 Found 653 hits with Last Name = 'silva' and Initial = 'j'
Found 653 hits with Last Name = 'silva' and Initial = 'j' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Coagulation factor XI
(Homo sapiens (Human)) | BDBM50598740
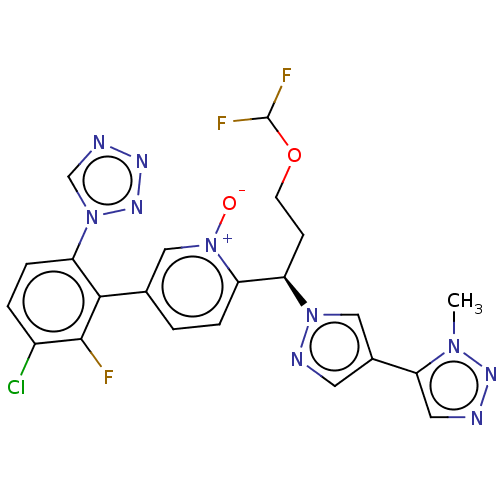
(CHEMBL5175227)Show SMILES Cn1nncc1-c1cnn(c1)[C@H](CCOC(F)F)c1ccc(c[n+]1[O-])-c1c(F)c(Cl)ccc1-n1cnnn1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00442
BindingDB Entry DOI: 10.7270/Q2C82FBD |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM50598738
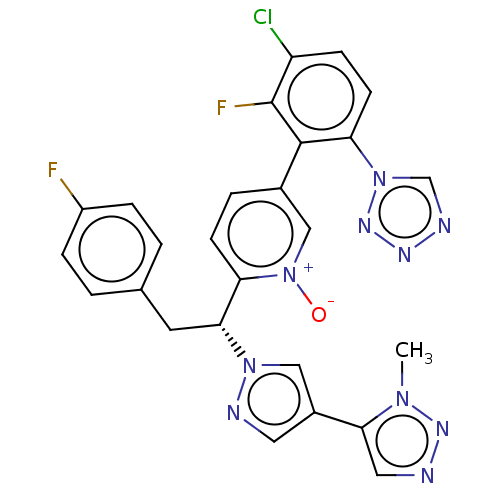
(CHEMBL5204065)Show SMILES Cn1nncc1-c1cnn(c1)[C@H](Cc1ccc(F)cc1)c1ccc(c[n+]1[O-])-c1c(F)c(Cl)ccc1-n1cnnn1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00442
BindingDB Entry DOI: 10.7270/Q2C82FBD |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM50598739

(CHEMBL5188215)Show SMILES COCC[C@H](c1ccc(c[n+]1[O-])-c1c(F)c(Cl)ccc1-n1cnnn1)n1cc(cn1)-c1cnnn1C |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00442
BindingDB Entry DOI: 10.7270/Q2C82FBD |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM50598724

(CHEMBL5170592)Show SMILES Cn1nncc1-c1cnn(c1)[C@H](CCOC(F)F)c1ccc(c[n+]1[O-])-c1c(F)c(Cl)ccc1-n1cc(Cl)nn1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00442
BindingDB Entry DOI: 10.7270/Q2C82FBD |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM50598737
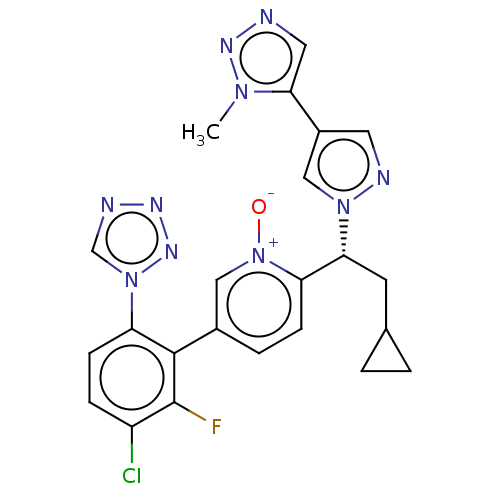
(CHEMBL5205631)Show SMILES Cn1nncc1-c1cnn(c1)[C@H](CC1CC1)c1ccc(c[n+]1[O-])-c1c(F)c(Cl)ccc1-n1cnnn1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00442
BindingDB Entry DOI: 10.7270/Q2C82FBD |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM50598741

(CHEMBL5204894)Show SMILES Cn1nncc1-c1cnn(c1)[C@H](Cc1ccc(F)cc1)c1ccc(c[n+]1[O-])-c1c(F)c(Cl)ccc1-n1cc(Cl)nn1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00442
BindingDB Entry DOI: 10.7270/Q2C82FBD |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM50598725

(CHEMBL5185397)Show SMILES COC(=O)Nc1ccc(cc1)-c1cnc([nH]1)C(CC1CC1)c1ccc(c[n+]1[O-])-c1cc(Cl)ccc1-n1cnnn1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00442
BindingDB Entry DOI: 10.7270/Q2C82FBD |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM50598743
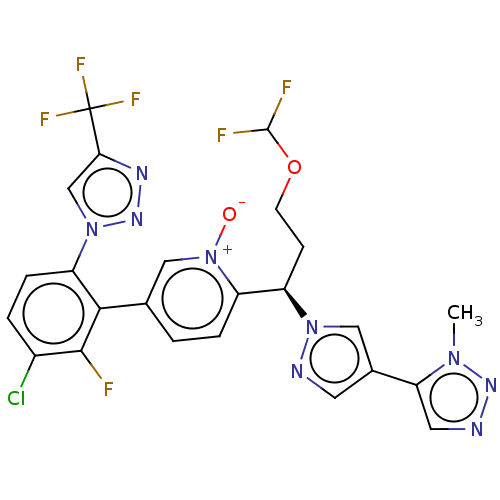
(CHEMBL5178223)Show SMILES Cn1nncc1-c1cnn(c1)[C@H](CCOC(F)F)c1ccc(c[n+]1[O-])-c1c(F)c(Cl)ccc1-n1cc(nn1)C(F)(F)F |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00442
BindingDB Entry DOI: 10.7270/Q2C82FBD |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM50598734
(CHEMBL5197480)Show SMILES Cc1ncsc1-c1cnn(c1)C(CC1CC1)c1ccc(c[n+]1[O-])-c1c(F)c(Cl)ccc1-n1cnnn1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00442
BindingDB Entry DOI: 10.7270/Q2C82FBD |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM50598745
(CHEMBL5198823)Show SMILES Cn1nncc1-c1cnn(c1)[C@H](Cc1ccn(n1)C(F)F)c1ccc(c[n+]1[O-])-c1c(F)c(Cl)ccc1-n1cc(nn1)C(F)(F)F |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00442
BindingDB Entry DOI: 10.7270/Q2C82FBD |
More data for this
Ligand-Target Pair | |
Aurora kinase B
(Homo sapiens (Human)) | BDBM50315769

(3-(4-(4-(2-(3-((dimethylamino)methyl)phenyl)-1H-py...)Show SMILES CCn1cc(c(n1)-c1ccc(NC(=O)N(C)C)cc1)-c1ccnc2[nH]c(cc12)-c1cccc(CN(C)C)c1 Show InChI InChI=1S/C30H33N7O/c1-6-37-19-26(28(34-37)21-10-12-23(13-11-21)32-30(38)36(4)5)24-14-15-31-29-25(24)17-27(33-29)22-9-7-8-20(16-22)18-35(2)3/h7-17,19H,6,18H2,1-5H3,(H,31,33)(H,32,38) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Competitive inhibition of human Aurora B ATP binding site by rapid dilution method |
J Med Chem 53: 3973-4001 (2010)
Article DOI: 10.1021/jm901870q
BindingDB Entry DOI: 10.7270/Q27082CK |
More data for this
Ligand-Target Pair | |
Aurora kinase B
(Homo sapiens (Human)) | BDBM50315769

(3-(4-(4-(2-(3-((dimethylamino)methyl)phenyl)-1H-py...)Show SMILES CCn1cc(c(n1)-c1ccc(NC(=O)N(C)C)cc1)-c1ccnc2[nH]c(cc12)-c1cccc(CN(C)C)c1 Show InChI InChI=1S/C30H33N7O/c1-6-37-19-26(28(34-37)21-10-12-23(13-11-21)32-30(38)36(4)5)24-14-15-31-29-25(24)17-27(33-29)22-9-7-8-20(16-22)18-35(2)3/h7-17,19H,6,18H2,1-5H3,(H,31,33)(H,32,38) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Competitive inhibition of Aurora B ATP binding site |
J Med Chem 53: 3973-4001 (2010)
Article DOI: 10.1021/jm901870q
BindingDB Entry DOI: 10.7270/Q27082CK |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM50598736

(CHEMBL5208095)Show SMILES Cn1cncc1-c1cnn(c1)[C@H](CC1CC1)c1ccc(c[n+]1[O-])-c1c(F)c(Cl)ccc1-n1cnnn1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00442
BindingDB Entry DOI: 10.7270/Q2C82FBD |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM50598744
(CHEMBL5190323)Show SMILES Cn1nncc1-c1cnn(c1)[C@H](Cn1cc(F)cn1)c1ccc(c[n+]1[O-])-c1c(F)c(Cl)ccc1-n1cc(Cl)nn1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00442
BindingDB Entry DOI: 10.7270/Q2C82FBD |
More data for this
Ligand-Target Pair | |
Prostaglandin D2 receptor
(Homo sapiens (Human)) | BDBM50205275
(CHEMBL426559 | [(3R)-4-(4-chlorobenzyl)-7-fluoro-5...)Show SMILES CS(=O)(=O)c1cc(F)cc2c3CC[C@H](CC(O)=O)c3n(Cc3ccc(Cl)cc3)c12 Show InChI InChI=1S/C21H19ClFNO4S/c1-29(27,28)18-10-15(23)9-17-16-7-4-13(8-19(25)26)20(16)24(21(17)18)11-12-2-5-14(22)6-3-12/h2-3,5-6,9-10,13H,4,7-8,11H2,1H3,(H,25,26)/t13-/m1/s1 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.570 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Canada & Co.
Curated by ChEMBL
| Assay Description
Binding affinity to human DP receptor expressed in HEK293 cells |
J Med Chem 50: 794-806 (2007)
Article DOI: 10.1021/jm0603668
BindingDB Entry DOI: 10.7270/Q20P0ZP2 |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Homo sapiens (Human)) | BDBM13534

(CHEMBL572878 | N-[4-({4-[(3-methyl-1H-pyrazol-5-yl...)Show SMILES CN1CCN(CC1)c1cc(Nc2cc(C)n[nH]2)nc(Sc2ccc(NC(=O)C3CC3)cc2)n1 Show InChI InChI=1S/C23H28N8OS/c1-15-13-20(29-28-15)25-19-14-21(31-11-9-30(2)10-12-31)27-23(26-19)33-18-7-5-17(6-8-18)24-22(32)16-3-4-16/h5-8,13-14,16H,3-4,9-12H2,1-2H3,(H,24,32)(H2,25,26,27,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of Aurora A |
J Med Chem 53: 3973-4001 (2010)
Article DOI: 10.1021/jm901870q
BindingDB Entry DOI: 10.7270/Q27082CK |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM50598732
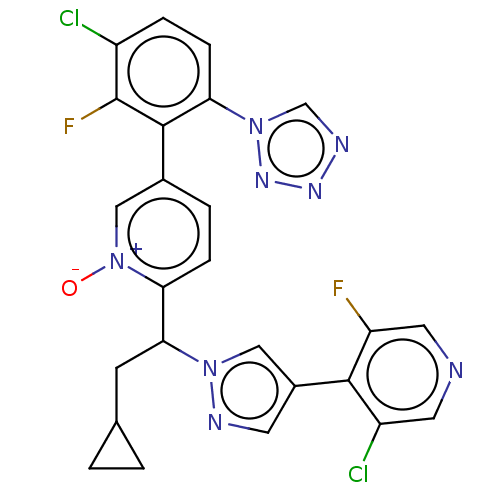
(CHEMBL5192284)Show SMILES [O-][n+]1cc(ccc1C(CC1CC1)n1cc(cn1)-c1c(F)cncc1Cl)-c1c(F)c(Cl)ccc1-n1cnnn1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00442
BindingDB Entry DOI: 10.7270/Q2C82FBD |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Thromboxane A2 receptor
(Homo sapiens (Human)) | BDBM50205274
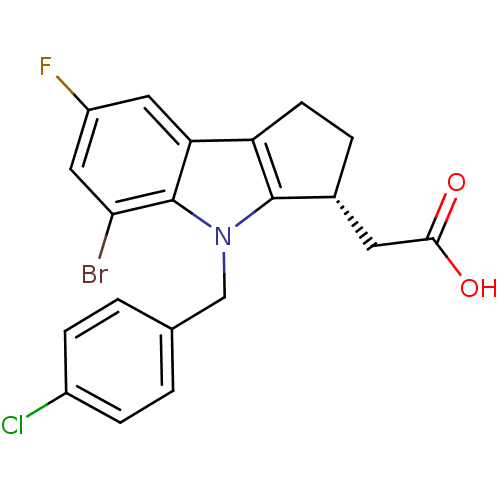
(CHEMBL426387 | [(3R)-5-bromo-4-(4-chlorobenzyl)-7-...)Show SMILES OC(=O)C[C@H]1CCc2c1n(Cc1ccc(Cl)cc1)c1c(Br)cc(F)cc21 Show InChI InChI=1S/C20H16BrClFNO2/c21-17-9-14(23)8-16-15-6-3-12(7-18(25)26)19(15)24(20(16)17)10-11-1-4-13(22)5-2-11/h1-2,4-5,8-9,12H,3,6-7,10H2,(H,25,26)/t12-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.840 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Canada & Co.
Curated by ChEMBL
| Assay Description
Binding affinity to human TP receptor expressed in HEK293 cells |
J Med Chem 50: 794-806 (2007)
Article DOI: 10.1021/jm0603668
BindingDB Entry DOI: 10.7270/Q20P0ZP2 |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM50598742
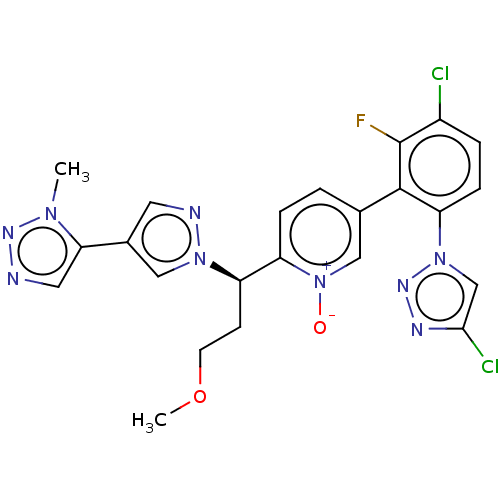
(CHEMBL5182855)Show SMILES COCC[C@H](c1ccc(c[n+]1[O-])-c1c(F)c(Cl)ccc1-n1cc(Cl)nn1)n1cc(cn1)-c1cnnn1C |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00442
BindingDB Entry DOI: 10.7270/Q2C82FBD |
More data for this
Ligand-Target Pair | |
Prostaglandin D2 receptor
(Homo sapiens (Human)) | BDBM50205276
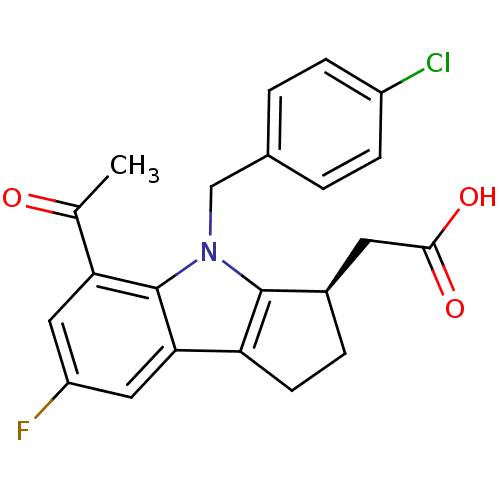
(CHEMBL385126 | [(3R)-4-(4-chlorobenzyl)-7-fluoro-5...)Show SMILES CC(=O)c1cc(F)cc2c3CC[C@H](CC(O)=O)c3n(Cc3ccc(Cl)cc3)c12 Show InChI InChI=1S/C22H19ClFNO3/c1-12(26)18-9-16(24)10-19-17-7-4-14(8-20(27)28)21(17)25(22(18)19)11-13-2-5-15(23)6-3-13/h2-3,5-6,9-10,14H,4,7-8,11H2,1H3,(H,27,28)/t14-/m1/s1 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Canada & Co.
Curated by ChEMBL
| Assay Description
Binding affinity to human DP receptor expressed in HEK293 cells |
J Med Chem 50: 794-806 (2007)
Article DOI: 10.1021/jm0603668
BindingDB Entry DOI: 10.7270/Q20P0ZP2 |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM50598729
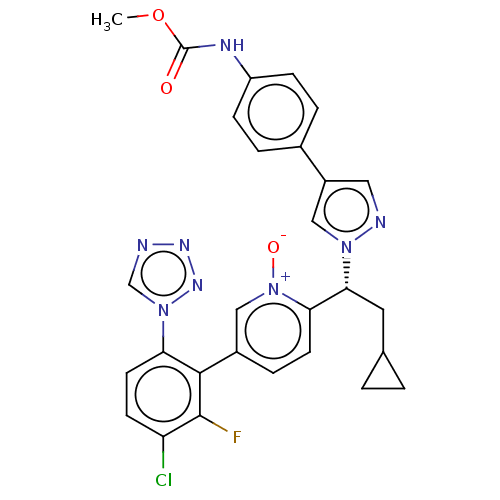
(CHEMBL5195600)Show SMILES COC(=O)Nc1ccc(cc1)-c1cnn(c1)[C@H](CC1CC1)c1ccc(c[n+]1[O-])-c1c(F)c(Cl)ccc1-n1cnnn1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00442
BindingDB Entry DOI: 10.7270/Q2C82FBD |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM50598727
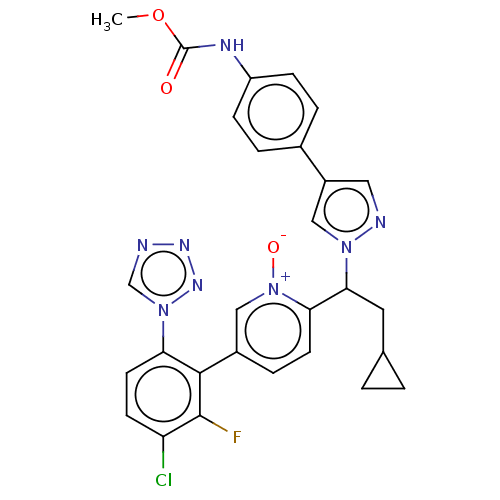
(CHEMBL5198338)Show SMILES COC(=O)Nc1ccc(cc1)-c1cnn(c1)C(CC1CC1)c1ccc(c[n+]1[O-])-c1c(F)c(Cl)ccc1-n1cnnn1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00442
BindingDB Entry DOI: 10.7270/Q2C82FBD |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Aurora kinase C
(Homo sapiens (Human)) | BDBM50315769

(3-(4-(4-(2-(3-((dimethylamino)methyl)phenyl)-1H-py...)Show SMILES CCn1cc(c(n1)-c1ccc(NC(=O)N(C)C)cc1)-c1ccnc2[nH]c(cc12)-c1cccc(CN(C)C)c1 Show InChI InChI=1S/C30H33N7O/c1-6-37-19-26(28(34-37)21-10-12-23(13-11-21)32-30(38)36(4)5)24-14-15-31-29-25(24)17-27(33-29)22-9-7-8-20(16-22)18-35(2)3/h7-17,19H,6,18H2,1-5H3,(H,31,33)(H,32,38) | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Competitive inhibition of human Aurora C ATP binding site |
J Med Chem 53: 3973-4001 (2010)
Article DOI: 10.1021/jm901870q
BindingDB Entry DOI: 10.7270/Q27082CK |
More data for this
Ligand-Target Pair | |
Prostaglandin D2 receptor
(Homo sapiens (Human)) | BDBM50205274

(CHEMBL426387 | [(3R)-5-bromo-4-(4-chlorobenzyl)-7-...)Show SMILES OC(=O)C[C@H]1CCc2c1n(Cc1ccc(Cl)cc1)c1c(Br)cc(F)cc21 Show InChI InChI=1S/C20H16BrClFNO2/c21-17-9-14(23)8-16-15-6-3-12(7-18(25)26)19(15)24(20(16)17)10-11-1-4-13(22)5-2-11/h1-2,4-5,8-9,12H,3,6-7,10H2,(H,25,26)/t12-/m1/s1 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Canada & Co.
Curated by ChEMBL
| Assay Description
Binding affinity to human DP receptor expressed in HEK293 cells |
J Med Chem 50: 794-806 (2007)
Article DOI: 10.1021/jm0603668
BindingDB Entry DOI: 10.7270/Q20P0ZP2 |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM50598735
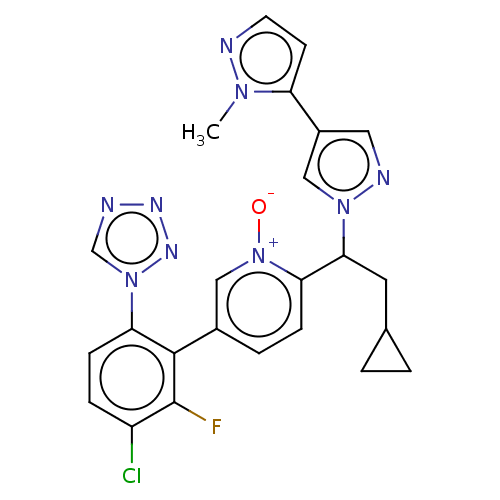
(CHEMBL5193267)Show SMILES Cn1nccc1-c1cnn(c1)C(CC1CC1)c1ccc(c[n+]1[O-])-c1c(F)c(Cl)ccc1-n1cnnn1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00442
BindingDB Entry DOI: 10.7270/Q2C82FBD |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Aurora kinase B
(Homo sapiens (Human)) | BDBM13534

(CHEMBL572878 | N-[4-({4-[(3-methyl-1H-pyrazol-5-yl...)Show SMILES CN1CCN(CC1)c1cc(Nc2cc(C)n[nH]2)nc(Sc2ccc(NC(=O)C3CC3)cc2)n1 Show InChI InChI=1S/C23H28N8OS/c1-15-13-20(29-28-15)25-19-14-21(31-11-9-30(2)10-12-31)27-23(26-19)33-18-7-5-17(6-8-18)24-22(32)16-3-4-16/h5-8,13-14,16H,3-4,9-12H2,1-2H3,(H,24,32)(H2,25,26,27,28,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Competitive inhibition of Aurora B ATP binding site |
J Med Chem 53: 3973-4001 (2010)
Article DOI: 10.1021/jm901870q
BindingDB Entry DOI: 10.7270/Q27082CK |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Prostaglandin D2 receptor
(Homo sapiens (Human)) | BDBM50184217
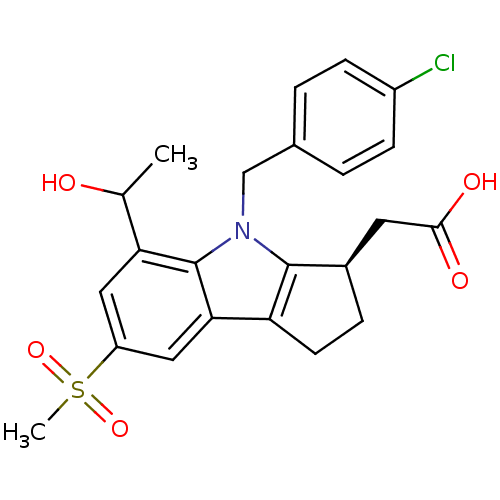
(2-((R)-4-(4-chlorobenzyl)-5-(1-hydroxyethyl)-7-(me...)Show SMILES CC(O)c1cc(cc2c3CC[C@H](CC(O)=O)c3n(Cc3ccc(Cl)cc3)c12)S(C)(=O)=O Show InChI InChI=1S/C23H24ClNO5S/c1-13(26)19-10-17(31(2,29)30)11-20-18-8-5-15(9-21(27)28)22(18)25(23(19)20)12-14-3-6-16(24)7-4-14/h3-4,6-7,10-11,13,15,26H,5,8-9,12H2,1-2H3,(H,27,28)/t13?,15-/m1/s1 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Canada & Co.
Curated by ChEMBL
| Assay Description
Binding affinity to human DP receptor expressed in HEK293 cells |
J Med Chem 50: 794-806 (2007)
Article DOI: 10.1021/jm0603668
BindingDB Entry DOI: 10.7270/Q20P0ZP2 |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM50598730

(CHEMBL5204289)Show SMILES Cc1nc(N)ccc1-c1cnn(c1)C(CC1CC1)c1ccc(c[n+]1[O-])-c1c(F)c(Cl)ccc1-n1cnnn1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00442
BindingDB Entry DOI: 10.7270/Q2C82FBD |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Prostaglandin D2 receptor
(Homo sapiens (Human)) | BDBM50184212
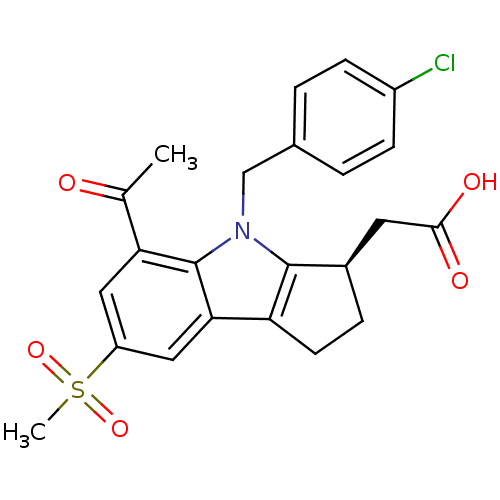
((R)-2-(4-(4-chlorobenzyl)-5-acetyl-7-(methylsulfon...)Show SMILES CC(=O)c1cc(cc2c3CC[C@H](CC(O)=O)c3n(Cc3ccc(Cl)cc3)c12)S(C)(=O)=O |r| Show InChI InChI=1S/C23H22ClNO5S/c1-13(26)19-10-17(31(2,29)30)11-20-18-8-5-15(9-21(27)28)22(18)25(23(19)20)12-14-3-6-16(24)7-4-14/h3-4,6-7,10-11,15H,5,8-9,12H2,1-2H3,(H,27,28)/t15-/m1/s1 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Canada & Co.
Curated by ChEMBL
| Assay Description
Binding affinity to human DP receptor expressed in HEK293 cells |
J Med Chem 50: 794-806 (2007)
Article DOI: 10.1021/jm0603668
BindingDB Entry DOI: 10.7270/Q20P0ZP2 |
More data for this
Ligand-Target Pair | |
Thromboxane A2 receptor
(Homo sapiens (Human)) | BDBM50205275

(CHEMBL426559 | [(3R)-4-(4-chlorobenzyl)-7-fluoro-5...)Show SMILES CS(=O)(=O)c1cc(F)cc2c3CC[C@H](CC(O)=O)c3n(Cc3ccc(Cl)cc3)c12 Show InChI InChI=1S/C21H19ClFNO4S/c1-29(27,28)18-10-15(23)9-17-16-7-4-13(8-19(25)26)20(16)24(21(17)18)11-12-2-5-14(22)6-3-12/h2-3,5-6,9-10,13H,4,7-8,11H2,1H3,(H,25,26)/t13-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Canada & Co.
Curated by ChEMBL
| Assay Description
Binding affinity to human TP receptor expressed in HEK293 cells |
J Med Chem 50: 794-806 (2007)
Article DOI: 10.1021/jm0603668
BindingDB Entry DOI: 10.7270/Q20P0ZP2 |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM50598731
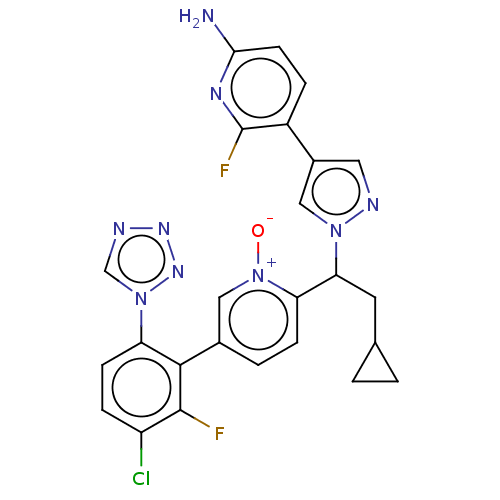
(CHEMBL5198972)Show SMILES Nc1ccc(-c2cnn(c2)C(CC2CC2)c2ccc(c[n+]2[O-])-c2c(F)c(Cl)ccc2-n2cnnn2)c(F)n1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 3.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00442
BindingDB Entry DOI: 10.7270/Q2C82FBD |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Aurora kinase C
(Homo sapiens (Human)) | BDBM13534

(CHEMBL572878 | N-[4-({4-[(3-methyl-1H-pyrazol-5-yl...)Show SMILES CN1CCN(CC1)c1cc(Nc2cc(C)n[nH]2)nc(Sc2ccc(NC(=O)C3CC3)cc2)n1 Show InChI InChI=1S/C23H28N8OS/c1-15-13-20(29-28-15)25-19-14-21(31-11-9-30(2)10-12-31)27-23(26-19)33-18-7-5-17(6-8-18)24-22(32)16-3-4-16/h5-8,13-14,16H,3-4,9-12H2,1-2H3,(H,24,32)(H2,25,26,27,28,29) | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 4.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Competitive inhibition of Aurora C ATP binding site |
J Med Chem 53: 3973-4001 (2010)
Article DOI: 10.1021/jm901870q
BindingDB Entry DOI: 10.7270/Q27082CK |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM50598733
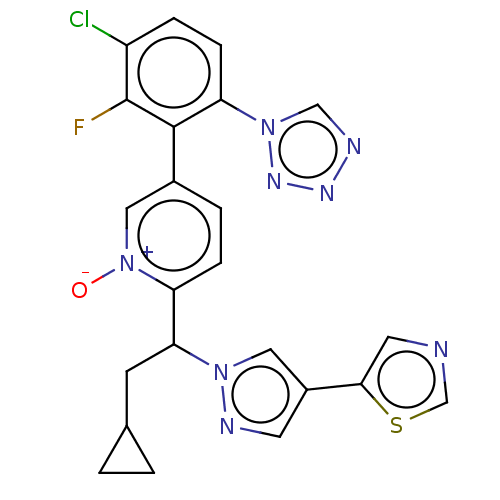
(CHEMBL5188316)Show SMILES [O-][n+]1cc(ccc1C(CC1CC1)n1cc(cn1)-c1cncs1)-c1c(F)c(Cl)ccc1-n1cnnn1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 6.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00442
BindingDB Entry DOI: 10.7270/Q2C82FBD |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM50598726
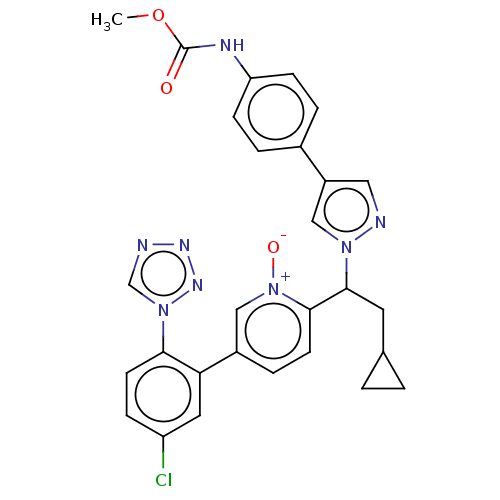
(CHEMBL5171252)Show SMILES COC(=O)Nc1ccc(cc1)-c1cnn(c1)C(CC1CC1)c1ccc(c[n+]1[O-])-c1cc(Cl)ccc1-n1cnnn1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 7.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00442
BindingDB Entry DOI: 10.7270/Q2C82FBD |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Prostaglandin D2 receptor
(Homo sapiens (Human)) | BDBM50205278
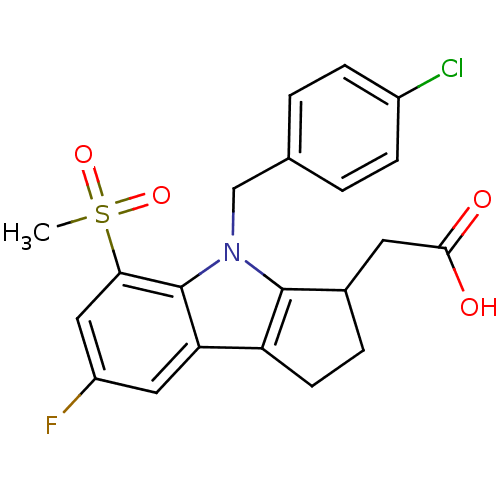
(2-(4-(4-chlorobenzyl)-7-fluoro-5-(methylsulfonyl)-...)Show SMILES CS(=O)(=O)c1cc(F)cc2c3CCC(CC(O)=O)c3n(Cc3ccc(Cl)cc3)c12 Show InChI InChI=1S/C21H19ClFNO4S/c1-29(27,28)18-10-15(23)9-17-16-7-4-13(8-19(25)26)20(16)24(21(17)18)11-12-2-5-14(22)6-3-12/h2-3,5-6,9-10,13H,4,7-8,11H2,1H3,(H,25,26) | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 8.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Canada & Co.
Curated by ChEMBL
| Assay Description
Binding affinity to human DP receptor expressed in HEK293 cells |
J Med Chem 50: 794-806 (2007)
Article DOI: 10.1021/jm0603668
BindingDB Entry DOI: 10.7270/Q20P0ZP2 |
More data for this
Ligand-Target Pair | |
Prostaglandin D2 receptor
(Homo sapiens (Human)) | BDBM50205277
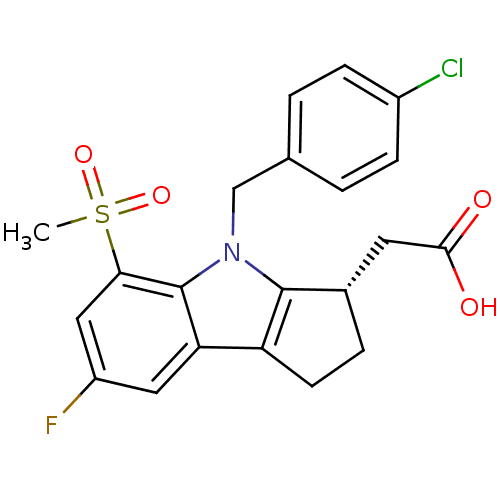
((S)-2-(4-(4-chlorobenzyl)-7-fluoro-5-(methylsulfon...)Show SMILES CS(=O)(=O)c1cc(F)cc2c3CC[C@@H](CC(O)=O)c3n(Cc3ccc(Cl)cc3)c12 Show InChI InChI=1S/C21H19ClFNO4S/c1-29(27,28)18-10-15(23)9-17-16-7-4-13(8-19(25)26)20(16)24(21(17)18)11-12-2-5-14(22)6-3-12/h2-3,5-6,9-10,13H,4,7-8,11H2,1H3,(H,25,26)/t13-/m0/s1 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 9.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Canada & Co.
Curated by ChEMBL
| Assay Description
Binding affinity to human DP receptor expressed in HEK293 cells |
J Med Chem 50: 794-806 (2007)
Article DOI: 10.1021/jm0603668
BindingDB Entry DOI: 10.7270/Q20P0ZP2 |
More data for this
Ligand-Target Pair | |
Thromboxane A2 receptor
(Homo sapiens (Human)) | BDBM50205276

(CHEMBL385126 | [(3R)-4-(4-chlorobenzyl)-7-fluoro-5...)Show SMILES CC(=O)c1cc(F)cc2c3CC[C@H](CC(O)=O)c3n(Cc3ccc(Cl)cc3)c12 Show InChI InChI=1S/C22H19ClFNO3/c1-12(26)18-9-16(24)10-19-17-7-4-14(8-20(27)28)21(17)25(22(18)19)11-13-2-5-15(23)6-3-13/h2-3,5-6,9-10,14H,4,7-8,11H2,1H3,(H,27,28)/t14-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Canada & Co.
Curated by ChEMBL
| Assay Description
Binding affinity to human TP receptor expressed in HEK293 cells |
J Med Chem 50: 794-806 (2007)
Article DOI: 10.1021/jm0603668
BindingDB Entry DOI: 10.7270/Q20P0ZP2 |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50135997

(CHEMBL3754471)Show SMILES [H][C@@]12CCC(=O)[C@@]1(C)CC[C@@]1([H])[C@@]2([H])C[C@H](C)C2=CC(=O)CC[C@]12C |r,t:19| Show InChI InChI=1S/C20H28O2/c1-12-10-14-15-4-5-18(22)20(15,3)9-7-16(14)19(2)8-6-13(21)11-17(12)19/h11-12,14-16H,4-10H2,1-3H3/t12-,14-,15-,16-,19+,20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Coimbra
Curated by ChEMBL
| Assay Description
Competitive irreversible inhibition of human placental microsome aromatase using varying levels of [1beta3H]-androstenedione as substrate measured af... |
J Med Chem 62: 3636-3657 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00157
BindingDB Entry DOI: 10.7270/Q2N301CW |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50135997

(CHEMBL3754471)Show SMILES [H][C@@]12CCC(=O)[C@@]1(C)CC[C@@]1([H])[C@@]2([H])C[C@H](C)C2=CC(=O)CC[C@]12C |r,t:19| Show InChI InChI=1S/C20H28O2/c1-12-10-14-15-4-5-18(22)20(15,3)9-7-16(14)19(2)8-6-13(21)11-17(12)19/h11-12,14-16H,4-10H2,1-3H3/t12-,14-,15-,16-,19+,20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Coimbra
Curated by ChEMBL
| Assay Description
Competitive reversible inhibition of human placental microsome aromatase using varying levels of [1beta3H]-androstenedione as substrate measured afte... |
J Med Chem 62: 3636-3657 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00157
BindingDB Entry DOI: 10.7270/Q2N301CW |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50398447
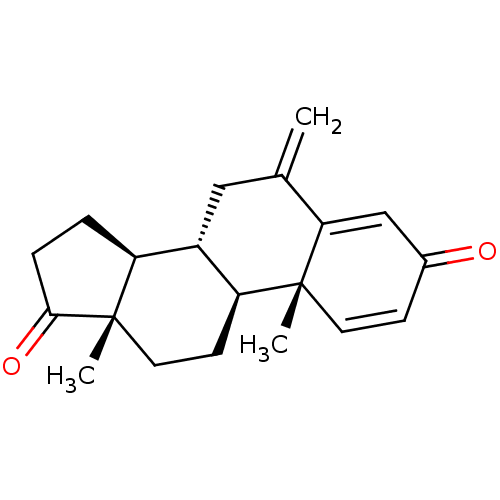
(Aromasin | EXEMESTANE)Show SMILES C[C@]12CC[C@H]3[C@@H](CC(=C)C4=CC(=O)C=C[C@]34C)[C@@H]1CCC2=O |r,c:13,t:9| Show InChI InChI=1S/C20H24O2/c1-12-10-14-15-4-5-18(22)20(15,3)9-7-16(14)19(2)8-6-13(21)11-17(12)19/h6,8,11,14-16H,1,4-5,7,9-10H2,2-3H3/t14-,15-,16-,19+,20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| DrugBank
PDB
Article
PubMed
| 26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Coimbra
Curated by ChEMBL
| Assay Description
Competitive inhibition of human placental microsome aromatase using varying levels of [1beta2beta3H]-androstenedione as substrate |
J Med Chem 62: 3636-3657 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00157
BindingDB Entry DOI: 10.7270/Q2N301CW |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Aromatase
(Homo sapiens (Human)) | BDBM50174541
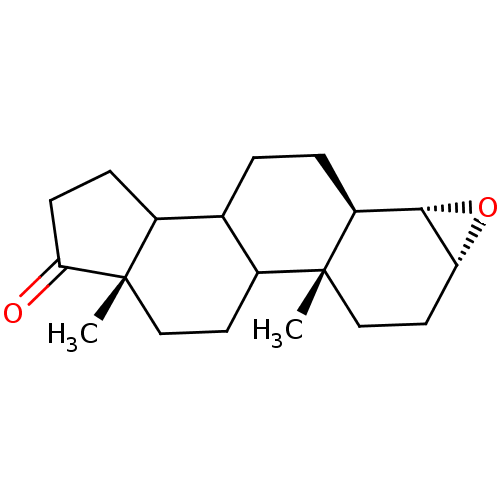
((3R,4S,5R,10R,13S)-10,13-Dimethyl-hexadecahydro-20...)Show SMILES C[C@]12CCC3C(CC[C@H]4[C@@H]5O[C@@H]5CC[C@]34C)C1CCC2=O Show InChI InChI=1S/C19H28O2/c1-18-10-8-15-17(21-15)14(18)4-3-11-12-5-6-16(20)19(12,2)9-7-13(11)18/h11-15,17H,3-10H2,1-2H3/t11?,12?,13?,14-,15+,17-,18+,19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 38 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oporto
Curated by ChEMBL
| Assay Description
Inhibition constant against aromatase protein from human placental microsomes using [1-beta-3H]-androstenedione; Competitive inhibition |
J Med Chem 48: 6379-85 (2005)
Article DOI: 10.1021/jm050129p
BindingDB Entry DOI: 10.7270/Q25M66GD |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50332805
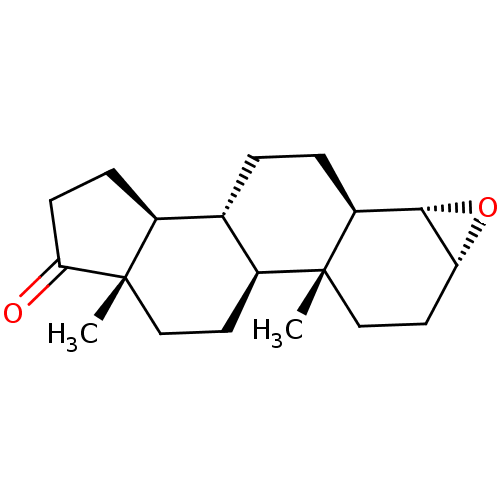
((3R,4S,5R,8R,9S,10R,13S,14S)-10,13-Dimethyl-hexade...)Show SMILES C[C@]12CC[C@H]3[C@@H](CC[C@H]4[C@@H]5O[C@@H]5CC[C@]34C)[C@@H]1CCC2=O |r| Show InChI InChI=1S/C19H28O2/c1-18-10-8-15-17(21-15)14(18)4-3-11-12-5-6-16(20)19(12,2)9-7-13(11)18/h11-15,17H,3-10H2,1-2H3/t11-,12-,13-,14-,15+,17-,18+,19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 38 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Coimbra
Curated by ChEMBL
| Assay Description
Inhibition of aromatase in human microsomes using [1beta-3H]androstenedione as substrate after 5 mins by Dixon plot analysis |
J Med Chem 55: 3992-4002 (2012)
Article DOI: 10.1021/jm300262w
BindingDB Entry DOI: 10.7270/Q22V2H52 |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50174544
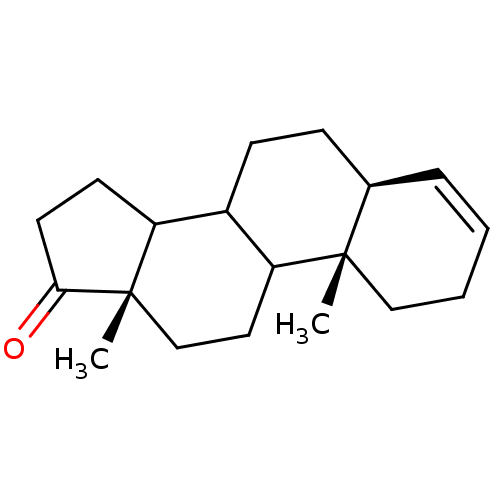
((5S,10S,13S)-10,13-Dimethyl-1,2,5,6,7,8,9,10,11,12...)Show SMILES C[C@]12CCC3C(CC[C@H]4C=CCC[C@]34C)C1CCC2=O |c:9| Show InChI InChI=1S/C19H28O/c1-18-11-4-3-5-13(18)6-7-14-15-8-9-17(20)19(15,2)12-10-16(14)18/h3,5,13-16H,4,6-12H2,1-2H3/t13-,14?,15?,16?,18+,19+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oporto
Curated by ChEMBL
| Assay Description
Inhibition constant against aromatase protein from human placental microsomes using [1-beta-3H]-androstenedione; Competitive inhibition |
J Med Chem 48: 6379-85 (2005)
Article DOI: 10.1021/jm050129p
BindingDB Entry DOI: 10.7270/Q25M66GD |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50332803

((5S,8R,9S,10S,13S,14S)-10,13-dimethyl-5,6,7,8,9,10...)Show SMILES C[C@]12CC[C@H]3[C@@H](CC[C@H]4C=CCC[C@]34C)[C@@H]1CCC2=O |r,c:9| Show InChI InChI=1S/C19H28O/c1-18-11-4-3-5-13(18)6-7-14-15-8-9-17(20)19(15,2)12-10-16(14)18/h3,5,13-16H,4,6-12H2,1-2H3/t13-,14+,15+,16+,18+,19+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Coimbra
Curated by ChEMBL
| Assay Description
Inhibition of aromatase in human microsomes using [1beta-3H]androstenedione as substrate after 5 mins by Dixon plot analysis |
J Med Chem 55: 3992-4002 (2012)
Article DOI: 10.1021/jm300262w
BindingDB Entry DOI: 10.7270/Q22V2H52 |
More data for this
Ligand-Target Pair | |
Xanthine dehydrogenase/oxidase
(Homo sapiens (Human)) | BDBM50135279

(CHEMBL3747521)Show SMILES Nc1nc2c(c[nH]c2c(=O)[nH]1)C(=O)c1cccc(c1)[N+]([O-])=O Show InChI InChI=1S/C13H9N5O4/c14-13-16-9-8(5-15-10(9)12(20)17-13)11(19)6-2-1-3-7(4-6)18(21)22/h1-5,15H,(H3,14,16,17,20) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 55 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Estadual de Campinas
Curated by ChEMBL
| Assay Description
Non-competitive inhibition of xanthine oxidase (unknown origin) by Lineweaver-Burk plot analysis |
Bioorg Med Chem 24: 226-31 (2016)
Article DOI: 10.1016/j.bmc.2015.12.006
BindingDB Entry DOI: 10.7270/Q2HX1FGM |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM50598728
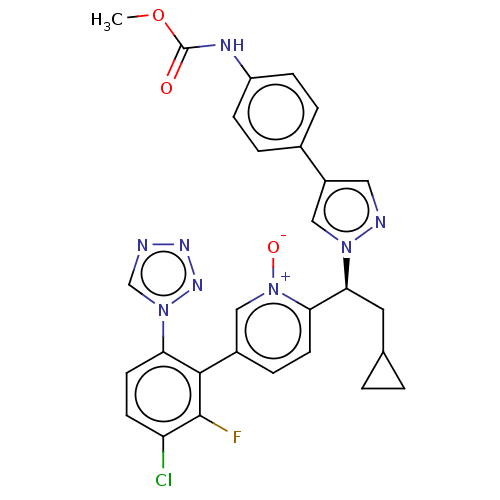
(CHEMBL5180591)Show SMILES COC(=O)Nc1ccc(cc1)-c1cnn(c1)[C@@H](CC1CC1)c1ccc(c[n+]1[O-])-c1c(F)c(Cl)ccc1-n1cnnn1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 61 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00442
BindingDB Entry DOI: 10.7270/Q2C82FBD |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50388393

(CHEMBL2058266)Show SMILES C[C@]12CC[C@H]3[C@@H](CC[C@@]45O[C@@H]4CCC[C@]35C)[C@@H]1CCC2=O |r| Show InChI InChI=1S/C19H28O2/c1-17-10-8-14-12(13(17)5-6-15(17)20)7-11-19-16(21-19)4-3-9-18(14,19)2/h12-14,16H,3-11H2,1-2H3/t12-,13-,14-,16+,17-,18+,19+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 86 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Coimbra
Curated by ChEMBL
| Assay Description
Inhibition of aromatase in human microsomes using [1beta-3H]androstenedione as substrate after 5 mins by Dixon plot analysis |
J Med Chem 55: 3992-4002 (2012)
Article DOI: 10.1021/jm300262w
BindingDB Entry DOI: 10.7270/Q22V2H52 |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50388396

(CHEMBL1077603)Show SMILES C[C@]12CC[C@H]3[C@@H](CCC4=C[C@@H](O)CC[C@]34C)[C@@H]1CCC2=O |r,t:8| Show InChI InChI=1S/C19H28O2/c1-18-9-7-13(20)11-12(18)3-4-14-15-5-6-17(21)19(15,2)10-8-16(14)18/h11,13-16,20H,3-10H2,1-2H3/t13-,14-,15-,16-,18-,19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Coimbra
Curated by ChEMBL
| Assay Description
Inhibition of aromatase in human microsomes using [1beta-3H]androstenedione as substrate after 5 mins by Dixon plot analysis |
J Med Chem 55: 3992-4002 (2012)
Article DOI: 10.1021/jm300262w
BindingDB Entry DOI: 10.7270/Q22V2H52 |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP2 subtype
(Homo sapiens (Human)) | BDBM50205275

(CHEMBL426559 | [(3R)-4-(4-chlorobenzyl)-7-fluoro-5...)Show SMILES CS(=O)(=O)c1cc(F)cc2c3CC[C@H](CC(O)=O)c3n(Cc3ccc(Cl)cc3)c12 Show InChI InChI=1S/C21H19ClFNO4S/c1-29(27,28)18-10-15(23)9-17-16-7-4-13(8-19(25)26)20(16)24(21(17)18)11-12-2-5-14(22)6-3-12/h2-3,5-6,9-10,13H,4,7-8,11H2,1H3,(H,25,26)/t13-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 136 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Canada & Co.
Curated by ChEMBL
| Assay Description
Binding affinity to human EP2 receptor expressed in HEK293 cells |
J Med Chem 50: 794-806 (2007)
Article DOI: 10.1021/jm0603668
BindingDB Entry DOI: 10.7270/Q20P0ZP2 |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50174543
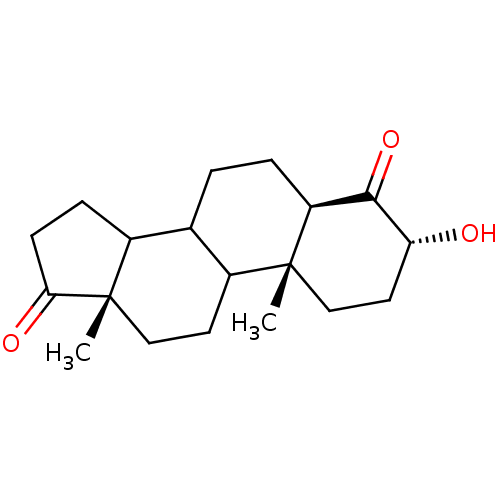
((3R,5R,10R,13S)-3-Hydroxy-10,13-dimethyl-tetradeca...)Show SMILES C[C@]12CCC3C(CC[C@H]4C(=O)[C@H](O)CC[C@]34C)C1CCC2=O Show InChI InChI=1S/C19H28O3/c1-18-10-8-15(20)17(22)14(18)4-3-11-12-5-6-16(21)19(12,2)9-7-13(11)18/h11-15,20H,3-10H2,1-2H3/t11?,12?,13?,14-,15+,18+,19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 147 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oporto
Curated by ChEMBL
| Assay Description
Inhibition constant against aromatase protein from human placental microsomes using [1-beta-3H]-androstenedione; Competitive inhibition |
J Med Chem 48: 6379-85 (2005)
Article DOI: 10.1021/jm050129p
BindingDB Entry DOI: 10.7270/Q25M66GD |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data