 Found 45246 hits with Last Name = 'lee' and Initial = 'j'
Found 45246 hits with Last Name = 'lee' and Initial = 'j' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50434903
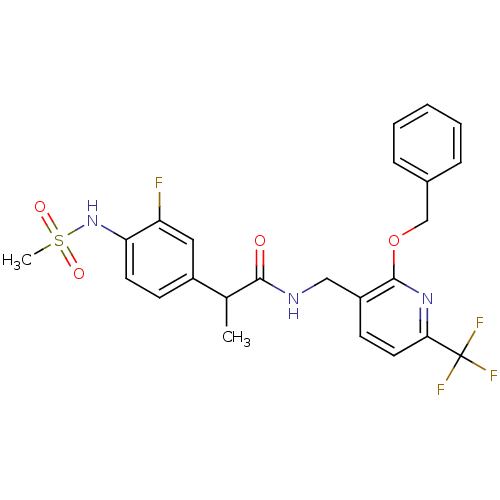
(CHEMBL2385223)Show SMILES CC(C(=O)NCc1ccc(nc1OCc1ccccc1)C(F)(F)F)c1ccc(NS(C)(=O)=O)c(F)c1 Show InChI InChI=1S/C24H23F4N3O4S/c1-15(17-8-10-20(19(25)12-17)31-36(2,33)34)22(32)29-13-18-9-11-21(24(26,27)28)30-23(18)35-14-16-6-4-3-5-7-16/h3-12,15,31H,13-14H2,1-2H3,(H,29,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.00100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University
Curated by ChEMBL
| Assay Description
Antagonist activity at human TRPV1 expressed in CHOK1 cells assessed as inhibition of N-arachidonoyldopamine-induced activity after 5 mins by FLIPR a... |
Eur J Med Chem 64: 589-602 (2013)
Article DOI: 10.1016/j.ejmech.2013.04.003
BindingDB Entry DOI: 10.7270/Q2BZ67FC |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4C
(Rattus norvegicus) | BDBM14361
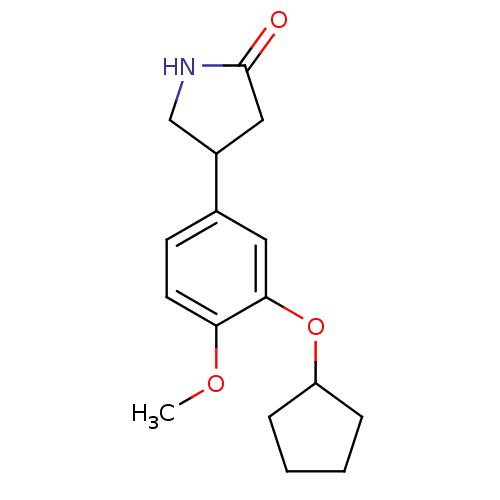
((R,S)-Rolipram | 4-(3-cyclopentyloxy-4-methoxy-phe...)Show InChI InChI=1S/C16H21NO3/c1-19-14-7-6-11(12-9-16(18)17-10-12)8-15(14)20-13-4-2-3-5-13/h6-8,12-13H,2-5,9-10H2,1H3,(H,17,18) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 0.00230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
LEAD GENE CO., LTD
Curated by ChEMBL
| Assay Description
Binding affinity towards rolipram binding site on phosphodiesterase 4 using [3H]rolipram as radioligand in crude rat brain homogenate |
Bioorg Med Chem Lett 13: 2355-8 (2003)
BindingDB Entry DOI: 10.7270/Q2CZ39CG |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4
(Rattus norvegicus (Rat)) | BDBM86812

(CAS_45263784 | NSC_45263784 | rac-2-(6-fluoro-5-(4...)Show SMILES [O-][N+](=O)c1ccc(cc1)-c1cc(cnc1F)C1CC2CCC1N2 |TLB:11:16:19.20:22| Show InChI InChI=1S/C17H16FN3O2/c18-17-15(10-1-4-13(5-2-10)21(22)23)7-11(9-19-17)14-8-12-3-6-16(14)20-12/h1-2,4-5,7,9,12,14,16,20H,3,6,8H2 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.00900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute
Curated by PDSP Ki Database
| |
Bioorg Med Chem 16: 746-54 (2008)
Article DOI: 10.1016/j.bmc.2007.10.027
BindingDB Entry DOI: 10.7270/Q2H130KC |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(Homo sapiens (Human)) | BDBM50095105
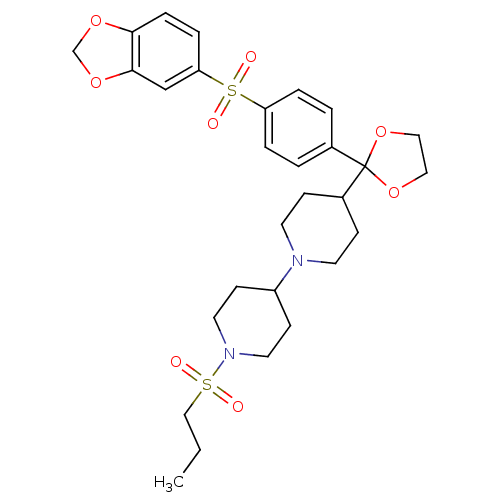
(4-{2-[4-(Benzo[1,3]dioxole-5-sulfonyl)-phenyl]-[1,...)Show SMILES CCCS(=O)(=O)N1CCC(CC1)N1CCC(CC1)C1(OCCO1)c1ccc(cc1)S(=O)(=O)c1ccc2OCOc2c1 Show InChI InChI=1S/C29H38N2O8S2/c1-2-19-40(32,33)31-15-11-24(12-16-31)30-13-9-23(10-14-30)29(38-17-18-39-29)22-3-5-25(6-4-22)41(34,35)26-7-8-27-28(20-26)37-21-36-27/h3-8,20,23-24H,2,9-19,21H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Antagonistic activity of the compound against Muscarinic acetylcholine receptor M2 |
Bioorg Med Chem Lett 11: 2311-4 (2001)
BindingDB Entry DOI: 10.7270/Q28S4P7Z |
More data for this
Ligand-Target Pair | |
Calcitonin gene-related peptide 1
(Homo sapiens (Human)) | BDBM50356281
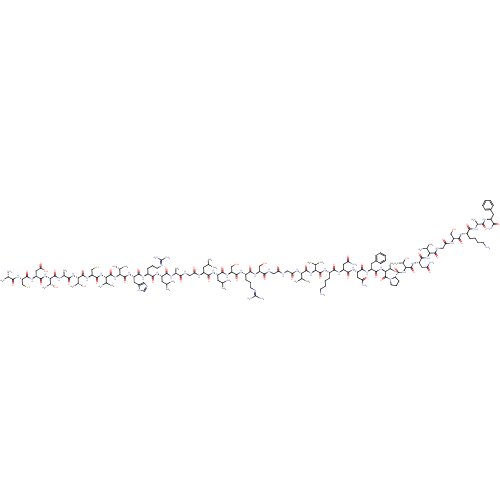
(CHEMBL1910953)Show SMILES CC(C)C[C@H](NC(=O)CNC(=O)[C@H](C)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@@H](NC(=O)[C@@H](NC(=O)[C@H](CS)NC(=O)[C@@H](NC(=O)[C@H](C)NC(=O)[C@@H](NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CS)NC(=O)[C@H](C)N)[C@@H](C)O)[C@@H](C)O)C(C)C)[C@@H](C)O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CO)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CO)C(=O)NCC(=O)NCC(=O)N[C@@H](C(C)C)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](C(C)C)C(=O)N1CCC[C@H]1C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](C(C)C)C(=O)NCC(=O)N[C@@H](CO)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](C)C(=O)N[C@@H](Cc1ccccc1)C(N)=O |r| Show InChI InChI=1S/C163H269N51O49S2/c1-73(2)52-97(186-116(226)65-179-131(233)82(18)183-139(241)98(53-74(3)4)193-137(239)94(44-35-49-176-162(171)172)188-142(244)101(57-91-62-175-72-182-91)199-159(261)128(88(24)221)213-156(258)123(79(13)14)207-151(253)110(71-265)204-160(262)126(86(22)219)210-133(235)84(20)185-157(259)125(85(21)218)211-147(249)105(61-119(229)230)198-150(252)109(70-264)203-130(232)81(17)166)140(242)194-99(54-75(5)6)141(243)202-108(69-217)149(251)190-95(45-36-50-177-163(173)174)138(240)201-106(67-215)134(236)180-63-115(225)178-64-118(228)205-121(77(9)10)155(257)208-122(78(11)12)154(256)191-93(43-32-34-48-165)136(238)196-102(58-112(167)222)144(246)197-103(59-113(168)223)143(245)195-100(56-90-40-29-26-30-41-90)145(247)209-124(80(15)16)161(263)214-51-37-46-111(214)152(254)212-127(87(23)220)158(260)200-104(60-114(169)224)146(248)206-120(76(7)8)153(255)181-66-117(227)187-107(68-216)148(250)189-92(42-31-33-47-164)135(237)184-83(19)132(234)192-96(129(170)231)55-89-38-27-25-28-39-89/h25-30,38-41,62,72-88,92-111,120-128,215-221,264-265H,31-37,42-61,63-71,164-166H2,1-24H3,(H2,167,222)(H2,168,223)(H2,169,224)(H2,170,231)(H,175,182)(H,178,225)(H,179,233)(H,180,236)(H,181,255)(H,183,241)(H,184,237)(H,185,259)(H,186,226)(H,187,227)(H,188,244)(H,189,250)(H,190,251)(H,191,256)(H,192,234)(H,193,239)(H,194,242)(H,195,245)(H,196,238)(H,197,246)(H,198,252)(H,199,261)(H,200,260)(H,201,240)(H,202,243)(H,203,232)(H,204,262)(H,205,228)(H,206,248)(H,207,253)(H,208,257)(H,209,247)(H,210,235)(H,211,249)(H,212,254)(H,213,258)(H,229,230)(H4,171,172,176)(H4,173,174,177)/t81-,82-,83-,84-,85+,86+,87+,88+,92-,93-,94-,95-,96-,97-,98-,99-,100-,101-,102-,103-,104-,105-,106-,107-,108-,109-,110-,111-,120-,121-,122-,123-,124-,125-,126-,127-,128-/m0/s1 | PDB
Reactome pathway
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [125I]adrenomedullin form CGRP receptor in human SK-N-MC cell membrane by competitive binding assay in the absence of MgCl2 |
Bioorg Med Chem Lett 21: 6705-8 (2011)
Article DOI: 10.1016/j.bmcl.2011.09.056
BindingDB Entry DOI: 10.7270/Q2ZK5H25 |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50398494
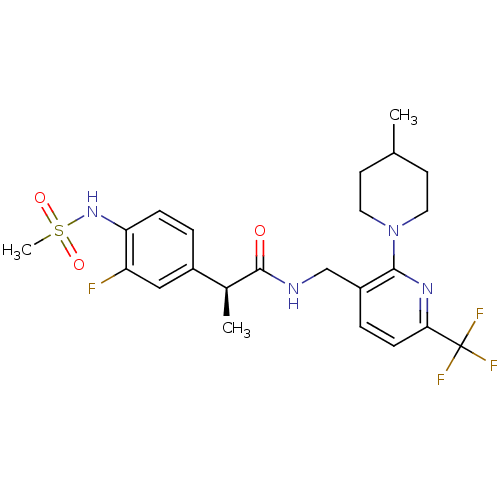
(CHEMBL2177429)Show SMILES C[C@H](C(=O)NCc1ccc(nc1N1CCC(C)CC1)C(F)(F)F)c1ccc(NS(C)(=O)=O)c(F)c1 |r| Show InChI InChI=1S/C23H28F4N4O3S/c1-14-8-10-31(11-9-14)21-17(5-7-20(29-21)23(25,26)27)13-28-22(32)15(2)16-4-6-19(18(24)12-16)30-35(3,33)34/h4-7,12,14-15,30H,8-11,13H2,1-3H3,(H,28,32)/t15-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University
Curated by ChEMBL
| Assay Description
Antagonist activity at human TRPV1 expressed in CHOK1 cells assessed as inhibition of N-acetyldopamine-induced activity after 5 mins by FLIPR assay |
Bioorg Med Chem 21: 6657-64 (2013)
Article DOI: 10.1016/j.bmc.2013.08.015
BindingDB Entry DOI: 10.7270/Q26Q1ZPN |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50144258

(4-[(8-Benzo[1,3]dioxol-5-ylmethyl-8-aza-bicyclo[3....)Show SMILES [#6]-[#6]-[#7](-[#6]-[#6])-[#6](=O)-c1ccc(cc1)-[#6](=[#6]-1/[#6]-[#6]-2-[#6]-[#6]-[#6](-[#6]-1)-[#7]-2-[#6]-c1ccc2-[#8]-[#6]-[#8]-c2c1)\c1ccccc1 Show InChI InChI=1S/C33H36N2O3/c1-3-34(4-2)33(36)26-13-11-25(12-14-26)32(24-8-6-5-7-9-24)27-19-28-15-16-29(20-27)35(28)21-23-10-17-30-31(18-23)38-22-37-30/h5-14,17-18,28-29H,3-4,15-16,19-22H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Binding affinity for delta opioid receptor |
Bioorg Med Chem Lett 14: 2109-12 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.051
BindingDB Entry DOI: 10.7270/Q25B01X4 |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50398494

(CHEMBL2177429)Show SMILES C[C@H](C(=O)NCc1ccc(nc1N1CCC(C)CC1)C(F)(F)F)c1ccc(NS(C)(=O)=O)c(F)c1 |r| Show InChI InChI=1S/C23H28F4N4O3S/c1-14-8-10-31(11-9-14)21-17(5-7-20(29-21)23(25,26)27)13-28-22(32)15(2)16-4-6-19(18(24)12-16)30-35(3,33)34/h4-7,12,14-15,30H,8-11,13H2,1-3H3,(H,28,32)/t15-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University
Curated by ChEMBL
| Assay Description
Antagonist activity at human TRPV1 expressed in CHOK1 cells assessed as inhibition of N-acetyldopamine-induced activity after 5 mins by FLIPR assay |
J Med Chem 55: 8392-408 (2012)
Article DOI: 10.1021/jm300780p
BindingDB Entry DOI: 10.7270/Q2TX3GH1 |
More data for this
Ligand-Target Pair | |
Glutamate carboxypeptidase 2
(Homo sapiens (Human)) | BDBM50246899
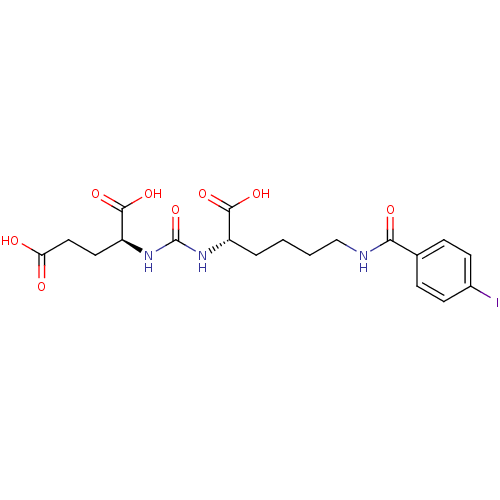
((S)-2-(3-((S)-1-carboxy-5-(4-iodobenzamido)pentyl)...)Show SMILES OC(=O)CC[C@H](NC(=O)N[C@@H](CCCCNC(=O)c1ccc(I)cc1)C(O)=O)C(O)=O |r| Show InChI InChI=1S/C19H24IN3O8/c20-12-6-4-11(5-7-12)16(26)21-10-2-1-3-13(17(27)28)22-19(31)23-14(18(29)30)8-9-15(24)25/h4-7,13-14H,1-3,8-10H2,(H,21,26)(H,24,25)(H,27,28)(H,29,30)(H2,22,23,31)/t13-,14-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yonsei University College of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of PSMA (unknown origin) |
Bioorg Med Chem Lett 28: 572-576 (2018)
Article DOI: 10.1016/j.bmcl.2018.01.047
BindingDB Entry DOI: 10.7270/Q2RN3BGJ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Calcitonin gene-related peptide 1
(Homo sapiens (Human)) | BDBM50356281

(CHEMBL1910953)Show SMILES CC(C)C[C@H](NC(=O)CNC(=O)[C@H](C)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@@H](NC(=O)[C@@H](NC(=O)[C@H](CS)NC(=O)[C@@H](NC(=O)[C@H](C)NC(=O)[C@@H](NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CS)NC(=O)[C@H](C)N)[C@@H](C)O)[C@@H](C)O)C(C)C)[C@@H](C)O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CO)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CO)C(=O)NCC(=O)NCC(=O)N[C@@H](C(C)C)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](C(C)C)C(=O)N1CCC[C@H]1C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](C(C)C)C(=O)NCC(=O)N[C@@H](CO)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](C)C(=O)N[C@@H](Cc1ccccc1)C(N)=O |r| Show InChI InChI=1S/C163H269N51O49S2/c1-73(2)52-97(186-116(226)65-179-131(233)82(18)183-139(241)98(53-74(3)4)193-137(239)94(44-35-49-176-162(171)172)188-142(244)101(57-91-62-175-72-182-91)199-159(261)128(88(24)221)213-156(258)123(79(13)14)207-151(253)110(71-265)204-160(262)126(86(22)219)210-133(235)84(20)185-157(259)125(85(21)218)211-147(249)105(61-119(229)230)198-150(252)109(70-264)203-130(232)81(17)166)140(242)194-99(54-75(5)6)141(243)202-108(69-217)149(251)190-95(45-36-50-177-163(173)174)138(240)201-106(67-215)134(236)180-63-115(225)178-64-118(228)205-121(77(9)10)155(257)208-122(78(11)12)154(256)191-93(43-32-34-48-165)136(238)196-102(58-112(167)222)144(246)197-103(59-113(168)223)143(245)195-100(56-90-40-29-26-30-41-90)145(247)209-124(80(15)16)161(263)214-51-37-46-111(214)152(254)212-127(87(23)220)158(260)200-104(60-114(169)224)146(248)206-120(76(7)8)153(255)181-66-117(227)187-107(68-216)148(250)189-92(42-31-33-47-164)135(237)184-83(19)132(234)192-96(129(170)231)55-89-38-27-25-28-39-89/h25-30,38-41,62,72-88,92-111,120-128,215-221,264-265H,31-37,42-61,63-71,164-166H2,1-24H3,(H2,167,222)(H2,168,223)(H2,169,224)(H2,170,231)(H,175,182)(H,178,225)(H,179,233)(H,180,236)(H,181,255)(H,183,241)(H,184,237)(H,185,259)(H,186,226)(H,187,227)(H,188,244)(H,189,250)(H,190,251)(H,191,256)(H,192,234)(H,193,239)(H,194,242)(H,195,245)(H,196,238)(H,197,246)(H,198,252)(H,199,261)(H,200,260)(H,201,240)(H,202,243)(H,203,232)(H,204,262)(H,205,228)(H,206,248)(H,207,253)(H,208,257)(H,209,247)(H,210,235)(H,211,249)(H,212,254)(H,213,258)(H,229,230)(H4,171,172,176)(H4,173,174,177)/t81-,82-,83-,84-,85+,86+,87+,88+,92-,93-,94-,95-,96-,97-,98-,99-,100-,101-,102-,103-,104-,105-,106-,107-,108-,109-,110-,111-,120-,121-,122-,123-,124-,125-,126-,127-,128-/m0/s1 | PDB
Reactome pathway
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [125I]adrenomedullin form CGRP receptor in human SK-N-MC cell membrane by competitive binding assay |
Bioorg Med Chem Lett 21: 6705-8 (2011)
Article DOI: 10.1016/j.bmcl.2011.09.056
BindingDB Entry DOI: 10.7270/Q2ZK5H25 |
More data for this
Ligand-Target Pair | |
Scytalone dehydratase
(Magnaporthe grisea) | BDBM50486975
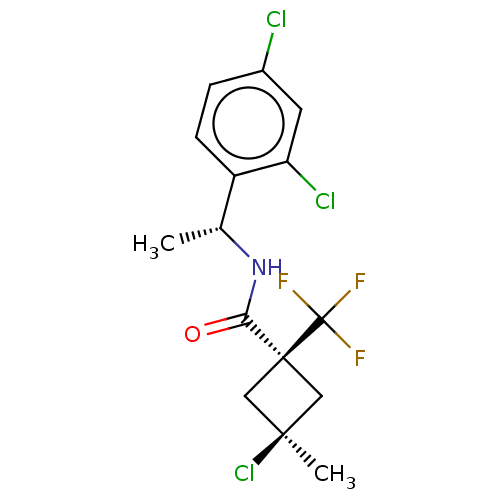
(CHEMBL2251221)Show SMILES C[C@@H](NC(=O)[C@@]1(C[C@@](C)(Cl)C1)C(F)(F)F)c1ccc(Cl)cc1Cl |r,wU:7.8,wD:5.4,1.0,(9.6,-20.54,;9.61,-22.08,;8.29,-22.87,;6.95,-22.11,;6.93,-20.57,;5.62,-22.9,;4.51,-23.96,;3.44,-22.85,;2.34,-23.93,;2.1,-22.07,;4.55,-21.79,;6.7,-23.98,;6.31,-25.47,;8.19,-23.58,;7.79,-25.07,;10.95,-22.84,;10.96,-24.38,;12.3,-25.13,;13.63,-24.35,;14.97,-25.1,;13.61,-22.8,;12.27,-22.05,;12.24,-20.51,)| Show InChI InChI=1S/C15H15Cl3F3NO/c1-8(10-4-3-9(16)5-11(10)17)22-12(23)14(15(19,20)21)6-13(2,18)7-14/h3-5,8H,6-7H2,1-2H3,(H,22,23)/t8-,13-,14+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Stine Haskell Research Center
Curated by ChEMBL
| Assay Description
Inhibition of Magnaporthe grisea scytalone dehydratase |
Bioorg Med Chem 8: 897-907 (2000)
Article DOI: 10.1016/s0968-0896(00)00034-1
BindingDB Entry DOI: 10.7270/Q2SX6H4B |
More data for this
Ligand-Target Pair | |
Scytalone dehydratase
(Magnaporthe grisea) | BDBM50486958
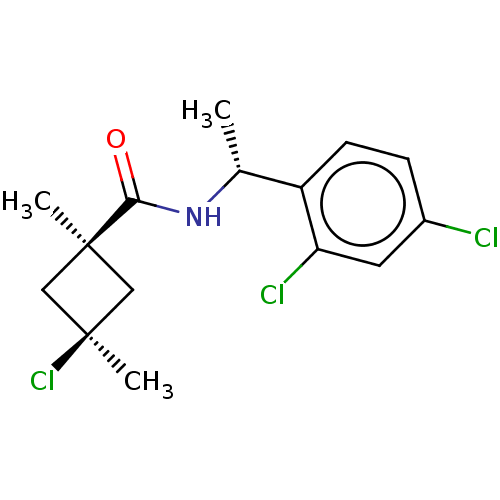
(CHEMBL2251869)Show SMILES C[C@@H](NC(=O)[C@@]1(C)C[C@@](C)(Cl)C1)c1ccc(Cl)cc1Cl |r,wU:5.4,8.9,wD:1.0,(10.61,-1.98,;10.62,-3.52,;9.3,-4.3,;7.96,-3.55,;7.94,-2.01,;6.63,-4.33,;6.62,-5.86,;5.55,-5.43,;4.45,-4.34,;3.11,-5.1,;3.35,-3.24,;5.54,-3.25,;11.97,-4.27,;11.97,-5.81,;13.31,-6.57,;14.64,-5.78,;15.98,-6.54,;14.62,-4.24,;13.28,-3.49,;13.25,-1.95,)| Show InChI InChI=1S/C15H18Cl3NO/c1-9(11-5-4-10(16)6-12(11)17)19-13(20)14(2)7-15(3,18)8-14/h4-6,9H,7-8H2,1-3H3,(H,19,20)/t9-,14-,15+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Stine Haskell Research Center
Curated by ChEMBL
| Assay Description
Inhibition of Magnaporthe grisea scytalone dehydratase |
Bioorg Med Chem 8: 897-907 (2000)
Article DOI: 10.1016/s0968-0896(00)00034-1
BindingDB Entry DOI: 10.7270/Q2SX6H4B |
More data for this
Ligand-Target Pair | |
Calcitonin gene-related peptide 1
(Homo sapiens (Human)) | BDBM50184069
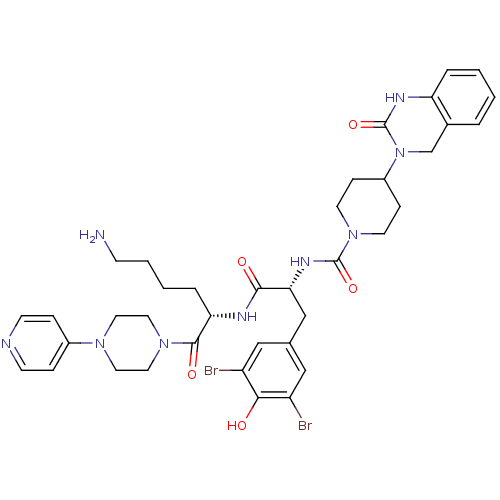
(CHEMBL207197 | N-((R)-1-((S)-6-amino-1-oxo-1-(4-(p...)Show SMILES NCCCC[C@H](NC(=O)[C@@H](Cc1cc(Br)c(O)c(Br)c1)NC(=O)N1CCC(CC1)N1Cc2ccccc2NC1=O)C(=O)N1CCN(CC1)c1ccncc1 Show InChI InChI=1S/C38H47Br2N9O5/c39-29-21-25(22-30(40)34(29)50)23-33(45-37(53)48-15-10-28(11-16-48)49-24-26-5-1-2-6-31(26)44-38(49)54)35(51)43-32(7-3-4-12-41)36(52)47-19-17-46(18-20-47)27-8-13-42-14-9-27/h1-2,5-6,8-9,13-14,21-22,28,32-33,50H,3-4,7,10-12,15-20,23-24,41H2,(H,43,51)(H,44,54)(H,45,53)/t32-,33+/m0/s1 | PDB
Reactome pathway
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.0140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [125I]adrenomedullin form CGRP receptor in human SK-N-MC cell membrane by competitive binding assay |
Bioorg Med Chem Lett 21: 6705-8 (2011)
Article DOI: 10.1016/j.bmcl.2011.09.056
BindingDB Entry DOI: 10.7270/Q2ZK5H25 |
More data for this
Ligand-Target Pair | |
Scytalone dehydratase
(Magnaporthe grisea) | BDBM50486969
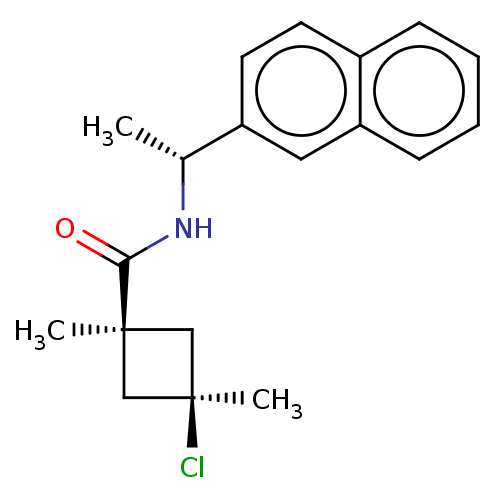
(CHEMBL2251871)Show SMILES C[C@@H](NC(=O)[C@@]1(C)C[C@@](C)(Cl)C1)c1ccc2ccccc2c1 |r,wU:5.4,8.9,wD:1.0,(40.37,-2.17,;40.39,-3.71,;39.06,-4.5,;37.72,-3.74,;37.71,-2.2,;36.4,-4.52,;36.39,-6.06,;35.31,-5.62,;34.22,-4.54,;32.88,-5.3,;33.12,-3.44,;35.3,-3.44,;41.73,-4.47,;41.74,-6.01,;43.08,-6.76,;44.41,-5.98,;45.75,-6.73,;47.08,-5.93,;47.05,-4.38,;45.7,-3.64,;44.38,-4.43,;43.04,-3.68,)| Show InChI InChI=1S/C19H22ClNO/c1-13(21-17(22)18(2)11-19(3,20)12-18)15-9-8-14-6-4-5-7-16(14)10-15/h4-10,13H,11-12H2,1-3H3,(H,21,22)/t13-,18-,19+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Stine Haskell Research Center
Curated by ChEMBL
| Assay Description
Inhibition of Magnaporthe grisea scytalone dehydratase |
Bioorg Med Chem 8: 897-907 (2000)
Article DOI: 10.1016/s0968-0896(00)00034-1
BindingDB Entry DOI: 10.7270/Q2SX6H4B |
More data for this
Ligand-Target Pair | |
Scytalone dehydratase
(Magnaporthe grisea) | BDBM50486961
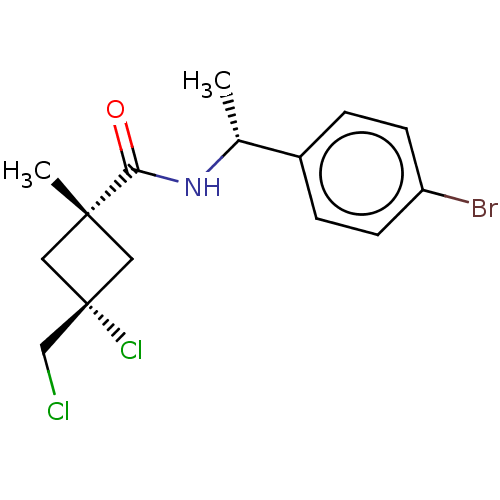
(CHEMBL2251863)Show SMILES C[C@@H](NC(=O)[C@]1(C)C[C@@](Cl)(CCl)C1)c1ccc(Br)cc1 |r,wU:8.9,wD:5.4,1.0,(9.94,-41.23,;9.96,-42.77,;8.63,-43.55,;7.29,-42.8,;7.27,-41.26,;5.97,-43.58,;5.95,-45.11,;4.88,-44.67,;3.78,-43.59,;2.68,-42.49,;2.44,-44.35,;1.11,-43.57,;4.87,-42.5,;11.3,-43.52,;11.31,-45.06,;12.65,-45.82,;13.97,-45.03,;15.32,-45.79,;13.95,-43.48,;12.61,-42.74,)| Show InChI InChI=1S/C15H18BrCl2NO/c1-10(11-3-5-12(16)6-4-11)19-13(20)14(2)7-15(18,8-14)9-17/h3-6,10H,7-9H2,1-2H3,(H,19,20)/t10-,14-,15+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Stine Haskell Research Center
Curated by ChEMBL
| Assay Description
Inhibition of Magnaporthe grisea scytalone dehydratase |
Bioorg Med Chem 8: 897-907 (2000)
Article DOI: 10.1016/s0968-0896(00)00034-1
BindingDB Entry DOI: 10.7270/Q2SX6H4B |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4C
(Rattus norvegicus) | BDBM50220995

(CHEMBL77788)Show InChI InChI=1S/C15H20N2O3/c1-11(18)17-16-10-12-7-8-14(19-2)15(9-12)20-13-5-3-4-6-13/h7-10,13H,3-6H2,1-2H3,(H,17,18)/b16-10+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
LEAD GENE CO., LTD
Curated by ChEMBL
| Assay Description
Binding affinity towards rolipram binding site on phosphodiesterase 4 using [3H]rolipram as radioligand in crude rat brain homogenate |
Bioorg Med Chem Lett 13: 2355-8 (2003)
BindingDB Entry DOI: 10.7270/Q2CZ39CG |
More data for this
Ligand-Target Pair | |
Dimer of Gag-Pol polyprotein [501-599]
(Human immunodeficiency virus type 1) | BDBM10173
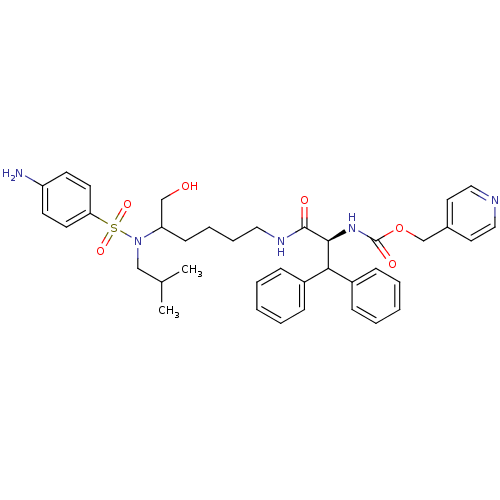
(lysine sulfonamide analogue 34 | pyridin-4-ylmethy...)Show SMILES CC(C)CN(C(CO)CCCCNC(=O)[C@@H](NC(=O)OCc1ccncc1)C(c1ccccc1)c1ccccc1)S(=O)(=O)c1ccc(N)cc1 |r| Show InChI InChI=1S/C38H47N5O6S/c1-28(2)25-43(50(47,48)34-18-16-32(39)17-19-34)33(26-44)15-9-10-22-41-37(45)36(42-38(46)49-27-29-20-23-40-24-21-29)35(30-11-5-3-6-12-30)31-13-7-4-8-14-31/h3-8,11-14,16-21,23-24,28,33,35-36,44H,9-10,15,22,25-27,39H2,1-2H3,(H,41,45)(H,42,46)/t33?,36-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0200 | n/a | 0.390 | n/a | n/a | n/a | n/a | n/a | n/a |
Ambrilia Biopharma Inc.
| Assay Description
HIV-1 protease activity was measured by a continuous fluorometric assay. The proteolytic activity can be monitored by the increase in fluorescence in... |
Bioorg Med Chem Lett 16: 3459-62 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.011
BindingDB Entry DOI: 10.7270/Q2RB72T4 |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4
(Rattus norvegicus (Rat)) | BDBM50100717
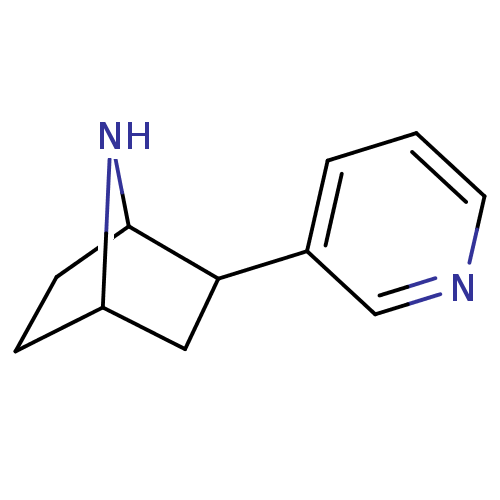
(2-(pyridin-3-yl)-7-aza-bicyclo[2.2.1]heptane | 2-P...)Show InChI InChI=1S/C11H14N2/c1-2-8(7-12-5-1)10-6-9-3-4-11(10)13-9/h1-2,5,7,9-11,13H,3-4,6H2 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute
Curated by PDSP Ki Database
| |
Bioorg Med Chem 16: 746-54 (2008)
Article DOI: 10.1016/j.bmc.2007.10.027
BindingDB Entry DOI: 10.7270/Q2H130KC |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50073160
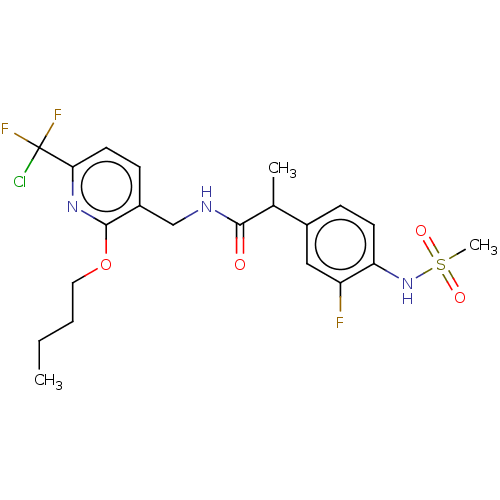
(CHEMBL3407762)Show SMILES CCCCOc1nc(ccc1CNC(=O)C(C)c1ccc(NS(C)(=O)=O)c(F)c1)C(F)(F)Cl Show InChI InChI=1S/C21H25ClF3N3O4S/c1-4-5-10-32-20-15(7-9-18(27-20)21(22,24)25)12-26-19(29)13(2)14-6-8-17(16(23)11-14)28-33(3,30)31/h6-9,11,13,28H,4-5,10,12H2,1-3H3,(H,26,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University
Curated by ChEMBL
| Assay Description
Antagonist activity at human TRPV1 expressed in CHO cells assessed as inhibition of N-arachidonoyl dopamine-induced activity by FLIPR assay |
Eur J Med Chem 93: 101-8 (2015)
Article DOI: 10.1016/j.ejmech.2015.02.001
BindingDB Entry DOI: 10.7270/Q2N0188S |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50073160

(CHEMBL3407762)Show SMILES CCCCOc1nc(ccc1CNC(=O)C(C)c1ccc(NS(C)(=O)=O)c(F)c1)C(F)(F)Cl Show InChI InChI=1S/C21H25ClF3N3O4S/c1-4-5-10-32-20-15(7-9-18(27-20)21(22,24)25)12-26-19(29)13(2)14-6-8-17(16(23)11-14)28-33(3,30)31/h6-9,11,13,28H,4-5,10,12H2,1-3H3,(H,26,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University
Curated by ChEMBL
| Assay Description
Antagonist activity at human TRPV1 expressed in CHO cells assessed as inhibition of N-arachidonoyl dopamine-induced activity by FLIPR assay |
Eur J Med Chem 93: 101-8 (2015)
Article DOI: 10.1016/j.ejmech.2015.02.001
BindingDB Entry DOI: 10.7270/Q2N0188S |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50049553
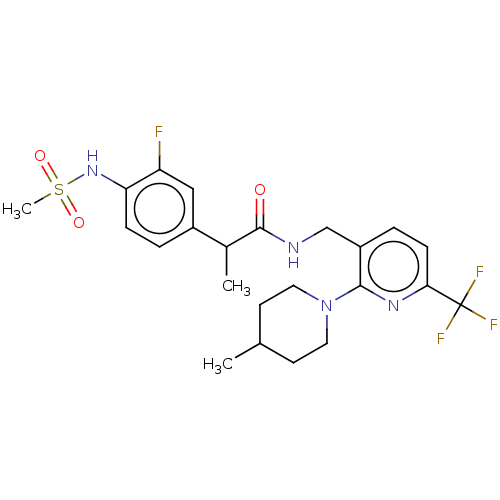
(CHEMBL2177428)Show SMILES CC(C(=O)NCc1ccc(nc1N1CCC(C)CC1)C(F)(F)F)c1ccc(NS(C)(=O)=O)c(F)c1 Show InChI InChI=1S/C23H28F4N4O3S/c1-14-8-10-31(11-9-14)21-17(5-7-20(29-21)23(25,26)27)13-28-22(32)15(2)16-4-6-19(18(24)12-16)30-35(3,33)34/h4-7,12,14-15,30H,8-11,13H2,1-3H3,(H,28,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University
Curated by ChEMBL
| Assay Description
Antagonist activity at human TRPV1 expressed in CHO cells assessed as inhibition of N-arachidonoyl dopamine-induced activity by FLIPR assay |
Eur J Med Chem 93: 101-8 (2015)
Article DOI: 10.1016/j.ejmech.2015.02.001
BindingDB Entry DOI: 10.7270/Q2N0188S |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50049553

(CHEMBL2177428)Show SMILES CC(C(=O)NCc1ccc(nc1N1CCC(C)CC1)C(F)(F)F)c1ccc(NS(C)(=O)=O)c(F)c1 Show InChI InChI=1S/C23H28F4N4O3S/c1-14-8-10-31(11-9-14)21-17(5-7-20(29-21)23(25,26)27)13-28-22(32)15(2)16-4-6-19(18(24)12-16)30-35(3,33)34/h4-7,12,14-15,30H,8-11,13H2,1-3H3,(H,28,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University
Curated by ChEMBL
| Assay Description
Antagonist activity at human TRPV1 expressed in CHO cells assessed as inhibition of N-arachidonoyl dopamine-induced activation by FLIPR assay |
Bioorg Med Chem Lett 25: 2326-30 (2015)
Article DOI: 10.1016/j.bmcl.2015.04.024
BindingDB Entry DOI: 10.7270/Q2Z60QSC |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50061298

(CHEMBL3393837)Show SMILES CC1CCN(CC1)c1nc(ccc1CNC(=O)Nc1cccc2cnccc12)C(F)(F)F Show InChI InChI=1S/C23H24F3N5O/c1-15-8-11-31(12-9-15)21-17(5-6-20(30-21)23(24,25)26)14-28-22(32)29-19-4-2-3-16-13-27-10-7-18(16)19/h2-7,10,13,15H,8-9,11-12,14H2,1H3,(H2,28,29,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shenyang Pharmaceutical University
Curated by ChEMBL
| Assay Description
Antagonist activity at human TRPV1 assessed as inhibition of NADA-induced effect at 1 uM by FLIPR assay |
Bioorg Med Chem Lett 25: 803-6 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.086
BindingDB Entry DOI: 10.7270/Q2JD4ZG3 |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50049553

(CHEMBL2177428)Show SMILES CC(C(=O)NCc1ccc(nc1N1CCC(C)CC1)C(F)(F)F)c1ccc(NS(C)(=O)=O)c(F)c1 Show InChI InChI=1S/C23H28F4N4O3S/c1-14-8-10-31(11-9-14)21-17(5-7-20(29-21)23(25,26)27)13-28-22(32)15(2)16-4-6-19(18(24)12-16)30-35(3,33)34/h4-7,12,14-15,30H,8-11,13H2,1-3H3,(H,28,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University
Curated by ChEMBL
| Assay Description
Antagonist activity at human TRPV1 expressed in CHO cells assessed as inhibition of N-arachidonoyl dopamine-induced activity by FLIPR assay |
Eur J Med Chem 93: 101-8 (2015)
Article DOI: 10.1016/j.ejmech.2015.02.001
BindingDB Entry DOI: 10.7270/Q2N0188S |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4/beta-2
(Rattus norvegicus (Rat)) | BDBM50162061

(2-(5-Ethynyl-pyridin-3-yl)-7-aza-bicyclo[2.2.1]hep...)Show SMILES C#Cc1cncc(c1)C1CC2CCC1N2 |THB:6:8:14:12.11| Show InChI InChI=1S/C13H14N2/c1-2-9-5-10(8-14-7-9)12-6-11-3-4-13(12)15-11/h1,5,7-8,11-13,15H,3-4,6H2 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute
Curated by ChEMBL
| Assay Description
Inhibition of [3H]epibatidine binding to Nicotinic acetylcholine receptor alpha4-beta2 in rat cerebral cortex |
J Med Chem 48: 1221-8 (2005)
Article DOI: 10.1021/jm040160b
BindingDB Entry DOI: 10.7270/Q26W9BV6 |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50061298

(CHEMBL3393837)Show SMILES CC1CCN(CC1)c1nc(ccc1CNC(=O)Nc1cccc2cnccc12)C(F)(F)F Show InChI InChI=1S/C23H24F3N5O/c1-15-8-11-31(12-9-15)21-17(5-6-20(30-21)23(24,25)26)14-28-22(32)29-19-4-2-3-16-13-27-10-7-18(16)19/h2-7,10,13,15H,8-9,11-12,14H2,1H3,(H2,28,29,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shenyang Pharmaceutical University
Curated by ChEMBL
| Assay Description
Antagonist activity at human TRPV1 assessed as inhibition of NADA-induced effect at 1 uM by FLIPR assay |
Bioorg Med Chem Lett 25: 803-6 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.086
BindingDB Entry DOI: 10.7270/Q2JD4ZG3 |
More data for this
Ligand-Target Pair | |
Scytalone dehydratase
(Magnaporthe grisea) | BDBM50486960

(CHEMBL2251864)Show SMILES CC[C@@]1(C[C@@](C)(Cl)C1)C(=O)N[C@H](C)c1ccc(Br)cc1 |r,wU:2.8,4.5,wD:11.12,(22.61,-45.48,;21.28,-44.7,;21.3,-43.16,;20.21,-44.26,;19.11,-43.18,;17.77,-43.94,;18.01,-42.08,;20.2,-42.08,;22.62,-42.38,;22.6,-40.84,;23.96,-43.14,;25.29,-42.36,;25.27,-40.82,;26.63,-43.11,;26.64,-44.65,;27.98,-45.4,;29.3,-44.62,;30.65,-45.37,;29.28,-43.07,;27.94,-42.32,)| Show InChI InChI=1S/C16H21BrClNO/c1-4-16(9-15(3,18)10-16)14(20)19-11(2)12-5-7-13(17)8-6-12/h5-8,11H,4,9-10H2,1-3H3,(H,19,20)/t11-,15-,16+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Stine Haskell Research Center
Curated by ChEMBL
| Assay Description
Inhibition of Magnaporthe grisea scytalone dehydratase |
Bioorg Med Chem 8: 897-907 (2000)
Article DOI: 10.1016/s0968-0896(00)00034-1
BindingDB Entry DOI: 10.7270/Q2SX6H4B |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50144236

(4-[(R)-(S)-8-Aza-bicyclo[3.2.1]oct-(3Z)-ylidene-ph...)Show SMILES CCN(CC)C(=O)c1ccc(cc1)C(=C1\C[C@@H]2CC[C@H](C1)N2)\c1ccccc1 Show InChI InChI=1S/C25H30N2O/c1-3-27(4-2)25(28)20-12-10-19(11-13-20)24(18-8-6-5-7-9-18)21-16-22-14-15-23(17-21)26-22/h5-13,22-23,26H,3-4,14-17H2,1-2H3/b24-21-/t22-,23+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Binding affinity for delta opioid receptor |
Bioorg Med Chem Lett 14: 2109-12 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.051
BindingDB Entry DOI: 10.7270/Q25B01X4 |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Rattus norvegicus (rat)) | BDBM50366620
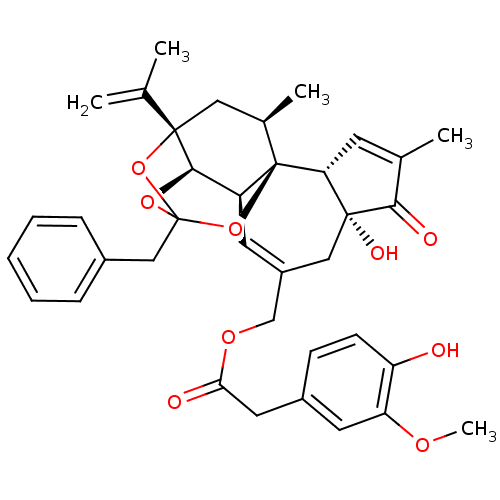
(RESINIFERATOXIN)Show SMILES COc1cc(CC(=O)OCC2=C[C@H]3[C@H]4OC5(Cc6ccccc6)O[C@@]4(C[C@@H](C)[C@]3(O5)[C@@H]3C=C(C)C(=O)[C@@]3(O)C2)C(C)=C)ccc1O |r,t:10,35,TLB:23:15:12:24.25.26,THB:16:15:12:24.25.26| Show InChI InChI=1S/C37H40O9/c1-21(2)35-17-23(4)37-27(33(35)44-36(45-35,46-37)19-24-9-7-6-8-10-24)14-26(18-34(41)30(37)13-22(3)32(34)40)20-43-31(39)16-25-11-12-28(38)29(15-25)42-5/h6-15,23,27,30,33,38,41H,1,16-20H2,2-5H3/t23-,27+,30-,33-,34-,35+,36?,37-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University
Curated by ChEMBL
| Assay Description
In vitro binding affinity towards vanilloid receptor by [3H]RTX displacement. |
Bioorg Med Chem Lett 9: 2909-14 (1999)
BindingDB Entry DOI: 10.7270/Q2HT2PTB |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Rattus norvegicus (rat)) | BDBM50366620

(RESINIFERATOXIN)Show SMILES COc1cc(CC(=O)OCC2=C[C@H]3[C@H]4OC5(Cc6ccccc6)O[C@@]4(C[C@@H](C)[C@]3(O5)[C@@H]3C=C(C)C(=O)[C@@]3(O)C2)C(C)=C)ccc1O |r,t:10,35,TLB:23:15:12:24.25.26,THB:16:15:12:24.25.26| Show InChI InChI=1S/C37H40O9/c1-21(2)35-17-23(4)37-27(33(35)44-36(45-35,46-37)19-24-9-7-6-8-10-24)14-26(18-34(41)30(37)13-22(3)32(34)40)20-43-31(39)16-25-11-12-28(38)29(15-25)42-5/h6-15,23,27,30,33,38,41H,1,16-20H2,2-5H3/t23-,27+,30-,33-,34-,35+,36?,37-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University
Curated by ChEMBL
| Assay Description
In vitro binding affinity towards vanilloid receptor by [3H]RTX displacement. |
Bioorg Med Chem Lett 9: 2909-14 (1999)
BindingDB Entry DOI: 10.7270/Q2HT2PTB |
More data for this
Ligand-Target Pair | |
Calcitonin gene-related peptide 1
(Homo sapiens (Human)) | BDBM50356282
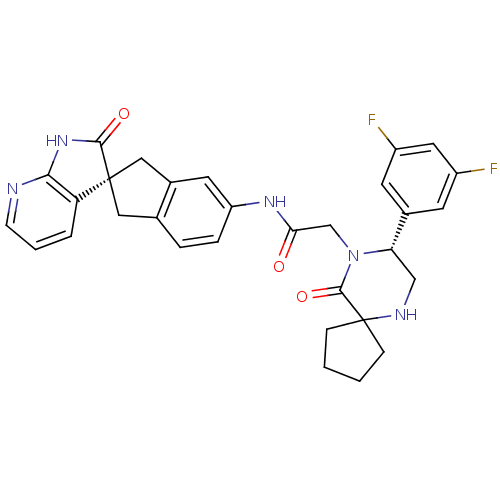
(CHEMBL1910936)Show SMILES Fc1cc(F)cc(c1)[C@@H]1CNC2(CCCC2)C(=O)N1CC(=O)Nc1ccc2C[C@]3(Cc2c1)C(=O)Nc1ncccc31 |r| Show InChI InChI=1S/C31H29F2N5O3/c32-21-10-19(11-22(33)13-21)25-16-35-31(7-1-2-8-31)29(41)38(25)17-26(39)36-23-6-5-18-14-30(15-20(18)12-23)24-4-3-9-34-27(24)37-28(30)40/h3-6,9-13,25,35H,1-2,7-8,14-17H2,(H,36,39)(H,34,37,40)/t25-,30+/m0/s1 | PDB
Reactome pathway
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [125I]adrenomedullin form CGRP receptor in human SK-N-MC cell membrane by competitive binding assay |
Bioorg Med Chem Lett 21: 6705-8 (2011)
Article DOI: 10.1016/j.bmcl.2011.09.056
BindingDB Entry DOI: 10.7270/Q2ZK5H25 |
More data for this
Ligand-Target Pair | |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Escherichia coli) | BDBM50493080
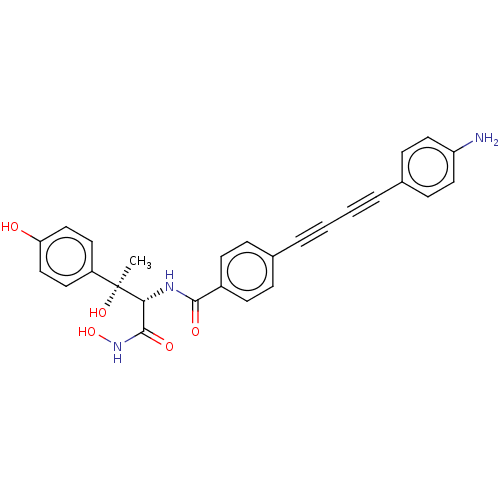
(CHEMBL2420203)Show SMILES C[C@@](O)([C@H](NC(=O)c1ccc(cc1)C#CC#Cc1ccc(N)cc1)C(=O)NO)c1ccc(O)cc1 |r| Show InChI InChI=1S/C27H23N3O5/c1-27(34,21-12-16-23(31)17-13-21)24(26(33)30-35)29-25(32)20-10-6-18(7-11-20)4-2-3-5-19-8-14-22(28)15-9-19/h6-17,24,31,34-35H,28H2,1H3,(H,29,32)(H,30,33)/t24-,27+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 0.0240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Duke University
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli LpxC enzyme using UDP-3-O-[(R)-3-hydroxymyristoyl]-N-acetyl glucosamine and [gamma-32P] UDP-3-O-[(R)-3-hydroxymyristoy... |
J Med Chem 56: 6954-6966 (2013)
Article DOI: 10.1021/jm4007774
BindingDB Entry DOI: 10.7270/Q28K7D11 |
More data for this
Ligand-Target Pair | |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Escherichia coli) | BDBM50493081
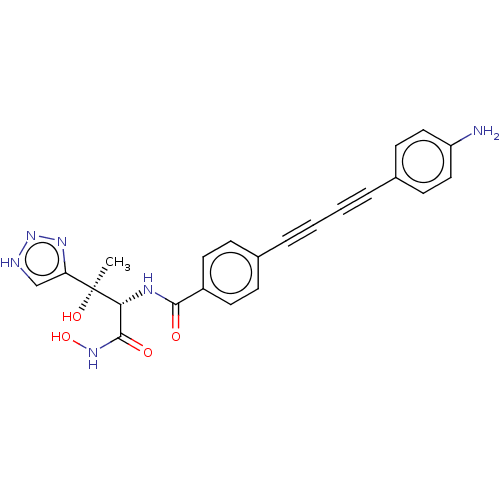
(CHEMBL2420205)Show SMILES C[C@@](O)([C@H](NC(=O)c1ccc(cc1)C#CC#Cc1ccc(N)cc1)C(=O)NO)c1c[nH]nn1 |r| Show InChI InChI=1S/C23H20N6O4/c1-23(32,19-14-25-29-27-19)20(22(31)28-33)26-21(30)17-10-6-15(7-11-17)4-2-3-5-16-8-12-18(24)13-9-16/h6-14,20,32-33H,24H2,1H3,(H,26,30)(H,28,31)(H,25,27,29)/t20-,23+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Duke University
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli LpxC enzyme using UDP-3-O-[(R)-3-hydroxymyristoyl]-N-acetyl glucosamine and [gamma-32P] UDP-3-O-[(R)-3-hydroxymyristoy... |
J Med Chem 56: 6954-6966 (2013)
Article DOI: 10.1021/jm4007774
BindingDB Entry DOI: 10.7270/Q28K7D11 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50144229
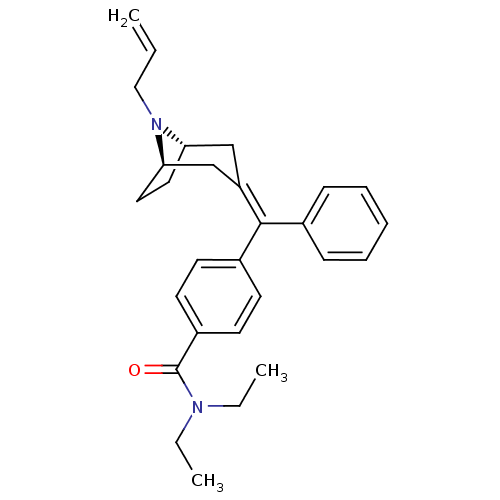
(4-{[(1S,5R)-8-Allyl-8-aza-bicyclo[3.2.1]oct-(3Z)-y...)Show SMILES CCN(CC)C(=O)c1ccc(cc1)C(=C1\C[C@@H]2CC[C@H](C1)N2CC=C)\c1ccccc1 |THB:22:21:14.15.20:17.18| Show InChI InChI=1S/C28H34N2O/c1-4-18-30-25-16-17-26(30)20-24(19-25)27(21-10-8-7-9-11-21)22-12-14-23(15-13-22)28(31)29(5-2)6-3/h4,7-15,25-26H,1,5-6,16-20H2,2-3H3/b27-24-/t25-,26+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Effective concentration against stimulation of [35S]-GTP-gammaS, binding in CHO cells transfected with the human opioid receptor delta 1 |
Bioorg Med Chem Lett 14: 2109-12 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.051
BindingDB Entry DOI: 10.7270/Q25B01X4 |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4
(Rattus norvegicus (Rat)) | BDBM50049757

(()-2-(6-Chloro-pyridin-3-yl)-7-aza-bicyclo[2.2.1]h...)Show InChI InChI=1S/C11H13ClN2/c12-11-4-1-7(6-13-11)9-5-8-2-3-10(9)14-8/h1,4,6,8-10,14H,2-3,5H2 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.0260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute
Curated by PDSP Ki Database
| |
Bioorg Med Chem 16: 746-54 (2008)
Article DOI: 10.1016/j.bmc.2007.10.027
BindingDB Entry DOI: 10.7270/Q2H130KC |
More data for this
Ligand-Target Pair | |
Scytalone dehydratase
(Magnaporthe grisea) | BDBM50486966

(CHEMBL2114191)Show SMILES C[C@@H](NC(=O)[C@]1(C[C@@](C)(Cl)C1)C(F)(F)F)c1ccc(Br)cc1 |wU:5.4,wD:7.7,1.0,(6.11,-1.45,;6.11,-2.99,;4.79,-3.76,;3.47,-2.99,;3.47,-1.45,;1.92,-2.99,;.83,-4.07,;-.25,-2.98,;-1.03,-1.65,;-1.34,-4.05,;.84,-1.9,;2.32,-4.47,;2.71,-5.97,;1.28,-5.9,;3.92,-5.2,;7.45,-3.76,;7.45,-5.3,;8.79,-6.07,;10.12,-5.3,;11.45,-6.07,;10.12,-3.76,;8.79,-2.99,)| Show InChI InChI=1S/C15H16BrClF3NO/c1-9(10-3-5-11(16)6-4-10)21-12(22)14(15(18,19)20)7-13(2,17)8-14/h3-6,9H,7-8H2,1-2H3,(H,21,22)/t9-,13-,14-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Stine Haskell Research Center
Curated by ChEMBL
| Assay Description
Inhibition of Magnaporthe grisea scytalone dehydratase |
Bioorg Med Chem 8: 897-907 (2000)
Article DOI: 10.1016/s0968-0896(00)00034-1
BindingDB Entry DOI: 10.7270/Q2SX6H4B |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4/beta-2
(Rattus norvegicus (Rat)) | BDBM50049757

(()-2-(6-Chloro-pyridin-3-yl)-7-aza-bicyclo[2.2.1]h...)Show InChI InChI=1S/C11H13ClN2/c12-11-4-1-7(6-13-11)9-5-8-2-3-10(9)14-8/h1,4,6,8-10,14H,2-3,5H2 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.0260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute
Curated by ChEMBL
| Assay Description
Inhibition of [3H]epibatidine binding to Nicotinic acetylcholine receptor alpha4-beta2 in rat cerebral cortex |
J Med Chem 48: 1221-8 (2005)
Article DOI: 10.1021/jm040160b
BindingDB Entry DOI: 10.7270/Q26W9BV6 |
More data for this
Ligand-Target Pair | |
Scytalone dehydratase
(Magnaporthe grisea) | BDBM50486974
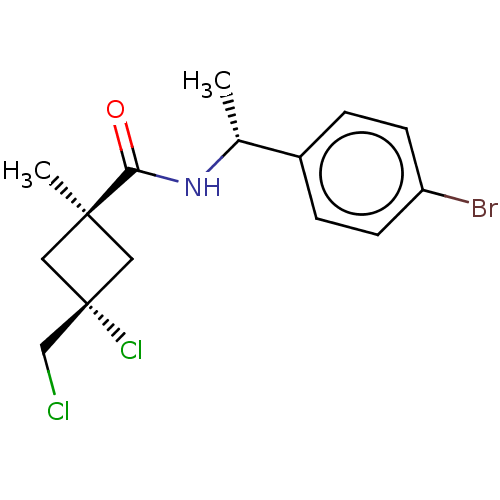
(CHEMBL2251862)Show SMILES C[C@@H](NC(=O)[C@@]1(C)C[C@@](Cl)(CCl)C1)c1ccc(Br)cc1 |r,wU:5.4,8.9,wD:1.0,(40.03,-33.88,;40.05,-35.42,;38.72,-36.2,;37.38,-35.45,;37.37,-33.91,;36.06,-36.23,;36.04,-37.76,;34.97,-37.32,;33.88,-36.24,;32.54,-37,;32.78,-35.14,;33.17,-33.65,;34.96,-35.15,;41.39,-36.17,;41.4,-37.71,;42.74,-38.47,;44.07,-37.68,;45.41,-38.44,;44.05,-36.13,;42.7,-35.39,)| Show InChI InChI=1S/C15H18BrCl2NO/c1-10(11-3-5-12(16)6-4-11)19-13(20)14(2)7-15(18,8-14)9-17/h3-6,10H,7-9H2,1-2H3,(H,19,20)/t10-,14-,15-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Stine Haskell Research Center
Curated by ChEMBL
| Assay Description
Inhibition of Magnaporthe grisea scytalone dehydratase |
Bioorg Med Chem 8: 897-907 (2000)
Article DOI: 10.1016/s0968-0896(00)00034-1
BindingDB Entry DOI: 10.7270/Q2SX6H4B |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4
(Rattus norvegicus (Rat)) | BDBM86815
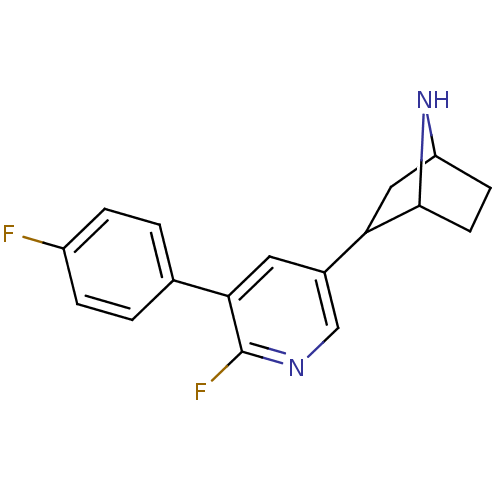
(CAS_45266019 | NSC_45266019 | rac-2-(6-fluoro-5-(4...)Show SMILES Fc1ccc(cc1)-c1cc(cnc1F)C1CC2CCC1N2 |TLB:9:14:17.18:20| Show InChI InChI=1S/C17H16F2N2/c18-12-3-1-10(2-4-12)15-7-11(9-20-17(15)19)14-8-13-5-6-16(14)21-13/h1-4,7,9,13-14,16,21H,5-6,8H2 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute
Curated by PDSP Ki Database
| |
Bioorg Med Chem 16: 746-54 (2008)
Article DOI: 10.1016/j.bmc.2007.10.027
BindingDB Entry DOI: 10.7270/Q2H130KC |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4/beta-2
(Rattus norvegicus (Rat)) | BDBM50162062

(2-(5-Iodo-pyridin-3-yl)-7-methyl-7-aza-bicyclo[2.2...)Show InChI InChI=1S/C12H15IN2/c1-15-10-2-3-12(15)11(5-10)8-4-9(13)7-14-6-8/h4,6-7,10-12H,2-3,5H2,1H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute
Curated by ChEMBL
| Assay Description
Inhibition of [3H]epibatidine binding to Nicotinic acetylcholine receptor alpha4-beta2 in rat cerebral cortex |
J Med Chem 48: 1221-8 (2005)
Article DOI: 10.1021/jm040160b
BindingDB Entry DOI: 10.7270/Q26W9BV6 |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50128922
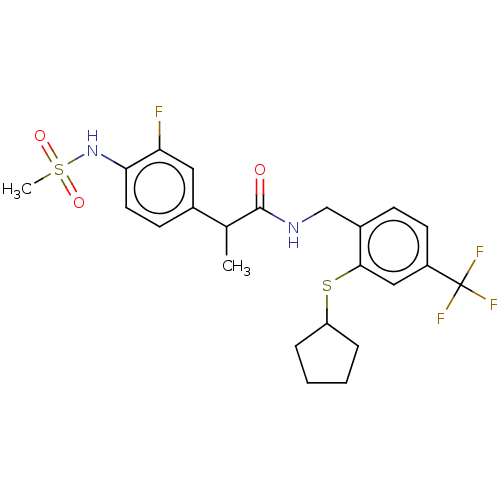
(CHEMBL3627950)Show SMILES CC(C(=O)NCc1ccc(cc1SC1CCCC1)C(F)(F)F)c1ccc(NS(C)(=O)=O)c(F)c1 Show InChI InChI=1S/C23H26F4N2O3S2/c1-14(15-8-10-20(19(24)11-15)29-34(2,31)32)22(30)28-13-16-7-9-17(23(25,26)27)12-21(16)33-18-5-3-4-6-18/h7-12,14,18,29H,3-6,13H2,1-2H3,(H,28,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University
Curated by ChEMBL
| Assay Description
Antagonist activity at human TRPV1 heterologously expressed in CHO cells assessed as inhibition of NADA-induced activation by FLIPR assay |
Bioorg Med Chem 23: 6844-54 (2015)
Article DOI: 10.1016/j.bmc.2015.10.001
BindingDB Entry DOI: 10.7270/Q2DZ0B4X |
More data for this
Ligand-Target Pair | |
Scytalone dehydratase
(Magnaporthe grisea) | BDBM50486972

(CHEMBL2251872)Show SMILES COc1cc(Br)ccc1C(C)NC(=O)[C@@]1(C)C[C@@](C)(Cl)C1 |r,wU:14.14,17.19,(13.59,-9.79,;12.27,-10.58,;12.29,-12.12,;13.63,-12.87,;13.65,-14.42,;15,-15.17,;12.33,-15.2,;10.99,-14.45,;10.98,-12.91,;9.64,-12.15,;9.62,-10.61,;8.31,-12.94,;6.97,-12.18,;6.95,-10.64,;5.65,-12.96,;5.63,-14.5,;4.56,-14.06,;3.46,-12.97,;2.12,-13.74,;2.36,-11.88,;4.55,-11.88,)| Show InChI InChI=1S/C16H21BrClNO2/c1-10(12-6-5-11(17)7-13(12)21-4)19-14(20)15(2)8-16(3,18)9-15/h5-7,10H,8-9H2,1-4H3,(H,19,20)/t10?,15-,16+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Stine Haskell Research Center
Curated by ChEMBL
| Assay Description
Inhibition of Magnaporthe grisea scytalone dehydratase |
Bioorg Med Chem 8: 897-907 (2000)
Article DOI: 10.1016/s0968-0896(00)00034-1
BindingDB Entry DOI: 10.7270/Q2SX6H4B |
More data for this
Ligand-Target Pair | |
Pyruvate decarboxylase
(Zymomonas mobilis subsp. mobilis (strain ATCC 3182...) | BDBM50589544
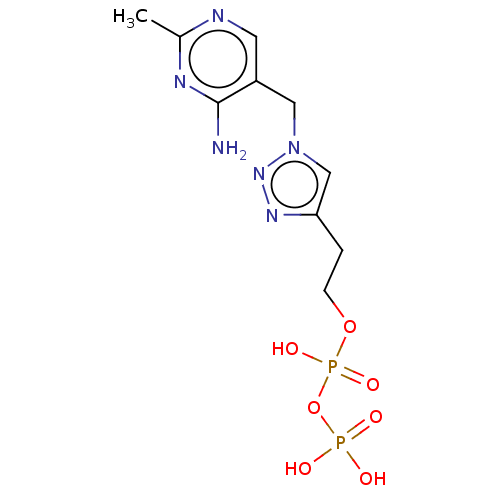
(CHEMBL3559521)Show SMILES Cc1ncc(Cn2cc(CCOP(O)(=O)OP(O)(O)=O)nn2)c(N)n1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1039/d2md00085g
BindingDB Entry DOI: 10.7270/Q2M90DMB |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50128911

(CHEMBL3627723)Show SMILES C[C@H](C(=O)NCc1ccc(cc1SC1CCCCC1)C(F)(F)F)c1ccc(NS(C)(=O)=O)c(F)c1 |r| Show InChI InChI=1S/C24H28F4N2O3S2/c1-15(16-9-11-21(20(25)12-16)30-35(2,32)33)23(31)29-14-17-8-10-18(24(26,27)28)13-22(17)34-19-6-4-3-5-7-19/h8-13,15,19,30H,3-7,14H2,1-2H3,(H,29,31)/t15-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University
Curated by ChEMBL
| Assay Description
Antagonist activity at human TRPV1 heterologously expressed in CHO cells assessed as inhibition of NADA-induced activation by FLIPR assay |
Bioorg Med Chem 23: 6844-54 (2015)
Article DOI: 10.1016/j.bmc.2015.10.001
BindingDB Entry DOI: 10.7270/Q2DZ0B4X |
More data for this
Ligand-Target Pair | |
Scytalone dehydratase
(Magnaporthe grisea) | BDBM50486967
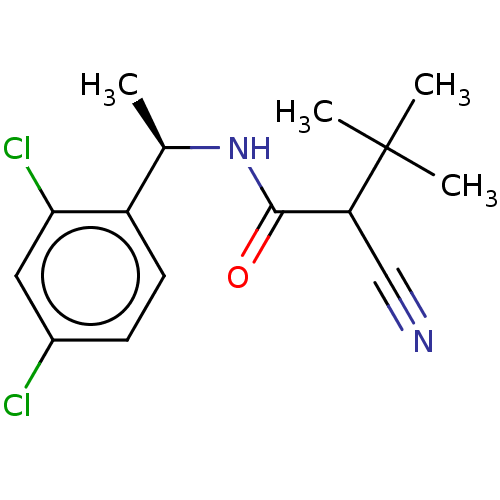
(CHEBI:81799 | DICLOCYMET)Show SMILES C[C@@H](NC(=O)C(C#N)C(C)(C)C)c1ccc(Cl)cc1Cl |r| Show InChI InChI=1S/C15H18Cl2N2O/c1-9(11-6-5-10(16)7-13(11)17)19-14(20)12(8-18)15(2,3)4/h5-7,9,12H,1-4H3,(H,19,20)/t9-,12?/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| 0.0360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Stine Haskell Research Center
Curated by ChEMBL
| Assay Description
Inhibition of Magnaporthe grisea scytalone dehydratase |
Bioorg Med Chem 8: 897-907 (2000)
Article DOI: 10.1016/s0968-0896(00)00034-1
BindingDB Entry DOI: 10.7270/Q2SX6H4B |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM176555
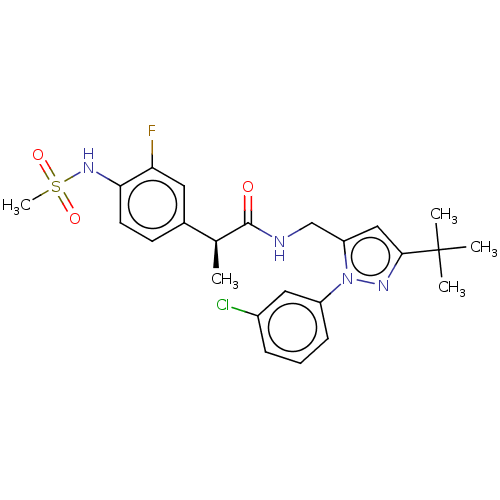
(US9120756, 17)Show SMILES C[C@H](C(=O)NCc1cc(nn1-c1cccc(Cl)c1)C(C)(C)C)c1ccc(NS(C)(=O)=O)c(F)c1 Show InChI InChI=1S/C24H28ClFN4O3S/c1-15(16-9-10-21(20(26)11-16)29-34(5,32)33)23(31)27-14-19-13-22(24(2,3)4)28-30(19)18-8-6-7-17(25)12-18/h6-13,15,29H,14H2,1-5H3,(H,27,31)/t15-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University
Curated by ChEMBL
| Assay Description
Antagonist activity at human TRPV1 expressed in CHO cells assessed as inhibition of NADA-induced intracellular calcium level preincubated with cells ... |
Bioorg Med Chem Lett 27: 4383-4388 (2017)
Article DOI: 10.1016/j.bmcl.2017.08.020
BindingDB Entry DOI: 10.7270/Q2CF9SPD |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4/beta-2
(Rattus norvegicus (Rat)) | BDBM50162063

(2-(5-Fluoro-pyridin-3-yl)-7-aza-bicyclo[2.2.1]hept...)Show InChI InChI=1S/C11H13FN2/c12-8-3-7(5-13-6-8)10-4-9-1-2-11(10)14-9/h3,5-6,9-11,14H,1-2,4H2 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute
Curated by ChEMBL
| Assay Description
Inhibition of [3H]epibatidine binding to Nicotinic acetylcholine receptor alpha4-beta2 in rat cerebral cortex |
J Med Chem 48: 1221-8 (2005)
Article DOI: 10.1021/jm040160b
BindingDB Entry DOI: 10.7270/Q26W9BV6 |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50073159
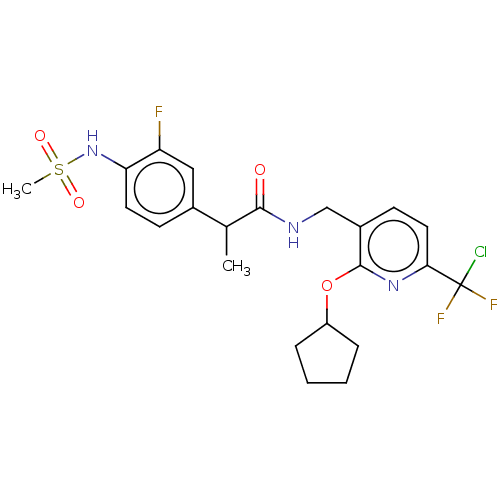
(CHEMBL3407765)Show SMILES CC(C(=O)NCc1ccc(nc1OC1CCCC1)C(F)(F)Cl)c1ccc(NS(C)(=O)=O)c(F)c1 Show InChI InChI=1S/C22H25ClF3N3O4S/c1-13(14-7-9-18(17(24)11-14)29-34(2,31)32)20(30)27-12-15-8-10-19(22(23,25)26)28-21(15)33-16-5-3-4-6-16/h7-11,13,16,29H,3-6,12H2,1-2H3,(H,27,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University
Curated by ChEMBL
| Assay Description
Antagonist activity at human TRPV1 expressed in CHO cells assessed as inhibition of N-arachidonoyl dopamine-induced activity by FLIPR assay |
Eur J Med Chem 93: 101-8 (2015)
Article DOI: 10.1016/j.ejmech.2015.02.001
BindingDB Entry DOI: 10.7270/Q2N0188S |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50073159

(CHEMBL3407765)Show SMILES CC(C(=O)NCc1ccc(nc1OC1CCCC1)C(F)(F)Cl)c1ccc(NS(C)(=O)=O)c(F)c1 Show InChI InChI=1S/C22H25ClF3N3O4S/c1-13(14-7-9-18(17(24)11-14)29-34(2,31)32)20(30)27-12-15-8-10-19(22(23,25)26)28-21(15)33-16-5-3-4-6-16/h7-11,13,16,29H,3-6,12H2,1-2H3,(H,27,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University
Curated by ChEMBL
| Assay Description
Antagonist activity at human TRPV1 expressed in CHO cells assessed as inhibition of N-arachidonoyl dopamine-induced activity by FLIPR assay |
Eur J Med Chem 93: 101-8 (2015)
Article DOI: 10.1016/j.ejmech.2015.02.001
BindingDB Entry DOI: 10.7270/Q2N0188S |
More data for this
Ligand-Target Pair | |
Scytalone dehydratase
(Magnaporthe grisea) | BDBM50078312
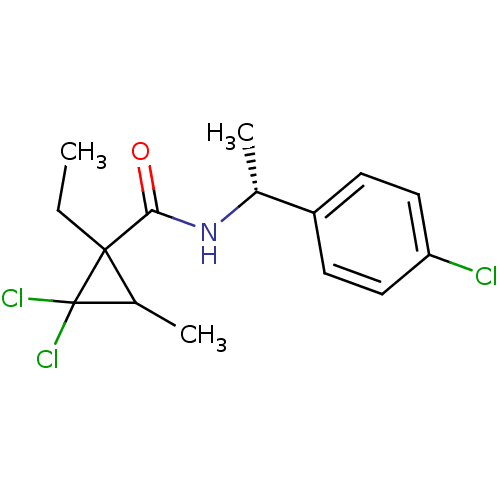
(2,2-Dichloro-1-ethyl-3-methyl-cyclopropanecarboxyl...)Show SMILES CCC1(C(C)C1(Cl)Cl)C(=O)N[C@H](C)c1ccc(Cl)cc1 Show InChI InChI=1S/C15H18Cl3NO/c1-4-14(10(3)15(14,17)18)13(20)19-9(2)11-5-7-12(16)8-6-11/h5-10H,4H2,1-3H3,(H,19,20)/t9-,10?,14?/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0410 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Stine Haskell Research Center
Curated by ChEMBL
| Assay Description
Inhibition of Magnaporthe grisea scytalone dehydratase |
Bioorg Med Chem 8: 897-907 (2000)
Article DOI: 10.1016/s0968-0896(00)00034-1
BindingDB Entry DOI: 10.7270/Q2SX6H4B |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data