 Found 7699 hits with Last Name = 'xia' and Initial = 'h'
Found 7699 hits with Last Name = 'xia' and Initial = 'h' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
ALK tyrosine kinase receptor
(Homo sapiens (Human)) | BDBM50519598
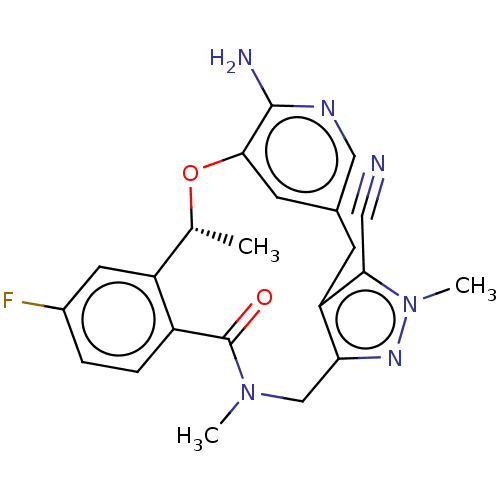
(CHEMBL4436406)Show SMILES C[C@H]1Oc2cc(Cc3c(CN(C)C(=O)c4ccc(F)cc14)nn(C)c3C#N)cnc2N |r| Show InChI InChI=1S/C22H21FN6O2/c1-12-16-8-14(23)4-5-15(16)22(30)28(2)11-18-17(19(9-24)29(3)27-18)6-13-7-20(31-12)21(25)26-10-13/h4-5,7-8,10,12H,6,11H2,1-3H3,(H2,25,26)/t12-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| <0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University
Curated by ChEMBL
| Assay Description
Inhibition of ALK (unknown origin) |
J Med Chem 62: 10927-10954 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00446
BindingDB Entry DOI: 10.7270/Q2S185WV |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM50245852
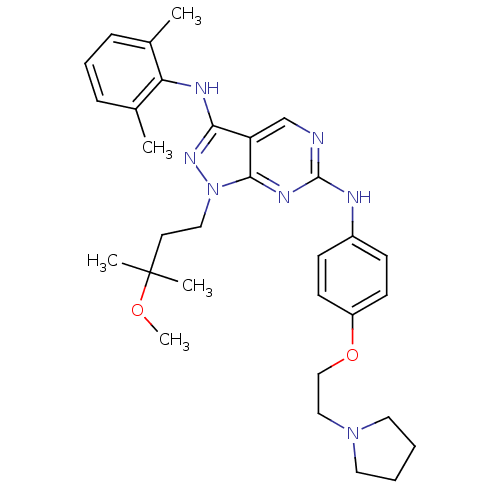
(CHEMBL458333 | N3-(2,6-dimethylphenyl)-1-(3-methox...)Show SMILES COC(C)(C)CCn1nc(Nc2c(C)cccc2C)c2cnc(Nc3ccc(OCCN4CCCC4)cc3)nc12 Show InChI InChI=1S/C31H41N7O2/c1-22-9-8-10-23(2)27(22)34-28-26-21-32-30(35-29(26)38(36-28)18-15-31(3,4)39-5)33-24-11-13-25(14-12-24)40-20-19-37-16-6-7-17-37/h8-14,21H,6-7,15-20H2,1-5H3,(H,34,36)(H,32,33,35) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of LCK (unknown origin) |
Bioorg Med Chem Lett 18: 6352-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.092
BindingDB Entry DOI: 10.7270/Q2B56JKZ |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase ROS
(Homo sapiens (Human)) | BDBM50519598

(CHEMBL4436406)Show SMILES C[C@H]1Oc2cc(Cc3c(CN(C)C(=O)c4ccc(F)cc14)nn(C)c3C#N)cnc2N |r| Show InChI InChI=1S/C22H21FN6O2/c1-12-16-8-14(23)4-5-15(16)22(30)28(2)11-18-17(19(9-24)29(3)27-18)6-13-7-20(31-12)21(25)26-10-13/h4-5,7-8,10,12H,6,11H2,1-3H3,(H2,25,26)/t12-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| <0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University
Curated by ChEMBL
| Assay Description
Inhibition of ROS1 (unknown origin) |
J Med Chem 62: 10927-10954 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00446
BindingDB Entry DOI: 10.7270/Q2S185WV |
More data for this
Ligand-Target Pair | |
Activated CDC42 kinase 1
(Homo sapiens (Human)) | BDBM50421256
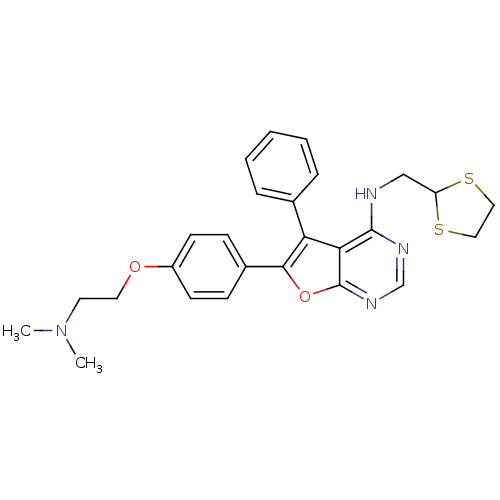
(CHEMBL2087874)Show SMILES CN(C)CCOc1ccc(cc1)-c1oc2ncnc(NCC3SCCS3)c2c1-c1ccccc1 Show InChI InChI=1S/C26H28N4O2S2/c1-30(2)12-13-31-20-10-8-19(9-11-20)24-22(18-6-4-3-5-7-18)23-25(28-17-29-26(23)32-24)27-16-21-33-14-15-34-21/h3-11,17,21H,12-16H2,1-2H3,(H,27,28,29) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal His6-tagged ACK1 expressed in baculovirus infected Hi5 cells assessed as inhibition of autophosphorylation after 2 hrs by EL... |
Bioorg Med Chem Lett 22: 6212-7 (2012)
Article DOI: 10.1016/j.bmcl.2012.08.020
BindingDB Entry DOI: 10.7270/Q2VH5Q4X |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50245852

(CHEMBL458333 | N3-(2,6-dimethylphenyl)-1-(3-methox...)Show SMILES COC(C)(C)CCn1nc(Nc2c(C)cccc2C)c2cnc(Nc3ccc(OCCN4CCCC4)cc3)nc12 Show InChI InChI=1S/C31H41N7O2/c1-22-9-8-10-23(2)27(22)34-28-26-21-32-30(35-29(26)38(36-28)18-15-31(3,4)39-5)33-24-11-13-25(14-12-24)40-20-19-37-16-6-7-17-37/h8-14,21H,6-7,15-20H2,1-5H3,(H,34,36)(H,32,33,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of BTK (unknown origin) |
Bioorg Med Chem Lett 18: 6352-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.092
BindingDB Entry DOI: 10.7270/Q2B56JKZ |
More data for this
Ligand-Target Pair | |
Activated CDC42 kinase 1
(Homo sapiens (Human)) | BDBM50421255
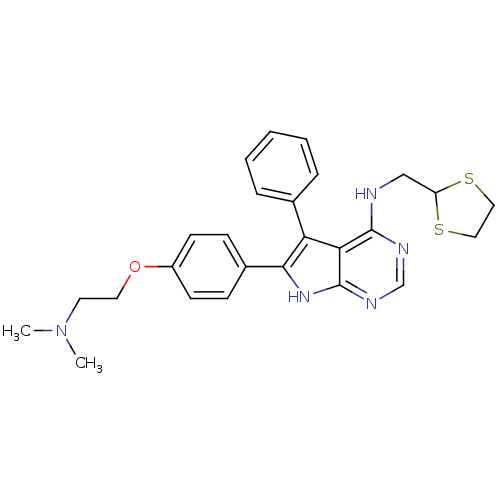
(CHEMBL2087873)Show SMILES CN(C)CCOc1ccc(cc1)-c1[nH]c2ncnc(NCC3SCCS3)c2c1-c1ccccc1 Show InChI InChI=1S/C26H29N5OS2/c1-31(2)12-13-32-20-10-8-19(9-11-20)24-22(18-6-4-3-5-7-18)23-25(28-17-29-26(23)30-24)27-16-21-33-14-15-34-21/h3-11,17,21H,12-16H2,1-2H3,(H2,27,28,29,30) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal His6-tagged ACK1 expressed in baculovirus infected Hi5 cells assessed as inhibition of autophosphorylation after 2 hrs by EL... |
Bioorg Med Chem Lett 22: 6212-7 (2012)
Article DOI: 10.1016/j.bmcl.2012.08.020
BindingDB Entry DOI: 10.7270/Q2VH5Q4X |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50348414
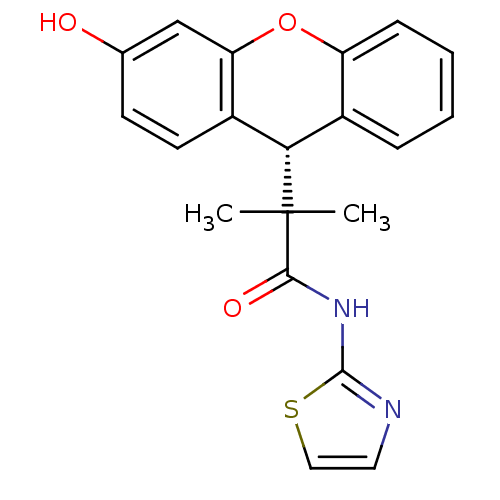
(CHEMBL1800984)Show SMILES CC(C)([C@H]1c2ccccc2Oc2cc(O)ccc12)C(=O)Nc1nccs1 |r| Show InChI InChI=1S/C20H18N2O3S/c1-20(2,18(24)22-19-21-9-10-26-19)17-13-5-3-4-6-15(13)25-16-11-12(23)7-8-14(16)17/h3-11,17,23H,1-2H3,(H,21,22,24)/t17-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Displacement of GS-red from GRapha by fluorescence polarization assay |
J Med Chem 53: 8241-51 (2010)
Article DOI: 10.1021/jm100957a
BindingDB Entry DOI: 10.7270/Q2HX1DPW |
More data for this
Ligand-Target Pair | |
Activated CDC42 kinase 1
(Homo sapiens (Human)) | BDBM50421257
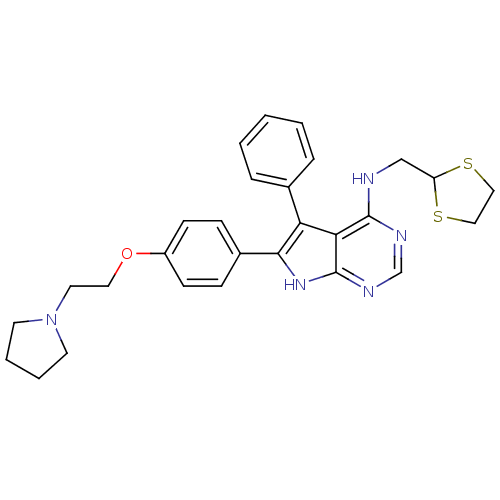
(CHEMBL2087875)Show SMILES C(CN1CCCC1)Oc1ccc(cc1)-c1[nH]c2ncnc(NCC3SCCS3)c2c1-c1ccccc1 Show InChI InChI=1S/C28H31N5OS2/c1-2-6-20(7-3-1)24-25-27(29-18-23-35-16-17-36-23)30-19-31-28(25)32-26(24)21-8-10-22(11-9-21)34-15-14-33-12-4-5-13-33/h1-3,6-11,19,23H,4-5,12-18H2,(H2,29,30,31,32) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal His6-tagged ACK1 expressed in baculovirus infected Hi5 cells assessed as inhibition of autophosphorylation after 2 hrs by EL... |
Bioorg Med Chem Lett 22: 6212-7 (2012)
Article DOI: 10.1016/j.bmcl.2012.08.020
BindingDB Entry DOI: 10.7270/Q2VH5Q4X |
More data for this
Ligand-Target Pair | |
Aurora kinase B
(Homo sapiens (Human)) | BDBM50315769

(3-(4-(4-(2-(3-((dimethylamino)methyl)phenyl)-1H-py...)Show SMILES CCn1cc(c(n1)-c1ccc(NC(=O)N(C)C)cc1)-c1ccnc2[nH]c(cc12)-c1cccc(CN(C)C)c1 Show InChI InChI=1S/C30H33N7O/c1-6-37-19-26(28(34-37)21-10-12-23(13-11-21)32-30(38)36(4)5)24-14-15-31-29-25(24)17-27(33-29)22-9-7-8-20(16-22)18-35(2)3/h7-17,19H,6,18H2,1-5H3,(H,31,33)(H,32,38) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Competitive inhibition of Aurora B ATP binding site |
J Med Chem 53: 3973-4001 (2010)
Article DOI: 10.1021/jm901870q
BindingDB Entry DOI: 10.7270/Q27082CK |
More data for this
Ligand-Target Pair | |
Aurora kinase B
(Homo sapiens (Human)) | BDBM50315769

(3-(4-(4-(2-(3-((dimethylamino)methyl)phenyl)-1H-py...)Show SMILES CCn1cc(c(n1)-c1ccc(NC(=O)N(C)C)cc1)-c1ccnc2[nH]c(cc12)-c1cccc(CN(C)C)c1 Show InChI InChI=1S/C30H33N7O/c1-6-37-19-26(28(34-37)21-10-12-23(13-11-21)32-30(38)36(4)5)24-14-15-31-29-25(24)17-27(33-29)22-9-7-8-20(16-22)18-35(2)3/h7-17,19H,6,18H2,1-5H3,(H,31,33)(H,32,38) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Competitive inhibition of human Aurora B ATP binding site by rapid dilution method |
J Med Chem 53: 3973-4001 (2010)
Article DOI: 10.1021/jm901870q
BindingDB Entry DOI: 10.7270/Q27082CK |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50348407
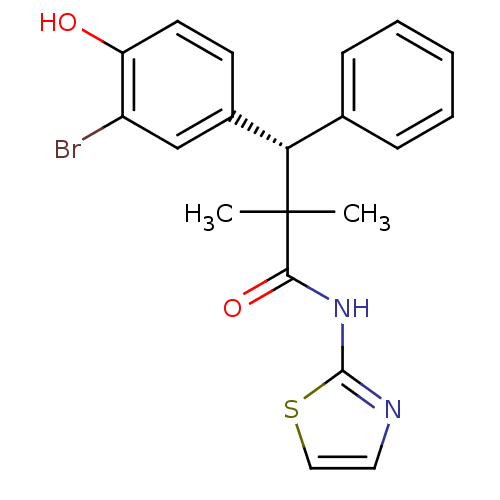
(CHEMBL1801006)Show SMILES CC(C)([C@@H](c1ccccc1)c1ccc(O)c(Br)c1)C(=O)Nc1nccs1 |r| Show InChI InChI=1S/C20H19BrN2O2S/c1-20(2,18(25)23-19-22-10-11-26-19)17(13-6-4-3-5-7-13)14-8-9-16(24)15(21)12-14/h3-12,17,24H,1-2H3,(H,22,23,25)/t17-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Displacement of GS-red from GRapha by fluorescence polarization assay |
J Med Chem 53: 8241-51 (2010)
Article DOI: 10.1021/jm100957a
BindingDB Entry DOI: 10.7270/Q2HX1DPW |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50348412
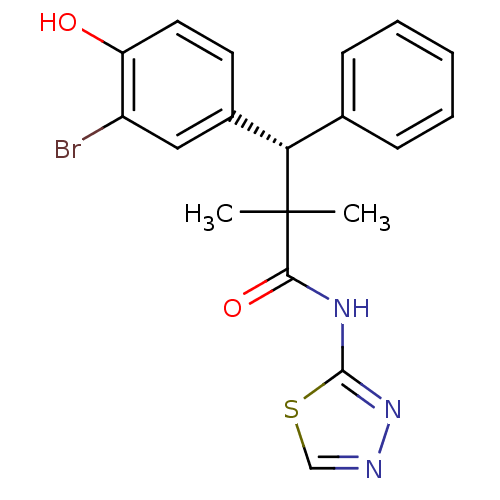
(CHEMBL1800982)Show SMILES CC(C)([C@@H](c1ccccc1)c1ccc(O)c(Br)c1)C(=O)Nc1nncs1 |r| Show InChI InChI=1S/C19H18BrN3O2S/c1-19(2,17(25)22-18-23-21-11-26-18)16(12-6-4-3-5-7-12)13-8-9-15(24)14(20)10-13/h3-11,16,24H,1-2H3,(H,22,23,25)/t16-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Displacement of GS-red from GRapha by fluorescence polarization assay |
J Med Chem 53: 8241-51 (2010)
Article DOI: 10.1021/jm100957a
BindingDB Entry DOI: 10.7270/Q2HX1DPW |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50440220
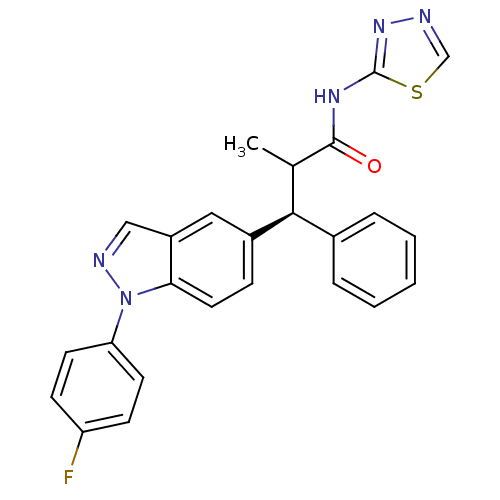
(CHEMBL2426624)Show SMILES CC([C@@H](c1ccccc1)c1ccc2n(ncc2c1)-c1ccc(F)cc1)C(=O)Nc1nncs1 |r| Show InChI InChI=1S/C25H20FN5OS/c1-16(24(32)29-25-30-27-15-33-25)23(17-5-3-2-4-6-17)18-7-12-22-19(13-18)14-28-31(22)21-10-8-20(26)9-11-21/h2-16,23H,1H3,(H,29,30,32)/t16?,23-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.480 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ironwood Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity to glucocorticoid receptor (unknown origin) by fluorescence polarization assay |
Bioorg Med Chem Lett 23: 5442-7 (2013)
Article DOI: 10.1016/j.bmcl.2013.06.089
BindingDB Entry DOI: 10.7270/Q29888FB |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50440220

(CHEMBL2426624)Show SMILES CC([C@@H](c1ccccc1)c1ccc2n(ncc2c1)-c1ccc(F)cc1)C(=O)Nc1nncs1 |r| Show InChI InChI=1S/C25H20FN5OS/c1-16(24(32)29-25-30-27-15-33-25)23(17-5-3-2-4-6-17)18-7-12-22-19(13-18)14-28-31(22)21-10-8-20(26)9-11-21/h2-16,23H,1H3,(H,29,30,32)/t16?,23-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.480 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ironwood Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity to glucocorticoid receptor (unknown origin) by fluorescence polarization assay |
Bioorg Med Chem Lett 23: 5442-7 (2013)
Article DOI: 10.1016/j.bmcl.2013.06.089
BindingDB Entry DOI: 10.7270/Q29888FB |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Homo sapiens (Human)) | BDBM13534

(CHEMBL572878 | N-[4-({4-[(3-methyl-1H-pyrazol-5-yl...)Show SMILES CN1CCN(CC1)c1cc(Nc2cc(C)n[nH]2)nc(Sc2ccc(NC(=O)C3CC3)cc2)n1 Show InChI InChI=1S/C23H28N8OS/c1-15-13-20(29-28-15)25-19-14-21(31-11-9-30(2)10-12-31)27-23(26-19)33-18-7-5-17(6-8-18)24-22(32)16-3-4-16/h5-8,13-14,16H,3-4,9-12H2,1-2H3,(H,24,32)(H2,25,26,27,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of Aurora A |
J Med Chem 53: 3973-4001 (2010)
Article DOI: 10.1021/jm901870q
BindingDB Entry DOI: 10.7270/Q27082CK |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50348404
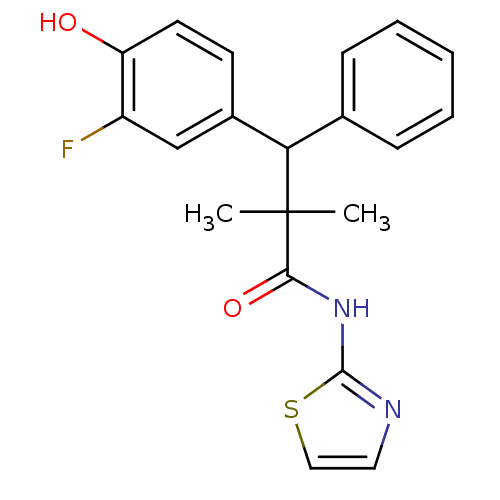
(CHEMBL1801009)Show SMILES CC(C)(C(c1ccccc1)c1ccc(O)c(F)c1)C(=O)Nc1nccs1 Show InChI InChI=1S/C20H19FN2O2S/c1-20(2,18(25)23-19-22-10-11-26-19)17(13-6-4-3-5-7-13)14-8-9-16(24)15(21)12-14/h3-12,17,24H,1-2H3,(H,22,23,25) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Displacement of GS-red from GRapha by fluorescence polarization assay |
J Med Chem 53: 8241-51 (2010)
Article DOI: 10.1021/jm100957a
BindingDB Entry DOI: 10.7270/Q2HX1DPW |
More data for this
Ligand-Target Pair | |
ALK tyrosine kinase receptor
(Homo sapiens (Human)) | BDBM50519598

(CHEMBL4436406)Show SMILES C[C@H]1Oc2cc(Cc3c(CN(C)C(=O)c4ccc(F)cc14)nn(C)c3C#N)cnc2N |r| Show InChI InChI=1S/C22H21FN6O2/c1-12-16-8-14(23)4-5-15(16)22(30)28(2)11-18-17(19(9-24)29(3)27-18)6-13-7-20(31-12)21(25)26-10-13/h4-5,7-8,10,12H,6,11H2,1-3H3,(H2,25,26)/t12-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University
Curated by ChEMBL
| Assay Description
Inhibition of ALK L1196M (unknown origin) |
J Med Chem 62: 10927-10954 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00446
BindingDB Entry DOI: 10.7270/Q2S185WV |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50348411
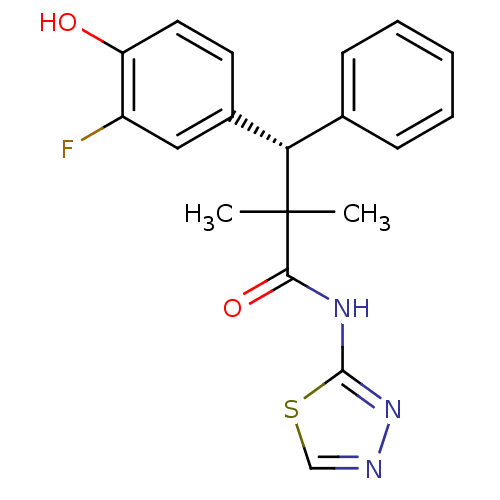
(CHEMBL1800981)Show SMILES CC(C)([C@@H](c1ccccc1)c1ccc(O)c(F)c1)C(=O)Nc1nncs1 |r| Show InChI InChI=1S/C19H18FN3O2S/c1-19(2,17(25)22-18-23-21-11-26-18)16(12-6-4-3-5-7-12)13-8-9-15(24)14(20)10-13/h3-11,16,24H,1-2H3,(H,22,23,25)/t16-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Displacement of GS-red from GRapha by fluorescence polarization assay |
J Med Chem 53: 8241-51 (2010)
Article DOI: 10.1021/jm100957a
BindingDB Entry DOI: 10.7270/Q2HX1DPW |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50348405
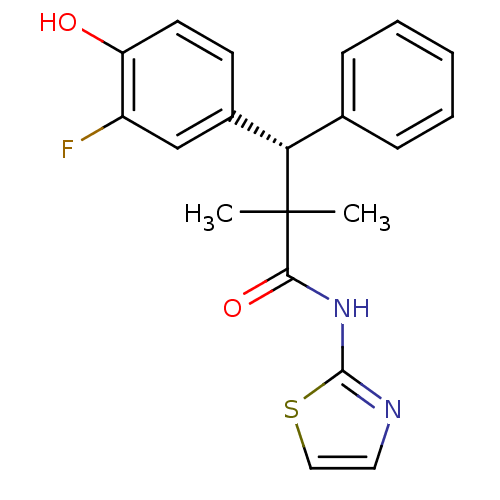
(CHEMBL1801008)Show SMILES CC(C)([C@@H](c1ccccc1)c1ccc(O)c(F)c1)C(=O)Nc1nccs1 |r| Show InChI InChI=1S/C20H19FN2O2S/c1-20(2,18(25)23-19-22-10-11-26-19)17(13-6-4-3-5-7-13)14-8-9-16(24)15(21)12-14/h3-12,17,24H,1-2H3,(H,22,23,25)/t17-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Displacement of GS-red from GRapha by fluorescence polarization assay |
J Med Chem 53: 8241-51 (2010)
Article DOI: 10.1021/jm100957a
BindingDB Entry DOI: 10.7270/Q2HX1DPW |
More data for this
Ligand-Target Pair | |
ALK tyrosine kinase receptor
(Homo sapiens (Human)) | BDBM192752
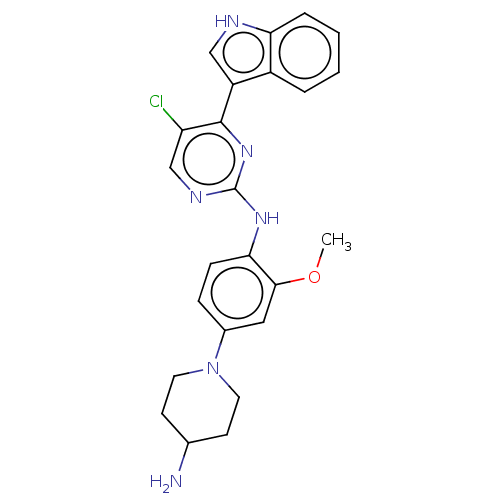
(AZD3463)Show SMILES COc1cc(ccc1Nc1ncc(Cl)c(n1)-c1c[nH]c2ccccc12)N1CCC(N)CC1 Show InChI InChI=1S/C24H25ClN6O/c1-32-22-12-16(31-10-8-15(26)9-11-31)6-7-21(22)29-24-28-14-19(25)23(30-24)18-13-27-20-5-3-2-4-17(18)20/h2-7,12-15,27H,8-11,26H2,1H3,(H,28,29,30) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 0.75 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University
Curated by ChEMBL
| Assay Description
Inhibition of ALK (unknown origin) |
J Med Chem 62: 10927-10954 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00446
BindingDB Entry DOI: 10.7270/Q2S185WV |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM19202
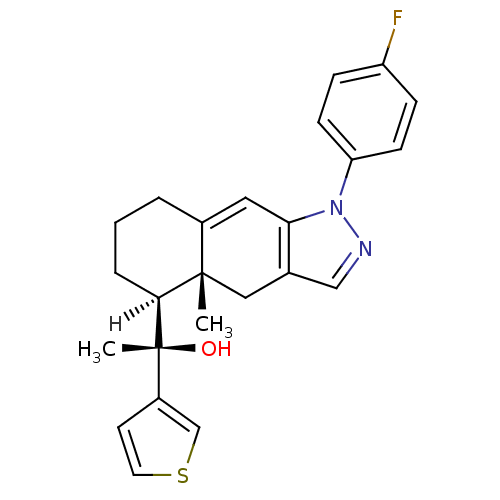
((1S)-1-[(4aR,5S)-1-(4-fluorophenyl)-4a-methyl-1H,4...)Show SMILES [H][C@@]1(CCCC2=Cc3c(C[C@]12C)cnn3-c1ccc(F)cc1)[C@](C)(O)c1ccsc1 |r,t:5| Show InChI InChI=1S/C24H25FN2OS/c1-23-13-16-14-26-27(20-8-6-19(25)7-9-20)21(16)12-17(23)4-3-5-22(23)24(2,28)18-10-11-29-15-18/h6-12,14-15,22,28H,3-5,13H2,1-2H3/t22-,23-,24+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Displacement of GS-red from human glucocorticoid receptor by fluorescent polarization assay |
J Med Chem 53: 1270-80 (2010)
Article DOI: 10.1021/jm901551w
BindingDB Entry DOI: 10.7270/Q2KS6SGW |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50348415
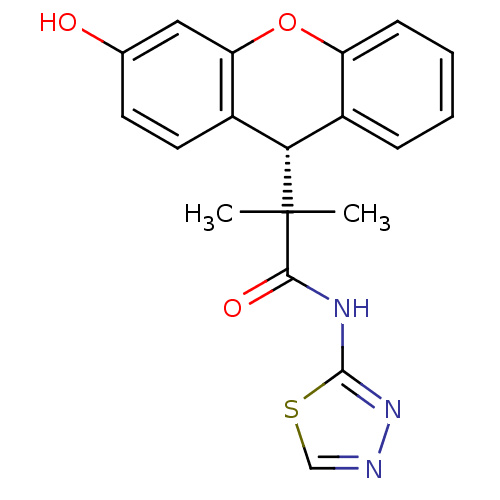
(CHEMBL1800985)Show SMILES CC(C)([C@H]1c2ccccc2Oc2cc(O)ccc12)C(=O)Nc1nncs1 |r| Show InChI InChI=1S/C19H17N3O3S/c1-19(2,17(24)21-18-22-20-10-26-18)16-12-5-3-4-6-14(12)25-15-9-11(23)7-8-13(15)16/h3-10,16,23H,1-2H3,(H,21,22,24)/t16-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Displacement of GS-red from GRapha by fluorescence polarization assay |
J Med Chem 53: 8241-51 (2010)
Article DOI: 10.1021/jm100957a
BindingDB Entry DOI: 10.7270/Q2HX1DPW |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50276642
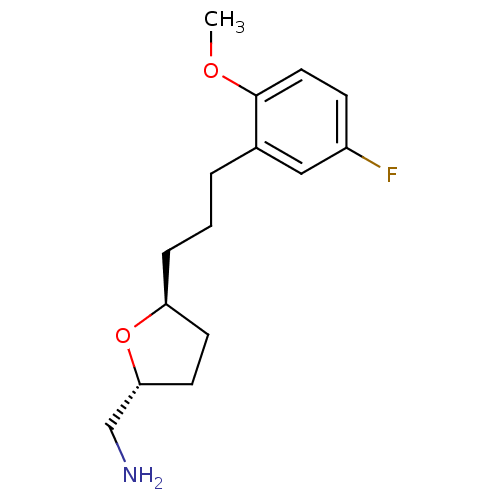
(CHEMBL460407 | trans-2-Aminomethyl-5-((2''-methoxy...)Show InChI InChI=1S/C15H22FNO2/c1-18-15-8-5-12(16)9-11(15)3-2-4-13-6-7-14(10-17)19-13/h5,8-9,13-14H,2-4,6-7,10,17H2,1H3/t13-,14+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Human BioMolecular Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [125I]RTI55 from human SERT expressed in HEK cells |
Bioorg Med Chem 17: 2047-68 (2009)
Article DOI: 10.1016/j.bmc.2009.01.023
BindingDB Entry DOI: 10.7270/Q2057GV0 |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50348408
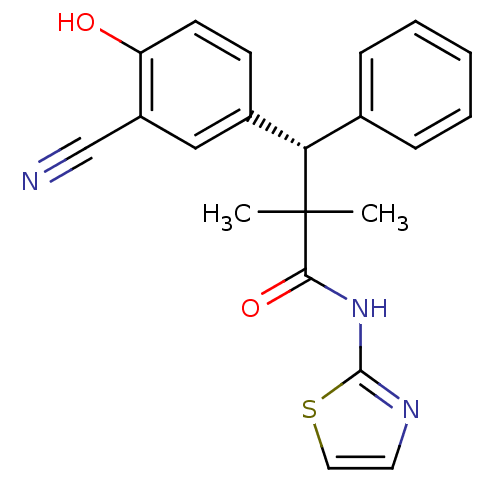
(CHEMBL1801005)Show SMILES CC(C)([C@@H](c1ccccc1)c1ccc(O)c(c1)C#N)C(=O)Nc1nccs1 |r| Show InChI InChI=1S/C21H19N3O2S/c1-21(2,19(26)24-20-23-10-11-27-20)18(14-6-4-3-5-7-14)15-8-9-17(25)16(12-15)13-22/h3-12,18,25H,1-2H3,(H,23,24,26)/t18-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Displacement of GS-red from GRapha by fluorescence polarization assay |
J Med Chem 53: 8241-51 (2010)
Article DOI: 10.1021/jm100957a
BindingDB Entry DOI: 10.7270/Q2HX1DPW |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50440216
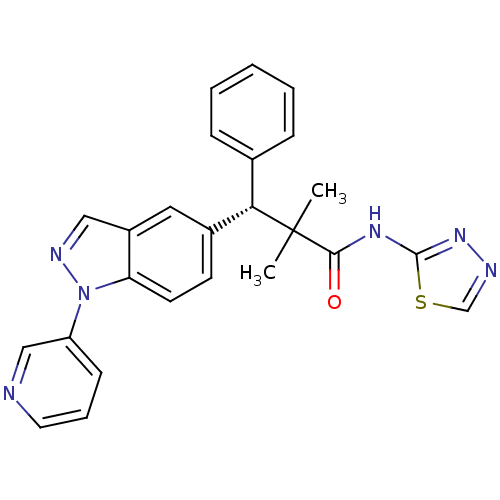
(CHEMBL2426648)Show SMILES CC(C)([C@@H](c1ccccc1)c1ccc2n(ncc2c1)-c1cccnc1)C(=O)Nc1nncs1 |r| Show InChI InChI=1S/C25H22N6OS/c1-25(2,23(32)29-24-30-27-16-33-24)22(17-7-4-3-5-8-17)18-10-11-21-19(13-18)14-28-31(21)20-9-6-12-26-15-20/h3-16,22H,1-2H3,(H,29,30,32)/t22-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ironwood Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity to glucocorticoid receptor (unknown origin) by fluorescence polarization assay |
Bioorg Med Chem Lett 23: 5442-7 (2013)
Article DOI: 10.1016/j.bmcl.2013.06.089
BindingDB Entry DOI: 10.7270/Q29888FB |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50303827
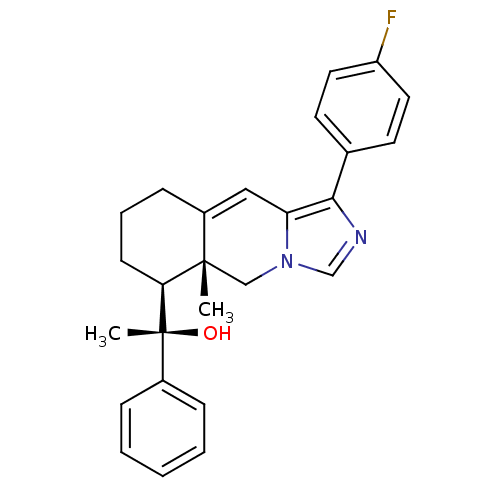
((S)-1-((5aR,6S)-1-(4-fluorophenyl)-5a-methyl-5,5a,...)Show SMILES C[C@](O)([C@H]1CCCC2=Cc3c(ncn3C[C@]12C)-c1ccc(F)cc1)c1ccccc1 |r,t:7| Show InChI InChI=1S/C26H27FN2O/c1-25-16-29-17-28-24(18-11-13-21(27)14-12-18)22(29)15-20(25)9-6-10-23(25)26(2,30)19-7-4-3-5-8-19/h3-5,7-8,11-15,17,23,30H,6,9-10,16H2,1-2H3/t23-,25-,26+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Displacement of GS-red from human glucocorticoid receptor by fluorescent polarization assay |
J Med Chem 53: 1270-80 (2010)
Article DOI: 10.1021/jm901551w
BindingDB Entry DOI: 10.7270/Q2KS6SGW |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50303831
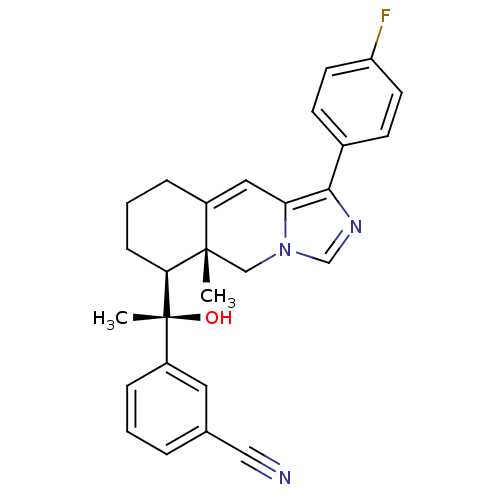
(3-((S)-1-((5aR,6S)-1-(4-fluorophenyl)-5a-methyl-5,...)Show SMILES C[C@](O)([C@H]1CCCC2=Cc3c(ncn3C[C@]12C)-c1ccc(F)cc1)c1cccc(c1)C#N |r,t:7| Show InChI InChI=1S/C27H26FN3O/c1-26-16-31-17-30-25(19-9-11-22(28)12-10-19)23(31)14-20(26)6-4-8-24(26)27(2,32)21-7-3-5-18(13-21)15-29/h3,5,7,9-14,17,24,32H,4,6,8,16H2,1-2H3/t24-,26-,27+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Displacement of GS-red from human glucocorticoid receptor by fluorescent polarization assay |
J Med Chem 53: 1270-80 (2010)
Article DOI: 10.1021/jm901551w
BindingDB Entry DOI: 10.7270/Q2KS6SGW |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50303813
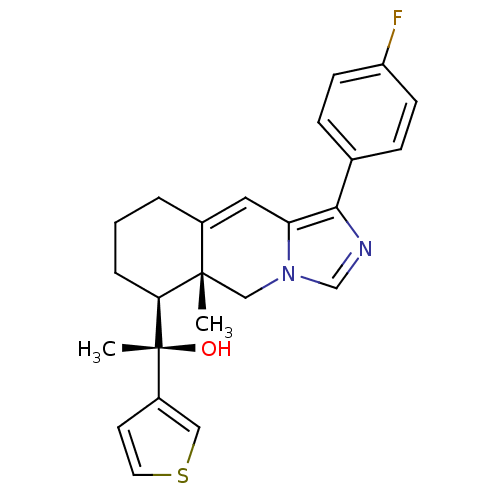
((S)-1-((5aR,6S)-1-(4-Fluorophenyl)-5a-methyl-5,5a,...)Show SMILES C[C@](O)([C@H]1CCCC2=Cc3c(ncn3C[C@]12C)-c1ccc(F)cc1)c1ccsc1 |r,t:7| Show InChI InChI=1S/C24H25FN2OS/c1-23-14-27-15-26-22(16-6-8-19(25)9-7-16)20(27)12-17(23)4-3-5-21(23)24(2,28)18-10-11-29-13-18/h6-13,15,21,28H,3-5,14H2,1-2H3/t21-,23-,24+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Displacement of GS-red from human glucocorticoid receptor by fluorescent polarization assay |
J Med Chem 53: 1270-80 (2010)
Article DOI: 10.1021/jm901551w
BindingDB Entry DOI: 10.7270/Q2KS6SGW |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50440488
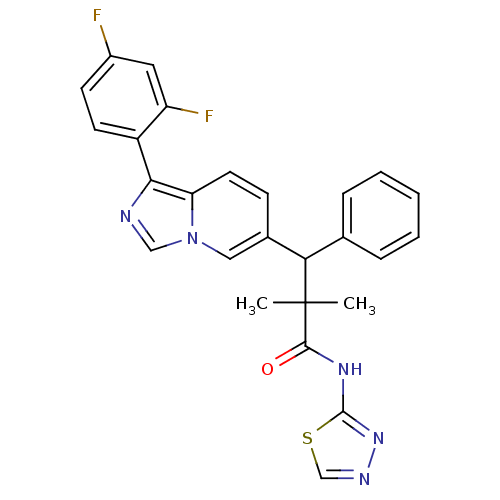
(CHEMBL2426147)Show SMILES CC(C)(C(c1ccccc1)c1ccc2c(ncn2c1)-c1ccc(F)cc1F)C(=O)Nc1nncs1 Show InChI InChI=1S/C26H21F2N5OS/c1-26(2,24(34)31-25-32-30-15-35-25)22(16-6-4-3-5-7-16)17-8-11-21-23(29-14-33(21)13-17)19-10-9-18(27)12-20(19)28/h3-15,22H,1-2H3,(H,31,32,34) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Binding affinity to glucocorticoid receptor-LBD (unknown origin) by fluorescence polarization assay |
Bioorg Med Chem Lett 23: 5571-4 (2013)
Article DOI: 10.1016/j.bmcl.2013.08.049
BindingDB Entry DOI: 10.7270/Q2QC04Z4 |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM30132
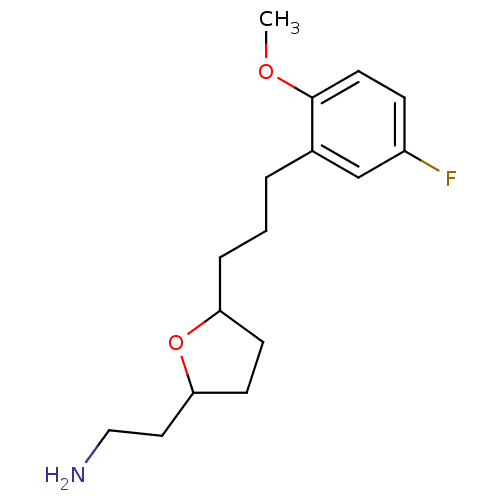
(CHEMBL450907 | tetrahydrofuranyl ethylamine, 15)Show InChI InChI=1S/C16H24FNO2/c1-19-16-8-5-13(17)11-12(16)3-2-4-14-6-7-15(20-14)9-10-18/h5,8,11,14-15H,2-4,6-7,9-10,18H2,1H3 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Human BioMolecular Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [125I]RTI55 from human SERT expressed in HEK cells |
Bioorg Med Chem 17: 2047-68 (2009)
Article DOI: 10.1016/j.bmc.2009.01.023
BindingDB Entry DOI: 10.7270/Q2057GV0 |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM30132

(CHEMBL450907 | tetrahydrofuranyl ethylamine, 15)Show InChI InChI=1S/C16H24FNO2/c1-19-16-8-5-13(17)11-12(16)3-2-4-14-6-7-15(20-14)9-10-18/h5,8,11,14-15H,2-4,6-7,9-10,18H2,1H3 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Human BioMolecular Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [125I]RTI55 from human SERT expressed in HEK cells |
Bioorg Med Chem 17: 2047-68 (2009)
Article DOI: 10.1016/j.bmc.2009.01.023
BindingDB Entry DOI: 10.7270/Q2057GV0 |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM18207

((1R,2S,10S,11S,13R,14R,15S,17S)-1-fluoro-14,17-dih...)Show SMILES [H][C@@]12C[C@@H](C)[C@](O)(C(=O)CO)[C@@]1(C)C[C@H](O)[C@@]1(F)[C@@]2([H])CCC2=CC(=O)C=C[C@]12C |c:28,t:24| Show InChI InChI=1S/C22H29FO5/c1-12-8-16-15-5-4-13-9-14(25)6-7-19(13,2)21(15,23)17(26)10-20(16,3)22(12,28)18(27)11-24/h6-7,9,12,15-17,24,26,28H,4-5,8,10-11H2,1-3H3/t12-,15+,16+,17+,19+,20+,21+,22+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Binding affinity to glucocorticoid receptor-LBD (unknown origin) by fluorescence polarization assay |
Bioorg Med Chem Lett 23: 5571-4 (2013)
Article DOI: 10.1016/j.bmcl.2013.08.049
BindingDB Entry DOI: 10.7270/Q2QC04Z4 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50440230

(CHEMBL2426639)Show SMILES CC(C)(C(Cc1ccccc1)c1ccc2n(ncc2c1)-c1ccc(F)cc1)C(=O)Nc1nncs1 Show InChI InChI=1S/C27H24FN5OS/c1-27(2,25(34)31-26-32-29-17-35-26)23(14-18-6-4-3-5-7-18)19-8-13-24-20(15-19)16-30-33(24)22-11-9-21(28)10-12-22/h3-13,15-17,23H,14H2,1-2H3,(H,31,32,34) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ironwood Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity to glucocorticoid receptor (unknown origin) by fluorescence polarization assay |
Bioorg Med Chem Lett 23: 5442-7 (2013)
Article DOI: 10.1016/j.bmcl.2013.06.089
BindingDB Entry DOI: 10.7270/Q29888FB |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50348410
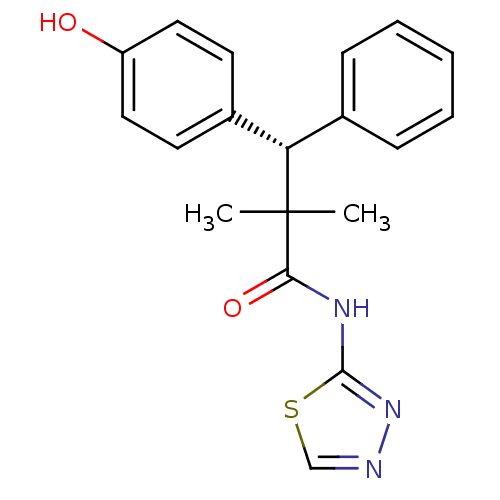
(CHEMBL1801004)Show SMILES CC(C)([C@@H](c1ccccc1)c1ccc(O)cc1)C(=O)Nc1nncs1 |r| Show InChI InChI=1S/C19H19N3O2S/c1-19(2,17(24)21-18-22-20-12-25-18)16(13-6-4-3-5-7-13)14-8-10-15(23)11-9-14/h3-12,16,23H,1-2H3,(H,21,22,24)/t16-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ironwood Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity to glucocorticoid receptor (unknown origin) by fluorescence polarization assay |
Bioorg Med Chem Lett 23: 5442-7 (2013)
Article DOI: 10.1016/j.bmcl.2013.06.089
BindingDB Entry DOI: 10.7270/Q29888FB |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM18207

((1R,2S,10S,11S,13R,14R,15S,17S)-1-fluoro-14,17-dih...)Show SMILES [H][C@@]12C[C@@H](C)[C@](O)(C(=O)CO)[C@@]1(C)C[C@H](O)[C@@]1(F)[C@@]2([H])CCC2=CC(=O)C=C[C@]12C |c:28,t:24| Show InChI InChI=1S/C22H29FO5/c1-12-8-16-15-5-4-13-9-14(25)6-7-19(13,2)21(15,23)17(26)10-20(16,3)22(12,28)18(27)11-24/h6-7,9,12,15-17,24,26,28H,4-5,8,10-11H2,1-3H3/t12-,15+,16+,17+,19+,20+,21+,22+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Displacement of GS-red from GRapha by fluorescence polarization assay |
J Med Chem 53: 8241-51 (2010)
Article DOI: 10.1021/jm100957a
BindingDB Entry DOI: 10.7270/Q2HX1DPW |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50348401
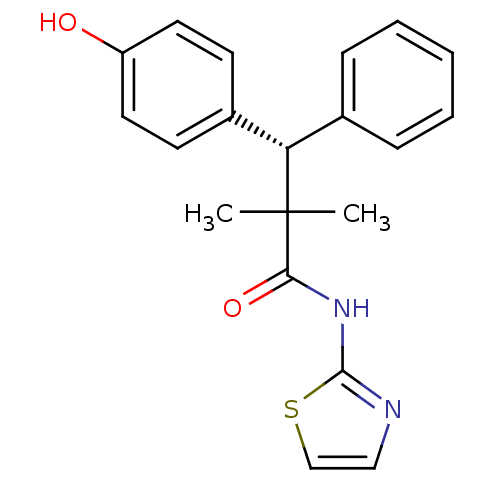
(CHEMBL1801012)Show SMILES CC(C)([C@@H](c1ccccc1)c1ccc(O)cc1)C(=O)Nc1nccs1 |r| Show InChI InChI=1S/C20H20N2O2S/c1-20(2,18(24)22-19-21-12-13-25-19)17(14-6-4-3-5-7-14)15-8-10-16(23)11-9-15/h3-13,17,23H,1-2H3,(H,21,22,24)/t17-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Displacement of GS-red from GRapha by fluorescence polarization assay |
J Med Chem 53: 8241-51 (2010)
Article DOI: 10.1021/jm100957a
BindingDB Entry DOI: 10.7270/Q2HX1DPW |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50348410

(CHEMBL1801004)Show SMILES CC(C)([C@@H](c1ccccc1)c1ccc(O)cc1)C(=O)Nc1nncs1 |r| Show InChI InChI=1S/C19H19N3O2S/c1-19(2,17(24)21-18-22-20-12-25-18)16(13-6-4-3-5-7-13)14-8-10-15(23)11-9-14/h3-12,16,23H,1-2H3,(H,21,22,24)/t16-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Displacement of GS-red from GRapha by fluorescence polarization assay |
J Med Chem 53: 8241-51 (2010)
Article DOI: 10.1021/jm100957a
BindingDB Entry DOI: 10.7270/Q2HX1DPW |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM18207

((1R,2S,10S,11S,13R,14R,15S,17S)-1-fluoro-14,17-dih...)Show SMILES [H][C@@]12C[C@@H](C)[C@](O)(C(=O)CO)[C@@]1(C)C[C@H](O)[C@@]1(F)[C@@]2([H])CCC2=CC(=O)C=C[C@]12C |c:28,t:24| Show InChI InChI=1S/C22H29FO5/c1-12-8-16-15-5-4-13-9-14(25)6-7-19(13,2)21(15,23)17(26)10-20(16,3)22(12,28)18(27)11-24/h6-7,9,12,15-17,24,26,28H,4-5,8,10-11H2,1-3H3/t12-,15+,16+,17+,19+,20+,21+,22+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Displacement of GS-red from human glucocorticoid receptor by fluorescent polarization assay |
J Med Chem 53: 1270-80 (2010)
Article DOI: 10.1021/jm901551w
BindingDB Entry DOI: 10.7270/Q2KS6SGW |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM18207

((1R,2S,10S,11S,13R,14R,15S,17S)-1-fluoro-14,17-dih...)Show SMILES [H][C@@]12C[C@@H](C)[C@](O)(C(=O)CO)[C@@]1(C)C[C@H](O)[C@@]1(F)[C@@]2([H])CCC2=CC(=O)C=C[C@]12C |c:28,t:24| Show InChI InChI=1S/C22H29FO5/c1-12-8-16-15-5-4-13-9-14(25)6-7-19(13,2)21(15,23)17(26)10-20(16,3)22(12,28)18(27)11-24/h6-7,9,12,15-17,24,26,28H,4-5,8,10-11H2,1-3H3/t12-,15+,16+,17+,19+,20+,21+,22+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ironwood Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity to glucocorticoid receptor (unknown origin) by fluorescence polarization assay |
Bioorg Med Chem Lett 23: 5442-7 (2013)
Article DOI: 10.1016/j.bmcl.2013.06.089
BindingDB Entry DOI: 10.7270/Q29888FB |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM19190
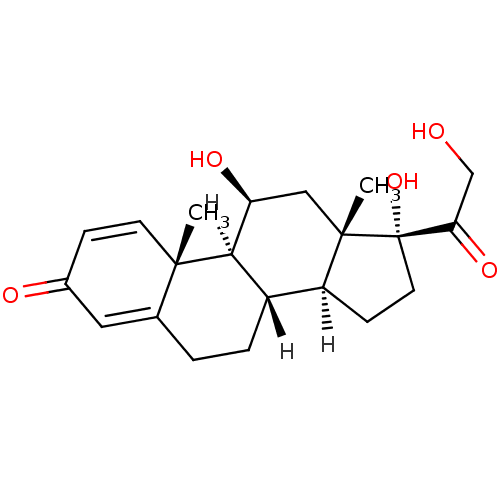
((1S,2R,10S,11S,14R,15S,17S)-14,17-dihydroxy-14-(2-...)Show SMILES [H][C@@]12CC[C@](O)(C(=O)CO)[C@@]1(C)C[C@H](O)[C@@]1([H])[C@@]2([H])CCC2=CC(=O)C=C[C@]12C |r,c:27,t:23| Show InChI InChI=1S/C21H28O5/c1-19-7-5-13(23)9-12(19)3-4-14-15-6-8-21(26,17(25)11-22)20(15,2)10-16(24)18(14)19/h5,7,9,14-16,18,22,24,26H,3-4,6,8,10-11H2,1-2H3/t14-,15-,16-,18+,19-,20-,21-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Displacement of GS-red from GRapha by fluorescence polarization assay |
J Med Chem 53: 8241-51 (2010)
Article DOI: 10.1021/jm100957a
BindingDB Entry DOI: 10.7270/Q2HX1DPW |
More data for this
Ligand-Target Pair | |
Aurora kinase C
(Homo sapiens (Human)) | BDBM50315769

(3-(4-(4-(2-(3-((dimethylamino)methyl)phenyl)-1H-py...)Show SMILES CCn1cc(c(n1)-c1ccc(NC(=O)N(C)C)cc1)-c1ccnc2[nH]c(cc12)-c1cccc(CN(C)C)c1 Show InChI InChI=1S/C30H33N7O/c1-6-37-19-26(28(34-37)21-10-12-23(13-11-21)32-30(38)36(4)5)24-14-15-31-29-25(24)17-27(33-29)22-9-7-8-20(16-22)18-35(2)3/h7-17,19H,6,18H2,1-5H3,(H,31,33)(H,32,38) | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Competitive inhibition of human Aurora C ATP binding site |
J Med Chem 53: 3973-4001 (2010)
Article DOI: 10.1021/jm901870q
BindingDB Entry DOI: 10.7270/Q27082CK |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50303811
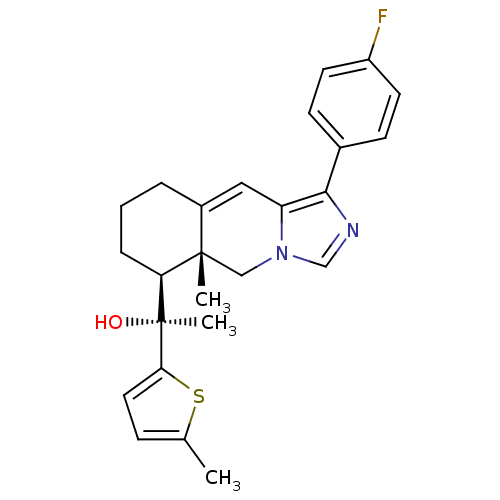
((S)-1-((5aR,6S)-1-(4-Fluorophenyl)-5a-methyl-5,5a,...)Show SMILES Cc1ccc(s1)[C@@](C)(O)[C@H]1CCCC2=Cc3c(ncn3C[C@]12C)-c1ccc(F)cc1 |r,t:14| Show InChI InChI=1S/C25H27FN2OS/c1-16-7-12-22(30-16)25(3,29)21-6-4-5-18-13-20-23(17-8-10-19(26)11-9-17)27-15-28(20)14-24(18,21)2/h7-13,15,21,29H,4-6,14H2,1-3H3/t21-,24-,25-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Displacement of GS-red from human glucocorticoid receptor by fluorescent polarization assay |
J Med Chem 53: 1270-80 (2010)
Article DOI: 10.1021/jm901551w
BindingDB Entry DOI: 10.7270/Q2KS6SGW |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50440233

(CHEMBL2426636)Show SMILES CCCC(c1ccc2n(ncc2c1)-c1ccc(F)cc1)C(C)(C)C(=O)Nc1nncs1 Show InChI InChI=1S/C23H24FN5OS/c1-4-5-19(23(2,3)21(30)27-22-28-25-14-31-22)15-6-11-20-16(12-15)13-26-29(20)18-9-7-17(24)8-10-18/h6-14,19H,4-5H2,1-3H3,(H,27,28,30) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ironwood Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity to glucocorticoid receptor (unknown origin) by fluorescence polarization assay |
Bioorg Med Chem Lett 23: 5442-7 (2013)
Article DOI: 10.1016/j.bmcl.2013.06.089
BindingDB Entry DOI: 10.7270/Q29888FB |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM19223
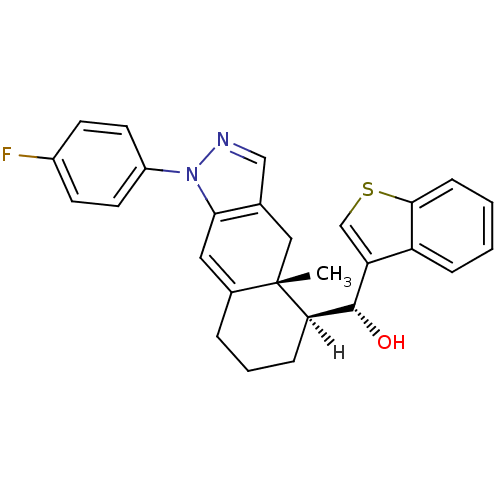
((R)-[(4aR,5S)-1-(4-fluorophenyl)-4a-methyl-1H,4H,4...)Show SMILES [H][C@@]1(CCCC2=Cc3c(C[C@]12C)cnn3-c1ccc(F)cc1)[C@@H](O)c1csc2ccccc12 |r,t:5| Show InChI InChI=1S/C27H25FN2OS/c1-27-14-17-15-29-30(20-11-9-19(28)10-12-20)24(17)13-18(27)5-4-7-23(27)26(31)22-16-32-25-8-3-2-6-21(22)25/h2-3,6,8-13,15-16,23,26,31H,4-5,7,14H2,1H3/t23-,26+,27+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Displacement of GS-red from human glucocorticoid receptor by fluorescent polarization assay |
J Med Chem 53: 1270-80 (2010)
Article DOI: 10.1021/jm901551w
BindingDB Entry DOI: 10.7270/Q2KS6SGW |
More data for this
Ligand-Target Pair | |
Aurora kinase B
(Homo sapiens (Human)) | BDBM13534

(CHEMBL572878 | N-[4-({4-[(3-methyl-1H-pyrazol-5-yl...)Show SMILES CN1CCN(CC1)c1cc(Nc2cc(C)n[nH]2)nc(Sc2ccc(NC(=O)C3CC3)cc2)n1 Show InChI InChI=1S/C23H28N8OS/c1-15-13-20(29-28-15)25-19-14-21(31-11-9-30(2)10-12-31)27-23(26-19)33-18-7-5-17(6-8-18)24-22(32)16-3-4-16/h5-8,13-14,16H,3-4,9-12H2,1-2H3,(H,24,32)(H2,25,26,27,28,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Competitive inhibition of Aurora B ATP binding site |
J Med Chem 53: 3973-4001 (2010)
Article DOI: 10.1021/jm901870q
BindingDB Entry DOI: 10.7270/Q27082CK |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50440214
(CHEMBL2426650)Show SMILES CC(C)(C(c1ccccc1)c1ccc2n(ncc2c1)-c1ccncc1)C(=O)Nc1nncs1 Show InChI InChI=1S/C25H22N6OS/c1-25(2,23(32)29-24-30-27-16-33-24)22(17-6-4-3-5-7-17)18-8-9-21-19(14-18)15-28-31(21)20-10-12-26-13-11-20/h3-16,22H,1-2H3,(H,29,30,32) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ironwood Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity to glucocorticoid receptor (unknown origin) by fluorescence polarization assay |
Bioorg Med Chem Lett 23: 5442-7 (2013)
Article DOI: 10.1016/j.bmcl.2013.06.089
BindingDB Entry DOI: 10.7270/Q29888FB |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50440490

(CHEMBL2426146)Show SMILES CC(C)(C(c1ccccc1)c1ccc2c(ncn2c1)-c1ccc(F)cc1F)C(=O)Nc1nccs1 Show InChI InChI=1S/C27H22F2N4OS/c1-27(2,25(34)32-26-30-12-13-35-26)23(17-6-4-3-5-7-17)18-8-11-22-24(31-16-33(22)15-18)20-10-9-19(28)14-21(20)29/h3-16,23H,1-2H3,(H,30,32,34) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Binding affinity to glucocorticoid receptor-LBD (unknown origin) by fluorescence polarization assay |
Bioorg Med Chem Lett 23: 5571-4 (2013)
Article DOI: 10.1016/j.bmcl.2013.08.049
BindingDB Entry DOI: 10.7270/Q2QC04Z4 |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50440501
(CHEMBL2426144)Show SMILES CC(C)(C(c1ccccc1)c1ccc2c(ncn2c1)-c1ccc(cc1)C#N)C(=O)Nc1nncs1 Show InChI InChI=1S/C27H22N6OS/c1-27(2,25(34)31-26-32-30-17-35-26)23(19-6-4-3-5-7-19)21-12-13-22-24(29-16-33(22)15-21)20-10-8-18(14-28)9-11-20/h3-13,15-17,23H,1-2H3,(H,31,32,34) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Binding affinity to glucocorticoid receptor-LBD (unknown origin) by fluorescence polarization assay |
Bioorg Med Chem Lett 23: 5571-4 (2013)
Article DOI: 10.1016/j.bmcl.2013.08.049
BindingDB Entry DOI: 10.7270/Q2QC04Z4 |
More data for this
Ligand-Target Pair | |
Hepatocyte growth factor receptor
(Homo sapiens (Human)) | BDBM50306682

((R)-3-(1-(2,6-dichloro-3-fluorophenyl)ethoxy)-5-(1...)Show SMILES C[C@@H](Oc1cc(cnc1N)-c1cnn(c1)C1CCNCC1)c1c(Cl)ccc(F)c1Cl |r| Show InChI InChI=1S/C21H22Cl2FN5O/c1-12(19-16(22)2-3-17(24)20(19)23)30-18-8-13(9-27-21(18)25)14-10-28-29(11-14)15-4-6-26-7-5-15/h2-3,8-12,15,26H,4-7H2,1H3,(H2,25,27)/t12-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University
Curated by ChEMBL
| Assay Description
Inhibition of c-Met (unknown origin) |
J Med Chem 62: 10927-10954 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00446
BindingDB Entry DOI: 10.7270/Q2S185WV |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Activated CDC42 kinase 1
(Homo sapiens (Human)) | BDBM50421286
(CHEMBL2087662)Show SMILES CN(C)C(=O)c1ccc(cc1)-c1[nH]c2ncnc(NC[C@@H]3CCCO3)c2c1-c1ccccc1 |r| Show InChI InChI=1S/C26H27N5O2/c1-31(2)26(32)19-12-10-18(11-13-19)23-21(17-7-4-3-5-8-17)22-24(28-16-29-25(22)30-23)27-15-20-9-6-14-33-20/h3-5,7-8,10-13,16,20H,6,9,14-15H2,1-2H3,(H2,27,28,29,30)/t20-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal His6-tagged ACK1 expressed in baculovirus infected Hi5 cells assessed as inhibition of autophosphorylation after 2 hrs by EL... |
Bioorg Med Chem Lett 22: 6212-7 (2012)
Article DOI: 10.1016/j.bmcl.2012.08.020
BindingDB Entry DOI: 10.7270/Q2VH5Q4X |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data