 Found 44 hits with Last Name = 'hasegawa' and Initial = 'h'
Found 44 hits with Last Name = 'hasegawa' and Initial = 'h' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Prostaglandin-H2 D-isomerase
(Homo sapiens (Human)) | BDBM50008805
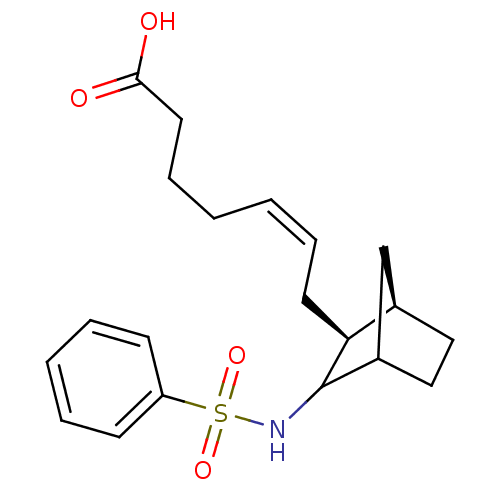
(7-(3-Benzenesulfonylamino-bicyclo[2.2.1]hept-2-yl)...)Show SMILES OC(=O)CCC\C=C/C[C@H]1[C@@H]2CCC(C2)C1NS(=O)(=O)c1ccccc1 Show InChI InChI=1S/C20H27NO4S/c22-19(23)11-7-2-1-6-10-18-15-12-13-16(14-15)20(18)21-26(24,25)17-8-4-3-5-9-17/h1,3-6,8-9,15-16,18,20-21H,2,7,10-14H2,(H,22,23)/b6-1-/t15-,16?,18+,20?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 298: 411-9 (2001)
BindingDB Entry DOI: 10.7270/Q2028Q3C |
More data for this
Ligand-Target Pair | |
Prostaglandin-H2 D-isomerase
(GUINEA PIG) | BDBM50008805

(7-(3-Benzenesulfonylamino-bicyclo[2.2.1]hept-2-yl)...)Show SMILES OC(=O)CCC\C=C/C[C@H]1[C@@H]2CCC(C2)C1NS(=O)(=O)c1ccccc1 Show InChI InChI=1S/C20H27NO4S/c22-19(23)11-7-2-1-6-10-18-15-12-13-16(14-15)20(18)21-26(24,25)17-8-4-3-5-9-17/h1,3-6,8-9,15-16,18,20-21H,2,7,10-14H2,(H,22,23)/b6-1-/t15-,16?,18+,20?/m1/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.490 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 298: 411-9 (2001)
BindingDB Entry DOI: 10.7270/Q2028Q3C |
More data for this
Ligand-Target Pair | |
Prostaglandin-H2 D-isomerase
(Homo sapiens (Human)) | BDBM50060462
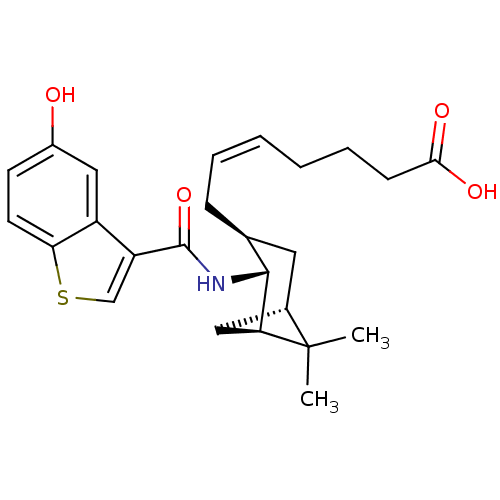
((Z)-7-{(1R,2R,3S,5S)-2-[(5-Hydroxy-benzo[b]thiophe...)Show SMILES CC1(C)[C@@H]2C[C@H]1[C@H](NC(=O)c1csc3ccc(O)cc13)[C@@H](C\C=C/CCCC(O)=O)C2 Show InChI InChI=1S/C25H31NO4S/c1-25(2)16-11-15(7-5-3-4-6-8-22(28)29)23(20(25)12-16)26-24(30)19-14-31-21-10-9-17(27)13-18(19)21/h3,5,9-10,13-16,20,23,27H,4,6-8,11-12H2,1-2H3,(H,26,30)(H,28,29)/b5-3-/t15-,16-,20-,23+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 298: 411-9 (2001)
BindingDB Entry DOI: 10.7270/Q2028Q3C |
More data for this
Ligand-Target Pair | |
Prostaglandin-H2 D-isomerase
(Homo sapiens (Human)) | BDBM50060462

((Z)-7-{(1R,2R,3S,5S)-2-[(5-Hydroxy-benzo[b]thiophe...)Show SMILES CC1(C)[C@@H]2C[C@H]1[C@H](NC(=O)c1csc3ccc(O)cc13)[C@@H](C\C=C/CCCC(O)=O)C2 Show InChI InChI=1S/C25H31NO4S/c1-25(2)16-11-15(7-5-3-4-6-8-22(28)29)23(20(25)12-16)26-24(30)19-14-31-21-10-9-17(27)13-18(19)21/h3,5,9-10,13-16,20,23,27H,4,6-8,11-12H2,1-2H3,(H,26,30)(H,28,29)/b5-3-/t15-,16-,20-,23+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 24.2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 298: 411-9 (2001)
BindingDB Entry DOI: 10.7270/Q2028Q3C |
More data for this
Ligand-Target Pair | |
Prostaglandin-H2 D-isomerase
(GUINEA PIG) | BDBM50060462

((Z)-7-{(1R,2R,3S,5S)-2-[(5-Hydroxy-benzo[b]thiophe...)Show SMILES CC1(C)[C@@H]2C[C@H]1[C@H](NC(=O)c1csc3ccc(O)cc13)[C@@H](C\C=C/CCCC(O)=O)C2 Show InChI InChI=1S/C25H31NO4S/c1-25(2)16-11-15(7-5-3-4-6-8-22(28)29)23(20(25)12-16)26-24(30)19-14-31-21-10-9-17(27)13-18(19)21/h3,5,9-10,13-16,20,23,27H,4,6-8,11-12H2,1-2H3,(H,26,30)(H,28,29)/b5-3-/t15-,16-,20-,23+/m0/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 193 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 298: 411-9 (2001)
BindingDB Entry DOI: 10.7270/Q2028Q3C |
More data for this
Ligand-Target Pair | |
Prostaglandin-H2 D-isomerase
(Homo sapiens (Human)) | BDBM85347
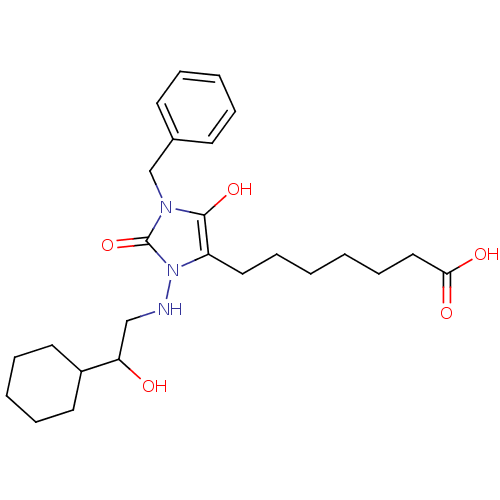
(BWA868C | CAS_122021 | NSC_122021)Show SMILES OC(CNn1c(CCCCCCC(O)=O)c(O)n(Cc2ccccc2)c1=O)C1CCCCC1 Show InChI InChI=1S/C25H37N3O5/c29-22(20-13-7-4-8-14-20)17-26-28-21(15-9-1-2-10-16-23(30)31)24(32)27(25(28)33)18-19-11-5-3-6-12-19/h3,5-6,11-12,20,22,26,29,32H,1-2,4,7-10,13-18H2,(H,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
Patents
| PubMed
| 2.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 298: 411-9 (2001)
BindingDB Entry DOI: 10.7270/Q2028Q3C |
More data for this
Ligand-Target Pair | |
Prostaglandin-H2 D-isomerase
(Homo sapiens (Human)) | BDBM50008805

(7-(3-Benzenesulfonylamino-bicyclo[2.2.1]hept-2-yl)...)Show SMILES OC(=O)CCC\C=C/C[C@H]1[C@@H]2CCC(C2)C1NS(=O)(=O)c1ccccc1 Show InChI InChI=1S/C20H27NO4S/c22-19(23)11-7-2-1-6-10-18-15-12-13-16(14-15)20(18)21-26(24,25)17-8-4-3-5-9-17/h1,3-6,8-9,15-16,18,20-21H,2,7,10-14H2,(H,22,23)/b6-1-/t15-,16?,18+,20?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 298: 411-9 (2001)
BindingDB Entry DOI: 10.7270/Q2028Q3C |
More data for this
Ligand-Target Pair | |
Endothelin-converting enzyme 1
(Rattus norvegicus (Rat)) | BDBM50112397

(CHEMBL22323 | Sodium;4-chloro-N-{[(4-cyano-3-methy...)Show SMILES Cc1nn(c(NC(=O)[N-]S(=O)(=O)c2ccc(Cl)cc2)c1C#N)-c1ccccc1 Show InChI InChI=1S/C18H14ClN5O3S/c1-12-16(11-20)17(24(22-12)14-5-3-2-4-6-14)21-18(25)23-28(26,27)15-9-7-13(19)8-10-15/h2-10H,1H3,(H2,21,22,23,25)/p-1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 42 | n/a | n/a | n/a | n/a | n/a | n/a |
Sumitomo Pharmaceuticals Co., Ltd.
Curated by ChEMBL
| Assay Description
Tested in vitro for inhibition of Endothelin-converting enzyme (ECE) of rat lung membrane |
Bioorg Med Chem Lett 12: 1275-8 (2002)
BindingDB Entry DOI: 10.7270/Q2R49RBT |
More data for this
Ligand-Target Pair | |
Endothelin-converting enzyme 1
(Rattus norvegicus (Rat)) | BDBM50040413

((S)-2-{(S)-2-[Hydroxy-(3,4,5-trihydroxy-6-methyl-t...)Show SMILES CC(C)C[C@H](NP(O)(=O)OC1OC(C)C(O)C(O)C1O)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(O)=O Show InChI InChI=1S/C23H34N3O10P/c1-11(2)8-16(26-37(33,34)36-23-20(29)19(28)18(27)12(3)35-23)21(30)25-17(22(31)32)9-13-10-24-15-7-5-4-6-14(13)15/h4-7,10-12,16-20,23-24,27-29H,8-9H2,1-3H3,(H,25,30)(H,31,32)(H2,26,33,34)/t12?,16-,17-,18?,19?,20?,23?/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 690 | n/a | n/a | n/a | n/a | n/a | n/a |
Sumitomo Pharmaceuticals Co., Ltd.
Curated by ChEMBL
| Assay Description
Tested in vitro for inhibition of Endothelin-converting enzyme (ECE) of rat lung membrane |
Bioorg Med Chem Lett 12: 1275-8 (2002)
BindingDB Entry DOI: 10.7270/Q2R49RBT |
More data for this
Ligand-Target Pair | |
Endothelin-converting enzyme 1
(Rattus norvegicus (Rat)) | BDBM50112394

(5-{(2Z)-2-[[(E)-(4-propylphenyl)diazenyl](phenyl)m...)Show SMILES CCCc1ccc(NN=C(N=Nc2nnnn2-c2ccccc2)c2ccccc2)cc1 |w:10.9,8.7| Show InChI InChI=1S/C23H22N8/c1-2-9-18-14-16-20(17-15-18)24-25-22(19-10-5-3-6-11-19)26-27-23-28-29-30-31(23)21-12-7-4-8-13-21/h3-8,10-17,24H,2,9H2,1H3 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sumitomo Pharmaceuticals Co., Ltd.
Curated by ChEMBL
| Assay Description
Tested in vitro for inhibition of Endothelin-converting enzyme (ECE) of rat lung membrane |
Bioorg Med Chem Lett 12: 1275-8 (2002)
BindingDB Entry DOI: 10.7270/Q2R49RBT |
More data for this
Ligand-Target Pair | |
Endothelin-converting enzyme 1
(Rattus norvegicus (Rat)) | BDBM50112393
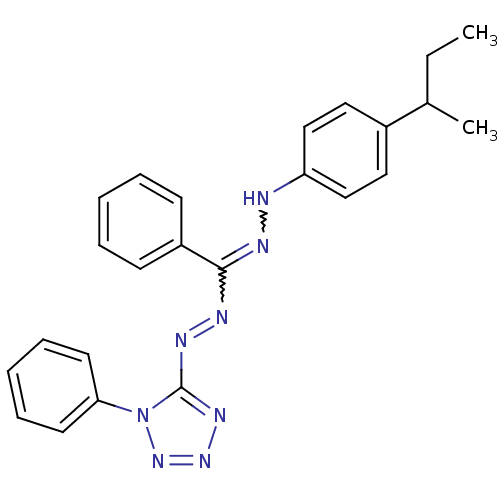
(5-{(2Z)-2-[[(E)-(4-secbutylphenyl)diazenyl](phenyl...)Show SMILES CCC(C)c1ccc(NN=C(N=Nc2nnnn2-c2ccccc2)c2ccccc2)cc1 |w:11.10,9.8| Show InChI InChI=1S/C24H24N8/c1-3-18(2)19-14-16-21(17-15-19)25-26-23(20-10-6-4-7-11-20)27-28-24-29-30-31-32(24)22-12-8-5-9-13-22/h4-18,25H,3H2,1-2H3 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sumitomo Pharmaceuticals Co., Ltd.
Curated by ChEMBL
| Assay Description
Tested in vitro for inhibition of Endothelin-converting enzyme (ECE) of rat lung membrane |
Bioorg Med Chem Lett 12: 1275-8 (2002)
BindingDB Entry DOI: 10.7270/Q2R49RBT |
More data for this
Ligand-Target Pair | |
Endothelin-converting enzyme 1
(Rattus norvegicus (Rat)) | BDBM50112401
(5-{(2Z)-2-[[(E)-(4-isopropylphenyl)diazenyl](pheny...)Show SMILES CC(C)c1ccc(NN=C(N=Nc2nnnn2-c2ccccc2)c2ccccc2)cc1 |w:10.9,8.7| Show InChI InChI=1S/C23H22N8/c1-17(2)18-13-15-20(16-14-18)24-25-22(19-9-5-3-6-10-19)26-27-23-28-29-30-31(23)21-11-7-4-8-12-21/h3-17,24H,1-2H3 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 3.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sumitomo Pharmaceuticals Co., Ltd.
Curated by ChEMBL
| Assay Description
Tested in vitro for inhibition of Endothelin-converting enzyme (ECE) of rat lung membrane |
Bioorg Med Chem Lett 12: 1275-8 (2002)
BindingDB Entry DOI: 10.7270/Q2R49RBT |
More data for this
Ligand-Target Pair | |
Endothelin-converting enzyme 1
(Rattus norvegicus (Rat)) | BDBM50112396

(5-{(2Z)-2-[[(E)-(4-cyclohexylphenyl)diazenyl](phen...)Show SMILES C1CCC(CC1)c1ccc(NN=C(N=Nc2nnnn2-c2ccccc2)c2ccccc2)cc1 |w:13.13,11.11| Show InChI InChI=1S/C26H26N8/c1-4-10-20(11-5-1)21-16-18-23(19-17-21)27-28-25(22-12-6-2-7-13-22)29-30-26-31-32-33-34(26)24-14-8-3-9-15-24/h2-3,6-9,12-20,27H,1,4-5,10-11H2 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 3.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sumitomo Pharmaceuticals Co., Ltd.
Curated by ChEMBL
| Assay Description
Tested in vitro for inhibition of Endothelin-converting enzyme (ECE) of rat lung membrane |
Bioorg Med Chem Lett 12: 1275-8 (2002)
BindingDB Entry DOI: 10.7270/Q2R49RBT |
More data for this
Ligand-Target Pair | |
Endothelin-converting enzyme 1
(Rattus norvegicus (Rat)) | BDBM50112400

(5-{(2Z)-2-[[(E)-(4-tertbutylphenyl)diazenyl](pheny...)Show SMILES CC(C)(C)c1ccc(NN=C(N=Nc2nnnn2-c2ccccc2)c2ccccc2)cc1 |w:11.10,9.8| Show InChI InChI=1S/C24H24N8/c1-24(2,3)19-14-16-20(17-15-19)25-26-22(18-10-6-4-7-11-18)27-28-23-29-30-31-32(23)21-12-8-5-9-13-21/h4-17,25H,1-3H3 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 3.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sumitomo Pharmaceuticals Co., Ltd.
Curated by ChEMBL
| Assay Description
Tested in vitro for inhibition of Endothelin-converting enzyme (ECE) of rat lung membrane |
Bioorg Med Chem Lett 12: 1275-8 (2002)
BindingDB Entry DOI: 10.7270/Q2R49RBT |
More data for this
Ligand-Target Pair | |
Endothelin-converting enzyme 1
(Rattus norvegicus (Rat)) | BDBM50112398

(5-{(2Z)-2-[[(E)-(4-ethylphenyl)diazenyl](phenyl)me...)Show SMILES CCc1ccc(NN=C(N=Nc2nnnn2-c2ccccc2)c2ccccc2)cc1 |w:9.8,7.6| Show InChI InChI=1S/C22H20N8/c1-2-17-13-15-19(16-14-17)23-24-21(18-9-5-3-6-10-18)25-26-22-27-28-29-30(22)20-11-7-4-8-12-20/h3-16,23H,2H2,1H3 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 3.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sumitomo Pharmaceuticals Co., Ltd.
Curated by ChEMBL
| Assay Description
Tested in vitro for inhibition of Endothelin-converting enzyme (ECE) of rat lung membrane |
Bioorg Med Chem Lett 12: 1275-8 (2002)
BindingDB Entry DOI: 10.7270/Q2R49RBT |
More data for this
Ligand-Target Pair | |
Endothelin-converting enzyme 1
(Rattus norvegicus (Rat)) | BDBM50112395

(5-{(2Z)-2-[[(E)-(4-methylphenyl)diazenyl](phenyl)m...)Show SMILES Cc1ccc(NN=C(N=Nc2nnnn2-c2ccccc2)c2ccccc2)cc1 |w:8.7,6.5| Show InChI InChI=1S/C21H18N8/c1-16-12-14-18(15-13-16)22-23-20(17-8-4-2-5-9-17)24-25-21-26-27-28-29(21)19-10-6-3-7-11-19/h2-15,22H,1H3 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 8.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sumitomo Pharmaceuticals Co., Ltd.
Curated by ChEMBL
| Assay Description
Tested in vitro for inhibition of Endothelin-converting enzyme (ECE) of rat lung membrane |
Bioorg Med Chem Lett 12: 1275-8 (2002)
BindingDB Entry DOI: 10.7270/Q2R49RBT |
More data for this
Ligand-Target Pair | |
Endothelin-converting enzyme 1
(Rattus norvegicus (Rat)) | BDBM50112399

(1-phenyl-5-((2Z)-2-{phenyl[(E)-phenyldiazenyl]meth...)Show SMILES N(N=C(N=Nc1nnnn1-c1ccccc1)c1ccccc1)c1ccccc1 |w:3.2,1.0| Show InChI InChI=1S/C20H16N8/c1-4-10-16(11-5-1)19(22-21-17-12-6-2-7-13-17)23-24-20-25-26-27-28(20)18-14-8-3-9-15-18/h1-15,21H | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sumitomo Pharmaceuticals Co., Ltd.
Curated by ChEMBL
| Assay Description
Tested in vitro for inhibition of Endothelin-converting enzyme (ECE) of rat lung membrane |
Bioorg Med Chem Lett 12: 1275-8 (2002)
BindingDB Entry DOI: 10.7270/Q2R49RBT |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-alpha
(Homo sapiens (Human)) | BDBM50179869
(CHEMBL203682 | N-methyl-N-[4-(2,2,2-trifluoro-1-hy...)Show SMILES CN(c1ccc(cc1)C(O)(C(F)(F)F)C(F)(F)F)S(=O)(=O)c1ccccc1 Show InChI InChI=1S/C16H13F6NO3S/c1-23(27(25,26)13-5-3-2-4-6-13)12-9-7-11(8-10-12)14(24,15(17,18)19)16(20,21)22/h2-10,24H,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 200 | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Activity against LXR alpha transiently transfected in HEK293 cells |
Bioorg Med Chem Lett 16: 1638-42 (2006)
Article DOI: 10.1016/j.bmcl.2005.12.015
BindingDB Entry DOI: 10.7270/Q21J99B2 |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-alpha
(Homo sapiens (Human)) | BDBM50179865

(2-cyano-N-(4-(1,1,1,3,3,3-hexafluoro-2-hydroxyprop...)Show SMILES CN(c1ccc(cc1)C(O)(C(F)(F)F)C(F)(F)F)S(=O)(=O)c1ccccc1C#N Show InChI InChI=1S/C17H12F6N2O3S/c1-25(29(27,28)14-5-3-2-4-11(14)10-24)13-8-6-12(7-9-13)15(26,16(18,19)20)17(21,22)23/h2-9,26H,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 700 | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Activity against LXR alpha transiently transfected in HEK293 cells |
Bioorg Med Chem Lett 16: 1638-42 (2006)
Article DOI: 10.1016/j.bmcl.2005.12.015
BindingDB Entry DOI: 10.7270/Q21J99B2 |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-alpha
(Homo sapiens (Human)) | BDBM50179866

(CHEMBL380851 | N-(4-(1,1,1,3,3,3-hexafluoro-2-hydr...)Show SMILES CC(C)CN(c1ccc(cc1)C(O)(C(F)(F)F)C(F)(F)F)S(=O)(=O)c1cccs1 Show InChI InChI=1S/C17H17F6NO3S2/c1-11(2)10-24(29(26,27)14-4-3-9-28-14)13-7-5-12(6-8-13)15(25,16(18,19)20)17(21,22)23/h3-9,11,25H,10H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 40 | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Activity against LXR alpha transiently transfected in HEK293 cells |
Bioorg Med Chem Lett 16: 1638-42 (2006)
Article DOI: 10.1016/j.bmcl.2005.12.015
BindingDB Entry DOI: 10.7270/Q21J99B2 |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-alpha
(Homo sapiens (Human)) | BDBM19993

(CHEMBL62136 | N-[4-(1,1,1,3,3,3-hexafluoro-2-hydro...)Show SMILES OC(c1ccc(cc1)N(CC(F)(F)F)S(=O)(=O)c1ccccc1)(C(F)(F)F)C(F)(F)F Show InChI InChI=1S/C17H12F9NO3S/c18-14(19,20)10-27(31(29,30)13-4-2-1-3-5-13)12-8-6-11(7-9-12)15(28,16(21,22)23)17(24,25)26/h1-9,28H,10H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | n/a | n/a | 10 | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Activity against LXR alpha transiently transfected in HEK293 cells |
Bioorg Med Chem Lett 16: 1638-42 (2006)
Article DOI: 10.1016/j.bmcl.2005.12.015
BindingDB Entry DOI: 10.7270/Q21J99B2 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Oxysterols receptor LXR-alpha
(Homo sapiens (Human)) | BDBM50179870

(CHEMBL206445 | N1-(4-(1,1,1,3,3,3-hexafluoro-2-hyd...)Show SMILES CN(c1ccc(cc1)C(O)(C(F)(F)F)C(F)(F)F)S(=O)(=O)c1cccc(c1)S(N)(=O)=O Show InChI InChI=1S/C16H14F6N2O5S2/c1-24(31(28,29)13-4-2-3-12(9-13)30(23,26)27)11-7-5-10(6-8-11)14(25,15(17,18)19)16(20,21)22/h2-9,25H,1H3,(H2,23,26,27) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 80 | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Activity against LXR alpha transiently transfected in HEK293 cells |
Bioorg Med Chem Lett 16: 1638-42 (2006)
Article DOI: 10.1016/j.bmcl.2005.12.015
BindingDB Entry DOI: 10.7270/Q21J99B2 |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-alpha
(Homo sapiens (Human)) | BDBM20164

((1S,2R,5S,10S,11S,14R,15R)-14-[(2R)-4-[(2S)-3,3-di...)Show SMILES [H][C@@]1(CC[C@@H](C)[C@@]2([H])CC[C@@]3([H])[C@]4([H])CC=C5C[C@@]([H])(O)CC[C@]5(C)[C@@]4([H])CC[C@]23C)OC1(C)C |r,t:15| Show InChI InChI=1S/C27H44O2/c1-17(6-11-24-25(2,3)29-24)21-9-10-22-20-8-7-18-16-19(28)12-14-26(18,4)23(20)13-15-27(21,22)5/h7,17,19-24,28H,6,8-16H2,1-5H3/t17-,19+,20+,21-,22+,23+,24+,26+,27-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 500 | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Activity against LXR alpha transiently transfected in HEK293 cells |
Bioorg Med Chem Lett 16: 1638-42 (2006)
Article DOI: 10.1016/j.bmcl.2005.12.015
BindingDB Entry DOI: 10.7270/Q21J99B2 |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-alpha
(Homo sapiens (Human)) | BDBM50179871
(CHEMBL383657 | N-methyl-N-(4-(1,1,1-trifluoro-2-hy...)Show SMILES CN(c1ccc(cc1)C(C)(O)C(F)(F)F)S(=O)(=O)c1ccccc1 Show InChI InChI=1S/C16H16F3NO3S/c1-15(21,16(17,18)19)12-8-10-13(11-9-12)20(2)24(22,23)14-6-4-3-5-7-14/h3-11,21H,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Activity against LXR alpha transiently transfected in HEK293 cells |
Bioorg Med Chem Lett 16: 1638-42 (2006)
Article DOI: 10.1016/j.bmcl.2005.12.015
BindingDB Entry DOI: 10.7270/Q21J99B2 |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-alpha
(Homo sapiens (Human)) | BDBM50179872

(CHEMBL206674 | biphenyl-3-sulfonic acid methyl-[4-...)Show SMILES CN(c1ccc(cc1)C(O)(C(F)(F)F)C(F)(F)F)S(=O)(=O)c1cccc(c1)-c1ccccc1 Show InChI InChI=1S/C22H17F6NO3S/c1-29(18-12-10-17(11-13-18)20(30,21(23,24)25)22(26,27)28)33(31,32)19-9-5-8-16(14-19)15-6-3-2-4-7-15/h2-14,30H,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 30 | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Activity against LXR alpha transiently transfected in HEK293 cells |
Bioorg Med Chem Lett 16: 1638-42 (2006)
Article DOI: 10.1016/j.bmcl.2005.12.015
BindingDB Entry DOI: 10.7270/Q21J99B2 |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-alpha
(Homo sapiens (Human)) | BDBM50179876

(1,1,1,3,3,3-hexafluoro-2-(4-(phenylsulfonyl)-3,4-d...)Show SMILES OC(c1ccc2N(CCOc2c1)S(=O)(=O)c1ccccc1)(C(F)(F)F)C(F)(F)F Show InChI InChI=1S/C17H13F6NO4S/c18-16(19,20)15(25,17(21,22)23)11-6-7-13-14(10-11)28-9-8-24(13)29(26,27)12-4-2-1-3-5-12/h1-7,10,25H,8-9H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | n/a | n/a | 100 | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Activity against LXR alpha transiently transfected in HEK293 cells |
Bioorg Med Chem Lett 16: 1638-42 (2006)
Article DOI: 10.1016/j.bmcl.2005.12.015
BindingDB Entry DOI: 10.7270/Q21J99B2 |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-alpha
(Homo sapiens (Human)) | BDBM50179877

(1,1,1,3,3,3-hexafluoro-2-(1-(phenylsulfonyl)-1,2,3...)Show SMILES OC(c1ccc2N(CCCc2c1)S(=O)(=O)c1ccccc1)(C(F)(F)F)C(F)(F)F Show InChI InChI=1S/C18H15F6NO3S/c19-17(20,21)16(26,18(22,23)24)13-8-9-15-12(11-13)5-4-10-25(15)29(27,28)14-6-2-1-3-7-14/h1-3,6-9,11,26H,4-5,10H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 30 | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Activity against LXR alpha transiently transfected in HEK293 cells |
Bioorg Med Chem Lett 16: 1638-42 (2006)
Article DOI: 10.1016/j.bmcl.2005.12.015
BindingDB Entry DOI: 10.7270/Q21J99B2 |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-alpha
(Homo sapiens (Human)) | BDBM50179873

(CHEMBL204282 | N-(3-(1,1,1,3,3,3-hexafluoro-2-hydr...)Show SMILES CN(c1cccc(c1)C(O)(C(F)(F)F)C(F)(F)F)S(=O)(=O)c1ccccc1 Show InChI InChI=1S/C16H13F6NO3S/c1-23(27(25,26)13-8-3-2-4-9-13)12-7-5-6-11(10-12)14(24,15(17,18)19)16(20,21)22/h2-10,24H,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Activity against LXR alpha transiently transfected in HEK293 cells |
Bioorg Med Chem Lett 16: 1638-42 (2006)
Article DOI: 10.1016/j.bmcl.2005.12.015
BindingDB Entry DOI: 10.7270/Q21J99B2 |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-alpha
(Homo sapiens (Human)) | BDBM50179878

(CHEMBL206192 | N-(4-(1,1,1,3,3,3-hexafluoro-2-hydr...)Show SMILES CN(c1ccc(cc1)C(O)(C(F)(F)F)C(F)(F)F)S(=O)(=O)c1cccs1 Show InChI InChI=1S/C14H11F6NO3S2/c1-21(26(23,24)11-3-2-8-25-11)10-6-4-9(5-7-10)12(22,13(15,16)17)14(18,19)20/h2-8,22H,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 600 | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Activity against LXR alpha transiently transfected in HEK293 cells |
Bioorg Med Chem Lett 16: 1638-42 (2006)
Article DOI: 10.1016/j.bmcl.2005.12.015
BindingDB Entry DOI: 10.7270/Q21J99B2 |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-alpha
(Homo sapiens (Human)) | BDBM50179874

(CHEMBL380249 | N-(2-chloro-4-(1,1,1,3,3,3-hexafluo...)Show SMILES CN(c1ccc(cc1Cl)C(O)(C(F)(F)F)C(F)(F)F)S(=O)(=O)c1ccccc1 Show InChI InChI=1S/C16H12ClF6NO3S/c1-24(28(26,27)11-5-3-2-4-6-11)13-8-7-10(9-12(13)17)14(25,15(18,19)20)16(21,22)23/h2-9,25H,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 200 | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Activity against LXR alpha transiently transfected in HEK293 cells |
Bioorg Med Chem Lett 16: 1638-42 (2006)
Article DOI: 10.1016/j.bmcl.2005.12.015
BindingDB Entry DOI: 10.7270/Q21J99B2 |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-alpha
(Homo sapiens (Human)) | BDBM50179875

(4-(1,1,1,3,3,3-hexafluoro-2-hydroxypropan-2-yl)-N-...)Show SMILES CN(c1ccccc1)S(=O)(=O)c1ccc(cc1)C(O)(C(F)(F)F)C(F)(F)F Show InChI InChI=1S/C16H13F6NO3S/c1-23(12-5-3-2-4-6-12)27(25,26)13-9-7-11(8-10-13)14(24,15(17,18)19)16(20,21)22/h2-10,24H,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 100 | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Activity against LXR alpha transiently transfected in HEK293 cells |
Bioorg Med Chem Lett 16: 1638-42 (2006)
Article DOI: 10.1016/j.bmcl.2005.12.015
BindingDB Entry DOI: 10.7270/Q21J99B2 |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-alpha
(Homo sapiens (Human)) | BDBM50179879

(4-(1,1,1,3,3,3-hexafluoro-2-hydroxypropan-2-yl)-N-...)Show SMILES CN(C(=O)c1ccc(cc1)C(O)(C(F)(F)F)C(F)(F)F)c1ccccc1 Show InChI InChI=1S/C17H13F6NO2/c1-24(13-5-3-2-4-6-13)14(25)11-7-9-12(10-8-11)15(26,16(18,19)20)17(21,22)23/h2-10,26H,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 200 | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Activity against LXR alpha transiently transfected in HEK293 cells |
Bioorg Med Chem Lett 16: 1638-42 (2006)
Article DOI: 10.1016/j.bmcl.2005.12.015
BindingDB Entry DOI: 10.7270/Q21J99B2 |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-alpha
(Homo sapiens (Human)) | BDBM50179880
(1,1,1,3,3,3-hexafluoro-2-(1-(phenylsulfonyl)indoli...)Show SMILES OC(c1ccc2N(CCc2c1)S(=O)(=O)c1ccccc1)(C(F)(F)F)C(F)(F)F Show InChI InChI=1S/C17H13F6NO3S/c18-16(19,20)15(25,17(21,22)23)12-6-7-14-11(10-12)8-9-24(14)28(26,27)13-4-2-1-3-5-13/h1-7,10,25H,8-9H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 50 | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Activity against LXR alpha transiently transfected in HEK293 cells |
Bioorg Med Chem Lett 16: 1638-42 (2006)
Article DOI: 10.1016/j.bmcl.2005.12.015
BindingDB Entry DOI: 10.7270/Q21J99B2 |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-alpha
(Homo sapiens (Human)) | BDBM50179857

(4-cyano-N-(4-(1,1,1,3,3,3-hexafluoro-2-hydroxyprop...)Show SMILES CN(c1ccc(cc1)C(O)(C(F)(F)F)C(F)(F)F)S(=O)(=O)c1ccc(cc1)C#N Show InChI InChI=1S/C17H12F6N2O3S/c1-25(29(27,28)14-8-2-11(10-24)3-9-14)13-6-4-12(5-7-13)15(26,16(18,19)20)17(21,22)23/h2-9,26H,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Activity against LXR alpha transiently transfected in HEK293 cells |
Bioorg Med Chem Lett 16: 1638-42 (2006)
Article DOI: 10.1016/j.bmcl.2005.12.015
BindingDB Entry DOI: 10.7270/Q21J99B2 |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-alpha
(Homo sapiens (Human)) | BDBM50179856

(CHEMBL205770 | N-(3-chloro-4-(1,1,1,3,3,3-hexafluo...)Show SMILES CN(c1ccc(c(Cl)c1)C(O)(C(F)(F)F)C(F)(F)F)S(=O)(=O)c1ccccc1 Show InChI InChI=1S/C16H12ClF6NO3S/c1-24(28(26,27)11-5-3-2-4-6-11)10-7-8-12(13(17)9-10)14(25,15(18,19)20)16(21,22)23/h2-9,25H,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | >5.00E+3 | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Activity against LXR alpha transiently transfected in HEK293 cells |
Bioorg Med Chem Lett 16: 1638-42 (2006)
Article DOI: 10.1016/j.bmcl.2005.12.015
BindingDB Entry DOI: 10.7270/Q21J99B2 |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-alpha
(Homo sapiens (Human)) | BDBM50179868
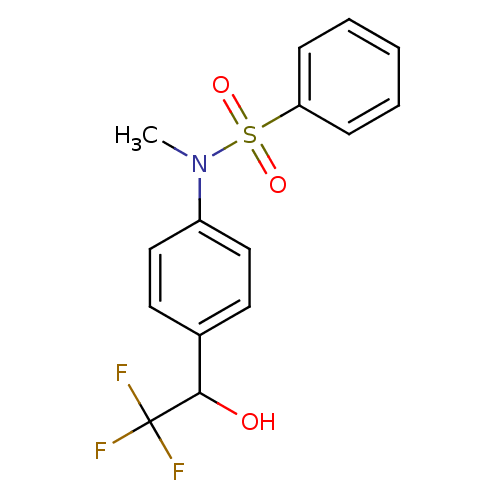
(CHEMBL203454 | N-methyl-N-(4-(2,2,2-trifluoro-1-hy...)Show SMILES CN(c1ccc(cc1)C(O)C(F)(F)F)S(=O)(=O)c1ccccc1 Show InChI InChI=1S/C15H14F3NO3S/c1-19(23(21,22)13-5-3-2-4-6-13)12-9-7-11(8-10-12)14(20)15(16,17)18/h2-10,14,20H,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | >5.00E+3 | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Activity against LXR alpha transiently transfected in HEK293 cells |
Bioorg Med Chem Lett 16: 1638-42 (2006)
Article DOI: 10.1016/j.bmcl.2005.12.015
BindingDB Entry DOI: 10.7270/Q21J99B2 |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-alpha
(Homo sapiens (Human)) | BDBM50179867
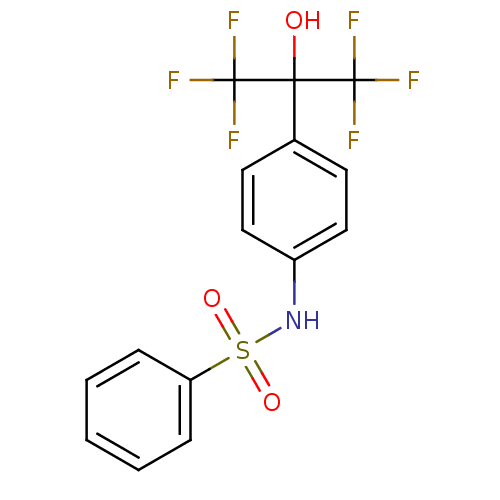
(CHEMBL382792 | N-(4-(1,1,1,3,3,3-hexafluoro-2-hydr...)Show SMILES OC(c1ccc(NS(=O)(=O)c2ccccc2)cc1)(C(F)(F)F)C(F)(F)F Show InChI InChI=1S/C15H11F6NO3S/c16-14(17,18)13(23,15(19,20)21)10-6-8-11(9-7-10)22-26(24,25)12-4-2-1-3-5-12/h1-9,22-23H | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Activity against LXR alpha transiently transfected in HEK293 cells |
Bioorg Med Chem Lett 16: 1638-42 (2006)
Article DOI: 10.1016/j.bmcl.2005.12.015
BindingDB Entry DOI: 10.7270/Q21J99B2 |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-alpha
(Homo sapiens (Human)) | BDBM50179864

(3-cyano-N-(4-(1,1,1,3,3,3-hexafluoro-2-hydroxyprop...)Show SMILES CN(c1ccc(cc1)C(O)(C(F)(F)F)C(F)(F)F)S(=O)(=O)c1cccc(c1)C#N Show InChI InChI=1S/C17H12F6N2O3S/c1-25(29(27,28)14-4-2-3-11(9-14)10-24)13-7-5-12(6-8-13)15(26,16(18,19)20)17(21,22)23/h2-9,26H,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 20 | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Activity against LXR alpha transiently transfected in HEK293 cells |
Bioorg Med Chem Lett 16: 1638-42 (2006)
Article DOI: 10.1016/j.bmcl.2005.12.015
BindingDB Entry DOI: 10.7270/Q21J99B2 |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-alpha
(Homo sapiens (Human)) | BDBM50179863

(CHEMBL379225 | N-(4-(1,1,1,3,3,3-hexafluoro-2-hydr...)Show SMILES CN(C(=O)c1ccccc1)c1ccc(cc1)C(O)(C(F)(F)F)C(F)(F)F Show InChI InChI=1S/C17H13F6NO2/c1-24(14(25)11-5-3-2-4-6-11)13-9-7-12(8-10-13)15(26,16(18,19)20)17(21,22)23/h2-10,26H,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 100 | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Activity against LXR alpha transiently transfected in HEK293 cells |
Bioorg Med Chem Lett 16: 1638-42 (2006)
Article DOI: 10.1016/j.bmcl.2005.12.015
BindingDB Entry DOI: 10.7270/Q21J99B2 |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-alpha
(Homo sapiens (Human)) | BDBM50179862
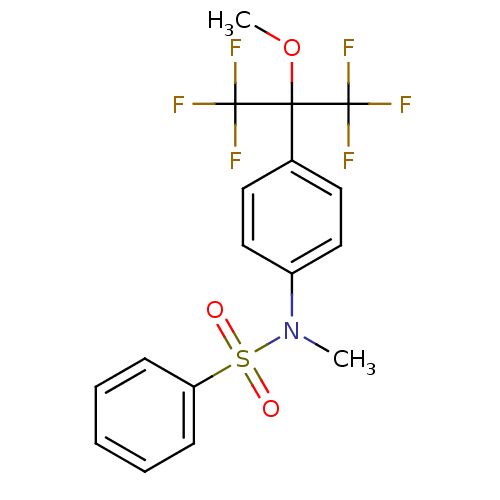
(CHEMBL203824 | N-(4-(1,1,1,3,3,3-hexafluoro-2-meth...)Show SMILES COC(c1ccc(cc1)N(C)S(=O)(=O)c1ccccc1)(C(F)(F)F)C(F)(F)F Show InChI InChI=1S/C17H15F6NO3S/c1-24(28(25,26)14-6-4-3-5-7-14)13-10-8-12(9-11-13)15(27-2,16(18,19)20)17(21,22)23/h3-11H,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Activity against LXR alpha transiently transfected in HEK293 cells |
Bioorg Med Chem Lett 16: 1638-42 (2006)
Article DOI: 10.1016/j.bmcl.2005.12.015
BindingDB Entry DOI: 10.7270/Q21J99B2 |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-alpha
(Homo sapiens (Human)) | BDBM50179861

(CHEMBL380711 | N-(4-(1,1,1,3,3,3-hexafluoro-2-hydr...)Show SMILES CN(Cc1ccc(cc1)C(O)(C(F)(F)F)C(F)(F)F)S(=O)(=O)c1ccccc1 Show InChI InChI=1S/C17H15F6NO3S/c1-24(28(26,27)14-5-3-2-4-6-14)11-12-7-9-13(10-8-12)15(25,16(18,19)20)17(21,22)23/h2-10,25H,11H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Activity against LXR alpha transiently transfected in HEK293 cells |
Bioorg Med Chem Lett 16: 1638-42 (2006)
Article DOI: 10.1016/j.bmcl.2005.12.015
BindingDB Entry DOI: 10.7270/Q21J99B2 |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-alpha
(Homo sapiens (Human)) | BDBM50179858

(1,1,1,3,3,3-hexafluoro-2-(1-(phenylsulfonyl)-2,3,4...)Show SMILES OC(c1ccc2N(CCCCc2c1)S(=O)(=O)c1ccccc1)(C(F)(F)F)C(F)(F)F Show InChI InChI=1S/C19H17F6NO3S/c20-18(21,22)17(27,19(23,24)25)14-9-10-16-13(12-14)6-4-5-11-26(16)30(28,29)15-7-2-1-3-8-15/h1-3,7-10,12,27H,4-6,11H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 10 | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Activity against LXR alpha transiently transfected in HEK293 cells |
Bioorg Med Chem Lett 16: 1638-42 (2006)
Article DOI: 10.1016/j.bmcl.2005.12.015
BindingDB Entry DOI: 10.7270/Q21J99B2 |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-alpha
(Homo sapiens (Human)) | BDBM50179859

(CHEMBL203606 | isobutyl 4-(1,1,1,3,3,3-hexafluoro-...)Show SMILES CC(C)COC(=O)N(C)c1ccc(cc1)C(O)(C(F)(F)F)C(F)(F)F Show InChI InChI=1S/C15H17F6NO3/c1-9(2)8-25-12(23)22(3)11-6-4-10(5-7-11)13(24,14(16,17)18)15(19,20)21/h4-7,9,24H,8H2,1-3H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | n/a | n/a | 100 | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Activity against LXR alpha transiently transfected in HEK293 cells |
Bioorg Med Chem Lett 16: 1638-42 (2006)
Article DOI: 10.1016/j.bmcl.2005.12.015
BindingDB Entry DOI: 10.7270/Q21J99B2 |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-alpha
(Homo sapiens (Human)) | BDBM50179860

(CHEMBL206738 | N-cyclopentyl-N-(4-(1,1,1,3,3,3-hex...)Show SMILES OC(c1ccc(cc1)N(C1CCCC1)S(=O)(=O)c1ccccc1)(C(F)(F)F)C(F)(F)F Show InChI InChI=1S/C20H19F6NO3S/c21-19(22,23)18(28,20(24,25)26)14-10-12-16(13-11-14)27(15-6-4-5-7-15)31(29,30)17-8-2-1-3-9-17/h1-3,8-13,15,28H,4-7H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 8 | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Activity against LXR alpha transiently transfected in HEK293 cells |
Bioorg Med Chem Lett 16: 1638-42 (2006)
Article DOI: 10.1016/j.bmcl.2005.12.015
BindingDB Entry DOI: 10.7270/Q21J99B2 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data