

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Voltage-dependent L-type calcium channel subunit alpha-1C/alpha-1D/alpha-1F/alpha-1S (Mus musculus) | BDBM50478489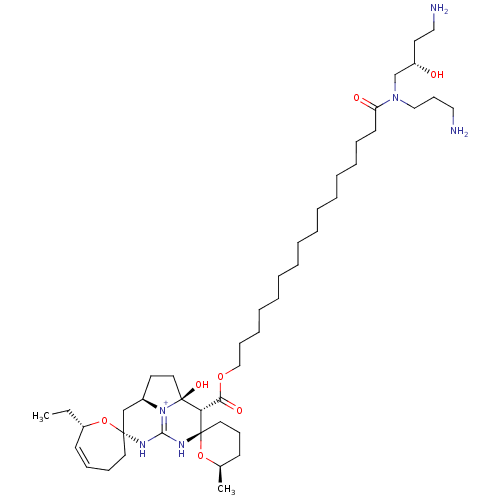 (CRAMBESCIDIN 816 HYDROCHLORIDE | Crambescidin 816 ...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Brussels Curated by ChEMBL | Assay Description Antagonist activity at voltage-dependent L-type calcium channel in mouse neurobalstoma-rat glioma NG 108-15 hybrid cells assessed as inhibition of KC... | J Nat Prod 56: 1007-15 (1993) Article DOI: 10.1021/np50097a004 BindingDB Entry DOI: 10.7270/Q2ZG6W1M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 3 (Homo sapiens (Human)) | BDBM50317647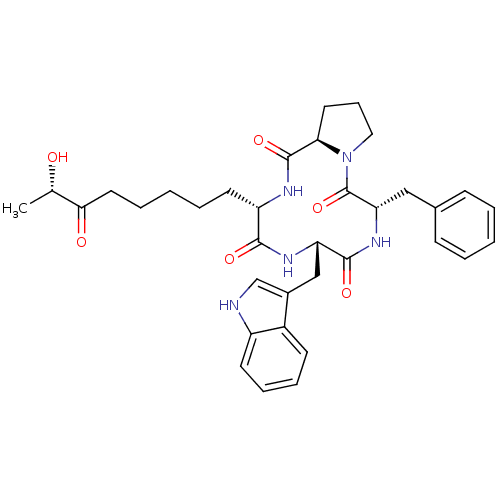 ((3S,6S,9S,14aR)-6-((1H-indol-3-yl)methyl)-9-benzyl...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Salerno Curated by ChEMBL | Assay Description Inhibition of full length HDAC3 expressed in HEK293 cells after 15 mins by fluorescence assay | Bioorg Med Chem 18: 3252-60 (2010) Article DOI: 10.1016/j.bmc.2010.03.022 BindingDB Entry DOI: 10.7270/Q2BC3ZP6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50317647 ((3S,6S,9S,14aR)-6-((1H-indol-3-yl)methyl)-9-benzyl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 68 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Salerno Curated by ChEMBL | Assay Description Inhibition of full length HDAC1 expressed in HEK293 cells after 15 mins by fluorescence assay | Bioorg Med Chem 18: 3252-60 (2010) Article DOI: 10.1016/j.bmc.2010.03.022 BindingDB Entry DOI: 10.7270/Q2BC3ZP6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50405790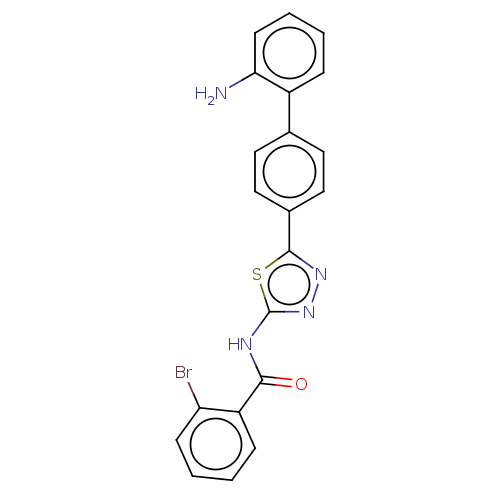 (CHEMBL5284121) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description Antagonist potency against carbachol induced inhibition of electrically stimulated guinea pig atria Muscarinic acetylcholine receptor | Citation and Details | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor alpha (Homo sapiens (Human)) | BDBM50540553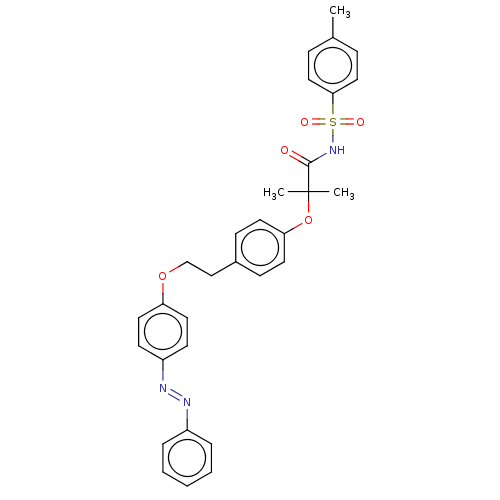 (CHEMBL4644726) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a | n/a |
"G. d'Annunzio" University of Chieti-Pescara Curated by ChEMBL | Assay Description Antagonist activity at GAL4-tagged human PPARalpha LBD expressed in human HepG2 cells assessed as inhibition of Wy14,643-induced receptor transactiva... | ACS Med Chem Lett 11: 624-632 (2020) Article DOI: 10.1021/acsmedchemlett.9b00666 BindingDB Entry DOI: 10.7270/Q2765JW1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 2 (Homo sapiens (Human)) | BDBM50317647 ((3S,6S,9S,14aR)-6-((1H-indol-3-yl)methyl)-9-benzyl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 179 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Salerno Curated by ChEMBL | Assay Description Inhibition of full length HDAC2 expressed in HEK293 cells after 15 mins by fluorescence assay | Bioorg Med Chem 18: 3252-60 (2010) Article DOI: 10.1016/j.bmc.2010.03.022 BindingDB Entry DOI: 10.7270/Q2BC3ZP6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50464480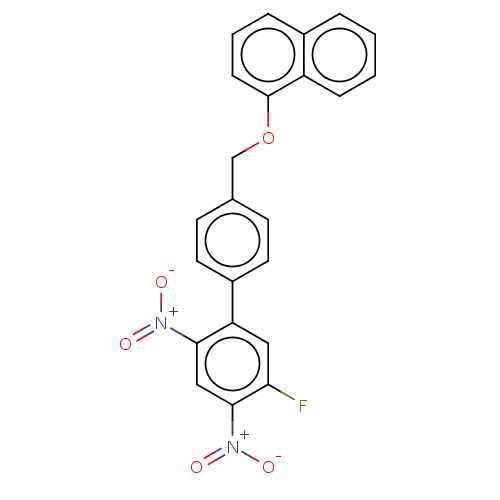 (CHEMBL4282628) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Salerno Curated by ChEMBL | Assay Description Inhibition of mPGES1 in IL-1beta-stimulated human A549 cell microsomes using PGH2 as substrate assessed as reduction in PGE2 production preincubated ... | Eur J Med Chem 143: 1419-1427 (2018) Article DOI: 10.1016/j.ejmech.2017.10.039 BindingDB Entry DOI: 10.7270/Q2G44SXG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50405760 (CHEMBL5284133) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description Antagonist potency against muscarinic receptors was assed by antagonism of carbachol induced inhibition of electrically stimulated guinea pig atria | Citation and Details | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor alpha (Homo sapiens (Human)) | BDBM50540555 (CHEMBL4642691) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 210 | n/a | n/a | n/a | n/a | n/a | n/a |
"G. d'Annunzio" University of Chieti-Pescara Curated by ChEMBL | Assay Description Antagonist activity at GAL4-tagged human PPARalpha LBD expressed in human HepG2 cells assessed as inhibition of Wy14,643-induced receptor transactiva... | ACS Med Chem Lett 11: 624-632 (2020) Article DOI: 10.1021/acsmedchemlett.9b00666 BindingDB Entry DOI: 10.7270/Q2765JW1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor alpha (Homo sapiens (Human)) | BDBM50540566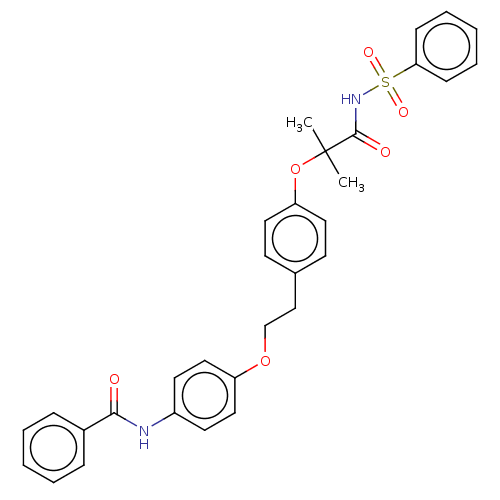 (CHEMBL4636567) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 240 | n/a | n/a | n/a | n/a | n/a | n/a |
"G. d'Annunzio" University of Chieti-Pescara Curated by ChEMBL | Assay Description Antagonist activity at GAL4-tagged human PPARalpha LBD expressed in human HepG2 cells assessed as inhibition of Wy14,643-induced receptor transactiva... | ACS Med Chem Lett 11: 624-632 (2020) Article DOI: 10.1021/acsmedchemlett.9b00666 BindingDB Entry DOI: 10.7270/Q2765JW1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50464484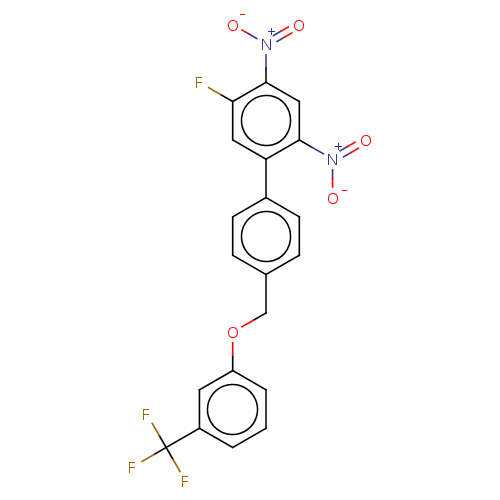 (CHEMBL4290910) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 260 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Salerno Curated by ChEMBL | Assay Description Inhibition of mPGES1 in IL-1beta-stimulated human A549 cell microsomes using PGH2 as substrate assessed as reduction in PGE2 production preincubated ... | Eur J Med Chem 143: 1419-1427 (2018) Article DOI: 10.1016/j.ejmech.2017.10.039 BindingDB Entry DOI: 10.7270/Q2G44SXG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor alpha (Homo sapiens (Human)) | BDBM50540554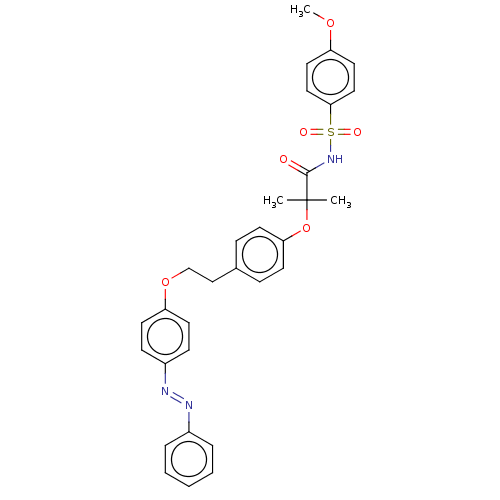 (CHEMBL4636810) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 330 | n/a | n/a | n/a | n/a | n/a | n/a |
"G. d'Annunzio" University of Chieti-Pescara Curated by ChEMBL | Assay Description Antagonist activity at GAL4-tagged human PPARalpha LBD expressed in human HepG2 cells assessed as inhibition of Wy14,643-induced receptor transactiva... | ACS Med Chem Lett 11: 624-632 (2020) Article DOI: 10.1021/acsmedchemlett.9b00666 BindingDB Entry DOI: 10.7270/Q2765JW1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50339570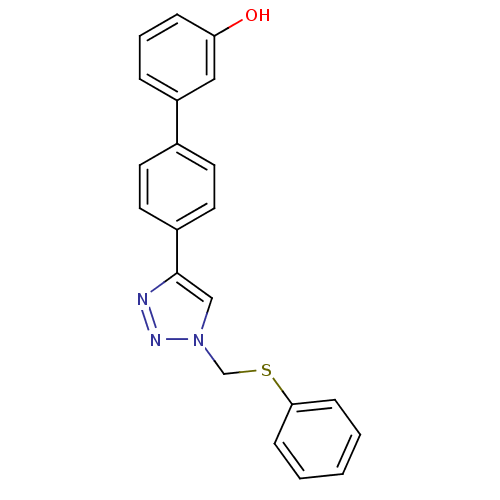 (4'-(1-Phenylsulfanylmethyl-1H-[1,2,3]triazol-4-yl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Salerno Curated by ChEMBL | Assay Description Inhibition of 5-lipoxygenase in human neutrophils | J Med Chem 54: 1565-75 (2011) Article DOI: 10.1021/jm101238d BindingDB Entry DOI: 10.7270/Q2SN098M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50061980 (CHEMBL3394323) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 410 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Salerno Curated by ChEMBL | Assay Description Inhibition of mPGES-1 in interleukin-1beta-stimulated human A549 cells microsomal membranes assessed as reduction in PGE2 formation incubated for 15 ... | ACS Med Chem Lett 6: 187-91 (2015) Article DOI: 10.1021/ml500433j BindingDB Entry DOI: 10.7270/Q2474CJH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50464486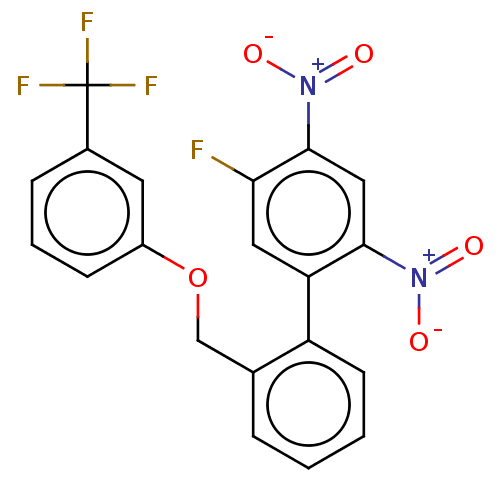 (CHEMBL4293214) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 540 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Salerno Curated by ChEMBL | Assay Description Inhibition of mPGES1 in IL-1beta-stimulated human A549 cell microsomes using PGH2 as substrate assessed as reduction in PGE2 production preincubated ... | Eur J Med Chem 143: 1419-1427 (2018) Article DOI: 10.1016/j.ejmech.2017.10.039 BindingDB Entry DOI: 10.7270/Q2G44SXG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50339571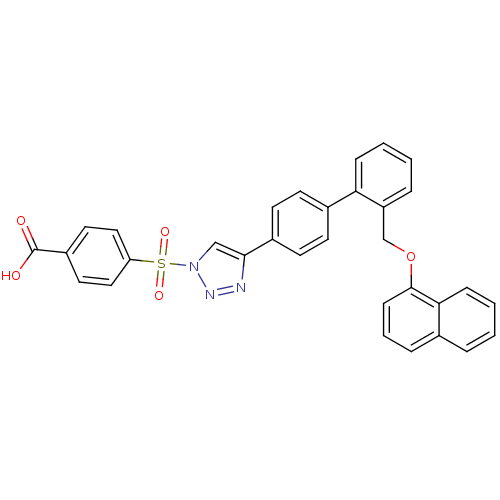 (4-{4-[2'-(Naphthalen-1-yloxymethyl)-biphenyl-4-yl]...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Salerno Curated by ChEMBL | Assay Description Inhibition of 5-lipoxygenase in human neutrophils | J Med Chem 54: 1565-75 (2011) Article DOI: 10.1021/jm101238d BindingDB Entry DOI: 10.7270/Q2SN098M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50464479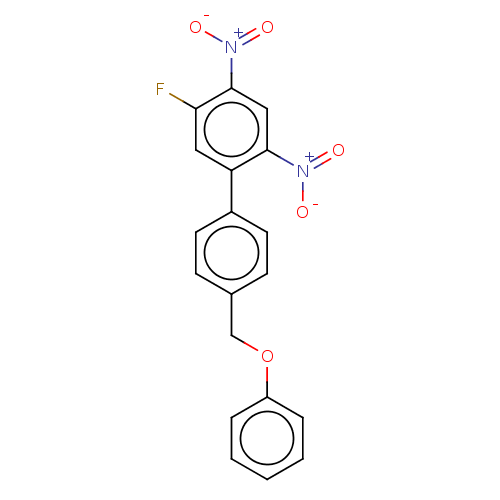 (CHEMBL4283041) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Salerno Curated by ChEMBL | Assay Description Inhibition of mPGES1 in IL-1beta-stimulated human A549 cell microsomes using PGH2 as substrate assessed as reduction in PGE2 production preincubated ... | Eur J Med Chem 143: 1419-1427 (2018) Article DOI: 10.1016/j.ejmech.2017.10.039 BindingDB Entry DOI: 10.7270/Q2G44SXG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor alpha (Homo sapiens (Human)) | BDBM50155330 (CHEMBL3781890) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 650 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£"G. d'Annunzio" Curated by ChEMBL | Assay Description Antagonist activity at PPARalpha (unknown origin) expressed in HEK293A cells assessed as inhibition of GW7647-induced transactivation after 16 to 18 ... | Eur J Med Chem 114: 191-200 (2016) Article DOI: 10.1016/j.ejmech.2016.02.064 BindingDB Entry DOI: 10.7270/Q2H9972K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50386165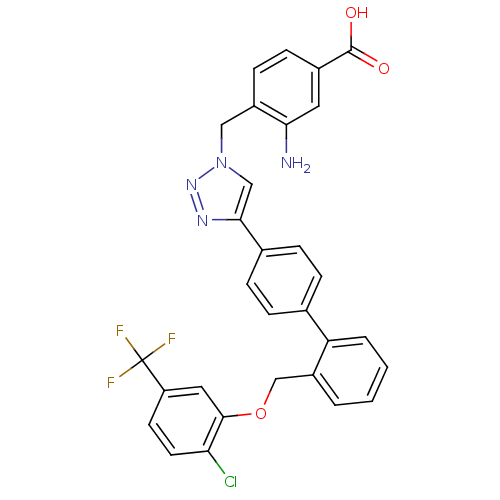 (CHEMBL2042365) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 680 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Salerno Curated by ChEMBL | Assay Description Inhibition of mPGES1 in IL-1beta-stimulated human A549 cell assessed as inhibition of PGE2 production preincubated for 15 mins before substrate addit... | Eur J Med Chem 54: 311-23 (2012) Article DOI: 10.1016/j.ejmech.2012.05.014 BindingDB Entry DOI: 10.7270/Q21J9BTR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor alpha (Homo sapiens (Human)) | BDBM50155326 (CHEMBL3780463) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 680 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£"G. d'Annunzio" Curated by ChEMBL | Assay Description Antagonist activity at PPARalpha (unknown origin) expressed in HEK293A cells assessed as inhibition of GW7647-induced transactivation after 16 to 18 ... | Eur J Med Chem 114: 191-200 (2016) Article DOI: 10.1016/j.ejmech.2016.02.064 BindingDB Entry DOI: 10.7270/Q2H9972K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50539779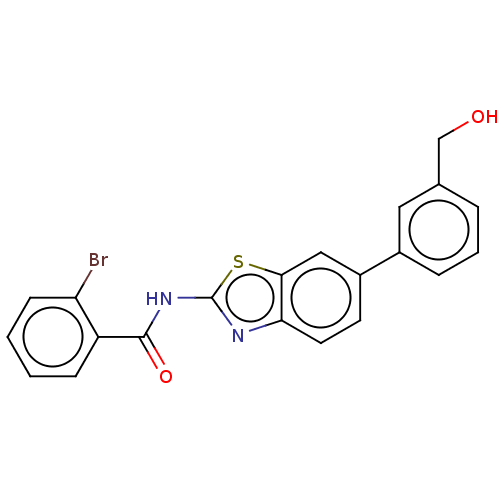 (CHEMBL4647475) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Salerno Curated by ChEMBL | Assay Description Inhibition of mPGES1 in human A549 cells assessed as reduction in IL-1beta induced PGE2 release using PGH2 as substrate preincubated for 15 mins foll... | ACS Med Chem Lett 11: 783-789 (2020) Article DOI: 10.1021/acsmedchemlett.9b00618 BindingDB Entry DOI: 10.7270/Q2Z32361 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor alpha (Homo sapiens (Human)) | BDBM50155332 (CHEMBL3781465) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£"G. d'Annunzio" Curated by ChEMBL | Assay Description Antagonist activity at PPARalpha (unknown origin) expressed in HEK293A cells assessed as inhibition of GW7647-induced transactivation after 16 to 18 ... | Eur J Med Chem 114: 191-200 (2016) Article DOI: 10.1016/j.ejmech.2016.02.064 BindingDB Entry DOI: 10.7270/Q2H9972K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50339571 (4-{4-[2'-(Naphthalen-1-yloxymethyl)-biphenyl-4-yl]...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Salerno Curated by ChEMBL | Assay Description Inhibition of human recombinant 5-lipooxygenase expressed in Escherichia coli MV1190 by cell free assay | J Med Chem 54: 1565-75 (2011) Article DOI: 10.1021/jm101238d BindingDB Entry DOI: 10.7270/Q2SN098M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor alpha (Homo sapiens (Human)) | BDBM50155329 (CHEMBL3779912) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 820 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£"G. d'Annunzio" Curated by ChEMBL | Assay Description Antagonist activity at PPARalpha (unknown origin) expressed in HEK293A cells assessed as inhibition of GW7647-induced transactivation after 16 to 18 ... | Eur J Med Chem 114: 191-200 (2016) Article DOI: 10.1016/j.ejmech.2016.02.064 BindingDB Entry DOI: 10.7270/Q2H9972K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor alpha (Homo sapiens (Human)) | BDBM50155324 (CHEMBL3780894) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 850 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£"G. d'Annunzio" Curated by ChEMBL | Assay Description Antagonist activity at PPARalpha (unknown origin) expressed in HEK293A cells assessed as inhibition of GW7647-induced transactivation after 16 to 18 ... | Eur J Med Chem 114: 191-200 (2016) Article DOI: 10.1016/j.ejmech.2016.02.064 BindingDB Entry DOI: 10.7270/Q2H9972K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50339572 (4'-(1-Phenylsulfanylmethyl-1H-[1,2,3]triazol-4-yl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 900 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Salerno Curated by ChEMBL | Assay Description Inhibition of 5-lipoxygenase in human neutrophils | J Med Chem 54: 1565-75 (2011) Article DOI: 10.1021/jm101238d BindingDB Entry DOI: 10.7270/Q2SN098M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50386165 (CHEMBL2042365) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 900 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Salerno Curated by ChEMBL | Assay Description Inhibition of human recombinant 5-lipoxygenase in cell-free system preincubated for 10 mins before substrate arachidonic acid addition measured after... | Eur J Med Chem 54: 311-23 (2012) Article DOI: 10.1016/j.ejmech.2012.05.014 BindingDB Entry DOI: 10.7270/Q21J9BTR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50390010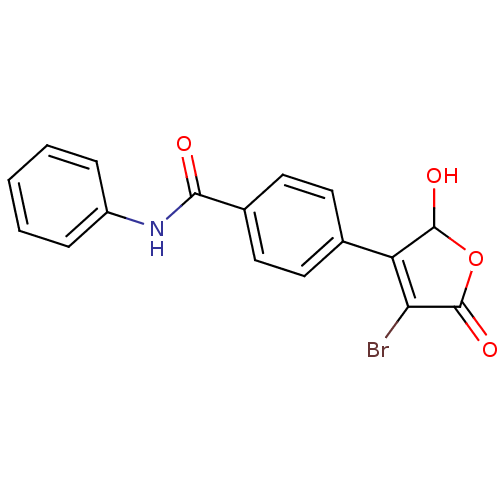 (CHEMBL2071516) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 900 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Salerno Curated by ChEMBL | Assay Description Inhibition of mPGES-1-mediated PGE2 formation in IL-1beta-stimulated human A549 cells preincubated for 10 mins measured after 1 min by RP-HPLC analys... | Bioorg Med Chem 20: 5012-6 (2012) Article DOI: 10.1016/j.bmc.2012.06.032 BindingDB Entry DOI: 10.7270/Q2GF0VKD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50386164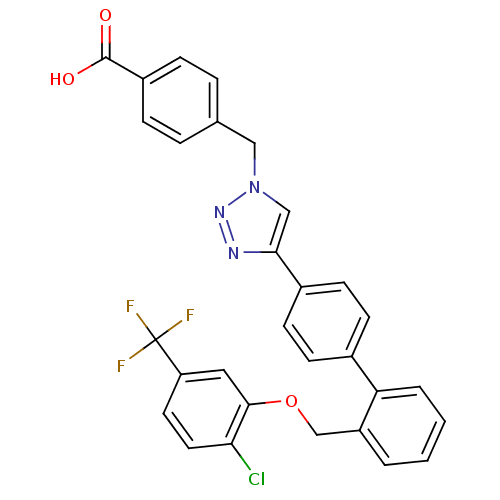 (CHEMBL2042366) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Salerno Curated by ChEMBL | Assay Description Inhibition of mPGES1 in IL-1beta-stimulated human A549 cell assessed as inhibition of PGE2 production preincubated for 15 mins before substrate addit... | Eur J Med Chem 54: 311-23 (2012) Article DOI: 10.1016/j.ejmech.2012.05.014 BindingDB Entry DOI: 10.7270/Q21J9BTR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor alpha (Homo sapiens (Human)) | BDBM50540556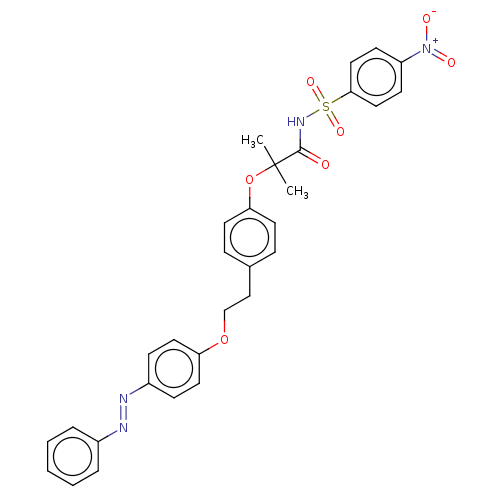 (CHEMBL4636520) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
"G. d'Annunzio" University of Chieti-Pescara Curated by ChEMBL | Assay Description Antagonist activity at GAL4-tagged human PPARalpha LBD expressed in human HepG2 cells assessed as inhibition of Wy14,643-induced receptor transactiva... | ACS Med Chem Lett 11: 624-632 (2020) Article DOI: 10.1021/acsmedchemlett.9b00666 BindingDB Entry DOI: 10.7270/Q2765JW1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50386154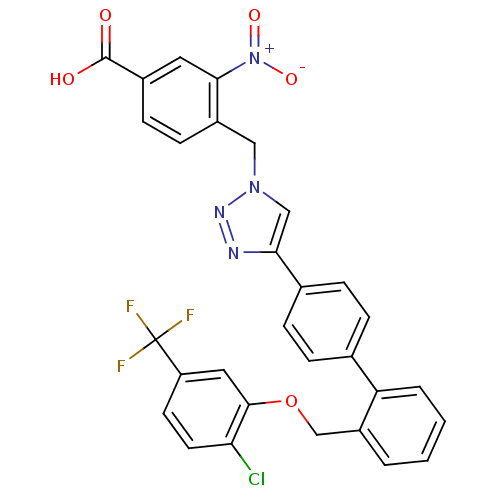 (CHEMBL2042361) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Salerno Curated by ChEMBL | Assay Description Inhibition of mPGES1 in IL-1beta-stimulated human A549 cell assessed as inhibition of PGE2 production preincubated for 15 mins before substrate addit... | Eur J Med Chem 54: 311-23 (2012) Article DOI: 10.1016/j.ejmech.2012.05.014 BindingDB Entry DOI: 10.7270/Q21J9BTR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50386163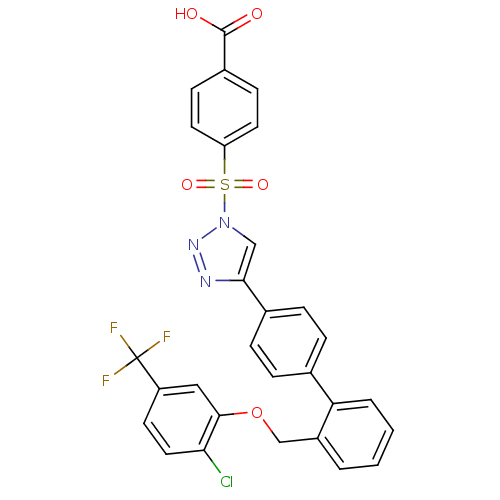 (CHEMBL2042367) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Salerno Curated by ChEMBL | Assay Description Inhibition of 5-lipoxygenase in human neutrophil preincubated for 15 mins before substrate arachidonic acid addition measured after 10 mins by HPLC m... | Eur J Med Chem 54: 311-23 (2012) Article DOI: 10.1016/j.ejmech.2012.05.014 BindingDB Entry DOI: 10.7270/Q21J9BTR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor alpha (Homo sapiens (Human)) | BDBM50540552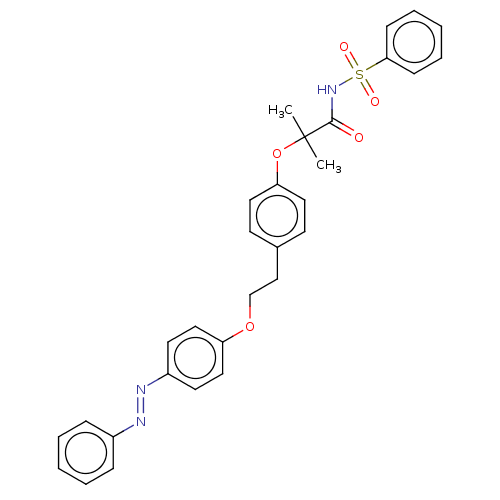 (CHEMBL4636990) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
"G. d'Annunzio" University of Chieti-Pescara Curated by ChEMBL | Assay Description Antagonist activity at GAL4-tagged human PPARalpha LBD expressed in human HepG2 cells assessed as inhibition of Wy14,643-induced receptor transactiva... | ACS Med Chem Lett 11: 624-632 (2020) Article DOI: 10.1021/acsmedchemlett.9b00666 BindingDB Entry DOI: 10.7270/Q2765JW1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific demethylase 6B (Homo sapiens (Human)) | BDBM50535443 (CHEMBL4591907) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Biomolecular Chemistry (ICB) Curated by ChEMBL | Assay Description Inhibition of recombinant human JMJD3 expressed in baculovirus infected Sf9 cells using biotinylated histone H3 peptide as substrate incubated for 15... | ACS Med Chem Lett 10: 601-605 (2019) Article DOI: 10.1021/acsmedchemlett.8b00589 BindingDB Entry DOI: 10.7270/Q2GF0Z01 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Voltage-dependent L-type calcium channel subunit alpha-1F (Mus musculus) | BDBM50101817 (Adalat | Adalat Cc | Afeditab Cr | BAY-A-1040 | CH...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Brussels Curated by ChEMBL | Assay Description Antagonist activity at voltage-dependent L-type calcium channel in mouse neurobalstoma-rat glioma NG 108-15 hybrid cells assessed as inhibition of KC... | J Nat Prod 56: 1007-15 (1993) Article DOI: 10.1021/np50097a004 BindingDB Entry DOI: 10.7270/Q2ZG6W1M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50539778 (CHEMBL4636106) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Salerno Curated by ChEMBL | Assay Description Inhibition of mPGES1 in human A549 cells assessed as reduction in IL-1beta induced PGE2 release using PGH2 as substrate preincubated for 15 mins foll... | ACS Med Chem Lett 11: 783-789 (2020) Article DOI: 10.1021/acsmedchemlett.9b00618 BindingDB Entry DOI: 10.7270/Q2Z32361 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50061981 (CHEMBL3394322) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Salerno Curated by ChEMBL | Assay Description Inhibition of mPGES-1 in interleukin-1beta-stimulated human A549 cells microsomal membranes assessed as reduction in PGE2 formation incubated for 15 ... | ACS Med Chem Lett 6: 187-91 (2015) Article DOI: 10.1021/ml500433j BindingDB Entry DOI: 10.7270/Q2474CJH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50386164 (CHEMBL2042366) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.47E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Salerno Curated by ChEMBL | Assay Description Inhibition of human recombinant 5-lipoxygenase in cell-free system preincubated for 10 mins before substrate arachidonic acid addition measured after... | Eur J Med Chem 54: 311-23 (2012) Article DOI: 10.1016/j.ejmech.2012.05.014 BindingDB Entry DOI: 10.7270/Q21J9BTR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor alpha (Homo sapiens (Human)) | BDBM50540557 (CHEMBL4642679) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
"G. d'Annunzio" University of Chieti-Pescara Curated by ChEMBL | Assay Description Antagonist activity at GAL4-tagged human PPARalpha LBD expressed in human HepG2 cells assessed as inhibition of Wy14,643-induced receptor transactiva... | ACS Med Chem Lett 11: 624-632 (2020) Article DOI: 10.1021/acsmedchemlett.9b00666 BindingDB Entry DOI: 10.7270/Q2765JW1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor alpha (Homo sapiens (Human)) | BDBM50540571 (CHEMBL4632967) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.52E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
"G. d'Annunzio" University of Chieti-Pescara Curated by ChEMBL | Assay Description Antagonist activity at GAL4-tagged human PPARalpha LBD expressed in human HepG2 cells assessed as inhibition of Wy14,643-induced receptor transactiva... | ACS Med Chem Lett 11: 624-632 (2020) Article DOI: 10.1021/acsmedchemlett.9b00666 BindingDB Entry DOI: 10.7270/Q2765JW1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50464485 (CHEMBL4279158) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.64E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Salerno Curated by ChEMBL | Assay Description Inhibition of mPGES1 in IL-1beta-stimulated human A549 cell microsomes using PGH2 as substrate assessed as reduction in PGE2 production preincubated ... | Eur J Med Chem 143: 1419-1427 (2018) Article DOI: 10.1016/j.ejmech.2017.10.039 BindingDB Entry DOI: 10.7270/Q2G44SXG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor alpha (Homo sapiens (Human)) | BDBM50540568 (CHEMBL4634884) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
"G. d'Annunzio" University of Chieti-Pescara Curated by ChEMBL | Assay Description Antagonist activity at GAL4-tagged human PPARalpha LBD expressed in human HepG2 cells assessed as inhibition of Wy14,643-induced receptor transactiva... | ACS Med Chem Lett 11: 624-632 (2020) Article DOI: 10.1021/acsmedchemlett.9b00666 BindingDB Entry DOI: 10.7270/Q2765JW1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50339573 (4'-[1-(2-o-Tolyl-ethyl)-1H-[1,2,3]triazol-4-yl]-bi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Salerno Curated by ChEMBL | Assay Description Inhibition of 5-lipoxygenase in human neutrophils | J Med Chem 54: 1565-75 (2011) Article DOI: 10.1021/jm101238d BindingDB Entry DOI: 10.7270/Q2SN098M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50405791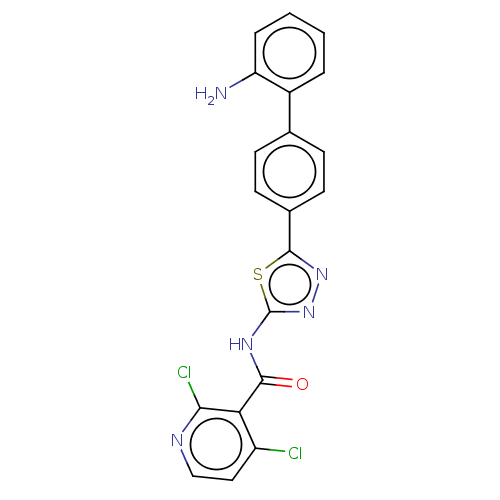 (CHEMBL5285589) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description Antagonist potency against carbachol induced inhibition of electrically stimulated guinea pig atria Muscarinic acetylcholine receptor | Citation and Details | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50539781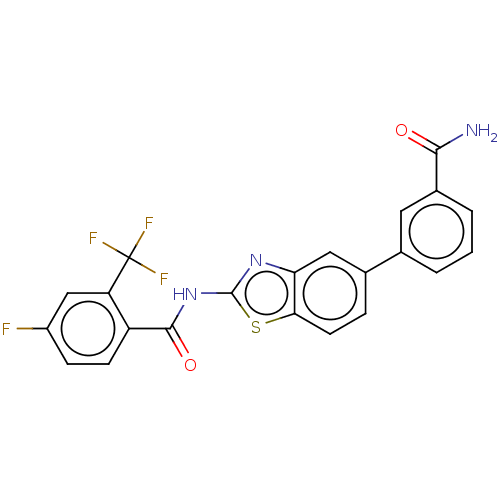 (CHEMBL4647634) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Salerno Curated by ChEMBL | Assay Description Inhibition of mPGES1 in human A549 cells assessed as reduction in IL-1beta induced PGE2 release using PGH2 as substrate preincubated for 15 mins foll... | ACS Med Chem Lett 11: 783-789 (2020) Article DOI: 10.1021/acsmedchemlett.9b00618 BindingDB Entry DOI: 10.7270/Q2Z32361 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50386163 (CHEMBL2042367) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.96E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Salerno Curated by ChEMBL | Assay Description Inhibition of human recombinant 5-lipoxygenase in cell-free system preincubated for 10 mins before substrate arachidonic acid addition measured after... | Eur J Med Chem 54: 311-23 (2012) Article DOI: 10.1016/j.ejmech.2012.05.014 BindingDB Entry DOI: 10.7270/Q21J9BTR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM50317649 (6-((3S,6S,9S,14aR)-6-((1H-indol-3-yl)methyl)-9-ben...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Salerno Curated by ChEMBL | Assay Description Inhibition of full length HDAC6 expressed in HEK293 cells after 15 mins by fluorescence assay | Bioorg Med Chem 18: 3252-60 (2010) Article DOI: 10.1016/j.bmc.2010.03.022 BindingDB Entry DOI: 10.7270/Q2BC3ZP6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50386164 (CHEMBL2042366) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Salerno Curated by ChEMBL | Assay Description Inhibition of 5-lipoxygenase in human neutrophil preincubated for 15 mins before substrate arachidonic acid addition measured after 10 mins by HPLC m... | Eur J Med Chem 54: 311-23 (2012) Article DOI: 10.1016/j.ejmech.2012.05.014 BindingDB Entry DOI: 10.7270/Q21J9BTR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50386154 (CHEMBL2042361) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Salerno Curated by ChEMBL | Assay Description Inhibition of human recombinant 5-lipoxygenase in cell-free system preincubated for 10 mins before substrate arachidonic acid addition measured after... | Eur J Med Chem 54: 311-23 (2012) Article DOI: 10.1016/j.ejmech.2012.05.014 BindingDB Entry DOI: 10.7270/Q21J9BTR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50386165 (CHEMBL2042365) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Salerno Curated by ChEMBL | Assay Description Inhibition of 5-lipoxygenase in human neutrophil preincubated for 15 mins before substrate arachidonic acid addition measured after 10 mins by HPLC m... | Eur J Med Chem 54: 311-23 (2012) Article DOI: 10.1016/j.ejmech.2012.05.014 BindingDB Entry DOI: 10.7270/Q21J9BTR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 236 total ) | Next | Last >> |