 Found 7453 hits with Last Name = 'nan' and Initial = 'g'
Found 7453 hits with Last Name = 'nan' and Initial = 'g' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Prothrombin
(Homo sapiens (Human)) | BDBM50292202
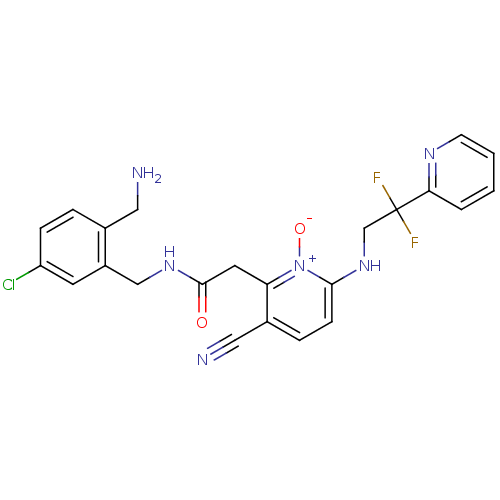
(CHEMBL382542 | N-(2-Aminomethyl-5-chloro-benzyl)-2...)Show SMILES NCc1ccc(Cl)cc1CNC(=O)Cc1c(ccc(NCC(F)(F)c2ccccn2)[n+]1[O-])C#N Show InChI InChI=1S/C23H21ClF2N6O2/c24-18-6-4-15(11-27)17(9-18)13-30-22(33)10-19-16(12-28)5-7-21(32(19)34)31-14-23(25,26)20-3-1-2-8-29-20/h1-9,31H,10-11,13-14,27H2,(H,30,33) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.00100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity against thrombin in human plasma |
Bioorg Med Chem Lett 15: 2771-5 (2005)
Article DOI: 10.1016/j.bmcl.2005.03.110
BindingDB Entry DOI: 10.7270/Q2PN96C6 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50147818
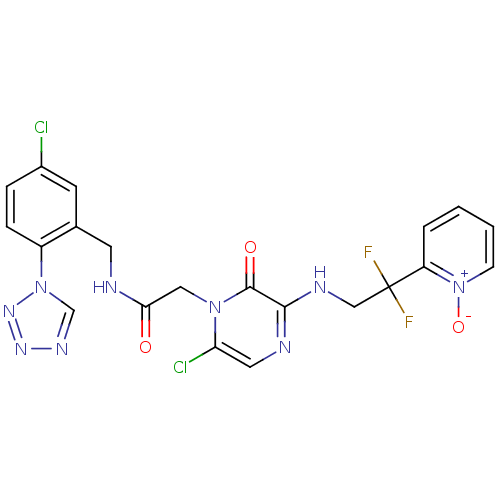
((2-[6-CHLORO-3-{[2,2-DIFLUORO-2-(1-OXIDOPYRIDIN-2-...)Show SMILES [O-][n+]1ccccc1C(F)(F)CNc1ncc(Cl)n(CC(=O)NCc2cc(Cl)ccc2-n2cnnn2)c1=O Show InChI InChI=1S/C21H17Cl2F2N9O3/c22-14-4-5-15(33-12-29-30-31-33)13(7-14)8-26-18(35)10-32-17(23)9-27-19(20(32)36)28-11-21(24,25)16-3-1-2-6-34(16)37/h1-7,9,12H,8,10-11H2,(H,26,35)(H,27,28) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.00140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory potency against human thrombin |
J Med Chem 47: 2995-3008 (2004)
Article DOI: 10.1021/jm030303e
BindingDB Entry DOI: 10.7270/Q270826B |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Prothrombin
(Homo sapiens (Human)) | BDBM50147824
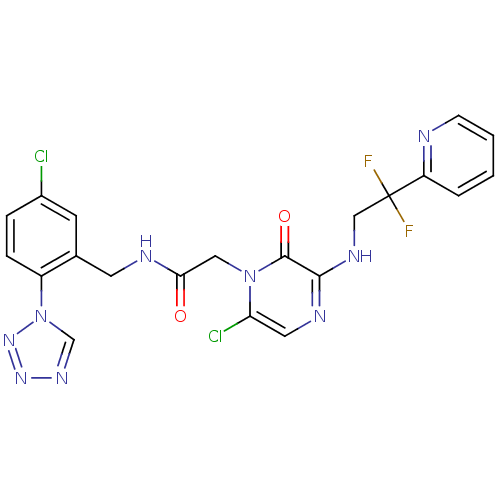
(2-[6-Chloro-3-(2,2-difluoro-2-pyridin-2-yl-ethylam...)Show SMILES FC(F)(CNc1ncc(Cl)n(CC(=O)NCc2cc(Cl)ccc2-n2cnnn2)c1=O)c1ccccn1 Show InChI InChI=1S/C21H17Cl2F2N9O2/c22-14-4-5-15(34-12-30-31-32-34)13(7-14)8-27-18(35)10-33-17(23)9-28-19(20(33)36)29-11-21(24,25)16-3-1-2-6-26-16/h1-7,9,12H,8,10-11H2,(H,27,35)(H,28,29) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.00150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory potency against human thrombin |
J Med Chem 47: 2995-3008 (2004)
Article DOI: 10.1021/jm030303e
BindingDB Entry DOI: 10.7270/Q270826B |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50292203
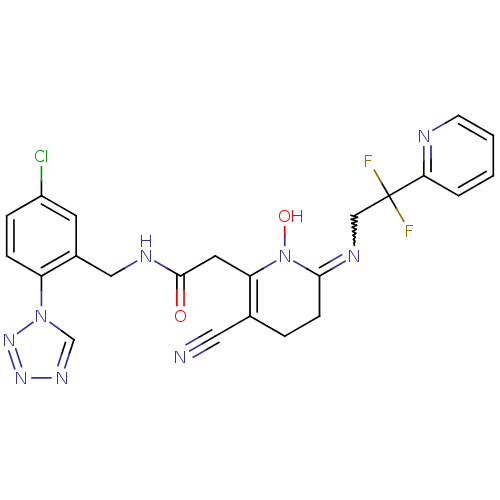
(CHEMBL196030 | N-(5-Chloro-2-tetrazol-1-yl-benzyl)...)Show SMILES ON1C(CCC(C#N)=C1CC(=O)NCc1cc(Cl)ccc1-n1cnnn1)=NCC(F)(F)c1ccccn1 |w:26.29,c:7| Show InChI InChI=1S/C23H20ClF2N9O2/c24-17-5-6-18(34-14-31-32-33-34)16(9-17)12-29-22(36)10-19-15(11-27)4-7-21(35(19)37)30-13-23(25,26)20-3-1-2-8-28-20/h1-3,5-6,8-9,14,37H,4,7,10,12-13H2,(H,29,36) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.00200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity against thrombin in human plasma |
Bioorg Med Chem Lett 15: 2771-5 (2005)
Article DOI: 10.1016/j.bmcl.2005.03.110
BindingDB Entry DOI: 10.7270/Q2PN96C6 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50133524
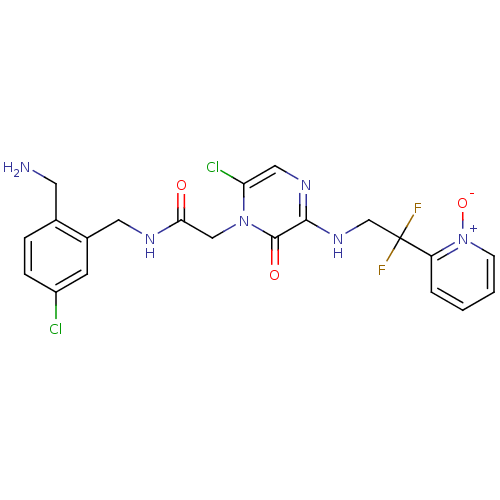
(CHEMBL419773 | N-(2-Aminomethyl-5-chloro-benzyl)-2...)Show SMILES NCc1ccc(Cl)cc1CNC(=O)Cn1c(Cl)cnc(NCC(F)(F)c2cccc[n+]2[O-])c1=O Show InChI InChI=1S/C21H20Cl2F2N6O3/c22-15-5-4-13(8-26)14(7-15)9-27-18(32)11-30-17(23)10-28-19(20(30)33)29-12-21(24,25)16-3-1-2-6-31(16)34/h1-7,10H,8-9,11-12,26H2,(H,27,32)(H,28,29) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.00340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory constant evaluated against thrombin (Factor IIa) |
Bioorg Med Chem Lett 13: 3477-82 (2003)
BindingDB Entry DOI: 10.7270/Q29P3119 |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM578
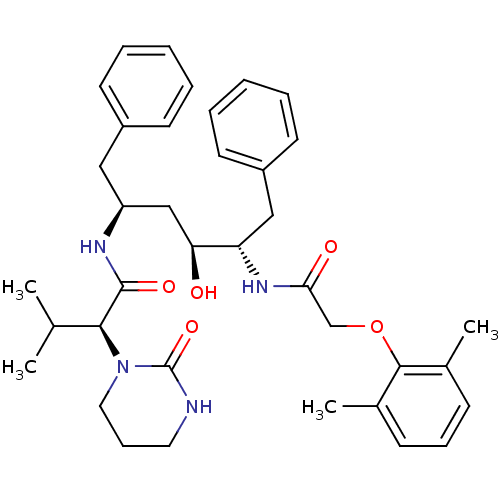
((2S)-N-[(2S,4S,5S)-5-[2-(2,6-dimethylphenoxy)aceta...)Show SMILES CC(C)[C@H](N1CCCNC1=O)C(=O)N[C@H](C[C@H](O)[C@H](Cc1ccccc1)NC(=O)COc1c(C)cccc1C)Cc1ccccc1 |r| Show InChI InChI=1S/C37H48N4O5/c1-25(2)34(41-20-12-19-38-37(41)45)36(44)39-30(21-28-15-7-5-8-16-28)23-32(42)31(22-29-17-9-6-10-18-29)40-33(43)24-46-35-26(3)13-11-14-27(35)4/h5-11,13-18,25,30-32,34,42H,12,19-24H2,1-4H3,(H,38,45)(H,39,44)(H,40,43)/t30-,31-,32-,34-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
PubMed
| 0.00400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Queensland
Curated by ChEMBL
| Assay Description
Binding affinity against ritonavir-resistant strains. |
J Med Chem 43: 305-41 (2000)
Checked by Author
BindingDB Entry DOI: 10.7270/Q2JD4XH4 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Neuronal acetylcholine receptor subunit alpha-4
(Rattus norvegicus (Rat)) | BDBM50049757

(()-2-(6-Chloro-pyridin-3-yl)-7-aza-bicyclo[2.2.1]h...)Show InChI InChI=1S/C11H13ClN2/c12-11-4-1-7(6-13-11)9-5-8-2-3-10(9)14-8/h1,4,6,8-10,14H,2-3,5H2 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.00800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse
Curated by PDSP Ki Database
| |
Mol Pharmacol 57: 642-9 (2000)
Article DOI: 10.1124/mol.57.3.642
BindingDB Entry DOI: 10.7270/Q21C1VF8 |
More data for this
Ligand-Target Pair | |
Nicotinic acetylcholine receptor
(RAT) | BDBM50049757

(()-2-(6-Chloro-pyridin-3-yl)-7-aza-bicyclo[2.2.1]h...)Show InChI InChI=1S/C11H13ClN2/c12-11-4-1-7(6-13-11)9-5-8-2-3-10(9)14-8/h1,4,6,8-10,14H,2-3,5H2 | UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.00900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse
Curated by PDSP Ki Database
| |
Mol Pharmacol 57: 642-9 (2000)
Article DOI: 10.1124/mol.57.3.642
BindingDB Entry DOI: 10.7270/Q21C1VF8 |
More data for this
Ligand-Target Pair | |
Nicotinic acetylcholine receptor
(RAT) | BDBM50049750
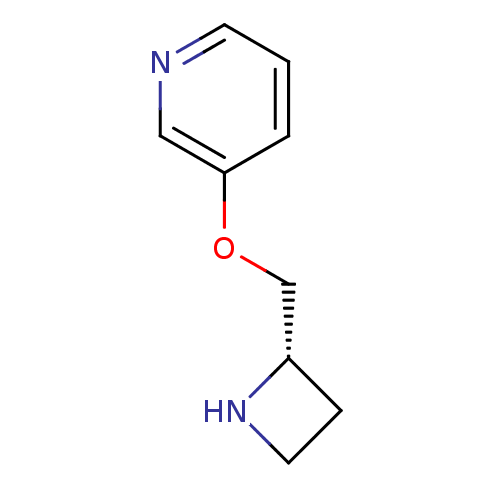
((S)-3-(azetidin-2-ylmethoxy)pyridine | 3-((S)-1-Az...)Show InChI InChI=1S/C9H12N2O/c1-2-9(6-10-4-1)12-7-8-3-5-11-8/h1-2,4,6,8,11H,3,5,7H2/t8-/m0/s1 | UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse
Curated by PDSP Ki Database
| |
Mol Pharmacol 57: 642-9 (2000)
Article DOI: 10.1124/mol.57.3.642
BindingDB Entry DOI: 10.7270/Q21C1VF8 |
More data for this
Ligand-Target Pair | |
Cathepsin D
(Homo sapiens (Human)) | BDBM912
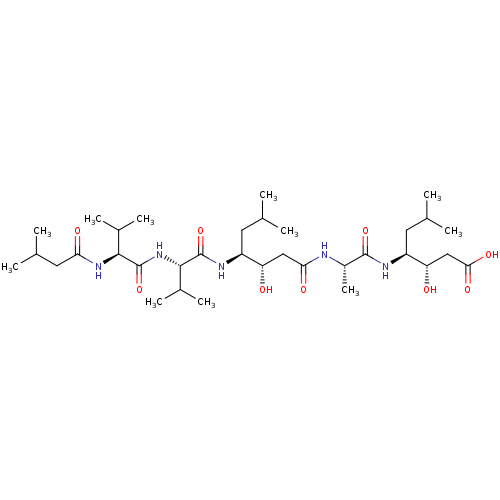
((3S,4S)-3-hydroxy-4-[(2S)-2-[(3S,4S)-3-hydroxy-6-m...)Show SMILES CC(C)C[C@H](NC(=O)[C@H](C)NC(=O)C[C@H](O)[C@H](CC(C)C)NC(=O)[C@@H](NC(=O)[C@@H](NC(=O)CC(C)C)C(C)C)C(C)C)[C@@H](O)CC(O)=O |r| Show InChI InChI=1S/C34H63N5O9/c1-17(2)12-23(37-33(47)31(21(9)10)39-34(48)30(20(7)8)38-27(42)14-19(5)6)25(40)15-28(43)35-22(11)32(46)36-24(13-18(3)4)26(41)16-29(44)45/h17-26,30-31,40-41H,12-16H2,1-11H3,(H,35,43)(H,36,46)(H,37,47)(H,38,42)(H,39,48)(H,44,45)/t22-,23-,24-,25-,26-,30-,31-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Queensland
Curated by ChEMBL
| Assay Description
Binding affinity against human cathepsin D |
J Med Chem 43: 305-41 (2000)
Checked by Author
BindingDB Entry DOI: 10.7270/Q2JD4XH4 |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4
(Rattus norvegicus (Rat)) | BDBM50066789
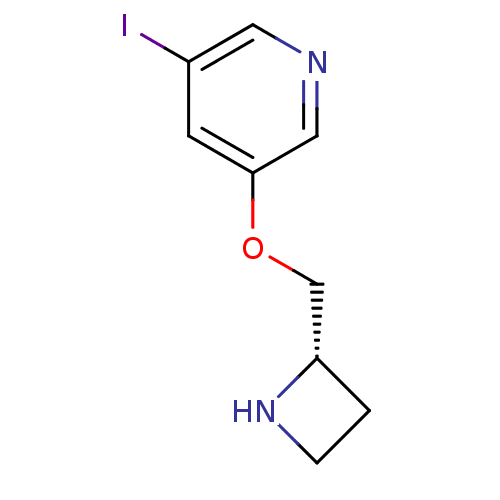
(3-((S)-1-Azetidin-2-ylmethoxy)-5-iodo-pyridine | A...)Show InChI InChI=1S/C9H11IN2O/c10-7-3-9(5-11-4-7)13-6-8-1-2-12-8/h3-5,8,12H,1-2,6H2/t8-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse
Curated by PDSP Ki Database
| |
Mol Pharmacol 57: 642-9 (2000)
Article DOI: 10.1124/mol.57.3.642
BindingDB Entry DOI: 10.7270/Q21C1VF8 |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4
(Rattus norvegicus (Rat)) | BDBM50049750

((S)-3-(azetidin-2-ylmethoxy)pyridine | 3-((S)-1-Az...)Show InChI InChI=1S/C9H12N2O/c1-2-9(6-10-4-1)12-7-8-3-5-11-8/h1-2,4,6,8,11H,3,5,7H2/t8-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse
Curated by PDSP Ki Database
| |
Mol Pharmacol 57: 642-9 (2000)
Article DOI: 10.1124/mol.57.3.642
BindingDB Entry DOI: 10.7270/Q21C1VF8 |
More data for this
Ligand-Target Pair | |
Nicotinic acetylcholine receptor
(RAT) | BDBM50066789

(3-((S)-1-Azetidin-2-ylmethoxy)-5-iodo-pyridine | A...)Show InChI InChI=1S/C9H11IN2O/c10-7-3-9(5-11-4-7)13-6-8-1-2-12-8/h3-5,8,12H,1-2,6H2/t8-/m0/s1 | UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse
Curated by PDSP Ki Database
| |
Mol Pharmacol 57: 642-9 (2000)
Article DOI: 10.1124/mol.57.3.642
BindingDB Entry DOI: 10.7270/Q21C1VF8 |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50036477

(2,6-Dichloro-3-(2-morpholin-4-yl-ethoxy)-benzoic a...)Show SMILES COc1cc2c(C(=O)N(COC(=O)c3c(Cl)ccc(OCCN4CCOCC4)c3Cl)S2(=O)=O)c(c1)C(C)C Show InChI InChI=1S/C25H28Cl2N2O8S/c1-15(2)17-12-16(34-3)13-20-21(17)24(30)29(38(20,32)33)14-37-25(31)22-18(26)4-5-19(23(22)27)36-11-8-28-6-9-35-10-7-28/h4-5,12-13,15H,6-11,14H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Queensland
Curated by ChEMBL
| Assay Description
Binding affinity against Elastase. |
J Med Chem 43: 305-41 (2000)
Checked by Author
BindingDB Entry DOI: 10.7270/Q2JD4XH4 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50147793
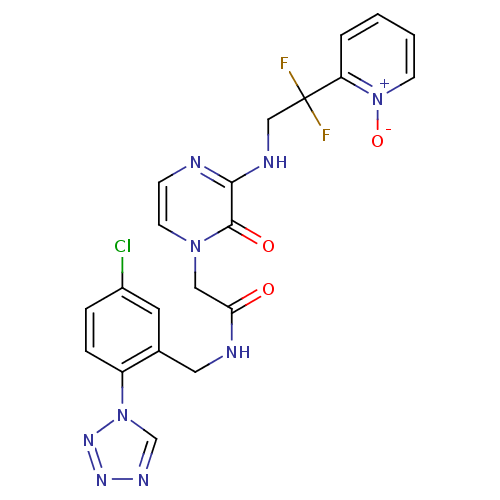
(CHEMBL323583 | N-(5-Chloro-2-tetrazol-1-yl-benzyl)...)Show SMILES [O-][n+]1ccccc1C(F)(F)CNc1nccn(CC(=O)NCc2cc(Cl)ccc2-n2cnnn2)c1=O Show InChI InChI=1S/C21H18ClF2N9O3/c22-15-4-5-16(32-13-28-29-30-32)14(9-15)10-26-18(34)11-31-8-6-25-19(20(31)35)27-12-21(23,24)17-3-1-2-7-33(17)36/h1-9,13H,10-12H2,(H,25,27)(H,26,34) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory potency against human thrombin |
J Med Chem 47: 2995-3008 (2004)
Article DOI: 10.1021/jm030303e
BindingDB Entry DOI: 10.7270/Q270826B |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Rattus norvegicus (rat)) | BDBM50160893
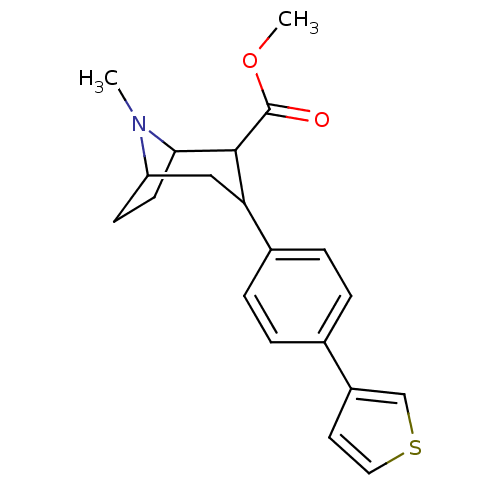
(8-Methyl-3-(4-thiophen-3-yl-phenyl)-8-aza-bicyclo[...)Show SMILES COC(=O)C1C2CCC(CC1c1ccc(cc1)-c1ccsc1)N2C |TLB:23:22:4.10.9:6.7,THB:2:4:22:6.7,11:10:22:6.7| Show InChI InChI=1S/C20H23NO2S/c1-21-16-7-8-18(21)19(20(22)23-2)17(11-16)14-5-3-13(4-6-14)15-9-10-24-12-15/h3-6,9-10,12,16-19H,7-8,11H2,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yale University
Curated by ChEMBL
| Assay Description
Displacement of [3H]paroxetine from serotonin transporter of rat cerebral cortex |
Bioorg Med Chem Lett 15: 1131-3 (2005)
Article DOI: 10.1016/j.bmcl.2004.12.014
BindingDB Entry DOI: 10.7270/Q2PK0GXK |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50147788

(CHEMBL103874 | N-(5-Chloro-2-tetrazol-1-yl-benzyl)...)Show SMILES Cc1ccc(NS(=O)(=O)Cc2ccccc2)c(=O)n1CC(=O)NCc1cc(Cl)ccc1-n1cnnn1 Show InChI InChI=1S/C23H22ClN7O4S/c1-16-7-9-20(27-36(34,35)14-17-5-3-2-4-6-17)23(33)30(16)13-22(32)25-12-18-11-19(24)8-10-21(18)31-15-26-28-29-31/h2-11,15,27H,12-14H2,1H3,(H,25,32) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory potency against human thrombin |
J Med Chem 47: 2995-3008 (2004)
Article DOI: 10.1021/jm030303e
BindingDB Entry DOI: 10.7270/Q270826B |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4
(Rattus norvegicus (Rat)) | BDBM50100712
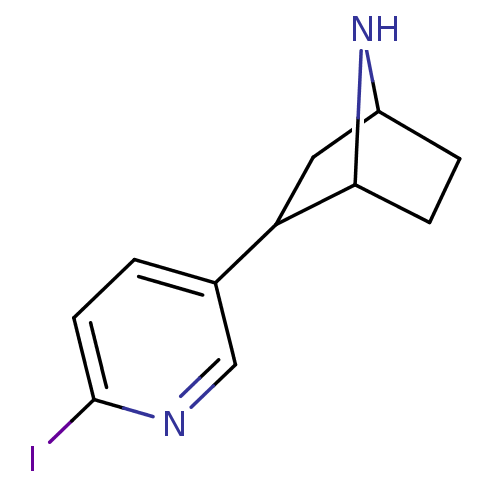
(2-(6-Iodo-pyridin-3-yl)-7-aza-bicyclo[2.2.1]heptan...)Show InChI InChI=1S/C11H13IN2/c12-11-4-1-7(6-13-11)9-5-8-2-3-10(9)14-8/h1,4,6,8-10,14H,2-3,5H2 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse
Curated by PDSP Ki Database
| |
Mol Pharmacol 57: 642-9 (2000)
Article DOI: 10.1124/mol.57.3.642
BindingDB Entry DOI: 10.7270/Q21C1VF8 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50131480
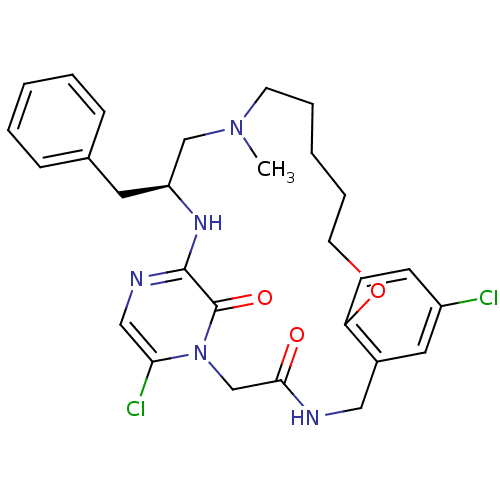
((S)-20-Benzyl-8,25-dichloro-18-methyl-12-oxa-1,4,1...)Show SMILES CN1CCCCCOc2ccc(Cl)cc2CNC(=O)Cn2c(Cl)cnc(N[C@@H](Cc3ccccc3)C1)c2=O Show InChI InChI=1S/C28H33Cl2N5O3/c1-34-12-6-3-7-13-38-24-11-10-22(29)15-21(24)16-31-26(36)19-35-25(30)17-32-27(28(35)37)33-23(18-34)14-20-8-4-2-5-9-20/h2,4-5,8-11,15,17,23H,3,6-7,12-14,16,18-19H2,1H3,(H,31,36)(H,32,33)/t23-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against thrombin IIa |
Bioorg Med Chem Lett 13: 2781-4 (2003)
BindingDB Entry DOI: 10.7270/Q2GF0V2P |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM729

((2S,4R)-N-tert-butyl-1-[(2R,3S)-2-hydroxy-3-[(2S)-...)Show SMILES CC(C)[C@H](NC(=O)c1ccc2ccccc2n1)C(=O)N[C@@H](Cc1ccccc1)[C@H](O)CN1CC[C@H](C[C@H]1C(=O)NC(C)(C)C)OCc1ccncc1 |r| Show InChI InChI=1S/C41H52N6O5/c1-27(2)37(45-38(49)33-16-15-30-13-9-10-14-32(30)43-33)40(51)44-34(23-28-11-7-6-8-12-28)36(48)25-47-22-19-31(52-26-29-17-20-42-21-18-29)24-35(47)39(50)46-41(3,4)5/h6-18,20-21,27,31,34-37,48H,19,22-26H2,1-5H3,(H,44,51)(H,45,49)(H,46,50)/t31-,34+,35+,36-,37+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.0310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Queensland
Curated by ChEMBL
| Assay Description
Binding affinity against HIV-1 protease . |
J Med Chem 43: 305-41 (2000)
Checked by Author
BindingDB Entry DOI: 10.7270/Q2JD4XH4 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50147809
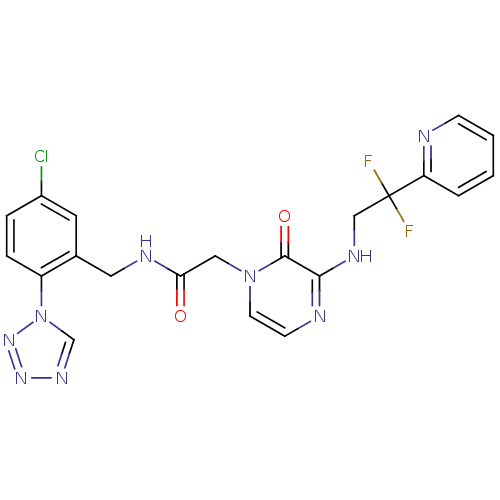
(CHEMBL103342 | N-(5-Chloro-2-tetrazol-1-yl-benzyl)...)Show SMILES FC(F)(CNc1nccn(CC(=O)NCc2cc(Cl)ccc2-n2cnnn2)c1=O)c1ccccn1 Show InChI InChI=1S/C21H18ClF2N9O2/c22-15-4-5-16(33-13-29-30-31-33)14(9-15)10-27-18(34)11-32-8-7-26-19(20(32)35)28-12-21(23,24)17-3-1-2-6-25-17/h1-9,13H,10-12H2,(H,26,28)(H,27,34) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory potency against human thrombin |
J Med Chem 47: 2995-3008 (2004)
Article DOI: 10.1021/jm030303e
BindingDB Entry DOI: 10.7270/Q270826B |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50082556
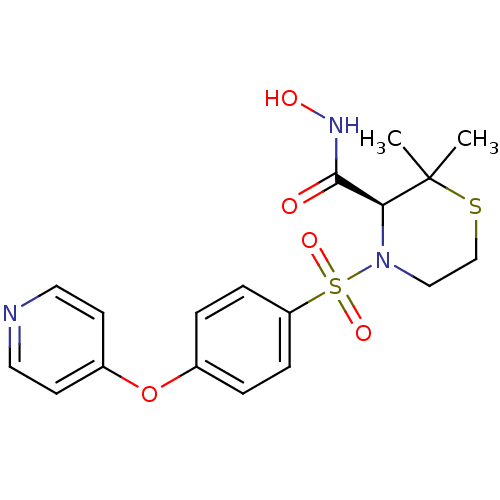
((S)-2,2-Dimethyl-4-[4-(pyridin-4-yloxy)-benzenesul...)Show SMILES CC1(C)SCCN([C@H]1C(=O)NO)S(=O)(=O)c1ccc(Oc2ccncc2)cc1 Show InChI InChI=1S/C18H21N3O5S2/c1-18(2)16(17(22)20-23)21(11-12-27-18)28(24,25)15-5-3-13(4-6-15)26-14-7-9-19-10-8-14/h3-10,16,23H,11-12H2,1-2H3,(H,20,22)/t16-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 0.0380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Queensland
Curated by ChEMBL
| Assay Description
Inhibition of matrix metalloprotease-13 (MMP-13). |
J Med Chem 43: 305-41 (2000)
Checked by Author
BindingDB Entry DOI: 10.7270/Q2JD4XH4 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50292196
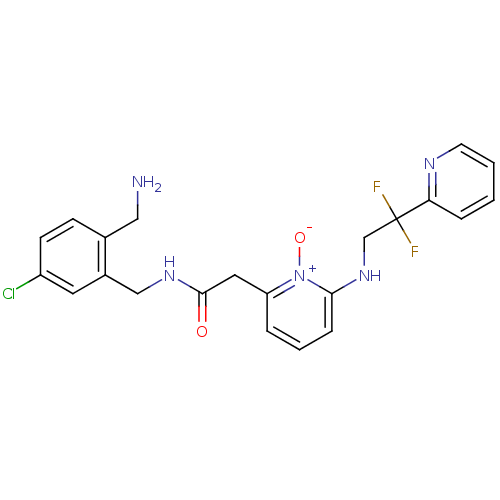
(CHEMBL195366 | N-(2-Aminomethyl-5-chloro-benzyl)-2...)Show SMILES NCc1ccc(Cl)cc1CNC(=O)Cc1cccc(NCC(F)(F)c2ccccn2)[n+]1[O-] Show InChI InChI=1S/C22H22ClF2N5O2/c23-17-8-7-15(12-26)16(10-17)13-28-21(31)11-18-4-3-6-20(30(18)32)29-14-22(24,25)19-5-1-2-9-27-19/h1-10,29H,11-14,26H2,(H,28,31) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity against thrombin in human plasma |
Bioorg Med Chem Lett 15: 2771-5 (2005)
Article DOI: 10.1016/j.bmcl.2005.03.110
BindingDB Entry DOI: 10.7270/Q2PN96C6 |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-3
(Rattus norvegicus (Rat)) | BDBM50049757

(()-2-(6-Chloro-pyridin-3-yl)-7-aza-bicyclo[2.2.1]h...)Show InChI InChI=1S/C11H13ClN2/c12-11-4-1-7(6-13-11)9-5-8-2-3-10(9)14-8/h1,4,6,8-10,14H,2-3,5H2 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse
Curated by PDSP Ki Database
| |
Mol Pharmacol 57: 642-9 (2000)
Article DOI: 10.1124/mol.57.3.642
BindingDB Entry DOI: 10.7270/Q21C1VF8 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50133529
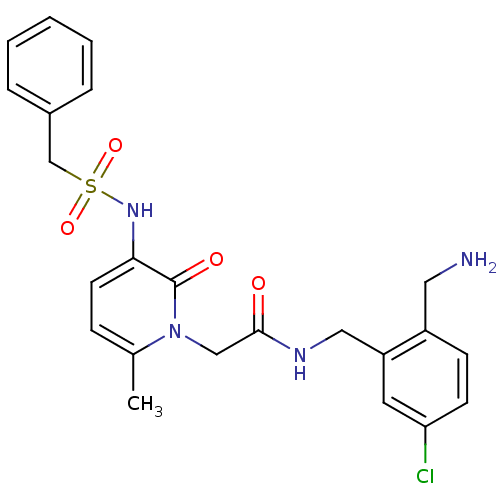
(CHEMBL420682 | N-(2-Aminomethyl-5-chloro-benzyl)-2...)Show SMILES Cc1ccc(NS(=O)(=O)Cc2ccccc2)c(=O)n1CC(=O)NCc1cc(Cl)ccc1CN Show InChI InChI=1S/C23H25ClN4O4S/c1-16-7-10-21(27-33(31,32)15-17-5-3-2-4-6-17)23(30)28(16)14-22(29)26-13-19-11-20(24)9-8-18(19)12-25/h2-11,27H,12-15,25H2,1H3,(H,26,29) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0460 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory constant evaluated against thrombin (Factor IIa) |
Bioorg Med Chem Lett 13: 3477-82 (2003)
BindingDB Entry DOI: 10.7270/Q29P3119 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50133521
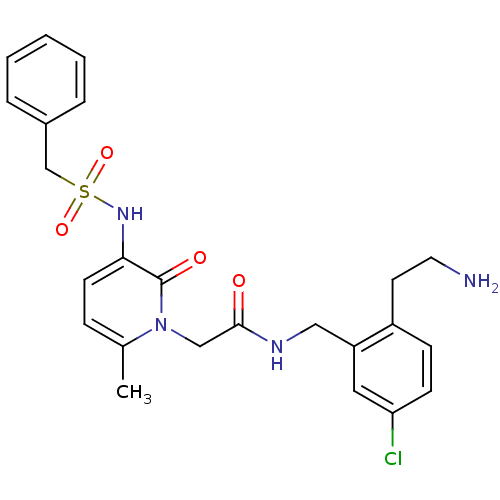
(CHEMBL116202 | N-[2-(2-Amino-ethyl)-5-chloro-benzy...)Show SMILES Cc1ccc(NS(=O)(=O)Cc2ccccc2)c(=O)n1CC(=O)NCc1cc(Cl)ccc1CCN Show InChI InChI=1S/C24H27ClN4O4S/c1-17-7-10-22(28-34(32,33)16-18-5-3-2-4-6-18)24(31)29(17)15-23(30)27-14-20-13-21(25)9-8-19(20)11-12-26/h2-10,13,28H,11-12,14-16,26H2,1H3,(H,27,30) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory constant evaluated against thrombin (Factor IIa) |
Bioorg Med Chem Lett 13: 3477-82 (2003)
BindingDB Entry DOI: 10.7270/Q29P3119 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50292200
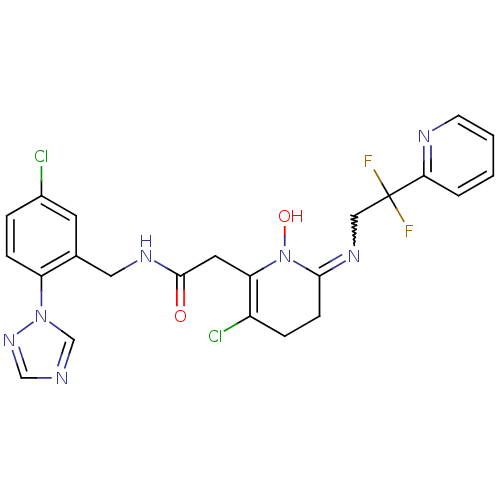
(2-[3-Chloro-6-(2,2-difluoro-2-pyridin-2-yl-ethylam...)Show SMILES ON1C(CCC(Cl)=C1CC(=O)NCc1cc(Cl)ccc1-n1cncn1)=NCC(F)(F)c1ccccn1 |w:25.28,c:6| Show InChI InChI=1S/C23H21Cl2F2N7O2/c24-16-4-6-18(33-14-28-13-32-33)15(9-16)11-30-22(35)10-19-17(25)5-7-21(34(19)36)31-12-23(26,27)20-3-1-2-8-29-20/h1-4,6,8-9,13-14,36H,5,7,10-12H2,(H,30,35) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity against thrombin in human plasma |
Bioorg Med Chem Lett 15: 2771-5 (2005)
Article DOI: 10.1016/j.bmcl.2005.03.110
BindingDB Entry DOI: 10.7270/Q2PN96C6 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50147812
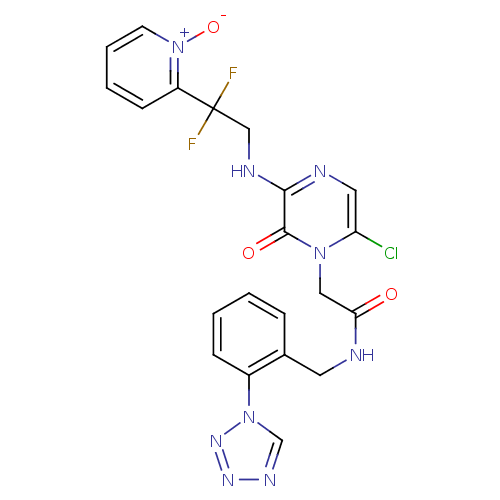
(2-{6-Chloro-3-[2,2-difluoro-2-(1-oxy-pyridin-2-yl)...)Show SMILES [O-][n+]1ccccc1C(F)(F)CNc1ncc(Cl)n(CC(=O)NCc2ccccc2-n2cnnn2)c1=O Show InChI InChI=1S/C21H18ClF2N9O3/c22-17-10-26-19(27-12-21(23,24)16-7-3-4-8-33(16)36)20(35)31(17)11-18(34)25-9-14-5-1-2-6-15(14)32-13-28-29-30-32/h1-8,10,13H,9,11-12H2,(H,25,34)(H,26,27) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory potency against human thrombin |
J Med Chem 47: 2995-3008 (2004)
Article DOI: 10.1021/jm030303e
BindingDB Entry DOI: 10.7270/Q270826B |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50131464
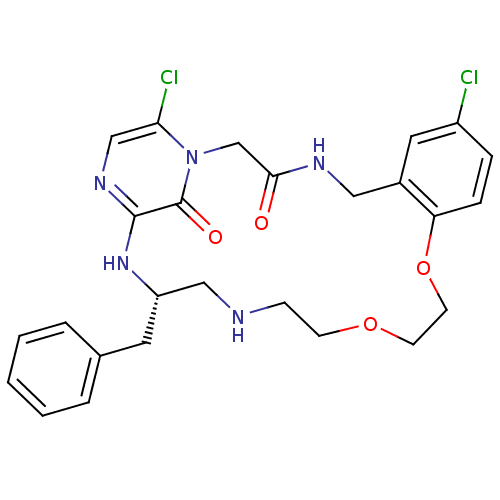
((S)-20-Benzyl-8,25-dichloro-12,15-dioxa-1,4,18,21,...)Show SMILES Clc1ccc2OCCOCCNC[C@H](Cc3ccccc3)Nc3ncc(Cl)n(CC(=O)NCc2c1)c3=O Show InChI InChI=1S/C26H29Cl2N5O4/c27-20-6-7-22-19(13-20)14-30-24(34)17-33-23(28)16-31-25(26(33)35)32-21(12-18-4-2-1-3-5-18)15-29-8-9-36-10-11-37-22/h1-7,13,16,21,29H,8-12,14-15,17H2,(H,30,34)(H,31,32)/t21-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against thrombin IIa |
Bioorg Med Chem Lett 13: 2781-4 (2003)
BindingDB Entry DOI: 10.7270/Q2GF0V2P |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50131460
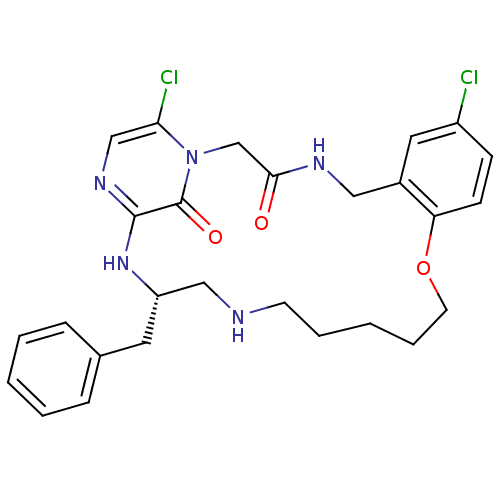
((S)-20-Benzyl-8,25-dichloro-12-oxa-1,4,18,21,23-pe...)Show SMILES Clc1ccc2OCCCCCNC[C@H](Cc3ccccc3)Nc3ncc(Cl)n(CC(=O)NCc2c1)c3=O Show InChI InChI=1S/C27H31Cl2N5O3/c28-21-9-10-23-20(14-21)15-31-25(35)18-34-24(29)17-32-26(27(34)36)33-22(13-19-7-3-1-4-8-19)16-30-11-5-2-6-12-37-23/h1,3-4,7-10,14,17,22,30H,2,5-6,11-13,15-16,18H2,(H,31,35)(H,32,33)/t22-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against thrombin IIa |
Bioorg Med Chem Lett 13: 2781-4 (2003)
BindingDB Entry DOI: 10.7270/Q2GF0V2P |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50057826

((S)-1-((R)-2-Amino-3,3-dicyclohexyl-propionyl)-pyr...)Show SMILES N[C@H](C(C1CCCCC1)C1CCCCC1)C(=O)N1CCC[C@H]1C(=O)NCC1CCC(N)CC1 |wU:21.24,wD:1.0,(8.57,-5.29,;9.89,-4.53,;9.89,-2.99,;11.24,-2.22,;12.55,-2.99,;13.9,-2.22,;13.9,-.68,;12.58,.09,;11.23,-.68,;8.57,-2.21,;7.23,-2.98,;5.9,-2.21,;5.9,-.67,;7.23,.1,;8.57,-.67,;11.24,-5.3,;11.24,-6.84,;12.72,-4.89,;13.26,-3.44,;14.8,-3.51,;15.22,-5,;13.92,-5.85,;13.86,-7.39,;12.49,-8.1,;15.15,-8.21,;16.52,-7.51,;17.82,-8.32,;17.78,-9.66,;18.86,-11.05,;17.78,-12.17,;18.86,-13.25,;17.85,-10.92,;16.76,-9.44,)| Show InChI InChI=1S/C27H48N4O2/c28-22-15-13-19(14-16-22)18-30-26(32)23-12-7-17-31(23)27(33)25(29)24(20-8-3-1-4-9-20)21-10-5-2-6-11-21/h19-25H,1-18,28-29H2,(H,30,32)/t19?,22?,23-,25+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0560 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Queensland
Curated by ChEMBL
| Assay Description
Binding affinity against Thrombin. |
J Med Chem 43: 305-41 (2000)
Checked by Author
BindingDB Entry DOI: 10.7270/Q2JD4XH4 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50131471
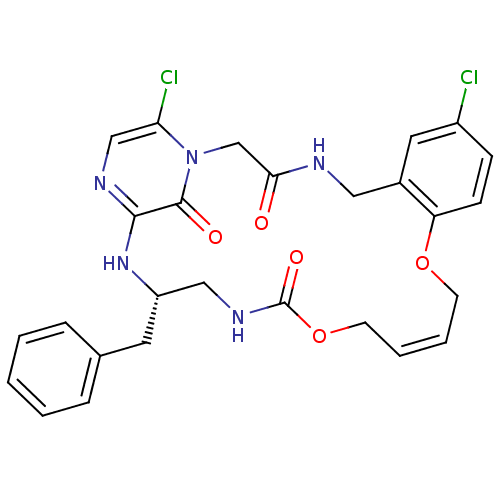
((14Z,21S)-21-benzyl-8,26-dichloro-12,17-dioxa-1,4,...)Show SMILES Clc1ccc2OC\C=C/COC(=O)NC[C@H](Cc3ccccc3)Nc3ncc(Cl)n(CC(=O)NCc2c1)c3=O |c:7| Show InChI InChI=1S/C27H27Cl2N5O5/c28-20-8-9-22-19(13-20)14-30-24(35)17-34-23(29)16-31-25(26(34)36)33-21(12-18-6-2-1-3-7-18)15-32-27(37)39-11-5-4-10-38-22/h1-9,13,16,21H,10-12,14-15,17H2,(H,30,35)(H,31,33)(H,32,37)/b5-4-/t21-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against thrombin IIa |
Bioorg Med Chem Lett 13: 2781-4 (2003)
BindingDB Entry DOI: 10.7270/Q2GF0V2P |
More data for this
Ligand-Target Pair | |
Sodium-dependent dopamine transporter
(Rattus norvegicus (rat)) | BDBM22165
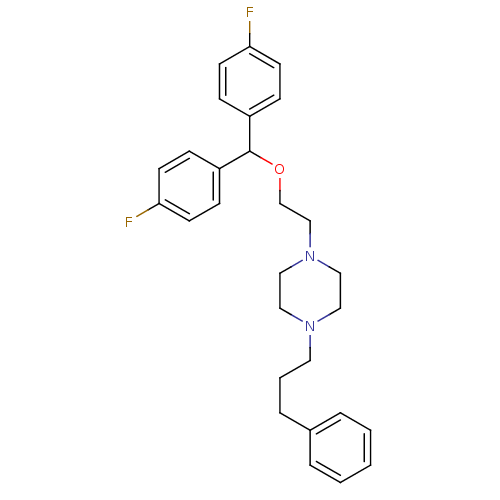
(1-{2-[bis(4-fluorophenyl)methoxy]ethyl}-4-(3-pheny...)Show SMILES Fc1ccc(cc1)C(OCCN1CCN(CCCc2ccccc2)CC1)c1ccc(F)cc1 Show InChI InChI=1S/C28H32F2N2O/c29-26-12-8-24(9-13-26)28(25-10-14-27(30)15-11-25)33-22-21-32-19-17-31(18-20-32)16-4-7-23-5-2-1-3-6-23/h1-3,5-6,8-15,28H,4,7,16-22H2 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Biochemicals International
Curated by ChEMBL
| Assay Description
In vitro binding affinity towards dopamine transporter in rat striatal membranes by [3H]GBR-12395 displacement. |
J Med Chem 39: 543-8 (1996)
Article DOI: 10.1021/jm9505324
BindingDB Entry DOI: 10.7270/Q2KD1ZJ5 |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Rattus norvegicus (rat)) | BDBM50106857
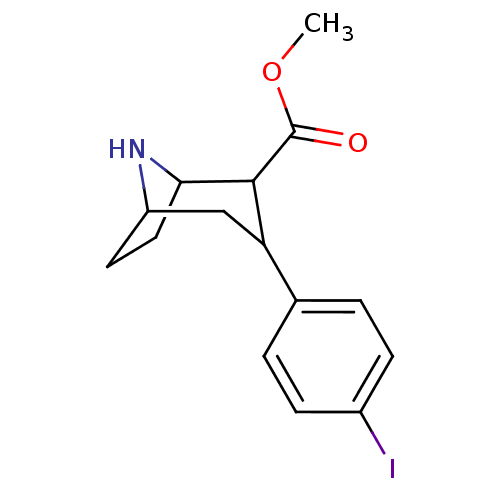
(3-(4-Iodo-phenyl)-8-aza-bicyclo[3.2.1]octane-2-car...)Show SMILES COC(=O)C1C2CCC(CC1c1ccc(I)cc1)N2 |THB:2:4:18:6.7| Show InChI InChI=1S/C15H18INO2/c1-19-15(18)14-12(8-11-6-7-13(14)17-11)9-2-4-10(16)5-3-9/h2-5,11-14,17H,6-8H2,1H3 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0620 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yale School of Medicine
Curated by ChEMBL
| Assay Description
Binding affinity to serotonin transporter (SERT) in rat forebrain tissue |
Bioorg Med Chem Lett 14: 2117-20 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.043
BindingDB Entry DOI: 10.7270/Q2WS8SQK |
More data for this
Ligand-Target Pair | |
Alpha-1A adrenergic receptor
(Homo sapiens (Human)) | BDBM50090035
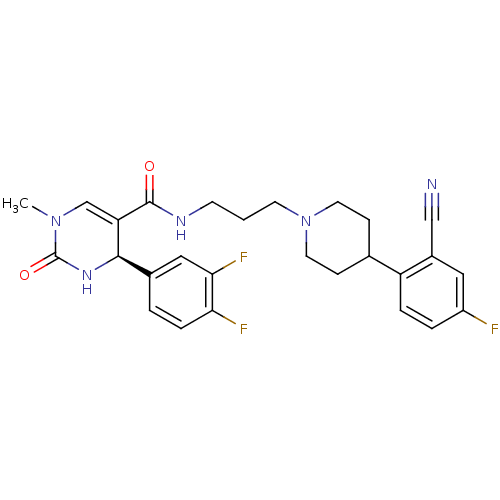
(4-(3,4-Difluoro-phenyl)-1-methyl-2-oxo-1,2,3,4-tet...)Show SMILES CN1C=C([C@H](NC1=O)c1ccc(F)c(F)c1)C(=O)NCCCN1CCC(CC1)c1ccc(F)cc1C#N |c:2| Show InChI InChI=1S/C27H28F3N5O2/c1-34-16-22(25(33-27(34)37)18-3-6-23(29)24(30)14-18)26(36)32-9-2-10-35-11-7-17(8-12-35)21-5-4-20(28)13-19(21)15-31/h3-6,13-14,16-17,25H,2,7-12H2,1H3,(H,32,36)(H,33,37)/t25-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0680 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Evaluated for the ability to displace [125I]- HEAT from human cloned Alpha-1A adrenergic receptor stably expressed in CHO cells. |
J Med Chem 43: 2703-18 (2000)
BindingDB Entry DOI: 10.7270/Q2XK8DTM |
More data for this
Ligand-Target Pair | |
Alpha-1A adrenergic receptor
(Homo sapiens (Human)) | BDBM50090023

(4-(3,4-Difluoro-phenyl)-2-oxo-1,2,3,4-tetrahydro-p...)Show SMILES Fc1ccc(C2CCN(CCCNC(=O)C3C=NC(=O)N[C@@H]3c3ccc(F)c(F)c3)CC2)c(c1)C#N |c:16| Show InChI InChI=1S/C26H26F3N5O2/c27-19-3-4-20(18(12-19)14-30)16-6-10-34(11-7-16)9-1-8-31-25(35)21-15-32-26(36)33-24(21)17-2-5-22(28)23(29)13-17/h2-5,12-13,15-16,21,24H,1,6-11H2,(H,31,35)(H,33,36)/t21?,24-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Evaluated for the ability to displace [125I]- HEAT from human cloned Alpha-1A adrenergic receptor stably expressed in CHO cells. |
J Med Chem 43: 2703-18 (2000)
BindingDB Entry DOI: 10.7270/Q2XK8DTM |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50220136

(3-[2-{1-[3,5-di(trifluoromethyl)phenyl]ethoxy}-3-(...)Show SMILES C[C@@H](O[C@H]1OCCN(Cc2n[nH]c(=O)[nH]2)[C@H]1c1ccc(F)cc1)c1cc(cc(c1)C(F)(F)F)C(F)(F)F |r| Show InChI InChI=1S/C23H21F7N4O3/c1-12(14-8-15(22(25,26)27)10-16(9-14)23(28,29)30)37-20-19(13-2-4-17(24)5-3-13)34(6-7-36-20)11-18-31-21(35)33-32-18/h2-5,8-10,12,19-20H,6-7,11H2,1H3,(H2,31,32,33,35)/t12-,19+,20-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 0.0794 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Montr£al
Curated by ChEMBL
| Assay Description
Binding affinity to NK1 receptor (unknown origin) |
Bioorg Med Chem Lett 24: 510-4 (2014)
Article DOI: 10.1016/j.bmcl.2013.12.033
BindingDB Entry DOI: 10.7270/Q2KS6T1G |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50292189
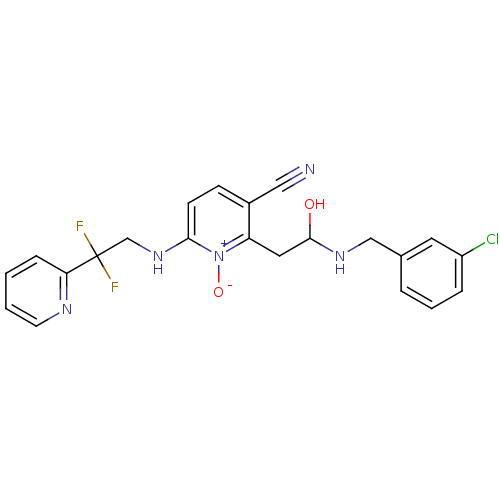
(2-[2-(3-Chloro-benzylamino)-2-hydroxy-ethyl]-6-(2,...)Show SMILES OC(Cc1c(ccc(NCC(F)(F)c2ccccn2)[n+]1[O-])C#N)NCc1cccc(Cl)c1 Show InChI InChI=1S/C22H20ClF2N5O2/c23-17-5-3-4-15(10-17)13-28-21(31)11-18-16(12-26)7-8-20(30(18)32)29-14-22(24,25)19-6-1-2-9-27-19/h1-10,21,28-29,31H,11,13-14H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity against thrombin in human plasma |
Bioorg Med Chem Lett 15: 2771-5 (2005)
Article DOI: 10.1016/j.bmcl.2005.03.110
BindingDB Entry DOI: 10.7270/Q2PN96C6 |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50082556

((S)-2,2-Dimethyl-4-[4-(pyridin-4-yloxy)-benzenesul...)Show SMILES CC1(C)SCCN([C@H]1C(=O)NO)S(=O)(=O)c1ccc(Oc2ccncc2)cc1 Show InChI InChI=1S/C18H21N3O5S2/c1-18(2)16(17(22)20-23)21(11-12-27-18)28(24,25)15-5-3-13(4-6-15)26-14-7-9-19-10-8-14/h3-10,16,23H,11-12H2,1-2H3,(H,20,22)/t16-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 0.0830 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Queensland
Curated by ChEMBL
| Assay Description
Inhibition of matrix metalloprotease-2 (MMP-2). |
J Med Chem 43: 305-41 (2000)
Checked by Author
BindingDB Entry DOI: 10.7270/Q2JD4XH4 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50147801
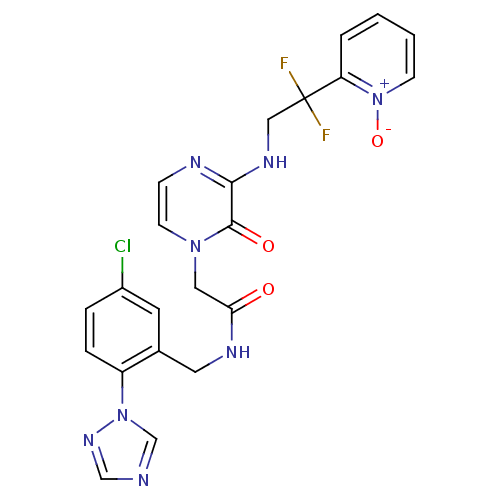
(CHEMBL102122 | N-(5-Chloro-2-[1,2,4]triazol-1-yl-b...)Show SMILES [O-][n+]1ccccc1C(F)(F)CNc1nccn(CC(=O)NCc2cc(Cl)ccc2-n2cncn2)c1=O Show InChI InChI=1S/C22H19ClF2N8O3/c23-16-4-5-17(32-14-26-13-30-32)15(9-16)10-28-19(34)11-31-8-6-27-20(21(31)35)29-12-22(24,25)18-3-1-2-7-33(18)36/h1-9,13-14H,10-12H2,(H,27,29)(H,28,34) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0850 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory potency against human thrombin |
J Med Chem 47: 2995-3008 (2004)
Article DOI: 10.1021/jm030303e
BindingDB Entry DOI: 10.7270/Q270826B |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50126304
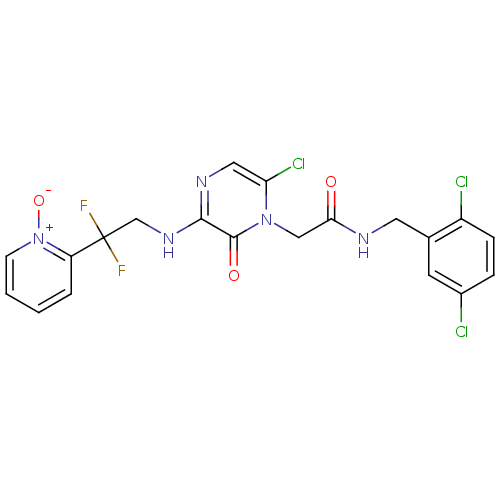
(2-{6-Chloro-3-[2,2-difluoro-2-(1-oxy-pyridin-2-yl)...)Show SMILES [O-][n+]1ccccc1C(F)(F)CNc1ncc(Cl)n(CC(=O)NCc2cc(Cl)ccc2Cl)c1=O Show InChI InChI=1S/C20H16Cl3F2N5O3/c21-13-4-5-14(22)12(7-13)8-26-17(31)10-29-16(23)9-27-18(19(29)32)28-11-20(24,25)15-3-1-2-6-30(15)33/h1-7,9H,8,10-11H2,(H,26,31)(H,27,28) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0870 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against human thrombin |
Bioorg Med Chem Lett 13: 1353-7 (2003)
BindingDB Entry DOI: 10.7270/Q2833RC9 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50131470
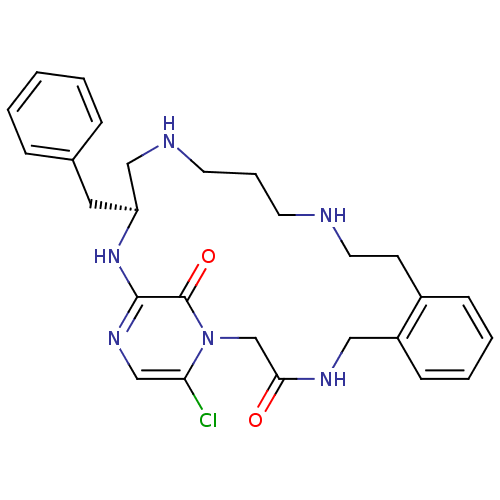
((11S)-11-BENZYL-6-CHLORO-1,2,10,11,12,13,14,15,16,...)Show SMILES Clc1cnc2N[C@@H](Cc3ccccc3)CNCCCNCCc3ccccc3CNC(=O)Cn1c2=O Show InChI InChI=1S/C27H33ClN6O2/c28-24-18-32-26-27(36)34(24)19-25(35)31-16-22-10-5-4-9-21(22)11-14-29-12-6-13-30-17-23(33-26)15-20-7-2-1-3-8-20/h1-5,7-10,18,23,29-30H,6,11-17,19H2,(H,31,35)(H,32,33)/t23-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
PubMed
| 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against thrombin IIa |
Bioorg Med Chem Lett 13: 2781-4 (2003)
BindingDB Entry DOI: 10.7270/Q2GF0V2P |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Prothrombin
(Homo sapiens (Human)) | BDBM50147821
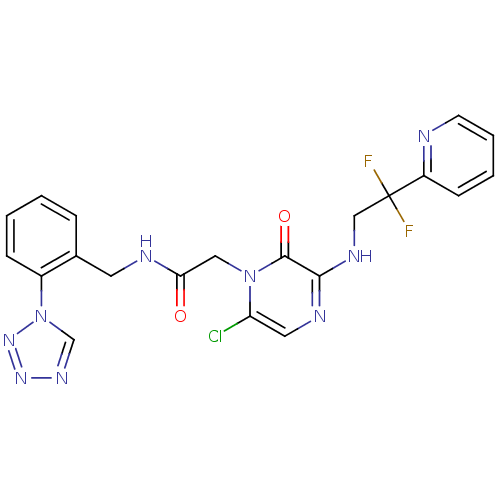
(2-[6-Chloro-3-(2,2-difluoro-2-pyridin-2-yl-ethylam...)Show SMILES FC(F)(CNc1ncc(Cl)n(CC(=O)NCc2ccccc2-n2cnnn2)c1=O)c1ccccn1 Show InChI InChI=1S/C21H18ClF2N9O2/c22-17-10-27-19(28-12-21(23,24)16-7-3-4-8-25-16)20(35)32(17)11-18(34)26-9-14-5-1-2-6-15(14)33-13-29-30-31-33/h1-8,10,13H,9,11-12H2,(H,26,34)(H,27,28) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0960 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory potency against human thrombin |
J Med Chem 47: 2995-3008 (2004)
Article DOI: 10.1021/jm030303e
BindingDB Entry DOI: 10.7270/Q270826B |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50084664

(({3-(5-Carbamimidoyl-2-hydroxy-phenoxy)-2,6-difluo...)Show SMILES CN(CC(O)=O)c1c(F)c(Oc2cccc(c2)C2=NCCN2C)cc(Oc2cc(ccc2O)C(N)=N)c1F |t:18| Show InChI InChI=1S/C26H25F2N5O5/c1-32-9-8-31-26(32)15-4-3-5-16(10-15)37-19-12-20(23(28)24(22(19)27)33(2)13-21(35)36)38-18-11-14(25(29)30)6-7-17(18)34/h3-7,10-12,34H,8-9,13H2,1-2H3,(H3,29,30)(H,35,36) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Queensland
Curated by ChEMBL
| Assay Description
Binding affinity against Coagulation factor X |
J Med Chem 43: 305-41 (2000)
Checked by Author
BindingDB Entry DOI: 10.7270/Q2JD4XH4 |
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 5
(Rattus norvegicus (Rat)) | BDBM50345553

(3-(2-(2-methylthiazol-4-yl)ethynyl)-5-bromobenzoni...)Show InChI InChI=1S/C13H7BrN2S/c1-9-16-13(8-17-9)3-2-10-4-11(7-15)6-12(14)5-10/h4-6,8H,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.106 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Neurodegenerative Disorders
Curated by ChEMBL
| Assay Description
Displacement of [3H]methoxy-PEPY from rat mGluR5 expressed in human HEK-293 cells by liquid scintillation counting |
Bioorg Med Chem Lett 21: 3243-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.04.047
BindingDB Entry DOI: 10.7270/Q290244M |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-3
(Rattus norvegicus (Rat)) | BDBM50100712

(2-(6-Iodo-pyridin-3-yl)-7-aza-bicyclo[2.2.1]heptan...)Show InChI InChI=1S/C11H13IN2/c12-11-4-1-7(6-13-11)9-5-8-2-3-10(9)14-8/h1,4,6,8-10,14H,2-3,5H2 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse
Curated by PDSP Ki Database
| |
Mol Pharmacol 57: 642-9 (2000)
Article DOI: 10.1124/mol.57.3.642
BindingDB Entry DOI: 10.7270/Q21C1VF8 |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Rattus norvegicus (rat)) | BDBM50160892
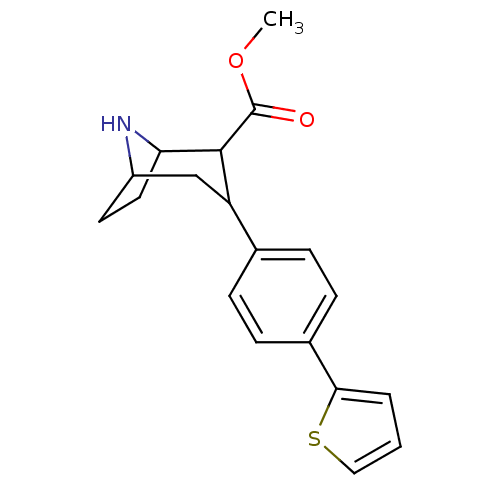
(3-(4-Thiophen-2-yl-phenyl)-8-aza-bicyclo[3.2.1]oct...)Show SMILES COC(=O)C1C2CCC(CC1c1ccc(cc1)-c1cccs1)N2 |THB:2:4:22:6.7,11:10:22:6.7| Show InChI InChI=1S/C19H21NO2S/c1-22-19(21)18-15(11-14-8-9-16(18)20-14)12-4-6-13(7-5-12)17-3-2-10-23-17/h2-7,10,14-16,18,20H,8-9,11H2,1H3 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yale University
Curated by ChEMBL
| Assay Description
Displacement of [3H]paroxetine from serotonin transporter of rat cerebral cortex |
Bioorg Med Chem Lett 15: 1131-3 (2005)
Article DOI: 10.1016/j.bmcl.2004.12.014
BindingDB Entry DOI: 10.7270/Q2PK0GXK |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Rattus norvegicus (rat)) | BDBM50048558
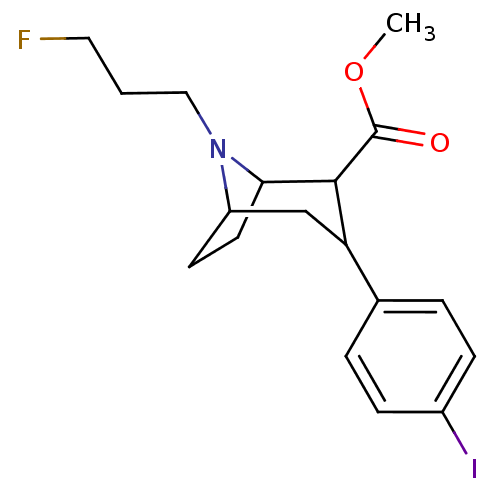
(8-(3-Fluoro-propyl)-3-(4-iodo-phenyl)-8-aza-bicycl...)Show SMILES COC(=O)C1C2CCC(CC1c1ccc(I)cc1)N2CCCF |TLB:11:10:18:6.7,THB:2:4:18:6.7| Show InChI InChI=1S/C18H23FINO2/c1-23-18(22)17-15(12-3-5-13(20)6-4-12)11-14-7-8-16(17)21(14)10-2-9-19/h3-6,14-17H,2,7-11H2,1H3 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Biochemicals International
Curated by ChEMBL
| Assay Description
In vitro binding affinity towards serotonin transporter in rat cerebral cortical homogenates by [3H]paroxetine displacement. |
J Med Chem 39: 543-8 (1996)
Article DOI: 10.1021/jm9505324
BindingDB Entry DOI: 10.7270/Q2KD1ZJ5 |
More data for this
Ligand-Target Pair | |
Alpha-1A adrenergic receptor
(Homo sapiens (Human)) | BDBM50090010
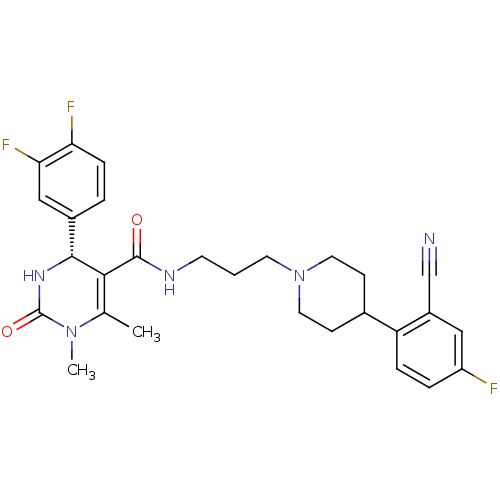
(4-(3,4-Difluoro-phenyl)-1,6-dimethyl-2-oxo-1,2,3,4...)Show SMILES CN1C(=O)N[C@@H](C(C(=O)NCCCN2CCC(CC2)c2ccc(F)cc2C#N)=C1C)c1ccc(F)c(F)c1 |c:29| Show InChI InChI=1S/C28H30F3N5O2/c1-17-25(26(34-28(38)35(17)2)19-4-7-23(30)24(31)15-19)27(37)33-10-3-11-36-12-8-18(9-13-36)22-6-5-21(29)14-20(22)16-32/h4-7,14-15,18,26H,3,8-13H2,1-2H3,(H,33,37)(H,34,38)/t26-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Evaluated for the ability to displace [125I]- HEAT from human cloned Alpha-1A adrenergic receptor stably expressed in CHO cells. |
J Med Chem 43: 2703-18 (2000)
BindingDB Entry DOI: 10.7270/Q2XK8DTM |
More data for this
Ligand-Target Pair | |
D(1A) dopamine receptor
(RAT) | BDBM82247
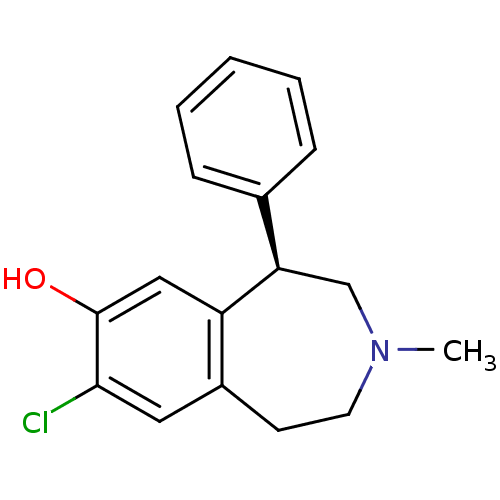
(8-Chloro-3-methyl-5-phenyl-2,3,4,5-tetrahydro-1H-b...)Show InChI InChI=1S/C17H18ClNO/c1-19-8-7-13-9-16(18)17(20)10-14(13)15(11-19)12-5-3-2-4-6-12/h2-6,9-10,15,20H,7-8,11H2,1H3/t15-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yale University
Curated by ChEMBL
| Assay Description
Compound was evaluated for affinity towards dopamine D1-like receptor in homogenate of caudateputamen tissue from rat brain |
Bioorg Med Chem Lett 10: 1113-5 (2000)
BindingDB Entry DOI: 10.7270/Q21N81NF |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data