 Found 39 hits with Last Name = 'chen' and Initial = 'is'
Found 39 hits with Last Name = 'chen' and Initial = 'is' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Nitric oxide synthase, inducible
(Mus musculus (mouse)) | BDBM50359989

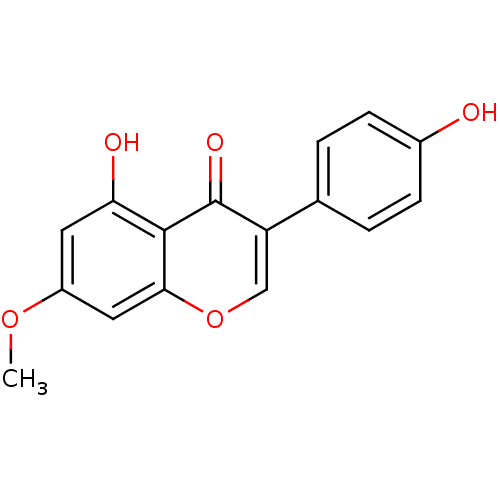
(CHEMBL1927943)Show SMILES Cc1coc2C[C@](C)(C=C)[C@@H]3[C@@H](OC(=O)C3=C)c12 |r| Show InChI InChI=1S/C15H16O3/c1-5-15(4)6-10-11(8(2)7-17-10)13-12(15)9(3)14(16)18-13/h5,7,12-13H,1,3,6H2,2,4H3/t12-,13-,15-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Kaohsiung Medical University
Curated by ChEMBL
| Assay Description
Inhibition of iNOS-mediated NO production in LPS-stimulated mouse RAW264.7 cells after 24 hrs by Griess reagent method |
J Nat Prod 74: 2489-96 (2011)
Article DOI: 10.1021/np100874f
BindingDB Entry DOI: 10.7270/Q2ST7Q8Z |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Mus musculus (mouse)) | BDBM50353025

(CHEMBL1821987)Show SMILES CC(C)CNC(=O)[C@@H]1C=C[C@@H]2C[C@H]3[C@H](Cc4ccc5OCOc5c4)[C@H]4C=C[C@@H]1[C@@H]2[C@@H]34 |r,c:8,27| Show InChI InChI=1S/C26H31NO3/c1-14(2)12-27-26(28)19-5-4-16-11-21-20(18-7-6-17(19)24(16)25(18)21)9-15-3-8-22-23(10-15)30-13-29-22/h3-8,10,14,16-21,24-25H,9,11-13H2,1-2H3,(H,27,28)/t16-,17+,18-,19-,20-,21+,24-,25-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9.59E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Kaohsiung Medical University
Curated by ChEMBL
| Assay Description
Inhibition of iNOS in mouse RAW264.7 cells assessed as anti-inflammatory activity by measuring maximum inhibition of LPS-induced nitric oxide product... |
J Nat Prod 74: 1875-80 (2011)
Article DOI: 10.1021/np200279r
BindingDB Entry DOI: 10.7270/Q2474B72 |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Mus musculus (mouse)) | BDBM50359990
(PRUNETIN)Show InChI InChI=1S/C16H12O5/c1-20-11-6-13(18)15-14(7-11)21-8-12(16(15)19)9-2-4-10(17)5-3-9/h2-8,17-18H,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.05E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Kaohsiung Medical University
Curated by ChEMBL
| Assay Description
Inhibition of iNOS-mediated NO production in LPS-stimulated mouse RAW264.7 cells after 24 hrs by Griess reagent method |
J Nat Prod 74: 2489-96 (2011)
Article DOI: 10.1021/np100874f
BindingDB Entry DOI: 10.7270/Q2ST7Q8Z |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Mus musculus (mouse)) | BDBM50353027

(CHEMBL1821990)Show SMILES CC(C)CNC(=O)[C@@H]1[C@H]2C[C@H]3[C@H](CCCc4ccc5OCOc5c4)[C@@H]4[C@H]3[C@@H]2C=C[C@H]14 |r,c:31,TLB:11:24:8.7:27.28,9:8:25.24:27.28,10:25:8.7:27.28,THB:11:10:26:7.29.24,5:7:25.24:27.28| Show InChI InChI=1S/C26H33NO3/c1-14(2)12-27-26(28)25-18-8-7-17-20(25)11-19-16(23(18)24(17)19)5-3-4-15-6-9-21-22(10-15)30-13-29-21/h6-10,14,16-20,23-25H,3-5,11-13H2,1-2H3,(H,27,28)/t16-,17+,18-,19-,20-,23-,24-,25-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.64E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Kaohsiung Medical University
Curated by ChEMBL
| Assay Description
Inhibition of iNOS in mouse RAW264.7 cells assessed as anti-inflammatory activity by measuring maximum inhibition of LPS-induced nitric oxide product... |
J Nat Prod 74: 1875-80 (2011)
Article DOI: 10.1021/np200279r
BindingDB Entry DOI: 10.7270/Q2474B72 |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Mus musculus (mouse)) | BDBM50359988
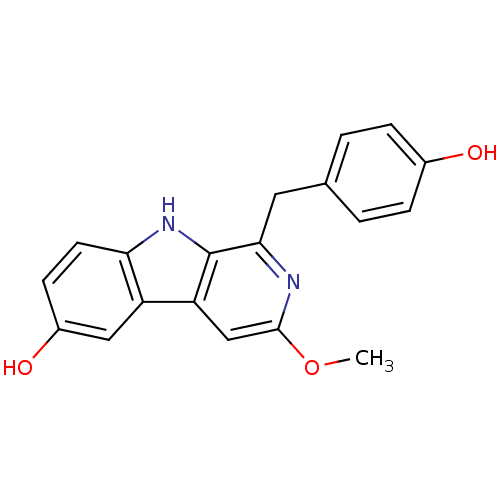
(CHEMBL1927942)Show InChI InChI=1S/C19H16N2O3/c1-24-18-10-15-14-9-13(23)6-7-16(14)21-19(15)17(20-18)8-11-2-4-12(22)5-3-11/h2-7,9-10,21-23H,8H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.84E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Kaohsiung Medical University
Curated by ChEMBL
| Assay Description
Inhibition of iNOS-mediated NO production in LPS-stimulated mouse RAW264.7 cells after 24 hrs by Griess reagent method |
J Nat Prod 74: 2489-96 (2011)
Article DOI: 10.1021/np100874f
BindingDB Entry DOI: 10.7270/Q2ST7Q8Z |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Mus musculus (mouse)) | BDBM50312648
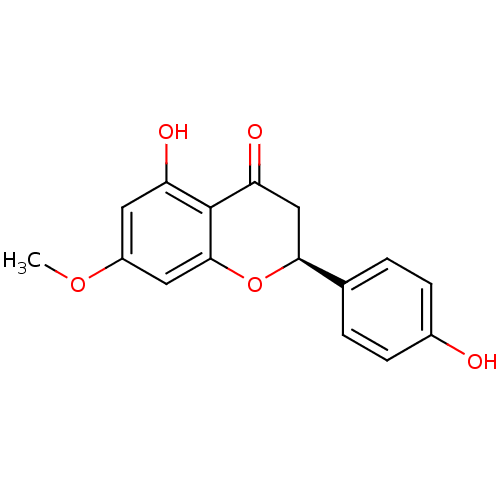
((2S)-5-hydroxy-2-(4-hydroxyphenyl)-7-methoxy-2,3-d...)Show SMILES COc1cc(O)c2C(=O)C[C@H](Oc2c1)c1ccc(O)cc1 |r| Show InChI InChI=1S/C16H14O5/c1-20-11-6-12(18)16-13(19)8-14(21-15(16)7-11)9-2-4-10(17)5-3-9/h2-7,14,17-18H,8H2,1H3/t14-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.96E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Kaohsiung Medical University
Curated by ChEMBL
| Assay Description
Inhibition of iNOS-mediated NO production in LPS-stimulated mouse RAW264.7 cells after 24 hrs by Griess reagent method |
J Nat Prod 74: 2489-96 (2011)
Article DOI: 10.1021/np100874f
BindingDB Entry DOI: 10.7270/Q2ST7Q8Z |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Mus musculus (mouse)) | BDBM50207159
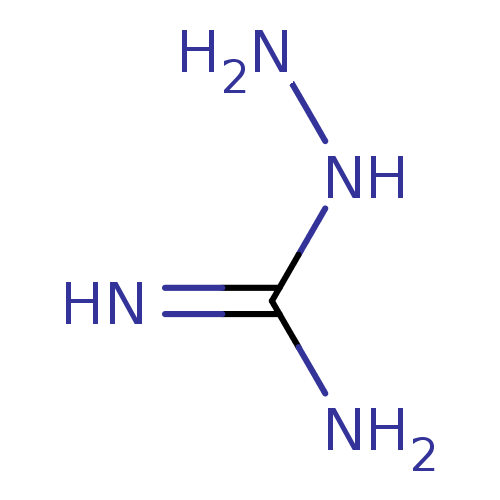
(2-aminoguanidine | 2-azanylguanidine | AMINOGUANID...)Show InChI InChI=1S/CH6N4/c2-1(3)5-4/h4H2,(H4,2,3,5) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
| DrugBank
MMDB
PDB
Article
PubMed
| n/a | n/a | 2.66E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Kaohsiung Medical University
Curated by ChEMBL
| Assay Description
Inhibition of iNOS in mouse RAW264.7 cells assessed as anti-inflammatory activity by measuring maximum inhibition of LPS-induced nitric oxide product... |
J Nat Prod 74: 1875-80 (2011)
Article DOI: 10.1021/np200279r
BindingDB Entry DOI: 10.7270/Q2474B72 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Nitric oxide synthase, inducible
(Mus musculus (mouse)) | BDBM50207159

(2-aminoguanidine | 2-azanylguanidine | AMINOGUANID...)Show InChI InChI=1S/CH6N4/c2-1(3)5-4/h4H2,(H4,2,3,5) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
| DrugBank
MMDB
PDB
Article
PubMed
| n/a | n/a | 2.85E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Kaohsiung Medical University
Curated by ChEMBL
| Assay Description
Inhibition of iNOS-mediated NO production in LPS-stimulated mouse RAW264.7 cells after 24 hrs by Griess reagent method |
J Nat Prod 74: 2489-96 (2011)
Article DOI: 10.1021/np100874f
BindingDB Entry DOI: 10.7270/Q2ST7Q8Z |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Protein kinase C gamma type
(Homo sapiens (Human)) | BDBM50292433
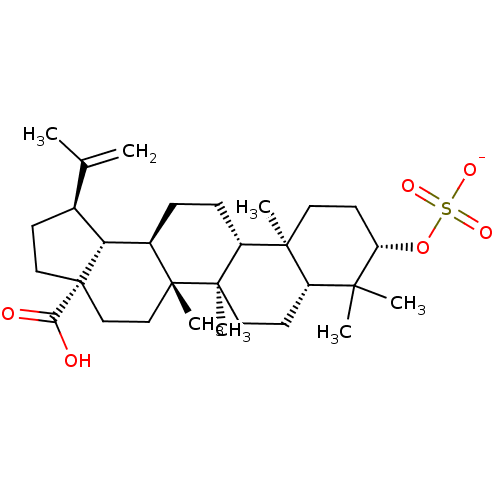
(CHEMBL502585 | betulinic acid 3-O-sulfonate potass...)Show SMILES CC(=C)[C@@H]1CC[C@@]2(CC[C@]3(C)[C@H](CC[C@@H]4[C@@]5(C)CC[C@H](OS([O-])(=O)=O)C(C)(C)[C@@H]5CC[C@@]34C)[C@@H]12)C(O)=O |r| Show InChI InChI=1S/C30H48O6S/c1-18(2)19-10-15-30(25(31)32)17-16-28(6)20(24(19)30)8-9-22-27(5)13-12-23(36-37(33,34)35)26(3,4)21(27)11-14-29(22,28)7/h19-24H,1,8-17H2,2-7H3,(H,31,32)(H,33,34,35)/p-1/t19-,20+,21-,22+,23-,24+,27-,28+,29+,30-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of PKC gamma |
J Nat Prod 57: 243-247 (1994)
Article DOI: 10.1021/np50104a008
BindingDB Entry DOI: 10.7270/Q2TT4R0Z |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Mus musculus (mouse)) | BDBM50353026

(CHEMBL1821989)Show SMILES OC(=O)\C=C\[C@@H]1[C@@H]2C[C@H]3[C@H](CCCCCc4ccc5OCOc5c4)[C@H]4[C@H]3[C@@H]2C=C[C@@H]14 |r,c:31,TLB:7:6:25.24:27.28,8:25:6.5:27.28,9:24:6.5:27.28,THB:4:5:25.24:27.28| Show InChI InChI=1S/C26H30O4/c27-24(28)11-9-16-18-7-8-19-20(16)13-21-17(25(18)26(19)21)5-3-1-2-4-15-6-10-22-23(12-15)30-14-29-22/h6-12,16-21,25-26H,1-5,13-14H2,(H,27,28)/b11-9+/t16-,17-,18-,19+,20-,21-,25+,26-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.96E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Kaohsiung Medical University
Curated by ChEMBL
| Assay Description
Inhibition of iNOS in mouse RAW264.7 cells assessed as anti-inflammatory activity by measuring maximum inhibition of LPS-induced nitric oxide product... |
J Nat Prod 74: 1875-80 (2011)
Article DOI: 10.1021/np200279r
BindingDB Entry DOI: 10.7270/Q2474B72 |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Mus musculus (mouse)) | BDBM50353024
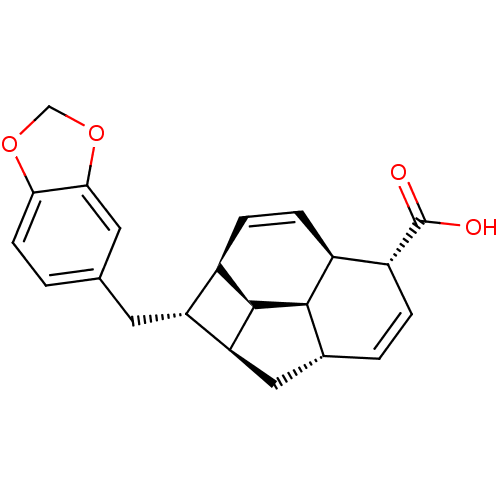
(CHEMBL1821986)Show SMILES OC(=O)[C@@H]1C=C[C@@H]2C[C@H]3[C@H](Cc4ccc5OCOc5c4)[C@H]4C=C[C@@H]1[C@@H]2[C@@H]34 |r,c:4,23| Show InChI InChI=1S/C22H22O4/c23-22(24)15-3-2-12-9-17-16(14-5-4-13(15)20(12)21(14)17)7-11-1-6-18-19(8-11)26-10-25-18/h1-6,8,12-17,20-21H,7,9-10H2,(H,23,24)/t12-,13+,14-,15-,16-,17+,20-,21-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.23E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Kaohsiung Medical University
Curated by ChEMBL
| Assay Description
Inhibition of iNOS in mouse RAW264.7 cells assessed as anti-inflammatory activity by measuring maximum inhibition of LPS-induced nitric oxide product... |
J Nat Prod 74: 1875-80 (2011)
Article DOI: 10.1021/np200279r
BindingDB Entry DOI: 10.7270/Q2474B72 |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Mus musculus (mouse)) | BDBM50353030
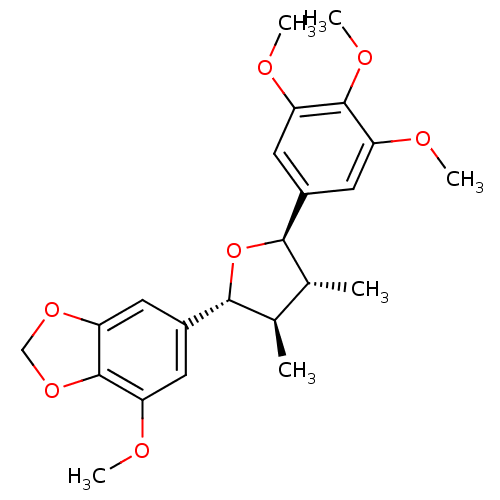
(CHEMBL1821993)Show SMILES COc1cc(cc2OCOc12)[C@@H]1O[C@H]([C@H](C)[C@H]1C)c1cc(OC)c(OC)c(OC)c1 |r| Show InChI InChI=1S/C23H28O7/c1-12-13(2)21(15-9-18(26-5)23-19(10-15)28-11-29-23)30-20(12)14-7-16(24-3)22(27-6)17(8-14)25-4/h7-10,12-13,20-21H,11H2,1-6H3/t12-,13-,20-,21-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Kaohsiung Medical University
Curated by ChEMBL
| Assay Description
Inhibition of iNOS in mouse RAW264.7 cells assessed as anti-inflammatory activity by measuring maximum inhibition of LPS-induced nitric oxide product... |
J Nat Prod 74: 1875-80 (2011)
Article DOI: 10.1021/np200279r
BindingDB Entry DOI: 10.7270/Q2474B72 |
More data for this
Ligand-Target Pair | |
Protein kinase C delta type
(Homo sapiens (Human)) | BDBM50292431
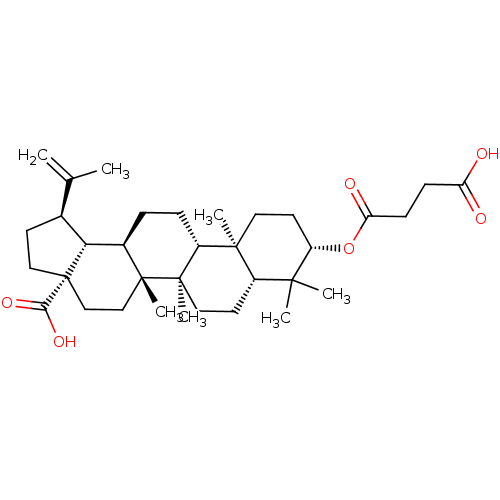
((1R,3aS,5aR,5bR,7aR,9S,11aR,11bR,13aR,13bR)-9-(3-c...)Show SMILES CC(=C)[C@@H]1CC[C@@]2(CC[C@]3(C)[C@H](CC[C@@H]4[C@@]5(C)CC[C@H](OC(=O)CCC(O)=O)C(C)(C)[C@@H]5CC[C@@]34C)[C@@H]12)C(O)=O |r| Show InChI InChI=1S/C34H52O6/c1-20(2)21-12-17-34(29(38)39)19-18-32(6)22(28(21)34)8-9-24-31(5)15-14-25(40-27(37)11-10-26(35)36)30(3,4)23(31)13-16-33(24,32)7/h21-25,28H,1,8-19H2,2-7H3,(H,35,36)(H,38,39)/t21-,22+,23-,24+,25-,28+,31-,32+,33+,34-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of PKC delta |
J Nat Prod 57: 243-247 (1994)
Article DOI: 10.1021/np50104a008
BindingDB Entry DOI: 10.7270/Q2TT4R0Z |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Mus musculus (mouse)) | BDBM50353023
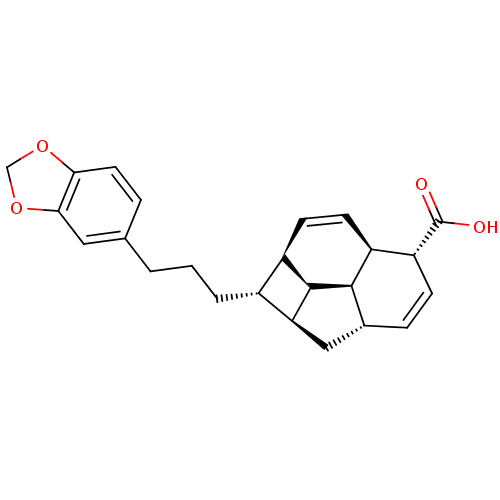
(CHEMBL1821985)Show SMILES OC(=O)[C@@H]1C=C[C@@H]2C[C@H]3[C@H](CCCc4ccc5OCOc5c4)[C@H]4C=C[C@@H]1[C@@H]2[C@@H]34 |r,c:4,25| Show InChI InChI=1S/C24H26O4/c25-24(26)18-6-5-14-11-19-15(16-7-8-17(18)22(14)23(16)19)3-1-2-13-4-9-20-21(10-13)28-12-27-20/h4-10,14-19,22-23H,1-3,11-12H2,(H,25,26)/t14-,15-,16-,17+,18-,19+,22-,23-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.96E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Kaohsiung Medical University
Curated by ChEMBL
| Assay Description
Inhibition of iNOS in mouse RAW264.7 cells assessed as anti-inflammatory activity by measuring maximum inhibition of LPS-induced nitric oxide product... |
J Nat Prod 74: 1875-80 (2011)
Article DOI: 10.1021/np200279r
BindingDB Entry DOI: 10.7270/Q2474B72 |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Mus musculus (mouse)) | BDBM50353029
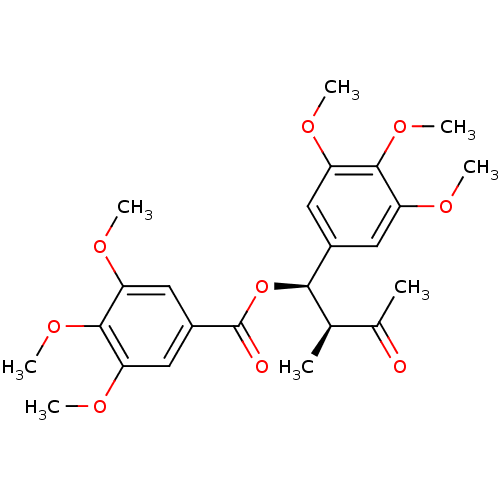
(CHEMBL1821992)Show SMILES COc1cc(cc(OC)c1OC)[C@H](OC(=O)c1cc(OC)c(OC)c(OC)c1)[C@H](C)C(C)=O |r| Show InChI InChI=1S/C24H30O9/c1-13(14(2)25)21(15-9-17(27-3)22(31-7)18(10-15)28-4)33-24(26)16-11-19(29-5)23(32-8)20(12-16)30-6/h9-13,21H,1-8H3/t13-,21-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.14E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Kaohsiung Medical University
Curated by ChEMBL
| Assay Description
Inhibition of iNOS in mouse RAW264.7 cells assessed as anti-inflammatory activity by measuring maximum inhibition of LPS-induced nitric oxide product... |
J Nat Prod 74: 1875-80 (2011)
Article DOI: 10.1021/np200279r
BindingDB Entry DOI: 10.7270/Q2474B72 |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Mus musculus (mouse)) | BDBM50353028

(CHEMBL1821991)Show SMILES COc1cc(cc2OCOc12)[C@@H]1O[C@H]([C@H](C)[C@H]1C)c1cc(O)c(OC)c(OC)c1 |r| Show InChI InChI=1S/C22H26O7/c1-11-12(2)20(14-8-17(25-4)22-18(9-14)27-10-28-22)29-19(11)13-6-15(23)21(26-5)16(7-13)24-3/h6-9,11-12,19-20,23H,10H2,1-5H3/t11-,12-,19-,20-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.15E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Kaohsiung Medical University
Curated by ChEMBL
| Assay Description
Inhibition of iNOS in mouse RAW264.7 cells assessed as anti-inflammatory activity by measuring maximum inhibition of LPS-induced nitric oxide product... |
J Nat Prod 74: 1875-80 (2011)
Article DOI: 10.1021/np200279r
BindingDB Entry DOI: 10.7270/Q2474B72 |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Mus musculus (mouse)) | BDBM50353031

(CHEMBL1821994)Show SMILES COc1cc(cc2OCOc12)[C@@H]1O[C@H]([C@H](C)[C@H]1C)c1cc2OCOc2c(OC)c1 |r| Show InChI InChI=1S/C22H24O7/c1-11-12(2)20(14-6-16(24-4)22-18(8-14)26-10-28-22)29-19(11)13-5-15(23-3)21-17(7-13)25-9-27-21/h5-8,11-12,19-20H,9-10H2,1-4H3/t11-,12-,19-,20-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.25E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Kaohsiung Medical University
Curated by ChEMBL
| Assay Description
Inhibition of iNOS in mouse RAW264.7 cells assessed as anti-inflammatory activity by measuring maximum inhibition of LPS-induced nitric oxide product... |
J Nat Prod 74: 1875-80 (2011)
Article DOI: 10.1021/np200279r
BindingDB Entry DOI: 10.7270/Q2474B72 |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Mus musculus (mouse)) | BDBM50353032

(CHEMBL1821988)Show SMILES OC(=O)[C@@H]1[C@H]2C[C@H]3[C@H](CCCc4ccc5OCOc5c4)[C@@H]4[C@H]3[C@@H]2C=C[C@H]14 |r,c:27,TLB:7:20:4.3:23.24,5:4:21.20:23.24,6:21:4.3:23.24,THB:23:22:5.6:3.25.20,1:3:21.20:23.24| Show InChI InChI=1S/C22H24O4/c23-22(24)21-14-6-5-13-16(21)9-15-12(19(14)20(13)15)3-1-2-11-4-7-17-18(8-11)26-10-25-17/h4-8,12-16,19-21H,1-3,9-10H2,(H,23,24)/t12-,13+,14-,15-,16-,19-,20-,21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.82E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Kaohsiung Medical University
Curated by ChEMBL
| Assay Description
Inhibition of iNOS in mouse RAW264.7 cells assessed as anti-inflammatory activity by measuring maximum inhibition of LPS-induced nitric oxide product... |
J Nat Prod 74: 1875-80 (2011)
Article DOI: 10.1021/np200279r
BindingDB Entry DOI: 10.7270/Q2474B72 |
More data for this
Ligand-Target Pair | |
Protein kinase C gamma type
(Homo sapiens (Human)) | BDBM50292432
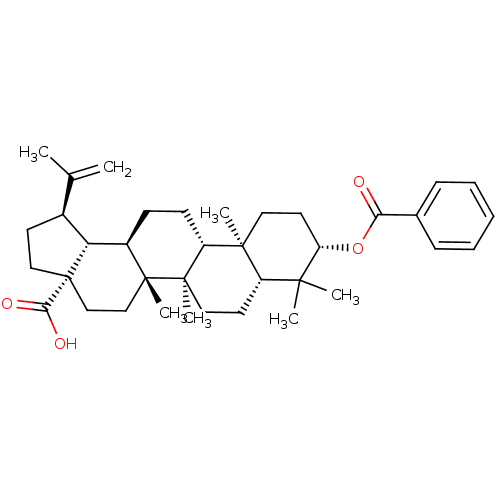
(Betulinic acid 3-O-benzoate | CHEMBL509553)Show SMILES CC(=C)[C@@H]1CC[C@@]2(CC[C@]3(C)[C@H](CC[C@@H]4[C@@]5(C)CC[C@H](OC(=O)c6ccccc6)C(C)(C)[C@@H]5CC[C@@]34C)[C@@H]12)C(O)=O |r| Show InChI InChI=1S/C37H52O4/c1-23(2)25-15-20-37(32(39)40)22-21-35(6)26(30(25)37)13-14-28-34(5)18-17-29(41-31(38)24-11-9-8-10-12-24)33(3,4)27(34)16-19-36(28,35)7/h8-12,25-30H,1,13-22H2,2-7H3,(H,39,40)/t25-,26+,27-,28+,29-,30+,34-,35+,36+,37-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of PKC gamma |
J Nat Prod 57: 243-247 (1994)
Article DOI: 10.1021/np50104a008
BindingDB Entry DOI: 10.7270/Q2TT4R0Z |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Mus musculus (mouse)) | BDBM50225106
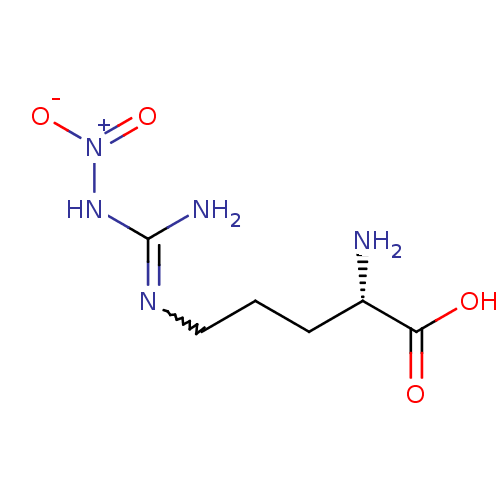
((2S)-2-amino-5-{[(E)-amino(nitroimino)methyl]amino...)Show SMILES N[C@@H](CCCNC(N)=N[N+]([O-])=O)C(O)=O |r,w:8.8| Show InChI InChI=1S/C6H13N5O4/c7-4(5(12)13)2-1-3-9-6(8)10-11(14)15/h4H,1-3,7H2,(H,12,13)(H3,8,9,10)/t4-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.44E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Kaohsiung Medical University
Curated by ChEMBL
| Assay Description
Inhibition of iNOS-mediated NO production in LPS-stimulated mouse RAW264.7 cells after 24 hrs by Griess reagent method |
J Nat Prod 74: 2489-96 (2011)
Article DOI: 10.1021/np100874f
BindingDB Entry DOI: 10.7270/Q2ST7Q8Z |
More data for this
Ligand-Target Pair | |
Protein kinase C epsilon type
(Homo sapiens (Human)) | BDBM50292433

(CHEMBL502585 | betulinic acid 3-O-sulfonate potass...)Show SMILES CC(=C)[C@@H]1CC[C@@]2(CC[C@]3(C)[C@H](CC[C@@H]4[C@@]5(C)CC[C@H](OS([O-])(=O)=O)C(C)(C)[C@@H]5CC[C@@]34C)[C@@H]12)C(O)=O |r| Show InChI InChI=1S/C30H48O6S/c1-18(2)19-10-15-30(25(31)32)17-16-28(6)20(24(19)30)8-9-22-27(5)13-12-23(36-37(33,34)35)26(3,4)21(27)11-14-29(22,28)7/h19-24H,1,8-17H2,2-7H3,(H,31,32)(H,33,34,35)/p-1/t19-,20+,21-,22+,23-,24+,27-,28+,29+,30-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of PKC epsilon |
J Nat Prod 57: 243-247 (1994)
Article DOI: 10.1021/np50104a008
BindingDB Entry DOI: 10.7270/Q2TT4R0Z |
More data for this
Ligand-Target Pair | |
Protein kinase C epsilon type
(Homo sapiens (Human)) | BDBM50292435
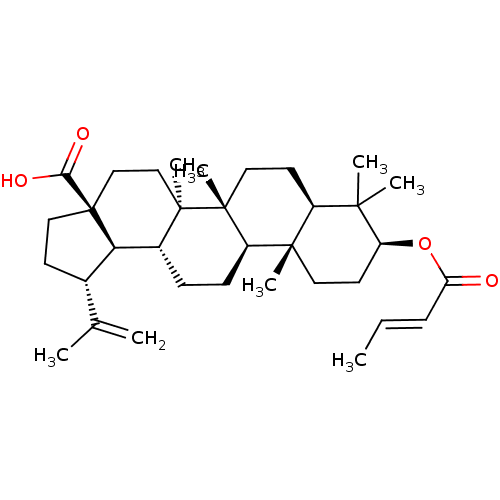
((1R,3aS,5aR,5bR,7aR,9S,11aR,11bR,13aR,13bR)-9-((E)...)Show SMILES C\C=C\C(=O)O[C@H]1CC[C@@]2(C)[C@@H](CC[C@]3(C)[C@@H]2CC[C@@H]2[C@H]4[C@@H](CC[C@@]4(CC[C@@]32C)C(O)=O)C(C)=C)C1(C)C |r| Show InChI InChI=1S/C34H52O4/c1-9-10-27(35)38-26-15-16-31(6)24(30(26,4)5)14-17-33(8)25(31)12-11-23-28-22(21(2)3)13-18-34(28,29(36)37)20-19-32(23,33)7/h9-10,22-26,28H,2,11-20H2,1,3-8H3,(H,36,37)/b10-9+/t22-,23+,24-,25+,26-,28+,31-,32+,33+,34-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of PKC epsilon |
J Nat Prod 57: 243-247 (1994)
Article DOI: 10.1021/np50104a008
BindingDB Entry DOI: 10.7270/Q2TT4R0Z |
More data for this
Ligand-Target Pair | |
Protein kinase C epsilon type
(Homo sapiens (Human)) | BDBM50292432

(Betulinic acid 3-O-benzoate | CHEMBL509553)Show SMILES CC(=C)[C@@H]1CC[C@@]2(CC[C@]3(C)[C@H](CC[C@@H]4[C@@]5(C)CC[C@H](OC(=O)c6ccccc6)C(C)(C)[C@@H]5CC[C@@]34C)[C@@H]12)C(O)=O |r| Show InChI InChI=1S/C37H52O4/c1-23(2)25-15-20-37(32(39)40)22-21-35(6)26(30(25)37)13-14-28-34(5)18-17-29(41-31(38)24-11-9-8-10-12-24)33(3,4)27(34)16-19-36(28,35)7/h8-12,25-30H,1,13-22H2,2-7H3,(H,39,40)/t25-,26+,27-,28+,29-,30+,34-,35+,36+,37-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of PKC epsilon |
J Nat Prod 57: 243-247 (1994)
Article DOI: 10.1021/np50104a008
BindingDB Entry DOI: 10.7270/Q2TT4R0Z |
More data for this
Ligand-Target Pair | |
Protein kinase C epsilon type
(Homo sapiens (Human)) | BDBM50103967
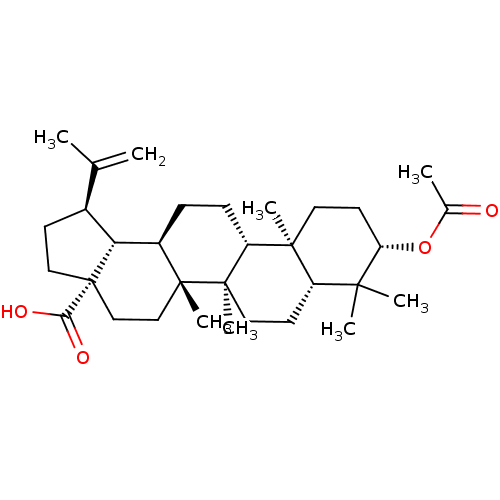
((3-O-acetyl)betulinic acid | 3-acetylbetulinic aci...)Show SMILES CC(=C)[C@@H]1CC[C@@]2(CC[C@]3(C)[C@H](CC[C@@H]4[C@@]5(C)CC[C@H](OC(C)=O)C(C)(C)[C@@H]5CC[C@@]34C)[C@@H]12)C(O)=O |r| Show InChI InChI=1S/C32H50O4/c1-19(2)21-11-16-32(27(34)35)18-17-30(7)22(26(21)32)9-10-24-29(6)14-13-25(36-20(3)33)28(4,5)23(29)12-15-31(24,30)8/h21-26H,1,9-18H2,2-8H3,(H,34,35)/t21-,22+,23-,24+,25-,26+,29-,30+,31+,32-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of PKC epsilon |
J Nat Prod 57: 243-247 (1994)
Article DOI: 10.1021/np50104a008
BindingDB Entry DOI: 10.7270/Q2TT4R0Z |
More data for this
Ligand-Target Pair | |
Protein kinase C epsilon type
(Homo sapiens (Human)) | BDBM50292434
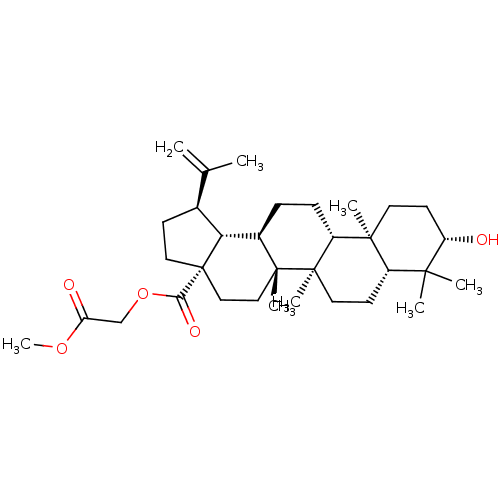
(CHEMBL509422 | betulinic acid 28-O-carboxymethylme...)Show SMILES COC(=O)COC(=O)[C@]12CC[C@H]([C@@H]1[C@H]1CC[C@@H]3[C@@]4(C)CC[C@H](O)C(C)(C)[C@@H]4CC[C@@]3(C)[C@]1(C)CC2)C(C)=C |r| Show InChI InChI=1S/C33H52O5/c1-20(2)21-11-16-33(28(36)38-19-26(35)37-8)18-17-31(6)22(27(21)33)9-10-24-30(5)14-13-25(34)29(3,4)23(30)12-15-32(24,31)7/h21-25,27,34H,1,9-19H2,2-8H3/t21-,22+,23-,24+,25-,27+,30-,31+,32+,33-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of PKC epsilon |
J Nat Prod 57: 243-247 (1994)
Article DOI: 10.1021/np50104a008
BindingDB Entry DOI: 10.7270/Q2TT4R0Z |
More data for this
Ligand-Target Pair | |
Protein kinase C epsilon type
(Homo sapiens (Human)) | BDBM23207
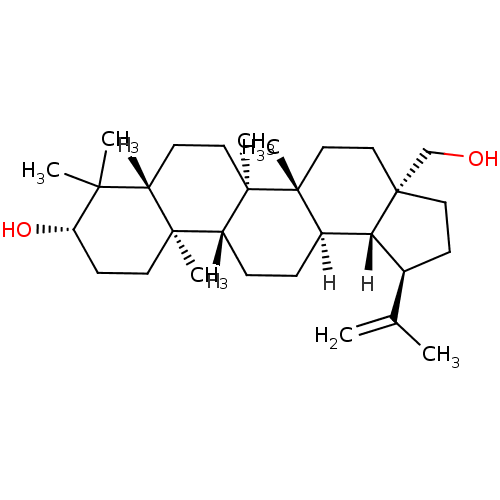
((1R,2R,5S,8R,9R,10R,13R,14R,17S,19R)-5-(hydroxymet...)Show SMILES [H][C@]12[C@@H](CC[C@]1(CO)CC[C@]1(C)[C@]2([H])CC[C@]2([H])[C@@]3(C)CC[C@H](O)C(C)(C)[C@]3([H])CC[C@@]12C)C(C)=C Show InChI InChI=1S/C30H50O2/c1-19(2)20-10-15-30(18-31)17-16-28(6)21(25(20)30)8-9-23-27(5)13-12-24(32)26(3,4)22(27)11-14-29(23,28)7/h20-25,31-32H,1,8-18H2,2-7H3/t20-,21+,22-,23+,24-,25+,27-,28+,29+,30+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of PKC epsilon |
J Nat Prod 57: 243-247 (1994)
Article DOI: 10.1021/np50104a008
BindingDB Entry DOI: 10.7270/Q2TT4R0Z |
More data for this
Ligand-Target Pair | |
Protein kinase C epsilon type
(Homo sapiens (Human)) | BDBM50103962
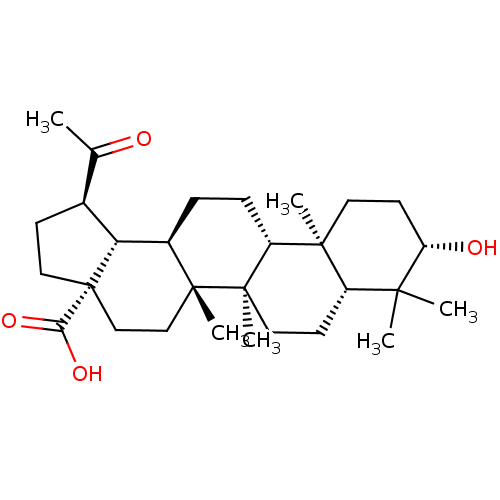
((1R,3aS,5aR,5bR,7aR,9S,11aR,11bR,13aR,13bS)-1-Acet...)Show SMILES CC(=O)[C@@H]1CC[C@@]2(CC[C@]3(C)[C@H](CC[C@@H]4[C@@]5(C)CC[C@H](O)C(C)(C)[C@@H]5CC[C@@]34C)[C@@H]12)C(O)=O |r| Show InChI InChI=1S/C29H46O4/c1-17(30)18-9-14-29(24(32)33)16-15-27(5)19(23(18)29)7-8-21-26(4)12-11-22(31)25(2,3)20(26)10-13-28(21,27)6/h18-23,31H,7-16H2,1-6H3,(H,32,33)/t18-,19+,20-,21+,22-,23+,26-,27+,28+,29-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of PKC epsilon |
J Nat Prod 57: 243-247 (1994)
Article DOI: 10.1021/np50104a008
BindingDB Entry DOI: 10.7270/Q2TT4R0Z |
More data for this
Ligand-Target Pair | |
Protein kinase C beta type
(Homo sapiens (Human)) | BDBM50002692

((AZT) 1-(4-Azido-5-hydroxymethyl-tetrahydro-furan-...)Show SMILES Cc1cn([C@H]2C[C@H](N=[N+]=[N-])[C@@H](CO)O2)c(=O)[nH]c1=O |r| Show InChI InChI=1S/C10H13N5O4/c1-5-3-15(10(18)12-9(5)17)8-2-6(13-14-11)7(4-16)19-8/h3,6-8,16H,2,4H2,1H3,(H,12,17,18)/t6-,7+,8+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of PKC beta2 |
J Nat Prod 57: 243-247 (1994)
Article DOI: 10.1021/np50104a008
BindingDB Entry DOI: 10.7270/Q2TT4R0Z |
More data for this
Ligand-Target Pair | |
Protein kinase C beta type
(Homo sapiens (Human)) | BDBM50292431

((1R,3aS,5aR,5bR,7aR,9S,11aR,11bR,13aR,13bR)-9-(3-c...)Show SMILES CC(=C)[C@@H]1CC[C@@]2(CC[C@]3(C)[C@H](CC[C@@H]4[C@@]5(C)CC[C@H](OC(=O)CCC(O)=O)C(C)(C)[C@@H]5CC[C@@]34C)[C@@H]12)C(O)=O |r| Show InChI InChI=1S/C34H52O6/c1-20(2)21-12-17-34(29(38)39)19-18-32(6)22(28(21)34)8-9-24-31(5)15-14-25(40-27(37)11-10-26(35)36)30(3,4)23(31)13-16-33(24,32)7/h21-25,28H,1,8-19H2,2-7H3,(H,35,36)(H,38,39)/t21-,22+,23-,24+,25-,28+,31-,32+,33+,34-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of PKC beta2 |
J Nat Prod 57: 243-247 (1994)
Article DOI: 10.1021/np50104a008
BindingDB Entry DOI: 10.7270/Q2TT4R0Z |
More data for this
Ligand-Target Pair | |
Protein kinase C beta type
(Homo sapiens (Human)) | BDBM50292433

(CHEMBL502585 | betulinic acid 3-O-sulfonate potass...)Show SMILES CC(=C)[C@@H]1CC[C@@]2(CC[C@]3(C)[C@H](CC[C@@H]4[C@@]5(C)CC[C@H](OS([O-])(=O)=O)C(C)(C)[C@@H]5CC[C@@]34C)[C@@H]12)C(O)=O |r| Show InChI InChI=1S/C30H48O6S/c1-18(2)19-10-15-30(25(31)32)17-16-28(6)20(24(19)30)8-9-22-27(5)13-12-23(36-37(33,34)35)26(3,4)21(27)11-14-29(22,28)7/h19-24H,1,8-17H2,2-7H3,(H,31,32)(H,33,34,35)/p-1/t19-,20+,21-,22+,23-,24+,27-,28+,29+,30-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of PKC beta2 |
J Nat Prod 57: 243-247 (1994)
Article DOI: 10.1021/np50104a008
BindingDB Entry DOI: 10.7270/Q2TT4R0Z |
More data for this
Ligand-Target Pair | |
Protein kinase C beta type
(Homo sapiens (Human)) | BDBM50292435

((1R,3aS,5aR,5bR,7aR,9S,11aR,11bR,13aR,13bR)-9-((E)...)Show SMILES C\C=C\C(=O)O[C@H]1CC[C@@]2(C)[C@@H](CC[C@]3(C)[C@@H]2CC[C@@H]2[C@H]4[C@@H](CC[C@@]4(CC[C@@]32C)C(O)=O)C(C)=C)C1(C)C |r| Show InChI InChI=1S/C34H52O4/c1-9-10-27(35)38-26-15-16-31(6)24(30(26,4)5)14-17-33(8)25(31)12-11-23-28-22(21(2)3)13-18-34(28,29(36)37)20-19-32(23,33)7/h9-10,22-26,28H,2,11-20H2,1,3-8H3,(H,36,37)/b10-9+/t22-,23+,24-,25+,26-,28+,31-,32+,33+,34-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of PKC beta2 |
J Nat Prod 57: 243-247 (1994)
Article DOI: 10.1021/np50104a008
BindingDB Entry DOI: 10.7270/Q2TT4R0Z |
More data for this
Ligand-Target Pair | |
Protein kinase C beta type
(Homo sapiens (Human)) | BDBM50292432

(Betulinic acid 3-O-benzoate | CHEMBL509553)Show SMILES CC(=C)[C@@H]1CC[C@@]2(CC[C@]3(C)[C@H](CC[C@@H]4[C@@]5(C)CC[C@H](OC(=O)c6ccccc6)C(C)(C)[C@@H]5CC[C@@]34C)[C@@H]12)C(O)=O |r| Show InChI InChI=1S/C37H52O4/c1-23(2)25-15-20-37(32(39)40)22-21-35(6)26(30(25)37)13-14-28-34(5)18-17-29(41-31(38)24-11-9-8-10-12-24)33(3,4)27(34)16-19-36(28,35)7/h8-12,25-30H,1,13-22H2,2-7H3,(H,39,40)/t25-,26+,27-,28+,29-,30+,34-,35+,36+,37-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of PKC beta2 |
J Nat Prod 57: 243-247 (1994)
Article DOI: 10.1021/np50104a008
BindingDB Entry DOI: 10.7270/Q2TT4R0Z |
More data for this
Ligand-Target Pair | |
Protein kinase C beta type
(Homo sapiens (Human)) | BDBM50103967

((3-O-acetyl)betulinic acid | 3-acetylbetulinic aci...)Show SMILES CC(=C)[C@@H]1CC[C@@]2(CC[C@]3(C)[C@H](CC[C@@H]4[C@@]5(C)CC[C@H](OC(C)=O)C(C)(C)[C@@H]5CC[C@@]34C)[C@@H]12)C(O)=O |r| Show InChI InChI=1S/C32H50O4/c1-19(2)21-11-16-32(27(34)35)18-17-30(7)22(26(21)32)9-10-24-29(6)14-13-25(36-20(3)33)28(4,5)23(29)12-15-31(24,30)8/h21-26H,1,9-18H2,2-8H3,(H,34,35)/t21-,22+,23-,24+,25-,26+,29-,30+,31+,32-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of PKC beta2 |
J Nat Prod 57: 243-247 (1994)
Article DOI: 10.1021/np50104a008
BindingDB Entry DOI: 10.7270/Q2TT4R0Z |
More data for this
Ligand-Target Pair | |
Protein kinase C beta type
(Homo sapiens (Human)) | BDBM50292434

(CHEMBL509422 | betulinic acid 28-O-carboxymethylme...)Show SMILES COC(=O)COC(=O)[C@]12CC[C@H]([C@@H]1[C@H]1CC[C@@H]3[C@@]4(C)CC[C@H](O)C(C)(C)[C@@H]4CC[C@@]3(C)[C@]1(C)CC2)C(C)=C |r| Show InChI InChI=1S/C33H52O5/c1-20(2)21-11-16-33(28(36)38-19-26(35)37-8)18-17-31(6)22(27(21)33)9-10-24-30(5)14-13-25(34)29(3,4)23(30)12-15-32(24,31)7/h21-25,27,34H,1,9-19H2,2-8H3/t21-,22+,23-,24+,25-,27+,30-,31+,32+,33-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of PKC beta2 |
J Nat Prod 57: 243-247 (1994)
Article DOI: 10.1021/np50104a008
BindingDB Entry DOI: 10.7270/Q2TT4R0Z |
More data for this
Ligand-Target Pair | |
Protein kinase C beta type
(Homo sapiens (Human)) | BDBM23207

((1R,2R,5S,8R,9R,10R,13R,14R,17S,19R)-5-(hydroxymet...)Show SMILES [H][C@]12[C@@H](CC[C@]1(CO)CC[C@]1(C)[C@]2([H])CC[C@]2([H])[C@@]3(C)CC[C@H](O)C(C)(C)[C@]3([H])CC[C@@]12C)C(C)=C Show InChI InChI=1S/C30H50O2/c1-19(2)20-10-15-30(18-31)17-16-28(6)21(25(20)30)8-9-23-27(5)13-12-24(32)26(3,4)22(27)11-14-29(23,28)7/h20-25,31-32H,1,8-18H2,2-7H3/t20-,21+,22-,23+,24-,25+,27-,28+,29+,30+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of PKC beta2 |
J Nat Prod 57: 243-247 (1994)
Article DOI: 10.1021/np50104a008
BindingDB Entry DOI: 10.7270/Q2TT4R0Z |
More data for this
Ligand-Target Pair | |
Protein kinase C beta type
(Homo sapiens (Human)) | BDBM50103962

((1R,3aS,5aR,5bR,7aR,9S,11aR,11bR,13aR,13bS)-1-Acet...)Show SMILES CC(=O)[C@@H]1CC[C@@]2(CC[C@]3(C)[C@H](CC[C@@H]4[C@@]5(C)CC[C@H](O)C(C)(C)[C@@H]5CC[C@@]34C)[C@@H]12)C(O)=O |r| Show InChI InChI=1S/C29H46O4/c1-17(30)18-9-14-29(24(32)33)16-15-27(5)19(23(18)29)7-8-21-26(4)12-11-22(31)25(2,3)20(26)10-13-28(21,27)6/h18-23,31H,7-16H2,1-6H3,(H,32,33)/t18-,19+,20-,21+,22-,23+,26-,27+,28+,29-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of PKC beta2 |
J Nat Prod 57: 243-247 (1994)
Article DOI: 10.1021/np50104a008
BindingDB Entry DOI: 10.7270/Q2TT4R0Z |
More data for this
Ligand-Target Pair | |
Protein kinase C epsilon type
(Homo sapiens (Human)) | BDBM50292431

((1R,3aS,5aR,5bR,7aR,9S,11aR,11bR,13aR,13bR)-9-(3-c...)Show SMILES CC(=C)[C@@H]1CC[C@@]2(CC[C@]3(C)[C@H](CC[C@@H]4[C@@]5(C)CC[C@H](OC(=O)CCC(O)=O)C(C)(C)[C@@H]5CC[C@@]34C)[C@@H]12)C(O)=O |r| Show InChI InChI=1S/C34H52O6/c1-20(2)21-12-17-34(29(38)39)19-18-32(6)22(28(21)34)8-9-24-31(5)15-14-25(40-27(37)11-10-26(35)36)30(3,4)23(31)13-16-33(24,32)7/h21-25,28H,1,8-19H2,2-7H3,(H,35,36)(H,38,39)/t21-,22+,23-,24+,25-,28+,31-,32+,33+,34-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of PKC epsilon |
J Nat Prod 57: 243-247 (1994)
Article DOI: 10.1021/np50104a008
BindingDB Entry DOI: 10.7270/Q2TT4R0Z |
More data for this
Ligand-Target Pair | |
Protein kinase C epsilon type
(Homo sapiens (Human)) | BDBM50002692

((AZT) 1-(4-Azido-5-hydroxymethyl-tetrahydro-furan-...)Show SMILES Cc1cn([C@H]2C[C@H](N=[N+]=[N-])[C@@H](CO)O2)c(=O)[nH]c1=O |r| Show InChI InChI=1S/C10H13N5O4/c1-5-3-15(10(18)12-9(5)17)8-2-6(13-14-11)7(4-16)19-8/h3,6-8,16H,2,4H2,1H3,(H,12,17,18)/t6-,7+,8+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of PKC epsilon |
J Nat Prod 57: 243-247 (1994)
Article DOI: 10.1021/np50104a008
BindingDB Entry DOI: 10.7270/Q2TT4R0Z |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Mus musculus (mouse)) | BDBM50225106

((2S)-2-amino-5-{[(E)-amino(nitroimino)methyl]amino...)Show SMILES N[C@@H](CCCNC(N)=N[N+]([O-])=O)C(O)=O |r,w:8.8| Show InChI InChI=1S/C6H13N5O4/c7-4(5(12)13)2-1-3-9-6(8)10-11(14)15/h4H,1-3,7H2,(H,12,13)(H3,8,9,10)/t4-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.52E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Kaohsiung Medical University
Curated by ChEMBL
| Assay Description
Inhibition of iNOS in mouse RAW264.7 cells assessed as anti-inflammatory activity by measuring maximum inhibition of LPS-induced nitric oxide product... |
J Nat Prod 74: 1875-80 (2011)
Article DOI: 10.1021/np200279r
BindingDB Entry DOI: 10.7270/Q2474B72 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data