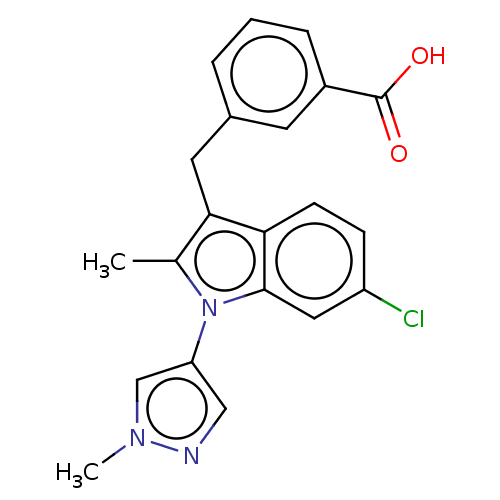
(CHEMBL4078251)Show SMILES Cc1c(Cc2cccc(c2)C(O)=O)c2ccc(Cl)cc2n1-c1cnn(C)c1 Show InChI InChI=1S/C21H18ClN3O2/c1-13-19(9-14-4-3-5-15(8-14)21(26)27)18-7-6-16(22)10-20(18)25(13)17-11-23-24(2)12-17/h3-8,10-12H,9H2,1-2H3,(H,26,27) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Strathclyde
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human ATX using bisP-nitrophenyl phosphate as substrate measured after 30 mins |
J Med Chem 60: 722-748 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01597
BindingDB Entry DOI: 10.7270/Q2MW2KCW |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 2
(Rattus norvegicus) | BDBM50605406

(CHEMBL5190935)Show SMILES [H][C@@]1(CC[C@@]2([H])C3[C@@H](O)C[C@]4([H])C[C@H](O)CC[C@]4(C)[C@@]3([H])CC[C@]12C)[C@H](C)CCC(=O)N1CCC(CC1)Oc1ccc(cc1)B(O)O |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00368
BindingDB Entry DOI: 10.7270/Q25H7MCC |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 2
(Rattus norvegicus) | BDBM50605406

(CHEMBL5190935)Show SMILES [H][C@@]1(CC[C@@]2([H])C3[C@@H](O)C[C@]4([H])C[C@H](O)CC[C@]4(C)[C@@]3([H])CC[C@]12C)[C@H](C)CCC(=O)N1CCC(CC1)Oc1ccc(cc1)B(O)O |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00368
BindingDB Entry DOI: 10.7270/Q25H7MCC |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 2
(Rattus norvegicus) | BDBM50605405

(CHEMBL5196449)Show SMILES [K;v0+].[H][C@@]1([#6]-[#6][C@@]2([H])[#6]3-[#6@@H](-[#8])-[#6][C@]4([H])[#6]-[#6@H](-[#8])-[#6]-[#6][C@]4([#6])[C@@]3([H])[#6]-[#6][C@]12[#6])[#6@H](-[#6])-[#6]-[#6]-[#6](=O)-[#7]-1-[#6]-[#6]-[#6](-[#6]-[#6]-1)-[#8]-c1ccc(cc1)[B-](F)(F)F |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 45 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00368
BindingDB Entry DOI: 10.7270/Q25H7MCC |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 2
(Rattus norvegicus) | BDBM50605401

(CHEMBL5181584)Show SMILES [H][C@@]1(CC[C@@]2([H])C3[C@@H](O)C[C@]4([H])C[C@H](O)CC[C@]4(C)[C@@]3([H])CC[C@]12C)[C@H](C)CCC(=O)NCCCOc1ccc(cc1)B(O)O |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00368
BindingDB Entry DOI: 10.7270/Q25H7MCC |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 2
(Rattus norvegicus) | BDBM50605405

(CHEMBL5196449)Show SMILES [K;v0+].[H][C@@]1([#6]-[#6][C@@]2([H])[#6]3-[#6@@H](-[#8])-[#6][C@]4([H])[#6]-[#6@H](-[#8])-[#6]-[#6][C@]4([#6])[C@@]3([H])[#6]-[#6][C@]12[#6])[#6@H](-[#6])-[#6]-[#6]-[#6](=O)-[#7]-1-[#6]-[#6]-[#6](-[#6]-[#6]-1)-[#8]-c1ccc(cc1)[B-](F)(F)F |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00368
BindingDB Entry DOI: 10.7270/Q25H7MCC |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 2
(Rattus norvegicus) | BDBM50605406

(CHEMBL5190935)Show SMILES [H][C@@]1(CC[C@@]2([H])C3[C@@H](O)C[C@]4([H])C[C@H](O)CC[C@]4(C)[C@@]3([H])CC[C@]12C)[C@H](C)CCC(=O)N1CCC(CC1)Oc1ccc(cc1)B(O)O |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 54 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00368
BindingDB Entry DOI: 10.7270/Q25H7MCC |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 2
(Rattus norvegicus) | BDBM50605407

(CHEMBL5205292)Show SMILES OB(O)c1ccc(COc2ccc(\C=C3\SC(=O)N(Cc4ccc(F)cc4)C3=O)cc2)cc1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00368
BindingDB Entry DOI: 10.7270/Q25H7MCC |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 2
(Rattus norvegicus) | BDBM50605399

(CHEMBL5198478)Show SMILES [H][C@@]1(CC[C@@]2([H])C3[C@@H](O)C[C@]4([H])C[C@H](O)CC[C@]4(C)[C@@]3([H])CC[C@]12C)[C@H](C)CCC(=O)NCCCOc1ccc(cc1)B1OC(C)(C)C(C)(C)O1 |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00368
BindingDB Entry DOI: 10.7270/Q25H7MCC |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 2
(Rattus norvegicus) | BDBM50605400

(CHEMBL5202425)Show SMILES [Na;v0+].[H][C@@]1([#6]-[#6][C@@]2([H])[#6]3-[#6@@H](-[#8])-[#6][C@]4([H])[#6]-[#6@H](-[#8])-[#6]-[#6][C@]4([#6])[C@@]3([H])[#6]-[#6][C@]12[#6])[#6@H](-[#6])-[#6]-[#6]-[#6](=O)-[#7]-[#6]-[#6]-[#6]-[#8]-c1ccc(cc1)[B-](F)(F)F |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00368
BindingDB Entry DOI: 10.7270/Q25H7MCC |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 2
(Rattus norvegicus) | BDBM50605405

(CHEMBL5196449)Show SMILES [K;v0+].[H][C@@]1([#6]-[#6][C@@]2([H])[#6]3-[#6@@H](-[#8])-[#6][C@]4([H])[#6]-[#6@H](-[#8])-[#6]-[#6][C@]4([#6])[C@@]3([H])[#6]-[#6][C@]12[#6])[#6@H](-[#6])-[#6]-[#6]-[#6](=O)-[#7]-1-[#6]-[#6]-[#6](-[#6]-[#6]-1)-[#8]-c1ccc(cc1)[B-](F)(F)F |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 72 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00368
BindingDB Entry DOI: 10.7270/Q25H7MCC |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 2
(Homo sapiens (Human)) | BDBM50233941
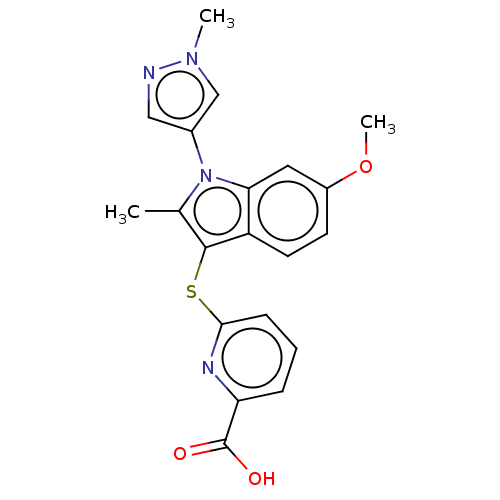
(CHEMBL4065406)Show SMILES COc1ccc2c(Sc3cccc(n3)C(O)=O)c(C)n(-c3cnn(C)c3)c2c1 Show InChI InChI=1S/C20H18N4O3S/c1-12-19(28-18-6-4-5-16(22-18)20(25)26)15-8-7-14(27-3)9-17(15)24(12)13-10-21-23(2)11-13/h4-11H,1-3H3,(H,25,26) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 75 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Strathclyde
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human ATX using bisP-nitrophenyl phosphate as substrate measured after 30 mins |
J Med Chem 60: 722-748 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01597
BindingDB Entry DOI: 10.7270/Q2MW2KCW |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 2
(Rattus norvegicus) | BDBM50605402

(CHEMBL5185387)Show SMILES [H][C@@]1(CC[C@@]2([H])C3[C@@H](O)C[C@]4([H])C[C@H](O)CC[C@]4(C)[C@@]3([H])CC[C@]12C)[C@H](C)CCC(=O)N1CCC(CC1)Oc1ccc(cc1)B1OC(C)(C)C(C)(C)O1 |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00368
BindingDB Entry DOI: 10.7270/Q25H7MCC |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 2
(Homo sapiens (Human)) | BDBM50233913

(CHEMBL4073215)Show SMILES Cc1c(Sc2cccc(c2)C(O)=O)c2ccc(Cl)cc2n1-c1ccccc1 Show InChI InChI=1S/C22H16ClNO2S/c1-14-21(27-18-9-5-6-15(12-18)22(25)26)19-11-10-16(23)13-20(19)24(14)17-7-3-2-4-8-17/h2-13H,1H3,(H,25,26) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 81 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Strathclyde
Curated by ChEMBL
| Assay Description
Inhibition of human ATX expressed in HEK293 Flp-In cells using LPC as substrate measured every 30 sec for 90 mins by fluorescence assay |
J Med Chem 60: 722-748 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01597
BindingDB Entry DOI: 10.7270/Q2MW2KCW |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 2
(Rattus norvegicus) | BDBM50605405

(CHEMBL5196449)Show SMILES [K;v0+].[H][C@@]1([#6]-[#6][C@@]2([H])[#6]3-[#6@@H](-[#8])-[#6][C@]4([H])[#6]-[#6@H](-[#8])-[#6]-[#6][C@]4([#6])[C@@]3([H])[#6]-[#6][C@]12[#6])[#6@H](-[#6])-[#6]-[#6]-[#6](=O)-[#7]-1-[#6]-[#6]-[#6](-[#6]-[#6]-1)-[#8]-c1ccc(cc1)[B-](F)(F)F |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 84 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00368
BindingDB Entry DOI: 10.7270/Q25H7MCC |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 2
(Homo sapiens (Human)) | BDBM50233921

(CHEMBL4086837)Show SMILES Cc1c(Sc2cccc(n2)C(O)=O)c2ccc(C)cc2n1-c1cnn(C)c1 Show InChI InChI=1S/C20H18N4O2S/c1-12-7-8-15-17(9-12)24(14-10-21-23(3)11-14)13(2)19(15)27-18-6-4-5-16(22-18)20(25)26/h4-11H,1-3H3,(H,25,26) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 85 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Strathclyde
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human ATX using bisP-nitrophenyl phosphate as substrate measured after 30 mins |
J Med Chem 60: 722-748 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01597
BindingDB Entry DOI: 10.7270/Q2MW2KCW |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 2
(Homo sapiens (Human)) | BDBM50233934
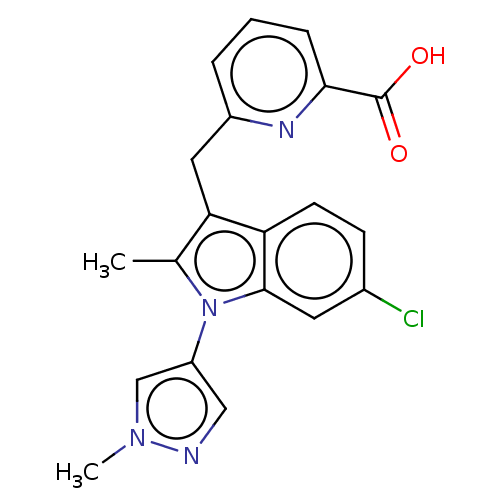
(CHEMBL4102520)Show SMILES Cc1c(Cc2cccc(n2)C(O)=O)c2ccc(Cl)cc2n1-c1cnn(C)c1 Show InChI InChI=1S/C20H17ClN4O2/c1-12-17(9-14-4-3-5-18(23-14)20(26)27)16-7-6-13(21)8-19(16)25(12)15-10-22-24(2)11-15/h3-8,10-11H,9H2,1-2H3,(H,26,27) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 89 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Strathclyde
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human ATX using bisP-nitrophenyl phosphate as substrate measured after 30 mins |
J Med Chem 60: 722-748 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01597
BindingDB Entry DOI: 10.7270/Q2MW2KCW |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 2
(Homo sapiens (Human)) | BDBM50501636

(CHEMBL4093316)Show SMILES [H][C@@]1(CC[C@@]2([H])[C@]3([H])[C@@H](O)C[C@]4([H])C[C@H](O)CC[C@]4(C)[C@@]3([H])CC[C@]12C)[C@H](C)CCC(=O)N1CCC(O)(CNC(=O)OCc2cc(Cl)cc(Cl)c2)CC1 |r| Show InChI InChI=1S/C38H56Cl2N2O6/c1-23(29-5-6-30-34-31(9-11-37(29,30)3)36(2)10-8-28(43)18-25(36)19-32(34)44)4-7-33(45)42-14-12-38(47,13-15-42)22-41-35(46)48-21-24-16-26(39)20-27(40)17-24/h16-17,20,23,25,28-32,34,43-44,47H,4-15,18-19,21-22H2,1-3H3,(H,41,46)/t23-,25+,28-,29-,30+,31+,32+,34+,36+,37-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
The Netherlands Institute
Curated by ChEMBL
| Assay Description
Inhibition of human ATX expressed in HEK293 Flp-In cells assessed as decrease in choline release from LPC measured every 30 secs for 90 mins by HVA b... |
J Med Chem 60: 2006-2017 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01743
BindingDB Entry DOI: 10.7270/Q22F7RGR |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 2
(Homo sapiens (Human)) | BDBM50233918
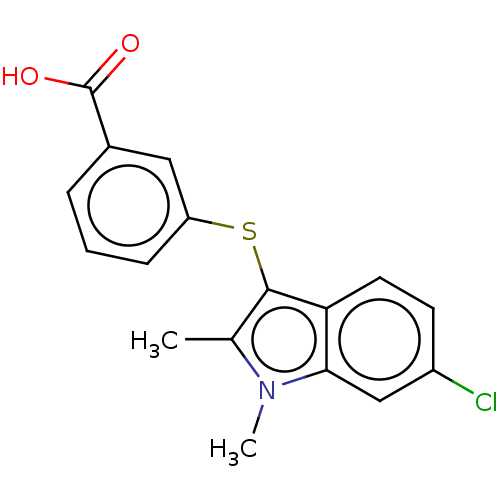
(CHEMBL4063573)Show InChI InChI=1S/C17H14ClNO2S/c1-10-16(14-7-6-12(18)9-15(14)19(10)2)22-13-5-3-4-11(8-13)17(20)21/h3-9H,1-2H3,(H,20,21) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Strathclyde
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human ATX using bisP-nitrophenyl phosphate as substrate measured after 30 mins |
J Med Chem 60: 722-748 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01597
BindingDB Entry DOI: 10.7270/Q2MW2KCW |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data