

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Coagulation factor X (Homo sapiens (Human)) | BDBM12396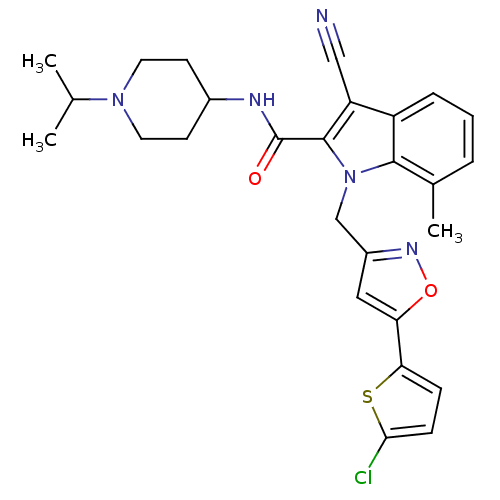 (1-[5-(5-Chlorothiophen-2-yl)isoxazol-3-ylmethyl]-3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharma | Assay Description The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrates S-2765. The hydrolysis rates of chromogenic s... | J Med Chem 48: 4511-25 (2005) Article DOI: 10.1021/jm0490540 BindingDB Entry DOI: 10.7270/Q21834RB | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM12389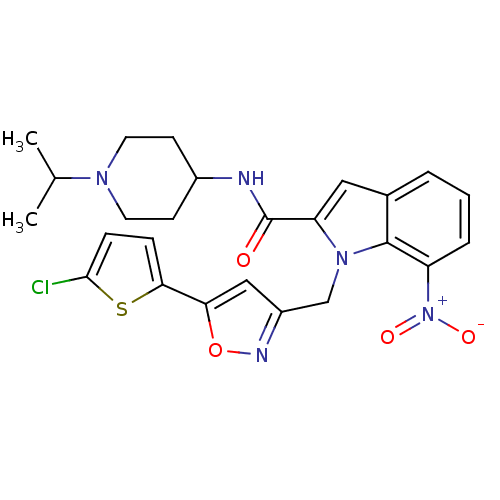 (1-[5-(5-Chloro-thiophen-2-yl)-isoxazol-3-ylmethyl]...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharma | Assay Description The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrates S-2765. The hydrolysis rates of chromogenic s... | J Med Chem 48: 4511-25 (2005) Article DOI: 10.1021/jm0490540 BindingDB Entry DOI: 10.7270/Q21834RB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM12387 (1-[5-(5-Chloro-thiophen-2-yl)-isoxazol-3-ylmethyl]...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 0.25 | -54.8 | n/a | n/a | n/a | n/a | n/a | 7.8 | 25 |
Aventis Pharma | Assay Description The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrates S-2765. The hydrolysis rates of chromogenic s... | J Med Chem 48: 4511-25 (2005) Article DOI: 10.1021/jm0490540 BindingDB Entry DOI: 10.7270/Q21834RB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM12395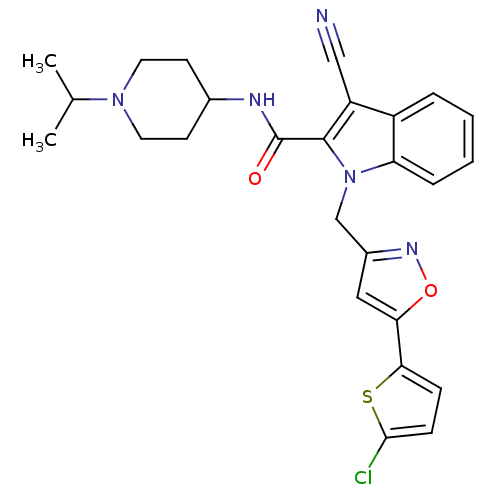 (1-[5-(5-Chloro-thiophen-2-yl)-isoxazol-3-ylmethyl]...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharma | Assay Description The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrates S-2765. The hydrolysis rates of chromogenic s... | J Med Chem 48: 4511-25 (2005) Article DOI: 10.1021/jm0490540 BindingDB Entry DOI: 10.7270/Q21834RB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50590085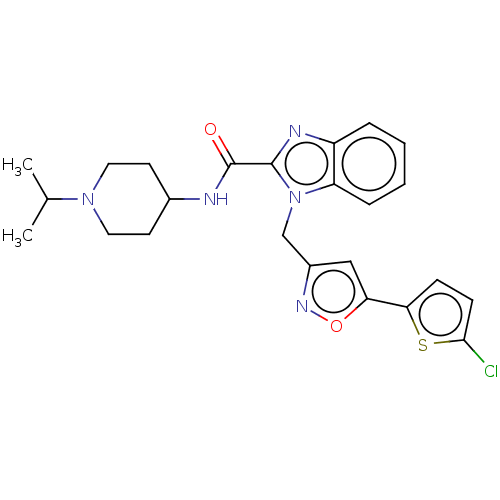 (CHEMBL5187102) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.2c00865 BindingDB Entry DOI: 10.7270/Q2BP06RZ | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM12384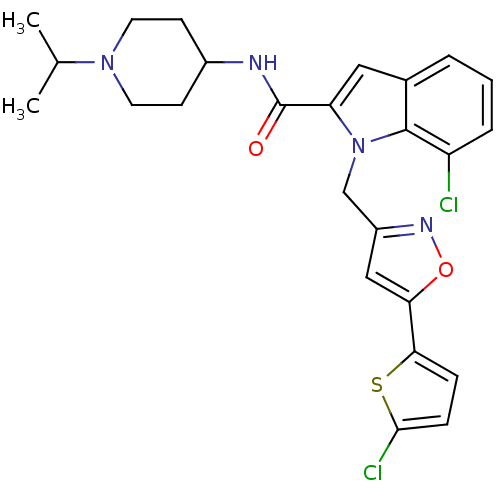 (2-Carboxyindole Scaffold 35 | 7-Chloro-1-[5-(5-chl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 0.700 | -52.3 | n/a | n/a | n/a | n/a | n/a | 7.8 | 25 |
Aventis Pharma | Assay Description The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrates S-2765. The hydrolysis rates of chromogenic s... | J Med Chem 48: 4511-25 (2005) Article DOI: 10.1021/jm0490540 BindingDB Entry DOI: 10.7270/Q21834RB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 12 (Homo sapiens (Human)) | BDBM50397165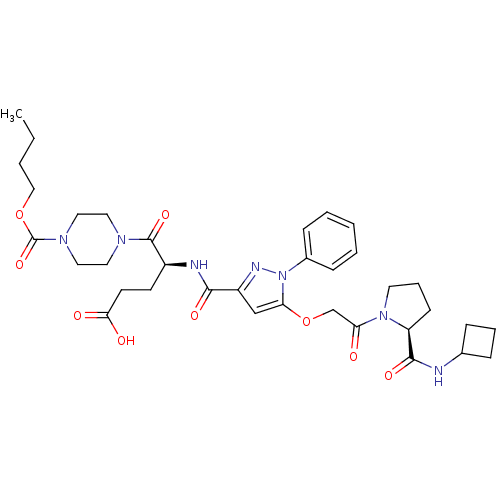 (CHEMBL2172149) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Aventis Deutschland GmbH Curated by ChEMBL | Assay Description Displacement of [33P]2-MeS-ADP from human recombinant P2Y12 receptor expressed in CHO cell membranes by scintillation counting method | J Med Chem 55: 8615-29 (2012) Article DOI: 10.1021/jm300771j BindingDB Entry DOI: 10.7270/Q2D50P3P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM15867 (1-{[(5-chloropyridin-2-yl)carbamoyl]methyl}-N-[1-(...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 1 | -51.4 | n/a | n/a | n/a | n/a | n/a | 7.8 | 25 |
Aventis Pharma Deutschland GmbH | Assay Description The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrates S-2765. The hydrolysis rates of chromogenic s... | Bioorg Med Chem Lett 14: 4191-5 (2004) Article DOI: 10.1016/j.bmcl.2004.06.020 BindingDB Entry DOI: 10.7270/Q22805WQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM12388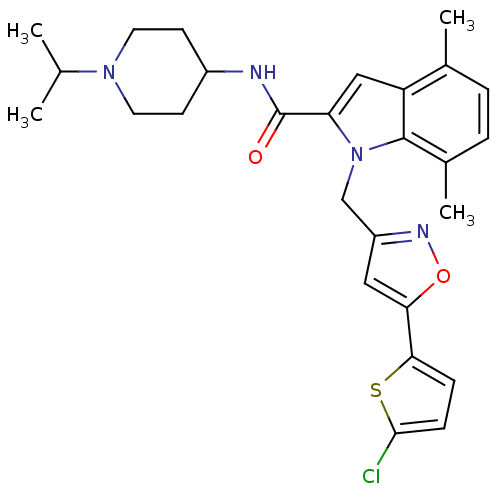 (1-[5-(5-Chloro-thiophen-2-yl)-isoxazol-3-ylmethyl]...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharma | Assay Description The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrates S-2765. The hydrolysis rates of chromogenic s... | J Med Chem 48: 4511-25 (2005) Article DOI: 10.1021/jm0490540 BindingDB Entry DOI: 10.7270/Q21834RB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM12381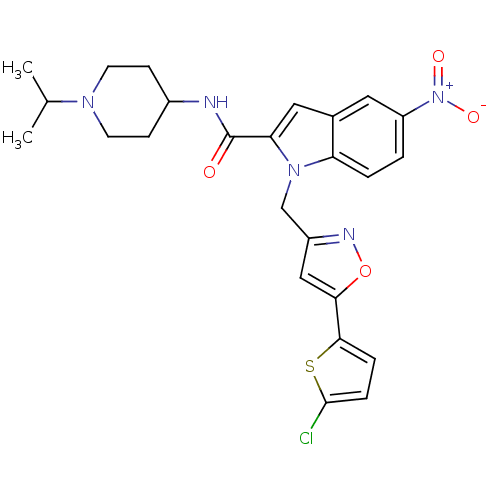 (1-[5-(5-Chloro-thiophen-2-yl)-isoxazol-3-ylmethyl]...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 1 | -51.4 | n/a | n/a | n/a | n/a | n/a | 7.8 | 25 |
Aventis Pharma | Assay Description The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrates S-2765. The hydrolysis rates of chromogenic s... | J Med Chem 48: 4511-25 (2005) Article DOI: 10.1021/jm0490540 BindingDB Entry DOI: 10.7270/Q21834RB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 12 (Homo sapiens (Human)) | BDBM50397156 (CHEMBL2172129) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Aventis Deutschland GmbH Curated by ChEMBL | Assay Description Displacement of [33P]2-MeS-ADP from human recombinant P2Y12 receptor expressed in CHO cell membranes by scintillation counting method | J Med Chem 55: 8615-29 (2012) Article DOI: 10.1021/jm300771j BindingDB Entry DOI: 10.7270/Q2D50P3P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM12379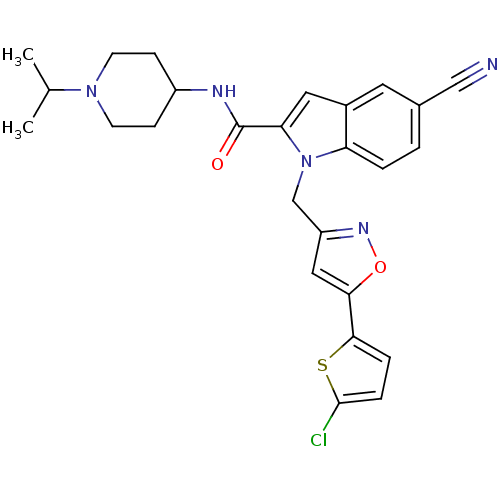 (1-[5-(5-Chloro-thiophen-2-yl)-isoxazol-3-ylmethyl]...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 2 | -49.7 | n/a | n/a | n/a | n/a | n/a | 7.8 | 25 |
Aventis Pharma | Assay Description The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrates S-2765. The hydrolysis rates of chromogenic s... | J Med Chem 48: 4511-25 (2005) Article DOI: 10.1021/jm0490540 BindingDB Entry DOI: 10.7270/Q21834RB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM12391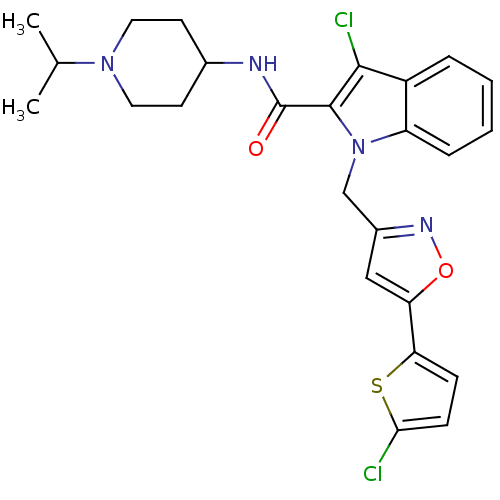 (2-Carboxyindole Scaffold 23 | 3-chloro-1-{[5-(5-ch...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharma | Assay Description The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrates S-2765. The hydrolysis rates of chromogenic s... | J Med Chem 48: 4511-25 (2005) Article DOI: 10.1021/jm0490540 BindingDB Entry DOI: 10.7270/Q21834RB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM12392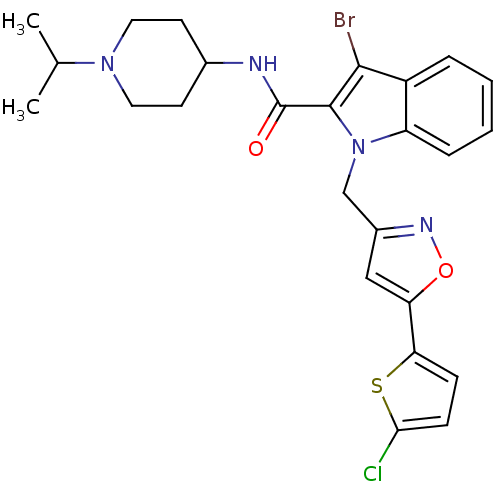 (2-Carboxyindole Scaffold 24 | 3-Bromo-1-[5-(5-chlo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharma | Assay Description The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrates S-2765. The hydrolysis rates of chromogenic s... | J Med Chem 48: 4511-25 (2005) Article DOI: 10.1021/jm0490540 BindingDB Entry DOI: 10.7270/Q21834RB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM12390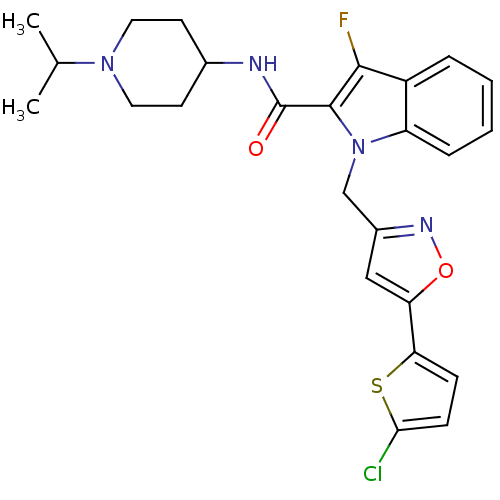 (1-[5-(5-Chlorothiophen-2-yl)isoxazol-3-ylmethyl]-3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharma | Assay Description The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrates S-2765. The hydrolysis rates of chromogenic s... | J Med Chem 48: 4511-25 (2005) Article DOI: 10.1021/jm0490540 BindingDB Entry DOI: 10.7270/Q21834RB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM12372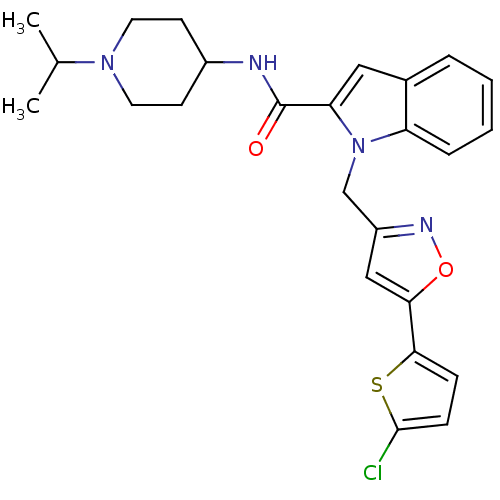 (1-{[5-(5-chlorothiophen-2-yl)-1,2-oxazol-3-yl]meth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.2c00865 BindingDB Entry DOI: 10.7270/Q2BP06RZ | ||||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50590072 (CHEMBL3904655) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.2c00865 BindingDB Entry DOI: 10.7270/Q2BP06RZ | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM12400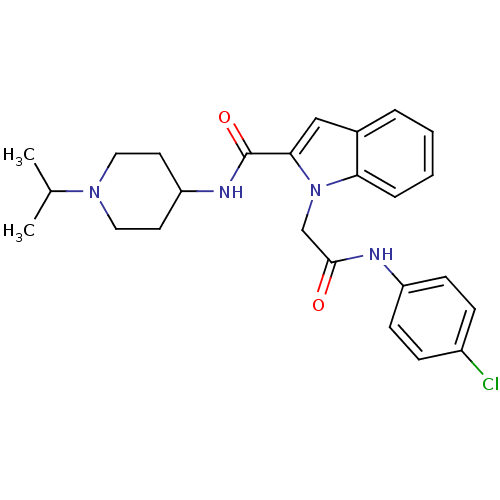 (1-[(4-Chloro-phenylcarbamoyl)-methyl]-1H-indole-2-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.2c00865 BindingDB Entry DOI: 10.7270/Q2BP06RZ | ||||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM12400 (1-[(4-Chloro-phenylcarbamoyl)-methyl]-1H-indole-2-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 3 | -48.6 | n/a | n/a | n/a | n/a | n/a | 7.8 | 25 |
Aventis Pharma Deutschland GmbH | Assay Description The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrates S-2765. The hydrolysis rates of chromogenic s... | Bioorg Med Chem Lett 14: 4191-5 (2004) Article DOI: 10.1016/j.bmcl.2004.06.020 BindingDB Entry DOI: 10.7270/Q22805WQ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM15862 (1-{[3-(5-chlorothiophen-2-yl)-1,2-oxazol-5-yl]meth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 3 | -48.6 | n/a | n/a | n/a | n/a | n/a | 7.8 | 25 |
Aventis Pharma Deutschland GmbH | Assay Description The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrates S-2765. The hydrolysis rates of chromogenic s... | Bioorg Med Chem Lett 14: 4191-5 (2004) Article DOI: 10.1016/j.bmcl.2004.06.020 BindingDB Entry DOI: 10.7270/Q22805WQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM12372 (1-{[5-(5-chlorothiophen-2-yl)-1,2-oxazol-3-yl]meth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 3 | -48.6 | n/a | n/a | n/a | n/a | n/a | 7.8 | 25 |
Aventis Pharma Deutschland GmbH | Assay Description The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrates S-2765. The hydrolysis rates of chromogenic s... | Bioorg Med Chem Lett 14: 4191-5 (2004) Article DOI: 10.1016/j.bmcl.2004.06.020 BindingDB Entry DOI: 10.7270/Q22805WQ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM12372 (1-{[5-(5-chlorothiophen-2-yl)-1,2-oxazol-3-yl]meth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 3 | -48.6 | n/a | n/a | n/a | n/a | n/a | 7.8 | 25 |
Aventis Pharma Deutschland GmbH | Assay Description The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrates S-2765. The hydrolysis rates of chromogenic s... | Bioorg Med Chem Lett 14: 4197-201 (2004) Article DOI: 10.1016/j.bmcl.2004.06.019 BindingDB Entry DOI: 10.7270/Q2610XKM | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM12400 (1-[(4-Chloro-phenylcarbamoyl)-methyl]-1H-indole-2-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharma | Assay Description The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrates S-2765. The hydrolysis rates of chromogenic s... | J Med Chem 48: 4511-25 (2005) Article DOI: 10.1021/jm0490540 BindingDB Entry DOI: 10.7270/Q21834RB | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM12393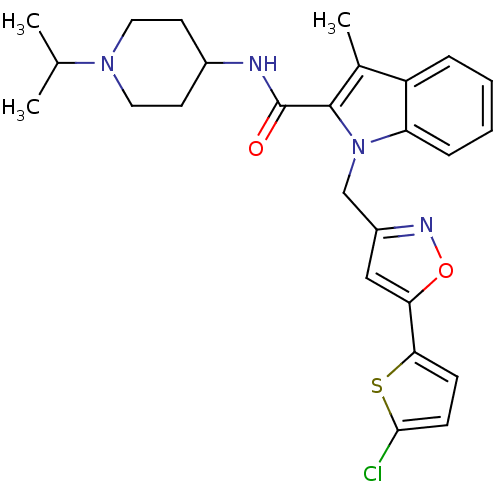 (1-[5-(5-Chlorothiophen-2-yl)isoxazol-3-ylmethyl]-3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharma | Assay Description The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrates S-2765. The hydrolysis rates of chromogenic s... | J Med Chem 48: 4511-25 (2005) Article DOI: 10.1021/jm0490540 BindingDB Entry DOI: 10.7270/Q21834RB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM12385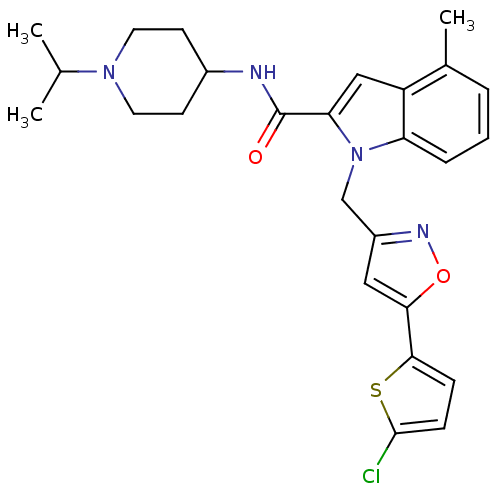 (1-[5-(5-Chloro-thiophen-2-yl)-isoxazol-3-ylmethyl]...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 3 | -48.6 | n/a | n/a | n/a | n/a | n/a | 7.8 | 25 |
Aventis Pharma | Assay Description The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrates S-2765. The hydrolysis rates of chromogenic s... | J Med Chem 48: 4511-25 (2005) Article DOI: 10.1021/jm0490540 BindingDB Entry DOI: 10.7270/Q21834RB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM12374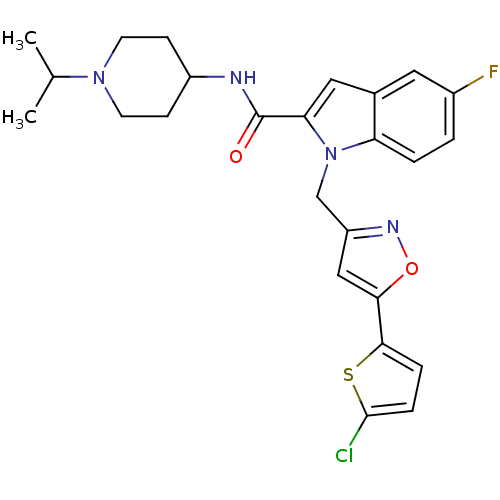 (1-[5-(5-Chloro-thiophen-2-yl)-isoxazol-3-ylmethyl]...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 3 | -48.6 | n/a | n/a | n/a | n/a | n/a | 7.8 | 25 |
Aventis Pharma | Assay Description The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrates S-2765. The hydrolysis rates of chromogenic s... | J Med Chem 48: 4511-25 (2005) Article DOI: 10.1021/jm0490540 BindingDB Entry DOI: 10.7270/Q21834RB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM12372 (1-{[5-(5-chlorothiophen-2-yl)-1,2-oxazol-3-yl]meth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 3 | -48.6 | n/a | n/a | n/a | n/a | n/a | 7.8 | 25 |
Aventis Pharma | Assay Description The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrates S-2765. The hydrolysis rates of chromogenic s... | J Med Chem 48: 4511-25 (2005) Article DOI: 10.1021/jm0490540 BindingDB Entry DOI: 10.7270/Q21834RB | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| P2Y purinoceptor 12 (Homo sapiens (Human)) | BDBM50397166 (CHEMBL2172148) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Aventis Deutschland GmbH Curated by ChEMBL | Assay Description Displacement of [33P]2-MeS-ADP from human recombinant P2Y12 receptor expressed in CHO cell membranes by scintillation counting method | J Med Chem 55: 8615-29 (2012) Article DOI: 10.1021/jm300771j BindingDB Entry DOI: 10.7270/Q2D50P3P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM15863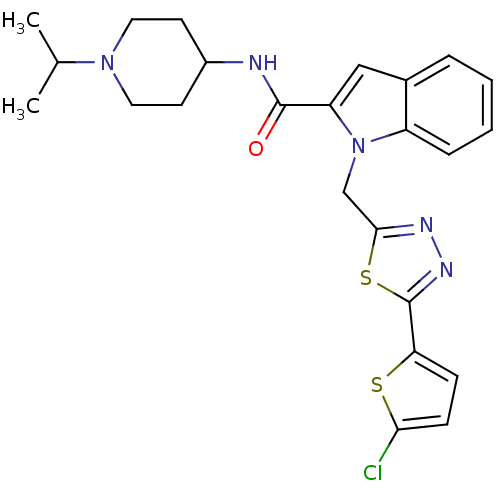 (1-{[5-(5-chlorothiophen-2-yl)-1,3,4-thiadiazol-2-y...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents | Article PubMed | 4 | -47.9 | n/a | n/a | n/a | n/a | n/a | 7.8 | 25 |
Aventis Pharma Deutschland GmbH | Assay Description The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrates S-2765. The hydrolysis rates of chromogenic s... | Bioorg Med Chem Lett 14: 4191-5 (2004) Article DOI: 10.1016/j.bmcl.2004.06.020 BindingDB Entry DOI: 10.7270/Q22805WQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50590086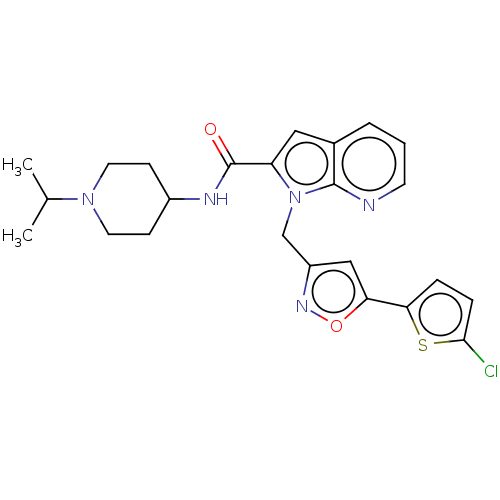 (CHEMBL5192536) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.2c00865 BindingDB Entry DOI: 10.7270/Q2BP06RZ | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 12 (Homo sapiens (Human)) | BDBM50397157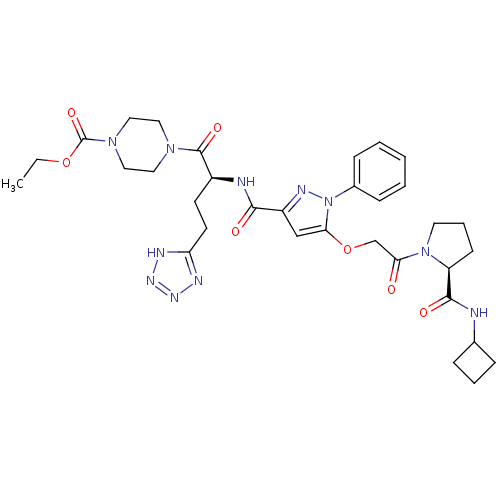 (CHEMBL2172128) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Aventis Deutschland GmbH Curated by ChEMBL | Assay Description Displacement of [33P]2-MeS-ADP from human recombinant P2Y12 receptor expressed in CHO cell membranes by scintillation counting method | J Med Chem 55: 8615-29 (2012) Article DOI: 10.1021/jm300771j BindingDB Entry DOI: 10.7270/Q2D50P3P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM12375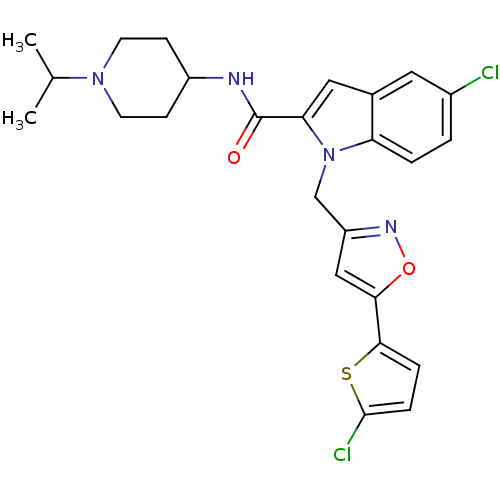 (2-Carboxyindole Scaffold 26 | 5-Chloro-1-[5-(5-chl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 5 | -47.4 | n/a | n/a | n/a | n/a | n/a | 7.8 | 25 |
Aventis Pharma | Assay Description The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrates S-2765. The hydrolysis rates of chromogenic s... | J Med Chem 48: 4511-25 (2005) Article DOI: 10.1021/jm0490540 BindingDB Entry DOI: 10.7270/Q21834RB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 12 (Homo sapiens (Human)) | BDBM50397164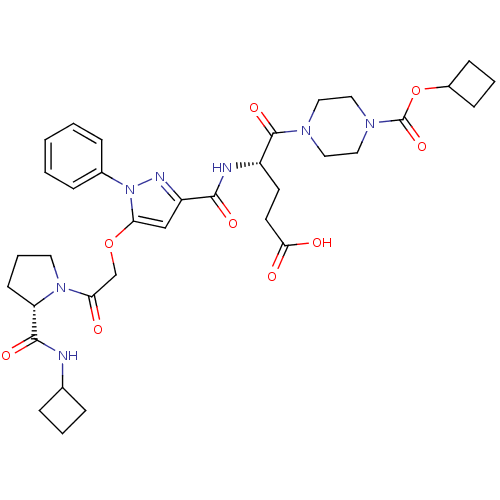 (CHEMBL2172150) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Aventis Deutschland GmbH Curated by ChEMBL | Assay Description Displacement of [33P]2-MeS-ADP from human recombinant P2Y12 receptor expressed in CHO cell membranes by scintillation counting method | J Med Chem 55: 8615-29 (2012) Article DOI: 10.1021/jm300771j BindingDB Entry DOI: 10.7270/Q2D50P3P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 12 (Homo sapiens (Human)) | BDBM50397168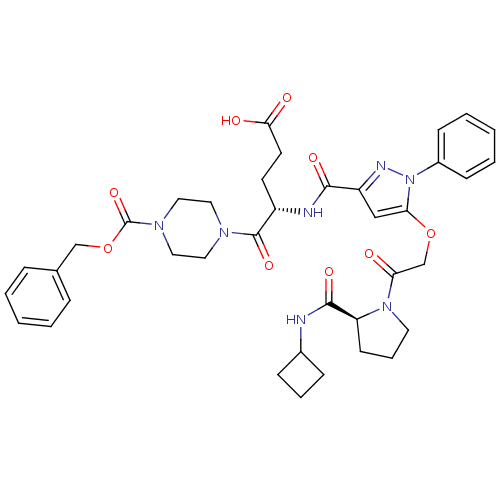 (CHEMBL2172146) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Aventis Deutschland GmbH Curated by ChEMBL | Assay Description Displacement of [33P]2-MeS-ADP from human recombinant P2Y12 receptor expressed in CHO cell membranes by scintillation counting method | J Med Chem 55: 8615-29 (2012) Article DOI: 10.1021/jm300771j BindingDB Entry DOI: 10.7270/Q2D50P3P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50590082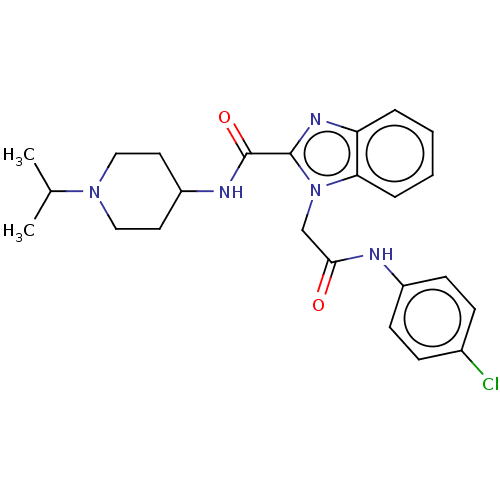 (CHEMBL5175184) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.2c00865 BindingDB Entry DOI: 10.7270/Q2BP06RZ | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 12 (Homo sapiens (Human)) | BDBM50397152 (CHEMBL2172133) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Aventis Deutschland GmbH Curated by ChEMBL | Assay Description Displacement of [33P]2-MeS-ADP from human recombinant P2Y12 receptor expressed in CHO cell membranes by scintillation counting method | J Med Chem 55: 8615-29 (2012) Article DOI: 10.1021/jm300771j BindingDB Entry DOI: 10.7270/Q2D50P3P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM12383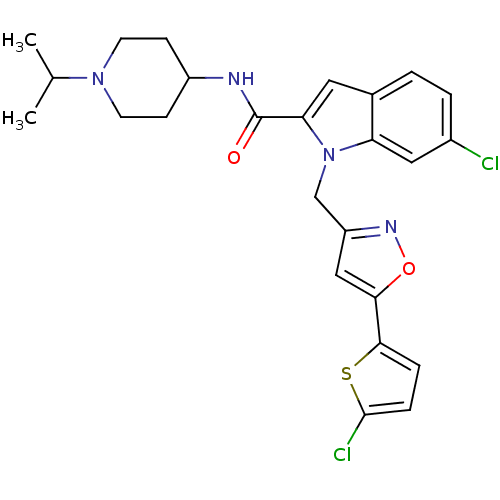 (2-Carboxyindole Scaffold 34 | 6-Chloro-1-[5-(5-chl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 7 | -46.5 | n/a | n/a | n/a | n/a | n/a | 7.8 | 25 |
Aventis Pharma | Assay Description The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrates S-2765. The hydrolysis rates of chromogenic s... | J Med Chem 48: 4511-25 (2005) Article DOI: 10.1021/jm0490540 BindingDB Entry DOI: 10.7270/Q21834RB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM12397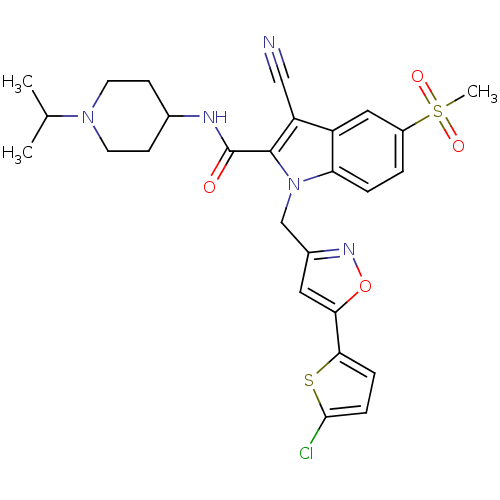 (1-[5-(5-Chloro-thiophen-2-yl)-isoxazol-3-ylmethyl]...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharma | Assay Description The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrates S-2765. The hydrolysis rates of chromogenic s... | J Med Chem 48: 4511-25 (2005) Article DOI: 10.1021/jm0490540 BindingDB Entry DOI: 10.7270/Q21834RB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50590073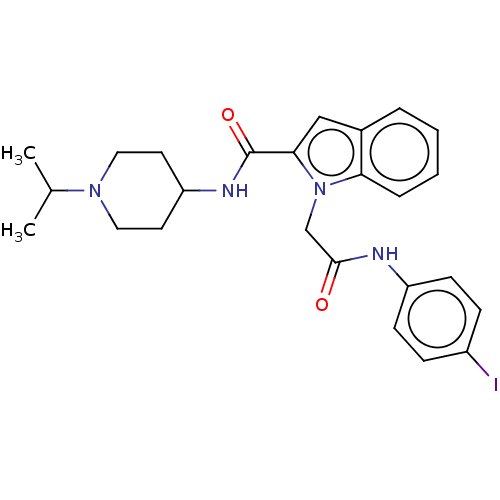 (CHEMBL5175600) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.2c00865 BindingDB Entry DOI: 10.7270/Q2BP06RZ | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM12382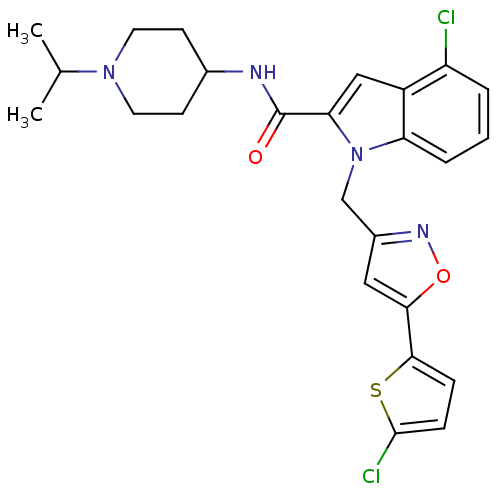 (2-Carboxyindole Scaffold 33 | 4-Chloro-1-[5-(5-chl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 7 | -46.5 | n/a | n/a | n/a | n/a | n/a | 7.8 | 25 |
Aventis Pharma | Assay Description The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrates S-2765. The hydrolysis rates of chromogenic s... | J Med Chem 48: 4511-25 (2005) Article DOI: 10.1021/jm0490540 BindingDB Entry DOI: 10.7270/Q21834RB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 12 (Homo sapiens (Human)) | BDBM50397144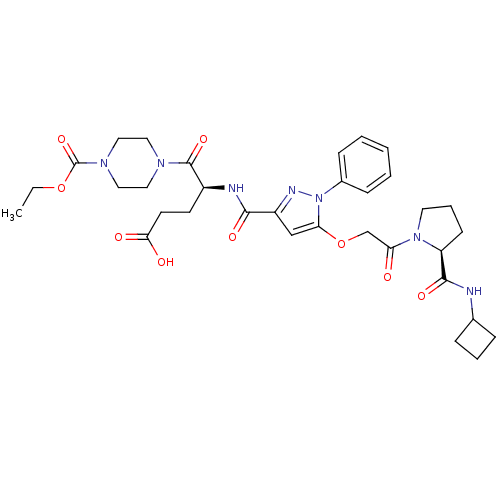 (CHEMBL2172272) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Aventis Deutschland GmbH Curated by ChEMBL | Assay Description Displacement of [33P]2-MeS-ADP from human recombinant P2Y12 receptor expressed in CHO cell membranes by scintillation counting method | J Med Chem 55: 8615-29 (2012) Article DOI: 10.1021/jm300771j BindingDB Entry DOI: 10.7270/Q2D50P3P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 12 (Homo sapiens (Human)) | BDBM50397143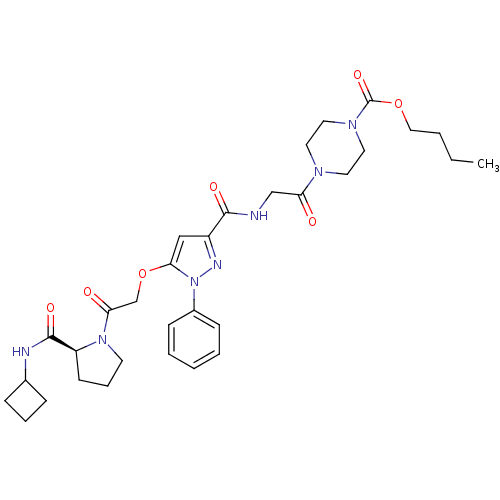 (CHEMBL2172140) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Aventis Deutschland GmbH Curated by ChEMBL | Assay Description Displacement of [33P]2-MeS-ADP from human recombinant P2Y12 receptor expressed in CHO cell membranes by scintillation counting method | J Med Chem 55: 8615-29 (2012) Article DOI: 10.1021/jm300771j BindingDB Entry DOI: 10.7270/Q2D50P3P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 12 (Homo sapiens (Human)) | BDBM50397163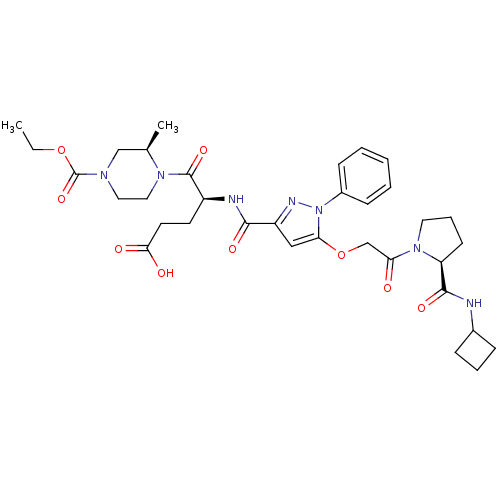 (CHEMBL2172151) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Aventis Deutschland GmbH Curated by ChEMBL | Assay Description Displacement of [33P]2-MeS-ADP from human recombinant P2Y12 receptor expressed in CHO cell membranes by scintillation counting method | J Med Chem 55: 8615-29 (2012) Article DOI: 10.1021/jm300771j BindingDB Entry DOI: 10.7270/Q2D50P3P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50590087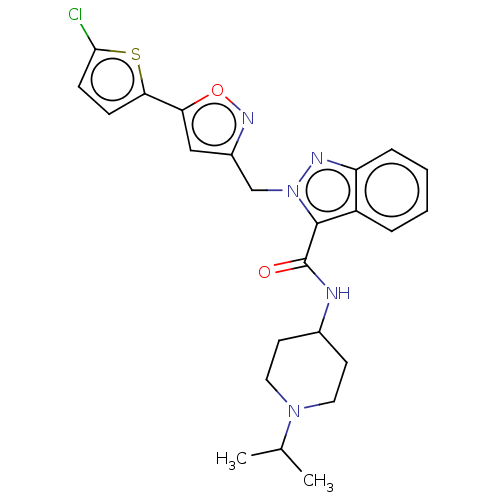 (CHEMBL5179837) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.2c00865 BindingDB Entry DOI: 10.7270/Q2BP06RZ | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM12378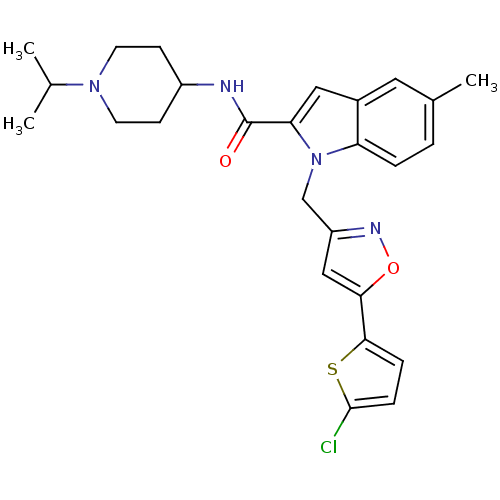 (1-[5-(5-Chloro-thiophen-2-yl)-isoxazol-3-ylmethyl]...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 9 | -45.9 | n/a | n/a | n/a | n/a | n/a | 7.8 | 25 |
Aventis Pharma | Assay Description The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrates S-2765. The hydrolysis rates of chromogenic s... | J Med Chem 48: 4511-25 (2005) Article DOI: 10.1021/jm0490540 BindingDB Entry DOI: 10.7270/Q21834RB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM12376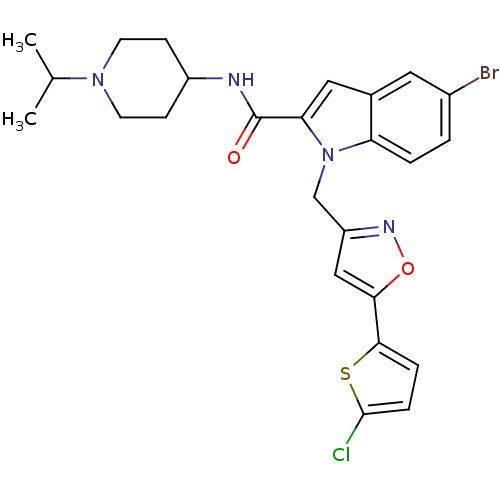 (2-Carboxyindole Scaffold 27 | 5-Bromo-1-[5-(5-chlo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 9 | -45.9 | n/a | n/a | n/a | n/a | n/a | 7.8 | 25 |
Aventis Pharma | Assay Description The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrates S-2765. The hydrolysis rates of chromogenic s... | J Med Chem 48: 4511-25 (2005) Article DOI: 10.1021/jm0490540 BindingDB Entry DOI: 10.7270/Q21834RB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50590097 (CHEMBL5187449) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.2c00865 BindingDB Entry DOI: 10.7270/Q2BP06RZ | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM13662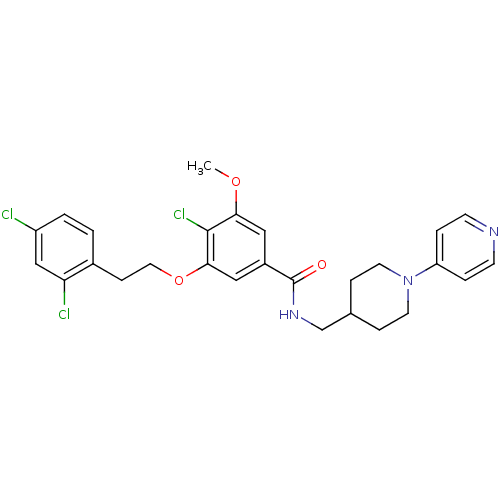 (3-Oxybenzamide 48 | 4-chloro-3-[2-(2,4-dichlorophe...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharma Deutschland GmbH Curated by ChEMBL | Assay Description Inhibitory activity against human Coagulation factor X | Bioorg Med Chem Lett 14: 2801-5 (2004) Article DOI: 10.1016/j.bmcl.2004.03.059 BindingDB Entry DOI: 10.7270/Q2XS5WW7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50590083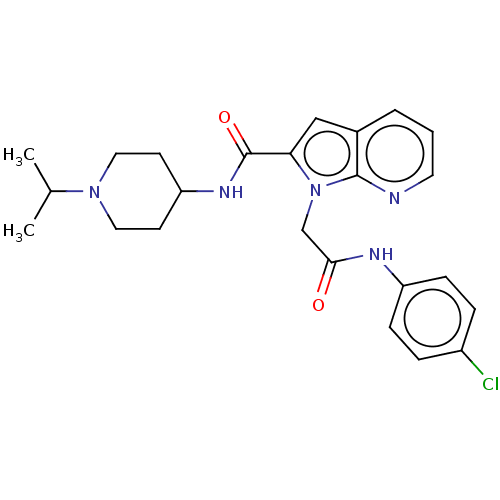 (CHEMBL5192808) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.2c00865 BindingDB Entry DOI: 10.7270/Q2BP06RZ | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor VII (Homo sapiens (Human)) | BDBM50148952 (CHEMBL429152 | N-[4-(1-{2-[3-(4-Carbamimidoyl-phen...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharma Deutschland GmbH Curated by ChEMBL | Assay Description In vitro inhibition of human coagulation factor VIIa | Bioorg Med Chem Lett 14: 3715-20 (2004) Article DOI: 10.1016/j.bmcl.2004.05.006 BindingDB Entry DOI: 10.7270/Q2PG1R6T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 781 total ) | Next | Last >> |