 Found 490 hits with Last Name = 'zimmermann' and Initial = 'j'
Found 490 hits with Last Name = 'zimmermann' and Initial = 'j' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Carbonyl reductase [NADPH] 1
(Homo sapiens (Human)) | BDBM50247677
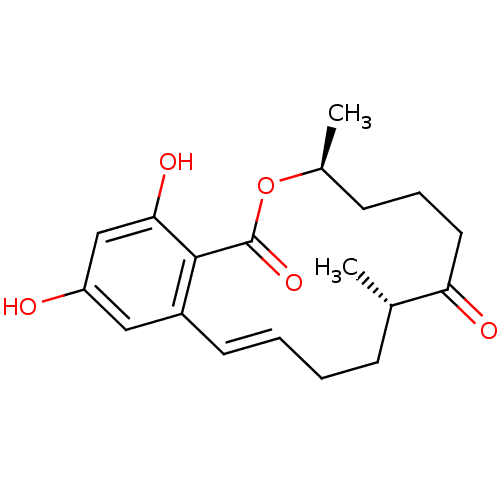
((3S,8S)-14,16-dihydroxy-3,8-dimethyl-3,4,5,6,9,10-...)Show SMILES C[C@H]1CCCC(=O)[C@@H](C)CC\C=C\c2cc(O)cc(O)c2C(=O)O1 |r,t:11| Show InChI InChI=1S/C19H24O5/c1-12-6-3-4-8-14-10-15(20)11-17(22)18(14)19(23)24-13(2)7-5-9-16(12)21/h4,8,10-13,20,22H,3,5-7,9H2,1-2H3/b8-4+/t12-,13-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universität Tübingen
Curated by ChEMBL
| Assay Description
Binding affinity to human recombinant carbonyl reductase 1 expressed in Escherichia coli assessed as NADPH oxidation using isatin as substrate |
Bioorg Med Chem 17: 530-6 (2009)
Article DOI: 10.1016/j.bmc.2008.11.076
BindingDB Entry DOI: 10.7270/Q2SJ1KFH |
More data for this
Ligand-Target Pair | |
Carbonyl reductase [NADPH] 1
(Homo sapiens (Human)) | BDBM8726

(5-chloro-2-(2,4-dichlorophenoxy)phenol | CHEMBL849...)Show InChI InChI=1S/C12H7Cl3O2/c13-7-1-3-11(9(15)5-7)17-12-4-2-8(14)6-10(12)16/h1-6,16H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universität Tübingen
Curated by ChEMBL
| Assay Description
Binding affinity to human recombinant carbonyl reductase 1 expressed in Escherichia coli assessed as NADPH oxidation using isatin as substrate |
Bioorg Med Chem 17: 530-6 (2009)
Article DOI: 10.1016/j.bmc.2008.11.076
BindingDB Entry DOI: 10.7270/Q2SJ1KFH |
More data for this
Ligand-Target Pair | |
Carbonyl reductase [NADPH] 1
(Homo sapiens (Human)) | BDBM7461
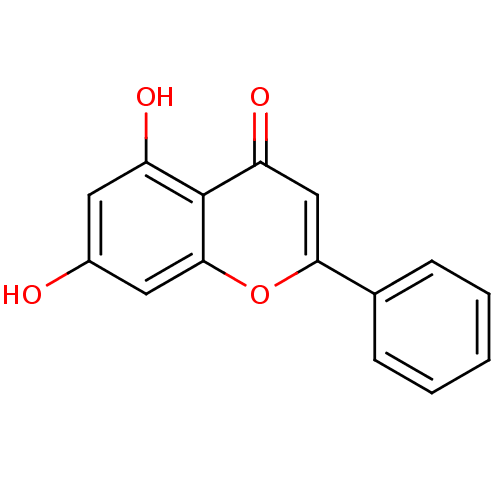
(5,7-dihydroxy-2-phenyl-4H-chromen-4-one | 5,7-dihy...)Show InChI InChI=1S/C15H10O4/c16-10-6-11(17)15-12(18)8-13(19-14(15)7-10)9-4-2-1-3-5-9/h1-8,16-17H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universität Tübingen
Curated by ChEMBL
| Assay Description
Binding affinity to human recombinant carbonyl reductase 1 expressed in Escherichia coli assessed as NADPH oxidation using isatin as substrate |
Bioorg Med Chem 17: 530-6 (2009)
Article DOI: 10.1016/j.bmc.2008.11.076
BindingDB Entry DOI: 10.7270/Q2SJ1KFH |
More data for this
Ligand-Target Pair | |
Carbonyl reductase [NADPH] 1
(Homo sapiens (Human)) | BDBM50073099
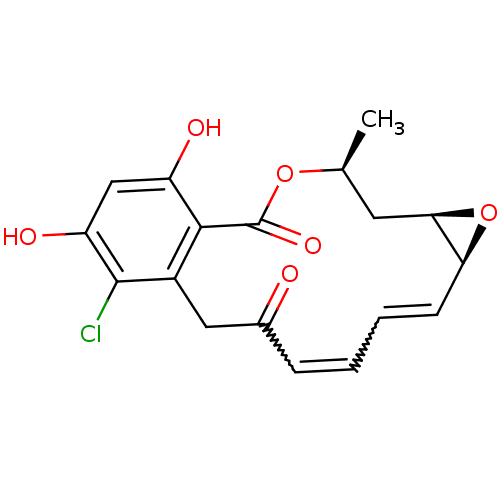
((9E,11E)-(4R,6R,8R)-16-chloro-17,19-dihydroxy-4-me...)Show SMILES C[C@H]1C[C@H]2O[C@H]2C=CC=CC(=O)Cc2c(Cl)c(O)cc(O)c2C(=O)O1 |w:7.8,9.10| Show InChI InChI=1S/C18H17ClO6/c1-9-6-15-14(25-15)5-3-2-4-10(20)7-11-16(18(23)24-9)12(21)8-13(22)17(11)19/h2-5,8-9,14-15,21-22H,6-7H2,1H3/t9-,14-,15+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 520 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universität Tübingen
Curated by ChEMBL
| Assay Description
Binding affinity to human recombinant carbonyl reductase 1 expressed in Escherichia coli assessed as NADPH oxidation using isatin as substrate |
Bioorg Med Chem 17: 530-6 (2009)
Article DOI: 10.1016/j.bmc.2008.11.076
BindingDB Entry DOI: 10.7270/Q2SJ1KFH |
More data for this
Ligand-Target Pair | |
Carbonyl reductase [NADPH] 1
(Homo sapiens (Human)) | BDBM50096619
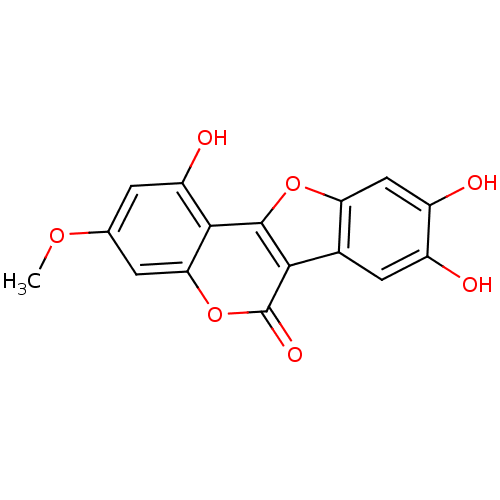
(1,8,9-Trihydroxy-3-methoxy-benzo[4,5]furo[3,2-c]ch...)Show InChI InChI=1S/C16H10O7/c1-21-6-2-10(19)14-12(3-6)23-16(20)13-7-4-8(17)9(18)5-11(7)22-15(13)14/h2-5,17-19H,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universität Tübingen
Curated by ChEMBL
| Assay Description
Binding affinity to human recombinant carbonyl reductase 1 expressed in Escherichia coli assessed as NADPH oxidation using isatin as substrate |
Bioorg Med Chem 17: 530-6 (2009)
Article DOI: 10.1016/j.bmc.2008.11.076
BindingDB Entry DOI: 10.7270/Q2SJ1KFH |
More data for this
Ligand-Target Pair | |
Carbonyl reductase [NADPH] 1
(Homo sapiens (Human)) | BDBM9461
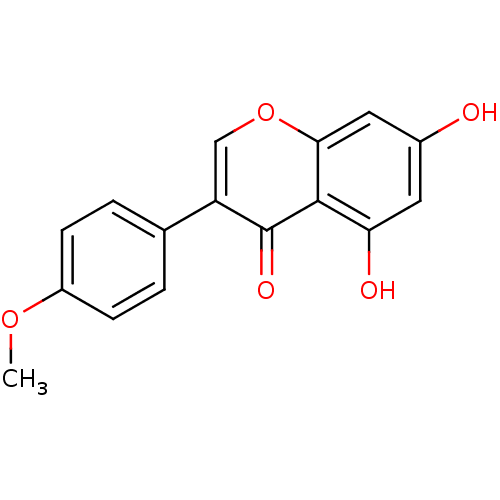
(5,7-dihydroxy-3-(4-methoxyphenyl)-4H-chromen-4-one...)Show InChI InChI=1S/C16H12O5/c1-20-11-4-2-9(3-5-11)12-8-21-14-7-10(17)6-13(18)15(14)16(12)19/h2-8,17-18H,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 2.03E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universität Tübingen
Curated by ChEMBL
| Assay Description
Binding affinity to human recombinant carbonyl reductase 1 expressed in Escherichia coli assessed as NADPH oxidation using isatin as substrate |
Bioorg Med Chem 17: 530-6 (2009)
Article DOI: 10.1016/j.bmc.2008.11.076
BindingDB Entry DOI: 10.7270/Q2SJ1KFH |
More data for this
Ligand-Target Pair | |
Carbonyl reductase [NADPH] 1
(Homo sapiens (Human)) | BDBM23419
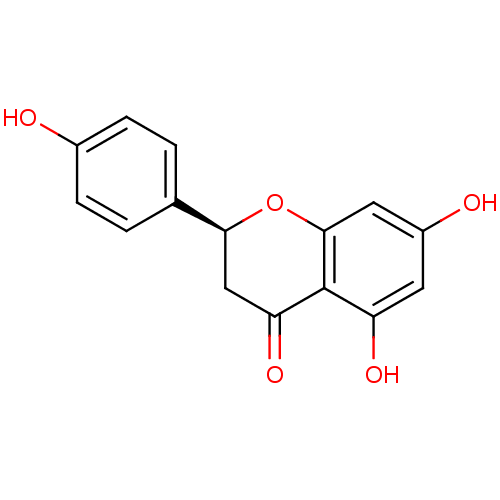
((2S)-5,7-dihydroxy-2-(4-hydroxyphenyl)-3,4-dihydro...)Show SMILES Oc1ccc(cc1)[C@@H]1CC(=O)c2c(O)cc(O)cc2O1 |r| Show InChI InChI=1S/C15H12O5/c16-9-3-1-8(2-4-9)13-7-12(19)15-11(18)5-10(17)6-14(15)20-13/h1-6,13,16-18H,7H2/t13-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 3.43E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universität Tübingen
Curated by ChEMBL
| Assay Description
Binding affinity to human recombinant carbonyl reductase 1 expressed in Escherichia coli assessed as NADPH oxidation using isatin as substrate |
Bioorg Med Chem 17: 530-6 (2009)
Article DOI: 10.1016/j.bmc.2008.11.076
BindingDB Entry DOI: 10.7270/Q2SJ1KFH |
More data for this
Ligand-Target Pair | |
Protein kinase C alpha type
(Homo sapiens (Human)) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of Protein kinase C alpha |
Bioorg Med Chem Lett 5: 497-500 (1995)
Article DOI: 10.1016/0960-894X(95)00060-7
BindingDB Entry DOI: 10.7270/Q24749T8 |
More data for this
Ligand-Target Pair | |
A5LHX3/O14818/P20618/P25786/P25787/P25788/P25789/P28062/P28065/P28066/P28070/P28072/P28074/P40306/P49720/P49721/P60900/Q8TAA3/Q99436
(Homo sapiens (Human)) | BDBM50469762
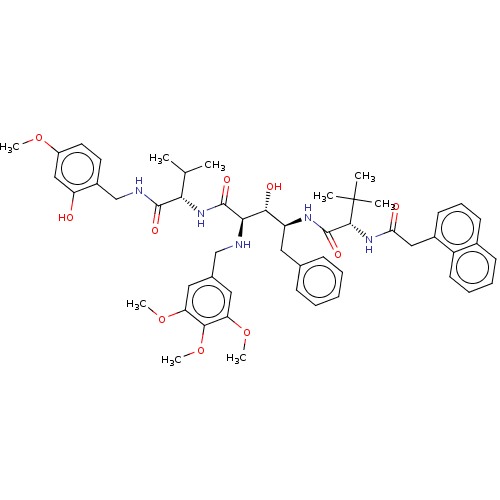
(CHEMBL432281)Show SMILES COc1ccc(CNC(=O)[C@@H](NC(=O)[C@H](NCc2cc(OC)c(OC)c(OC)c2)[C@H](O)[C@H](Cc2ccccc2)NC(=O)[C@@H](NC(=O)Cc2cccc3ccccc23)C(C)(C)C)C(C)C)c(O)c1 Show InChI InChI=1S/C52H65N5O10/c1-31(2)44(49(61)54-30-36-22-23-37(64-6)28-40(36)58)57-50(62)45(53-29-33-25-41(65-7)47(67-9)42(26-33)66-8)46(60)39(24-32-16-11-10-12-17-32)55-51(63)48(52(3,4)5)56-43(59)27-35-20-15-19-34-18-13-14-21-38(34)35/h10-23,25-26,28,31,39,44-46,48,53,58,60H,24,27,29-30H2,1-9H3,(H,54,61)(H,55,63)(H,56,59)(H,57,62)/t39-,44-,45+,46+,48+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma Inc.
Curated by ChEMBL
| Assay Description
Tested in vitro for inhibition of chymotrypsin like activity of purified human 20S proteasome |
Bioorg Med Chem Lett 12: 1331-4 (2002)
Article DOI: 10.1016/s0960-894x(02)00178-6
BindingDB Entry DOI: 10.7270/Q27D2XV2 |
More data for this
Ligand-Target Pair | |
Platelet-derived growth factor receptor alpha/beta
(Homo sapiens (Human)) | BDBM50175491

(4-Methyl-N-[4-methyl-3-(4-pyridin-3-yl-pyrimidin-2...)Show SMILES Cc1ccc(cc1)C(=O)Nc1ccc(C)c(Nc2nccc(n2)-c2cccnc2)c1 Show InChI InChI=1S/C24H21N5O/c1-16-5-8-18(9-6-16)23(30)27-20-10-7-17(2)22(14-20)29-24-26-13-11-21(28-24)19-4-3-12-25-15-19/h3-15H,1-2H3,(H,27,30)(H,26,28,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of the platelet-derived growth factor receptor. |
Bioorg Med Chem Lett 7: 187-192 (1997)
Article DOI: 10.1016/S0960-894X(96)00601-4
BindingDB Entry DOI: 10.7270/Q23X86MF |
More data for this
Ligand-Target Pair | |
Protein kinase C alpha type
(Homo sapiens (Human)) | BDBM2683
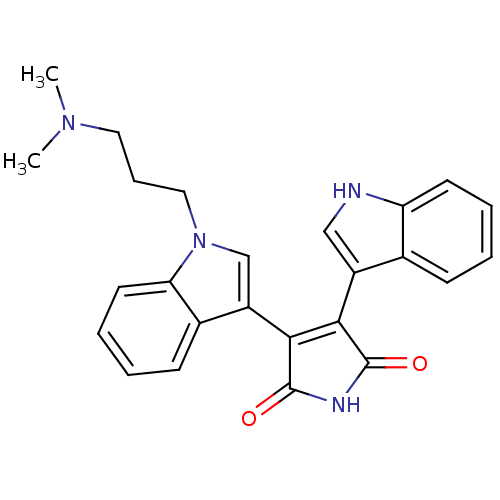
(2-[1-(3-dimethylaminopropyl)-indol-3-yl]-3-(indol-...)Show SMILES CN(C)CCCn1cc(C2=C(C(=O)NC2=O)c2c[nH]c3ccccc23)c2ccccc12 |t:9| Show InChI InChI=1S/C25H24N4O2/c1-28(2)12-7-13-29-15-19(17-9-4-6-11-21(17)29)23-22(24(30)27-25(23)31)18-14-26-20-10-5-3-8-16(18)20/h3-6,8-11,14-15,26H,7,12-13H2,1-2H3,(H,27,30,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of Protein kinase C alpha |
Bioorg Med Chem Lett 5: 497-500 (1995)
Article DOI: 10.1016/0960-894X(95)00060-7
BindingDB Entry DOI: 10.7270/Q24749T8 |
More data for this
Ligand-Target Pair | |
Platelet-derived growth factor receptor alpha/beta
(Homo sapiens (Human)) | BDBM50287090

(4-Chloro-N-[4-methyl-3-(4-pyridin-3-yl-pyrimidin-2...)Show SMILES Cc1ccc(NC(=O)c2ccc(Cl)cc2)cc1Nc1nccc(n1)-c1cccnc1 Show InChI InChI=1S/C23H18ClN5O/c1-15-4-9-19(27-22(30)16-5-7-18(24)8-6-16)13-21(15)29-23-26-12-10-20(28-23)17-3-2-11-25-14-17/h2-14H,1H3,(H,27,30)(H,26,28,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of the platelet-derived growth factor receptor. |
Bioorg Med Chem Lett 7: 187-192 (1997)
Article DOI: 10.1016/S0960-894X(96)00601-4
BindingDB Entry DOI: 10.7270/Q23X86MF |
More data for this
Ligand-Target Pair | |
Platelet-derived growth factor receptor alpha/beta
(Homo sapiens (Human)) | BDBM50287090

(4-Chloro-N-[4-methyl-3-(4-pyridin-3-yl-pyrimidin-2...)Show SMILES Cc1ccc(NC(=O)c2ccc(Cl)cc2)cc1Nc1nccc(n1)-c1cccnc1 Show InChI InChI=1S/C23H18ClN5O/c1-15-4-9-19(27-22(30)16-5-7-18(24)8-6-16)13-21(15)29-23-26-12-10-20(28-23)17-3-2-11-25-14-17/h2-14H,1H3,(H,27,30)(H,26,28,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of tyrosine kinase(PDGF-R) |
Bioorg Med Chem Lett 6: 1221-1226 (1996)
Article DOI: 10.1016/0960-894X(96)00197-7
BindingDB Entry DOI: 10.7270/Q2348KBG |
More data for this
Ligand-Target Pair | |
Platelet-derived growth factor receptor alpha/beta
(Homo sapiens (Human)) | BDBM50175491

(4-Methyl-N-[4-methyl-3-(4-pyridin-3-yl-pyrimidin-2...)Show SMILES Cc1ccc(cc1)C(=O)Nc1ccc(C)c(Nc2nccc(n2)-c2cccnc2)c1 Show InChI InChI=1S/C24H21N5O/c1-16-5-8-18(9-6-16)23(30)27-20-10-7-17(2)22(14-20)29-24-26-13-11-21(28-24)19-4-3-12-25-15-19/h3-15H,1-2H3,(H,27,30)(H,26,28,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of tyrosine kinase(PDGF-R) |
Bioorg Med Chem Lett 6: 1221-1226 (1996)
Article DOI: 10.1016/0960-894X(96)00197-7
BindingDB Entry DOI: 10.7270/Q2348KBG |
More data for this
Ligand-Target Pair | |
A5LHX3/O14818/P20618/P25786/P25787/P25788/P25789/P28062/P28065/P28066/P28070/P28072/P28074/P40306/P49720/P49721/P60900/Q8TAA3/Q99436
(Homo sapiens (Human)) | BDBM50469758
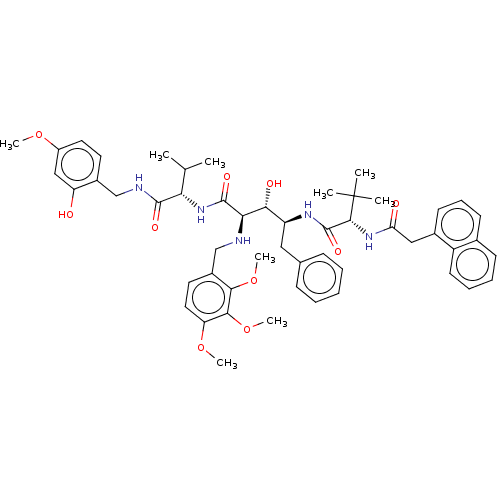
(CHEMBL27225)Show SMILES COc1ccc(CNC(=O)[C@@H](NC(=O)[C@H](NCc2ccc(OC)c(OC)c2OC)[C@H](O)[C@H](Cc2ccccc2)NC(=O)[C@@H](NC(=O)Cc2cccc3ccccc23)C(C)(C)C)C(C)C)c(O)c1 Show InChI InChI=1S/C52H65N5O10/c1-31(2)43(49(61)54-29-35-22-24-37(64-6)28-40(35)58)57-50(62)44(53-30-36-23-25-41(65-7)47(67-9)46(36)66-8)45(60)39(26-32-16-11-10-12-17-32)55-51(63)48(52(3,4)5)56-42(59)27-34-20-15-19-33-18-13-14-21-38(33)34/h10-25,28,31,39,43-45,48,53,58,60H,26-27,29-30H2,1-9H3,(H,54,61)(H,55,63)(H,56,59)(H,57,62)/t39-,43-,44+,45+,48+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma Inc.
Curated by ChEMBL
| Assay Description
Tested in vitro for inhibition of chymotrypsin like activity of purified human 20S proteasome |
Bioorg Med Chem Lett 12: 1331-4 (2002)
Article DOI: 10.1016/s0960-894x(02)00178-6
BindingDB Entry DOI: 10.7270/Q27D2XV2 |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 1
(Homo sapiens (Human)) | BDBM50073765
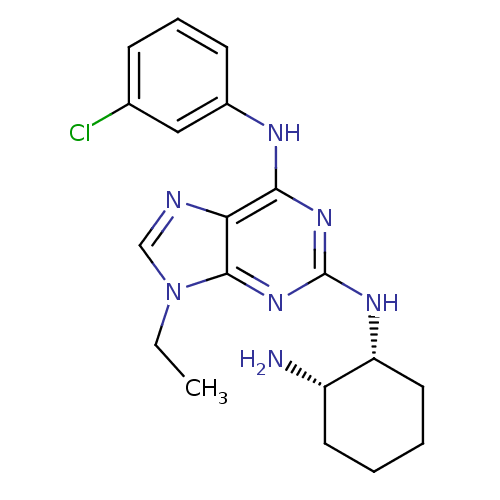
(CHEMBL367625 | N*2*-((1R,2S)-2-Amino-cyclohexyl)-N...)Show SMILES CCn1cnc2c(Nc3cccc(Cl)c3)nc(N[C@@H]3CCCC[C@@H]3N)nc12 Show InChI InChI=1S/C19H24ClN7/c1-2-27-11-22-16-17(23-13-7-5-6-12(20)10-13)25-19(26-18(16)27)24-15-9-4-3-8-14(15)21/h5-7,10-11,14-15H,2-4,8-9,21H2,1H3,(H2,23,24,25,26)/t14-,15+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
PubMed
| n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory activity against cyclin-dependent kinase 1 (CDK1) was determined |
Bioorg Med Chem Lett 9: 91-6 (1999)
BindingDB Entry DOI: 10.7270/Q24Q7VHT |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cyclin-dependent kinase 1
(Homo sapiens (Human)) | BDBM50073795
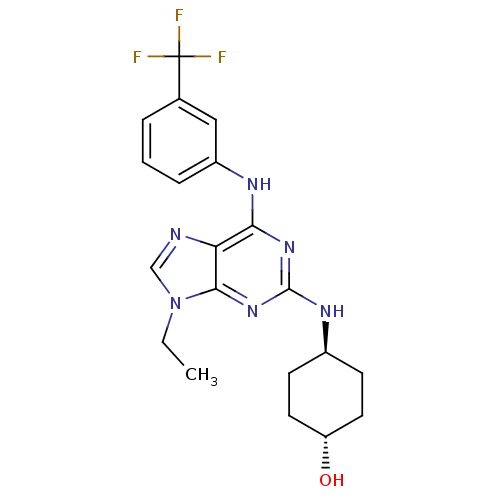
(4-[9-Ethyl-6-(3-trifluoromethyl-phenylamino)-9H-pu...)Show SMILES CCn1cnc2c(Nc3cccc(c3)C(F)(F)F)nc(N[C@H]3CC[C@H](O)CC3)nc12 |wU:21.21,wD:24.25,(7.96,-5.98,;6.45,-5.65,;5.98,-4.18,;6.88,-2.95,;5.98,-1.71,;4.53,-2.18,;3.2,-1.42,;3.2,.12,;4.53,.89,;5.86,.12,;7.19,.89,;7.19,2.44,;5.86,3.21,;4.53,2.43,;5.84,4.74,;5.84,6.15,;7.09,5.76,;4.6,5.76,;1.87,-2.18,;1.85,-3.72,;.52,-4.49,;-.81,-3.72,;-2.14,-4.49,;-3.47,-3.72,;-3.47,-2.18,;-4.8,-1.4,;-2.12,-1.41,;-.81,-2.18,;3.2,-4.5,;4.53,-3.72,)| Show InChI InChI=1S/C20H23F3N6O/c1-2-29-11-24-16-17(25-14-5-3-4-12(10-14)20(21,22)23)27-19(28-18(16)29)26-13-6-8-15(30)9-7-13/h3-5,10-11,13,15,30H,2,6-9H2,1H3,(H2,25,26,27,28)/t13-,15- | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory activity against cyclin-dependent kinase 1 (CDK1) was determined |
Bioorg Med Chem Lett 9: 91-6 (1999)
BindingDB Entry DOI: 10.7270/Q24Q7VHT |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 1
(Homo sapiens (Human)) | BDBM50073763
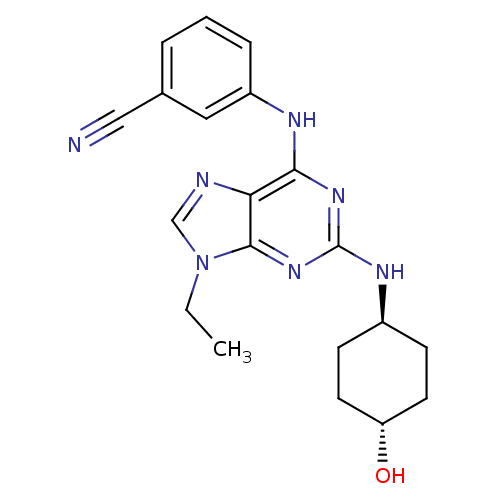
(3-[9-Ethyl-2-(4-hydroxy-cyclohexylamino)-9H-purin-...)Show SMILES CCn1cnc2c(Nc3cccc(c3)C#N)nc(N[C@H]3CC[C@H](O)CC3)nc12 |wU:19.19,wD:22.23,(7.96,-5.98,;6.45,-5.65,;5.98,-4.18,;6.88,-2.95,;5.98,-1.71,;4.53,-2.18,;3.2,-1.42,;3.2,.12,;4.53,.89,;5.86,.12,;7.19,.89,;7.19,2.44,;5.86,3.21,;4.53,2.43,;5.86,4.74,;5.86,6.28,;1.87,-2.18,;1.85,-3.72,;.52,-4.49,;-.81,-3.72,;-2.14,-4.49,;-3.47,-3.72,;-3.47,-2.18,;-4.8,-1.4,;-2.12,-1.41,;-.81,-2.18,;3.2,-4.5,;4.53,-3.72,)| Show InChI InChI=1S/C20H23N7O/c1-2-27-12-22-17-18(23-15-5-3-4-13(10-15)11-21)25-20(26-19(17)27)24-14-6-8-16(28)9-7-14/h3-5,10,12,14,16,28H,2,6-9H2,1H3,(H2,23,24,25,26)/t14-,16- | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory activity against cyclin-dependent kinase 1 (CDK1) was determined |
Bioorg Med Chem Lett 9: 91-6 (1999)
BindingDB Entry DOI: 10.7270/Q24Q7VHT |
More data for this
Ligand-Target Pair | |
A5LHX3/O14818/P20618/P25786/P25787/P25788/P25789/P28062/P28065/P28066/P28070/P28072/P28074/P40306/P49720/P49721/P60900/Q8TAA3/Q99436
(Homo sapiens (Human)) | BDBM50469765
(CHEMBL28320)Show SMILES COc1ccc(CNC(=O)[C@@H](NC(=O)[C@H](NCc2cc(OC)c(OC)cc2OC)[C@H](O)[C@H](Cc2ccccc2)NC(=O)[C@@H](NC(=O)Cc2cccc3ccccc23)C(C)(C)C)C(C)C)c(O)c1 Show InChI InChI=1S/C52H65N5O10/c1-31(2)45(49(61)54-29-35-22-23-37(64-6)27-40(35)58)57-50(62)46(53-30-36-25-42(66-8)43(67-9)28-41(36)65-7)47(60)39(24-32-16-11-10-12-17-32)55-51(63)48(52(3,4)5)56-44(59)26-34-20-15-19-33-18-13-14-21-38(33)34/h10-23,25,27-28,31,39,45-48,53,58,60H,24,26,29-30H2,1-9H3,(H,54,61)(H,55,63)(H,56,59)(H,57,62)/t39-,45-,46+,47+,48+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma Inc.
Curated by ChEMBL
| Assay Description
Tested in vitro for inhibition of chymotrypsin like activity of purified human 20S proteasome |
Bioorg Med Chem Lett 12: 1331-4 (2002)
Article DOI: 10.1016/s0960-894x(02)00178-6
BindingDB Entry DOI: 10.7270/Q27D2XV2 |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 1
(Homo sapiens (Human)) | BDBM50073796
(4-[6-(3-Chloro-phenylamino)-9-ethyl-9H-purin-2-yla...)Show SMILES CCn1cnc2c(Nc3cccc(Cl)c3)nc(N[C@H]3CC[C@H](O)CC3)nc12 |wU:18.18,wD:21.22,(7.96,-5.98,;6.45,-5.65,;5.98,-4.18,;6.88,-2.95,;5.98,-1.71,;4.53,-2.18,;3.2,-1.42,;3.2,.12,;4.53,.89,;5.86,.12,;7.19,.89,;7.19,2.44,;5.86,3.21,;5.86,4.74,;4.53,2.43,;1.87,-2.18,;1.85,-3.72,;.52,-4.49,;-.81,-3.72,;-2.14,-4.49,;-3.47,-3.72,;-3.47,-2.18,;-4.8,-1.4,;-2.12,-1.41,;-.81,-2.18,;3.2,-4.5,;4.53,-3.72,)| Show InChI InChI=1S/C19H23ClN6O/c1-2-26-11-21-16-17(22-14-5-3-4-12(20)10-14)24-19(25-18(16)26)23-13-6-8-15(27)9-7-13/h3-5,10-11,13,15,27H,2,6-9H2,1H3,(H2,22,23,24,25)/t13-,15- | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory activity against cyclin-dependent kinase 1 (CDK1) was determined |
Bioorg Med Chem Lett 9: 91-6 (1999)
BindingDB Entry DOI: 10.7270/Q24Q7VHT |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 1
(Homo sapiens (Human)) | BDBM50073755
(4-[6-(3-Chloro-phenylamino)-9-ethyl-9H-purin-2-yla...)Show SMILES CCn1cnc2c(Nc3cccc(Cl)c3)nc(N[C@@H]3CC[C@H](O)CC3)nc12 |wU:18.18,21.22,(18.21,-17.29,;16.71,-16.96,;16.22,-15.49,;17.13,-14.26,;16.24,-13.02,;14.77,-13.49,;13.44,-12.72,;13.44,-11.18,;14.78,-10.41,;16.11,-11.18,;17.45,-10.41,;17.45,-8.87,;16.11,-8.1,;16.1,-6.56,;14.78,-8.87,;12.11,-13.49,;12.11,-15.03,;10.78,-15.8,;9.43,-15.03,;9.43,-13.49,;8.12,-12.72,;6.77,-13.49,;5.44,-12.72,;6.77,-15.03,;8.12,-15.8,;13.44,-15.8,;14.77,-15.03,)| Show InChI InChI=1S/C19H23ClN6O/c1-2-26-11-21-16-17(22-14-5-3-4-12(20)10-14)24-19(25-18(16)26)23-13-6-8-15(27)9-7-13/h3-5,10-11,13,15,27H,2,6-9H2,1H3,(H2,22,23,24,25)/t13-,15+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of Cyclin-dependent kinase 1, CDK1-related human bladder carcinoma (T24) cell protliferation |
Bioorg Med Chem Lett 9: 91-6 (1999)
BindingDB Entry DOI: 10.7270/Q24Q7VHT |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 1
(Homo sapiens (Human)) | BDBM50073765

(CHEMBL367625 | N*2*-((1R,2S)-2-Amino-cyclohexyl)-N...)Show SMILES CCn1cnc2c(Nc3cccc(Cl)c3)nc(N[C@@H]3CCCC[C@@H]3N)nc12 Show InChI InChI=1S/C19H24ClN7/c1-2-27-11-22-16-17(23-13-7-5-6-12(20)10-13)25-19(26-18(16)27)24-15-9-4-3-8-14(15)21/h5-7,10-11,14-15H,2-4,8-9,21H2,1H3,(H2,23,24,25,26)/t14-,15+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
PubMed
| n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory activity against cyclin-dependent kinase 1 (CDK1) was determined |
Bioorg Med Chem Lett 9: 91-6 (1999)
BindingDB Entry DOI: 10.7270/Q24Q7VHT |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cyclin-dependent kinase 1
(Homo sapiens (Human)) | BDBM50073764
(CHEMBL171891 | N*2*-(4-Amino-cyclohexyl)-9-ethyl-N...)Show SMILES CCn1cnc2c(Nc3ccc(F)cc3)nc(N[C@H]3CC[C@H](N)CC3)nc12 |wU:18.18,wD:21.22,(7.96,-5.98,;6.45,-5.65,;5.98,-4.18,;6.88,-2.95,;5.98,-1.71,;4.53,-2.18,;3.2,-1.42,;3.2,.12,;4.53,.89,;5.86,.12,;7.19,.89,;7.19,2.44,;8.52,3.21,;5.86,3.21,;4.53,2.43,;1.87,-2.18,;1.85,-3.72,;.52,-4.49,;-.81,-3.72,;-2.14,-4.49,;-3.47,-3.72,;-3.47,-2.18,;-4.8,-1.4,;-2.12,-1.41,;-.81,-2.18,;3.2,-4.5,;4.53,-3.72,)| Show InChI InChI=1S/C19H24FN7/c1-2-27-11-22-16-17(23-14-7-3-12(20)4-8-14)25-19(26-18(16)27)24-15-9-5-13(21)6-10-15/h3-4,7-8,11,13,15H,2,5-6,9-10,21H2,1H3,(H2,23,24,25,26)/t13-,15- | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory activity against cyclin-dependent kinase 1 (CDK1) was determined |
Bioorg Med Chem Lett 9: 91-6 (1999)
BindingDB Entry DOI: 10.7270/Q24Q7VHT |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 1
(Homo sapiens (Human)) | BDBM50073765

(CHEMBL367625 | N*2*-((1R,2S)-2-Amino-cyclohexyl)-N...)Show SMILES CCn1cnc2c(Nc3cccc(Cl)c3)nc(N[C@@H]3CCCC[C@@H]3N)nc12 Show InChI InChI=1S/C19H24ClN7/c1-2-27-11-22-16-17(23-13-7-5-6-12(20)10-13)25-19(26-18(16)27)24-15-9-4-3-8-14(15)21/h5-7,10-11,14-15H,2-4,8-9,21H2,1H3,(H2,23,24,25,26)/t14-,15+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
PubMed
| n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory activity against cyclin-dependent kinase 1 (CDK1) was determined |
Bioorg Med Chem Lett 9: 91-6 (1999)
BindingDB Entry DOI: 10.7270/Q24Q7VHT |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cyclin-dependent kinase 1
(Homo sapiens (Human)) | BDBM50073767
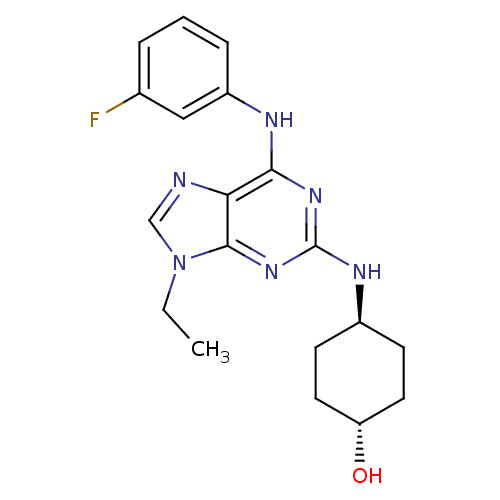
(4-[9-Ethyl-6-(3-fluoro-phenylamino)-9H-purin-2-yla...)Show SMILES CCn1cnc2c(Nc3cccc(F)c3)nc(N[C@H]3CC[C@H](O)CC3)nc12 |wU:18.18,wD:21.22,(7.96,-5.98,;6.45,-5.65,;5.98,-4.18,;6.88,-2.95,;5.98,-1.71,;4.53,-2.18,;3.2,-1.42,;3.2,.12,;4.53,.89,;5.86,.12,;7.19,.89,;7.19,2.44,;5.86,3.21,;5.86,4.75,;4.53,2.43,;1.87,-2.18,;1.85,-3.72,;.52,-4.49,;-.81,-3.72,;-2.14,-4.49,;-3.47,-3.72,;-3.47,-2.18,;-4.8,-1.4,;-2.12,-1.41,;-.81,-2.18,;3.2,-4.5,;4.53,-3.72,)| Show InChI InChI=1S/C19H23FN6O/c1-2-26-11-21-16-17(22-14-5-3-4-12(20)10-14)24-19(25-18(16)26)23-13-6-8-15(27)9-7-13/h3-5,10-11,13,15,27H,2,6-9H2,1H3,(H2,22,23,24,25)/t13-,15- | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory activity against cyclin-dependent kinase 1 (CDK1) was determined |
Bioorg Med Chem Lett 9: 91-6 (1999)
BindingDB Entry DOI: 10.7270/Q24Q7VHT |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM13530

(4-[(4-methylpiperazin-1-yl)methyl]-N-[4-methyl-3-[...)Show SMILES CN1CCN(Cc2ccc(cc2)C(=O)Nc2ccc(C)c(Nc3nccc(n3)-c3cccnc3)c2)CC1 Show InChI InChI=1S/C29H31N7O/c1-21-5-10-25(18-27(21)34-29-31-13-11-26(33-29)24-4-3-12-30-19-24)32-28(37)23-8-6-22(7-9-23)20-36-16-14-35(2)15-17-36/h3-13,18-19H,14-17,20H2,1-2H3,(H,32,37)(H,31,33,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
| n/a | n/a | 38 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of v-Abl tyrosine kinase. |
Bioorg Med Chem Lett 7: 187-192 (1997)
Article DOI: 10.1016/S0960-894X(96)00601-4
BindingDB Entry DOI: 10.7270/Q23X86MF |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cyclin-dependent kinase 1
(Homo sapiens (Human)) | BDBM50073794
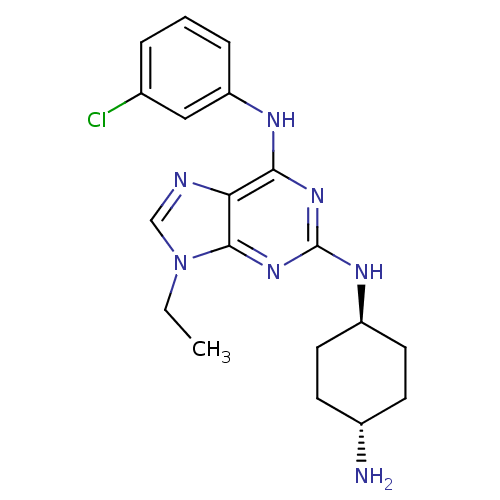
(CHEMBL367691 | N*2*-(4-Amino-cyclohexyl)-N*6*-(3-c...)Show SMILES CCn1cnc2c(Nc3cccc(Cl)c3)nc(N[C@H]3CC[C@H](N)CC3)nc12 |wU:18.18,wD:21.22,(7.96,-5.98,;6.45,-5.65,;5.98,-4.18,;6.88,-2.95,;5.98,-1.71,;4.53,-2.18,;3.2,-1.42,;3.2,.12,;4.53,.89,;5.86,.12,;7.19,.89,;7.19,2.44,;5.86,3.21,;5.86,4.75,;4.53,2.43,;1.87,-2.18,;1.85,-3.72,;.52,-4.49,;-.81,-3.72,;-2.14,-4.49,;-3.47,-3.72,;-3.47,-2.18,;-4.8,-1.4,;-2.12,-1.41,;-.81,-2.18,;3.2,-4.5,;4.53,-3.72,)| Show InChI InChI=1S/C19H24ClN7/c1-2-27-11-22-16-17(23-15-5-3-4-12(20)10-15)25-19(26-18(16)27)24-14-8-6-13(21)7-9-14/h3-5,10-11,13-14H,2,6-9,21H2,1H3,(H2,23,24,25,26)/t13-,14- | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 39 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory activity against cyclin-dependent kinase 1 (CDK1) was determined |
Bioorg Med Chem Lett 9: 91-6 (1999)
BindingDB Entry DOI: 10.7270/Q24Q7VHT |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 1
(Homo sapiens (Human)) | BDBM50073759
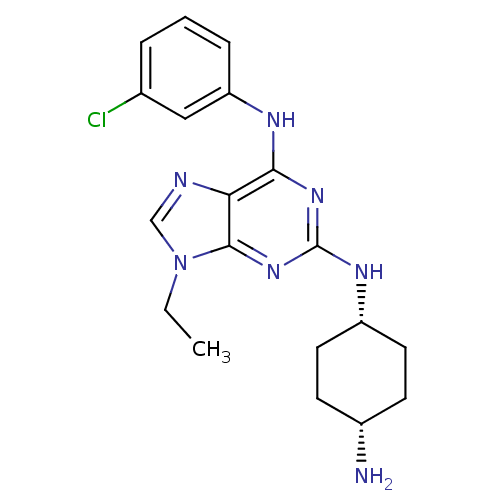
(CHEMBL173459 | N*2*-(4-Amino-cyclohexyl)-N*6*-(3-c...)Show SMILES CCn1cnc2c(Nc3cccc(Cl)c3)nc(N[C@@H]3CC[C@H](N)CC3)nc12 |wU:18.18,21.22,(9.76,-3.77,;8.25,-3.46,;7.77,-1.99,;8.68,-.75,;7.79,.49,;6.32,.02,;4.99,.79,;4.99,2.33,;6.32,3.1,;7.65,2.33,;9,3.1,;9,4.65,;7.65,5.42,;7.65,6.98,;6.32,4.65,;3.66,.02,;3.65,-1.52,;2.31,-2.29,;.97,-1.52,;.98,.02,;-.34,.8,;-1.68,.04,;-3.03,.81,;-1.7,-1.5,;-.37,-2.29,;4.99,-2.3,;6.32,-1.5,)| Show InChI InChI=1S/C19H24ClN7/c1-2-27-11-22-16-17(23-15-5-3-4-12(20)10-15)25-19(26-18(16)27)24-14-8-6-13(21)7-9-14/h3-5,10-11,13-14H,2,6-9,21H2,1H3,(H2,23,24,25,26)/t13-,14+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 39 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory activity against cyclin-dependent kinase 1 (CDK1) was determined |
Bioorg Med Chem Lett 9: 91-6 (1999)
BindingDB Entry DOI: 10.7270/Q24Q7VHT |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 1
(Homo sapiens (Human)) | BDBM50073766
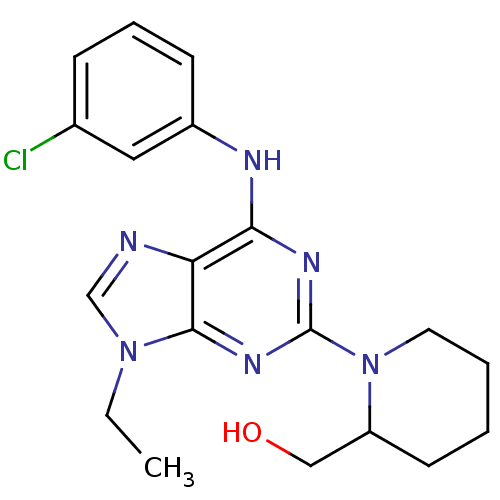
(CHEMBL176559 | {1-[6-(3-Chloro-phenylamino)-9-ethy...)Show InChI InChI=1S/C19H23ClN6O/c1-2-25-12-21-16-17(22-14-7-5-6-13(20)10-14)23-19(24-18(16)25)26-9-4-3-8-15(26)11-27/h5-7,10,12,15,27H,2-4,8-9,11H2,1H3,(H,22,23,24) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 39 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory activity against cyclin-dependent kinase 1 (CDK1) was determined |
Bioorg Med Chem Lett 9: 91-6 (1999)
BindingDB Entry DOI: 10.7270/Q24Q7VHT |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 1
(Homo sapiens (Human)) | BDBM50073759

(CHEMBL173459 | N*2*-(4-Amino-cyclohexyl)-N*6*-(3-c...)Show SMILES CCn1cnc2c(Nc3cccc(Cl)c3)nc(N[C@@H]3CC[C@H](N)CC3)nc12 |wU:18.18,21.22,(9.76,-3.77,;8.25,-3.46,;7.77,-1.99,;8.68,-.75,;7.79,.49,;6.32,.02,;4.99,.79,;4.99,2.33,;6.32,3.1,;7.65,2.33,;9,3.1,;9,4.65,;7.65,5.42,;7.65,6.98,;6.32,4.65,;3.66,.02,;3.65,-1.52,;2.31,-2.29,;.97,-1.52,;.98,.02,;-.34,.8,;-1.68,.04,;-3.03,.81,;-1.7,-1.5,;-.37,-2.29,;4.99,-2.3,;6.32,-1.5,)| Show InChI InChI=1S/C19H24ClN7/c1-2-27-11-22-16-17(23-15-5-3-4-12(20)10-15)25-19(26-18(16)27)24-14-8-6-13(21)7-9-14/h3-5,10-11,13-14H,2,6-9,21H2,1H3,(H2,23,24,25,26)/t13-,14+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 39 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory activity against cyclin-dependent kinase 1 (CDK1) was determined |
Bioorg Med Chem Lett 9: 91-6 (1999)
BindingDB Entry DOI: 10.7270/Q24Q7VHT |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 1
(Homo sapiens (Human)) | BDBM50073798
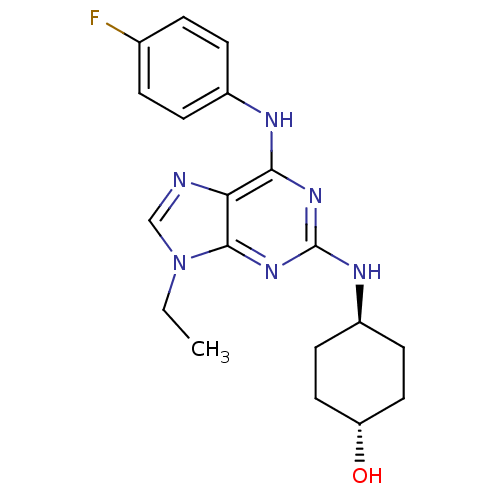
(4-[9-Ethyl-6-(4-fluoro-phenylamino)-9H-purin-2-yla...)Show SMILES CCn1cnc2c(Nc3ccc(F)cc3)nc(N[C@H]3CC[C@H](O)CC3)nc12 |wU:18.18,wD:21.22,(7.96,-5.98,;6.45,-5.65,;5.98,-4.18,;6.88,-2.95,;5.98,-1.71,;4.53,-2.18,;3.2,-1.42,;3.2,.12,;4.53,.89,;4.53,2.43,;5.86,3.21,;7.19,2.44,;8.52,3.21,;7.19,.89,;5.86,.12,;1.87,-2.18,;1.85,-3.72,;.52,-4.49,;-.81,-3.72,;-2.14,-4.49,;-3.47,-3.72,;-3.47,-2.18,;-4.8,-1.4,;-2.12,-1.41,;-.81,-2.18,;3.2,-4.5,;4.53,-3.72,)| Show InChI InChI=1S/C19H23FN6O/c1-2-26-11-21-16-17(22-13-5-3-12(20)4-6-13)24-19(25-18(16)26)23-14-7-9-15(27)10-8-14/h3-6,11,14-15,27H,2,7-10H2,1H3,(H2,22,23,24,25)/t14-,15- | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory activity against cyclin-dependent kinase 1 (CDK1) was determined |
Bioorg Med Chem Lett 9: 91-6 (1999)
BindingDB Entry DOI: 10.7270/Q24Q7VHT |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 1
(Homo sapiens (Human)) | BDBM50073777
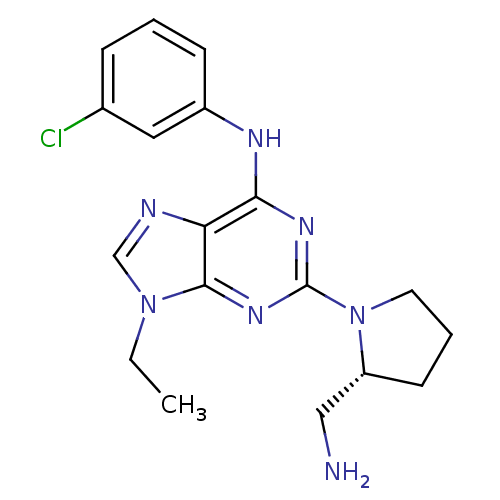
(CHEMBL368351 | [2-((R)-2-Aminomethyl-pyrrolidin-1-...)Show SMILES CCn1cnc2c(Nc3cccc(Cl)c3)nc(nc12)N1CCC[C@@H]1CN Show InChI InChI=1S/C18H22ClN7/c1-2-25-11-21-15-16(22-13-6-3-5-12(19)9-13)23-18(24-17(15)25)26-8-4-7-14(26)10-20/h3,5-6,9,11,14H,2,4,7-8,10,20H2,1H3,(H,22,23,24)/t14-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 41 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory activity against cyclin-dependent kinase 1 (CDK1) was determined |
Bioorg Med Chem Lett 9: 91-6 (1999)
BindingDB Entry DOI: 10.7270/Q24Q7VHT |
More data for this
Ligand-Target Pair | |
Platelet-derived growth factor receptor alpha/beta
(Homo sapiens (Human)) | BDBM50287091

(CHEMBL20252 | Naphthalene-2-carboxylic acid [4-met...)Show SMILES Cc1ccc(NC(=O)c2ccc3ccccc3c2)cc1Nc1nccc(n1)-c1cccnc1 Show InChI InChI=1S/C27H21N5O/c1-18-8-11-23(30-26(33)21-10-9-19-5-2-3-6-20(19)15-21)16-25(18)32-27-29-14-12-24(31-27)22-7-4-13-28-17-22/h2-17H,1H3,(H,30,33)(H,29,31,32) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of the platelet-derived growth factor receptor. |
Bioorg Med Chem Lett 7: 187-192 (1997)
Article DOI: 10.1016/S0960-894X(96)00601-4
BindingDB Entry DOI: 10.7270/Q23X86MF |
More data for this
Ligand-Target Pair | |
Platelet-derived growth factor receptor alpha/beta
(Homo sapiens (Human)) | BDBM13530

(4-[(4-methylpiperazin-1-yl)methyl]-N-[4-methyl-3-[...)Show SMILES CN1CCN(Cc2ccc(cc2)C(=O)Nc2ccc(C)c(Nc3nccc(n3)-c3cccnc3)c2)CC1 Show InChI InChI=1S/C29H31N7O/c1-21-5-10-25(18-27(21)34-29-31-13-11-26(33-29)24-4-3-12-30-19-24)32-28(37)23-8-6-22(7-9-23)20-36-16-14-35(2)15-17-36/h3-13,18-19H,14-17,20H2,1-2H3,(H,32,37)(H,31,33,34) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of the platelet-derived growth factor receptor. |
Bioorg Med Chem Lett 7: 187-192 (1997)
Article DOI: 10.1016/S0960-894X(96)00601-4
BindingDB Entry DOI: 10.7270/Q23X86MF |
More data for this
Ligand-Target Pair | |
Platelet-derived growth factor receptor alpha/beta
(Homo sapiens (Human)) | BDBM50287091

(CHEMBL20252 | Naphthalene-2-carboxylic acid [4-met...)Show SMILES Cc1ccc(NC(=O)c2ccc3ccccc3c2)cc1Nc1nccc(n1)-c1cccnc1 Show InChI InChI=1S/C27H21N5O/c1-18-8-11-23(30-26(33)21-10-9-19-5-2-3-6-20(19)15-21)16-25(18)32-27-29-14-12-24(31-27)22-7-4-13-28-17-22/h2-17H,1H3,(H,30,33)(H,29,31,32) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of tyrosine kinase(PDGF-R) |
Bioorg Med Chem Lett 6: 1221-1226 (1996)
Article DOI: 10.1016/0960-894X(96)00197-7
BindingDB Entry DOI: 10.7270/Q2348KBG |
More data for this
Ligand-Target Pair | |
A5LHX3/O14818/P20618/P25786/P25787/P25788/P25789/P28062/P28065/P28066/P28070/P28072/P28074/P40306/P49720/P49721/P60900/Q8TAA3/Q99436
(Homo sapiens (Human)) | BDBM50469764
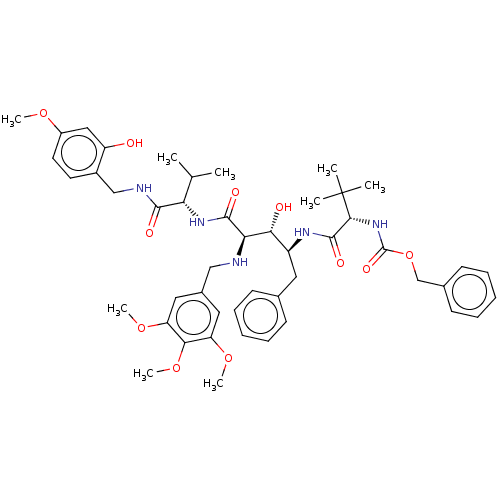
(CHEMBL27214)Show SMILES COc1ccc(CNC(=O)[C@@H](NC(=O)[C@H](NCc2cc(OC)c(OC)c(OC)c2)[C@H](O)[C@H](Cc2ccccc2)NC(=O)[C@@H](NC(=O)OCc2ccccc2)C(C)(C)C)C(C)C)c(O)c1 Show InChI InChI=1S/C48H63N5O11/c1-29(2)39(44(56)50-27-33-20-21-34(60-6)25-36(33)54)52-45(57)40(49-26-32-23-37(61-7)42(63-9)38(24-32)62-8)41(55)35(22-30-16-12-10-13-17-30)51-46(58)43(48(3,4)5)53-47(59)64-28-31-18-14-11-15-19-31/h10-21,23-25,29,35,39-41,43,49,54-55H,22,26-28H2,1-9H3,(H,50,56)(H,51,58)(H,52,57)(H,53,59)/t35-,39-,40+,41+,43+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma Inc.
Curated by ChEMBL
| Assay Description
Tested in vitro for inhibition of chymotrypsin like activity of purified human 20S proteasome |
Bioorg Med Chem Lett 12: 1331-4 (2002)
Article DOI: 10.1016/s0960-894x(02)00178-6
BindingDB Entry DOI: 10.7270/Q27D2XV2 |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 1
(Homo sapiens (Human)) | BDBM50073758
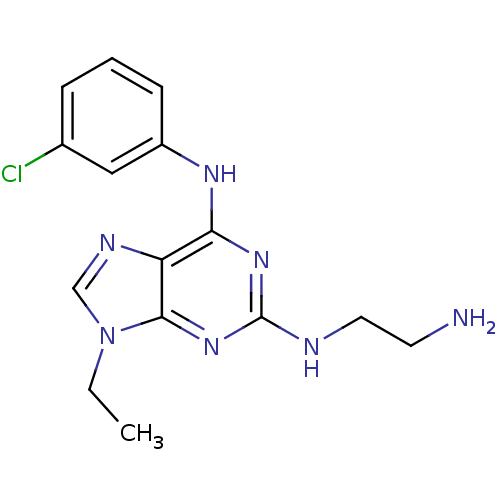
(CHEMBL173647 | N*2*-(2-Amino-ethyl)-N*6*-(3-chloro...)Show InChI InChI=1S/C15H18ClN7/c1-2-23-9-19-12-13(20-11-5-3-4-10(16)8-11)21-15(18-7-6-17)22-14(12)23/h3-5,8-9H,2,6-7,17H2,1H3,(H2,18,20,21,22) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory activity against cyclin-dependent kinase 1 (CDK1) was determined |
Bioorg Med Chem Lett 9: 91-6 (1999)
BindingDB Entry DOI: 10.7270/Q24Q7VHT |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 1
(Homo sapiens (Human)) | BDBM50073762
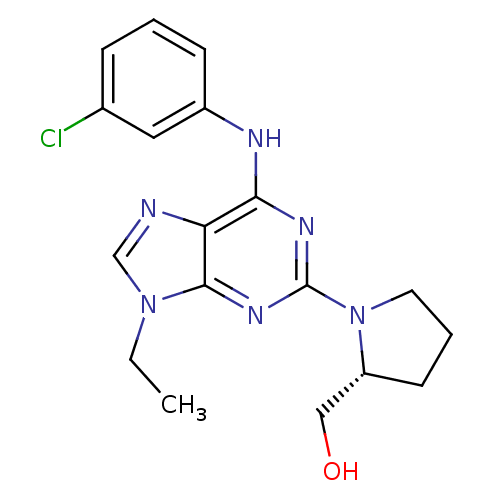
(CHEMBL366734 | {(R)-1-[6-(3-Chloro-phenylamino)-9-...)Show SMILES CCn1cnc2c(Nc3cccc(Cl)c3)nc(nc12)N1CCC[C@@H]1CO Show InChI InChI=1S/C18H21ClN6O/c1-2-24-11-20-15-16(21-13-6-3-5-12(19)9-13)22-18(23-17(15)24)25-8-4-7-14(25)10-26/h3,5-6,9,11,14,26H,2,4,7-8,10H2,1H3,(H,21,22,23)/t14-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory activity against cyclin-dependent kinase 1 (CDK1) was determined |
Bioorg Med Chem Lett 9: 91-6 (1999)
BindingDB Entry DOI: 10.7270/Q24Q7VHT |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 1
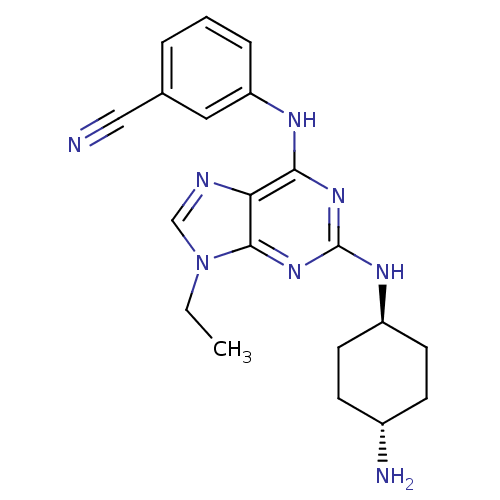
(Homo sapiens (Human)) | BDBM50073771
(3-[2-(4-Amino-cyclohexylamino)-9-ethyl-9H-purin-6-...)Show SMILES CCn1cnc2c(Nc3cccc(c3)C#N)nc(N[C@H]3CC[C@H](N)CC3)nc12 |wU:19.19,wD:22.23,(7.96,-5.98,;6.45,-5.65,;5.98,-4.18,;6.88,-2.95,;5.98,-1.71,;4.53,-2.18,;3.2,-1.42,;3.2,.12,;4.53,.89,;5.86,.12,;7.19,.89,;7.19,2.44,;5.86,3.21,;4.53,2.43,;5.86,4.74,;5.86,6.28,;1.87,-2.18,;1.85,-3.72,;.52,-4.49,;-.81,-3.72,;-2.14,-4.49,;-3.47,-3.72,;-3.47,-2.18,;-4.8,-1.4,;-2.12,-1.41,;-.81,-2.18,;3.2,-4.5,;4.53,-3.72,)| Show InChI InChI=1S/C20H24N8/c1-2-28-12-23-17-18(24-16-5-3-4-13(10-16)11-21)26-20(27-19(17)28)25-15-8-6-14(22)7-9-15/h3-5,10,12,14-15H,2,6-9,22H2,1H3,(H2,24,25,26,27)/t14-,15- | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 85 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory activity against cyclin-dependent kinase 1 (CDK1) was determined |
Bioorg Med Chem Lett 9: 91-6 (1999)
BindingDB Entry DOI: 10.7270/Q24Q7VHT |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 1
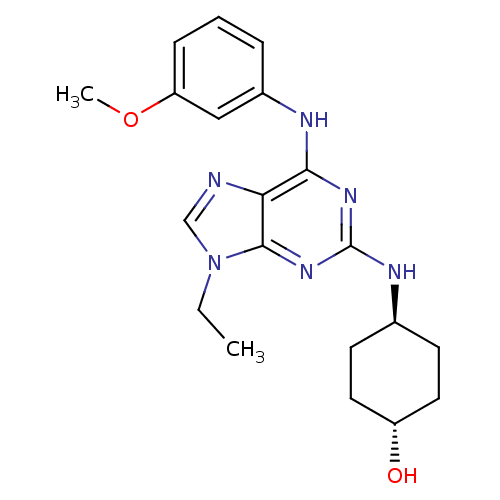
(Homo sapiens (Human)) | BDBM50073787
(4-[9-Ethyl-6-(3-methoxy-phenylamino)-9H-purin-2-yl...)Show SMILES CCn1cnc2c(Nc3cccc(OC)c3)nc(N[C@H]3CC[C@H](O)CC3)nc12 |wU:19.19,wD:22.23,(7.96,-5.98,;6.45,-5.65,;5.98,-4.18,;6.88,-2.95,;5.98,-1.71,;4.53,-2.18,;3.2,-1.42,;3.2,.12,;4.53,.89,;5.86,.12,;7.19,.89,;7.19,2.44,;5.86,3.21,;5.86,4.74,;4.53,5.52,;4.53,2.43,;1.87,-2.18,;1.85,-3.72,;.52,-4.49,;-.81,-3.72,;-2.14,-4.49,;-3.47,-3.72,;-3.47,-2.18,;-4.8,-1.4,;-2.12,-1.41,;-.81,-2.18,;3.2,-4.5,;4.53,-3.72,)| Show InChI InChI=1S/C20H26N6O2/c1-3-26-12-21-17-18(22-14-5-4-6-16(11-14)28-2)24-20(25-19(17)26)23-13-7-9-15(27)10-8-13/h4-6,11-13,15,27H,3,7-10H2,1-2H3,(H2,22,23,24,25)/t13-,15- | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory activity against cyclin-dependent kinase 1 (CDK1) was determined |
Bioorg Med Chem Lett 9: 91-6 (1999)
BindingDB Entry DOI: 10.7270/Q24Q7VHT |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 1
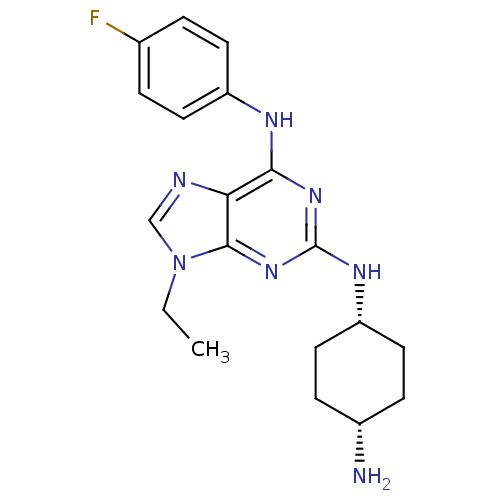
(Homo sapiens (Human)) | BDBM50073757
(CHEMBL173619 | N*2*-(4-Amino-cyclohexyl)-9-ethyl-N...)Show SMILES CCn1cnc2c(Nc3ccc(F)cc3)nc(N[C@@H]3CC[C@H](N)CC3)nc12 |wU:18.18,21.22,(7.96,-5.98,;6.45,-5.65,;5.98,-4.18,;6.88,-2.95,;5.98,-1.71,;4.53,-2.18,;3.2,-1.42,;3.2,.12,;4.53,.89,;5.86,.12,;7.19,.89,;7.19,2.44,;8.52,3.21,;5.86,3.21,;4.53,2.43,;1.87,-2.18,;1.85,-3.72,;.52,-4.49,;-.81,-3.72,;-.81,-2.18,;-2.12,-1.41,;-3.47,-2.18,;-4.8,-1.4,;-3.47,-3.72,;-2.14,-4.49,;3.2,-4.5,;4.53,-3.72,)| Show InChI InChI=1S/C19H24FN7/c1-2-27-11-22-16-17(23-14-7-3-12(20)4-8-14)25-19(26-18(16)27)24-15-9-5-13(21)6-10-15/h3-4,7-8,11,13,15H,2,5-6,9-10,21H2,1H3,(H2,23,24,25,26)/t13-,15+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory activity against cyclin-dependent kinase 1 (CDK1) was determined |
Bioorg Med Chem Lett 9: 91-6 (1999)
BindingDB Entry DOI: 10.7270/Q24Q7VHT |
More data for this
Ligand-Target Pair | |
A5LHX3/O14818/P20618/P25786/P25787/P25788/P25789/P28062/P28065/P28066/P28070/P28072/P28074/P40306/P49720/P49721/P60900/Q8TAA3/Q99436
(Homo sapiens (Human)) | BDBM50469763
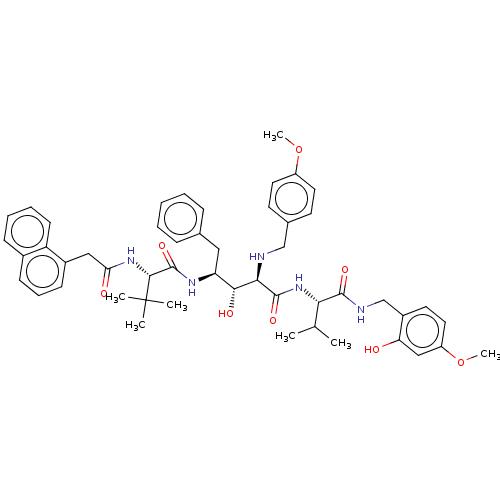
(CHEMBL279444)Show SMILES COc1ccc(CN[C@H]([C@H](O)[C@H](Cc2ccccc2)NC(=O)[C@@H](NC(=O)Cc2cccc3ccccc23)C(C)(C)C)C(=O)N[C@@H](C(C)C)C(=O)NCc2ccc(OC)cc2O)cc1 Show InChI InChI=1S/C50H61N5O8/c1-31(2)43(47(59)52-30-36-22-25-38(63-7)28-41(36)56)55-48(60)44(51-29-33-20-23-37(62-6)24-21-33)45(58)40(26-32-14-9-8-10-15-32)53-49(61)46(50(3,4)5)54-42(57)27-35-18-13-17-34-16-11-12-19-39(34)35/h8-25,28,31,40,43-46,51,56,58H,26-27,29-30H2,1-7H3,(H,52,59)(H,53,61)(H,54,57)(H,55,60)/t40-,43-,44+,45+,46+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma Inc.
Curated by ChEMBL
| Assay Description
Tested in vitro for inhibition of chymotrypsin like activity of purified human 20S proteasome |
Bioorg Med Chem Lett 12: 1331-4 (2002)
Article DOI: 10.1016/s0960-894x(02)00178-6
BindingDB Entry DOI: 10.7270/Q27D2XV2 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM50290422

(4-(4-Methyl-piperazin-1-ylmethyl)-N-[3-(4-pyridin-...)Show SMILES CN1CCN(Cc2ccc(cc2)C(=O)Nc2cccc(Nc3nccc(n3)-c3cccnc3)c2)CC1 Show InChI InChI=1S/C28H29N7O/c1-34-14-16-35(17-15-34)20-21-7-9-22(10-8-21)27(36)31-24-5-2-6-25(18-24)32-28-30-13-11-26(33-28)23-4-3-12-29-19-23/h2-13,18-19H,14-17,20H2,1H3,(H,31,36)(H,30,32,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of v-Abl tyrosine kinase. |
Bioorg Med Chem Lett 7: 187-192 (1997)
Article DOI: 10.1016/S0960-894X(96)00601-4
BindingDB Entry DOI: 10.7270/Q23X86MF |
More data for this
Ligand-Target Pair | |
A5LHX3/O14818/P20618/P25786/P25787/P25788/P25789/P28062/P28065/P28066/P28070/P28072/P28074/P40306/P49720/P49721/P60900/Q8TAA3/Q99436
(Homo sapiens (Human)) | BDBM50469761
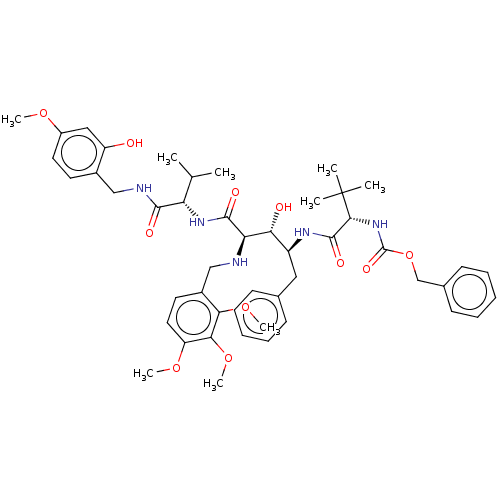
(CHEMBL283263)Show SMILES COc1ccc(CNC(=O)[C@@H](NC(=O)[C@H](NCc2ccc(OC)c(OC)c2OC)[C@H](O)[C@H](Cc2ccccc2)NC(=O)[C@@H](NC(=O)OCc2ccccc2)C(C)(C)C)C(C)C)c(O)c1 Show InChI InChI=1S/C48H63N5O11/c1-29(2)38(44(56)50-26-32-20-22-34(60-6)25-36(32)54)52-45(57)39(49-27-33-21-23-37(61-7)42(63-9)41(33)62-8)40(55)35(24-30-16-12-10-13-17-30)51-46(58)43(48(3,4)5)53-47(59)64-28-31-18-14-11-15-19-31/h10-23,25,29,35,38-40,43,49,54-55H,24,26-28H2,1-9H3,(H,50,56)(H,51,58)(H,52,57)(H,53,59)/t35-,38-,39+,40+,43+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma Inc.
Curated by ChEMBL
| Assay Description
Tested in vitro for inhibition of chymotrypsin like activity of purified human 20S proteasome |
Bioorg Med Chem Lett 12: 1331-4 (2002)
Article DOI: 10.1016/s0960-894x(02)00178-6
BindingDB Entry DOI: 10.7270/Q27D2XV2 |
More data for this
Ligand-Target Pair | |
Platelet-derived growth factor receptor alpha/beta
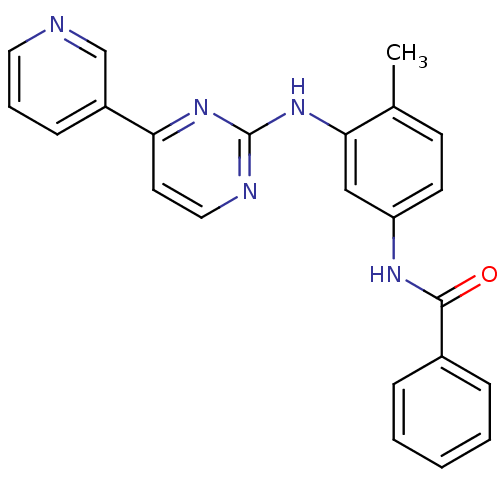
(Homo sapiens (Human)) | BDBM50240407
(CHEMBL20926 | N-(4-methyl-3-(4-(pyridin-3-yl)pyrim...)Show SMILES Cc1ccc(NC(=O)c2ccccc2)cc1Nc1nccc(n1)-c1cccnc1 Show InChI InChI=1S/C23H19N5O/c1-16-9-10-19(26-22(29)17-6-3-2-4-7-17)14-21(16)28-23-25-13-11-20(27-23)18-8-5-12-24-15-18/h2-15H,1H3,(H,26,29)(H,25,27,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of the platelet-derived growth factor receptor. |
Bioorg Med Chem Lett 7: 187-192 (1997)
Article DOI: 10.1016/S0960-894X(96)00601-4
BindingDB Entry DOI: 10.7270/Q23X86MF |
More data for this
Ligand-Target Pair | |
Platelet-derived growth factor receptor alpha/beta
(Homo sapiens (Human)) | BDBM50240407

(CHEMBL20926 | N-(4-methyl-3-(4-(pyridin-3-yl)pyrim...)Show SMILES Cc1ccc(NC(=O)c2ccccc2)cc1Nc1nccc(n1)-c1cccnc1 Show InChI InChI=1S/C23H19N5O/c1-16-9-10-19(26-22(29)17-6-3-2-4-7-17)14-21(16)28-23-25-13-11-20(27-23)18-8-5-12-24-15-18/h2-15H,1H3,(H,26,29)(H,25,27,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of tyrosine kinase(PDGF-R) |
Bioorg Med Chem Lett 6: 1221-1226 (1996)
Article DOI: 10.1016/0960-894X(96)00197-7
BindingDB Entry DOI: 10.7270/Q2348KBG |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 1
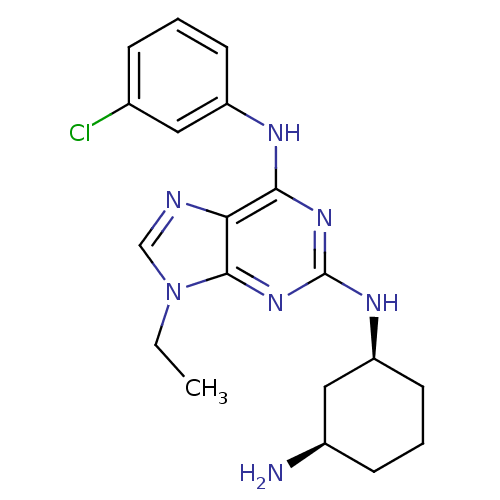
(Homo sapiens (Human)) | BDBM50073761
(CHEMBL368278 | N*2*-((1S,3R)-3-Amino-cyclohexyl)-N...)Show SMILES CCn1cnc2c(Nc3cccc(Cl)c3)nc(N[C@H]3CCC[C@@H](N)C3)nc12 Show InChI InChI=1S/C19H24ClN7/c1-2-27-11-22-16-17(23-14-7-3-5-12(20)9-14)25-19(26-18(16)27)24-15-8-4-6-13(21)10-15/h3,5,7,9,11,13,15H,2,4,6,8,10,21H2,1H3,(H2,23,24,25,26)/t13-,15+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory activity against cyclin-dependent kinase 1 (CDK1) was determined |
Bioorg Med Chem Lett 9: 91-6 (1999)
BindingDB Entry DOI: 10.7270/Q24Q7VHT |
More data for this
Ligand-Target Pair | |
Acyl-protein thioesterase 2
(Homo sapiens (Human)) | BDBM50282567
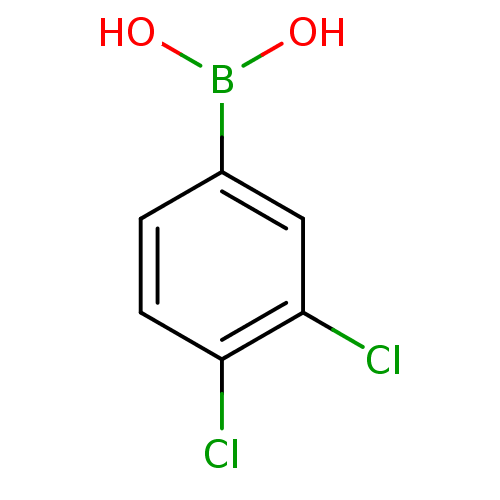
(3,4-Dichlorophenyl boronic acid | 3,4-dichloro ben...)Show InChI InChI=1S/C6H5BCl2O2/c8-5-2-1-4(7(10)11)3-6(5)9/h1-3,10-11H | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 138 | n/a | n/a | n/a | n/a | n/a | n/a |
Technical University of Dortmund
| Assay Description
The enzyme activities were determined by measuring the release of fluorescent 6,8-difluoro-4-methylumbelliferone (DiFMU) by the APT hydrolysis of DiF... |
Chembiochem 14: 115-22 (2013)
Article DOI: 10.1002/cbic.201200571
BindingDB Entry DOI: 10.7270/Q2NS0SG7 |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 1
(Homo sapiens (Human)) | BDBM50073790
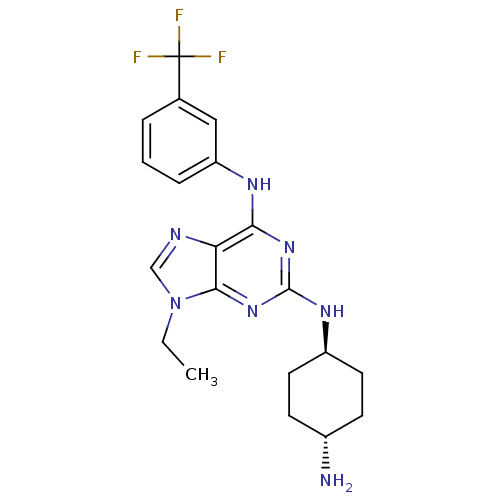
(CHEMBL173608 | N*2*-(4-Amino-cyclohexyl)-9-ethyl-N...)Show SMILES CCn1cnc2c(Nc3cccc(c3)C(F)(F)F)nc(N[C@H]3CC[C@H](N)CC3)nc12 |wU:21.21,wD:24.25,(7.96,-5.98,;6.45,-5.65,;5.98,-4.18,;6.88,-2.95,;5.98,-1.71,;4.53,-2.18,;3.2,-1.42,;3.2,.12,;4.53,.89,;5.86,.12,;7.19,.89,;7.19,2.44,;5.86,3.21,;4.53,2.43,;5.84,4.74,;5.84,6.15,;7.09,5.76,;4.6,5.76,;1.87,-2.18,;1.85,-3.72,;.52,-4.49,;-.81,-3.72,;-2.14,-4.49,;-3.47,-3.72,;-3.47,-2.18,;-4.8,-1.4,;-2.12,-1.41,;-.81,-2.18,;3.2,-4.5,;4.53,-3.72,)| Show InChI InChI=1S/C20H24F3N7/c1-2-30-11-25-16-17(26-15-5-3-4-12(10-15)20(21,22)23)28-19(29-18(16)30)27-14-8-6-13(24)7-9-14/h3-5,10-11,13-14H,2,6-9,24H2,1H3,(H2,26,27,28,29)/t13-,14- | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory activity against cyclin-dependent kinase 1 (CDK1) was determined |
Bioorg Med Chem Lett 9: 91-6 (1999)
BindingDB Entry DOI: 10.7270/Q24Q7VHT |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 1
(Homo sapiens (Human)) | BDBM50073784
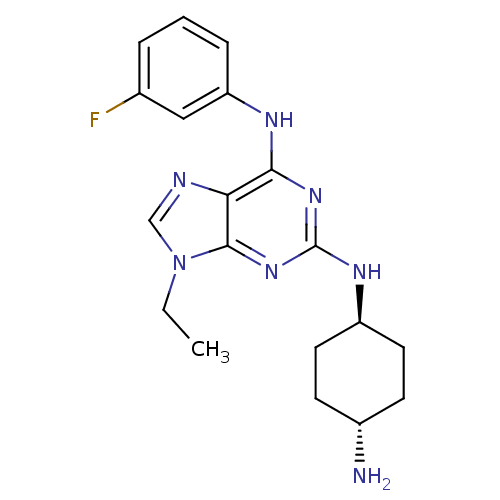
(CHEMBL366642 | N*2*-(4-Amino-cyclohexyl)-9-ethyl-N...)Show SMILES CCn1cnc2c(Nc3cccc(F)c3)nc(N[C@H]3CC[C@H](N)CC3)nc12 |wU:18.18,wD:21.22,(7.96,-5.98,;6.45,-5.65,;5.98,-4.18,;6.88,-2.95,;5.98,-1.71,;4.53,-2.18,;3.2,-1.42,;3.2,.12,;4.53,.89,;5.86,.12,;7.19,.89,;7.19,2.44,;5.86,3.21,;5.86,4.75,;4.53,2.43,;1.87,-2.18,;1.85,-3.72,;.52,-4.49,;-.81,-3.72,;-2.14,-4.49,;-3.47,-3.72,;-3.47,-2.18,;-4.8,-1.4,;-2.12,-1.41,;-.81,-2.18,;3.2,-4.5,;4.53,-3.72,)| Show InChI InChI=1S/C19H24FN7/c1-2-27-11-22-16-17(23-15-5-3-4-12(20)10-15)25-19(26-18(16)27)24-14-8-6-13(21)7-9-14/h3-5,10-11,13-14H,2,6-9,21H2,1H3,(H2,23,24,25,26)/t13-,14- | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory activity against cyclin-dependent kinase 1 (CDK1) was determined |
Bioorg Med Chem Lett 9: 91-6 (1999)
BindingDB Entry DOI: 10.7270/Q24Q7VHT |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data