

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM50379086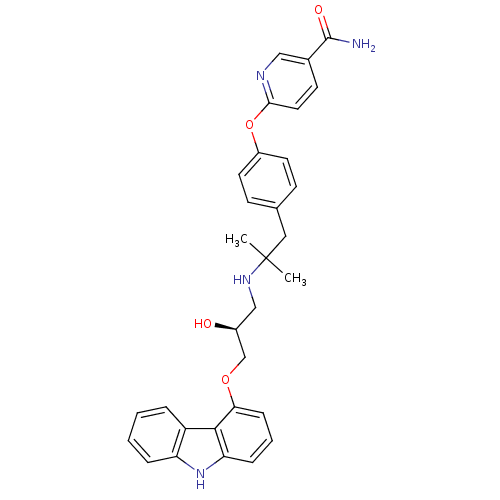 (CHEMBL2012521 | CHEMBL2012522 | LY-377604) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 0.0120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [125I]Iodocyanopindolol from human adrenergic beta2 receptor expressed in insect sf9 cells by scintillation counting | ACS Med Chem Lett 2: 583-586 (2011) Article DOI: 10.1021/ml200071k BindingDB Entry DOI: 10.7270/Q20R9QDP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-1 adrenergic receptor (Homo sapiens (Human)) | BDBM50379086 (CHEMBL2012521 | CHEMBL2012522 | LY-377604) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 0.0230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [125I]Iodocyanopindolol from human adrenergic beta1 receptor expressed in insect sf9 cells by scintillation counting | ACS Med Chem Lett 2: 583-586 (2011) Article DOI: 10.1021/ml200071k BindingDB Entry DOI: 10.7270/Q20R9QDP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vitamin D3 receptor (Homo sapiens (Human)) | BDBM50200182 ((1S,3R,5Z,7E)-9,10-secocholesta-5,7,10(19)-triene-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University Curated by ChEMBL | Assay Description Binding affinity to VDR ligand binding domain by fluorescence polarization competition assay | Bioorg Med Chem 18: 4119-37 (2010) Article DOI: 10.1016/j.bmc.2010.03.078 BindingDB Entry DOI: 10.7270/Q2154J17 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vitamin D3 receptor (Homo sapiens (Human)) | BDBM50320744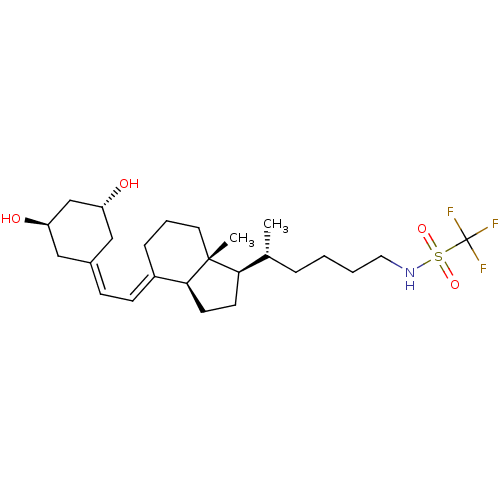 (CHEMBL1164227 | N-((R)-5-((1R,3aS,7aR,E)-4-(2-((3R...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University Curated by ChEMBL | Assay Description Binding affinity to VDR ligand binding domain by fluorescence polarization competition assay | Bioorg Med Chem 18: 4119-37 (2010) Article DOI: 10.1016/j.bmc.2010.03.078 BindingDB Entry DOI: 10.7270/Q2154J17 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vitamin D3 receptor (Homo sapiens (Human)) | BDBM50320746 (CHEMBL1165164 | N-((R)-5-((1R,3aS,7aR,E)-4-(2-((3R...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University Curated by ChEMBL | Assay Description Binding affinity to VDR ligand binding domain by fluorescence polarization competition assay | Bioorg Med Chem 18: 4119-37 (2010) Article DOI: 10.1016/j.bmc.2010.03.078 BindingDB Entry DOI: 10.7270/Q2154J17 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM50365264 (CHEMBL1738926) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 15.5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D Curated by ChEMBL | Assay Description Displacement of tetra-acetylated H4 peptide from human Brd4 bromodomain BD12 after 1 hr by FRET analysis | J Med Chem 54: 3827-38 (2011) Article DOI: 10.1021/jm200108t BindingDB Entry DOI: 10.7270/Q2GB2541 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Bromodomain-containing protein 3 (Homo sapiens (Human)) | BDBM50365264 (CHEMBL1738926) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 28.4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D Curated by ChEMBL | Assay Description Displacement of tetra-acetylated H4 peptide from human Brd3 bromodomain BD12 after 1 hr by FRET analysis | J Med Chem 54: 3827-38 (2011) Article DOI: 10.1021/jm200108t BindingDB Entry DOI: 10.7270/Q2GB2541 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vitamin D3 receptor (Homo sapiens (Human)) | BDBM50320745 (CHEMBL1165082 | N-((R)-5-((1R,3aS,7aR,E)-4-(2-((3R...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University Curated by ChEMBL | Assay Description Binding affinity to VDR ligand binding domain by fluorescence polarization competition assay | Bioorg Med Chem 18: 4119-37 (2010) Article DOI: 10.1016/j.bmc.2010.03.078 BindingDB Entry DOI: 10.7270/Q2154J17 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 2 (Homo sapiens (Human)) | BDBM50365264 (CHEMBL1738926) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 29.9 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D Curated by ChEMBL | Assay Description Displacement of tetra-acetylated H4 peptide from human Brd2 bromodomain BD12 after 1 hr by FRET analysis | J Med Chem 54: 3827-38 (2011) Article DOI: 10.1021/jm200108t BindingDB Entry DOI: 10.7270/Q2GB2541 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 2 (Homo sapiens (Human)) | BDBM50365463 (CHEMBL1232461) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 32.5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D Curated by ChEMBL | Assay Description Displacement of tetra-acetylated H4 peptide from human Brd2 bromodomain BD12 after 1 hr by FRET analysis | J Med Chem 54: 3827-38 (2011) Article DOI: 10.1021/jm200108t BindingDB Entry DOI: 10.7270/Q2GB2541 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 3 (Homo sapiens (Human)) | BDBM19149 (CHEMBL98 | N-hydroxy-N'-phenyloctanediamide | SAHA...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank US Patent | n/a | n/a | 33.8 | n/a | n/a | n/a | n/a | 8.1 | 37 |
The Royal Institution for the Advancement of Learning/McGill University US Patent | Assay Description Boc-Lys(Ac)-7-amino-4-methylcoumarin (Boc-Lys(Ac)-AMC) was used as substrate for the HDAC assays. Substrate solution was prepared as follow: Boc(Lys-... | US Patent US9428447 (2016) BindingDB Entry DOI: 10.7270/Q2TX3D9H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vitamin D3 receptor (Homo sapiens (Human)) | BDBM50320738 (CHEMBL1164212 | N-((R)-5-((1R,3aS,7aR,E)-4-(2-((3R...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University Curated by ChEMBL | Assay Description Binding affinity to VDR ligand binding domain by fluorescence polarization competition assay | Bioorg Med Chem 18: 4119-37 (2010) Article DOI: 10.1016/j.bmc.2010.03.078 BindingDB Entry DOI: 10.7270/Q2154J17 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM50365463 (CHEMBL1232461) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 36.1 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D Curated by ChEMBL | Assay Description Displacement of tetra-acetylated H4 peptide from human Brd4 bromodomain BD12 after 1 hr by FRET analysis | J Med Chem 54: 3827-38 (2011) Article DOI: 10.1021/jm200108t BindingDB Entry DOI: 10.7270/Q2GB2541 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Bromodomain-containing protein 3 (Homo sapiens (Human)) | BDBM50365463 (CHEMBL1232461) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 42.4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D Curated by ChEMBL | Assay Description Displacement of tetra-acetylated H4 peptide from human Brd3 bromodomain BD12 after 1 hr by FRET analysis | J Med Chem 54: 3827-38 (2011) Article DOI: 10.1021/jm200108t BindingDB Entry DOI: 10.7270/Q2GB2541 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vitamin D3 receptor (Homo sapiens (Human)) | BDBM50320735 (CHEMBL1164992 | triciferol) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 87 | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University Curated by ChEMBL | Assay Description Binding affinity to VDR ligand binding domain by fluorescence polarization competition assay | Bioorg Med Chem 18: 4119-37 (2010) Article DOI: 10.1016/j.bmc.2010.03.078 BindingDB Entry DOI: 10.7270/Q2154J17 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vitamin D3 receptor (Homo sapiens (Human)) | BDBM50329614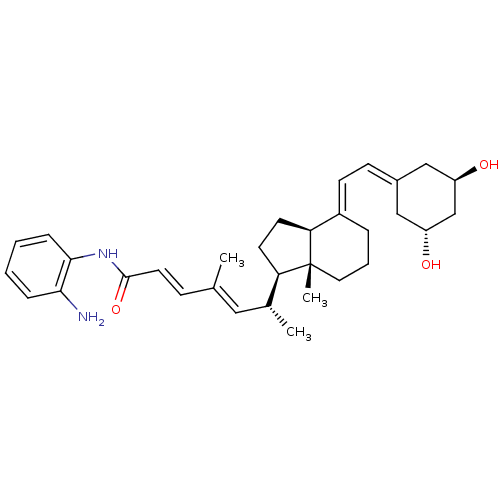 ((6R)-N-(2-aminophenyl)-6-((1R,7aR)-4-(2-((3R,5S)-3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 107 | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University Curated by ChEMBL | Assay Description Binding affinity to VDR-LBD by fluorescence polarization competition assay | J Med Chem 53: 7461-5 (2010) Article DOI: 10.1021/jm1007159 BindingDB Entry DOI: 10.7270/Q2V9889V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 3 (Homo sapiens (Human)) | BDBM19149 (CHEMBL98 | N-hydroxy-N'-phenyloctanediamide | SAHA...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University Curated by ChEMBL | Assay Description Inhibition of HDAC3 after 10 mins by fluorometric assay | Bioorg Med Chem 18: 4119-37 (2010) Article DOI: 10.1016/j.bmc.2010.03.078 BindingDB Entry DOI: 10.7270/Q2154J17 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vitamin D3 receptor (Homo sapiens (Human)) | BDBM50320737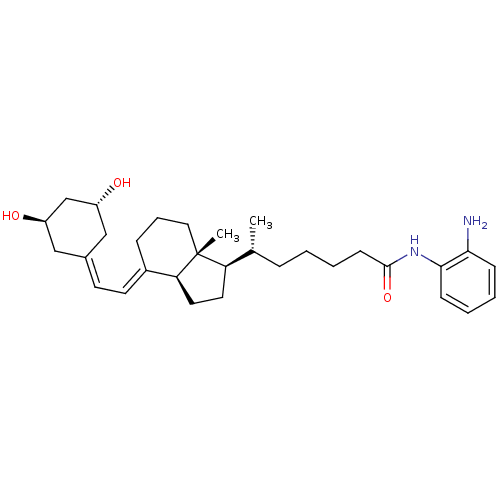 ((R)-N-(2-Aminophenyl)-6-((1R,3aS,7aR,E)-4-(2-((3R,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 185 | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University Curated by ChEMBL | Assay Description Binding affinity to VDR ligand binding domain by fluorescence polarization competition assay | Bioorg Med Chem 18: 4119-37 (2010) Article DOI: 10.1016/j.bmc.2010.03.078 BindingDB Entry DOI: 10.7270/Q2154J17 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vitamin D3 receptor (Homo sapiens (Human)) | BDBM50320748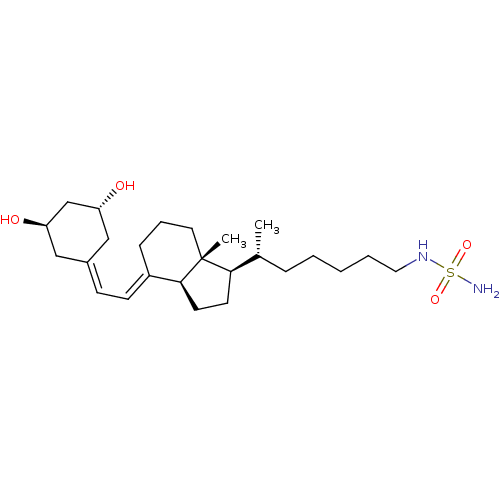 (CHEMBL1164243 | N-((R)-5-((1R,3aS,7aR,E)-4-(2-((3R...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 196 | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University Curated by ChEMBL | Assay Description Binding affinity to VDR ligand binding domain by fluorescence polarization competition assay | Bioorg Med Chem 18: 4119-37 (2010) Article DOI: 10.1016/j.bmc.2010.03.078 BindingDB Entry DOI: 10.7270/Q2154J17 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 2 (Homo sapiens (Human)) | BDBM19149 (CHEMBL98 | N-hydroxy-N'-phenyloctanediamide | SAHA...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | n/a | n/a | 210 | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University Curated by ChEMBL | Assay Description Inhibition of HDAC2 after 10 mins by fluorometric assay | Bioorg Med Chem 18: 4119-37 (2010) Article DOI: 10.1016/j.bmc.2010.03.078 BindingDB Entry DOI: 10.7270/Q2154J17 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Vitamin D3 receptor (Homo sapiens (Human)) | BDBM50320742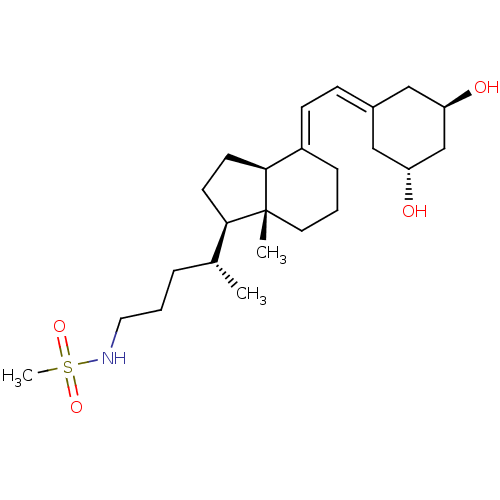 (CHEMBL1164225 | N-((R)-4-((1R,3aS,7aR,E)-4-(2-((3R...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 213 | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University Curated by ChEMBL | Assay Description Binding affinity to VDR ligand binding domain by fluorescence polarization competition assay | Bioorg Med Chem 18: 4119-37 (2010) Article DOI: 10.1016/j.bmc.2010.03.078 BindingDB Entry DOI: 10.7270/Q2154J17 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vitamin D3 receptor (Homo sapiens (Human)) | BDBM50320736 ((R)-5-((1R,3aS,7aR,E)-4-(2-((3R,5R)-3,5-Dihydroxyc...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 248 | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University Curated by ChEMBL | Assay Description Binding affinity to VDR ligand binding domain by fluorescence polarization competition assay | Bioorg Med Chem 18: 4119-37 (2010) Article DOI: 10.1016/j.bmc.2010.03.078 BindingDB Entry DOI: 10.7270/Q2154J17 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vitamin D3 receptor (Homo sapiens (Human)) | BDBM50320737 ((R)-N-(2-Aminophenyl)-6-((1R,3aS,7aR,E)-4-(2-((3R,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 248 | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University Curated by ChEMBL | Assay Description Binding affinity to VDR-LBD by fluorescence polarization competition assay | J Med Chem 53: 7461-5 (2010) Article DOI: 10.1021/jm1007159 BindingDB Entry DOI: 10.7270/Q2V9889V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vitamin D3 receptor (Homo sapiens (Human)) | BDBM50320747 (CHEMBL1164241 | N-((R)-5-((1R,3aS,7aR,E)-4-(2-((3R...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 280 | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University Curated by ChEMBL | Assay Description Binding affinity to VDR ligand binding domain by fluorescence polarization competition assay | Bioorg Med Chem 18: 4119-37 (2010) Article DOI: 10.1016/j.bmc.2010.03.078 BindingDB Entry DOI: 10.7270/Q2154J17 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vitamin D3 receptor (Homo sapiens (Human)) | BDBM50320743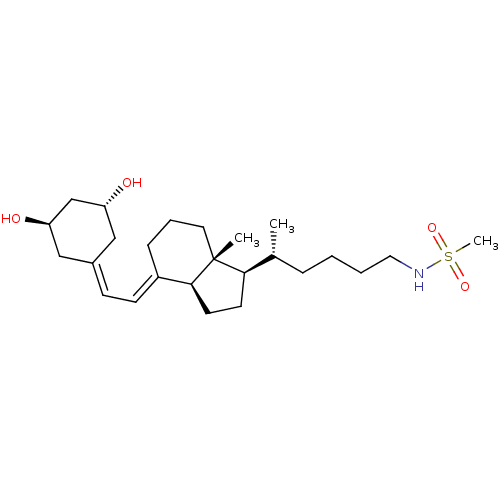 (CHEMBL1164226 | N-((R)-5-((1R,3aS,7aR,E)-4-(2-((3R...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 321 | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University Curated by ChEMBL | Assay Description Binding affinity to VDR ligand binding domain by fluorescence polarization competition assay | Bioorg Med Chem 18: 4119-37 (2010) Article DOI: 10.1016/j.bmc.2010.03.078 BindingDB Entry DOI: 10.7270/Q2154J17 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM19149 (CHEMBL98 | N-hydroxy-N'-phenyloctanediamide | SAHA...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | n/a | n/a | 350 | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University Curated by ChEMBL | Assay Description Inhibition of HDAC6 after 10 mins by fluorometric assay | Bioorg Med Chem 18: 4119-37 (2010) Article DOI: 10.1016/j.bmc.2010.03.078 BindingDB Entry DOI: 10.7270/Q2154J17 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vitamin D3 receptor (Homo sapiens (Human)) | BDBM50320741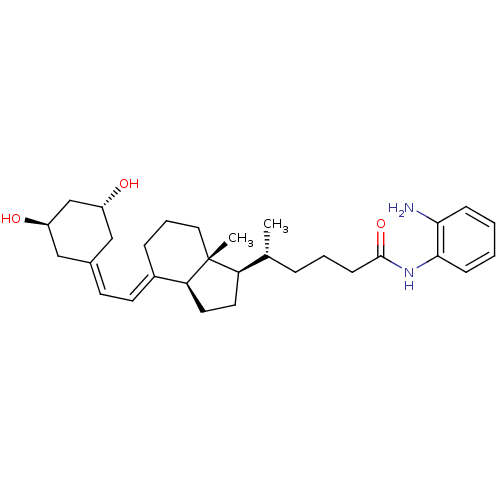 ((R)-N-(2-Aminophenyl)-5-((1R,3aS,7aR,E)-4-(2-((3R,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 524 | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University Curated by ChEMBL | Assay Description Binding affinity to VDR ligand binding domain by fluorescence polarization competition assay | Bioorg Med Chem 18: 4119-37 (2010) Article DOI: 10.1016/j.bmc.2010.03.078 BindingDB Entry DOI: 10.7270/Q2154J17 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM242915 (US9428447, DK-406) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 557 | n/a | n/a | n/a | n/a | 8.1 | 37 |
The Royal Institution for the Advancement of Learning/McGill University US Patent | Assay Description Boc-Lys(Ac)-7-amino-4-methylcoumarin (Boc-Lys(Ac)-AMC) was used as substrate for the HDAC assays. Substrate solution was prepared as follow: Boc(Lys-... | US Patent US9428447 (2016) BindingDB Entry DOI: 10.7270/Q2TX3D9H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM50320735 (CHEMBL1164992 | triciferol) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 580 | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University Curated by ChEMBL | Assay Description Inhibition of HDAC6 after 10 mins by fluorometric assay | Bioorg Med Chem 18: 4119-37 (2010) Article DOI: 10.1016/j.bmc.2010.03.078 BindingDB Entry DOI: 10.7270/Q2154J17 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM19149 (CHEMBL98 | N-hydroxy-N'-phenyloctanediamide | SAHA...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank US Patent | n/a | n/a | 580 | n/a | n/a | n/a | n/a | 8.1 | 37 |
The Royal Institution for the Advancement of Learning/McGill University US Patent | Assay Description Boc-Lys(Ac)-7-amino-4-methylcoumarin (Boc-Lys(Ac)-AMC) was used as substrate for the HDAC assays. Substrate solution was prepared as follow: Boc(Lys-... | US Patent US9428447 (2016) BindingDB Entry DOI: 10.7270/Q2TX3D9H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM242910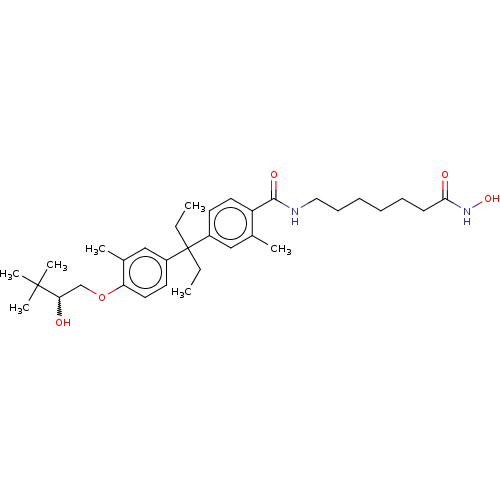 (US9428447, DK-362) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.08E+3 | n/a | n/a | n/a | n/a | 8.1 | 37 |
The Royal Institution for the Advancement of Learning/McGill University US Patent | Assay Description Boc-Lys(Ac)-7-amino-4-methylcoumarin (Boc-Lys(Ac)-AMC) was used as substrate for the HDAC assays. Substrate solution was prepared as follow: Boc(Lys-... | US Patent US9428447 (2016) BindingDB Entry DOI: 10.7270/Q2TX3D9H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM242912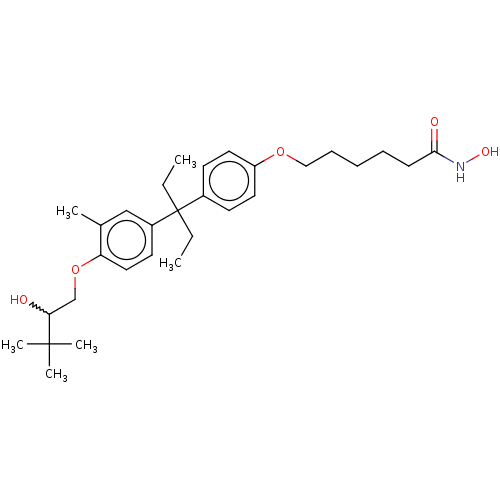 (US9428447, DK-367) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.57E+3 | n/a | n/a | n/a | n/a | 8.1 | 37 |
The Royal Institution for the Advancement of Learning/McGill University US Patent | Assay Description Boc-Lys(Ac)-7-amino-4-methylcoumarin (Boc-Lys(Ac)-AMC) was used as substrate for the HDAC assays. Substrate solution was prepared as follow: Boc(Lys-... | US Patent US9428447 (2016) BindingDB Entry DOI: 10.7270/Q2TX3D9H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM50320738 (CHEMBL1164212 | N-((R)-5-((1R,3aS,7aR,E)-4-(2-((3R...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.75E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University Curated by ChEMBL | Assay Description Inhibition of HDAC6 after 10 mins by fluorometric assay | Bioorg Med Chem 18: 4119-37 (2010) Article DOI: 10.1016/j.bmc.2010.03.078 BindingDB Entry DOI: 10.7270/Q2154J17 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM242914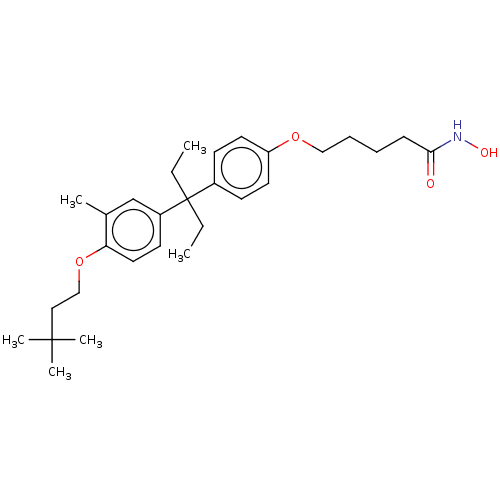 (US9428447, DK-405) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.97E+3 | n/a | n/a | n/a | n/a | 8.1 | 37 |
The Royal Institution for the Advancement of Learning/McGill University US Patent | Assay Description Boc-Lys(Ac)-7-amino-4-methylcoumarin (Boc-Lys(Ac)-AMC) was used as substrate for the HDAC assays. Substrate solution was prepared as follow: Boc(Lys-... | US Patent US9428447 (2016) BindingDB Entry DOI: 10.7270/Q2TX3D9H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM242906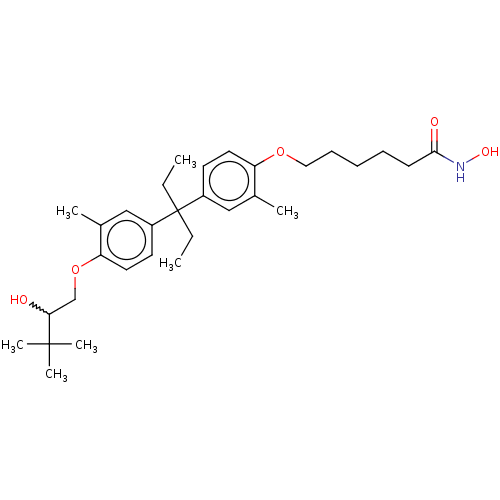 (US9428447, DK-319) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.65E+3 | n/a | n/a | n/a | n/a | 8.1 | 37 |
The Royal Institution for the Advancement of Learning/McGill University US Patent | Assay Description Boc-Lys(Ac)-7-amino-4-methylcoumarin (Boc-Lys(Ac)-AMC) was used as substrate for the HDAC assays. Substrate solution was prepared as follow: Boc(Lys-... | US Patent US9428447 (2016) BindingDB Entry DOI: 10.7270/Q2TX3D9H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM50320736 ((R)-5-((1R,3aS,7aR,E)-4-(2-((3R,5R)-3,5-Dihydroxyc...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University Curated by ChEMBL | Assay Description Inhibition of HDAC6 after 10 mins by fluorometric assay | Bioorg Med Chem 18: 4119-37 (2010) Article DOI: 10.1016/j.bmc.2010.03.078 BindingDB Entry DOI: 10.7270/Q2154J17 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM242913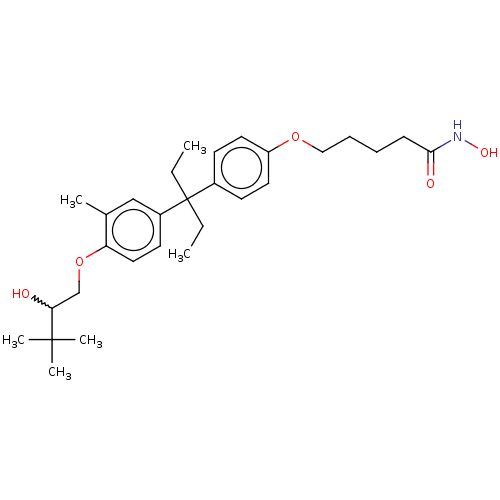 (US9428447, DK-381) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.13E+3 | n/a | n/a | n/a | n/a | 8.1 | 37 |
The Royal Institution for the Advancement of Learning/McGill University US Patent | Assay Description Boc-Lys(Ac)-7-amino-4-methylcoumarin (Boc-Lys(Ac)-AMC) was used as substrate for the HDAC assays. Substrate solution was prepared as follow: Boc(Lys-... | US Patent US9428447 (2016) BindingDB Entry DOI: 10.7270/Q2TX3D9H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 3 (Homo sapiens (Human)) | BDBM50320740 ((R)-5-((1R,3aS,7aR,E)-4-(2-((3R,5R)-3,5-Dihydroxyc...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University Curated by ChEMBL | Assay Description Inhibition of HDAC3 after 10 mins by fluorometric assay | Bioorg Med Chem 18: 4119-37 (2010) Article DOI: 10.1016/j.bmc.2010.03.078 BindingDB Entry DOI: 10.7270/Q2154J17 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM242909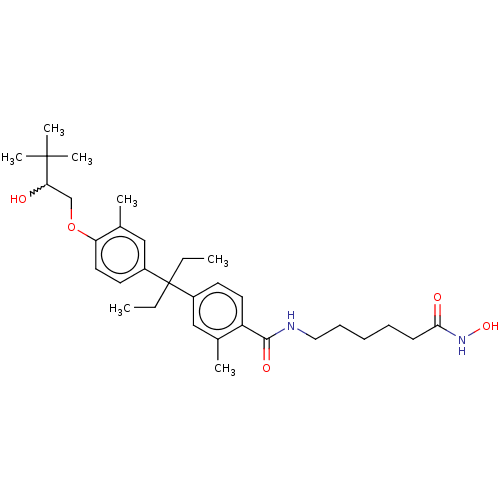 (US9428447, DK-361) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.40E+3 | n/a | n/a | n/a | n/a | 8.1 | 37 |
The Royal Institution for the Advancement of Learning/McGill University US Patent | Assay Description Boc-Lys(Ac)-7-amino-4-methylcoumarin (Boc-Lys(Ac)-AMC) was used as substrate for the HDAC assays. Substrate solution was prepared as follow: Boc(Lys-... | US Patent US9428447 (2016) BindingDB Entry DOI: 10.7270/Q2TX3D9H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM242908 (US9428447, DK-347) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.94E+3 | n/a | n/a | n/a | n/a | 8.1 | 37 |
The Royal Institution for the Advancement of Learning/McGill University US Patent | Assay Description Boc-Lys(Ac)-7-amino-4-methylcoumarin (Boc-Lys(Ac)-AMC) was used as substrate for the HDAC assays. Substrate solution was prepared as follow: Boc(Lys-... | US Patent US9428447 (2016) BindingDB Entry DOI: 10.7270/Q2TX3D9H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM242916 (US9428447, JF-B06) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4.70E+3 | n/a | n/a | n/a | n/a | 8.1 | 37 |
The Royal Institution for the Advancement of Learning/McGill University US Patent | Assay Description Boc-Lys(Ac)-7-amino-4-methylcoumarin (Boc-Lys(Ac)-AMC) was used as substrate for the HDAC assays. Substrate solution was prepared as follow: Boc(Lys-... | US Patent US9428447 (2016) BindingDB Entry DOI: 10.7270/Q2TX3D9H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM242907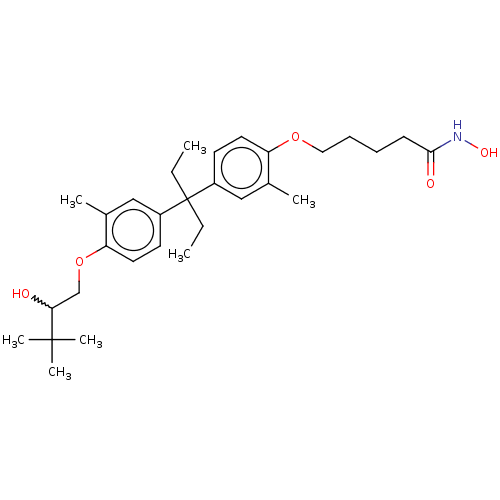 (US9428447, DK-320) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5.03E+3 | n/a | n/a | n/a | n/a | 8.1 | 37 |
The Royal Institution for the Advancement of Learning/McGill University US Patent | Assay Description Boc-Lys(Ac)-7-amino-4-methylcoumarin (Boc-Lys(Ac)-AMC) was used as substrate for the HDAC assays. Substrate solution was prepared as follow: Boc(Lys-... | US Patent US9428447 (2016) BindingDB Entry DOI: 10.7270/Q2TX3D9H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM242911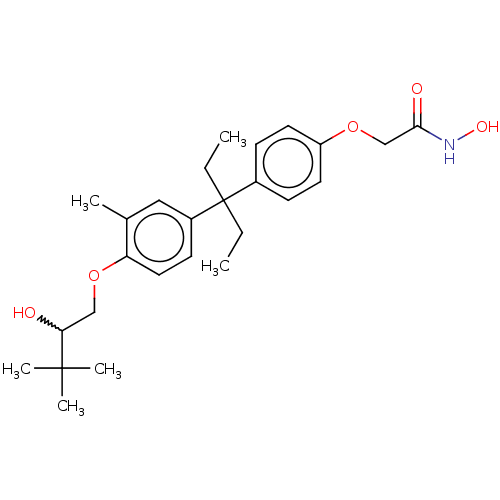 (US9428447, DK-366) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 7.40E+3 | n/a | n/a | n/a | n/a | 8.1 | 37 |
The Royal Institution for the Advancement of Learning/McGill University US Patent | Assay Description Boc-Lys(Ac)-7-amino-4-methylcoumarin (Boc-Lys(Ac)-AMC) was used as substrate for the HDAC assays. Substrate solution was prepared as follow: Boc(Lys-... | US Patent US9428447 (2016) BindingDB Entry DOI: 10.7270/Q2TX3D9H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM50320740 ((R)-5-((1R,3aS,7aR,E)-4-(2-((3R,5R)-3,5-Dihydroxyc...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 9.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University Curated by ChEMBL | Assay Description Inhibition of HDAC6 after 10 mins by fluorometric assay | Bioorg Med Chem 18: 4119-37 (2010) Article DOI: 10.1016/j.bmc.2010.03.078 BindingDB Entry DOI: 10.7270/Q2154J17 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 3 (Homo sapiens (Human)) | BDBM242901 (US9428447, JFD-15) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 9.83E+3 | n/a | n/a | n/a | n/a | 8.1 | 37 |
The Royal Institution for the Advancement of Learning/McGill University US Patent | Assay Description Boc-Lys(Ac)-7-amino-4-methylcoumarin (Boc-Lys(Ac)-AMC) was used as substrate for the HDAC assays. Substrate solution was prepared as follow: Boc(Lys-... | US Patent US9428447 (2016) BindingDB Entry DOI: 10.7270/Q2TX3D9H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 2 (Homo sapiens (Human)) | BDBM50320735 (CHEMBL1164992 | triciferol) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.04E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University Curated by ChEMBL | Assay Description Inhibition of HDAC2 after 10 mins by fluorometric assay | Bioorg Med Chem 18: 4119-37 (2010) Article DOI: 10.1016/j.bmc.2010.03.078 BindingDB Entry DOI: 10.7270/Q2154J17 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 3 (Homo sapiens (Human)) | BDBM50320735 (CHEMBL1164992 | triciferol) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.33E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University Curated by ChEMBL | Assay Description Inhibition of HDAC3 after 10 mins by fluorometric assay | Bioorg Med Chem 18: 4119-37 (2010) Article DOI: 10.1016/j.bmc.2010.03.078 BindingDB Entry DOI: 10.7270/Q2154J17 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 3 (Homo sapiens (Human)) | BDBM50320736 ((R)-5-((1R,3aS,7aR,E)-4-(2-((3R,5R)-3,5-Dihydroxyc...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.45E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University Curated by ChEMBL | Assay Description Inhibition of HDAC3 after 10 mins by fluorometric assay | Bioorg Med Chem 18: 4119-37 (2010) Article DOI: 10.1016/j.bmc.2010.03.078 BindingDB Entry DOI: 10.7270/Q2154J17 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 2 (Homo sapiens (Human)) | BDBM50320736 ((R)-5-((1R,3aS,7aR,E)-4-(2-((3R,5R)-3,5-Dihydroxyc...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.65E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University Curated by ChEMBL | Assay Description Inhibition of HDAC2 after 10 mins by fluorometric assay | Bioorg Med Chem 18: 4119-37 (2010) Article DOI: 10.1016/j.bmc.2010.03.078 BindingDB Entry DOI: 10.7270/Q2154J17 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 3 (Homo sapiens (Human)) | BDBM242899 (US9428447, JF-B53) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.70E+4 | n/a | n/a | n/a | n/a | 8.1 | 37 |
The Royal Institution for the Advancement of Learning/McGill University US Patent | Assay Description Boc-Lys(Ac)-7-amino-4-methylcoumarin (Boc-Lys(Ac)-AMC) was used as substrate for the HDAC assays. Substrate solution was prepared as follow: Boc(Lys-... | US Patent US9428447 (2016) BindingDB Entry DOI: 10.7270/Q2TX3D9H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 103 total ) | Next | Last >> |