

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cannabinoid receptor 2 (Rattus norvegicus (Rat)) | BDBM50006250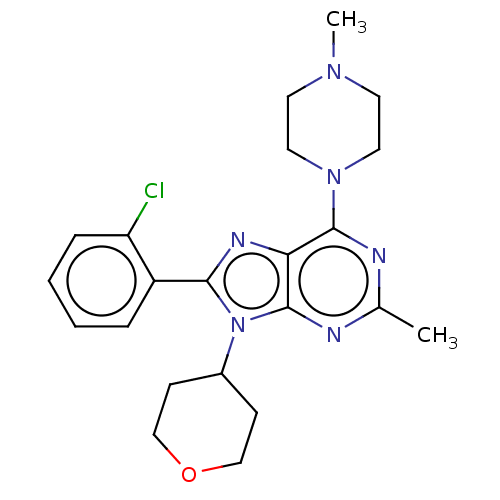 (CHEMBL3139186) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 37 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Binding affinity to rat CB2 receptor | Bioorg Med Chem Lett 24: 5572-5 (2014) Article DOI: 10.1016/j.bmcl.2014.11.006 BindingDB Entry DOI: 10.7270/Q2R78GVW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50006250 (CHEMBL3139186) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Binding affinity to human CB2 receptor | Bioorg Med Chem Lett 24: 5572-5 (2014) Article DOI: 10.1016/j.bmcl.2014.11.006 BindingDB Entry DOI: 10.7270/Q2R78GVW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM50112584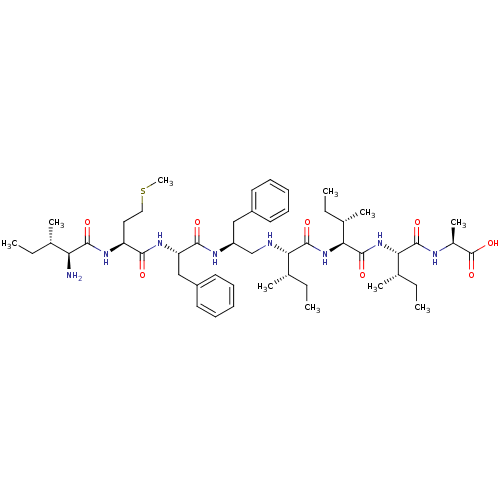 (2-(2-{2-[2-(2-{2-[2-(2-Amino-3-methyl-pentanoylami...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Carlsberg Laboratory Curated by ChEMBL | Assay Description Inhibition of Leishmania mexicana Cysteine protease B (CPB2.8deltaCTE) was obtained in a solution-phase assay | J Med Chem 45: 1971-82 (2002) BindingDB Entry DOI: 10.7270/Q29887QH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM50370072 (CHEMBL1790422) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Carlsberg Laboratory Curated by ChEMBL | Assay Description Inhibition of Leishmania mexicana Cysteine protease B (CPB2.8deltaCTE) was obtained in a solution-phase assay | J Med Chem 45: 1971-82 (2002) BindingDB Entry DOI: 10.7270/Q29887QH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM50112580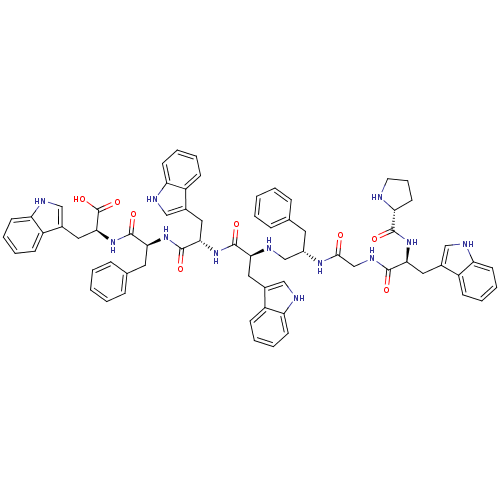 (CHEMBL268148 | PWGF(CH2NH)WWFW) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Carlsberg Laboratory Curated by ChEMBL | Assay Description Inhibition of Leishmania mexicana Cysteine protease B (CPB2.8deltaCTE) was obtained in a solution-phase assay | J Med Chem 45: 1971-82 (2002) BindingDB Entry DOI: 10.7270/Q29887QH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM50112597 (2-{2-[2-(2-{2-[2-[2-(2-Amino-3-carboxy-propionylam...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Carlsberg Laboratory Curated by ChEMBL | Assay Description Inhibition of Leishmania mexicana Cysteine protease B (CPB2.8deltaCTE) was obtained in a solution-phase assay | J Med Chem 45: 1971-82 (2002) BindingDB Entry DOI: 10.7270/Q29887QH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM50112581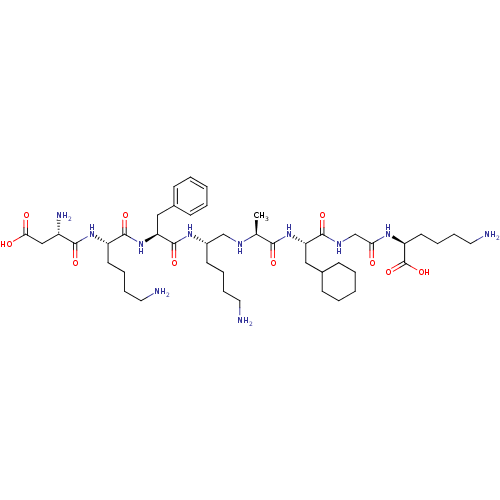 (6-Amino-2-(2-{2-[2-(6-amino-2-{2-[6-amino-2-(2-ami...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Carlsberg Laboratory Curated by ChEMBL | Assay Description Inhibition of Leishmania mexicana Cysteine protease B (CPB2.8deltaCTE) was obtained in a solution-phase assay | J Med Chem 45: 1971-82 (2002) BindingDB Entry DOI: 10.7270/Q29887QH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM50112596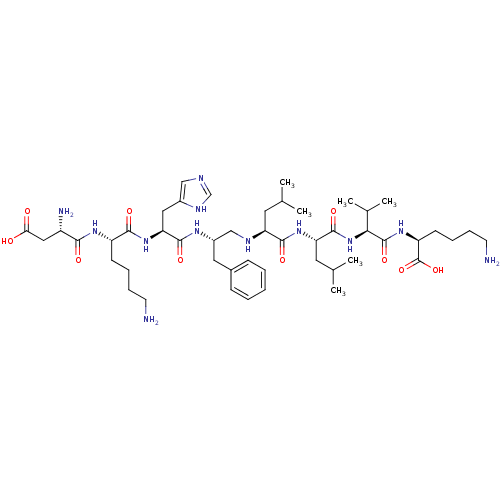 (6-Amino-2-{2-[2-(2-{2-[2-[6-amino-2-(2-amino-3-car...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Carlsberg Laboratory Curated by ChEMBL | Assay Description Inhibition of Leishmania mexicana Cysteine protease B (CPB2.8deltaCTE) was obtained in a solution-phase assay | J Med Chem 45: 1971-82 (2002) BindingDB Entry DOI: 10.7270/Q29887QH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM50112594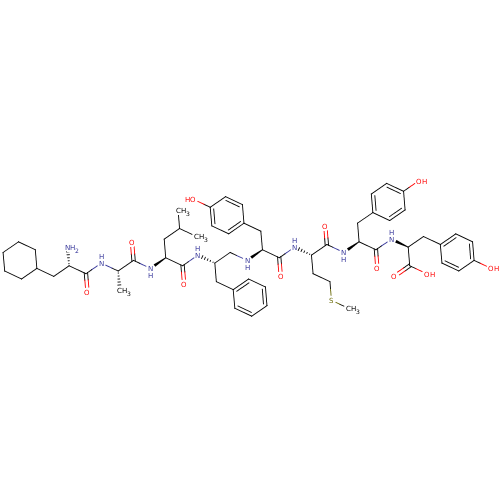 (CHEMBL266692 | ChaALF(CH2NH)YMYY) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Carlsberg Laboratory Curated by ChEMBL | Assay Description Inhibition of Leishmania mexicana Cysteine protease B (CPB2.8deltaCTE) was obtained in a solution-phase assay | J Med Chem 45: 1971-82 (2002) BindingDB Entry DOI: 10.7270/Q29887QH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM50112583 (1-{2-[2-[2-(6-Amino-2-{2-[2-(2-amino-3-methyl-pent...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 5.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Carlsberg Laboratory Curated by ChEMBL | Assay Description Inhibition of Leishmania mexicana Cysteine protease B (CPB2.8deltaCTE) was obtained in a solution-phase assay | J Med Chem 45: 1971-82 (2002) BindingDB Entry DOI: 10.7270/Q29887QH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM50112579 (6-Amino-2-(2-{2-[2-(6-amino-2-{2-[2-(2-amino-3-cyc...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 6.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Carlsberg Laboratory Curated by ChEMBL | Assay Description Inhibition of Leishmania mexicana Cysteine protease B (CPB2.8deltaCTE) was obtained in a solution-phase assay | J Med Chem 45: 1971-82 (2002) BindingDB Entry DOI: 10.7270/Q29887QH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM50112591 (2-(2-{2-[2-(2-{2-[2-(2-Amino-4-methylsulfanyl-buty...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 6.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Carlsberg Laboratory Curated by ChEMBL | Assay Description Inhibition of Leishmania mexicana Cysteine protease B (CPB2.8deltaCTE) was obtained in a solution-phase assay | J Med Chem 45: 1971-82 (2002) BindingDB Entry DOI: 10.7270/Q29887QH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM50112585 (2-[3-Cyclohexyl-2-(2-{2-[2-(3-(3H-imidazol-4-yl)-2...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 6.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Carlsberg Laboratory Curated by ChEMBL | Assay Description Inhibition of Leishmania mexicana Cysteine protease B (CPB2.8deltaCTE) was obtained in a solution-phase assay | J Med Chem 45: 1971-82 (2002) BindingDB Entry DOI: 10.7270/Q29887QH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM50112582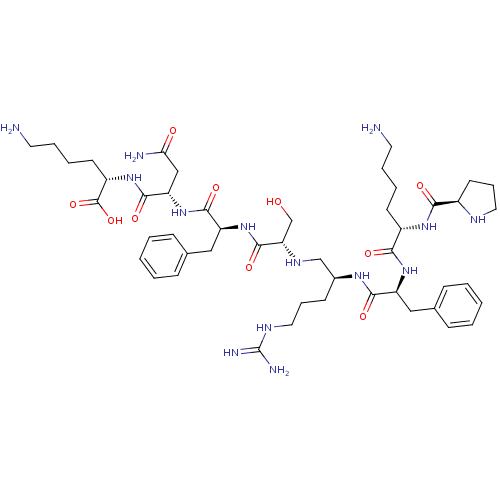 (6-Amino-2-[2-(2-{2-[2-(2-{6-amino-2-[(pyrrolidine-...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 8.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Carlsberg Laboratory Curated by ChEMBL | Assay Description Inhibition of Leishmania mexicana Cysteine protease B (CPB2.8deltaCTE) was obtained in a solution-phase assay | J Med Chem 45: 1971-82 (2002) BindingDB Entry DOI: 10.7270/Q29887QH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM50370073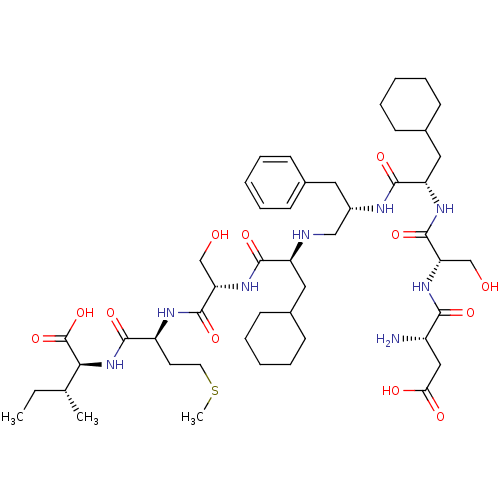 (CHEMBL1790421) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.01E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Carlsberg Laboratory Curated by ChEMBL | Assay Description Inhibition of Leishmania mexicana Cysteine protease B (CPB2.8deltaCTE) was obtained in a solution-phase assay | J Med Chem 45: 1971-82 (2002) BindingDB Entry DOI: 10.7270/Q29887QH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM50370068 (CHEMBL1790423) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Carlsberg Laboratory Curated by ChEMBL | Assay Description Inhibition of Leishmania mexicana Cysteine protease B (CPB2.8deltaCTE) was obtained in a solution-phase assay | J Med Chem 45: 1971-82 (2002) BindingDB Entry DOI: 10.7270/Q29887QH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM50370070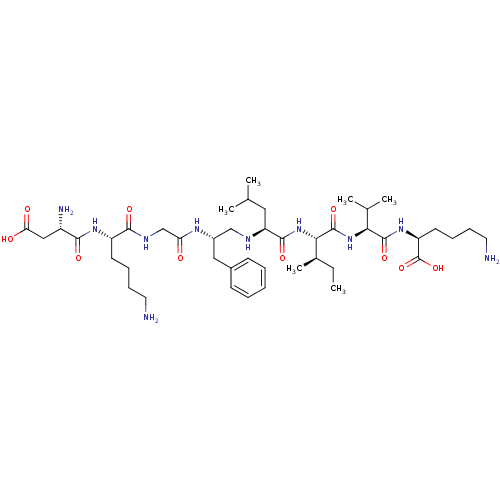 (CHEMBL1790419) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.86E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Carlsberg Laboratory Curated by ChEMBL | Assay Description Inhibition of Leishmania mexicana Cysteine protease B (CPB2.8deltaCTE) was obtained in a solution-phase assay | J Med Chem 45: 1971-82 (2002) BindingDB Entry DOI: 10.7270/Q29887QH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM50370074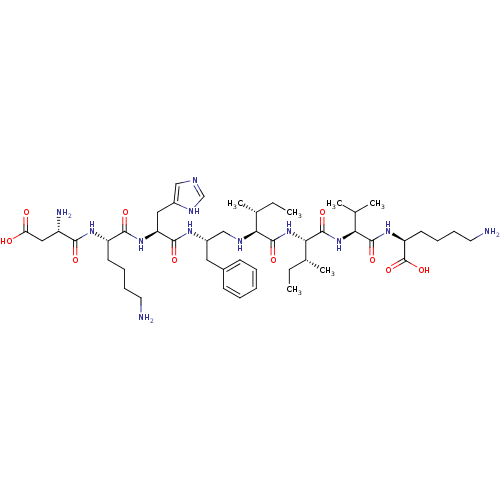 (CHEMBL1790420) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Carlsberg Laboratory Curated by ChEMBL | Assay Description Inhibition of Leishmania mexicana Cysteine protease B (CPB2.8deltaCTE) was obtained in a solution-phase assay | J Med Chem 45: 1971-82 (2002) BindingDB Entry DOI: 10.7270/Q29887QH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM50370069 (CHEMBL1790418) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Carlsberg Laboratory Curated by ChEMBL | Assay Description Inhibition of Leishmania mexicana Cysteine protease B (CPB2.8deltaCTE) was obtained in a solution-phase assay | J Med Chem 45: 1971-82 (2002) BindingDB Entry DOI: 10.7270/Q29887QH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM50112592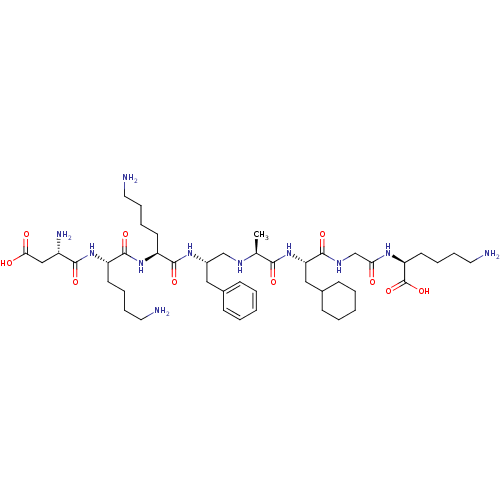 (6-Amino-2-(2-{2-[2-(2-{6-amino-2-[6-amino-2-(2-ami...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.11E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Carlsberg Laboratory Curated by ChEMBL | Assay Description Inhibition of Leishmania mexicana Cysteine protease B (CPB2.8deltaCTE) was obtained in a solution-phase assay | J Med Chem 45: 1971-82 (2002) BindingDB Entry DOI: 10.7270/Q29887QH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM50112587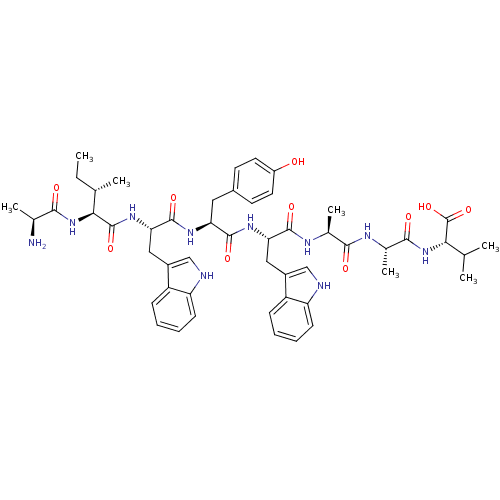 (2-(2-{2-[2-[2-[2-[2-(2-Amino-propionylamino)-3-met...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 4.47E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Carlsberg Laboratory Curated by ChEMBL | Assay Description Inhibition of Leishmania mexicana Cysteine protease B (CPB2.8deltaCTE) was obtained in a solution-phase assay | J Med Chem 45: 1971-82 (2002) BindingDB Entry DOI: 10.7270/Q29887QH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM50370071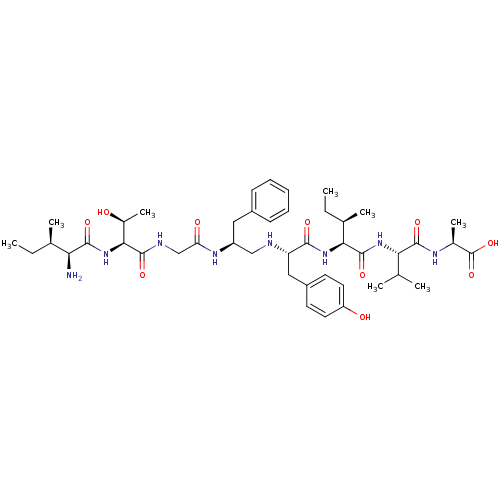 (CHEMBL1790424) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 5.33E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Carlsberg Laboratory Curated by ChEMBL | Assay Description Inhibition of Leishmania mexicana Cysteine protease B (CPB2.8deltaCTE) was obtained in a solution-phase assay | J Med Chem 45: 1971-82 (2002) BindingDB Entry DOI: 10.7270/Q29887QH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM50112588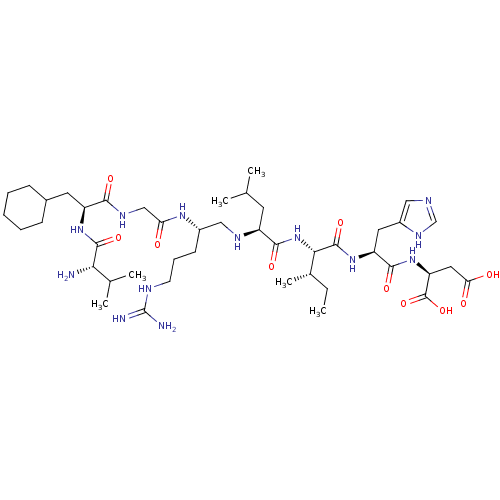 (2-[2-{2-[2-(2-{2-[2-(2-Amino-3-methyl-butyrylamino...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 7.16E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Carlsberg Laboratory Curated by ChEMBL | Assay Description Inhibition of Leishmania mexicana Cysteine protease B (CPB2.8deltaCTE) was obtained in a solution-phase assay | J Med Chem 45: 1971-82 (2002) BindingDB Entry DOI: 10.7270/Q29887QH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM50112578 (2-(2-{2-[2-(6-Amino-2-{2-[2-(2-amino-3-methyl-pent...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Carlsberg Laboratory Curated by ChEMBL | Assay Description Inhibition of Leishmania mexicana Cysteine protease B (CPB2.8deltaCTE) was obtained in a solution-phase assay | J Med Chem 45: 1971-82 (2002) BindingDB Entry DOI: 10.7270/Q29887QH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen activator inhibitor 1 (Homo sapiens (Human)) | BDBM50111316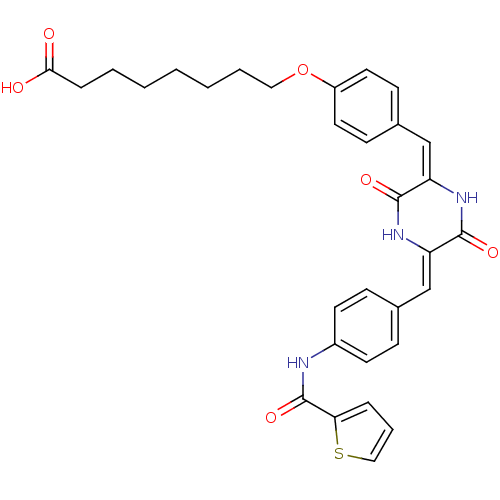 (8-[4-(3,6-Dioxo-5-{4-[(thiophene-2-carbonyl)-amino...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Xenova Limited Curated by ChEMBL | Assay Description Inhibitory activity against human plasminogen activator inhibitor-1(PAI-1) evaluated by chromogenic assay. | Bioorg Med Chem Lett 12: 1063-6 (2002) BindingDB Entry DOI: 10.7270/Q2W958HQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen activator inhibitor 1 (Homo sapiens (Human)) | BDBM50111273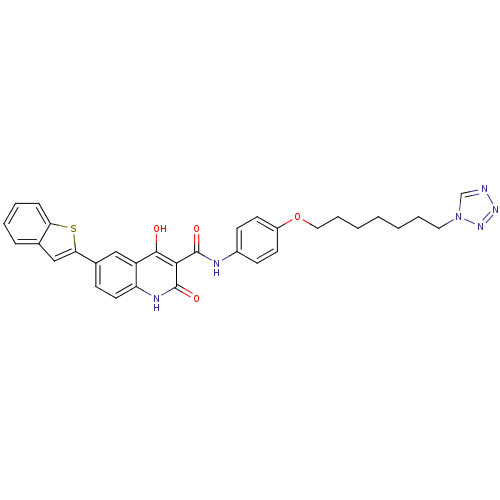 (6-Benzo[b]thiophen-2-yl-4-hydroxy-2-oxo-1,2-dihydr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 290 | n/a | n/a | n/a | n/a | n/a | n/a |
Xenova Limited Curated by ChEMBL | Assay Description Inhibitory activity against human plasminogen activator inhibitor-1(PAI-1) evaluated by complex assay. | Bioorg Med Chem Lett 12: 1063-6 (2002) BindingDB Entry DOI: 10.7270/Q2W958HQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen activator inhibitor 1 (Homo sapiens (Human)) | BDBM50111280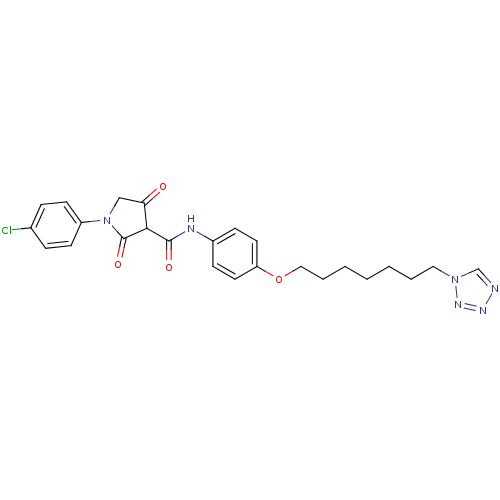 (1-(4-Chloro-phenyl)-4-hydroxy-2-oxo-2,5-dihydro-1H...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Xenova Limited Curated by ChEMBL | Assay Description Inhibitory activity against human plasminogen activator inhibitor-1(PAI-1) evaluated by complex assay. | Bioorg Med Chem Lett 12: 1063-6 (2002) BindingDB Entry DOI: 10.7270/Q2W958HQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen activator inhibitor 1 (Homo sapiens (Human)) | BDBM50111298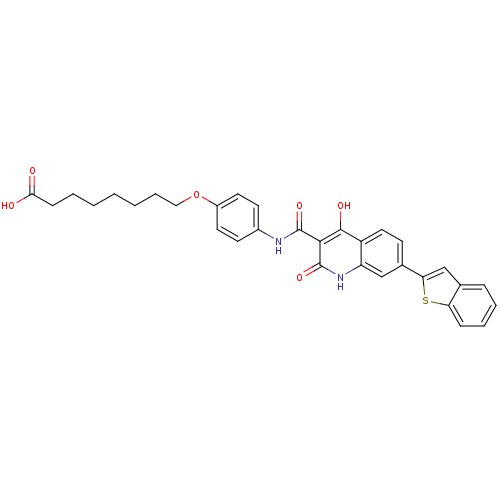 (8-{4-[(7-Benzo[b]thiophen-2-yl-4-hydroxy-2-oxo-1,2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Xenova Limited Curated by ChEMBL | Assay Description Inhibitory activity against human plasminogen activator inhibitor-1(PAI-1) evaluated by chromogenic assay. | Bioorg Med Chem Lett 12: 1063-6 (2002) BindingDB Entry DOI: 10.7270/Q2W958HQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen activator inhibitor 1 (Homo sapiens (Human)) | BDBM50111292 (8-{4-[(6-Benzo[b]thiophen-2-yl-4-hydroxy-2-oxo-1,2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
Xenova Limited Curated by ChEMBL | Assay Description Inhibitory activity against human plasminogen activator inhibitor-1(PAI-1) evaluated by chromogenic assay. | Bioorg Med Chem Lett 12: 1063-6 (2002) BindingDB Entry DOI: 10.7270/Q2W958HQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen activator inhibitor 1 (Homo sapiens (Human)) | BDBM50111309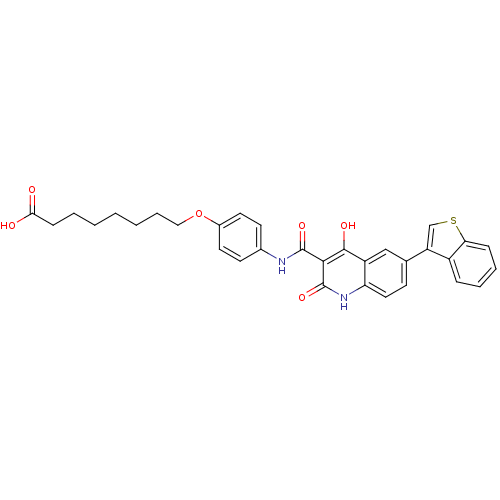 (8-{4-[(6-Benzo[b]thiophen-3-yl-4-hydroxy-2-oxo-1,2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 510 | n/a | n/a | n/a | n/a | n/a | n/a |
Xenova Limited Curated by ChEMBL | Assay Description Inhibitory activity against human plasminogen activator inhibitor-1(PAI-1) evaluated by chromogenic assay. | Bioorg Med Chem Lett 12: 1063-6 (2002) BindingDB Entry DOI: 10.7270/Q2W958HQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen activator inhibitor 1 (Homo sapiens (Human)) | BDBM50111316 (8-[4-(3,6-Dioxo-5-{4-[(thiophene-2-carbonyl)-amino...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 510 | n/a | n/a | n/a | n/a | n/a | n/a |
Xenova Limited Curated by ChEMBL | Assay Description Inhibitory activity against human plasminogen activator inhibitor-1(PAI-1) evaluated by complex assay. | Bioorg Med Chem Lett 12: 1063-6 (2002) BindingDB Entry DOI: 10.7270/Q2W958HQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen activator inhibitor 1 (Homo sapiens (Human)) | BDBM50111281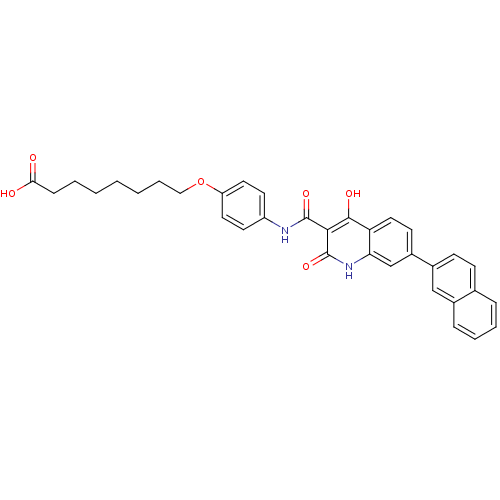 (8-{4-[(4-Hydroxy-7-naphthalen-2-yl-2-oxo-1,2-dihyd...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
Xenova Limited Curated by ChEMBL | Assay Description Inhibitory activity against human plasminogen activator inhibitor-1(PAI-1) evaluated by chromogenic assay. | Bioorg Med Chem Lett 12: 1063-6 (2002) BindingDB Entry DOI: 10.7270/Q2W958HQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen activator inhibitor 1 (Homo sapiens (Human)) | BDBM50111282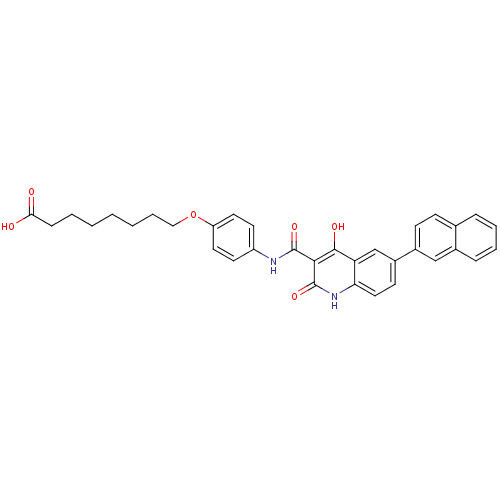 (8-{4-[(4-Hydroxy-6-naphthalen-2-yl-2-oxo-1,2-dihyd...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 670 | n/a | n/a | n/a | n/a | n/a | n/a |
Xenova Limited Curated by ChEMBL | Assay Description Inhibitory activity against human plasminogen activator inhibitor-1(PAI-1) evaluated by chromogenic assay. | Bioorg Med Chem Lett 12: 1063-6 (2002) BindingDB Entry DOI: 10.7270/Q2W958HQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen activator inhibitor 1 (Homo sapiens (Human)) | BDBM50111280 (1-(4-Chloro-phenyl)-4-hydroxy-2-oxo-2,5-dihydro-1H...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 690 | n/a | n/a | n/a | n/a | n/a | n/a |
Xenova Limited Curated by ChEMBL | Assay Description Inhibitory activity against human plasminogen activator inhibitor-1(PAI-1) evaluated by chromogenic assay. | Bioorg Med Chem Lett 12: 1063-6 (2002) BindingDB Entry DOI: 10.7270/Q2W958HQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen activator inhibitor 1 (Homo sapiens (Human)) | BDBM50111279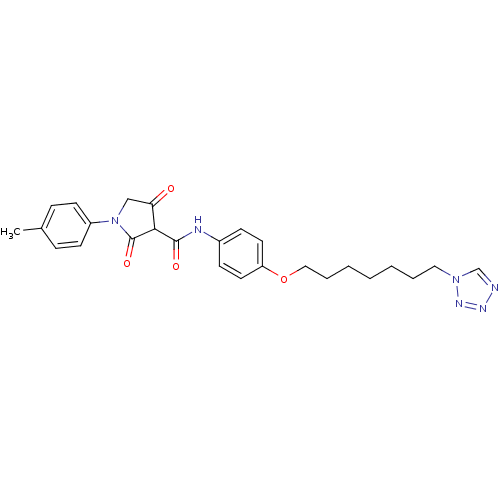 (4-Hydroxy-2-oxo-1-p-tolyl-2,5-dihydro-1H-pyrrole-3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 830 | n/a | n/a | n/a | n/a | n/a | n/a |
Xenova Limited Curated by ChEMBL | Assay Description Inhibitory activity against human plasminogen activator inhibitor-1(PAI-1) evaluated by chromogenic assay. | Bioorg Med Chem Lett 12: 1063-6 (2002) BindingDB Entry DOI: 10.7270/Q2W958HQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen activator inhibitor 1 (Homo sapiens (Human)) | BDBM50111279 (4-Hydroxy-2-oxo-1-p-tolyl-2,5-dihydro-1H-pyrrole-3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 890 | n/a | n/a | n/a | n/a | n/a | n/a |
Xenova Limited Curated by ChEMBL | Assay Description Inhibitory activity against human plasminogen activator inhibitor-1(PAI-1) evaluated by chromogenic assay. | Bioorg Med Chem Lett 12: 1063-6 (2002) BindingDB Entry DOI: 10.7270/Q2W958HQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen activator inhibitor 1 (Homo sapiens (Human)) | BDBM50111286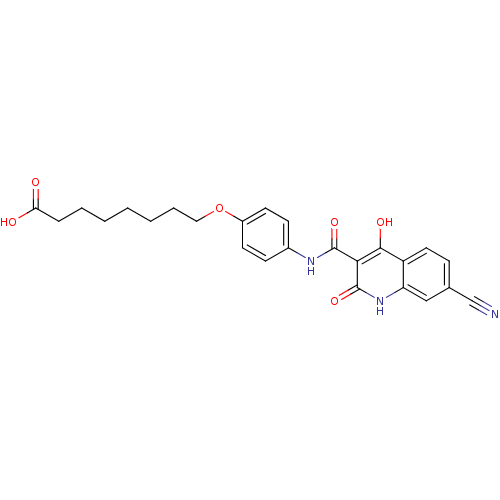 (8-{4-[(7-Cyano-4-hydroxy-2-oxo-1,2-dihydro-quinoli...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 900 | n/a | n/a | n/a | n/a | n/a | n/a |
Xenova Limited Curated by ChEMBL | Assay Description Inhibitory activity against human plasminogen activator inhibitor-1(PAI-1) evaluated by chromogenic assay. | Bioorg Med Chem Lett 12: 1063-6 (2002) BindingDB Entry DOI: 10.7270/Q2W958HQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen activator inhibitor 1 (Homo sapiens (Human)) | BDBM50111309 (8-{4-[(6-Benzo[b]thiophen-3-yl-4-hydroxy-2-oxo-1,2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 940 | n/a | n/a | n/a | n/a | n/a | n/a |
Xenova Limited Curated by ChEMBL | Assay Description Inhibitory activity against human plasminogen activator inhibitor-1(PAI-1) evaluated by complex assay. | Bioorg Med Chem Lett 12: 1063-6 (2002) BindingDB Entry DOI: 10.7270/Q2W958HQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen activator inhibitor 1 (Homo sapiens (Human)) | BDBM50111273 (6-Benzo[b]thiophen-2-yl-4-hydroxy-2-oxo-1,2-dihydr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.01E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Xenova Limited Curated by ChEMBL | Assay Description Inhibitory activity against human plasminogen activator inhibitor-1(PAI-1) evaluated by complex assay. | Bioorg Med Chem Lett 12: 1063-6 (2002) BindingDB Entry DOI: 10.7270/Q2W958HQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen activator inhibitor 1 (Homo sapiens (Human)) | BDBM50111284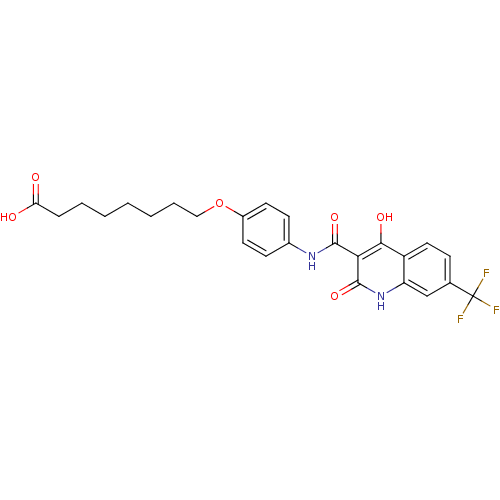 (8-{4-[(4-Hydroxy-2-oxo-7-trifluoromethyl-1,2-dihyd...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.03E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Xenova Limited Curated by ChEMBL | Assay Description Inhibitory activity against human plasminogen activator inhibitor-1(PAI-1) evaluated by chromogenic assay. | Bioorg Med Chem Lett 12: 1063-6 (2002) BindingDB Entry DOI: 10.7270/Q2W958HQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen activator inhibitor 1 (Homo sapiens (Human)) | BDBM50111299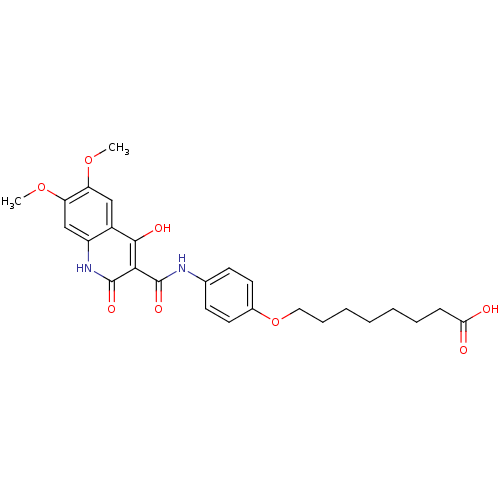 (8-{4-[(4-Hydroxy-6,7-dimethoxy-2-oxo-1,2-dihydro-q...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.65E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Xenova Limited Curated by ChEMBL | Assay Description Inhibitory activity against human plasminogen activator inhibitor-1(PAI-1) evaluated by chromogenic assay. | Bioorg Med Chem Lett 12: 1063-6 (2002) BindingDB Entry DOI: 10.7270/Q2W958HQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen activator inhibitor 1 (Homo sapiens (Human)) | BDBM50111319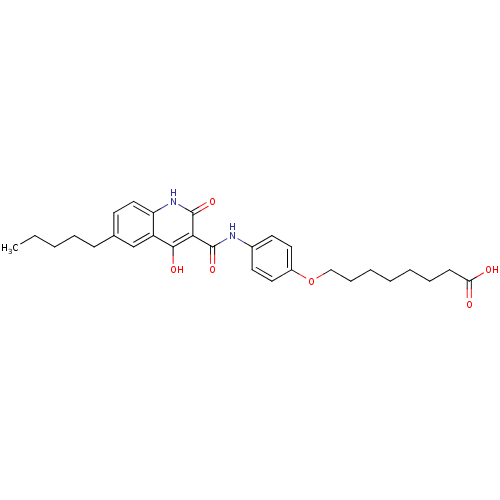 (8-{4-[(4-Hydroxy-2-oxo-6-pentyl-1,2-dihydro-quinol...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Xenova Limited Curated by ChEMBL | Assay Description Inhibitory activity against human plasminogen activator inhibitor-1(PAI-1) evaluated by chromogenic assay. | Bioorg Med Chem Lett 12: 1063-6 (2002) BindingDB Entry DOI: 10.7270/Q2W958HQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen activator inhibitor 1 (Homo sapiens (Human)) | BDBM50111306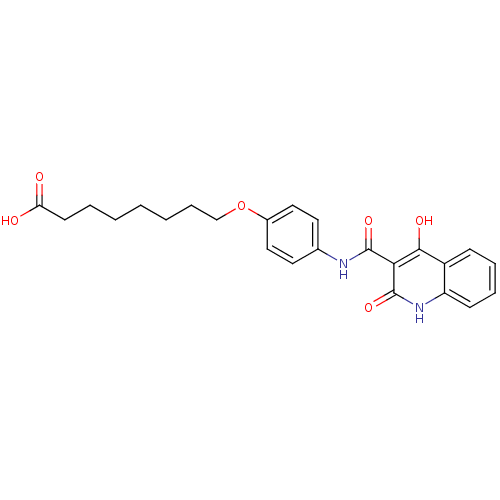 (8-{4-[(4-Hydroxy-2-oxo-1,2-dihydro-quinoline-3-car...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.85E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Xenova Limited Curated by ChEMBL | Assay Description Inhibitory activity against human plasminogen activator inhibitor-1(PAI-1) evaluated by chromogenic assay. | Bioorg Med Chem Lett 12: 1063-6 (2002) BindingDB Entry DOI: 10.7270/Q2W958HQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen activator inhibitor 1 (Homo sapiens (Human)) | BDBM50111313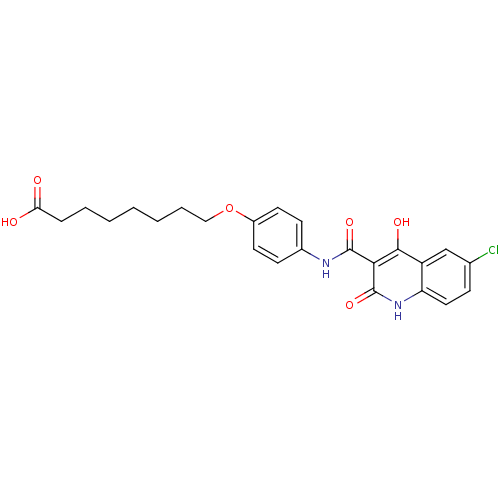 (8-{4-[(6-Chloro-4-hydroxy-2-oxo-1,2-dihydro-quinol...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.95E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Xenova Limited Curated by ChEMBL | Assay Description Inhibitory activity against human plasminogen activator inhibitor-1(PAI-1) evaluated by chromogenic assay. | Bioorg Med Chem Lett 12: 1063-6 (2002) BindingDB Entry DOI: 10.7270/Q2W958HQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen activator inhibitor 1 (Homo sapiens (Human)) | BDBM50111285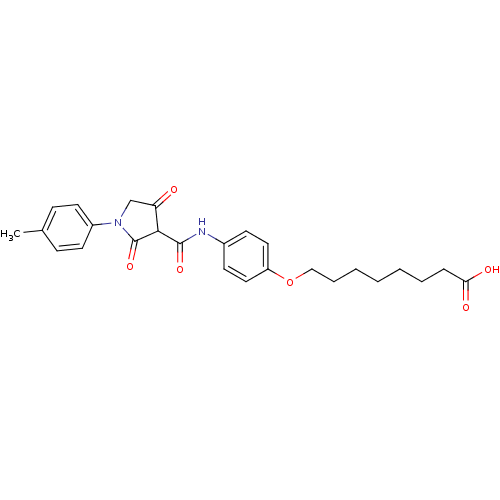 (8-(4-(4-hydroxy-2-oxo-1-p-tolyl-2,5-dihydro-1H-pyr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.95E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Xenova Limited Curated by ChEMBL | Assay Description Inhibitory activity against human plasminogen activator inhibitor-1(PAI-1) evaluated by chromogenic assay. | Bioorg Med Chem Lett 12: 1063-6 (2002) BindingDB Entry DOI: 10.7270/Q2W958HQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen activator inhibitor 1 (Homo sapiens (Human)) | BDBM50111288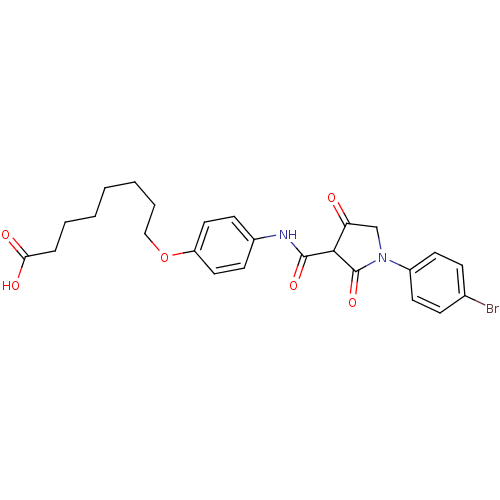 (8-(4-{[1-(4-Bromo-phenyl)-4-hydroxy-2-oxo-2,5-dihy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.99E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Xenova Limited Curated by ChEMBL | Assay Description Inhibitory activity against human plasminogen activator inhibitor-1(PAI-1) evaluated by chromogenic assay. | Bioorg Med Chem Lett 12: 1063-6 (2002) BindingDB Entry DOI: 10.7270/Q2W958HQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen activator inhibitor 1 (Homo sapiens (Human)) | BDBM50111295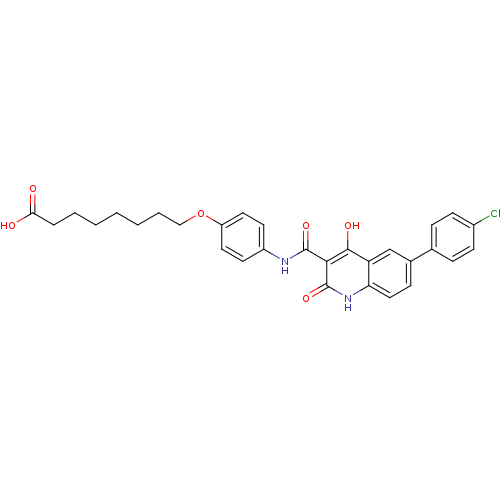 (8-(4-{[6-(4-Chloro-phenyl)-4-hydroxy-2-oxo-1,2-dih...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Xenova Limited Curated by ChEMBL | Assay Description Inhibitory activity against human plasminogen activator inhibitor-1(PAI-1) evaluated by chromogenic assay. | Bioorg Med Chem Lett 12: 1063-6 (2002) BindingDB Entry DOI: 10.7270/Q2W958HQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen activator inhibitor 1 (Homo sapiens (Human)) | BDBM50111308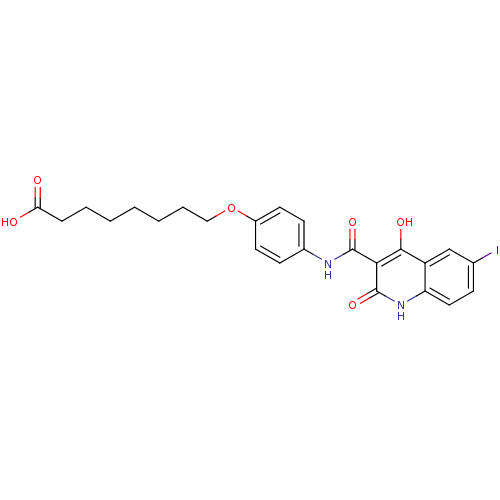 (8-{4-[(4-Hydroxy-6-iodo-2-oxo-1,2-dihydro-quinolin...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Xenova Limited Curated by ChEMBL | Assay Description Inhibitory activity against human plasminogen activator inhibitor-1(PAI-1) evaluated by chromogenic assay. | Bioorg Med Chem Lett 12: 1063-6 (2002) BindingDB Entry DOI: 10.7270/Q2W958HQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen activator inhibitor 1 (Homo sapiens (Human)) | BDBM50111296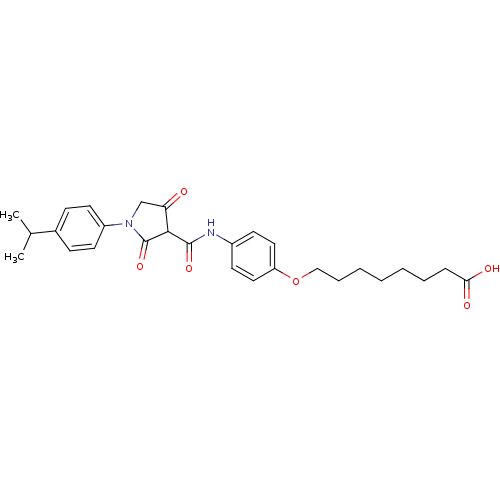 (8-(4-{[4-Hydroxy-1-(4-isopropyl-phenyl)-2-oxo-2,5-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.35E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Xenova Limited Curated by ChEMBL | Assay Description Inhibitory activity against human plasminogen activator inhibitor-1(PAI-1) evaluated by chromogenic assay. | Bioorg Med Chem Lett 12: 1063-6 (2002) BindingDB Entry DOI: 10.7270/Q2W958HQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen activator inhibitor 1 (Homo sapiens (Human)) | BDBM50111314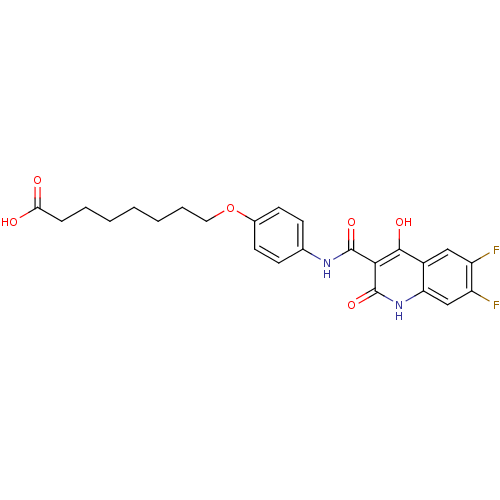 (8-{4-[(6,7-Difluoro-4-hydroxy-2-oxo-1,2-dihydro-qu...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.35E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Xenova Limited Curated by ChEMBL | Assay Description Inhibitory activity against human plasminogen activator inhibitor-1(PAI-1) evaluated by chromogenic assay. | Bioorg Med Chem Lett 12: 1063-6 (2002) BindingDB Entry DOI: 10.7270/Q2W958HQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 103 total ) | Next | Last >> |