

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50366764 (CHEMBL1790045 | MCL-117) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.00300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from human mu opioid receptor expressed in CHO cells | J Med Chem 49: 256-62 (2006) Article DOI: 10.1021/jm050577x BindingDB Entry DOI: 10.7270/Q2HQ40QF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50366764 (CHEMBL1790045 | MCL-117) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.00300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Binding affinity towards guinea pig Opioid receptor kappa 1 using radioligand [3H]U-69593 | Bioorg Med Chem Lett 11: 2735-40 (2001) BindingDB Entry DOI: 10.7270/Q25T3M01 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50366764 (CHEMBL1790045 | MCL-117) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.00300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Displacement of [3H]U69593 from human kappa opioid receptor expressed in CHO cells | J Med Chem 49: 256-62 (2006) Article DOI: 10.1021/jm050577x BindingDB Entry DOI: 10.7270/Q2HQ40QF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (GUINEA PIG) | BDBM50366764 (CHEMBL1790045 | MCL-117) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.00320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Binding affinity towards Opioid receptor mu 1 in guinea pig brain membranes using [3H]DAMGO as radioligand | Bioorg Med Chem Lett 11: 2735-40 (2001) BindingDB Entry DOI: 10.7270/Q25T3M01 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (GUINEA PIG) | BDBM50451302 (CHEMBL2115245) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.00480 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Binding affinity towards Opioid receptor mu 1 in guinea pig brain membranes using [3H]DAMGO as radioligand | Bioorg Med Chem Lett 11: 2735-40 (2001) BindingDB Entry DOI: 10.7270/Q25T3M01 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50180190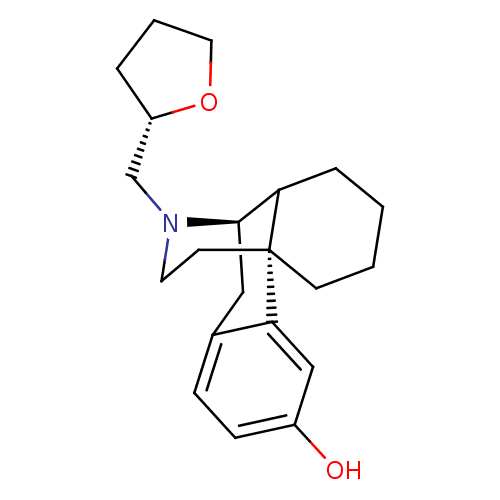 ((1R,9R)-17-[(2S)-oxolan-2-ylmethyl]-17-azatetracyc...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from human mu opioid receptor expressed in CHO cells | J Med Chem 49: 256-62 (2006) Article DOI: 10.1021/jm050577x BindingDB Entry DOI: 10.7270/Q2HQ40QF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (GUINEA PIG) | BDBM50180190 ((1R,9R)-17-[(2S)-oxolan-2-ylmethyl]-17-azatetracyc...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Binding affinity towards Opioid receptor mu 1 by displacing the radioligand [3H]DAMGO from guinea pig brain membrane | J Med Chem 43: 114-22 (2000) BindingDB Entry DOI: 10.7270/Q20V8DGH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM50514081 (CHEMBL4471466) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Philipps University Marburg Curated by ChEMBL | Assay Description Inhibition of human plasma kallikrein by Michaelis-menten analysis | J Med Chem 63: 1445-1472 (2020) Article DOI: 10.1021/acs.jmedchem.9b01060 BindingDB Entry DOI: 10.7270/Q2VQ361P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50303629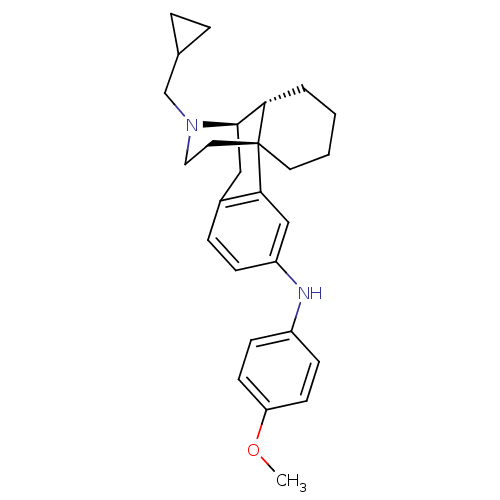 (17-(Cyclopropylmethyl)-N-(4-methoxyphenyl)morphina...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from human mu opioid receptor expressed in CHO cells | J Med Chem 53: 402-18 (2010) Article DOI: 10.1021/jm9013482 BindingDB Entry DOI: 10.7270/Q2668D84 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50303629 (17-(Cyclopropylmethyl)-N-(4-methoxyphenyl)morphina...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Displacement of [3H]U69593 from human kappa opioid receptor expressed in CHO cells | J Med Chem 53: 402-18 (2010) Article DOI: 10.1021/jm9013482 BindingDB Entry DOI: 10.7270/Q2668D84 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50105483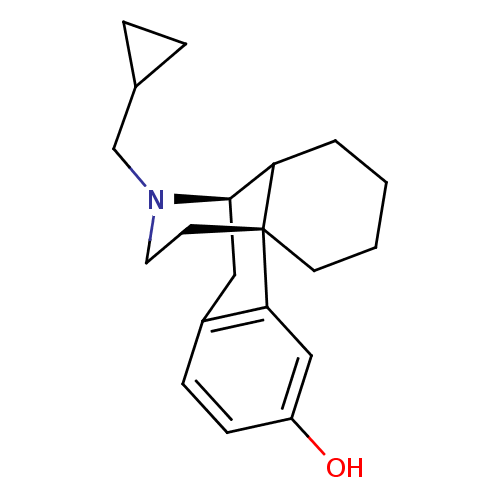 ((-)-cyclorphan | 17-cyclopropylmethyl-(1R,9R)-17-a...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Agonistic activity against kappa opioid receptor in Chinese hamster ovary membranes | J Med Chem 47: 1886-8 (2004) Article DOI: 10.1021/jm049978n BindingDB Entry DOI: 10.7270/Q2N58N4X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50135800 ((-)-3-Hydroxy-N-cycloproypylmethylmorphinan Mandel...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Displacement of [3H]U-69593 from human kappa opioid receptors expressed in CHO cell membrane | Bioorg Med Chem Lett 17: 1508-11 (2007) Article DOI: 10.1016/j.bmcl.2007.01.013 BindingDB Entry DOI: 10.7270/Q2WS8V2W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50135800 ((-)-3-Hydroxy-N-cycloproypylmethylmorphinan Mandel...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Displacement of [3H]-U69,593 from human kappa opioid receptor expressed in CHO cells after 60 mins by scintillation counting | J Med Chem 55: 3878-90 (2012) Article DOI: 10.1021/jm3001086 BindingDB Entry DOI: 10.7270/Q28053P6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50135800 ((-)-3-Hydroxy-N-cycloproypylmethylmorphinan Mandel...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Inhibitory activity against Opioid receptor kappa 1 in chinese Hamster Ovary (CHO) cell membranes was determined using [3H]U-69593 radioligand | J Med Chem 46: 5162-70 (2003) Article DOI: 10.1021/jm030139v BindingDB Entry DOI: 10.7270/Q2GX4C9C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50135800 ((-)-3-Hydroxy-N-cycloproypylmethylmorphinan Mandel...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Displacement of [3H]U69593 from human kappa opioid receptor expressed in CHO cells | J Med Chem 53: 402-18 (2010) Article DOI: 10.1021/jm9013482 BindingDB Entry DOI: 10.7270/Q2668D84 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A)/D(1B)/D(2)/D(3)/D(4) dopamine receptor (Rattus norvegicus (rat)-RAT-Rattus norvegicus (Rat...) | BDBM50368145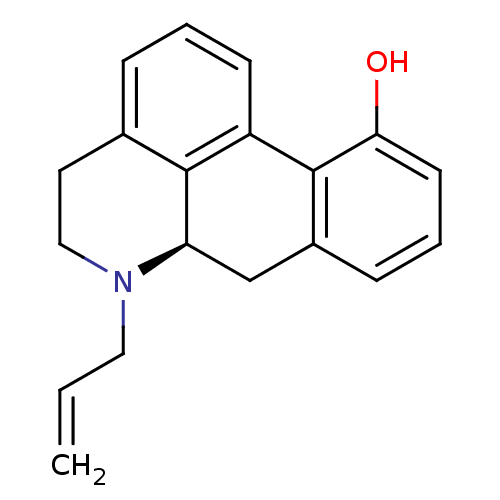 (CHEMBL1788212) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University Curated by ChEMBL | Assay Description Compound was evaluated for its ability to inhibit Striatal Dopamine Receptor in rat brain through radioreceptor assay carried out with agonist ligand... | J Med Chem 34: 24-8 (1991) BindingDB Entry DOI: 10.7270/Q24Q7VKQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50135800 ((-)-3-Hydroxy-N-cycloproypylmethylmorphinan Mandel...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Displacement of [3H]U69,593 from human kappa opioid receptor expressed in CHO membrane | Bioorg Med Chem 15: 4106-12 (2007) Article DOI: 10.1016/j.bmc.2007.03.076 BindingDB Entry DOI: 10.7270/Q2D50MN1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50135800 ((-)-3-Hydroxy-N-cycloproypylmethylmorphinan Mandel...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Displacement of [3H]U69593 from human kappa opioid receptor expressed in CHO cells after 60 mins by scintillation counting | J Med Chem 54: 1903-13 (2011) Article DOI: 10.1021/jm101542c BindingDB Entry DOI: 10.7270/Q2028SJ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50135800 ((-)-3-Hydroxy-N-cycloproypylmethylmorphinan Mandel...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Binding affinity against opioid receptor kappa 1 using [3H]-U-69,593 as radioligand in guinea pig brain membranes. | J Med Chem 47: 165-74 (2003) Article DOI: 10.1021/jm0304156 BindingDB Entry DOI: 10.7270/Q2KD1XB2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50105483 ((-)-cyclorphan | 17-cyclopropylmethyl-(1R,9R)-17-a...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Displacement of [3H]U69593 from human kappa opioid receptor expressed in CHO cells | J Med Chem 49: 256-62 (2006) Article DOI: 10.1021/jm050577x BindingDB Entry DOI: 10.7270/Q2HQ40QF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50451302 (CHEMBL2115245) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0370 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Binding affinity towards guinea pig Opioid receptor kappa 1 using radioligand [3H]U-69593 | Bioorg Med Chem Lett 11: 2735-40 (2001) BindingDB Entry DOI: 10.7270/Q25T3M01 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50204451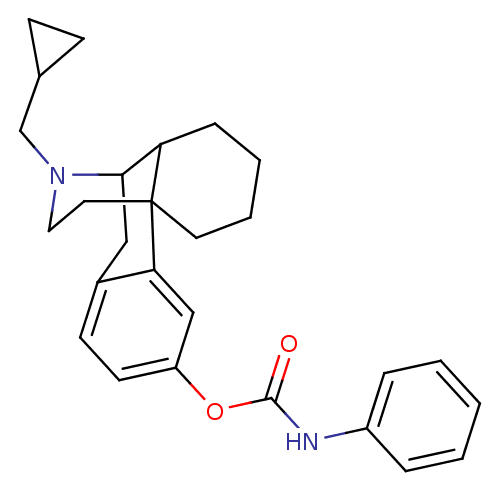 (CHEMBL397035 | MCL-429) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0460 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Displacement of [3H]U-69593 from human kappa opioid receptors expressed in CHO cell membrane | Bioorg Med Chem Lett 17: 1508-11 (2007) Article DOI: 10.1016/j.bmcl.2007.01.013 BindingDB Entry DOI: 10.7270/Q2WS8V2W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50303630 (17-(Cyclopropylmethyl)-N-phenylmorphinan-3-amine |...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0470 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Displacement of [3H]U69593 from human kappa opioid receptor expressed in CHO cells | J Med Chem 53: 402-18 (2010) Article DOI: 10.1021/jm9013482 BindingDB Entry DOI: 10.7270/Q2668D84 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50272298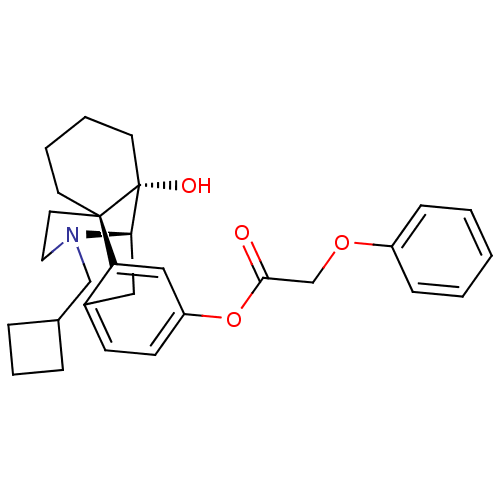 ((-)-N-cyclobutylmethylmorphinan-3-yl-14-ol phenoxy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0480 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Displacement of [3H]U69593 from human kappa opioid receptor expressed in CHO cell membrane | Bioorg Med Chem Lett 18: 4474-6 (2008) Article DOI: 10.1016/j.bmcl.2008.07.054 BindingDB Entry DOI: 10.7270/Q2K07423 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50143602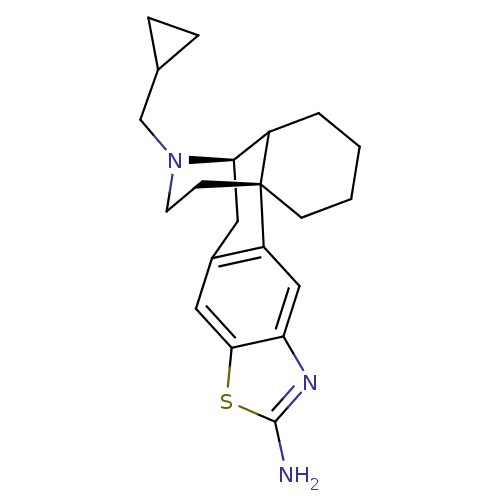 (20-cyclopropylmethyl-(1R,12R)-7-thia-5,20-diazapen...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0490 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Inhibition of [3H]U-69593 binding to Opioid receptor kappa 1 of Chinese hamster ovary membrane | J Med Chem 47: 1886-8 (2004) Article DOI: 10.1021/jm049978n BindingDB Entry DOI: 10.7270/Q2N58N4X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50135806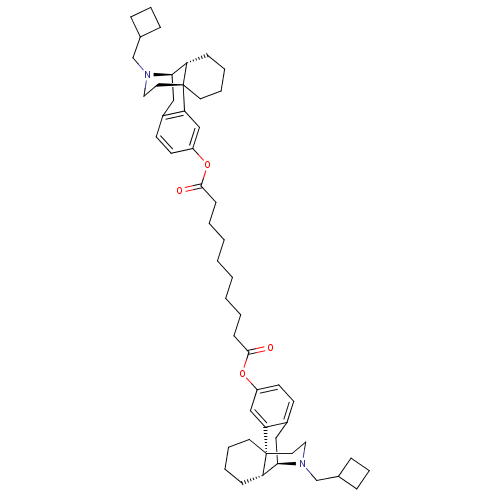 (CHEMBL147511 | MCL-144 | di[17-cyclobutylmethyl-(1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0490 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Agonist activity at human kappa opioid receptor expressed in CHO cells assessed as stimulation of [35S]GTPgammaS binding | J Med Chem 52: 7389-96 (2009) Article DOI: 10.1021/jm900379p BindingDB Entry DOI: 10.7270/Q29P31Q8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50210557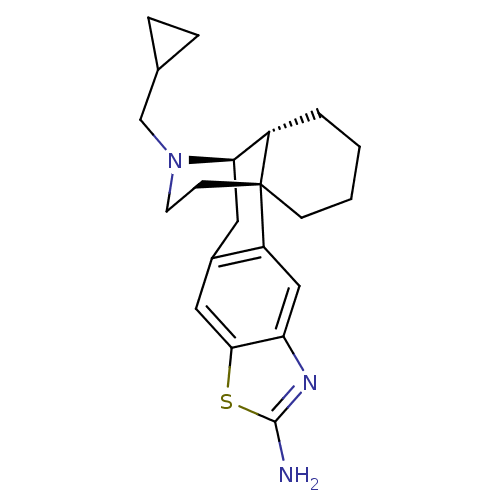 (CHEMBL242756 | MCL-147) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0490 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Antagonist activity at human kappa-opioid receptor expressed in CHO cells assessed as inhibition of U69593-induced [35S]GTPgammaS binding after 60 mi... | Bioorg Med Chem 19: 2808-16 (2011) Article DOI: 10.1016/j.bmc.2011.03.052 BindingDB Entry DOI: 10.7270/Q2KW5GB1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50135806 (CHEMBL147511 | MCL-144 | di[17-cyclobutylmethyl-(1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0490 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Antagonist activity at human kappa-opioid receptor expressed in CHO cells assessed as inhibition of U69593-induced [35S]GTPgammaS binding after 60 mi... | Bioorg Med Chem 19: 2808-16 (2011) Article DOI: 10.1016/j.bmc.2011.03.052 BindingDB Entry DOI: 10.7270/Q2KW5GB1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50210557 (CHEMBL242756 | MCL-147) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0490 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Displacement of [3H]U69593 from human kappa opioid receptor expressed in CHO cells after 60 mins by scintillation counting | J Med Chem 54: 1903-13 (2011) Article DOI: 10.1021/jm101542c BindingDB Entry DOI: 10.7270/Q2028SJ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50135806 (CHEMBL147511 | MCL-144 | di[17-cyclobutylmethyl-(1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0490 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Displacement of [3H]U69593 from human kappa opioid receptor expressed in CHO cells | J Med Chem 49: 256-62 (2006) Article DOI: 10.1021/jm050577x BindingDB Entry DOI: 10.7270/Q2HQ40QF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50210557 (CHEMBL242756 | MCL-147) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0490 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Displacement of [3H]U69,593 from human kappa opioid receptor expressed in CHO membrane | Bioorg Med Chem 15: 4106-12 (2007) Article DOI: 10.1016/j.bmc.2007.03.076 BindingDB Entry DOI: 10.7270/Q2D50MN1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50135806 (CHEMBL147511 | MCL-144 | di[17-cyclobutylmethyl-(1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Inhibitory activity against Opioid receptor kappa 1 in chinese Hamster Ovary (CHO) cell membranes was determined using [3H]U-69593 radioligand | J Med Chem 46: 5162-70 (2003) Article DOI: 10.1021/jm030139v BindingDB Entry DOI: 10.7270/Q2GX4C9C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50204450 (CHEMBL242048 | MCL-428) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0510 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Displacement of [3H]U-69593 from human kappa opioid receptors expressed in CHO cell membrane | Bioorg Med Chem Lett 17: 1508-11 (2007) Article DOI: 10.1016/j.bmcl.2007.01.013 BindingDB Entry DOI: 10.7270/Q2WS8V2W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50001023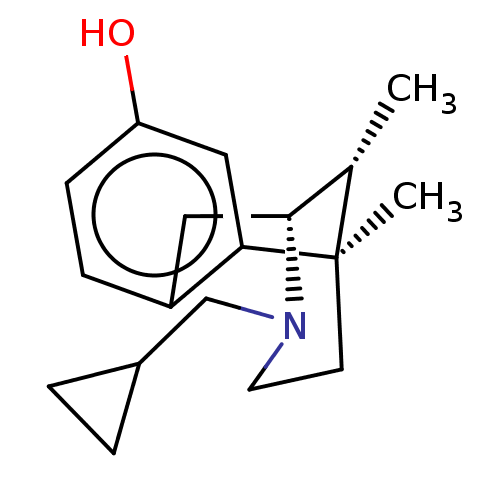 ((2R,6R,11R)-3-Cyclopropylmethyl-6,11-dimethyl-1,2,...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.0520 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Binding affinity towards Opioid receptor kappa 1 by displacing the radioligand [3H]U-69593 from guinea pig brain membrane | J Med Chem 43: 114-22 (2000) BindingDB Entry DOI: 10.7270/Q20V8DGH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50135800 ((-)-3-Hydroxy-N-cycloproypylmethylmorphinan Mandel...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.0530 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Binding affinity towards Opioid receptor kappa 1 by displacing the radioligand [3H]U-69593 from guinea pig brain membrane | J Med Chem 43: 114-22 (2000) BindingDB Entry DOI: 10.7270/Q20V8DGH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM52987 ((6aR)-6-propyl-5,6,6a,7-tetrahydro-4H-dibenzo[de,g...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0530 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Displacement of [3H]raclopride from rat dopamine D2 receptor | J Med Chem 50: 171-81 (2007) Article DOI: 10.1021/jm060959i BindingDB Entry DOI: 10.7270/Q22J6CPJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM52987 ((6aR)-6-propyl-5,6,6a,7-tetrahydro-4H-dibenzo[de,g...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0530 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University Curated by ChEMBL | Assay Description Binding affinity at rat striatal Dopamine receptor D2 using [3H]- piperone radioligand | J Med Chem 33: 1800-5 (1990) BindingDB Entry DOI: 10.7270/Q2K35SMD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50135800 ((-)-3-Hydroxy-N-cycloproypylmethylmorphinan Mandel...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.0530 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Binding affinity towards Opioid receptor kappa 1 in guinea pig brain membranes using [3H]U-69593 as radioligand | Bioorg Med Chem Lett 11: 2735-40 (2001) BindingDB Entry DOI: 10.7270/Q25T3M01 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50135800 ((-)-3-Hydroxy-N-cycloproypylmethylmorphinan Mandel...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Inhibitory activity against Opioid receptor mu 1 in chinese Hamster Ovary (CHO) cells membranes was determined using [3H]-DAMGO radioligand | J Med Chem 46: 5162-70 (2003) Article DOI: 10.1021/jm030139v BindingDB Entry DOI: 10.7270/Q2GX4C9C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (GUINEA PIG) | BDBM50135800 ((-)-3-Hydroxy-N-cycloproypylmethylmorphinan Mandel...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Binding affinity against opioid receptor mu 1 using [3H]-DAMGO as radioligand in guinea pig brain membranes. | J Med Chem 47: 165-74 (2003) Article DOI: 10.1021/jm0304156 BindingDB Entry DOI: 10.7270/Q2KD1XB2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (GUINEA PIG) | BDBM50137996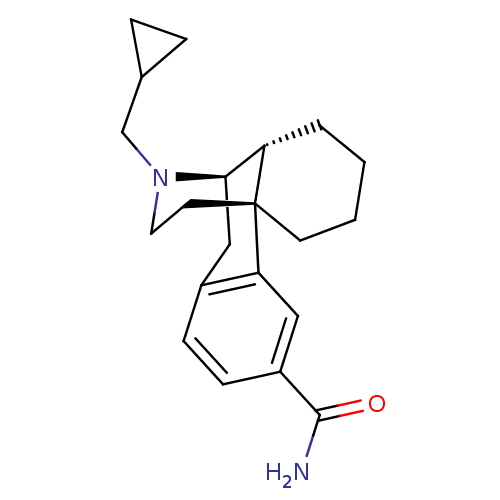 (3-Carboxamido-N-cyclopropylmethylmorphinan | CHEMB...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Binding affinity against opioid receptor mu 1 using [3H]-DAMGO as radioligand in guinea pig brain membranes. | J Med Chem 47: 165-74 (2003) Article DOI: 10.1021/jm0304156 BindingDB Entry DOI: 10.7270/Q2KD1XB2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50106857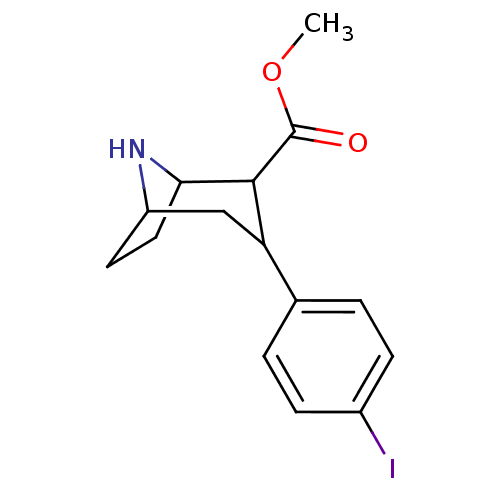 (3-(4-Iodo-phenyl)-8-aza-bicyclo[3.2.1]octane-2-car...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Alcohol and Drug Abuse Research Center Curated by ChEMBL | Assay Description Ability to displace [3H]- paroxetine from Serotonin transporter in rat cerebral cortical homogenate | Bioorg Med Chem Lett 11: 3049-53 (2001) BindingDB Entry DOI: 10.7270/Q23N22PN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent dopamine transporter (Rattus norvegicus (rat)) | BDBM22165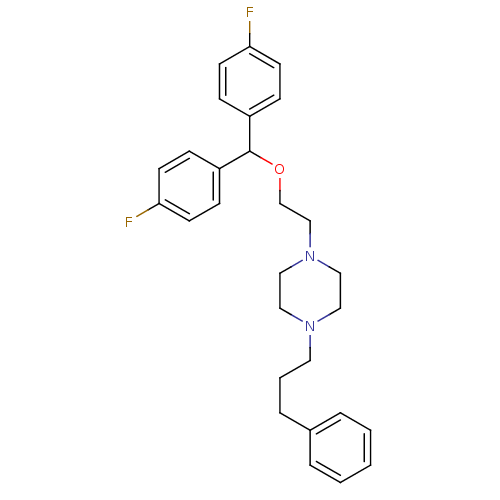 (1-{2-[bis(4-fluorophenyl)methoxy]ethyl}-4-(3-pheny...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Biochemicals International Curated by ChEMBL | Assay Description In vitro binding affinity towards dopamine transporter in rat striatal membranes by [3H]GBR-12395 displacement. | J Med Chem 39: 543-8 (1996) Article DOI: 10.1021/jm9505324 BindingDB Entry DOI: 10.7270/Q2KD1ZJ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM21397 (8-[4-(4-fluorophenyl)-4-keto-butyl]-1-phenyl-1,3,8...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PubMed | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Biochemicals Inc. Curated by ChEMBL | Assay Description Binding affinity against dopamine receptor D2 by using [3H]spiperone as radioligand in caudate-putamen of monkey | J Med Chem 34: 3235-41 (1991) BindingDB Entry DOI: 10.7270/Q2KS6S5V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50272298 ((-)-N-cyclobutylmethylmorphinan-3-yl-14-ol phenoxy...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0610 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from human mu opioid receptor expressed in CHO cell membrane | Bioorg Med Chem Lett 18: 4474-6 (2008) Article DOI: 10.1016/j.bmcl.2008.07.054 BindingDB Entry DOI: 10.7270/Q2K07423 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50135800 ((-)-3-Hydroxy-N-cycloproypylmethylmorphinan Mandel...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0620 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Displacement of [3H]-DAMGO from human mu opioid receptor expressed in CHO cells after 60 mins by scintillation counting | J Med Chem 55: 3878-90 (2012) Article DOI: 10.1021/jm3001086 BindingDB Entry DOI: 10.7270/Q28053P6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50105483 ((-)-cyclorphan | 17-cyclopropylmethyl-(1R,9R)-17-a...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0620 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from human mu opioid receptor expressed in CHO cells | J Med Chem 49: 256-62 (2006) Article DOI: 10.1021/jm050577x BindingDB Entry DOI: 10.7270/Q2HQ40QF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50135800 ((-)-3-Hydroxy-N-cycloproypylmethylmorphinan Mandel...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0620 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from human mu opioid receptor expressed in CHO cells | J Med Chem 53: 402-18 (2010) Article DOI: 10.1021/jm9013482 BindingDB Entry DOI: 10.7270/Q2668D84 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50135800 ((-)-3-Hydroxy-N-cycloproypylmethylmorphinan Mandel...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0620 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from human mu opioid receptor expressed in CHO membrane | Bioorg Med Chem 15: 4106-12 (2007) Article DOI: 10.1016/j.bmc.2007.03.076 BindingDB Entry DOI: 10.7270/Q2D50MN1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50135800 ((-)-3-Hydroxy-N-cycloproypylmethylmorphinan Mandel...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0620 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Agonist activity at human mu opioid receptor expressed in CHO cells assessed as [35S]GTPgammaS binding by scintillation counting | J Med Chem 54: 1903-13 (2011) Article DOI: 10.1021/jm101542c BindingDB Entry DOI: 10.7270/Q2028SJ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 6425 total ) | Next | Last >> |