

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sodium-dependent dopamine transporter (Homo sapiens (Human)) | BDBM50101075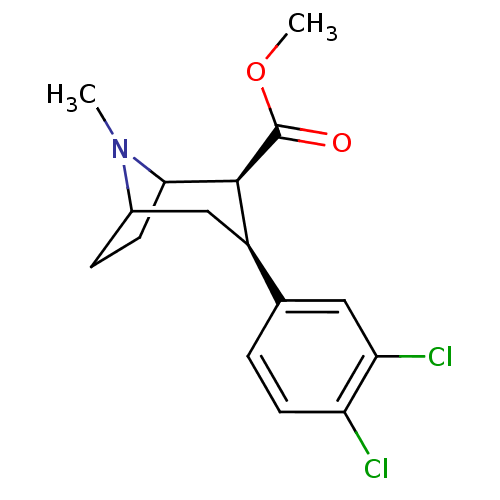 ((2S,3S)-3-(3,4-Dichloro-phenyl)-2-methoxycarbonyl-...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse-Intramural Research Program Curated by ChEMBL | Assay Description Displacement of [3H]CFT from human DAT expressed in COS7 cells | J Med Chem 48: 7513-6 (2005) Article DOI: 10.1021/jm050431y BindingDB Entry DOI: 10.7270/Q27M07HM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent dopamine transporter (Homo sapiens (Human)) | BDBM50101075 ((2S,3S)-3-(3,4-Dichloro-phenyl)-2-methoxycarbonyl-...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse-Intramural Research Program Curated by ChEMBL | Assay Description Displacement of [3H]dopamine from human DAT expressed in COS7 cells | J Med Chem 48: 7513-6 (2005) Article DOI: 10.1021/jm050431y BindingDB Entry DOI: 10.7270/Q27M07HM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent noradrenaline transporter (Homo sapiens (Human)) | BDBM50101075 ((2S,3S)-3-(3,4-Dichloro-phenyl)-2-methoxycarbonyl-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse-Intramural Research Program Curated by ChEMBL | Assay Description Displacement of [3H]dopamine from human NET expressed in COS7 cells | J Med Chem 48: 7513-6 (2005) Article DOI: 10.1021/jm050431y BindingDB Entry DOI: 10.7270/Q27M07HM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-1 (Homo sapiens (Human)) | BDBM50383265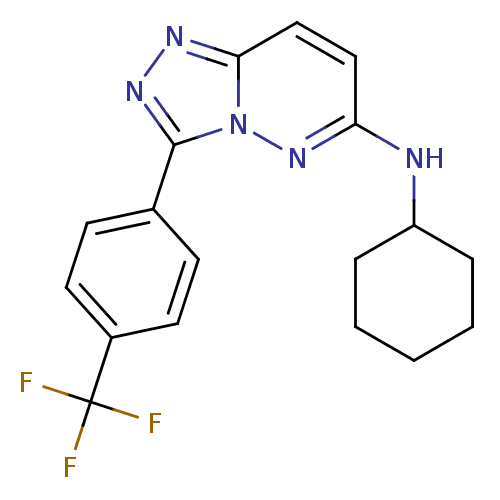 (CHEMBL2032368) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genzyme Corp. Curated by ChEMBL | Assay Description Inhibition of human Pim-1 using 5-FAM-RSRHSSYPAGT-CONH2 as substrate preincubated for 15 mins prior substrate addition measured after 45 mins by fluo... | J Med Chem 55: 2641-8 (2012) Article DOI: 10.1021/jm2014698 BindingDB Entry DOI: 10.7270/Q2Q2418B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Homo sapiens (Human)) | BDBM50101075 ((2S,3S)-3-(3,4-Dichloro-phenyl)-2-methoxycarbonyl-...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse-Intramural Research Program Curated by ChEMBL | Assay Description Displacement of [3H]5HT from human SERT expressed in COS7 cells | J Med Chem 48: 7513-6 (2005) Article DOI: 10.1021/jm050431y BindingDB Entry DOI: 10.7270/Q27M07HM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent dopamine transporter (Homo sapiens (Human)) | BDBM50177767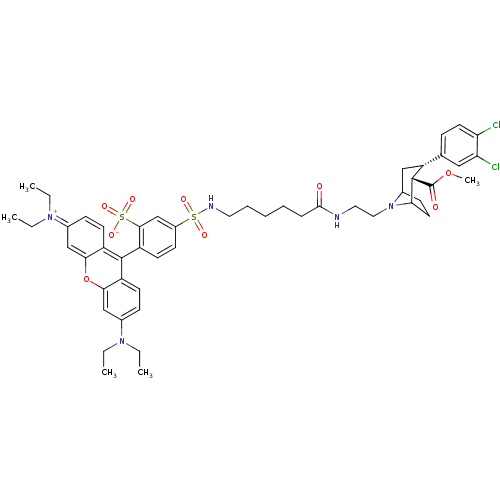 (2-(3-sec-Butylimino-6-diethylamino-3H-xanthen-9-yl...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse-Intramural Research Program Curated by ChEMBL | Assay Description Displacement of [3H]CFT from human DAT expressed in COS7 cells | J Med Chem 48: 7513-6 (2005) Article DOI: 10.1021/jm050431y BindingDB Entry DOI: 10.7270/Q27M07HM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent dopamine transporter (Homo sapiens (Human)) | BDBM50177766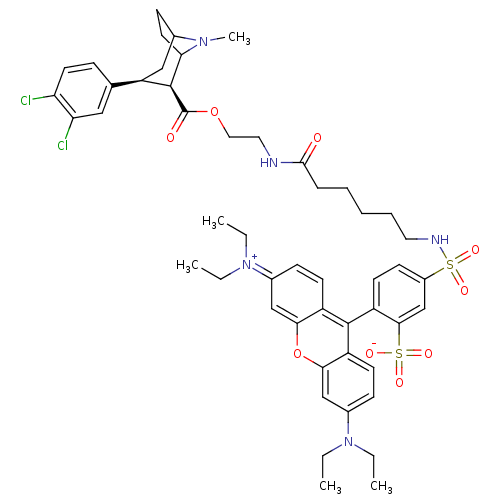 (2-(3-sec-Butylimino-6-diethylamino-3H-xanthen-9-yl...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse-Intramural Research Program Curated by ChEMBL | Assay Description Displacement of [3H]CFT from human DAT expressed in COS7 cells | J Med Chem 48: 7513-6 (2005) Article DOI: 10.1021/jm050431y BindingDB Entry DOI: 10.7270/Q27M07HM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent dopamine transporter (Homo sapiens (Human)) | BDBM50177769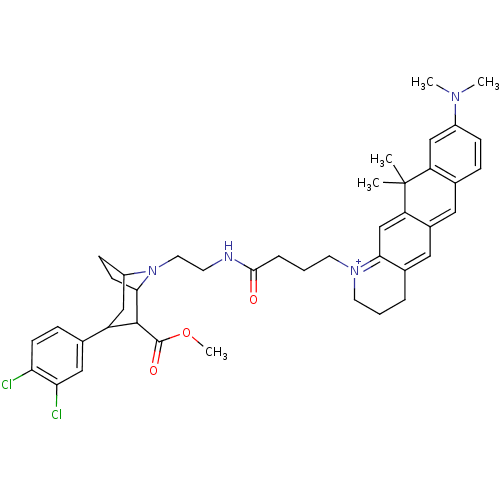 (CHEMBL198842 | [1-(3-{2-[3-(3,4-Dichloro-phenyl)-2...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse-Intramural Research Program Curated by ChEMBL | Assay Description Displacement of [3H]CFT from human DAT expressed in COS7 cells | J Med Chem 48: 7513-6 (2005) Article DOI: 10.1021/jm050431y BindingDB Entry DOI: 10.7270/Q27M07HM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent dopamine transporter (Homo sapiens (Human)) | BDBM50177767 (2-(3-sec-Butylimino-6-diethylamino-3H-xanthen-9-yl...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 62 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse-Intramural Research Program Curated by ChEMBL | Assay Description Displacement of [3H]dopamine from human DAT expressed in COS7 cells | J Med Chem 48: 7513-6 (2005) Article DOI: 10.1021/jm050431y BindingDB Entry DOI: 10.7270/Q27M07HM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent dopamine transporter (Homo sapiens (Human)) | BDBM50177766 (2-(3-sec-Butylimino-6-diethylamino-3H-xanthen-9-yl...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 72 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse-Intramural Research Program Curated by ChEMBL | Assay Description Displacement of [3H]dopamine from human DAT expressed in COS7 cells | J Med Chem 48: 7513-6 (2005) Article DOI: 10.1021/jm050431y BindingDB Entry DOI: 10.7270/Q27M07HM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent noradrenaline transporter (Homo sapiens (Human)) | BDBM50177766 (2-(3-sec-Butylimino-6-diethylamino-3H-xanthen-9-yl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 177 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse-Intramural Research Program Curated by ChEMBL | Assay Description Displacement of [3H]dopamine from human NET expressed in COS7 cells | J Med Chem 48: 7513-6 (2005) Article DOI: 10.1021/jm050431y BindingDB Entry DOI: 10.7270/Q27M07HM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent noradrenaline transporter (Homo sapiens (Human)) | BDBM50177767 (2-(3-sec-Butylimino-6-diethylamino-3H-xanthen-9-yl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 194 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse-Intramural Research Program Curated by ChEMBL | Assay Description Displacement of [3H]dopamine from human NET expressed in COS7 cells | J Med Chem 48: 7513-6 (2005) Article DOI: 10.1021/jm050431y BindingDB Entry DOI: 10.7270/Q27M07HM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Homo sapiens (Human)) | BDBM50177769 (CHEMBL198842 | [1-(3-{2-[3-(3,4-Dichloro-phenyl)-2...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 227 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse-Intramural Research Program Curated by ChEMBL | Assay Description Displacement of [3H]5HT from human SERT expressed in COS7 cells | J Med Chem 48: 7513-6 (2005) Article DOI: 10.1021/jm050431y BindingDB Entry DOI: 10.7270/Q27M07HM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent dopamine transporter (Homo sapiens (Human)) | BDBM50177769 (CHEMBL198842 | [1-(3-{2-[3-(3,4-Dichloro-phenyl)-2...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse-Intramural Research Program Curated by ChEMBL | Assay Description Displacement of [3H]dopamine from human DAT expressed in COS7 cells | J Med Chem 48: 7513-6 (2005) Article DOI: 10.1021/jm050431y BindingDB Entry DOI: 10.7270/Q27M07HM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent noradrenaline transporter (Homo sapiens (Human)) | BDBM50177769 (CHEMBL198842 | [1-(3-{2-[3-(3,4-Dichloro-phenyl)-2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 308 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse-Intramural Research Program Curated by ChEMBL | Assay Description Displacement of [3H]dopamine from human NET expressed in COS7 cells | J Med Chem 48: 7513-6 (2005) Article DOI: 10.1021/jm050431y BindingDB Entry DOI: 10.7270/Q27M07HM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Homo sapiens (Human)) | BDBM50177767 (2-(3-sec-Butylimino-6-diethylamino-3H-xanthen-9-yl...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 392 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse-Intramural Research Program Curated by ChEMBL | Assay Description Displacement of [3H]5HT from human SERT expressed in COS7 cells | J Med Chem 48: 7513-6 (2005) Article DOI: 10.1021/jm050431y BindingDB Entry DOI: 10.7270/Q27M07HM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent dopamine transporter (Homo sapiens (Human)) | BDBM50177768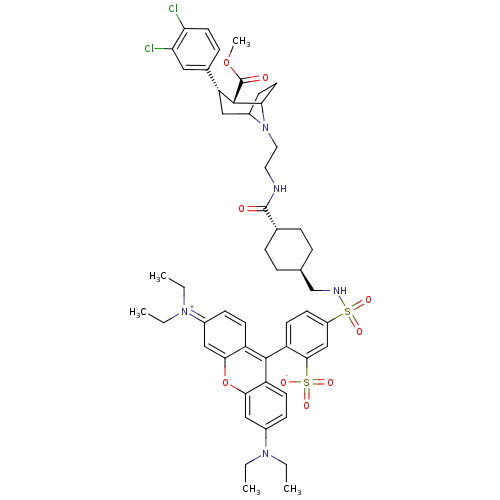 (2-(3-sec-Butylimino-6-diethylamino-3H-xanthen-9-yl...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 436 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse-Intramural Research Program Curated by ChEMBL | Assay Description Displacement of [3H]dopamine from human DAT expressed in COS7 cells | J Med Chem 48: 7513-6 (2005) Article DOI: 10.1021/jm050431y BindingDB Entry DOI: 10.7270/Q27M07HM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent noradrenaline transporter (Homo sapiens (Human)) | BDBM50177768 (2-(3-sec-Butylimino-6-diethylamino-3H-xanthen-9-yl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 627 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse-Intramural Research Program Curated by ChEMBL | Assay Description Displacement of [3H]dopamine from human NET expressed in COS7 cells | J Med Chem 48: 7513-6 (2005) Article DOI: 10.1021/jm050431y BindingDB Entry DOI: 10.7270/Q27M07HM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Homo sapiens (Human)) | BDBM50177768 (2-(3-sec-Butylimino-6-diethylamino-3H-xanthen-9-yl...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 705 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse-Intramural Research Program Curated by ChEMBL | Assay Description Displacement of [3H]5HT from human SERT expressed in COS7 cells | J Med Chem 48: 7513-6 (2005) Article DOI: 10.1021/jm050431y BindingDB Entry DOI: 10.7270/Q27M07HM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Homo sapiens (Human)) | BDBM50177766 (2-(3-sec-Butylimino-6-diethylamino-3H-xanthen-9-yl...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.49E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse-Intramural Research Program Curated by ChEMBL | Assay Description Displacement of [3H]5HT from human SERT expressed in COS7 cells | J Med Chem 48: 7513-6 (2005) Article DOI: 10.1021/jm050431y BindingDB Entry DOI: 10.7270/Q27M07HM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-1 (Homo sapiens (Human)) | BDBM50383272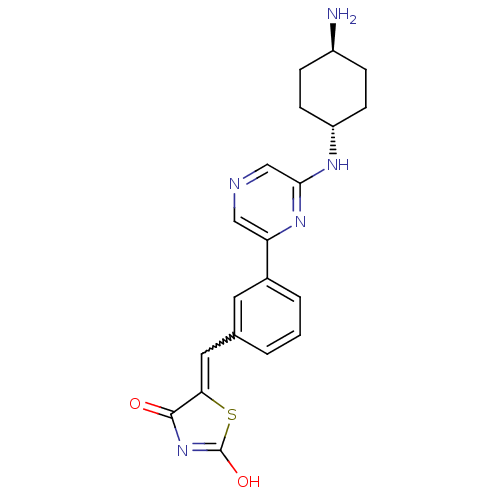 (CHEMBL2032375) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.470 | n/a | n/a | n/a | n/a | n/a | n/a |
Genzyme Corp. Curated by ChEMBL | Assay Description Inhibition of human Pim-1 using 5-FAM-RSRHSSYPAGT-CONH2 as substrate preincubated for 15 mins prior substrate addition measured after 45 mins by fluo... | J Med Chem 55: 2641-8 (2012) Article DOI: 10.1021/jm2014698 BindingDB Entry DOI: 10.7270/Q2Q2418B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-1 (Homo sapiens (Human)) | BDBM50345259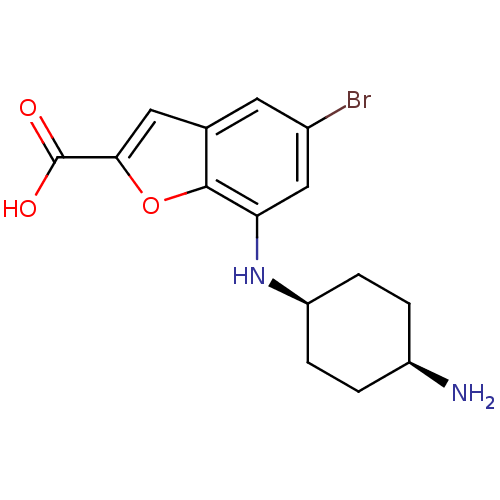 (CHEMBL1782530 | cis-7-(4-aminocyclohexylamino)-5-b...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Genzyme Corp. Curated by ChEMBL | Assay Description Inhibition of human Pim1 using 5-FAM-RSRHSSYPAGT-CONH2 as substrate after 45 mins by off-chip mobility shift method | Bioorg Med Chem Lett 21: 3050-6 (2011) Article DOI: 10.1016/j.bmcl.2011.03.030 BindingDB Entry DOI: 10.7270/Q2ZP46GG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-2 (Homo sapiens (Human)) | BDBM50345259 (CHEMBL1782530 | cis-7-(4-aminocyclohexylamino)-5-b...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Genzyme Corp. Curated by ChEMBL | Assay Description Inhibition of human Pim2 using 5-FAM-RSRHSSYPAGT-CONH2 as substrate after 90 mins by off-chip mobility shift method | Bioorg Med Chem Lett 21: 3050-6 (2011) Article DOI: 10.1016/j.bmcl.2011.03.030 BindingDB Entry DOI: 10.7270/Q2ZP46GG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine kinase 1 (Homo sapiens (Human)) | BDBM50323215 ((2S,3S)-N-((S)-1-(4-(5-(2-cyclohexylethyl)-1,2,4-o...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Genzyme Corporation Curated by ChEMBL | Assay Description Inhibition of sphingosine kinase 1 | Bioorg Med Chem Lett 20: 4550-4 (2010) Article DOI: 10.1016/j.bmcl.2010.06.019 BindingDB Entry DOI: 10.7270/Q23T9HDT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-1 (Homo sapiens (Human)) | BDBM50345260 (CHEMBL1782531 | trans-7-(4-aminocyclohexylamino)-5...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Genzyme Corp. Curated by ChEMBL | Assay Description Inhibition of human Pim1 using 5-FAM-RSRHSSYPAGT-CONH2 as substrate after 45 mins by off-chip mobility shift method | Bioorg Med Chem Lett 21: 3050-6 (2011) Article DOI: 10.1016/j.bmcl.2011.03.030 BindingDB Entry DOI: 10.7270/Q2ZP46GG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-1 (Homo sapiens (Human)) | BDBM50345250 (5-bromo-7-(piperidin-4-ylmethylamino)benzofuran-2-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Genzyme Corp. Curated by ChEMBL | Assay Description Inhibition of human Pim1 using 5-FAM-RSRHSSYPAGT-CONH2 as substrate after 45 mins by off-chip mobility shift method | Bioorg Med Chem Lett 21: 3050-6 (2011) Article DOI: 10.1016/j.bmcl.2011.03.030 BindingDB Entry DOI: 10.7270/Q2ZP46GG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine kinase 1 (Homo sapiens (Human)) | BDBM50323216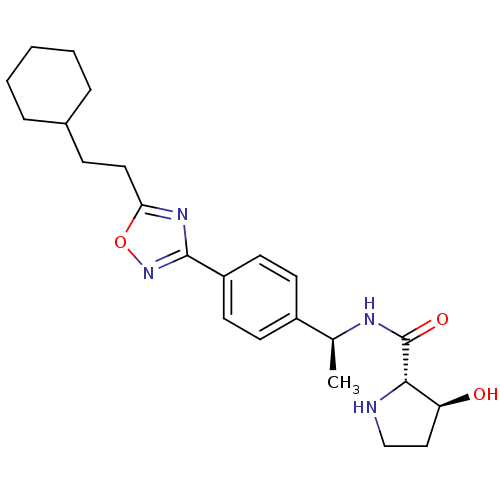 ((2S,3S)-N-((S)-1-(4-(5-(2-cyclohexylethyl)-1,2,4-o...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Genzyme Corporation Curated by ChEMBL | Assay Description Inhibition of sphingosine kinase 1 | Bioorg Med Chem Lett 20: 4550-4 (2010) Article DOI: 10.1016/j.bmcl.2010.06.019 BindingDB Entry DOI: 10.7270/Q23T9HDT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-2 (Homo sapiens (Human)) | BDBM50345245 (CHEMBL1782516 | trans-5-(6-(4-(aminomethyl)cyclohe...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Genzyme Corp. Curated by ChEMBL | Assay Description Inhibition of human Pim2 using 5-FAM-RSRHSSYPAGT-CONH2 as substrate after 90 mins by off-chip mobility shift method | Bioorg Med Chem Lett 21: 3050-6 (2011) Article DOI: 10.1016/j.bmcl.2011.03.030 BindingDB Entry DOI: 10.7270/Q2ZP46GG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-1 (Homo sapiens (Human)) | BDBM26626 ((5Z)-5-{[3-(trifluoromethyl)phenyl]methylidene}-1,...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Genzyme Corp. Curated by ChEMBL | Assay Description Inhibition of human Pim-1 using 5-FAM-RSRHSSYPAGT-CONH2 as substrate preincubated for 15 mins prior substrate addition measured after 45 mins by fluo... | J Med Chem 55: 2641-8 (2012) Article DOI: 10.1021/jm2014698 BindingDB Entry DOI: 10.7270/Q2Q2418B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine kinase 1 (Homo sapiens (Human)) | BDBM50323223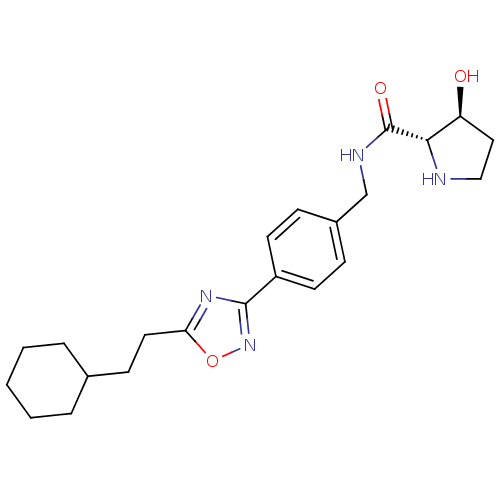 ((2S,3S)-N-(4-(5-(2-cyclohexylethyl)-1,2,4-oxadiazo...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Genzyme Corporation Curated by ChEMBL | Assay Description Inhibition of sphingosine kinase 1 | Bioorg Med Chem Lett 20: 4550-4 (2010) Article DOI: 10.1016/j.bmcl.2010.06.019 BindingDB Entry DOI: 10.7270/Q23T9HDT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine kinase 1 (Homo sapiens (Human)) | BDBM50323219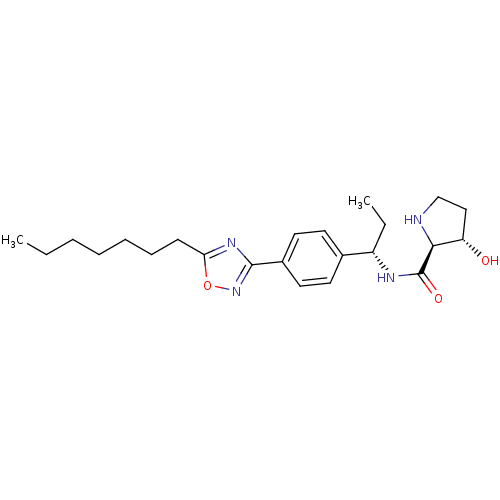 ((2S,3S)-N-((S)-1-(4-(5-heptyl-1,2,4-oxadiazol-3-yl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Genzyme Corporation Curated by ChEMBL | Assay Description Inhibition of sphingosine kinase 1 | Bioorg Med Chem Lett 20: 4550-4 (2010) Article DOI: 10.1016/j.bmcl.2010.06.019 BindingDB Entry DOI: 10.7270/Q23T9HDT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-1 (Homo sapiens (Human)) | BDBM50345253 (5-bromo-7-(2-(piperidin-4-yl)ethylamino)benzofuran...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Genzyme Corp. Curated by ChEMBL | Assay Description Inhibition of human Pim1 using 5-FAM-RSRHSSYPAGT-CONH2 as substrate after 45 mins by off-chip mobility shift method | Bioorg Med Chem Lett 21: 3050-6 (2011) Article DOI: 10.1016/j.bmcl.2011.03.030 BindingDB Entry DOI: 10.7270/Q2ZP46GG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine kinase 1 (Homo sapiens (Human)) | BDBM50323220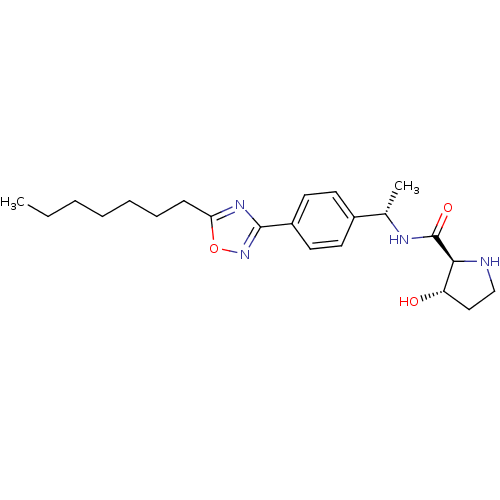 ((2S,3S)-N-((S)-1-(4-(5-heptyl-1,2,4-oxadiazol-3-yl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 48 | n/a | n/a | n/a | n/a | n/a | n/a |
Genzyme Corporation Curated by ChEMBL | Assay Description Inhibition of sphingosine kinase 1 | Bioorg Med Chem Lett 20: 4550-4 (2010) Article DOI: 10.1016/j.bmcl.2010.06.019 BindingDB Entry DOI: 10.7270/Q23T9HDT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine kinase 1 (Homo sapiens (Human)) | BDBM50323231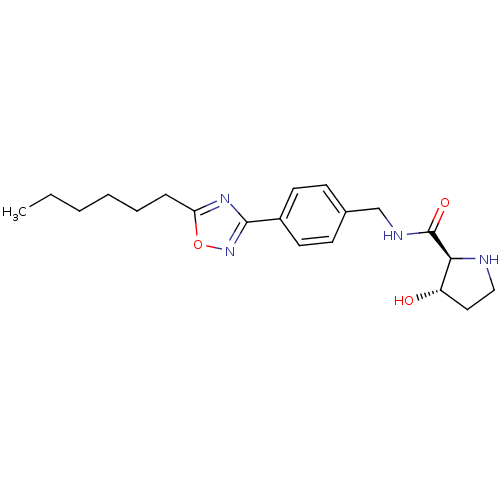 ((2S,3S)-N-(4-(5-hexyl-1,2,4-oxadiazol-3-yl)benzyl)...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 49 | n/a | n/a | n/a | n/a | n/a | n/a |
Genzyme Corporation Curated by ChEMBL | Assay Description Inhibition of sphingosine kinase 1 | Bioorg Med Chem Lett 20: 4550-4 (2010) Article DOI: 10.1016/j.bmcl.2010.06.019 BindingDB Entry DOI: 10.7270/Q23T9HDT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine kinase 1 (Homo sapiens (Human)) | BDBM50299323 ((S)-2-amino-4-hydroxy-N-(4-octylphenyl)butanamide ...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Genzyme Corporation Curated by ChEMBL | Assay Description Inhibition of human sphingosine kinase 1 by off chip mobility shift assay | Bioorg Med Chem Lett 19: 6119-21 (2009) Article DOI: 10.1016/j.bmcl.2009.09.022 BindingDB Entry DOI: 10.7270/Q29Z94ZP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-1 (Homo sapiens (Human)) | BDBM50209188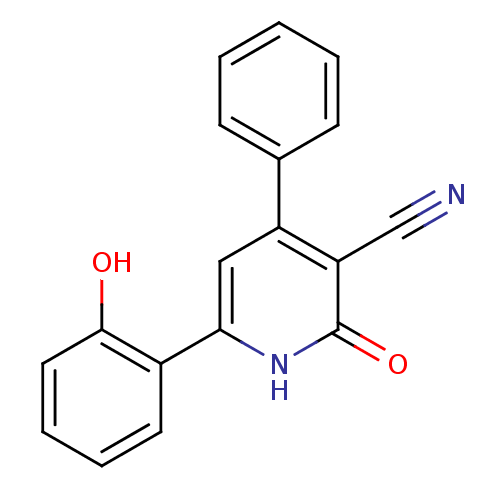 (6-(2-Hydroxy-phenyl)-2-oxo-4-phenyl-1,2-dihydro-py...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Genzyme Corp. Curated by ChEMBL | Assay Description Inhibition of human Pim-1 using 5-FAM-RSRHSSYPAGT-CONH2 as substrate preincubated for 15 mins prior substrate addition measured after 45 mins by fluo... | J Med Chem 55: 2641-8 (2012) Article DOI: 10.1021/jm2014698 BindingDB Entry DOI: 10.7270/Q2Q2418B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-1 (Homo sapiens (Human)) | BDBM50345251 (5-bromo-7-(2-(piperidin-4-yl)ethoxy)benzofuran-2-c...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 51 | n/a | n/a | n/a | n/a | n/a | n/a |
Genzyme Corp. Curated by ChEMBL | Assay Description Inhibition of human Pim1 using 5-FAM-RSRHSSYPAGT-CONH2 as substrate after 45 mins by off-chip mobility shift method | Bioorg Med Chem Lett 21: 3050-6 (2011) Article DOI: 10.1016/j.bmcl.2011.03.030 BindingDB Entry DOI: 10.7270/Q2ZP46GG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-1 (Homo sapiens (Human)) | BDBM50345245 (CHEMBL1782516 | trans-5-(6-(4-(aminomethyl)cyclohe...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 53 | n/a | n/a | n/a | n/a | n/a | n/a |
Genzyme Corp. Curated by ChEMBL | Assay Description Inhibition of human Pim1 using 5-FAM-RSRHSSYPAGT-CONH2 as substrate after 45 mins by off-chip mobility shift method | Bioorg Med Chem Lett 21: 3050-6 (2011) Article DOI: 10.1016/j.bmcl.2011.03.030 BindingDB Entry DOI: 10.7270/Q2ZP46GG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-2 (Homo sapiens (Human)) | BDBM50345242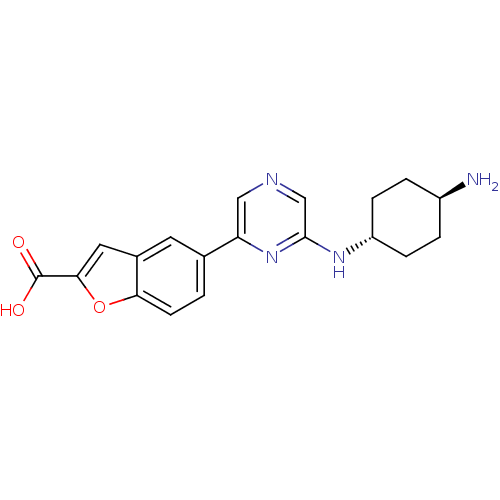 (CHEMBL1782513 | trans-5-(6-(4-aminocyclohexylamino...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 54 | n/a | n/a | n/a | n/a | n/a | n/a |
Genzyme Corp. Curated by ChEMBL | Assay Description Inhibition of human Pim2 using 5-FAM-RSRHSSYPAGT-CONH2 as substrate after 90 mins by off-chip mobility shift method | Bioorg Med Chem Lett 21: 3050-6 (2011) Article DOI: 10.1016/j.bmcl.2011.03.030 BindingDB Entry DOI: 10.7270/Q2ZP46GG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-1 (Homo sapiens (Human)) | BDBM50345255 (5-bromo-7-(2-(piperidin-4-yl)acetamido)benzofuran-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 55 | n/a | n/a | n/a | n/a | n/a | n/a |
Genzyme Corp. Curated by ChEMBL | Assay Description Inhibition of human Pim1 using 5-FAM-RSRHSSYPAGT-CONH2 as substrate after 45 mins by off-chip mobility shift method | Bioorg Med Chem Lett 21: 3050-6 (2011) Article DOI: 10.1016/j.bmcl.2011.03.030 BindingDB Entry DOI: 10.7270/Q2ZP46GG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine kinase 1 (Homo sapiens (Human)) | BDBM50323242 ((2S,3S)-N-((S)-1-(4-(5-(2-cyclopentylethyl)-1,2,4-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 56 | n/a | n/a | n/a | n/a | n/a | n/a |
Genzyme Corporation Curated by ChEMBL | Assay Description Inhibition of sphingosine kinase 1 | Bioorg Med Chem Lett 20: 4550-4 (2010) Article DOI: 10.1016/j.bmcl.2010.06.019 BindingDB Entry DOI: 10.7270/Q23T9HDT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-1 (Homo sapiens (Human)) | BDBM28394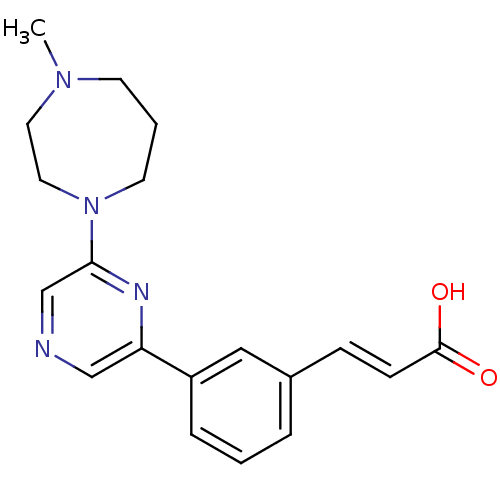 ((2E)-3-{3-[6-(4-methyl-1,4-diazepan-1-yl)pyrazin-2...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 57 | n/a | n/a | n/a | n/a | n/a | n/a |
Genzyme Corp. Curated by ChEMBL | Assay Description Inhibition of human Pim-1 using 5-FAM-RSRHSSYPAGT-CONH2 as substrate preincubated for 15 mins prior substrate addition measured after 45 mins by fluo... | J Med Chem 55: 2641-8 (2012) Article DOI: 10.1021/jm2014698 BindingDB Entry DOI: 10.7270/Q2Q2418B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine kinase 1 (Homo sapiens (Human)) | BDBM50323217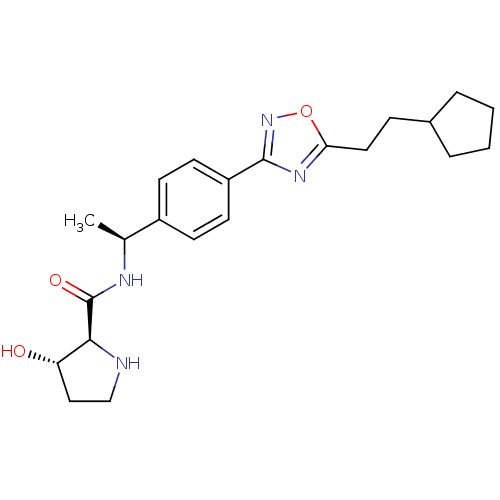 ((2S,3S)-N-((S)-1-(4-(5-(2-cyclopentylethyl)-1,2,4-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 58 | n/a | n/a | n/a | n/a | n/a | n/a |
Genzyme Corporation Curated by ChEMBL | Assay Description Inhibition of sphingosine kinase 1 | Bioorg Med Chem Lett 20: 4550-4 (2010) Article DOI: 10.1016/j.bmcl.2010.06.019 BindingDB Entry DOI: 10.7270/Q23T9HDT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-1 (Homo sapiens (Human)) | BDBM50345247 (5-bromo-7-(piperidin-4-ylmethoxy)benzofuran-2-carb...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Genzyme Corp. Curated by ChEMBL | Assay Description Inhibition of human Pim1 using 5-FAM-RSRHSSYPAGT-CONH2 as substrate after 45 mins by off-chip mobility shift method | Bioorg Med Chem Lett 21: 3050-6 (2011) Article DOI: 10.1016/j.bmcl.2011.03.030 BindingDB Entry DOI: 10.7270/Q2ZP46GG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine kinase 1 (Homo sapiens (Human)) | BDBM50323234 ((2S,3S)-N-(4-(3-heptyl-1,2,4-oxadiazol-5-yl)benzyl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 61 | n/a | n/a | n/a | n/a | n/a | n/a |
Genzyme Corporation Curated by ChEMBL | Assay Description Inhibition of sphingosine kinase 1 | Bioorg Med Chem Lett 20: 4550-4 (2010) Article DOI: 10.1016/j.bmcl.2010.06.019 BindingDB Entry DOI: 10.7270/Q23T9HDT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine kinase 1 (Homo sapiens (Human)) | BDBM50299328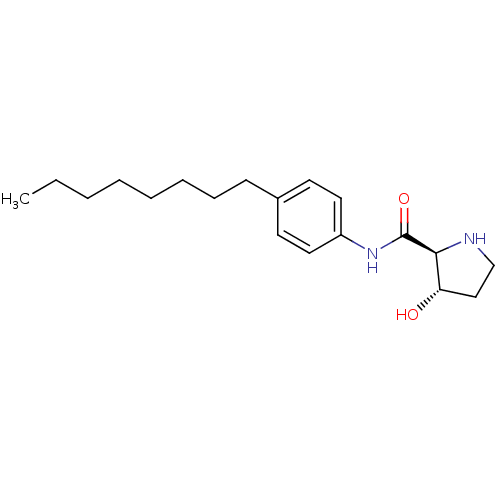 ((2S,3S)-3-hydroxy-N-(4-octylphenyl)pyrrolidine-2-c...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 62 | n/a | n/a | n/a | n/a | n/a | n/a |
Genzyme Corporation Curated by ChEMBL | Assay Description Inhibition of sphingosine kinase 1 | Bioorg Med Chem Lett 20: 4550-4 (2010) Article DOI: 10.1016/j.bmcl.2010.06.019 BindingDB Entry DOI: 10.7270/Q23T9HDT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine kinase 1 (Homo sapiens (Human)) | BDBM50299328 ((2S,3S)-3-hydroxy-N-(4-octylphenyl)pyrrolidine-2-c...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 62 | n/a | n/a | n/a | n/a | n/a | n/a |
Genzyme Corporation Curated by ChEMBL | Assay Description Inhibition of human sphingosine kinase 1 by off chip mobility shift assay | Bioorg Med Chem Lett 19: 6119-21 (2009) Article DOI: 10.1016/j.bmcl.2009.09.022 BindingDB Entry DOI: 10.7270/Q29Z94ZP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-2 (Homo sapiens (Human)) | BDBM50345260 (CHEMBL1782531 | trans-7-(4-aminocyclohexylamino)-5...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 66 | n/a | n/a | n/a | n/a | n/a | n/a |
Genzyme Corp. Curated by ChEMBL | Assay Description Inhibition of human Pim2 using 5-FAM-RSRHSSYPAGT-CONH2 as substrate after 90 mins by off-chip mobility shift method | Bioorg Med Chem Lett 21: 3050-6 (2011) Article DOI: 10.1016/j.bmcl.2011.03.030 BindingDB Entry DOI: 10.7270/Q2ZP46GG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine kinase 1 (Homo sapiens (Human)) | BDBM50323240 ((2S,3S)-N-(4-(5-heptyl-1,2,4-oxadiazol-3-yl)benzyl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 68 | n/a | n/a | n/a | n/a | n/a | n/a |
Genzyme Corporation Curated by ChEMBL | Assay Description Inhibition of sphingosine kinase 1 | Bioorg Med Chem Lett 20: 4550-4 (2010) Article DOI: 10.1016/j.bmcl.2010.06.019 BindingDB Entry DOI: 10.7270/Q23T9HDT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine kinase 1 (Homo sapiens (Human)) | BDBM50323225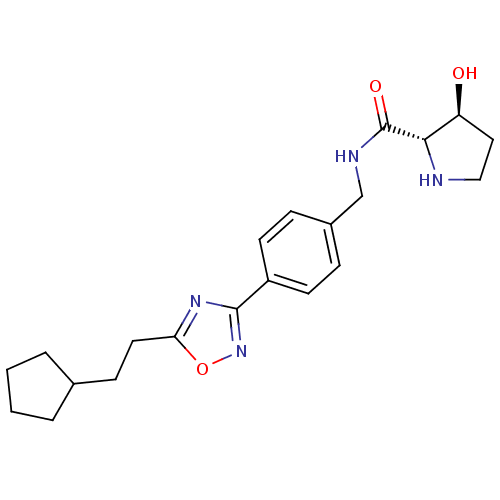 ((2S,3S)-N-(4-(5-(2-cyclopentylethyl)-1,2,4-oxadiaz...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 68 | n/a | n/a | n/a | n/a | n/a | n/a |
Genzyme Corporation Curated by ChEMBL | Assay Description Inhibition of sphingosine kinase 1 | Bioorg Med Chem Lett 20: 4550-4 (2010) Article DOI: 10.1016/j.bmcl.2010.06.019 BindingDB Entry DOI: 10.7270/Q23T9HDT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 185 total ) | Next | Last >> |