

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Histone acetyltransferase p300 (Homo sapiens (Human)) | BDBM50456444 (CHEMBL3734823) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University Curated by ChEMBL | Assay Description Inhibition of VMA intein chitin binding domain-fused p300 HAT domain (1287 to 1652 residues) (unknown origin) expressed in Escherichia coli BL21(RIL)... | Eur J Med Chem 138: 320-327 (2017) Article DOI: 10.1016/j.ejmech.2017.06.037 BindingDB Entry DOI: 10.7270/Q2ZW1PHN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50364189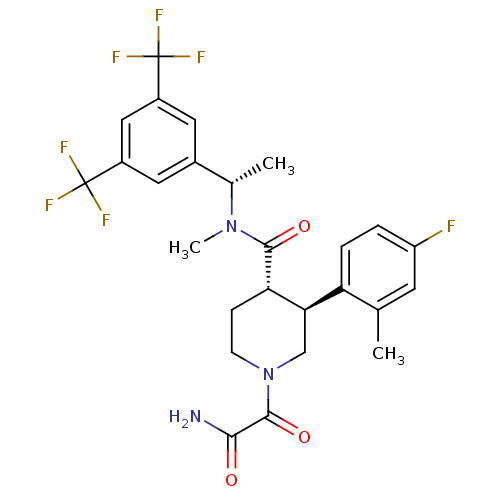 (CHEMBL1951813) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0170 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Co. Ltd Curated by ChEMBL | Assay Description Displacement of [125I]BH-SP from tachykinin NK1 receptor in human IM9 cells after 30 mins by scintillation counting | Bioorg Med Chem 20: 962-77 (2012) Article DOI: 10.1016/j.bmc.2011.11.048 BindingDB Entry DOI: 10.7270/Q2NP24WG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50364190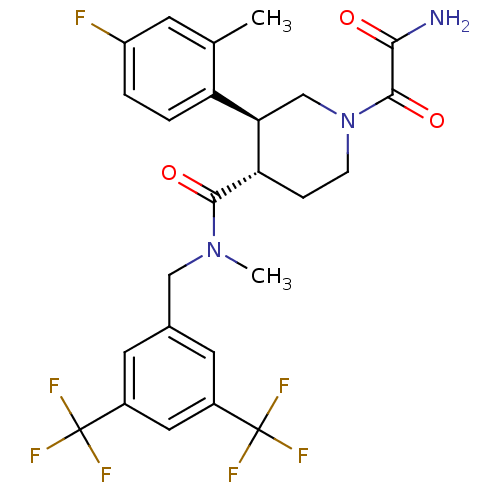 (CHEMBL1951810) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Co. Ltd Curated by ChEMBL | Assay Description Displacement of [125I]BH-SP from tachykinin NK1 receptor in human IM9 cells after 30 mins by scintillation counting | Bioorg Med Chem 20: 962-77 (2012) Article DOI: 10.1016/j.bmc.2011.11.048 BindingDB Entry DOI: 10.7270/Q2NP24WG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50364190 (CHEMBL1951810) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Co. Ltd Curated by ChEMBL | Assay Description Displacement of [125I]BH-SP from tachykinin NK1 receptor in human IM9 cells after 30 mins by scintillation counting | Bioorg Med Chem 20: 962-77 (2012) Article DOI: 10.1016/j.bmc.2011.11.048 BindingDB Entry DOI: 10.7270/Q2NP24WG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM47085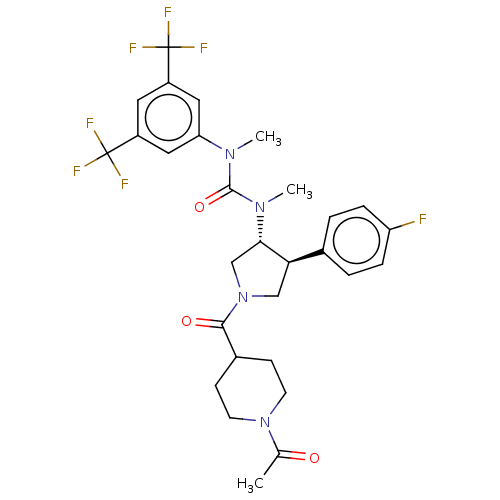 (US8592454, 71b) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.0270 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited US Patent | Assay Description Radioligand receptor binding inhibitory assay using human NK receptor. | US Patent US8592454 (2013) BindingDB Entry DOI: 10.7270/Q2XD10B0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM106978 (US8592454, 176) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.0290 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited US Patent | Assay Description Radioligand receptor binding inhibitory assay using human NK receptor. | US Patent US8592454 (2013) BindingDB Entry DOI: 10.7270/Q2XD10B0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM106983 (US8592454, 420) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited US Patent | Assay Description Radioligand receptor binding inhibitory assay using human NK receptor. | US Patent US8592454 (2013) BindingDB Entry DOI: 10.7270/Q2XD10B0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM106974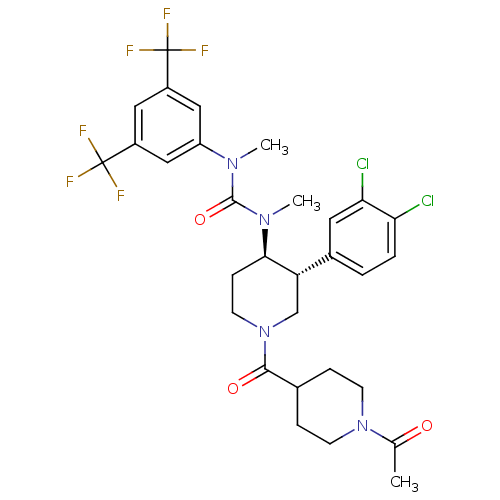 (US8592454, 25) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.0310 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited US Patent | Assay Description Radioligand receptor binding inhibitory assay using human NK receptor. | US Patent US8592454 (2013) BindingDB Entry DOI: 10.7270/Q2XD10B0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM106979 (US8592454, 192) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.0320 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited US Patent | Assay Description Radioligand receptor binding inhibitory assay using human NK receptor. | US Patent US8592454 (2013) BindingDB Entry DOI: 10.7270/Q2XD10B0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50364202 (CHEMBL1951626) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0320 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Co. Ltd Curated by ChEMBL | Assay Description Displacement of [125I]BH-SP from tachykinin NK1 receptor in human IM9 cells after 30 mins by scintillation counting | Bioorg Med Chem 20: 962-77 (2012) Article DOI: 10.1016/j.bmc.2011.11.048 BindingDB Entry DOI: 10.7270/Q2NP24WG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50364195 (CHEMBL1951633) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0350 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Co. Ltd Curated by ChEMBL | Assay Description Displacement of [125I]BH-SP from tachykinin NK1 receptor in human IM9 cells after 30 mins by scintillation counting | Bioorg Med Chem 20: 962-77 (2012) Article DOI: 10.1016/j.bmc.2011.11.048 BindingDB Entry DOI: 10.7270/Q2NP24WG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM106982 (US8592454, 378) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.0390 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited US Patent | Assay Description Radioligand receptor binding inhibitory assay using human NK receptor. | US Patent US8592454 (2013) BindingDB Entry DOI: 10.7270/Q2XD10B0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50364197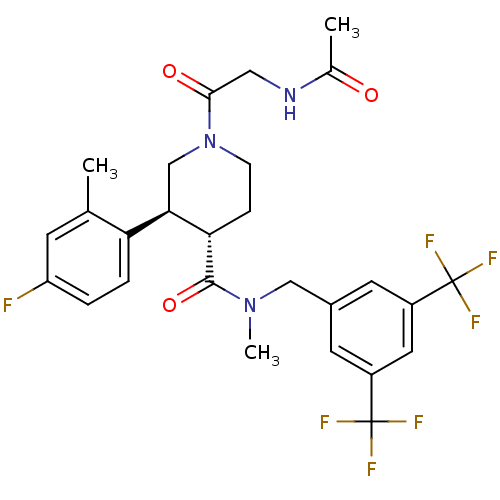 (CHEMBL1951631) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0430 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Co. Ltd Curated by ChEMBL | Assay Description Displacement of [125I]BH-SP from tachykinin NK1 receptor in human IM9 cells after 30 mins by scintillation counting | Bioorg Med Chem 20: 962-77 (2012) Article DOI: 10.1016/j.bmc.2011.11.048 BindingDB Entry DOI: 10.7270/Q2NP24WG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50364192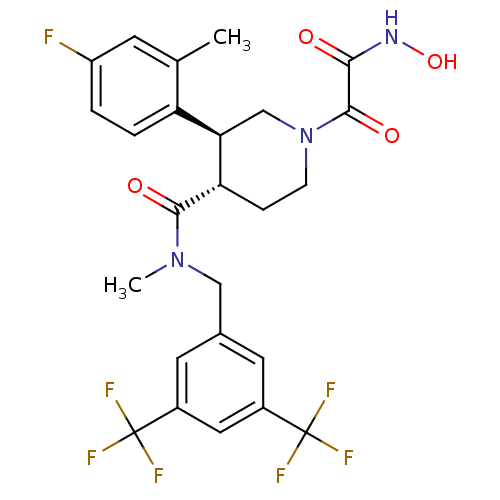 (CHEMBL1951811) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0460 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Co. Ltd Curated by ChEMBL | Assay Description Displacement of [125I]BH-SP from tachykinin NK1 receptor in human IM9 cells after 30 mins by scintillation counting | Bioorg Med Chem 20: 962-77 (2012) Article DOI: 10.1016/j.bmc.2011.11.048 BindingDB Entry DOI: 10.7270/Q2NP24WG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50364198 (CHEMBL1951630) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0470 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Co. Ltd Curated by ChEMBL | Assay Description Displacement of [125I]BH-SP from tachykinin NK1 receptor in human IM9 cells after 30 mins by scintillation counting | Bioorg Med Chem 20: 962-77 (2012) Article DOI: 10.1016/j.bmc.2011.11.048 BindingDB Entry DOI: 10.7270/Q2NP24WG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50364196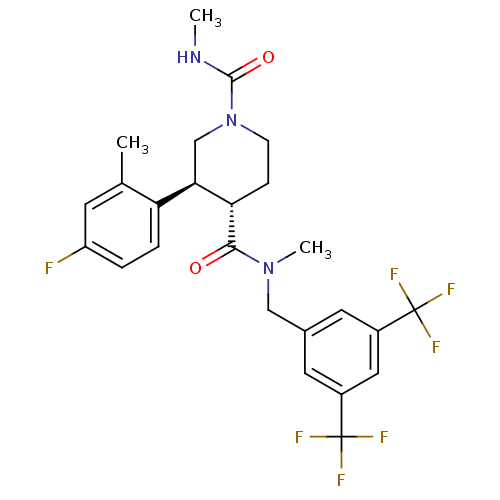 (CHEMBL1951632) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0480 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Co. Ltd Curated by ChEMBL | Assay Description Displacement of [125I]BH-SP from tachykinin NK1 receptor in human IM9 cells after 30 mins by scintillation counting | Bioorg Med Chem 20: 962-77 (2012) Article DOI: 10.1016/j.bmc.2011.11.048 BindingDB Entry DOI: 10.7270/Q2NP24WG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM106980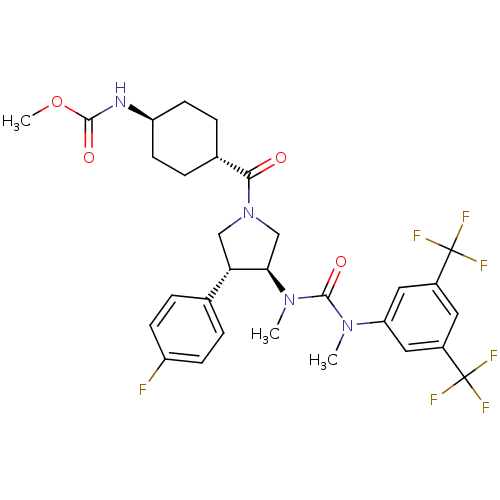 (US8592454, 233) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.0490 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited US Patent | Assay Description Radioligand receptor binding inhibitory assay using human NK receptor. | US Patent US8592454 (2013) BindingDB Entry DOI: 10.7270/Q2XD10B0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50364199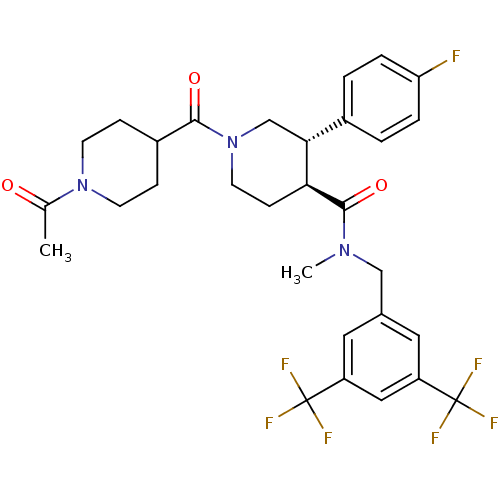 (CHEMBL1951629) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0520 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Co. Ltd Curated by ChEMBL | Assay Description Displacement of [125I]BH-SP from tachykinin NK1 receptor in human IM9 cells after 30 mins by scintillation counting | Bioorg Med Chem 20: 962-77 (2012) Article DOI: 10.1016/j.bmc.2011.11.048 BindingDB Entry DOI: 10.7270/Q2NP24WG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50364201 (CHEMBL1951627) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0640 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Co. Ltd Curated by ChEMBL | Assay Description Displacement of [125I]BH-SP from tachykinin NK1 receptor in human IM9 cells after 30 mins by scintillation counting | Bioorg Med Chem 20: 962-77 (2012) Article DOI: 10.1016/j.bmc.2011.11.048 BindingDB Entry DOI: 10.7270/Q2NP24WG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM106981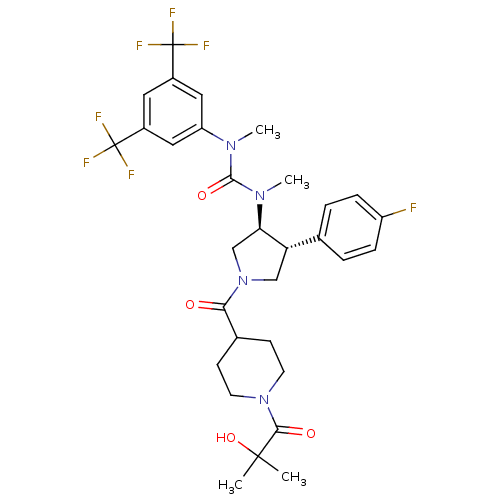 (US8592454, 308) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited US Patent | Assay Description Radioligand receptor binding inhibitory assay using human NK receptor. | US Patent US8592454 (2013) BindingDB Entry DOI: 10.7270/Q2XD10B0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50364194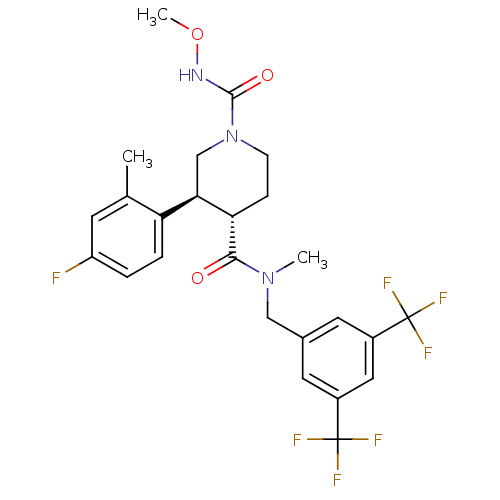 (CHEMBL1951634) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0730 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Co. Ltd Curated by ChEMBL | Assay Description Displacement of [125I]BH-SP from tachykinin NK1 receptor in human IM9 cells after 30 mins by scintillation counting | Bioorg Med Chem 20: 962-77 (2012) Article DOI: 10.1016/j.bmc.2011.11.048 BindingDB Entry DOI: 10.7270/Q2NP24WG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM106977 (US8592454, 104) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.0730 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited US Patent | Assay Description Radioligand receptor binding inhibitory assay using human NK receptor. | US Patent US8592454 (2013) BindingDB Entry DOI: 10.7270/Q2XD10B0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM106976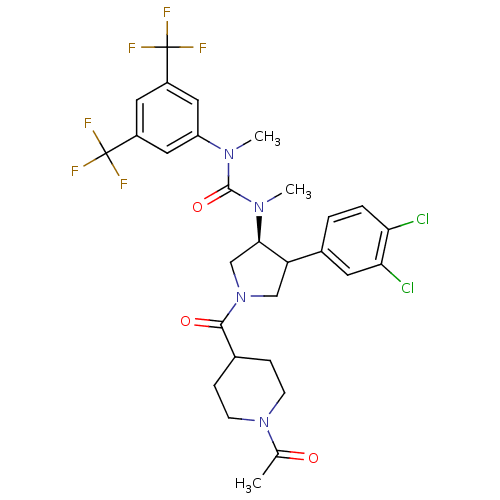 (US8592454, 66) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.0750 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited US Patent | Assay Description Radioligand receptor binding inhibitory assay using human NK receptor. | US Patent US8592454 (2013) BindingDB Entry DOI: 10.7270/Q2XD10B0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM97478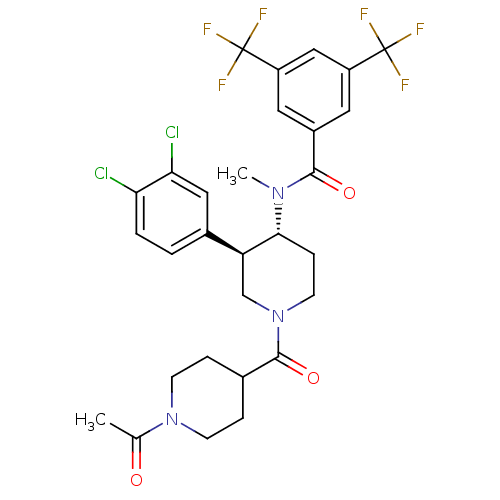 (US8470816, 91) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.0920 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Takeda Pharmaceutical Company Limited US Patent | Assay Description Radioligan receptor binding inhibitory activity using membrane fraction of CHO cell expressing human NK receptors. | US Patent US8470816 (2013) BindingDB Entry DOI: 10.7270/Q2NZ8688 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50364207 (CHEMBL1951621) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0980 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Co. Ltd Curated by ChEMBL | Assay Description Displacement of [125I]BH-SP from tachykinin NK1 receptor in human IM9 cells after 30 mins by scintillation counting | Bioorg Med Chem 20: 962-77 (2012) Article DOI: 10.1016/j.bmc.2011.11.048 BindingDB Entry DOI: 10.7270/Q2NP24WG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50364193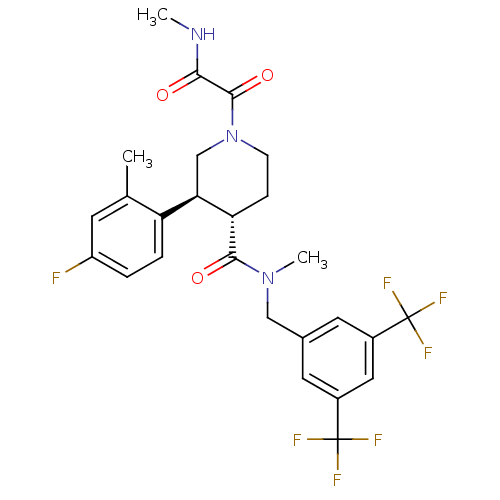 (CHEMBL1951635) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Co. Ltd Curated by ChEMBL | Assay Description Displacement of [125I]BH-SP from tachykinin NK1 receptor in human IM9 cells after 30 mins by scintillation counting | Bioorg Med Chem 20: 962-77 (2012) Article DOI: 10.1016/j.bmc.2011.11.048 BindingDB Entry DOI: 10.7270/Q2NP24WG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM106973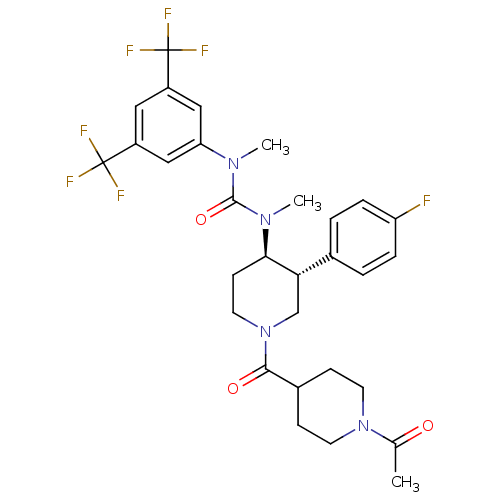 (US8592454, 4) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited US Patent | Assay Description Radioligand receptor binding inhibitory assay using human NK receptor. | US Patent US8592454 (2013) BindingDB Entry DOI: 10.7270/Q2XD10B0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50364203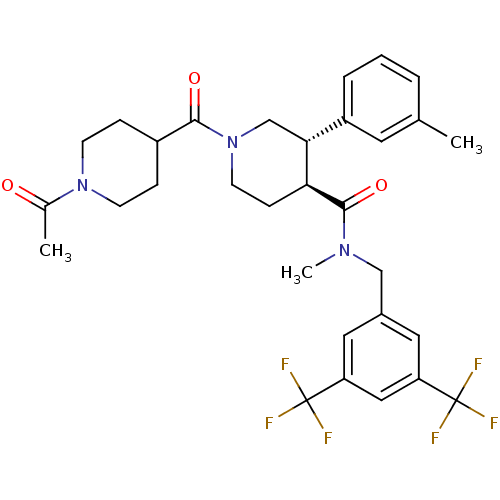 (CHEMBL1951625) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Co. Ltd Curated by ChEMBL | Assay Description Displacement of [125I]BH-SP from tachykinin NK1 receptor in human IM9 cells after 30 mins by scintillation counting | Bioorg Med Chem 20: 962-77 (2012) Article DOI: 10.1016/j.bmc.2011.11.048 BindingDB Entry DOI: 10.7270/Q2NP24WG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50364205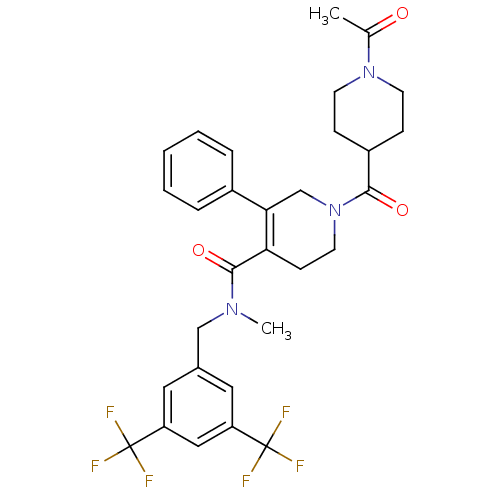 (CHEMBL1951623) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Co. Ltd Curated by ChEMBL | Assay Description Displacement of [125I]BH-SP from tachykinin NK1 receptor in human IM9 cells after 30 mins by scintillation counting | Bioorg Med Chem 20: 962-77 (2012) Article DOI: 10.1016/j.bmc.2011.11.048 BindingDB Entry DOI: 10.7270/Q2NP24WG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Matrix metalloproteinase-9 (Homo sapiens (Human)) | BDBM50062351 ((R)-N*4*-Hydroxy-N*1*-[(S)-2-(1H-indol-3-yl)-1-met...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 0.177 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human MMP-9 by fluorometric assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01215 BindingDB Entry DOI: 10.7270/Q2Q81HPF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50364204 (CHEMBL1951624) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Co. Ltd Curated by ChEMBL | Assay Description Displacement of [125I]BH-SP from tachykinin NK1 receptor in human IM9 cells after 30 mins by scintillation counting | Bioorg Med Chem 20: 962-77 (2012) Article DOI: 10.1016/j.bmc.2011.11.048 BindingDB Entry DOI: 10.7270/Q2NP24WG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50364188 (CHEMBL1951814) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Co. Ltd Curated by ChEMBL | Assay Description Displacement of [125I]BH-SP from tachykinin NK1 receptor in human IM9 cells after 30 mins by scintillation counting | Bioorg Med Chem 20: 962-77 (2012) Article DOI: 10.1016/j.bmc.2011.11.048 BindingDB Entry DOI: 10.7270/Q2NP24WG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50364200 (CHEMBL1951628) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.210 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Co. Ltd Curated by ChEMBL | Assay Description Displacement of [125I]BH-SP from tachykinin NK1 receptor in human IM9 cells after 30 mins by scintillation counting | Bioorg Med Chem 20: 962-77 (2012) Article DOI: 10.1016/j.bmc.2011.11.048 BindingDB Entry DOI: 10.7270/Q2NP24WG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA gyrase subunit A/B (Escherichia coli (strain K12)) | BDBM50554205 (CHEMBL4757242) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.210 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of Escherichia coli DNA gyrase by measuring supercoiling activity incubated for 60 mins by fluorescence based assay | Citation and Details Article DOI: 10.1016/j.bmc.2020.115776 BindingDB Entry DOI: 10.7270/Q2W66QFJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Homo sapiens (Human)) | BDBM97485 (US8470816, 458) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.220 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Takeda Pharmaceutical Company Limited US Patent | Assay Description Radioligan receptor binding inhibitory activity using membrane fraction of CHO cell expressing human NK receptors. | US Patent US8470816 (2013) BindingDB Entry DOI: 10.7270/Q2NZ8688 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Homo sapiens (Human)) | BDBM97486 (US8470816, 464) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.220 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Takeda Pharmaceutical Company Limited US Patent | Assay Description Radioligan receptor binding inhibitory activity using membrane fraction of CHO cell expressing human NK receptors. | US Patent US8470816 (2013) BindingDB Entry DOI: 10.7270/Q2NZ8688 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Homo sapiens (Human)) | BDBM97489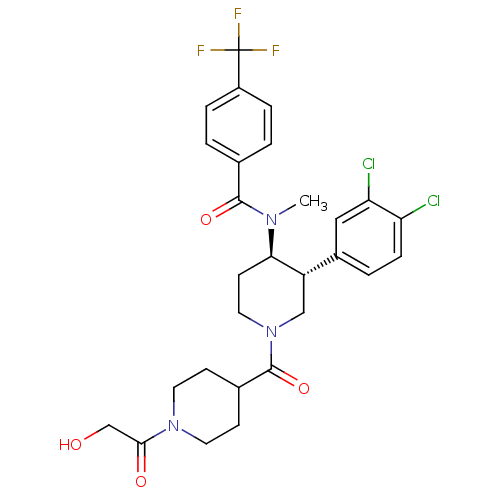 (US8470816, 632) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.220 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Takeda Pharmaceutical Company Limited US Patent | Assay Description Radioligan receptor binding inhibitory activity using membrane fraction of CHO cell expressing human NK receptors. | US Patent US8470816 (2013) BindingDB Entry DOI: 10.7270/Q2NZ8688 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Homo sapiens (Human)) | BDBM97481 (US8470816, 265) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.230 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Takeda Pharmaceutical Company Limited US Patent | Assay Description Radioligan receptor binding inhibitory activity using membrane fraction of CHO cell expressing human NK receptors. | US Patent US8470816 (2013) BindingDB Entry DOI: 10.7270/Q2NZ8688 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Homo sapiens (Human)) | BDBM97490 (US8470816, 633) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.240 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Takeda Pharmaceutical Company Limited US Patent | Assay Description Radioligan receptor binding inhibitory activity using membrane fraction of CHO cell expressing human NK receptors. | US Patent US8470816 (2013) BindingDB Entry DOI: 10.7270/Q2NZ8688 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Homo sapiens (Human)) | BDBM97483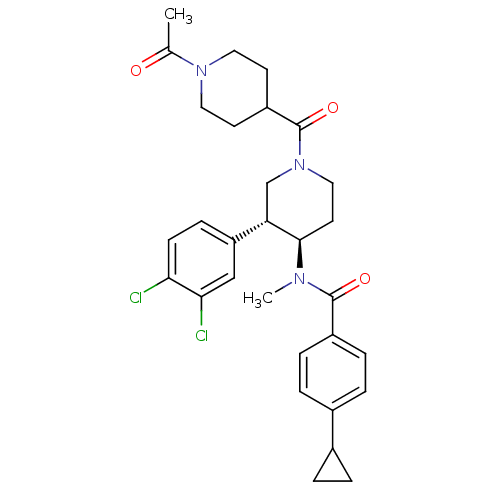 (US8470816, 292) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.25 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Takeda Pharmaceutical Company Limited US Patent | Assay Description Radioligan receptor binding inhibitory activity using membrane fraction of CHO cell expressing human NK receptors. | US Patent US8470816 (2013) BindingDB Entry DOI: 10.7270/Q2NZ8688 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA gyrase subunit A/B (Escherichia coli (strain K12)) | BDBM50554208 (CHEMBL4784687) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.260 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of Escherichia coli DNA gyrase by measuring supercoiling activity incubated for 60 mins by fluorescence based assay | Citation and Details Article DOI: 10.1016/j.bmc.2020.115776 BindingDB Entry DOI: 10.7270/Q2W66QFJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA gyrase subunit A/B (Escherichia coli (strain K12)) | BDBM50554207 (CHEMBL4758336) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.290 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of Escherichia coli DNA gyrase by measuring supercoiling activity incubated for 60 mins by fluorescence based assay | Citation and Details Article DOI: 10.1016/j.bmc.2020.115776 BindingDB Entry DOI: 10.7270/Q2W66QFJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Homo sapiens (Human)) | BDBM97479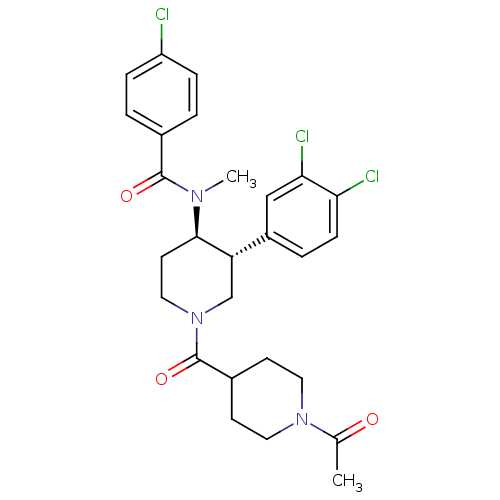 (US8470816, 251a) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.310 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Takeda Pharmaceutical Company Limited US Patent | Assay Description Radioligan receptor binding inhibitory activity using membrane fraction of CHO cell expressing human NK receptors. | US Patent US8470816 (2013) BindingDB Entry DOI: 10.7270/Q2NZ8688 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA gyrase subunit A/B (Escherichia coli (strain K12)) | BDBM50554204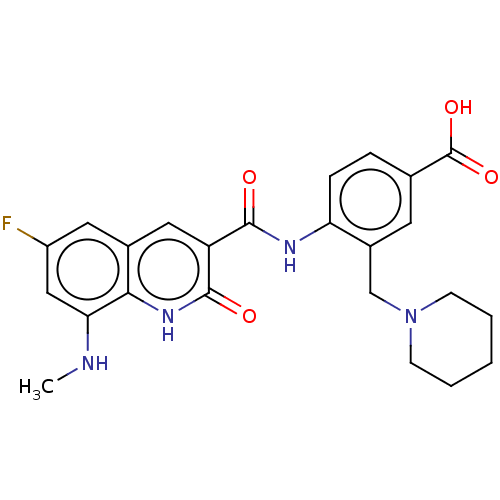 (CHEMBL4743844) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.310 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of Escherichia coli DNA gyrase by measuring supercoiling activity incubated for 60 mins by fluorescence based assay | Citation and Details Article DOI: 10.1016/j.bmc.2020.115776 BindingDB Entry DOI: 10.7270/Q2W66QFJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Homo sapiens (Human)) | BDBM97487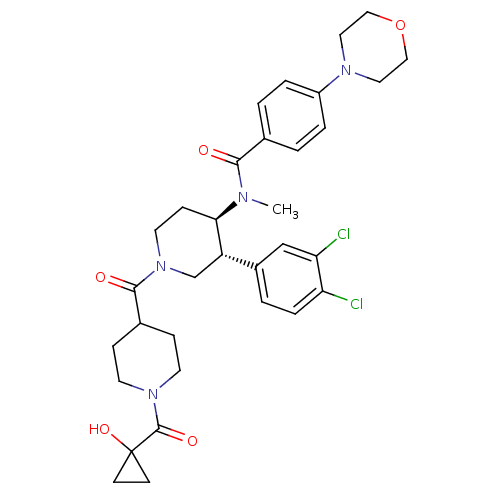 (US8470816, 623) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.310 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Takeda Pharmaceutical Company Limited US Patent | Assay Description Radioligan receptor binding inhibitory activity using membrane fraction of CHO cell expressing human NK receptors. | US Patent US8470816 (2013) BindingDB Entry DOI: 10.7270/Q2NZ8688 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Homo sapiens (Human)) | BDBM97491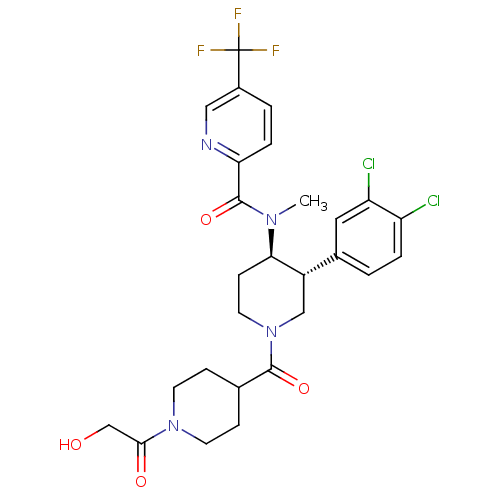 (US8470816, 635) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.360 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Takeda Pharmaceutical Company Limited US Patent | Assay Description Radioligan receptor binding inhibitory activity using membrane fraction of CHO cell expressing human NK receptors. | US Patent US8470816 (2013) BindingDB Entry DOI: 10.7270/Q2NZ8688 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA gyrase subunit A/B (Escherichia coli (strain K12)) | BDBM50554200 (CHEMBL4764566) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 0.360 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of Escherichia coli DNA gyrase by measuring supercoiling activity incubated for 60 mins by fluorescence based assay | Citation and Details Article DOI: 10.1016/j.bmc.2020.115776 BindingDB Entry DOI: 10.7270/Q2W66QFJ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Substance-K receptor (Homo sapiens (Human)) | BDBM97482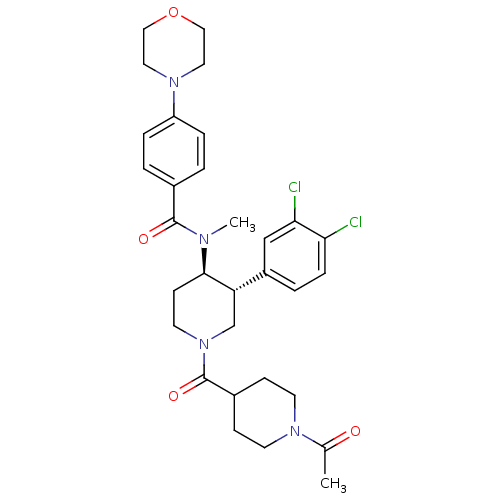 (US8470816, 268) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.420 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Takeda Pharmaceutical Company Limited US Patent | Assay Description Radioligan receptor binding inhibitory activity using membrane fraction of CHO cell expressing human NK receptors. | US Patent US8470816 (2013) BindingDB Entry DOI: 10.7270/Q2NZ8688 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 72 kDa type IV collagenase (Homo sapiens (Human)) | BDBM50062351 ((R)-N*4*-Hydroxy-N*1*-[(S)-2-(1H-indol-3-yl)-1-met...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 0.422 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human MMP-2 by fluorometric assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01215 BindingDB Entry DOI: 10.7270/Q2Q81HPF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA gyrase subunit A/B (Escherichia coli (strain K12)) | BDBM50554197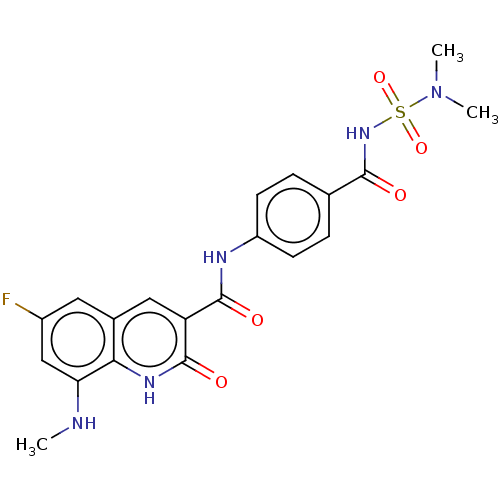 (CHEMBL4776319) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.430 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of Escherichia coli DNA gyrase by measuring supercoiling activity incubated for 60 mins by fluorescence based assay | Citation and Details Article DOI: 10.1016/j.bmc.2020.115776 BindingDB Entry DOI: 10.7270/Q2W66QFJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 352 total ) | Next | Last >> |