

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM50439646 (CHEMBL2419600 | US8993586, 110) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human ACC2 using acetyl-CoA as substrate assessed as [14C]malonyl-CoA synthesis preincubated for 10 mins prior to substrate addition me... | J Med Chem 56: 7110-9 (2013) Article DOI: 10.1021/jm401033t BindingDB Entry DOI: 10.7270/Q2JW8G9D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 1 (Homo sapiens (Human)) | BDBM50439646 (CHEMBL2419600 | US8993586, 110) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human ACC1 using acetyl-CoA as substrate assessed as [14C]malonyl-CoA synthesis preincubated for 10 mins prior to substrate addition me... | J Med Chem 56: 7110-9 (2013) Article DOI: 10.1021/jm401033t BindingDB Entry DOI: 10.7270/Q2JW8G9D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Free fatty acid receptor 1 (Homo sapiens (Human)) | BDBM50277775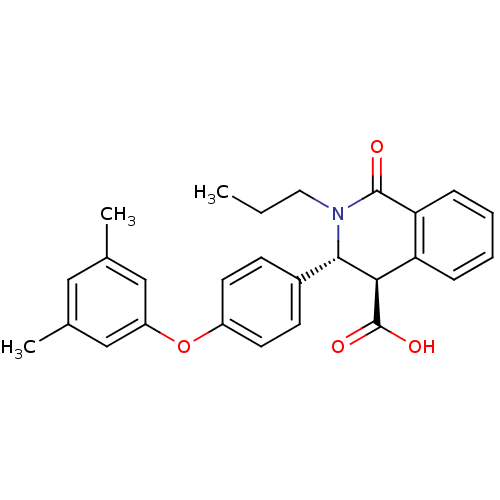 ((3R,4R)-3-(4-(3,5-dimethylphenoxy)phenyl)-1-oxo-2-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global R&D Curated by ChEMBL | Assay Description Antagonist activity at human GPR40 expressed HEK293 cells assessed as effect on intracellular calcium concentration by FLIPR assay | Bioorg Med Chem Lett 19: 2400-3 (2009) Article DOI: 10.1016/j.bmcl.2009.03.082 BindingDB Entry DOI: 10.7270/Q23N238P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM50439642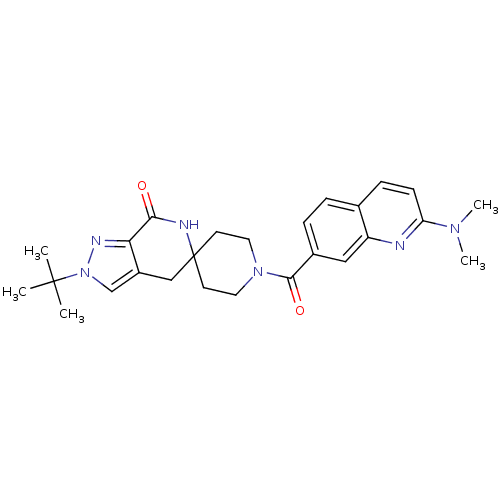 (CHEMBL2419589 | US8993586, 105) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human ACC2 using acetyl-CoA as substrate assessed as [14C]malonyl-CoA synthesis preincubated for 10 mins prior to substrate addition me... | J Med Chem 56: 7110-9 (2013) Article DOI: 10.1021/jm401033t BindingDB Entry DOI: 10.7270/Q2JW8G9D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Free fatty acid receptor 1 (Homo sapiens (Human)) | BDBM50277776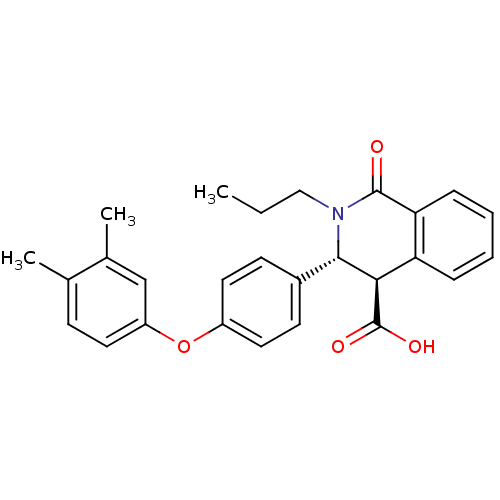 ((3R,4R)-3-(4-(3,4-dimethylphenoxy)phenyl)-1-oxo-2-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global R&D Curated by ChEMBL | Assay Description Antagonist activity at human GPR40 expressed HEK293 cells assessed as effect on intracellular calcium concentration by FLIPR assay | Bioorg Med Chem Lett 19: 2400-3 (2009) Article DOI: 10.1016/j.bmcl.2009.03.082 BindingDB Entry DOI: 10.7270/Q23N238P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM50439644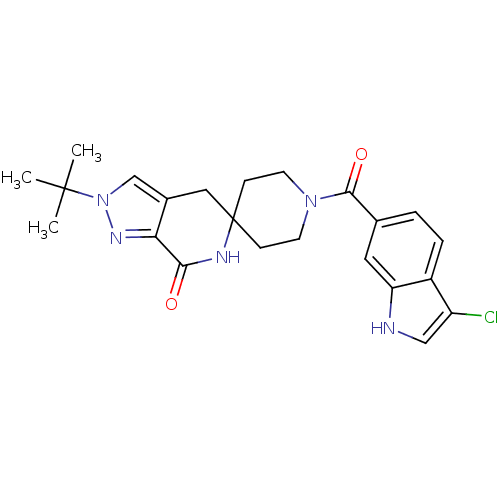 (CHEMBL2419593 | US8993586, 86) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human ACC2 using acetyl-CoA as substrate assessed as [14C]malonyl-CoA synthesis preincubated for 10 mins prior to substrate addition me... | J Med Chem 56: 7110-9 (2013) Article DOI: 10.1021/jm401033t BindingDB Entry DOI: 10.7270/Q2JW8G9D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM50439634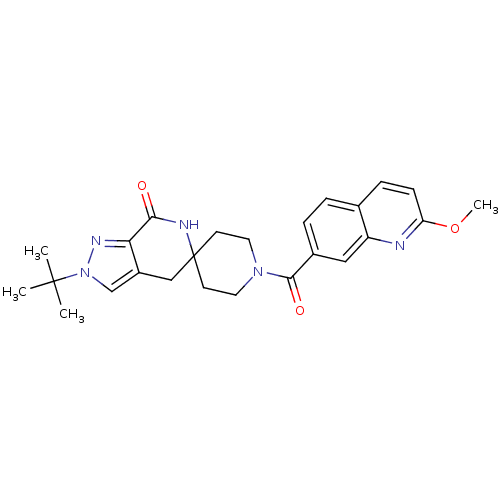 (CHEMBL2419596 | US8993586, 71) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human ACC2 using acetyl-CoA as substrate assessed as [14C]malonyl-CoA synthesis preincubated for 10 mins prior to substrate addition me... | J Med Chem 56: 7110-9 (2013) Article DOI: 10.1021/jm401033t BindingDB Entry DOI: 10.7270/Q2JW8G9D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Free fatty acid receptor 1 (Homo sapiens (Human)) | BDBM50277814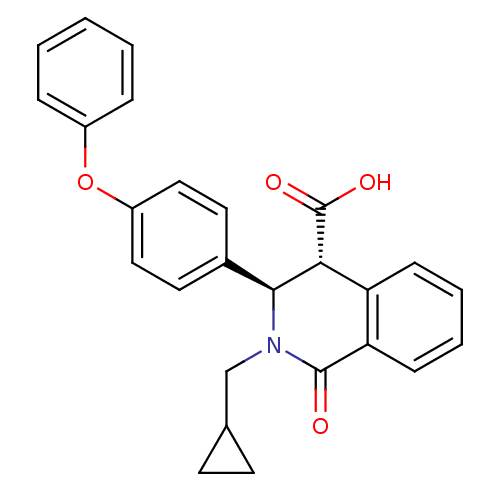 ((3R,4R)-2-(cyclopropylmethyl)-1-oxo-3-(4-phenoxyph...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global R&D Curated by ChEMBL | Assay Description Antagonist activity at human GPR40 expressed HEK293 cells assessed as effect on intracellular calcium concentration by FLIPR assay | Bioorg Med Chem Lett 19: 2400-3 (2009) Article DOI: 10.1016/j.bmcl.2009.03.082 BindingDB Entry DOI: 10.7270/Q23N238P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM50439643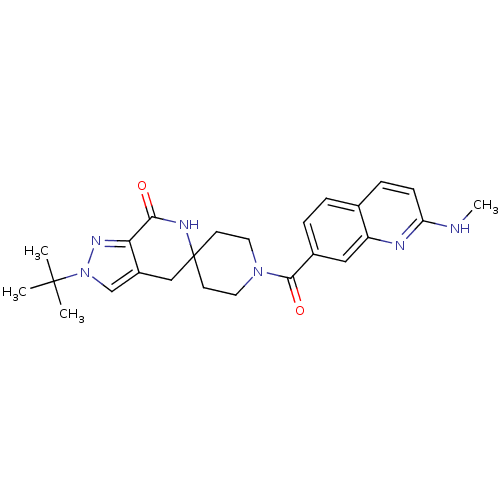 (CHEMBL2419598 | US8993586, 76) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human ACC2 using acetyl-CoA as substrate assessed as [14C]malonyl-CoA synthesis preincubated for 10 mins prior to substrate addition me... | J Med Chem 56: 7110-9 (2013) Article DOI: 10.1021/jm401033t BindingDB Entry DOI: 10.7270/Q2JW8G9D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM50439641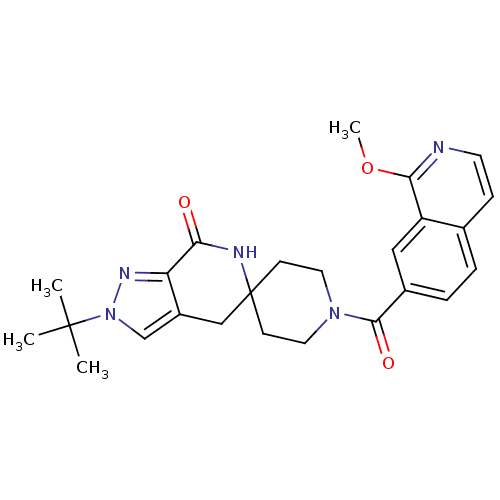 (CHEMBL2419597 | US8993586, 55) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human ACC2 using acetyl-CoA as substrate assessed as [14C]malonyl-CoA synthesis preincubated for 10 mins prior to substrate addition me... | J Med Chem 56: 7110-9 (2013) Article DOI: 10.1021/jm401033t BindingDB Entry DOI: 10.7270/Q2JW8G9D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM50439645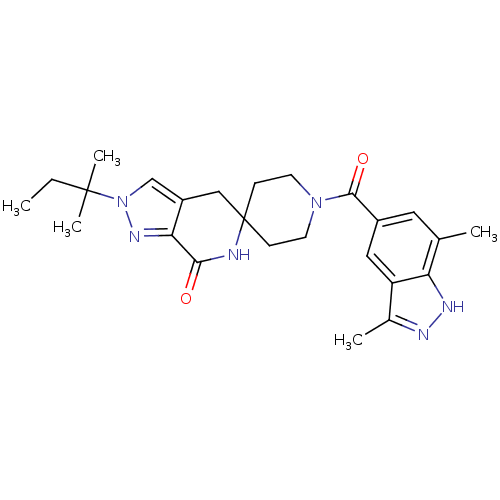 (CHEMBL2419607) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human ACC2 using acetyl-CoA as substrate assessed as [14C]malonyl-CoA synthesis preincubated for 10 mins prior to substrate addition me... | J Med Chem 56: 7110-9 (2013) Article DOI: 10.1021/jm401033t BindingDB Entry DOI: 10.7270/Q2JW8G9D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM50439635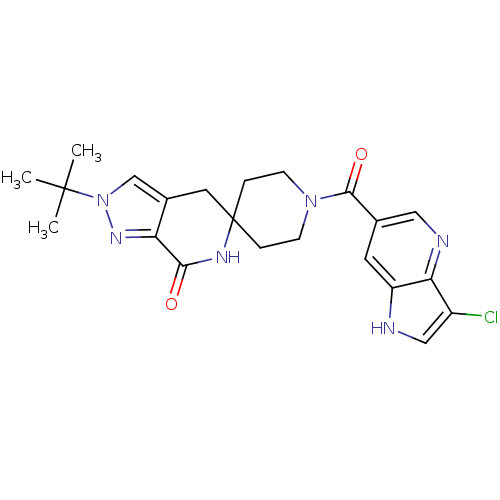 (CHEMBL2419594 | US8993586, 88) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human ACC2 using acetyl-CoA as substrate assessed as [14C]malonyl-CoA synthesis preincubated for 10 mins prior to substrate addition me... | J Med Chem 56: 7110-9 (2013) Article DOI: 10.1021/jm401033t BindingDB Entry DOI: 10.7270/Q2JW8G9D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 1 (Homo sapiens (Human)) | BDBM50439642 (CHEMBL2419589 | US8993586, 105) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human ACC1 using acetyl-CoA as substrate assessed as [14C]malonyl-CoA synthesis preincubated for 10 mins prior to substrate addition me... | J Med Chem 56: 7110-9 (2013) Article DOI: 10.1021/jm401033t BindingDB Entry DOI: 10.7270/Q2JW8G9D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM50546612 (CHEMBL4753907) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human ACC2 using acetyl coA as substrate incubated for 1 hr by transcreener fluorescence polarization assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00640 BindingDB Entry DOI: 10.7270/Q2RR22VT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM50546612 (CHEMBL4753907) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human ACC2 using acetyl coA as substrate incubated for 1 hr by transcreener fluorescence polarization assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00640 BindingDB Entry DOI: 10.7270/Q2RR22VT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM50546612 (CHEMBL4753907) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human ACC2 using acetyl coA as substrate incubated for 1 hr by transcreener fluorescence polarization assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00640 BindingDB Entry DOI: 10.7270/Q2RR22VT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM50546612 (CHEMBL4753907) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human ACC2 using acetyl coA as substrate incubated for 1 hr by transcreener fluorescence polarization assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00640 BindingDB Entry DOI: 10.7270/Q2RR22VT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 1 (Homo sapiens (Human)) | BDBM50439643 (CHEMBL2419598 | US8993586, 76) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human ACC1 using acetyl-CoA as substrate assessed as [14C]malonyl-CoA synthesis preincubated for 10 mins prior to substrate addition me... | J Med Chem 56: 7110-9 (2013) Article DOI: 10.1021/jm401033t BindingDB Entry DOI: 10.7270/Q2JW8G9D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Extracellular calcium-sensing receptor (Homo sapiens (Human)) | BDBM50346031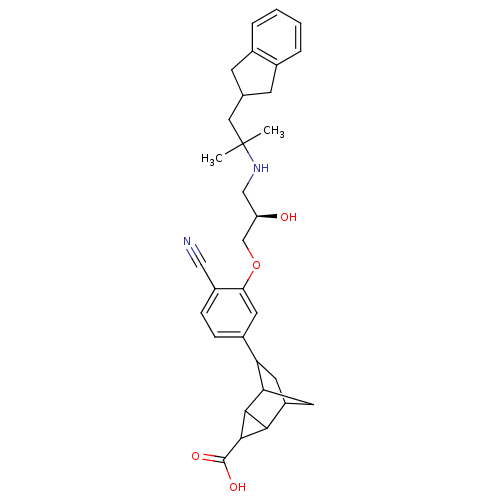 (6-{4-Cyano-3-[(R)-2-hydroxy-3-(2-indan-2-yl-1,1-di...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Negative allosteric modulator activity at human recombinant calcium sensing receptor expressed in HEK cells | Bioorg Med Chem Lett 19: 3328-32 (2009) Article DOI: 10.1016/j.bmcl.2009.04.044 BindingDB Entry DOI: 10.7270/Q2K937V7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM50439638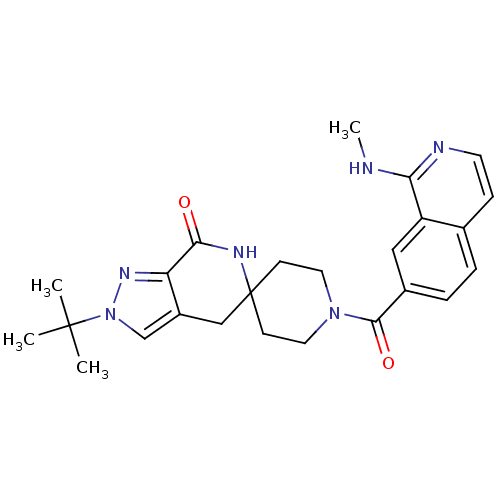 (CHEMBL2419599 | US8993586, 82) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human ACC2 using acetyl-CoA as substrate assessed as [14C]malonyl-CoA synthesis preincubated for 10 mins prior to substrate addition me... | J Med Chem 56: 7110-9 (2013) Article DOI: 10.1021/jm401033t BindingDB Entry DOI: 10.7270/Q2JW8G9D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM50439633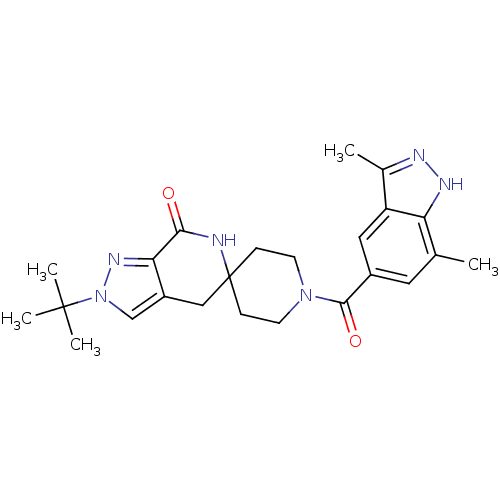 (CHEMBL2419604) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human ACC2 using acetyl-CoA as substrate assessed as [14C]malonyl-CoA synthesis preincubated for 10 mins prior to substrate addition me... | J Med Chem 56: 7110-9 (2013) Article DOI: 10.1021/jm401033t BindingDB Entry DOI: 10.7270/Q2JW8G9D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-tyrosine kinase 2-beta (Homo sapiens (Human)) | BDBM50402996 (CHEMBL2207440) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant C-terminal His-tagged PYK2 using ATP as substrate incubated for 1 hr prior to substrate addition | Bioorg Med Chem Lett 22: 7523-9 (2012) Article DOI: 10.1016/j.bmcl.2012.10.039 BindingDB Entry DOI: 10.7270/Q2319X2C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM50439639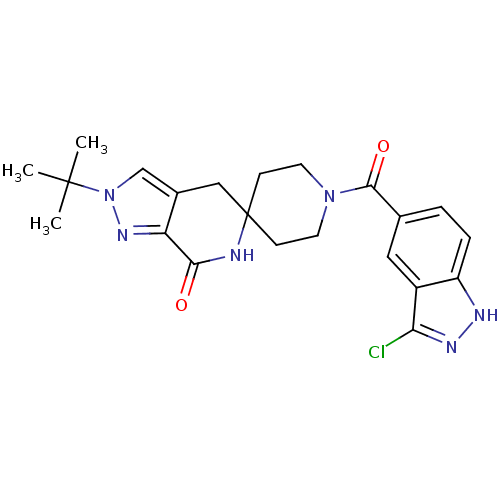 (CHEMBL2419591 | US8993586, 64) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human ACC2 using acetyl-CoA as substrate assessed as [14C]malonyl-CoA synthesis preincubated for 10 mins prior to substrate addition me... | J Med Chem 56: 7110-9 (2013) Article DOI: 10.1021/jm401033t BindingDB Entry DOI: 10.7270/Q2JW8G9D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM50439636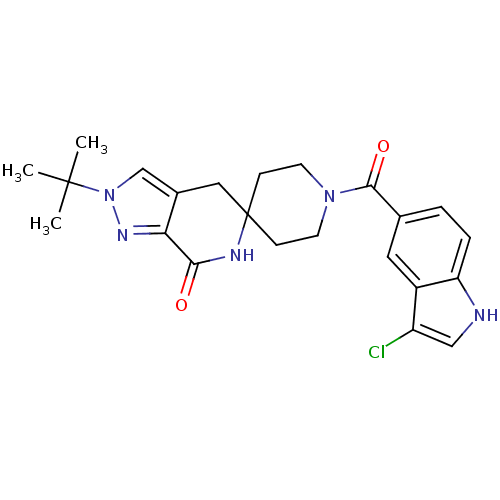 (CHEMBL2419592 | US8993586, 85) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human ACC2 using acetyl-CoA as substrate assessed as [14C]malonyl-CoA synthesis preincubated for 10 mins prior to substrate addition me... | J Med Chem 56: 7110-9 (2013) Article DOI: 10.1021/jm401033t BindingDB Entry DOI: 10.7270/Q2JW8G9D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 1 (Homo sapiens (Human)) | BDBM50439644 (CHEMBL2419593 | US8993586, 86) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human ACC1 using acetyl-CoA as substrate assessed as [14C]malonyl-CoA synthesis preincubated for 10 mins prior to substrate addition me... | J Med Chem 56: 7110-9 (2013) Article DOI: 10.1021/jm401033t BindingDB Entry DOI: 10.7270/Q2JW8G9D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 1 (Rattus norvegicus (Rat)) | BDBM50511112 (CHEMBL4567446) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of rat ACC1 using acetyl-CoA as substrate incubated for 20 mins in presence of [14C]O3 by liquid scintillation counting method | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00640 BindingDB Entry DOI: 10.7270/Q2RR22VT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 1 (Rattus norvegicus (Rat)) | BDBM50511112 (CHEMBL4567446) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of rat ACC1 using acetyl-CoA as substrate incubated for 20 mins in presence of [14C]O3 by liquid scintillation counting method | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00640 BindingDB Entry DOI: 10.7270/Q2RR22VT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 1 (Homo sapiens (Human)) | BDBM50546612 (CHEMBL4753907) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human ACC1 using acetyl coA as substrate incubated for 1 hr by transcreener fluorescence polarization assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00640 BindingDB Entry DOI: 10.7270/Q2RR22VT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 1 (Homo sapiens (Human)) | BDBM50546612 (CHEMBL4753907) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human ACC1 using acetyl coA as substrate incubated for 1 hr by transcreener fluorescence polarization assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00640 BindingDB Entry DOI: 10.7270/Q2RR22VT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 1 (Homo sapiens (Human)) | BDBM50546612 (CHEMBL4753907) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human ACC1 using acetyl coA as substrate incubated for 1 hr by transcreener fluorescence polarization assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00640 BindingDB Entry DOI: 10.7270/Q2RR22VT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 1 (Homo sapiens (Human)) | BDBM50546612 (CHEMBL4753907) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human ACC1 using acetyl coA as substrate incubated for 1 hr by transcreener fluorescence polarization assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00640 BindingDB Entry DOI: 10.7270/Q2RR22VT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM50511112 (CHEMBL4567446) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Uncompetitive inhibition of human ACC2 incubated for 15 mins in presence of ATP by Line weaver-Burk plot analysis | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00640 BindingDB Entry DOI: 10.7270/Q2RR22VT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM50511112 (CHEMBL4567446) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Uncompetitive inhibition of human ACC2 incubated for 15 mins in presence of ATP by Line weaver-Burk plot analysis | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00640 BindingDB Entry DOI: 10.7270/Q2RR22VT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM50546611 (CHEMBL4762472) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 9.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human ACC2 using acetyl coA as substrate incubated for 1 hr by transcreener fluorescence polarization assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00640 BindingDB Entry DOI: 10.7270/Q2RR22VT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM50546611 (CHEMBL4762472) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 9.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human ACC2 using acetyl coA as substrate incubated for 1 hr by transcreener fluorescence polarization assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00640 BindingDB Entry DOI: 10.7270/Q2RR22VT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM50439637 (CHEMBL2419601) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant ACC2 after 1 hr by transcreener assay | J Med Chem 57: 10512-26 (2014) Article DOI: 10.1021/jm5016022 BindingDB Entry DOI: 10.7270/Q2SN0BJM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM50439626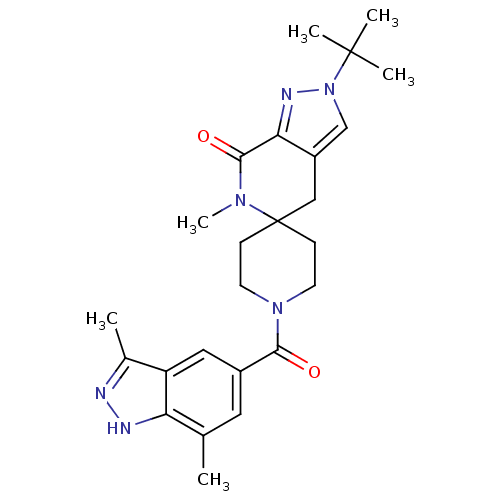 (CHEMBL2419610) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human ACC2 using acetyl-CoA as substrate assessed as [14C]malonyl-CoA synthesis preincubated for 10 mins prior to substrate addition me... | J Med Chem 56: 7110-9 (2013) Article DOI: 10.1021/jm401033t BindingDB Entry DOI: 10.7270/Q2JW8G9D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM50439637 (CHEMBL2419601) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human ACC2 using acetyl-CoA as substrate assessed as [14C]malonyl-CoA synthesis preincubated for 10 mins prior to substrate addition me... | J Med Chem 56: 7110-9 (2013) Article DOI: 10.1021/jm401033t BindingDB Entry DOI: 10.7270/Q2JW8G9D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Free fatty acid receptor 1 (Homo sapiens (Human)) | BDBM50277728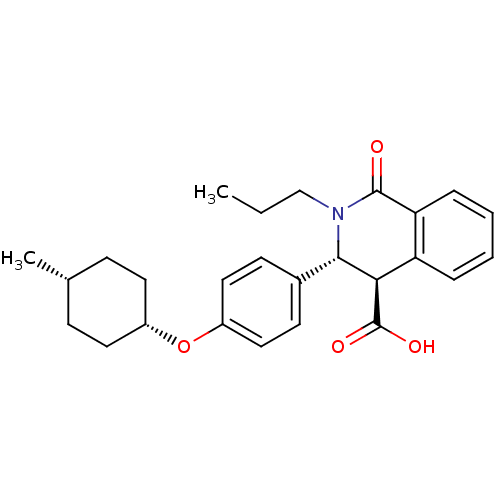 ((3R,4R)-3-(4-((cis)-4-methylcyclohexyloxy)phenyl)-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global R&D Curated by ChEMBL | Assay Description Antagonist activity at human GPR40 expressed HEK293 cells assessed as effect on intracellular calcium concentration by FLIPR assay | Bioorg Med Chem Lett 19: 2400-3 (2009) Article DOI: 10.1016/j.bmcl.2009.03.082 BindingDB Entry DOI: 10.7270/Q23N238P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM50546611 (CHEMBL4762472) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human ACC2 using acetyl coA as substrate incubated for 1 hr by transcreener fluorescence polarization assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00640 BindingDB Entry DOI: 10.7270/Q2RR22VT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 1 (Homo sapiens (Human)) | BDBM50439634 (CHEMBL2419596 | US8993586, 71) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human ACC1 using acetyl-CoA as substrate assessed as [14C]malonyl-CoA synthesis preincubated for 10 mins prior to substrate addition me... | J Med Chem 56: 7110-9 (2013) Article DOI: 10.1021/jm401033t BindingDB Entry DOI: 10.7270/Q2JW8G9D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Free fatty acid receptor 1 (Homo sapiens (Human)) | BDBM50277853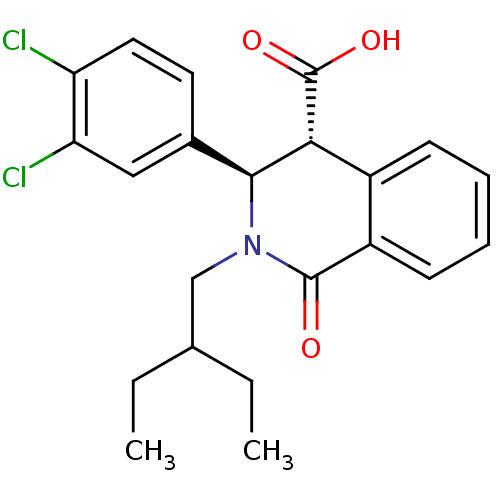 ((3R,4R)-3-(3,4-dichlorophenyl)-2-(2-ethylbutyl)-1-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global R&D Curated by ChEMBL | Assay Description Antagonist activity at human GPR40 expressed HEK293 cells assessed as effect on intracellular calcium concentration by FLIPR assay | Bioorg Med Chem Lett 19: 2400-3 (2009) Article DOI: 10.1016/j.bmcl.2009.03.082 BindingDB Entry DOI: 10.7270/Q23N238P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM50546611 (CHEMBL4762472) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human ACC2 using acetyl coA as substrate incubated for 1 hr by transcreener fluorescence polarization assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00640 BindingDB Entry DOI: 10.7270/Q2RR22VT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Free fatty acid receptor 1 (Homo sapiens (Human)) | BDBM50277887 ((3R,4R)-3-(benzo[d]thiazol-2-yl)-2-(2-ethylbutyl)-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global R&D Curated by ChEMBL | Assay Description Antagonist activity at human GPR40 expressed HEK293 cells assessed as effect on intracellular calcium concentration by FLIPR assay | Bioorg Med Chem Lett 19: 2400-3 (2009) Article DOI: 10.1016/j.bmcl.2009.03.082 BindingDB Entry DOI: 10.7270/Q23N238P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 1 (Homo sapiens (Human)) | BDBM50439641 (CHEMBL2419597 | US8993586, 55) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human ACC1 using acetyl-CoA as substrate assessed as [14C]malonyl-CoA synthesis preincubated for 10 mins prior to substrate addition me... | J Med Chem 56: 7110-9 (2013) Article DOI: 10.1021/jm401033t BindingDB Entry DOI: 10.7270/Q2JW8G9D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 1 (Homo sapiens (Human)) | BDBM50439635 (CHEMBL2419594 | US8993586, 88) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human ACC1 using acetyl-CoA as substrate assessed as [14C]malonyl-CoA synthesis preincubated for 10 mins prior to substrate addition me... | J Med Chem 56: 7110-9 (2013) Article DOI: 10.1021/jm401033t BindingDB Entry DOI: 10.7270/Q2JW8G9D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Free fatty acid receptor 1 (Homo sapiens (Human)) | BDBM50277729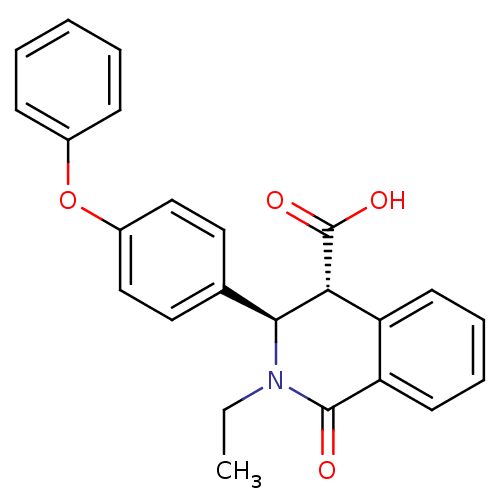 ((3R,4R)-2-ethyl-1-oxo-3-(4-phenoxyphenyl)-1,2,3,4-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global R&D Curated by ChEMBL | Assay Description Antagonist activity at human GPR40 expressed HEK293 cells assessed as effect on intracellular calcium concentration by FLIPR assay | Bioorg Med Chem Lett 19: 2400-3 (2009) Article DOI: 10.1016/j.bmcl.2009.03.082 BindingDB Entry DOI: 10.7270/Q23N238P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 1 (Homo sapiens (Human)) | BDBM50511112 (CHEMBL4567446) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Uncompetitive inhibition of human ACC1 incubated for 15 mins in presence of ATP by Line weaver-Burk plot analysis | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00640 BindingDB Entry DOI: 10.7270/Q2RR22VT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 1 (Homo sapiens (Human)) | BDBM50511112 (CHEMBL4567446) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Uncompetitive inhibition of human ACC1 incubated for 15 mins in presence of ATP by Line weaver-Burk plot analysis | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00640 BindingDB Entry DOI: 10.7270/Q2RR22VT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM50546609 (CHEMBL4743101) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human ACC2 using acetyl coA as substrate incubated for 1 hr by transcreener fluorescence polarization assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00640 BindingDB Entry DOI: 10.7270/Q2RR22VT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 304 total ) | Next | Last >> |