

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cathepsin S (Mus musculus (Mouse)) | BDBM50444314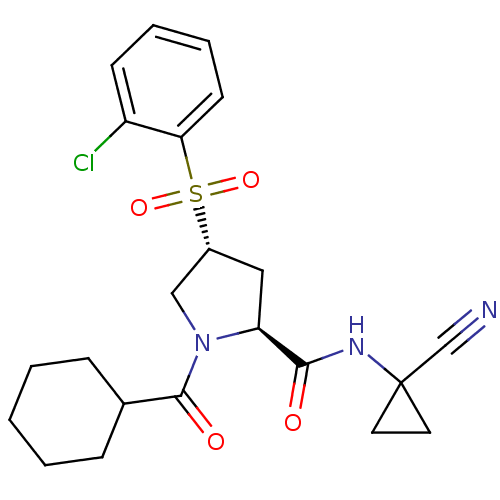 (CHEMBL3093935) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharma Research and Early Curated by ChEMBL | Assay Description Inhibition of mouse cathepsin-S using Z-Val-Val-Arg-AMC as substrate up to 20 mins by fluorescence assay | J Med Chem 56: 9789-801 (2014) Article DOI: 10.1021/jm401528k BindingDB Entry DOI: 10.7270/Q2N58NTW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Mus musculus (Mouse)) | BDBM50444318 (CHEMBL3093940) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharma Research and Early Curated by ChEMBL | Assay Description Inhibition of mouse cathepsin-S using Z-Val-Val-Arg-AMC as substrate up to 20 mins by fluorescence assay | J Med Chem 56: 9789-801 (2014) Article DOI: 10.1021/jm401528k BindingDB Entry DOI: 10.7270/Q2N58NTW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Mus musculus (Mouse)) | BDBM50444315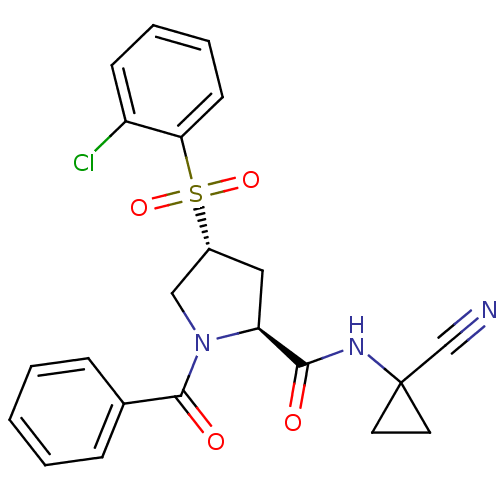 (CHEMBL3093934) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharma Research and Early Curated by ChEMBL | Assay Description Inhibition of mouse cathepsin-S using Z-Val-Val-Arg-AMC as substrate up to 20 mins by fluorescence assay | J Med Chem 56: 9789-801 (2014) Article DOI: 10.1021/jm401528k BindingDB Entry DOI: 10.7270/Q2N58NTW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM210877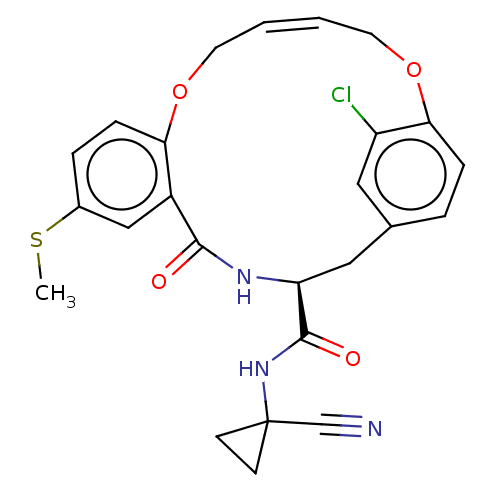 (US9290467, 47) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | 6.5 | n/a |
HOFFMANN—LA ROCHE INC. US Patent | Assay Description Enzyme activity is measured by observing the increase in fluorescence intensity caused by cleavage of a peptide substrate containing a fluorophore wh... | US Patent US9290467 (2016) BindingDB Entry DOI: 10.7270/Q2H130VZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM210881 (US9290467, 51) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | 6.5 | n/a |
HOFFMANN—LA ROCHE INC. US Patent | Assay Description Enzyme activity is measured by observing the increase in fluorescence intensity caused by cleavage of a peptide substrate containing a fluorophore wh... | US Patent US9290467 (2016) BindingDB Entry DOI: 10.7270/Q2H130VZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Mus musculus (Mouse)) | BDBM50444313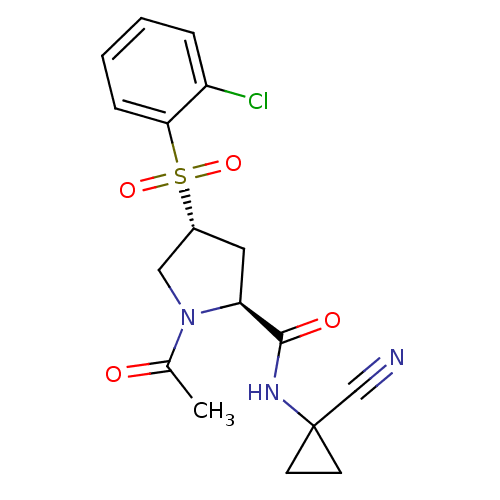 (CHEMBL3093936) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharma Research and Early Curated by ChEMBL | Assay Description Inhibition of mouse cathepsin-S using Z-Val-Val-Arg-AMC as substrate up to 20 mins by fluorescence assay | J Med Chem 56: 9789-801 (2014) Article DOI: 10.1021/jm401528k BindingDB Entry DOI: 10.7270/Q2N58NTW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM210873 (US9290467, 43) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | 6.5 | n/a |
HOFFMANN—LA ROCHE INC. US Patent | Assay Description Enzyme activity is measured by observing the increase in fluorescence intensity caused by cleavage of a peptide substrate containing a fluorophore wh... | US Patent US9290467 (2016) BindingDB Entry DOI: 10.7270/Q2H130VZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM210875 (US9290467, 45) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4.30 | n/a | n/a | n/a | n/a | 6.5 | n/a |
HOFFMANN—LA ROCHE INC. US Patent | Assay Description Enzyme activity is measured by observing the increase in fluorescence intensity caused by cleavage of a peptide substrate containing a fluorophore wh... | US Patent US9290467 (2016) BindingDB Entry DOI: 10.7270/Q2H130VZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM210871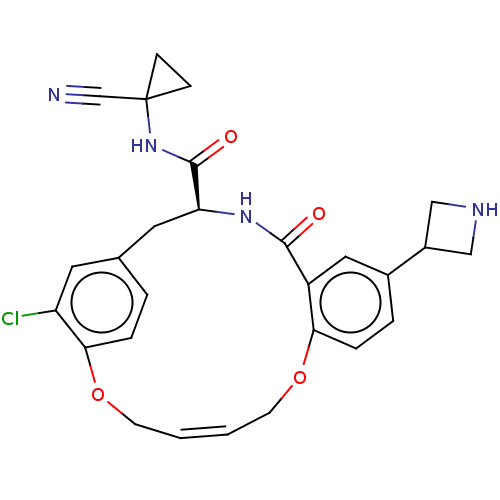 (US9290467, 41) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB US Patent | n/a | n/a | 5.20 | n/a | n/a | n/a | n/a | 6.5 | n/a |
HOFFMANN—LA ROCHE INC. US Patent | Assay Description Enzyme activity is measured by observing the increase in fluorescence intensity caused by cleavage of a peptide substrate containing a fluorophore wh... | US Patent US9290467 (2016) BindingDB Entry DOI: 10.7270/Q2H130VZ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cathepsin S (Mus musculus (Mouse)) | BDBM50444317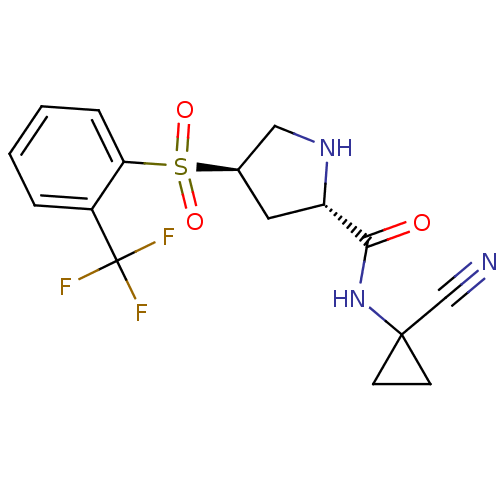 (CHEMBL3093932) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharma Research and Early Curated by ChEMBL | Assay Description Inhibition of mouse cathepsin-S using Z-Val-Val-Arg-AMC as substrate up to 20 mins by fluorescence assay | J Med Chem 56: 9789-801 (2014) Article DOI: 10.1021/jm401528k BindingDB Entry DOI: 10.7270/Q2N58NTW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM210870 (US9290467, 40) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 6.90 | n/a | n/a | n/a | n/a | 6.5 | n/a |
HOFFMANN—LA ROCHE INC. US Patent | Assay Description Enzyme activity is measured by observing the increase in fluorescence intensity caused by cleavage of a peptide substrate containing a fluorophore wh... | US Patent US9290467 (2016) BindingDB Entry DOI: 10.7270/Q2H130VZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholesteryl ester transfer protein (Homo sapiens (Human)) | BDBM50348226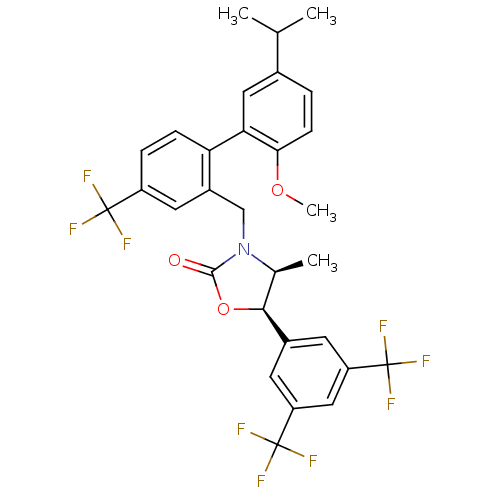 (CHEMBL1800622) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant CETP (unknown origin) assessed as inhibition of transfer of [3H]cholesteryl oleate or [3H]triolein using exogenous LDL and ... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00959 BindingDB Entry DOI: 10.7270/Q27M0CSV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM210867 (US9290467, 37) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 9 | n/a | n/a | n/a | n/a | 6.5 | n/a |
HOFFMANN—LA ROCHE INC. US Patent | Assay Description Enzyme activity is measured by observing the increase in fluorescence intensity caused by cleavage of a peptide substrate containing a fluorophore wh... | US Patent US9290467 (2016) BindingDB Entry DOI: 10.7270/Q2H130VZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholesteryl ester transfer protein (Homo sapiens (Human)) | BDBM50578772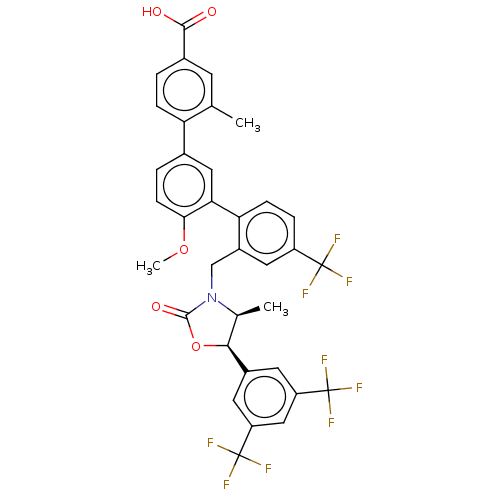 (CHEMBL4873416) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant CETP (unknown origin) assessed as inhibition of transfer of [3H]cholesteryl oleate or [3H]triolein using exogenous LDL and ... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00959 BindingDB Entry DOI: 10.7270/Q27M0CSV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM210864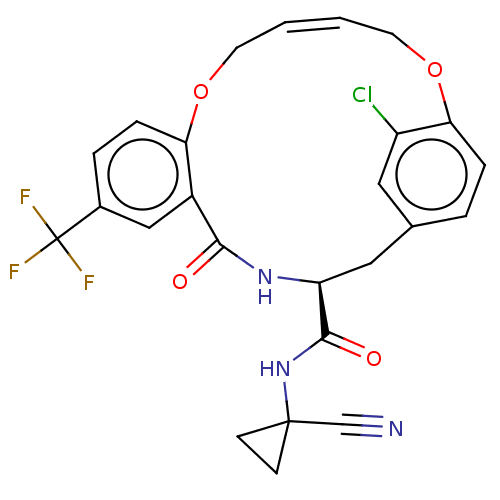 (US9290467, 34) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 9 | n/a | n/a | n/a | n/a | 6.5 | n/a |
HOFFMANN—LA ROCHE INC. US Patent | Assay Description Enzyme activity is measured by observing the increase in fluorescence intensity caused by cleavage of a peptide substrate containing a fluorophore wh... | US Patent US9290467 (2016) BindingDB Entry DOI: 10.7270/Q2H130VZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM210874 (US9290467, 44) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 9.20 | n/a | n/a | n/a | n/a | 6.5 | n/a |
HOFFMANN—LA ROCHE INC. US Patent | Assay Description Enzyme activity is measured by observing the increase in fluorescence intensity caused by cleavage of a peptide substrate containing a fluorophore wh... | US Patent US9290467 (2016) BindingDB Entry DOI: 10.7270/Q2H130VZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM210835 (US9290467, 5) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 9.60 | n/a | n/a | n/a | n/a | 6.5 | n/a |
HOFFMANN—LA ROCHE INC. US Patent | Assay Description Enzyme activity is measured by observing the increase in fluorescence intensity caused by cleavage of a peptide substrate containing a fluorophore wh... | US Patent US9290467 (2016) BindingDB Entry DOI: 10.7270/Q2H130VZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM210868 (US9290467, 38) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 10 | n/a | n/a | n/a | n/a | 6.5 | n/a |
HOFFMANN—LA ROCHE INC. US Patent | Assay Description Enzyme activity is measured by observing the increase in fluorescence intensity caused by cleavage of a peptide substrate containing a fluorophore wh... | US Patent US9290467 (2016) BindingDB Entry DOI: 10.7270/Q2H130VZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM210839 (US9290467, 9) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 10 | n/a | n/a | n/a | n/a | 6.5 | n/a |
HOFFMANN—LA ROCHE INC. US Patent | Assay Description Enzyme activity is measured by observing the increase in fluorescence intensity caused by cleavage of a peptide substrate containing a fluorophore wh... | US Patent US9290467 (2016) BindingDB Entry DOI: 10.7270/Q2H130VZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM210840 (US9290467, 10) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 10.3 | n/a | n/a | n/a | n/a | 6.5 | n/a |
HOFFMANN—LA ROCHE INC. US Patent | Assay Description Enzyme activity is measured by observing the increase in fluorescence intensity caused by cleavage of a peptide substrate containing a fluorophore wh... | US Patent US9290467 (2016) BindingDB Entry DOI: 10.7270/Q2H130VZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM210847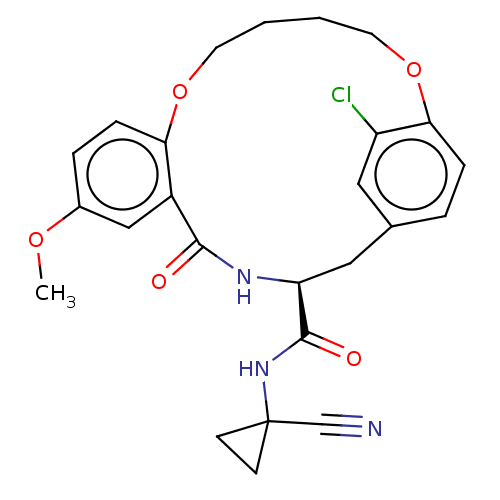 (US9290467, 17) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | US Patent | n/a | n/a | 10.4 | n/a | n/a | n/a | n/a | 6.5 | n/a |
HOFFMANN—LA ROCHE INC. US Patent | Assay Description Enzyme activity is measured by observing the increase in fluorescence intensity caused by cleavage of a peptide substrate containing a fluorophore wh... | US Patent US9290467 (2016) BindingDB Entry DOI: 10.7270/Q2H130VZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM210858 (US9290467, 28) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 11 | n/a | n/a | n/a | n/a | 6.5 | n/a |
HOFFMANN—LA ROCHE INC. US Patent | Assay Description Enzyme activity is measured by observing the increase in fluorescence intensity caused by cleavage of a peptide substrate containing a fluorophore wh... | US Patent US9290467 (2016) BindingDB Entry DOI: 10.7270/Q2H130VZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM210846 (US9290467, 16) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 11.4 | n/a | n/a | n/a | n/a | 6.5 | n/a |
HOFFMANN—LA ROCHE INC. US Patent | Assay Description Enzyme activity is measured by observing the increase in fluorescence intensity caused by cleavage of a peptide substrate containing a fluorophore wh... | US Patent US9290467 (2016) BindingDB Entry DOI: 10.7270/Q2H130VZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM210841 (US9290467, 11) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 11.6 | n/a | n/a | n/a | n/a | 6.5 | n/a |
HOFFMANN—LA ROCHE INC. US Patent | Assay Description Enzyme activity is measured by observing the increase in fluorescence intensity caused by cleavage of a peptide substrate containing a fluorophore wh... | US Patent US9290467 (2016) BindingDB Entry DOI: 10.7270/Q2H130VZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM210861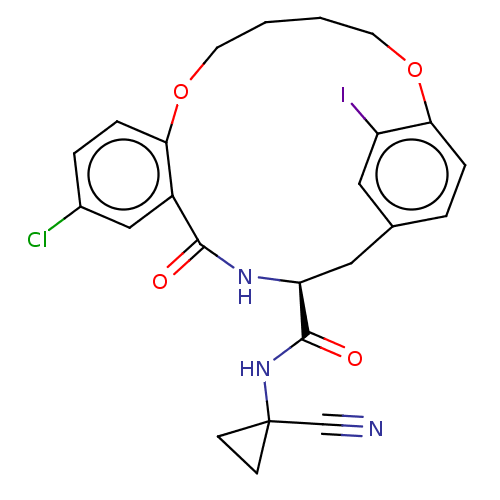 (US9290467, 31) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 12 | n/a | n/a | n/a | n/a | 6.5 | n/a |
HOFFMANN—LA ROCHE INC. US Patent | Assay Description Enzyme activity is measured by observing the increase in fluorescence intensity caused by cleavage of a peptide substrate containing a fluorophore wh... | US Patent US9290467 (2016) BindingDB Entry DOI: 10.7270/Q2H130VZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholesteryl ester transfer protein (Homo sapiens (Human)) | BDBM50578733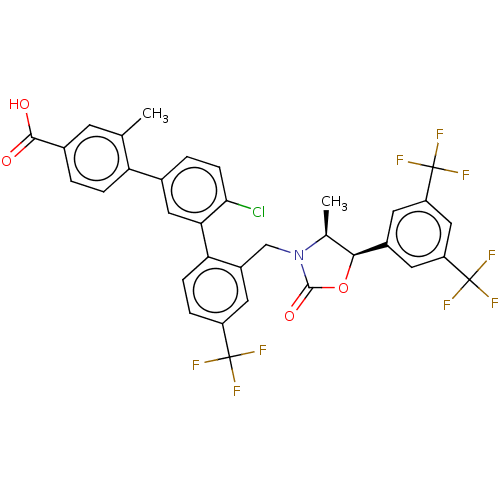 (CHEMBL4875851) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant CETP (unknown origin) assessed as inhibition of transfer of [3H]cholesteryl oleate or [3H]triolein between exogenous [3H]LD... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00959 BindingDB Entry DOI: 10.7270/Q27M0CSV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholesteryl ester transfer protein (Homo sapiens (Human)) | BDBM50578733 (CHEMBL4875851) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant CETP (unknown origin) assessed as inhibition of transfer of [3H]cholesteryl oleate or [3H]triolein using exogenous LDL and ... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00959 BindingDB Entry DOI: 10.7270/Q27M0CSV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM210859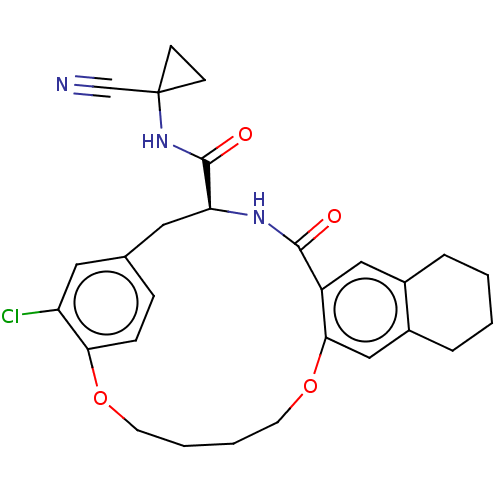 (US9290467, 29) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 13 | n/a | n/a | n/a | n/a | 6.5 | n/a |
HOFFMANN—LA ROCHE INC. US Patent | Assay Description Enzyme activity is measured by observing the increase in fluorescence intensity caused by cleavage of a peptide substrate containing a fluorophore wh... | US Patent US9290467 (2016) BindingDB Entry DOI: 10.7270/Q2H130VZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM210880 (US9290467, 50) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 13.5 | n/a | n/a | n/a | n/a | 6.5 | n/a |
HOFFMANN—LA ROCHE INC. US Patent | Assay Description Enzyme activity is measured by observing the increase in fluorescence intensity caused by cleavage of a peptide substrate containing a fluorophore wh... | US Patent US9290467 (2016) BindingDB Entry DOI: 10.7270/Q2H130VZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM210834 (US9290467, 4) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB US Patent | n/a | n/a | 13.8 | n/a | n/a | n/a | n/a | 6.5 | n/a |
HOFFMANN—LA ROCHE INC. US Patent | Assay Description Enzyme activity is measured by observing the increase in fluorescence intensity caused by cleavage of a peptide substrate containing a fluorophore wh... | US Patent US9290467 (2016) BindingDB Entry DOI: 10.7270/Q2H130VZ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM210857 (US9290467, 27) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 16 | n/a | n/a | n/a | n/a | 6.5 | n/a |
HOFFMANN—LA ROCHE INC. US Patent | Assay Description Enzyme activity is measured by observing the increase in fluorescence intensity caused by cleavage of a peptide substrate containing a fluorophore wh... | US Patent US9290467 (2016) BindingDB Entry DOI: 10.7270/Q2H130VZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM210862 (US9290467, 32) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 16 | n/a | n/a | n/a | n/a | 6.5 | n/a |
HOFFMANN—LA ROCHE INC. US Patent | Assay Description Enzyme activity is measured by observing the increase in fluorescence intensity caused by cleavage of a peptide substrate containing a fluorophore wh... | US Patent US9290467 (2016) BindingDB Entry DOI: 10.7270/Q2H130VZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholesteryl ester transfer protein (Homo sapiens (Human)) | BDBM50348228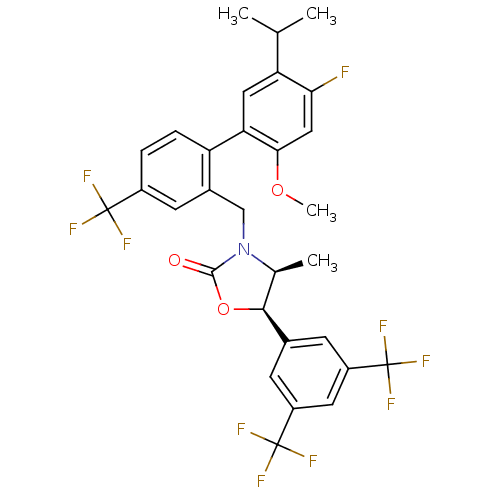 (CHEMBL1800807) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant CETP (unknown origin) assessed as inhibition of transfer of [3H]cholesteryl oleate or [3H]triolein using exogenous LDL and ... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00959 BindingDB Entry DOI: 10.7270/Q27M0CSV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholesteryl ester transfer protein (Homo sapiens (Human)) | BDBM264531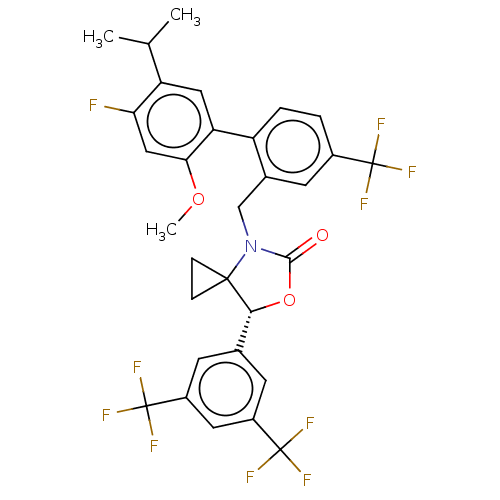 ((7R)-7-[3,5- bis(trifluoromethyl)phenyl]- 4-{[4'-f...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant CETP (unknown origin) assessed as inhibition of transfer of [3H]cholesteryl oleate or [3H]triolein between exogenous [3H]LD... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00959 BindingDB Entry DOI: 10.7270/Q27M0CSV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM210879 (US9290467, 49) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 18.3 | n/a | n/a | n/a | n/a | 6.5 | n/a |
HOFFMANN—LA ROCHE INC. US Patent | Assay Description Enzyme activity is measured by observing the increase in fluorescence intensity caused by cleavage of a peptide substrate containing a fluorophore wh... | US Patent US9290467 (2016) BindingDB Entry DOI: 10.7270/Q2H130VZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholesteryl ester transfer protein (Homo sapiens (Human)) | BDBM50578756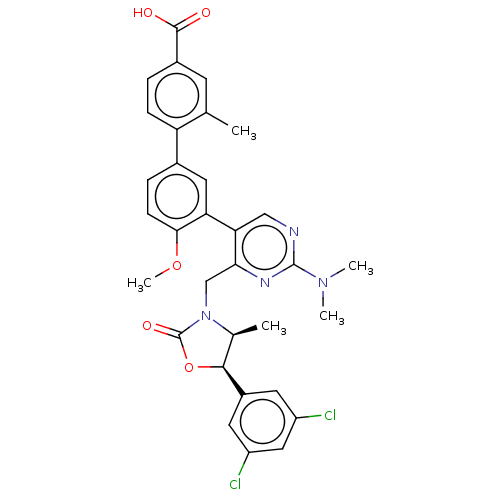 (CHEMBL4876430) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant CETP (unknown origin) assessed as inhibition of transfer of [3H]cholesteryl oleate or [3H]triolein between exogenous [3H]LD... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00959 BindingDB Entry DOI: 10.7270/Q27M0CSV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholesteryl ester transfer protein (Homo sapiens (Human)) | BDBM50578737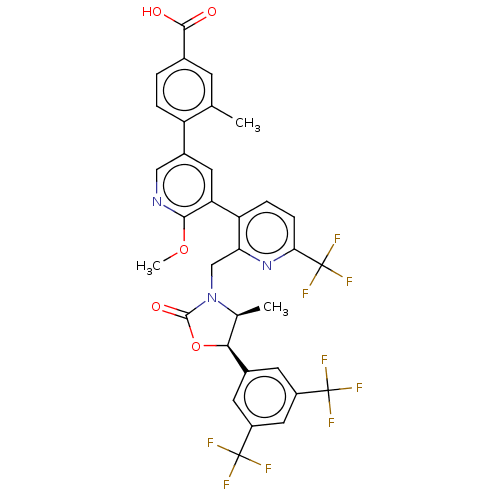 (CHEMBL4852134) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant CETP (unknown origin) assessed as inhibition of transfer of [3H]cholesteryl oleate or [3H]triolein between exogenous [3H]LD... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00959 BindingDB Entry DOI: 10.7270/Q27M0CSV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 8 (Homo sapiens (Human)) | BDBM50423246 (CHEMBL245568 | SB-649701) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Antagonist activity at human recombinant CCR8 expressed in RBL cells assessed as inhibition of I-309-induced intracellular calcium mobilization by FL... | Bioorg Med Chem Lett 17: 1722-5 (2007) Article DOI: 10.1016/j.bmcl.2006.12.076 BindingDB Entry DOI: 10.7270/Q2668FG7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholesteryl ester transfer protein (Homo sapiens (Human)) | BDBM138247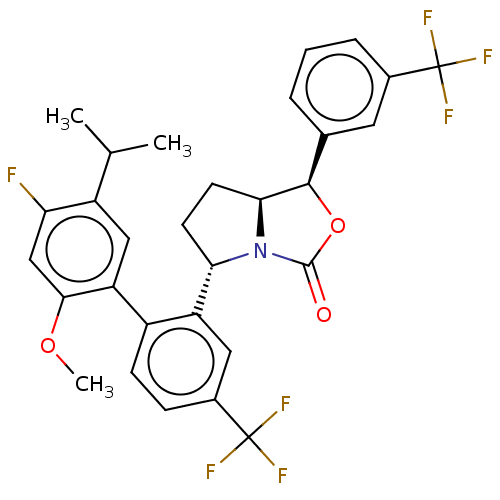 (US8871738, 30) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant CETP (unknown origin) assessed as inhibition of transfer of [3H]cholesteryl oleate or [3H]triolein between exogenous [3H]LD... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00959 BindingDB Entry DOI: 10.7270/Q27M0CSV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholesteryl ester transfer protein (Homo sapiens (Human)) | BDBM50578735 (CHEMBL4853626) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant CETP (unknown origin) assessed as inhibition of transfer of [3H]cholesteryl oleate or [3H]triolein between exogenous [3H]LD... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00959 BindingDB Entry DOI: 10.7270/Q27M0CSV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM210865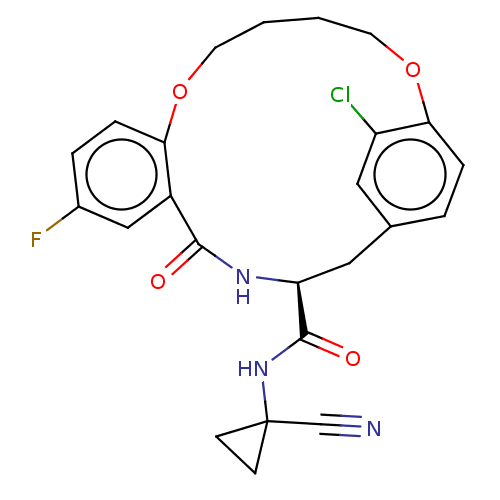 (US9290467, 35) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 23 | n/a | n/a | n/a | n/a | 6.5 | n/a |
HOFFMANN—LA ROCHE INC. US Patent | Assay Description Enzyme activity is measured by observing the increase in fluorescence intensity caused by cleavage of a peptide substrate containing a fluorophore wh... | US Patent US9290467 (2016) BindingDB Entry DOI: 10.7270/Q2H130VZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM210838 (US9290467, 8) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 24.8 | n/a | n/a | n/a | n/a | 6.5 | n/a |
HOFFMANN—LA ROCHE INC. US Patent | Assay Description Enzyme activity is measured by observing the increase in fluorescence intensity caused by cleavage of a peptide substrate containing a fluorophore wh... | US Patent US9290467 (2016) BindingDB Entry DOI: 10.7270/Q2H130VZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholesteryl ester transfer protein (Homo sapiens (Human)) | BDBM50578754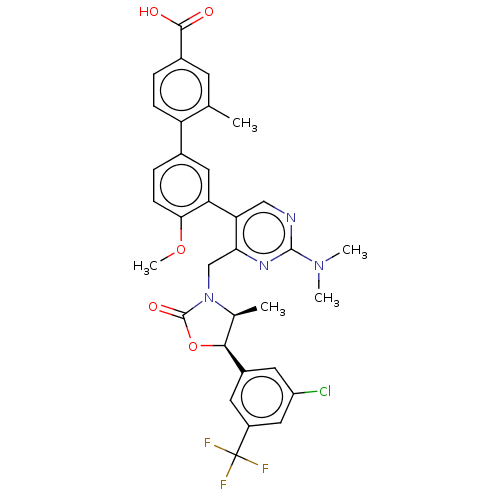 (CHEMBL4856008) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant CETP (unknown origin) assessed as inhibition of transfer of [3H]cholesteryl oleate or [3H]triolein between exogenous [3H]LD... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00959 BindingDB Entry DOI: 10.7270/Q27M0CSV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 8 (Homo sapiens (Human)) | BDBM50423246 (CHEMBL245568 | SB-649701) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Antagonist activity at human recombinant CCR8 expressed in RBL cells assessed as inhibition of I-309-induced intracellular calcium mobilization by FL... | Bioorg Med Chem Lett 17: 1722-5 (2007) Article DOI: 10.1016/j.bmcl.2006.12.076 BindingDB Entry DOI: 10.7270/Q2668FG7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholesteryl ester transfer protein (Homo sapiens (Human)) | BDBM50348226 (CHEMBL1800622) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant CETP (unknown origin) assessed as inhibition of transfer of [3H]cholesteryl oleate or [3H]triolein between exogenous [3H]LD... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00959 BindingDB Entry DOI: 10.7270/Q27M0CSV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM210872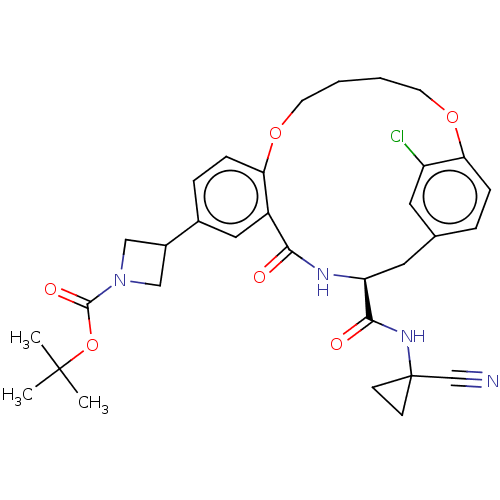 (US9290467, 42) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 25.5 | n/a | n/a | n/a | n/a | 6.5 | n/a |
HOFFMANN—LA ROCHE INC. US Patent | Assay Description Enzyme activity is measured by observing the increase in fluorescence intensity caused by cleavage of a peptide substrate containing a fluorophore wh... | US Patent US9290467 (2016) BindingDB Entry DOI: 10.7270/Q2H130VZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM210849 (US9290467, 19) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 26 | n/a | n/a | n/a | n/a | 6.5 | n/a |
HOFFMANN—LA ROCHE INC. US Patent | Assay Description Enzyme activity is measured by observing the increase in fluorescence intensity caused by cleavage of a peptide substrate containing a fluorophore wh... | US Patent US9290467 (2016) BindingDB Entry DOI: 10.7270/Q2H130VZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM210869 (US9290467, 39) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 26.2 | n/a | n/a | n/a | n/a | 6.5 | n/a |
HOFFMANN—LA ROCHE INC. US Patent | Assay Description Enzyme activity is measured by observing the increase in fluorescence intensity caused by cleavage of a peptide substrate containing a fluorophore wh... | US Patent US9290467 (2016) BindingDB Entry DOI: 10.7270/Q2H130VZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholesteryl ester transfer protein (Homo sapiens (Human)) | BDBM50578770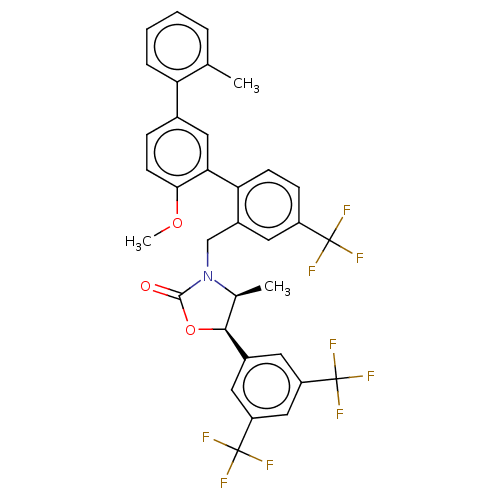 (CHEMBL4859118) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant CETP (unknown origin) assessed as inhibition of transfer of [3H]cholesteryl oleate or [3H]triolein using exogenous LDL and ... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00959 BindingDB Entry DOI: 10.7270/Q27M0CSV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Mus musculus (Mouse)) | BDBM50444316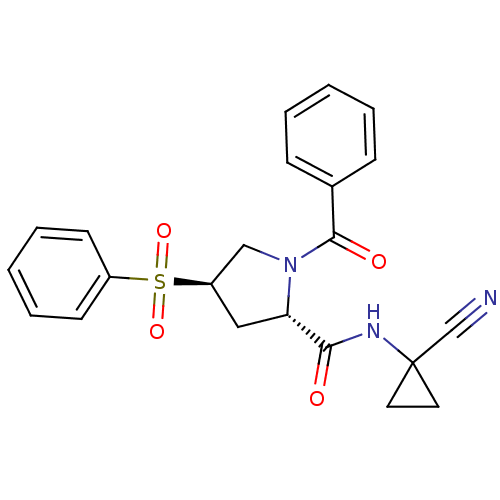 (CHEMBL3093933) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharma Research and Early Curated by ChEMBL | Assay Description Inhibition of mouse cathepsin-S using Z-Val-Val-Arg-AMC as substrate up to 20 mins by fluorescence assay | J Med Chem 56: 9789-801 (2014) Article DOI: 10.1021/jm401528k BindingDB Entry DOI: 10.7270/Q2N58NTW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 225 total ) | Next | Last >> |