 Found 216 hits with Last Name = 'yli-kauhaluoma' and Initial = 'j'
Found 216 hits with Last Name = 'yli-kauhaluoma' and Initial = 'j' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Protein kinase C delta type
(Homo sapiens (Human)) | BDBM50258529

(CHEMBL449158 | bryostatin 1)Show SMILES CCC\C=C\C=C\C(=O)O[C@H]1\C(C[C@H]2C[C@@H](OC(=O)C[C@H](O)C[C@@H]3C[C@H](OC(C)=O)C(C)(C)[C@](O)(C[C@@H]4C\C(C[C@@H](O4)\C=C\C(C)(C)[C@]1(O)O2)=C\C(=O)OC)O3)[C@@H](C)O)=C\C(=O)OC |r,t:43| Show InChI InChI=1S/C47H68O17/c1-10-11-12-13-14-15-39(51)62-43-31(22-41(53)58-9)21-34-25-37(28(2)48)61-42(54)24-32(50)23-35-26-38(59-29(3)49)45(6,7)46(55,63-35)27-36-19-30(20-40(52)57-8)18-33(60-36)16-17-44(4,5)47(43,56)64-34/h12-17,20,22,28,32-38,43,48,50,55-56H,10-11,18-19,21,23-27H2,1-9H3/b13-12+,15-14+,17-16+,30-20+,31-22+/t28-,32-,33+,34+,35-,36+,37-,38+,43+,46+,47-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.440 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Helsinki
Curated by ChEMBL
| Assay Description
Displacement of [3H]PDBu from human recombinant PKCdelta expressed in Sf9 cells by liquid scintillation counting |
J Med Chem 52: 3969-81 (2009)
Article DOI: 10.1021/jm900229p
BindingDB Entry DOI: 10.7270/Q2ZK5GJ1 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50000092

((-)-(etorphine) | (-)-morphine | (1S,5R,13R,14S)-1...)Show SMILES CN1CC[C@@]23[C@H]4Oc5c2c(C[C@@H]1[C@@H]3C=C[C@@H]4O)ccc5O |r,c:16,TLB:13:12:8.9.10:3.2.1| Show InChI InChI=1S/C17H19NO3/c1-18-7-6-17-10-3-5-13(20)16(17)21-15-12(19)4-2-9(14(15)17)8-11(10)18/h2-5,10-11,13,16,19-20H,6-8H2,1H3/t10-,11+,13-,16-,17-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Helsinki
Curated by ChEMBL
| Assay Description
Displacement of [3H]diprenorphine from human MOR expressed in African green monkey COS1 cell membranes incubated for 1 hr by scintillation counting m... |
Eur J Med Chem 151: 495-507 (2018)
Article DOI: 10.1016/j.ejmech.2018.02.074
BindingDB Entry DOI: 10.7270/Q2NK3HKB |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
UDP-glucuronosyltransferase 2B7
(Homo sapiens (Human)) | BDBM50211740
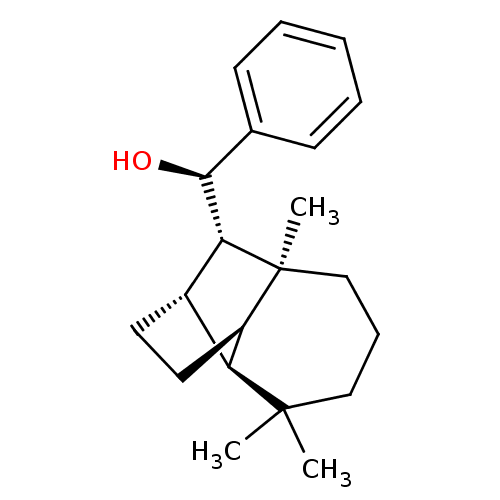
((1R)-phenyl-[(1R,2S,7S,8S,9S)-3,3,7-trimethyltricy...)Show SMILES C[C@]12CCCC(C)(C)[C@H]3[C@H](CC[C@@H]13)[C@@H]2[C@@H](O)c1ccccc1 |TLB:2:1:10.11:8,0:1:10.11:8,THB:5:8:10.11:13.1,14:13:10.11:8| Show InChI InChI=1S/C21H30O/c1-20(2)12-7-13-21(3)16-11-10-15(17(16)20)18(21)19(22)14-8-5-4-6-9-14/h4-6,8-9,15-19,22H,7,10-13H2,1-3H3/t15-,16+,17-,18+,19-,21-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 18.4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Helsinki
Curated by ChEMBL
| Assay Description
Inhibition of human UGT2B7 assessed as reduction of estriol glucuronidation |
J Med Chem 50: 2655-64 (2007)
Article DOI: 10.1021/jm061204e
BindingDB Entry DOI: 10.7270/Q23F4QF2 |
More data for this
Ligand-Target Pair | |
Protein kinase C alpha type
(Homo sapiens (Human)) | BDBM50015677
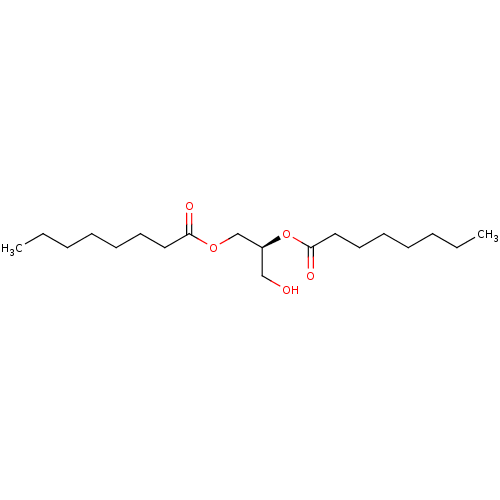
((S)-1-hydroxymethyl-2-octanoyloxy-ethyl ester | 1,...)Show InChI InChI=1S/C19H36O5/c1-3-5-7-9-11-13-18(21)23-16-17(15-20)24-19(22)14-12-10-8-6-4-2/h17,20H,3-16H2,1-2H3/t17-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Helsinki
Curated by ChEMBL
| Assay Description
Displacement of [3H]PDBu from human recombinant PKCalpha expressed in Sf9 cells by liquid scintillation counting |
J Med Chem 52: 3969-81 (2009)
Article DOI: 10.1021/jm900229p
BindingDB Entry DOI: 10.7270/Q2ZK5GJ1 |
More data for this
Ligand-Target Pair | |
Protein kinase C alpha type
(Homo sapiens (Human)) | BDBM50258472
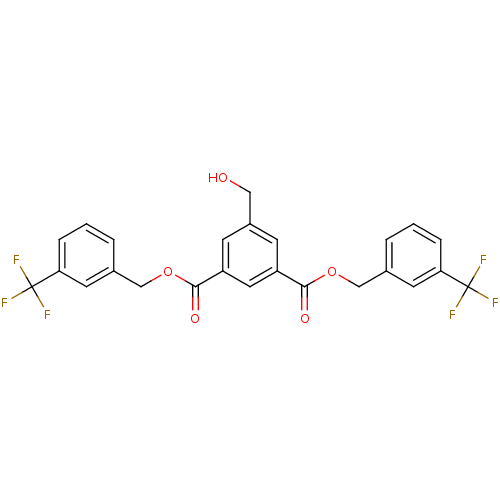
(CHEMBL466211 | bis(3-(trifluoromethyl)benzyl)5-(hy...)Show SMILES OCc1cc(cc(c1)C(=O)OCc1cccc(c1)C(F)(F)F)C(=O)OCc1cccc(c1)C(F)(F)F Show InChI InChI=1S/C25H18F6O5/c26-24(27,28)20-5-1-3-15(9-20)13-35-22(33)18-7-17(12-32)8-19(11-18)23(34)36-14-16-4-2-6-21(10-16)25(29,30)31/h1-11,32H,12-14H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 205 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Helsinki
Curated by ChEMBL
| Assay Description
Displacement of [3H]PDBu from human recombinant PKCalpha expressed in Sf9 cells by liquid scintillation counting |
J Med Chem 52: 3969-81 (2009)
Article DOI: 10.1021/jm900229p
BindingDB Entry DOI: 10.7270/Q2ZK5GJ1 |
More data for this
Ligand-Target Pair | |
Protein kinase C alpha type
(Homo sapiens (Human)) | BDBM50258322

(CHEMBL494361 | diheptan-3-yl 5-(hydroxymethyl)isop...)Show SMILES CCCCC(CC)OC(=O)c1cc(CO)cc(c1)C(=O)OC(CC)CCCC Show InChI InChI=1S/C23H36O5/c1-5-9-11-20(7-3)27-22(25)18-13-17(16-24)14-19(15-18)23(26)28-21(8-4)12-10-6-2/h13-15,20-21,24H,5-12,16H2,1-4H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 319 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Helsinki
Curated by ChEMBL
| Assay Description
Displacement of [3H]PDBu from human recombinant PKCalpha expressed in Sf9 cells by liquid scintillation counting |
J Med Chem 52: 3969-81 (2009)
Article DOI: 10.1021/jm900229p
BindingDB Entry DOI: 10.7270/Q2ZK5GJ1 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50000092

((-)-(etorphine) | (-)-morphine | (1S,5R,13R,14S)-1...)Show SMILES CN1CC[C@@]23[C@H]4Oc5c2c(C[C@@H]1[C@@H]3C=C[C@@H]4O)ccc5O |r,c:16,TLB:13:12:8.9.10:3.2.1| Show InChI InChI=1S/C17H19NO3/c1-18-7-6-17-10-3-5-13(20)16(17)21-15-12(19)4-2-9(14(15)17)8-11(10)18/h2-5,10-11,13,16,19-20H,6-8H2,1H3/t10-,11+,13-,16-,17-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Helsinki
Curated by ChEMBL
| Assay Description
Displacement of [3H]diprenorphine from human DOR expressed in African green monkey COS1 cell membranes incubated for 1 hr by scintillation counting m... |
Eur J Med Chem 151: 495-507 (2018)
Article DOI: 10.1016/j.ejmech.2018.02.074
BindingDB Entry DOI: 10.7270/Q2NK3HKB |
More data for this
Ligand-Target Pair | |
Protein kinase C delta type
(Homo sapiens (Human)) | BDBM50258322

(CHEMBL494361 | diheptan-3-yl 5-(hydroxymethyl)isop...)Show SMILES CCCCC(CC)OC(=O)c1cc(CO)cc(c1)C(=O)OC(CC)CCCC Show InChI InChI=1S/C23H36O5/c1-5-9-11-20(7-3)27-22(25)18-13-17(16-24)14-19(15-18)23(26)28-21(8-4)12-10-6-2/h13-15,20-21,24H,5-12,16H2,1-4H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 529 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Helsinki
Curated by ChEMBL
| Assay Description
Displacement of [3H]PDBu from human recombinant PKCdelta expressed in Sf9 cells by liquid scintillation counting |
J Med Chem 52: 3969-81 (2009)
Article DOI: 10.1021/jm900229p
BindingDB Entry DOI: 10.7270/Q2ZK5GJ1 |
More data for this
Ligand-Target Pair | |
Protein kinase C delta type
(Homo sapiens (Human)) | BDBM50258472

(CHEMBL466211 | bis(3-(trifluoromethyl)benzyl)5-(hy...)Show SMILES OCc1cc(cc(c1)C(=O)OCc1cccc(c1)C(F)(F)F)C(=O)OCc1cccc(c1)C(F)(F)F Show InChI InChI=1S/C25H18F6O5/c26-24(27,28)20-5-1-3-15(9-20)13-35-22(33)18-7-17(12-32)8-19(11-18)23(34)36-14-16-4-2-6-21(10-16)25(29,30)31/h1-11,32H,12-14H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 590 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Helsinki
Curated by ChEMBL
| Assay Description
Displacement of [3H]PDBu from human recombinant PKCdelta expressed in Sf9 cells by liquid scintillation counting |
J Med Chem 52: 3969-81 (2009)
Article DOI: 10.1021/jm900229p
BindingDB Entry DOI: 10.7270/Q2ZK5GJ1 |
More data for this
Ligand-Target Pair | |
Protein kinase C alpha type
(Homo sapiens (Human)) | BDBM50258321
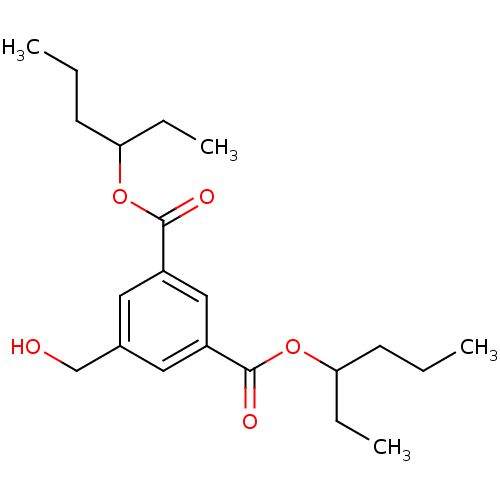
(CHEMBL523053 | dihexan-3-yl 5-(hydroxymethyl)isoph...)Show InChI InChI=1S/C21H32O5/c1-5-9-18(7-3)25-20(23)16-11-15(14-22)12-17(13-16)21(24)26-19(8-4)10-6-2/h11-13,18-19,22H,5-10,14H2,1-4H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 661 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Helsinki
Curated by ChEMBL
| Assay Description
Displacement of [3H]PDBu from human recombinant PKCalpha expressed in Sf9 cells by liquid scintillation counting |
J Med Chem 52: 3969-81 (2009)
Article DOI: 10.1021/jm900229p
BindingDB Entry DOI: 10.7270/Q2ZK5GJ1 |
More data for this
Ligand-Target Pair | |
Botulinum neurotoxin type A
(Clostridium botulinum) | BDBM50340404
(28-Hemisuccinylbetulin | CHEMBL1761333)Show SMILES CC(=C)[C@@H]1CC[C@]2(COC(=O)CCC(O)=O)CC[C@]3(C)[C@H](CC[C@@H]4[C@@]5(C)CC[C@H](O)C(C)(C)[C@@H]5CC[C@@]34C)[C@@H]12 |r| Show InChI InChI=1S/C34H54O5/c1-21(2)22-12-17-34(20-39-28(38)11-10-27(36)37)19-18-32(6)23(29(22)34)8-9-25-31(5)15-14-26(35)30(3,4)24(31)13-16-33(25,32)7/h22-26,29,35H,1,8-20H2,2-7H3,(H,36,37)/t22-,23+,24-,25+,26-,29+,31-,32+,33+,34+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of Clostridium BoNT/A protease light chain |
Bioorg Med Chem Lett 21: 2229-31 (2011)
Article DOI: 10.1016/j.bmcl.2011.02.115
BindingDB Entry DOI: 10.7270/Q2KS6RWV |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50370401
(CHEMBL4168822)Show SMILES [H][C@@]12Oc3c4c(C[C@@]5([H])N(CC[C@@]14[C@@]5([H])C=C[C@@H]2O)C(=O)OC)ccc3O |r,c:18,THB:19:9:13:4.5.6| Show InChI InChI=1S/C18H19NO5/c1-23-17(22)19-7-6-18-10-3-5-13(21)16(18)24-15-12(20)4-2-9(14(15)18)8-11(10)19/h2-5,10-11,13,16,20-21H,6-8H2,1H3/t10-,11+,13-,16-,18-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 814 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Helsinki
Curated by ChEMBL
| Assay Description
Displacement of [3H]diprenorphine from human MOR expressed in African green monkey COS1 cell membranes incubated for 1 hr by scintillation counting m... |
Eur J Med Chem 151: 495-507 (2018)
Article DOI: 10.1016/j.ejmech.2018.02.074
BindingDB Entry DOI: 10.7270/Q2NK3HKB |
More data for this
Ligand-Target Pair | |
Protein kinase C delta type
(Homo sapiens (Human)) | BDBM50258321

(CHEMBL523053 | dihexan-3-yl 5-(hydroxymethyl)isoph...)Show InChI InChI=1S/C21H32O5/c1-5-9-18(7-3)25-20(23)16-11-15(14-22)12-17(13-16)21(24)26-19(8-4)10-6-2/h11-13,18-19,22H,5-10,14H2,1-4H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 915 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Helsinki
Curated by ChEMBL
| Assay Description
Displacement of [3H]PDBu from human recombinant PKCdelta expressed in Sf9 cells by liquid scintillation counting |
J Med Chem 52: 3969-81 (2009)
Article DOI: 10.1021/jm900229p
BindingDB Entry DOI: 10.7270/Q2ZK5GJ1 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50370393
(CHEMBL4168247)Show SMILES [H][C@@]12Oc3c4c(C[C@@]5([H])N(CC[C@@]14[C@@]5([H])C(=O)[C@@H](O)[C@@H]2O)C(=O)OC)ccc3O |r,THB:21:9:13:4.5.6| Show InChI InChI=1S/C18H19NO7/c1-25-17(24)19-5-4-18-10-7-2-3-9(20)15(10)26-16(18)14(23)13(22)12(21)11(18)8(19)6-7/h2-3,8,11,13-14,16,20,22-23H,4-6H2,1H3/t8-,11-,13-,14+,16+,18-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.08E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Helsinki
Curated by ChEMBL
| Assay Description
Displacement of [3H]diprenorphine from human MOR expressed in African green monkey COS1 cell membranes incubated for 1 hr by scintillation counting m... |
Eur J Med Chem 151: 495-507 (2018)
Article DOI: 10.1016/j.ejmech.2018.02.074
BindingDB Entry DOI: 10.7270/Q2NK3HKB |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50370393

(CHEMBL4168247)Show SMILES [H][C@@]12Oc3c4c(C[C@@]5([H])N(CC[C@@]14[C@@]5([H])C(=O)[C@@H](O)[C@@H]2O)C(=O)OC)ccc3O |r,THB:21:9:13:4.5.6| Show InChI InChI=1S/C18H19NO7/c1-25-17(24)19-5-4-18-10-7-2-3-9(20)15(10)26-16(18)14(23)13(22)12(21)11(18)8(19)6-7/h2-3,8,11,13-14,16,20,22-23H,4-6H2,1H3/t8-,11-,13-,14+,16+,18-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.13E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Helsinki
Curated by ChEMBL
| Assay Description
Displacement of [3H]diprenorphine from human DOR expressed in African green monkey COS1 cell membranes incubated for 1 hr by scintillation counting m... |
Eur J Med Chem 151: 495-507 (2018)
Article DOI: 10.1016/j.ejmech.2018.02.074
BindingDB Entry DOI: 10.7270/Q2NK3HKB |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50370401

(CHEMBL4168822)Show SMILES [H][C@@]12Oc3c4c(C[C@@]5([H])N(CC[C@@]14[C@@]5([H])C=C[C@@H]2O)C(=O)OC)ccc3O |r,c:18,THB:19:9:13:4.5.6| Show InChI InChI=1S/C18H19NO5/c1-23-17(22)19-7-6-18-10-3-5-13(21)16(18)24-15-12(20)4-2-9(14(15)18)8-11(10)19/h2-5,10-11,13,16,20-21H,6-8H2,1H3/t10-,11+,13-,16-,18-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.69E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Helsinki
Curated by ChEMBL
| Assay Description
Displacement of [3H]diprenorphine from human DOR expressed in African green monkey COS1 cell membranes incubated for 1 hr by scintillation counting m... |
Eur J Med Chem 151: 495-507 (2018)
Article DOI: 10.1016/j.ejmech.2018.02.074
BindingDB Entry DOI: 10.7270/Q2NK3HKB |
More data for this
Ligand-Target Pair | |
Orexin/Hypocretin receptor type 1
(Homo sapiens (Human)) | BDBM50468969
(CHEMBL4295121)Show SMILES CCOc1ccc(cc1)-c1cc(C(=O)c2ccccc2)c2ccc(ccc12)C(O)=O Show InChI InChI=1S/C26H20O4/c1-2-30-20-12-8-17(9-13-20)23-16-24(25(27)18-6-4-3-5-7-18)22-15-11-19(26(28)29)10-14-21(22)23/h3-16H,2H2,1H3,(H,28,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Helsinki
Curated by ChEMBL
| Assay Description
Antagonist activity at human OX1 receptor expressed in CHOK1 cells assessed as inhibition of orexin-A-induced calcium accumulation preincubated for 3... |
Eur J Med Chem 157: 88-100 (2018)
Article DOI: 10.1016/j.ejmech.2018.07.040
BindingDB Entry DOI: 10.7270/Q2TQ647C |
More data for this
Ligand-Target Pair | |
Orexin receptor type 2
(Homo sapiens (Human)) | BDBM50468969

(CHEMBL4295121)Show SMILES CCOc1ccc(cc1)-c1cc(C(=O)c2ccccc2)c2ccc(ccc12)C(O)=O Show InChI InChI=1S/C26H20O4/c1-2-30-20-12-8-17(9-13-20)23-16-24(25(27)18-6-4-3-5-7-18)22-15-11-19(26(28)29)10-14-21(22)23/h3-16H,2H2,1H3,(H,28,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Helsinki
Curated by ChEMBL
| Assay Description
Antagonist activity at human OX2 receptor expressed in CHOK1 cells assessed as inhibition of orexin-A-induced calcium accumulation preincubated for 3... |
Eur J Med Chem 157: 88-100 (2018)
Article DOI: 10.1016/j.ejmech.2018.07.040
BindingDB Entry DOI: 10.7270/Q2TQ647C |
More data for this
Ligand-Target Pair | |
Orexin receptor type 2
(Homo sapiens (Human)) | BDBM50468970

(CHEMBL4281212)Show InChI InChI=1S/C13H9IO3/c1-17-13(16)8-3-2-4-10-9(7-15)6-12(14)11(10)5-8/h2-7H,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Helsinki
Curated by ChEMBL
| Assay Description
Antagonist activity at human OX2 receptor expressed in CHOK1 cells assessed as inhibition of orexin-A-induced calcium accumulation preincubated for 3... |
Eur J Med Chem 157: 88-100 (2018)
Article DOI: 10.1016/j.ejmech.2018.07.040
BindingDB Entry DOI: 10.7270/Q2TQ647C |
More data for this
Ligand-Target Pair | |
Orexin receptor type 2
(Homo sapiens (Human)) | BDBM50468971
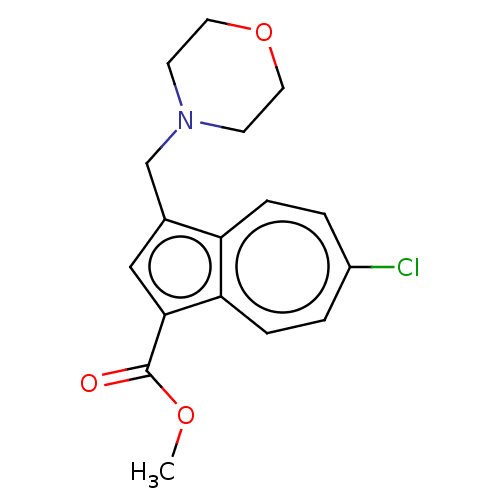
(CHEMBL4291497)Show InChI InChI=1S/C17H18ClNO3/c1-21-17(20)16-10-12(11-19-6-8-22-9-7-19)14-4-2-13(18)3-5-15(14)16/h2-5,10H,6-9,11H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 4.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Helsinki
Curated by ChEMBL
| Assay Description
Antagonist activity at human OX2 receptor expressed in CHOK1 cells assessed as inhibition of orexin-A-induced calcium accumulation preincubated for 3... |
Eur J Med Chem 157: 88-100 (2018)
Article DOI: 10.1016/j.ejmech.2018.07.040
BindingDB Entry DOI: 10.7270/Q2TQ647C |
More data for this
Ligand-Target Pair | |
Orexin/Hypocretin receptor type 1
(Homo sapiens (Human)) | BDBM50468970

(CHEMBL4281212)Show InChI InChI=1S/C13H9IO3/c1-17-13(16)8-3-2-4-10-9(7-15)6-12(14)11(10)5-8/h2-7H,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 4.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Helsinki
Curated by ChEMBL
| Assay Description
Antagonist activity at human OX1 receptor expressed in CHOK1 cells assessed as inhibition of orexin-A-induced calcium accumulation preincubated for 3... |
Eur J Med Chem 157: 88-100 (2018)
Article DOI: 10.1016/j.ejmech.2018.07.040
BindingDB Entry DOI: 10.7270/Q2TQ647C |
More data for this
Ligand-Target Pair | |
Orexin/Hypocretin receptor type 1
(Homo sapiens (Human)) | BDBM50468971

(CHEMBL4291497)Show InChI InChI=1S/C17H18ClNO3/c1-21-17(20)16-10-12(11-19-6-8-22-9-7-19)14-4-2-13(18)3-5-15(14)16/h2-5,10H,6-9,11H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 5.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Helsinki
Curated by ChEMBL
| Assay Description
Antagonist activity at human OX1 receptor expressed in CHOK1 cells assessed as inhibition of orexin-A-induced calcium accumulation preincubated for 3... |
Eur J Med Chem 157: 88-100 (2018)
Article DOI: 10.1016/j.ejmech.2018.07.040
BindingDB Entry DOI: 10.7270/Q2TQ647C |
More data for this
Ligand-Target Pair | |
Botulinum neurotoxin type A
(Clostridium botulinum) | BDBM50340405

(Betulinyl 28-carboxymethoxycarvacrolate | CHEMBL10...)Show SMILES CC(C)c1ccc(C)c(OCC(=O)OC[C@]23CC[C@H]([C@@H]2[C@H]2CC[C@@H]4[C@@]5(C)CC[C@H](O)C(C)(C)[C@@H]5CC[C@@]4(C)[C@]2(C)CC3)C(C)=C)c1 |r| Show InChI InChI=1S/C42H64O4/c1-26(2)29-12-11-28(5)32(23-29)45-24-36(44)46-25-42-20-15-30(27(3)4)37(42)31-13-14-34-39(8)18-17-35(43)38(6,7)33(39)16-19-41(34,10)40(31,9)21-22-42/h11-12,23,26,30-31,33-35,37,43H,3,13-22,24-25H2,1-2,4-10H3/t30-,31+,33-,34+,35-,37+,39-,40+,41+,42+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 1.33E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of Clostridium BoNT/A protease light chain |
Bioorg Med Chem Lett 21: 2229-31 (2011)
Article DOI: 10.1016/j.bmcl.2011.02.115
BindingDB Entry DOI: 10.7270/Q2KS6RWV |
More data for this
Ligand-Target Pair | |
Botulinum neurotoxin type A
(Clostridium botulinum) | BDBM23208
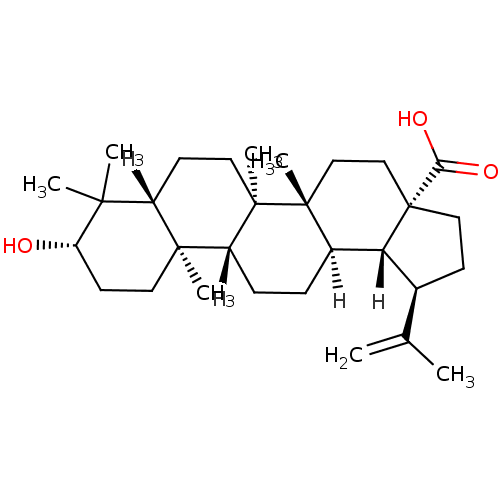
((1R,2R,5S,8R,9R,10R,13R,14R,17S,19R)-17-hydroxy-1,...)Show SMILES [H][C@]12[C@@H](CC[C@@]1(CC[C@]1(C)[C@]2([H])CC[C@]2([H])[C@@]3(C)CC[C@H](O)C(C)(C)[C@]3([H])CC[C@@]12C)C(O)=O)C(C)=C Show InChI InChI=1S/C30H48O3/c1-18(2)19-10-15-30(25(32)33)17-16-28(6)20(24(19)30)8-9-22-27(5)13-12-23(31)26(3,4)21(27)11-14-29(22,28)7/h19-24,31H,1,8-17H2,2-7H3,(H,32,33)/t19-,20+,21-,22+,23-,24+,27-,28+,29+,30-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 1.43E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of Clostridium BoNT/A protease light chain |
Bioorg Med Chem Lett 21: 2229-31 (2011)
Article DOI: 10.1016/j.bmcl.2011.02.115
BindingDB Entry DOI: 10.7270/Q2KS6RWV |
More data for this
Ligand-Target Pair | |
UDP-glucuronosyltransferase 2B17
(Homo sapiens (Human)) | BDBM50211740

((1R)-phenyl-[(1R,2S,7S,8S,9S)-3,3,7-trimethyltricy...)Show SMILES C[C@]12CCCC(C)(C)[C@H]3[C@H](CC[C@@H]13)[C@@H]2[C@@H](O)c1ccccc1 |TLB:2:1:10.11:8,0:1:10.11:8,THB:5:8:10.11:13.1,14:13:10.11:8| Show InChI InChI=1S/C21H30O/c1-20(2)12-7-13-21(3)16-11-10-15(17(16)20)18(21)19(22)14-8-5-4-6-9-14/h4-6,8-9,15-19,22H,7,10-13H2,1-3H3/t15-,16+,17-,18+,19-,21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.18E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Helsinki
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant UGT2B17 assessed as reduction of scopoletin glucuronidation |
J Med Chem 50: 2655-64 (2007)
Article DOI: 10.1021/jm061204e
BindingDB Entry DOI: 10.7270/Q23F4QF2 |
More data for this
Ligand-Target Pair | |
UDP-glucuronosyltransferase 2B17
(Homo sapiens (Human)) | BDBM50183611
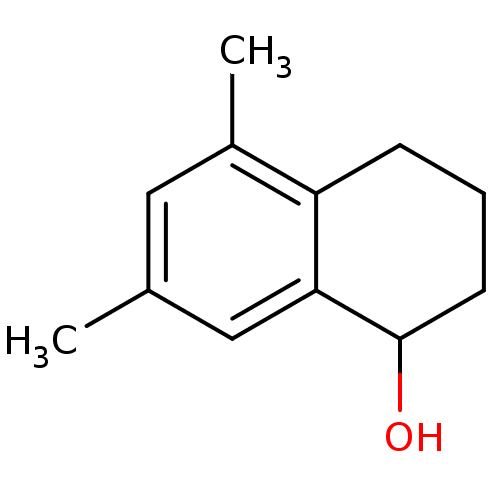
((rac)-5,7-dimethyl-1,2,3,4-tetrahydronaphthalen-1-...)Show InChI InChI=1S/C12H16O/c1-8-6-9(2)10-4-3-5-12(13)11(10)7-8/h6-7,12-13H,3-5H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.78E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Helsinki
Curated by ChEMBL
| Assay Description
Competitive inhibition of UGT2B17-catalyzed scopoletin glucuronidation |
J Med Chem 49: 1818-27 (2006)
Article DOI: 10.1021/jm051142c
BindingDB Entry DOI: 10.7270/Q2B857QP |
More data for this
Ligand-Target Pair | |
UDP-glucuronosyltransferase 2B7
(Homo sapiens (Human)) | BDBM50183611

((rac)-5,7-dimethyl-1,2,3,4-tetrahydronaphthalen-1-...)Show InChI InChI=1S/C12H16O/c1-8-6-9(2)10-4-3-5-12(13)11(10)7-8/h6-7,12-13H,3-5H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.01E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Helsinki
Curated by ChEMBL
| Assay Description
Competitive inhibition of UGT2B7-catalyzed scopoletin glucuronidation |
J Med Chem 49: 1818-27 (2006)
Article DOI: 10.1021/jm051142c
BindingDB Entry DOI: 10.7270/Q2B857QP |
More data for this
Ligand-Target Pair | |
UDP-glucuronosyltransferase 2B7
(Homo sapiens (Human)) | BDBM50183598

((rac)-6-methyl-3,4-dihydro-2H-chromen-4-ol | CHEMB...)Show InChI InChI=1S/C10H12O2/c1-7-2-3-10-8(6-7)9(11)4-5-12-10/h2-3,6,9,11H,4-5H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.13E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Helsinki
Curated by ChEMBL
| Assay Description
Competitive inhibition of UGT2B7-catalyzed scopoletin glucuronidation |
J Med Chem 49: 1818-27 (2006)
Article DOI: 10.1021/jm051142c
BindingDB Entry DOI: 10.7270/Q2B857QP |
More data for this
Ligand-Target Pair | |
UDP-glucuronosyltransferase 2B17
(Homo sapiens (Human)) | BDBM50183611

((rac)-5,7-dimethyl-1,2,3,4-tetrahydronaphthalen-1-...)Show InChI InChI=1S/C12H16O/c1-8-6-9(2)10-4-3-5-12(13)11(10)7-8/h6-7,12-13H,3-5H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.90E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Helsinki
Curated by ChEMBL
| Assay Description
Non competitive inhibition of UGT2B17-catalyzed scopoletin glucuronidation |
J Med Chem 49: 1818-27 (2006)
Article DOI: 10.1021/jm051142c
BindingDB Entry DOI: 10.7270/Q2B857QP |
More data for this
Ligand-Target Pair | |
UDP-glucuronosyltransferase 2B7
(Homo sapiens (Human)) | BDBM50183611

((rac)-5,7-dimethyl-1,2,3,4-tetrahydronaphthalen-1-...)Show InChI InChI=1S/C12H16O/c1-8-6-9(2)10-4-3-5-12(13)11(10)7-8/h6-7,12-13H,3-5H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.92E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Helsinki
Curated by ChEMBL
| Assay Description
Non competitive inhibition of UGT2B7-catalyzed scopoletin glucuronidation |
J Med Chem 49: 1818-27 (2006)
Article DOI: 10.1021/jm051142c
BindingDB Entry DOI: 10.7270/Q2B857QP |
More data for this
Ligand-Target Pair | |
UDP-glucuronosyltransferase 2B17
(Homo sapiens (Human)) | BDBM50183598

((rac)-6-methyl-3,4-dihydro-2H-chromen-4-ol | CHEMB...)Show InChI InChI=1S/C10H12O2/c1-7-2-3-10-8(6-7)9(11)4-5-12-10/h2-3,6,9,11H,4-5H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.01E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Helsinki
Curated by ChEMBL
| Assay Description
Competitive inhibition of UGT2B17-catalyzed scopoletin glucuronidation |
J Med Chem 49: 1818-27 (2006)
Article DOI: 10.1021/jm051142c
BindingDB Entry DOI: 10.7270/Q2B857QP |
More data for this
Ligand-Target Pair | |
UDP-glucuronosyltransferase 2B7
(Homo sapiens (Human)) | BDBM50183598

((rac)-6-methyl-3,4-dihydro-2H-chromen-4-ol | CHEMB...)Show InChI InChI=1S/C10H12O2/c1-7-2-3-10-8(6-7)9(11)4-5-12-10/h2-3,6,9,11H,4-5H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.58E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Helsinki
Curated by ChEMBL
| Assay Description
Non competitive inhibition of UGT2B7-catalyzed scopoletin glucuronidation |
J Med Chem 49: 1818-27 (2006)
Article DOI: 10.1021/jm051142c
BindingDB Entry DOI: 10.7270/Q2B857QP |
More data for this
Ligand-Target Pair | |
UDP-glucuronosyltransferase 2B7
(Homo sapiens (Human)) | BDBM50183609

((rac)-6-methyl-2,3-dihydro-1H-inden-1-ol | CHEMBL2...)Show InChI InChI=1S/C10H12O/c1-7-2-3-8-4-5-10(11)9(8)6-7/h2-3,6,10-11H,4-5H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3.01E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Helsinki
Curated by ChEMBL
| Assay Description
Competitive inhibition of UGT2B7-catalyzed scopoletin glucuronidation |
J Med Chem 49: 1818-27 (2006)
Article DOI: 10.1021/jm051142c
BindingDB Entry DOI: 10.7270/Q2B857QP |
More data for this
Ligand-Target Pair | |
UDP-glucuronosyltransferase 2B17
(Homo sapiens (Human)) | BDBM50183609

((rac)-6-methyl-2,3-dihydro-1H-inden-1-ol | CHEMBL2...)Show InChI InChI=1S/C10H12O/c1-7-2-3-8-4-5-10(11)9(8)6-7/h2-3,6,10-11H,4-5H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 4.27E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Helsinki
Curated by ChEMBL
| Assay Description
Competitive inhibition of UGT2B17-catalyzed scopoletin glucuronidation |
J Med Chem 49: 1818-27 (2006)
Article DOI: 10.1021/jm051142c
BindingDB Entry DOI: 10.7270/Q2B857QP |
More data for this
Ligand-Target Pair | |
UDP-glucuronosyltransferase 2B17
(Homo sapiens (Human)) | BDBM50183598

((rac)-6-methyl-3,4-dihydro-2H-chromen-4-ol | CHEMB...)Show InChI InChI=1S/C10H12O2/c1-7-2-3-10-8(6-7)9(11)4-5-12-10/h2-3,6,9,11H,4-5H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 6.08E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Helsinki
Curated by ChEMBL
| Assay Description
Non competitive inhibition of UGT2B17-catalyzed scopoletin glucuronidation |
J Med Chem 49: 1818-27 (2006)
Article DOI: 10.1021/jm051142c
BindingDB Entry DOI: 10.7270/Q2B857QP |
More data for this
Ligand-Target Pair | |
UDP-glucuronosyltransferase 2B7
(Homo sapiens (Human)) | BDBM50183609

((rac)-6-methyl-2,3-dihydro-1H-inden-1-ol | CHEMBL2...)Show InChI InChI=1S/C10H12O/c1-7-2-3-8-4-5-10(11)9(8)6-7/h2-3,6,10-11H,4-5H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 6.24E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Helsinki
Curated by ChEMBL
| Assay Description
Non competitive inhibition of UGT2B7-catalyzed scopoletin glucuronidation |
J Med Chem 49: 1818-27 (2006)
Article DOI: 10.1021/jm051142c
BindingDB Entry DOI: 10.7270/Q2B857QP |
More data for this
Ligand-Target Pair | |
UDP-glucuronosyltransferase 2B17
(Homo sapiens (Human)) | BDBM50183609

((rac)-6-methyl-2,3-dihydro-1H-inden-1-ol | CHEMBL2...)Show InChI InChI=1S/C10H12O/c1-7-2-3-8-4-5-10(11)9(8)6-7/h2-3,6,10-11H,4-5H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.44E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Helsinki
Curated by ChEMBL
| Assay Description
Non competitive inhibition of UGT2B17-catalyzed scopoletin glucuronidation |
J Med Chem 49: 1818-27 (2006)
Article DOI: 10.1021/jm051142c
BindingDB Entry DOI: 10.7270/Q2B857QP |
More data for this
Ligand-Target Pair | |
Phosphatidylserine lipase ABHD16A
(Homo sapiens (Human)) | BDBM50507489
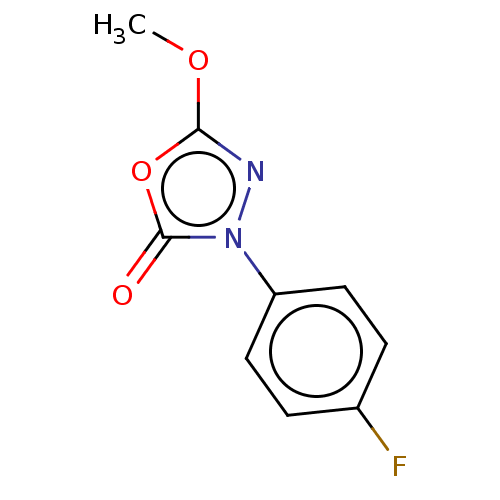
(CHEMBL4564841)Show InChI InChI=1S/C9H7FN2O3/c1-14-8-11-12(9(13)15-8)7-4-2-6(10)3-5-7/h2-5H,1H3 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Helsinki
Curated by ChEMBL
| Assay Description
Inhibition of ABHD16A (unknown origin) |
ACS Med Chem Lett 9: 1269-1273 (2018)
Article DOI: 10.1021/acsmedchemlett.8b00442
BindingDB Entry DOI: 10.7270/Q2KH0RM7 |
More data for this
Ligand-Target Pair | |
UDP-glucuronosyltransferase 2B7
(Homo sapiens (Human)) | BDBM50211740

((1R)-phenyl-[(1R,2S,7S,8S,9S)-3,3,7-trimethyltricy...)Show SMILES C[C@]12CCCC(C)(C)[C@H]3[C@H](CC[C@@H]13)[C@@H]2[C@@H](O)c1ccccc1 |TLB:2:1:10.11:8,0:1:10.11:8,THB:5:8:10.11:13.1,14:13:10.11:8| Show InChI InChI=1S/C21H30O/c1-20(2)12-7-13-21(3)16-11-10-15(17(16)20)18(21)19(22)14-8-5-4-6-9-14/h4-6,8-9,15-19,22H,7,10-13H2,1-3H3/t15-,16+,17-,18+,19-,21-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 47 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Helsinki
Curated by ChEMBL
| Assay Description
Inhibition of human UGT2B7 by substrate-independent inhibition assay |
J Med Chem 50: 2655-64 (2007)
Article DOI: 10.1021/jm061204e
BindingDB Entry DOI: 10.7270/Q23F4QF2 |
More data for this
Ligand-Target Pair | |
UDP-glucuronosyltransferase 2B7
(Homo sapiens (Human)) | BDBM50211740

((1R)-phenyl-[(1R,2S,7S,8S,9S)-3,3,7-trimethyltricy...)Show SMILES C[C@]12CCCC(C)(C)[C@H]3[C@H](CC[C@@H]13)[C@@H]2[C@@H](O)c1ccccc1 |TLB:2:1:10.11:8,0:1:10.11:8,THB:5:8:10.11:13.1,14:13:10.11:8| Show InChI InChI=1S/C21H30O/c1-20(2)12-7-13-21(3)16-11-10-15(17(16)20)18(21)19(22)14-8-5-4-6-9-14/h4-6,8-9,15-19,22H,7,10-13H2,1-3H3/t15-,16+,17-,18+,19-,21-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 54 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Helsinki
Curated by ChEMBL
| Assay Description
Inhibition of human UGT2B7 assessed as reduction of estriol glucuronidation |
J Med Chem 50: 2655-64 (2007)
Article DOI: 10.1021/jm061204e
BindingDB Entry DOI: 10.7270/Q23F4QF2 |
More data for this
Ligand-Target Pair | |
Phosphatidylserine lipase ABHD16A
(Homo sapiens (Human)) | BDBM50336546

(CHEMBL1673415 | Palmostatin B)Show SMILES CCCCCCCCCC[C@H]1[C@H](CCc2ccc(OC)c(OC)c2)OC1=O |r| Show InChI InChI=1S/C23H36O4/c1-4-5-6-7-8-9-10-11-12-19-20(27-23(19)24)15-13-18-14-16-21(25-2)22(17-18)26-3/h14,16-17,19-20H,4-13,15H2,1-3H3/t19-,20-/m0/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 57 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Helsinki
Curated by ChEMBL
| Assay Description
Reversible inhibition of human ABHD16A expressed in HEK293 cells preincubated for 30 mins followed by 40 fold compound dilution and subsequent 1-LG s... |
ACS Med Chem Lett 9: 1269-1273 (2018)
Article DOI: 10.1021/acsmedchemlett.8b00442
BindingDB Entry DOI: 10.7270/Q2KH0RM7 |
More data for this
Ligand-Target Pair | |
Phosphatidylserine lipase ABHD16A
(Homo sapiens (Human)) | BDBM195603
(KC01)Show InChI InChI=1S/C22H39NO3/c1-2-3-4-5-6-7-8-9-10-11-14-17-20-19(22(25)26-20)16-13-12-15-18-21(23)24/h16,20H,2-15,17-18H2,1H3,(H2,23,24)/b19-16- | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Helsinki
Curated by ChEMBL
| Assay Description
Inhibition of ABHD16A (unknown origin) |
ACS Med Chem Lett 9: 1269-1273 (2018)
Article DOI: 10.1021/acsmedchemlett.8b00442
BindingDB Entry DOI: 10.7270/Q2KH0RM7 |
More data for this
Ligand-Target Pair | |
Phosphatidylserine lipase ABHD16A
(Homo sapiens (Human)) | BDBM50336546

(CHEMBL1673415 | Palmostatin B)Show SMILES CCCCCCCCCC[C@H]1[C@H](CCc2ccc(OC)c(OC)c2)OC1=O |r| Show InChI InChI=1S/C23H36O4/c1-4-5-6-7-8-9-10-11-12-19-20(27-23(19)24)15-13-18-14-16-21(25-2)22(17-18)26-3/h14,16-17,19-20H,4-13,15H2,1-3H3/t19-,20-/m0/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 99 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Helsinki
Curated by ChEMBL
| Assay Description
Inhibition of human ABHD16A expressed in HEK293 cells preincubated for 30 mins followed by 1-LG substrate addition and measured after 90 to 120 mins ... |
ACS Med Chem Lett 9: 1269-1273 (2018)
Article DOI: 10.1021/acsmedchemlett.8b00442
BindingDB Entry DOI: 10.7270/Q2KH0RM7 |
More data for this
Ligand-Target Pair | |
UDP-glucuronosyltransferase 2B7
(Homo sapiens (Human)) | BDBM50211775

(((1S,3aR,4S,8aR,9S)-4,8,8-trimethyl-decahydro-1,4-...)Show SMILES C[C@]12CCCC(C)(C)[C@H]3[C@H](CC[C@@H]13)[C@@H]2CO |TLB:2:1:10.11:8,THB:11:12:9.13:2.3.5.4,5:8:10.11:13.1,14:13:10.11:8,0:1:10.11:8| Show InChI InChI=1S/C15H26O/c1-14(2)7-4-8-15(3)11-6-5-10(13(11)14)12(15)9-16/h10-13,16H,4-9H2,1-3H3/t10-,11-,12+,13+,15+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Helsinki
Curated by ChEMBL
| Assay Description
Inhibition of human UGT2B7 assessed as reduction of estriol glucuronidation |
J Med Chem 50: 2655-64 (2007)
Article DOI: 10.1021/jm061204e
BindingDB Entry DOI: 10.7270/Q23F4QF2 |
More data for this
Ligand-Target Pair | |
Phosphatidylserine lipase ABHD16A
(Homo sapiens (Human)) | BDBM50336546

(CHEMBL1673415 | Palmostatin B)Show SMILES CCCCCCCCCC[C@H]1[C@H](CCc2ccc(OC)c(OC)c2)OC1=O |r| Show InChI InChI=1S/C23H36O4/c1-4-5-6-7-8-9-10-11-12-19-20(27-23(19)24)15-13-18-14-16-21(25-2)22(17-18)26-3/h14,16-17,19-20H,4-13,15H2,1-3H3/t19-,20-/m0/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Helsinki
Curated by ChEMBL
| Assay Description
Inhibition of ABHD16A (unknown origin) |
ACS Med Chem Lett 9: 1269-1273 (2018)
Article DOI: 10.1021/acsmedchemlett.8b00442
BindingDB Entry DOI: 10.7270/Q2KH0RM7 |
More data for this
Ligand-Target Pair | |
Phosphatidylserine lipase ABHD16A
(Homo sapiens (Human)) | BDBM24567

((2S)-1-[(2S,3S)-3-hexyl-4-oxooxetan-2-yl]tridecan-...)Show SMILES CCCCCCCCCCC[C@@H](C[C@@H]1OC(=O)[C@H]1CCCCCC)OC(=O)[C@H](CC(C)C)NC=O |r| Show InChI InChI=1S/C29H53NO5/c1-5-7-9-11-12-13-14-15-16-18-24(34-29(33)26(30-22-31)20-23(3)4)21-27-25(28(32)35-27)19-17-10-8-6-2/h22-27H,5-21H2,1-4H3,(H,30,31)/t24-,25-,26-,27-/m0/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Helsinki
Curated by ChEMBL
| Assay Description
Inhibition of ABHD16A (unknown origin) |
ACS Med Chem Lett 9: 1269-1273 (2018)
Article DOI: 10.1021/acsmedchemlett.8b00442
BindingDB Entry DOI: 10.7270/Q2KH0RM7 |
More data for this
Ligand-Target Pair | |
UDP-glucuronosyltransferase 2B7
(Homo sapiens (Human)) | BDBM50211763
((1R)-2-chloro-1-[(1R,2S,7S,8S,9S)-3,3,7-trimethylt...)Show SMILES C[C@]12CCCC(C)(C)[C@H]3[C@H](CC[C@@H]13)[C@@H]2[C@@H](O)CCl |TLB:2:1:10.11:8,0:1:10.11:8,THB:5:8:10.11:13.1,14:13:10.11:8| Show InChI InChI=1S/C16H27ClO/c1-15(2)7-4-8-16(3)11-6-5-10(13(11)15)14(16)12(18)9-17/h10-14,18H,4-9H2,1-3H3/t10-,11+,12-,13-,14+,16-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Helsinki
Curated by ChEMBL
| Assay Description
Inhibition of human UGT2B7 assessed as reduction of estriol glucuronidation |
J Med Chem 50: 2655-64 (2007)
Article DOI: 10.1021/jm061204e
BindingDB Entry DOI: 10.7270/Q23F4QF2 |
More data for this
Ligand-Target Pair | |
UDP-glucuronosyltransferase 2B7
(Homo sapiens (Human)) | BDBM50211780

((1S)-2,2-dimethyl-1-[(1R,2S,7S,8S,9S)-3,3,7-trimet...)Show SMILES CC(C)(C)[C@@H](O)[C@H]1[C@H]2CC[C@@H]3[C@H]2C(C)(C)CCC[C@]13C |TLB:4:6:8.9:11,THB:17:18:8.9:11,12:11:8.9:6.18,19:18:8.9:11| Show InChI InChI=1S/C19H34O/c1-17(2,3)16(20)15-12-8-9-13-14(12)18(4,5)10-7-11-19(13,15)6/h12-16,20H,7-11H2,1-6H3/t12-,13+,14-,15+,16?,19-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Helsinki
Curated by ChEMBL
| Assay Description
Inhibition of human UGT2B7 assessed as reduction of estriol glucuronidation |
J Med Chem 50: 2655-64 (2007)
Article DOI: 10.1021/jm061204e
BindingDB Entry DOI: 10.7270/Q23F4QF2 |
More data for this
Ligand-Target Pair | |
UDP-glucuronosyltransferase 2B7
(Homo sapiens (Human)) | BDBM50211762
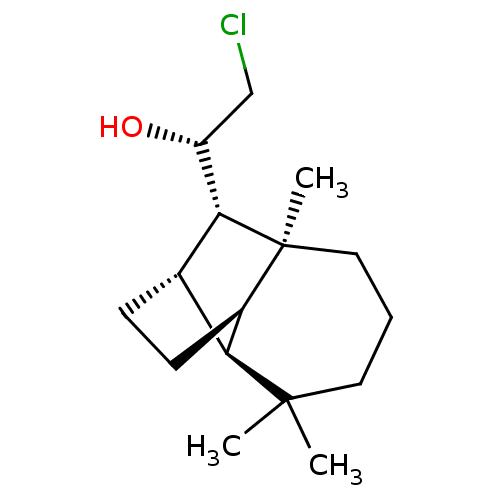
((1S)-2-chloro-1-[(1R,2S,7S,8S,9S)-3,3,7-trimethylt...)Show SMILES C[C@]12CCCC(C)(C)[C@H]3[C@H](CC[C@@H]13)[C@@H]2[C@H](O)CCl |TLB:2:1:10.11:8,0:1:10.11:8,THB:5:8:10.11:13.1,14:13:10.11:8| Show InChI InChI=1S/C16H27ClO/c1-15(2)7-4-8-16(3)11-6-5-10(13(11)15)14(16)12(18)9-17/h10-14,18H,4-9H2,1-3H3/t10-,11+,12+,13-,14+,16-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Helsinki
Curated by ChEMBL
| Assay Description
Inhibition of human UGT2B7 assessed as reduction of estriol glucuronidation |
J Med Chem 50: 2655-64 (2007)
Article DOI: 10.1021/jm061204e
BindingDB Entry DOI: 10.7270/Q23F4QF2 |
More data for this
Ligand-Target Pair | |
UDP-glucuronosyltransferase 2B7
(Homo sapiens (Human)) | BDBM50211777

((1S)-1-[(1R,2S,7S,8S,9S)-3,3,7-trimethyltricyclo[5...)Show SMILES C[C@]12CCCC(C)(C)[C@H]3[C@H](CC[C@@H]13)[C@@H]2[C@@H](O)CC=C |TLB:2:1:10.11:8,0:1:10.11:8,THB:5:8:10.11:13.1,14:13:10.11:8| Show InChI InChI=1S/C18H30O/c1-5-7-14(19)16-12-8-9-13-15(12)17(2,3)10-6-11-18(13,16)4/h5,12-16,19H,1,6-11H2,2-4H3/t12-,13+,14-,15-,16+,18-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Helsinki
Curated by ChEMBL
| Assay Description
Inhibition of human UGT2B7 assessed as reduction of estriol glucuronidation |
J Med Chem 50: 2655-64 (2007)
Article DOI: 10.1021/jm061204e
BindingDB Entry DOI: 10.7270/Q23F4QF2 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data