 Found 308 hits with Last Name = 'humes' and Initial = 'jl'
Found 308 hits with Last Name = 'humes' and Initial = 'jl' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50164777
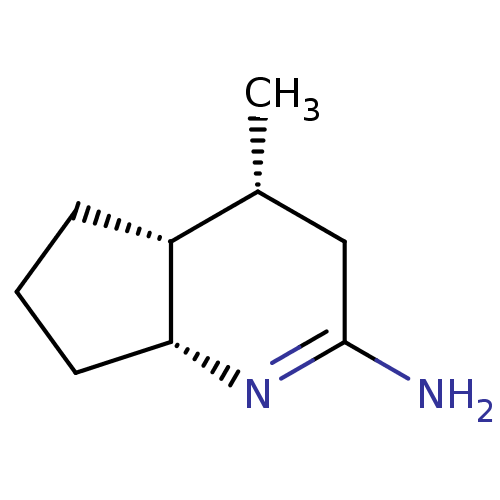
((4R,4aR,7aR)-4-Methyl-octahydro-[1]pyrindin-(2E)-y...)Show InChI InChI=1S/C9H16N2/c1-6-5-9(10)11-8-4-2-3-7(6)8/h6-8H,2-5H2,1H3,(H2,10,11)/t6-,7-,8-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against human Inducible nitric oxide synthase (iNOS) |
Bioorg Med Chem Lett 15: 1997-2001 (2005)
Article DOI: 10.1016/j.bmcl.2005.02.067
BindingDB Entry DOI: 10.7270/Q2XK8F3M |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50164777

((4R,4aR,7aR)-4-Methyl-octahydro-[1]pyrindin-(2E)-y...)Show InChI InChI=1S/C9H16N2/c1-6-5-9(10)11-8-4-2-3-7(6)8/h6-8H,2-5H2,1H3,(H2,10,11)/t6-,7-,8-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against human Inducible nitric oxide synthase (iNOS) |
Bioorg Med Chem Lett 15: 1997-2001 (2005)
Article DOI: 10.1016/j.bmcl.2005.02.067
BindingDB Entry DOI: 10.7270/Q2XK8F3M |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM50062133
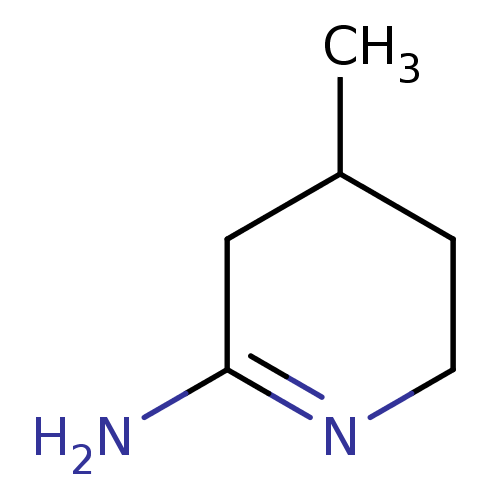
(4-Methyl-piperidin-(2E)-ylideneamine | 4-Methyl-pi...)Show InChI InChI=1S/C6H12N2/c1-5-2-3-8-6(7)4-5/h5H,2-4H2,1H3,(H2,7,8) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against human Neuronal nitric oxide synthase (nNOS) |
Bioorg Med Chem Lett 15: 1997-2001 (2005)
Article DOI: 10.1016/j.bmcl.2005.02.067
BindingDB Entry DOI: 10.7270/Q2XK8F3M |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50062133

(4-Methyl-piperidin-(2E)-ylideneamine | 4-Methyl-pi...)Show InChI InChI=1S/C6H12N2/c1-5-2-3-8-6(7)4-5/h5H,2-4H2,1H3,(H2,7,8) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Rahway
Curated by ChEMBL
| Assay Description
Inhibitory activity against inducible nitric oxide synthase (iNOS) |
Bioorg Med Chem Lett 10: 1975-8 (2001)
BindingDB Entry DOI: 10.7270/Q2319V30 |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50066778
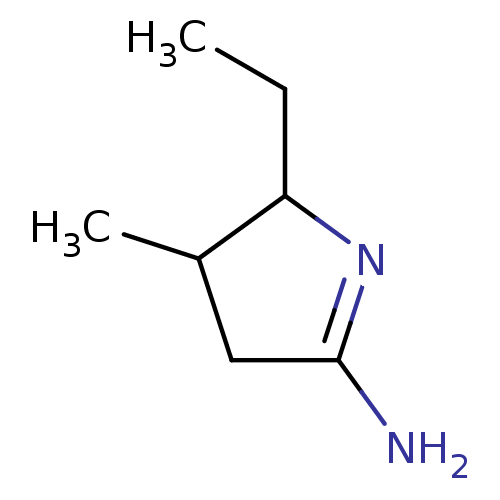
(5-Ethyl-4-methyl-pyrrolidin-(2E)-ylideneamine; hyd...)Show InChI InChI=1S/C7H14N2/c1-3-6-5(2)4-7(8)9-6/h5-6H,3-4H2,1-2H3,(H2,8,9) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratory
Curated by ChEMBL
| Assay Description
Inhibitory activity against Inducible nitric oxide synthase |
Bioorg Med Chem Lett 14: 4539-44 (2004)
Article DOI: 10.1016/j.bmcl.2004.06.033
BindingDB Entry DOI: 10.7270/Q218377V |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50164782

((4R,4aR,5R)-4-Methyl-octahydro-quinolin-(2E)-ylide...)Show InChI InChI=1S/C10H18N2/c1-7-6-10(11)12-9-5-3-2-4-8(7)9/h7-9H,2-6H2,1H3,(H2,11,12)/t7-,8-,9-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against human Inducible nitric oxide synthase (iNOS) |
Bioorg Med Chem Lett 15: 1997-2001 (2005)
Article DOI: 10.1016/j.bmcl.2005.02.067
BindingDB Entry DOI: 10.7270/Q2XK8F3M |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50091801

(6-Isobutyl-4-methyl-pyridin-2-ylamine | 6-isobutyl...)Show InChI InChI=1S/C10H16N2/c1-7(2)4-9-5-8(3)6-10(11)12-9/h5-7H,4H2,1-3H3,(H2,11,12) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Rahway
Curated by ChEMBL
| Assay Description
Inhibitory activity against inducible nitric oxide synthase (iNOS) |
Bioorg Med Chem Lett 10: 1975-8 (2001)
BindingDB Entry DOI: 10.7270/Q2319V30 |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM50164777

((4R,4aR,7aR)-4-Methyl-octahydro-[1]pyrindin-(2E)-y...)Show InChI InChI=1S/C9H16N2/c1-6-5-9(10)11-8-4-2-3-7(6)8/h6-8H,2-5H2,1H3,(H2,10,11)/t6-,7-,8-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against human Neuronal nitric oxide synthase (nNOS) |
Bioorg Med Chem Lett 15: 1997-2001 (2005)
Article DOI: 10.1016/j.bmcl.2005.02.067
BindingDB Entry DOI: 10.7270/Q2XK8F3M |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM50164777

((4R,4aR,7aR)-4-Methyl-octahydro-[1]pyrindin-(2E)-y...)Show InChI InChI=1S/C9H16N2/c1-6-5-9(10)11-8-4-2-3-7(6)8/h6-8H,2-5H2,1H3,(H2,10,11)/t6-,7-,8-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against human Neuronal nitric oxide synthase (nNOS) |
Bioorg Med Chem Lett 15: 1997-2001 (2005)
Article DOI: 10.1016/j.bmcl.2005.02.067
BindingDB Entry DOI: 10.7270/Q2XK8F3M |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM50164784
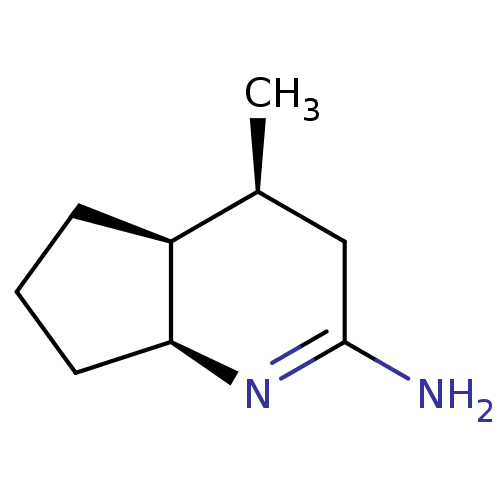
((4S,7S)-4-Methyl-octahydro-[1]pyrindin-(2E)-yliden...)Show InChI InChI=1S/C9H16N2/c1-6-5-9(10)11-8-4-2-3-7(6)8/h6-8H,2-5H2,1H3,(H2,10,11)/t6-,7-,8-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against human Neuronal nitric oxide synthase (nNOS) |
Bioorg Med Chem Lett 15: 1997-2001 (2005)
Article DOI: 10.1016/j.bmcl.2005.02.067
BindingDB Entry DOI: 10.7270/Q2XK8F3M |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50062133

(4-Methyl-piperidin-(2E)-ylideneamine | 4-Methyl-pi...)Show InChI InChI=1S/C6H12N2/c1-5-2-3-8-6(7)4-5/h5H,2-4H2,1H3,(H2,7,8) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against human Inducible nitric oxide synthase (iNOS) |
Bioorg Med Chem Lett 15: 1997-2001 (2005)
Article DOI: 10.1016/j.bmcl.2005.02.067
BindingDB Entry DOI: 10.7270/Q2XK8F3M |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50164779
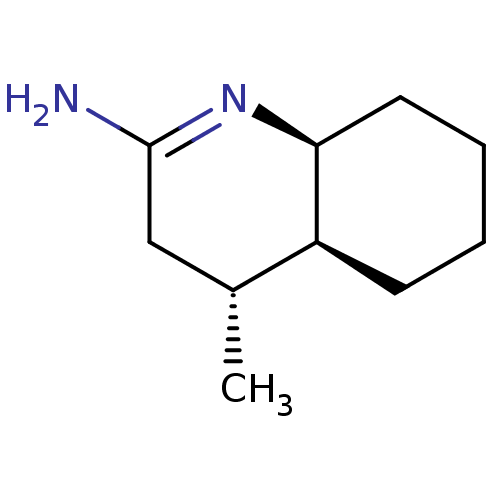
((4R,4aS,5S)-4-Methyl-octahydro-quinolin-(2E)-ylide...)Show InChI InChI=1S/C10H18N2/c1-7-6-10(11)12-9-5-3-2-4-8(7)9/h7-9H,2-6H2,1H3,(H2,11,12)/t7-,8+,9+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 38 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against human Inducible nitric oxide synthase (iNOS) |
Bioorg Med Chem Lett 15: 1997-2001 (2005)
Article DOI: 10.1016/j.bmcl.2005.02.067
BindingDB Entry DOI: 10.7270/Q2XK8F3M |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, endothelial
(Homo sapiens (Human)) | BDBM50091805

(2-amino-4,6-dimethylpyridine | 4,6-Dimethyl-pyridi...)Show InChI InChI=1S/C7H10N2/c1-5-3-6(2)9-7(8)4-5/h3-4H,1-2H3,(H2,8,9) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 45 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Rahway
Curated by ChEMBL
| Assay Description
Inhibitory activity against endothelial nitric oxide synthase (eNOS) |
Bioorg Med Chem Lett 10: 1975-8 (2001)
BindingDB Entry DOI: 10.7270/Q2319V30 |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50091811
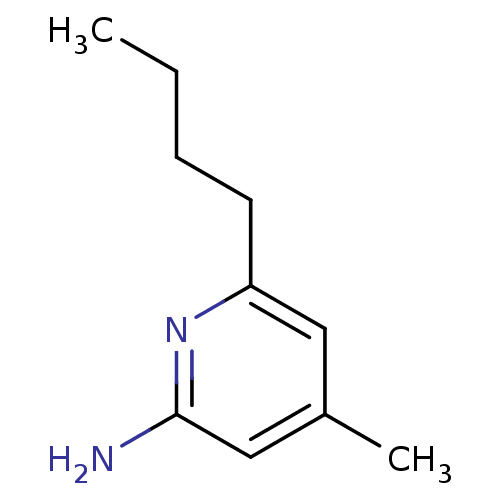
(6-Butyl-4-methyl-pyridin-2-ylamine | CHEMBL292507)Show InChI InChI=1S/C10H16N2/c1-3-4-5-9-6-8(2)7-10(11)12-9/h6-7H,3-5H2,1-2H3,(H2,11,12) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 46 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Rahway
Curated by ChEMBL
| Assay Description
Inhibitory activity against inducible nitric oxide synthase (iNOS) |
Bioorg Med Chem Lett 10: 1975-8 (2001)
BindingDB Entry DOI: 10.7270/Q2319V30 |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, endothelial
(Homo sapiens (Human)) | BDBM50091792
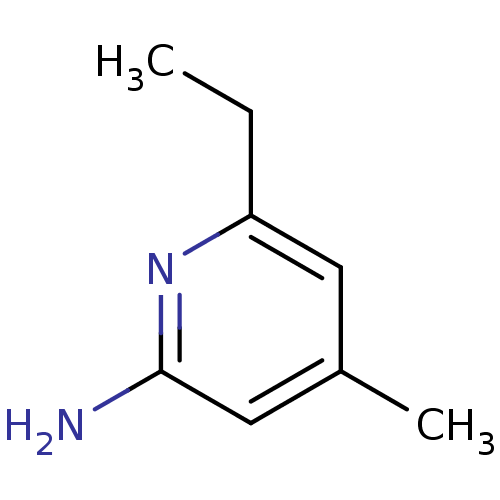
(6-Ethyl-4-methyl-pyridin-2-ylamine | CHEMBL294084)Show InChI InChI=1S/C8H12N2/c1-3-7-4-6(2)5-8(9)10-7/h4-5H,3H2,1-2H3,(H2,9,10) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 49 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Rahway
Curated by ChEMBL
| Assay Description
Inhibitory activity against endothelial nitric oxide synthase (eNOS) |
Bioorg Med Chem Lett 10: 1975-8 (2001)
BindingDB Entry DOI: 10.7270/Q2319V30 |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM50164782

((4R,4aR,5R)-4-Methyl-octahydro-quinolin-(2E)-ylide...)Show InChI InChI=1S/C10H18N2/c1-7-6-10(11)12-9-5-3-2-4-8(7)9/h7-9H,2-6H2,1H3,(H2,11,12)/t7-,8-,9-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 53 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against human Neuronal nitric oxide synthase (nNOS) |
Bioorg Med Chem Lett 15: 1997-2001 (2005)
Article DOI: 10.1016/j.bmcl.2005.02.067
BindingDB Entry DOI: 10.7270/Q2XK8F3M |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50091791
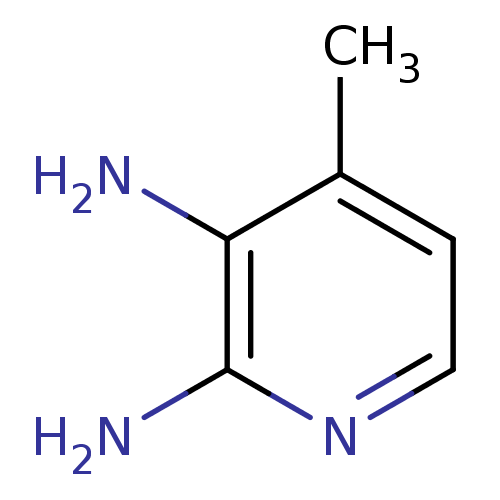
(4-Methyl-pyridine-2,3-diamine | CHEMBL61427)Show InChI InChI=1S/C6H9N3/c1-4-2-3-9-6(8)5(4)7/h2-3H,7H2,1H3,(H2,8,9) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
| PubMed
| n/a | n/a | 59 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Rahway
Curated by ChEMBL
| Assay Description
Inhibitory activity against inducible nitric oxide synthase (iNOS) |
Bioorg Med Chem Lett 10: 1975-8 (2001)
BindingDB Entry DOI: 10.7270/Q2319V30 |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, endothelial
(Homo sapiens (Human)) | BDBM50091800
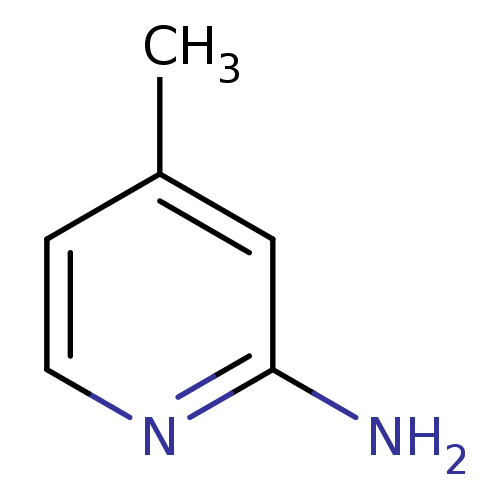
(2-amino-4-methylpyridine | 4-METHYLPYRIDIN-2-AMINE...)Show InChI InChI=1S/C6H8N2/c1-5-2-3-8-6(7)4-5/h2-4H,1H3,(H2,7,8) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 72 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Rahway
Curated by ChEMBL
| Assay Description
Inhibitory activity against endothelial nitric oxide synthase (eNOS) |
Bioorg Med Chem Lett 10: 1975-8 (2001)
BindingDB Entry DOI: 10.7270/Q2319V30 |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM50091800

(2-amino-4-methylpyridine | 4-METHYLPYRIDIN-2-AMINE...)Show InChI InChI=1S/C6H8N2/c1-5-2-3-8-6(7)4-5/h2-4H,1H3,(H2,7,8) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 75 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Rahway
Curated by ChEMBL
| Assay Description
Inhibitory activity against neuronal nitric oxide synthase |
Bioorg Med Chem Lett 10: 1975-8 (2001)
BindingDB Entry DOI: 10.7270/Q2319V30 |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50091809
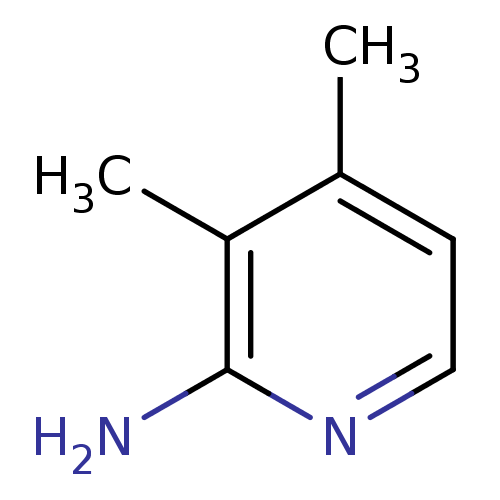
(3,4-Dimethyl-pyridin-2-ylamine | CHEMBL61939)Show InChI InChI=1S/C7H10N2/c1-5-3-4-9-7(8)6(5)2/h3-4H,1-2H3,(H2,8,9) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 76 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Rahway
Curated by ChEMBL
| Assay Description
Inhibitory activity against inducible nitric oxide synthase (iNOS) |
Bioorg Med Chem Lett 10: 1975-8 (2001)
BindingDB Entry DOI: 10.7270/Q2319V30 |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50091803

(4-Methyl-6-(3-methyl-butyl)-pyridin-2-ylamine | CH...)Show InChI InChI=1S/C11H18N2/c1-8(2)4-5-10-6-9(3)7-11(12)13-10/h6-8H,4-5H2,1-3H3,(H2,12,13) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 76 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Rahway
Curated by ChEMBL
| Assay Description
Inhibitory activity against inducible nitric oxide synthase (iNOS) |
Bioorg Med Chem Lett 10: 1975-8 (2001)
BindingDB Entry DOI: 10.7270/Q2319V30 |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM50066778

(5-Ethyl-4-methyl-pyrrolidin-(2E)-ylideneamine; hyd...)Show InChI InChI=1S/C7H14N2/c1-3-6-5(2)4-7(8)9-6/h5-6H,3-4H2,1-2H3,(H2,8,9) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratory
Curated by ChEMBL
| Assay Description
Inhibitory activity against Neuronal nitric oxide synthase |
Bioorg Med Chem Lett 14: 4539-44 (2004)
Article DOI: 10.1016/j.bmcl.2004.06.033
BindingDB Entry DOI: 10.7270/Q218377V |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, endothelial
(Homo sapiens (Human)) | BDBM50091791

(4-Methyl-pyridine-2,3-diamine | CHEMBL61427)Show InChI InChI=1S/C6H9N3/c1-4-2-3-9-6(8)5(4)7/h2-3H,7H2,1H3,(H2,8,9) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
| PubMed
| n/a | n/a | 81 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Rahway
Curated by ChEMBL
| Assay Description
Inhibitory activity against endothelial nitric oxide synthase (eNOS) |
Bioorg Med Chem Lett 10: 1975-8 (2001)
BindingDB Entry DOI: 10.7270/Q2319V30 |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM50091817
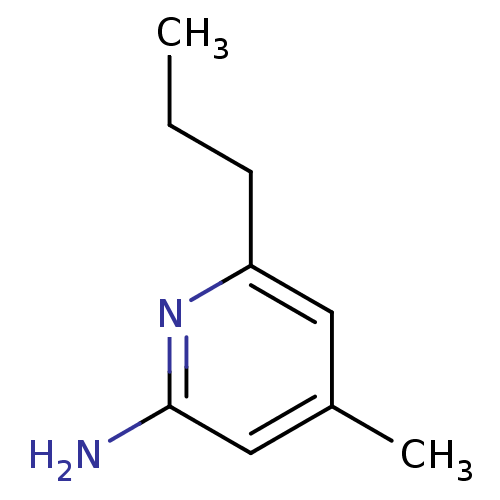
(4-Methyl-6-propyl-pyridin-2-ylamine | 4-Methyl-6-p...)Show InChI InChI=1S/C9H14N2/c1-3-4-8-5-7(2)6-9(10)11-8/h5-6H,3-4H2,1-2H3,(H2,10,11) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Rahway
Curated by ChEMBL
| Assay Description
Inhibitory activity against neuronal nitric oxide synthase |
Bioorg Med Chem Lett 10: 1975-8 (2001)
BindingDB Entry DOI: 10.7270/Q2319V30 |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM50091801

(6-Isobutyl-4-methyl-pyridin-2-ylamine | 6-isobutyl...)Show InChI InChI=1S/C10H16N2/c1-7(2)4-9-5-8(3)6-10(11)12-9/h5-7H,4H2,1-3H3,(H2,11,12) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Rahway
Curated by ChEMBL
| Assay Description
Inhibitory activity against neuronal nitric oxide synthase |
Bioorg Med Chem Lett 10: 1975-8 (2001)
BindingDB Entry DOI: 10.7270/Q2319V30 |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50091805

(2-amino-4,6-dimethylpyridine | 4,6-Dimethyl-pyridi...)Show InChI InChI=1S/C7H10N2/c1-5-3-6(2)9-7(8)4-5/h3-4H,1-2H3,(H2,8,9) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Rahway
Curated by ChEMBL
| Assay Description
Inhibitory activity against inducible nitric oxide synthase (iNOS) |
Bioorg Med Chem Lett 10: 1975-8 (2001)
BindingDB Entry DOI: 10.7270/Q2319V30 |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50091817

(4-Methyl-6-propyl-pyridin-2-ylamine | 4-Methyl-6-p...)Show InChI InChI=1S/C9H14N2/c1-3-4-8-5-7(2)6-9(10)11-8/h5-6H,3-4H2,1-2H3,(H2,10,11) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
PubMed
| n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Rahway
Curated by ChEMBL
| Assay Description
Inhibitory activity against inducible nitric oxide synthase (iNOS) |
Bioorg Med Chem Lett 10: 1975-8 (2001)
BindingDB Entry DOI: 10.7270/Q2319V30 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50091798
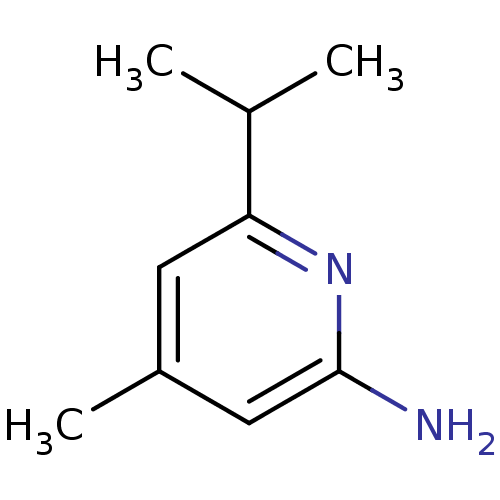
(6-Isopropyl-4-methyl-pyridin-2-ylamine | CHEMBL299...)Show InChI InChI=1S/C9H14N2/c1-6(2)8-4-7(3)5-9(10)11-8/h4-6H,1-3H3,(H2,10,11) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Rahway
Curated by ChEMBL
| Assay Description
Inhibitory activity against inducible nitric oxide synthase (iNOS) |
Bioorg Med Chem Lett 10: 1975-8 (2001)
BindingDB Entry DOI: 10.7270/Q2319V30 |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM50150924
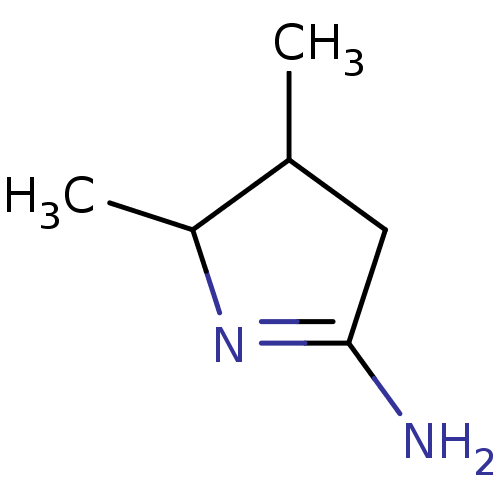
(4,5-Dimethyl-pyrrolidin-(2Z)-ylideneamine | CHEMBL...)Show InChI InChI=1S/C6H12N2/c1-4-3-6(7)8-5(4)2/h4-5H,3H2,1-2H3,(H2,7,8) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratory
Curated by ChEMBL
| Assay Description
Inhibitory activity against Neuronal nitric oxide synthase |
Bioorg Med Chem Lett 14: 4539-44 (2004)
Article DOI: 10.1016/j.bmcl.2004.06.033
BindingDB Entry DOI: 10.7270/Q218377V |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50164784

((4S,7S)-4-Methyl-octahydro-[1]pyrindin-(2E)-yliden...)Show InChI InChI=1S/C9H16N2/c1-6-5-9(10)11-8-4-2-3-7(6)8/h6-8H,2-5H2,1H3,(H2,10,11)/t6-,7-,8-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 133 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against human Inducible nitric oxide synthase (iNOS) |
Bioorg Med Chem Lett 15: 1997-2001 (2005)
Article DOI: 10.1016/j.bmcl.2005.02.067
BindingDB Entry DOI: 10.7270/Q2XK8F3M |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM50150933
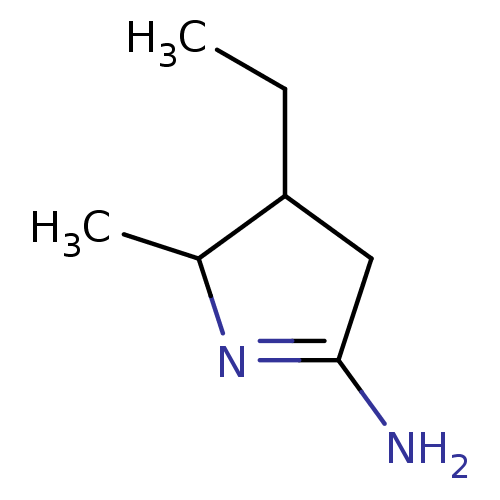
(4-Ethyl-5-methyl-pyrrolidin-(2Z)-ylideneamine | CH...)Show InChI InChI=1S/C7H14N2/c1-3-6-4-7(8)9-5(6)2/h5-6H,3-4H2,1-2H3,(H2,8,9) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratory
Curated by ChEMBL
| Assay Description
Inhibitory activity against Neuronal nitric oxide synthase |
Bioorg Med Chem Lett 14: 4539-44 (2004)
Article DOI: 10.1016/j.bmcl.2004.06.033
BindingDB Entry DOI: 10.7270/Q218377V |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, endothelial
(Homo sapiens (Human)) | BDBM50062133

(4-Methyl-piperidin-(2E)-ylideneamine | 4-Methyl-pi...)Show InChI InChI=1S/C6H12N2/c1-5-2-3-8-6(7)4-5/h5H,2-4H2,1H3,(H2,7,8) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 146 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against human Endothelial nitric oxide synthase (eNOS) |
Bioorg Med Chem Lett 15: 1997-2001 (2005)
Article DOI: 10.1016/j.bmcl.2005.02.067
BindingDB Entry DOI: 10.7270/Q2XK8F3M |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, endothelial
(Homo sapiens (Human)) | BDBM50091809

(3,4-Dimethyl-pyridin-2-ylamine | CHEMBL61939)Show InChI InChI=1S/C7H10N2/c1-5-3-4-9-7(8)6(5)2/h3-4H,1-2H3,(H2,8,9) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Rahway
Curated by ChEMBL
| Assay Description
Inhibitory activity against endothelial nitric oxide synthase (eNOS) |
Bioorg Med Chem Lett 10: 1975-8 (2001)
BindingDB Entry DOI: 10.7270/Q2319V30 |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, endothelial
(Homo sapiens (Human)) | BDBM50091801

(6-Isobutyl-4-methyl-pyridin-2-ylamine | 6-isobutyl...)Show InChI InChI=1S/C10H16N2/c1-7(2)4-9-5-8(3)6-10(11)12-9/h5-7H,4H2,1-3H3,(H2,11,12) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Rahway
Curated by ChEMBL
| Assay Description
Inhibitory activity against endothelial nitric oxide synthase (eNOS) |
Bioorg Med Chem Lett 10: 1975-8 (2001)
BindingDB Entry DOI: 10.7270/Q2319V30 |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM50155790
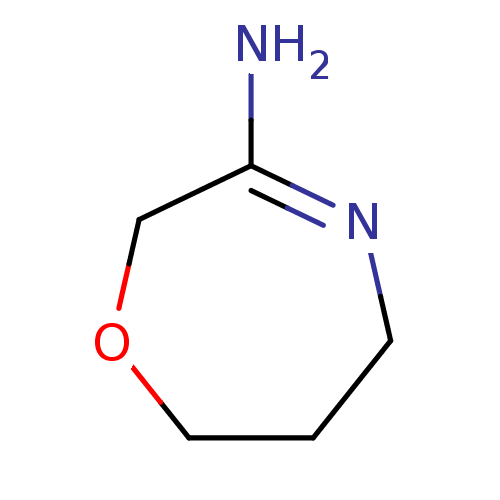
(CHEMBL362772 | [1,4]Oxazepan-(3E)-ylideneamine)Show InChI InChI=1S/C5H10N2O/c6-5-4-8-3-1-2-7-5/h1-4H2,(H2,6,7) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory concentration against Neuronal nitric oxide synthase |
Bioorg Med Chem Lett 14: 5907-11 (2004)
Article DOI: 10.1016/j.bmcl.2004.09.019
BindingDB Entry DOI: 10.7270/Q2JQ10HX |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50155790

(CHEMBL362772 | [1,4]Oxazepan-(3E)-ylideneamine)Show InChI InChI=1S/C5H10N2O/c6-5-4-8-3-1-2-7-5/h1-4H2,(H2,6,7) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory concentration against Inducible nitric oxide synthase |
Bioorg Med Chem Lett 14: 5907-11 (2004)
Article DOI: 10.1016/j.bmcl.2004.09.019
BindingDB Entry DOI: 10.7270/Q2JQ10HX |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50150933

(4-Ethyl-5-methyl-pyrrolidin-(2Z)-ylideneamine | CH...)Show InChI InChI=1S/C7H14N2/c1-3-6-4-7(8)9-5(6)2/h5-6H,3-4H2,1-2H3,(H2,8,9) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratory
Curated by ChEMBL
| Assay Description
Inhibitory activity against Inducible nitric oxide synthase |
Bioorg Med Chem Lett 14: 4539-44 (2004)
Article DOI: 10.1016/j.bmcl.2004.06.033
BindingDB Entry DOI: 10.7270/Q218377V |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50091800

(2-amino-4-methylpyridine | 4-METHYLPYRIDIN-2-AMINE...)Show InChI InChI=1S/C6H8N2/c1-5-2-3-8-6(7)4-5/h2-4H,1H3,(H2,7,8) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
PubMed
| n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Rahway
Curated by ChEMBL
| Assay Description
Inhibitory activity against inducible nitric oxide synthase (iNOS) |
Bioorg Med Chem Lett 10: 1975-8 (2001)
BindingDB Entry DOI: 10.7270/Q2319V30 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50164781
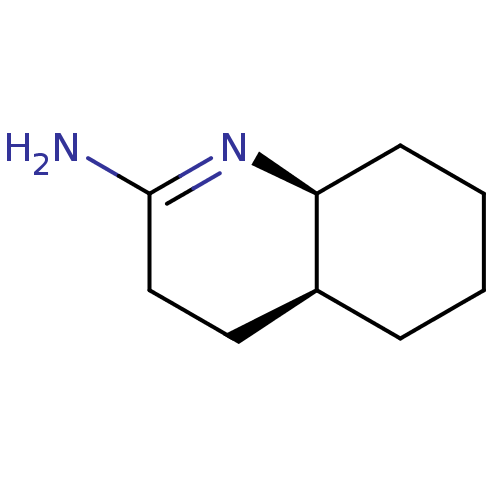
((5S,6S)-[Octahydro-quinolin-(2E)-ylidene]amine | C...)Show InChI InChI=1S/C9H16N2/c10-9-6-5-7-3-1-2-4-8(7)11-9/h7-8H,1-6H2,(H2,10,11)/t7-,8-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 186 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against human Inducible nitric oxide synthase (iNOS) |
Bioorg Med Chem Lett 15: 1997-2001 (2005)
Article DOI: 10.1016/j.bmcl.2005.02.067
BindingDB Entry DOI: 10.7270/Q2XK8F3M |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM50164779

((4R,4aS,5S)-4-Methyl-octahydro-quinolin-(2E)-ylide...)Show InChI InChI=1S/C10H18N2/c1-7-6-10(11)12-9-5-3-2-4-8(7)9/h7-9H,2-6H2,1H3,(H2,11,12)/t7-,8+,9+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against human Neuronal nitric oxide synthase (nNOS) |
Bioorg Med Chem Lett 15: 1997-2001 (2005)
Article DOI: 10.1016/j.bmcl.2005.02.067
BindingDB Entry DOI: 10.7270/Q2XK8F3M |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50155789
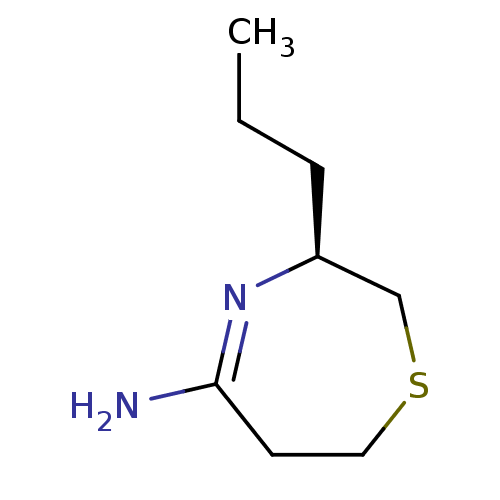
((S)-3-Propyl-[1,4]thiazepan-(5E)-ylideneamine | CH...)Show InChI InChI=1S/C8H16N2S/c1-2-3-7-6-11-5-4-8(9)10-7/h7H,2-6H2,1H3,(H2,9,10)/t7-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory concentration against Inducible nitric oxide synthase |
Bioorg Med Chem Lett 14: 5907-11 (2004)
Article DOI: 10.1016/j.bmcl.2004.09.019
BindingDB Entry DOI: 10.7270/Q2JQ10HX |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, endothelial
(Homo sapiens (Human)) | BDBM50091798

(6-Isopropyl-4-methyl-pyridin-2-ylamine | CHEMBL299...)Show InChI InChI=1S/C9H14N2/c1-6(2)8-4-7(3)5-9(10)11-8/h4-6H,1-3H3,(H2,10,11) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Rahway
Curated by ChEMBL
| Assay Description
Inhibitory activity against endothelial nitric oxide synthase (eNOS) |
Bioorg Med Chem Lett 10: 1975-8 (2001)
BindingDB Entry DOI: 10.7270/Q2319V30 |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50066780

(4-Methyl-pyrrolidin-(2E)-ylideneamine; hydrochlori...)Show InChI InChI=1S/C5H10N2/c1-4-2-5(6)7-3-4/h4H,2-3H2,1H3,(H2,6,7) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratory
Curated by ChEMBL
| Assay Description
Inhibitory activity against Inducible nitric oxide synthase |
Bioorg Med Chem Lett 14: 4539-44 (2004)
Article DOI: 10.1016/j.bmcl.2004.06.033
BindingDB Entry DOI: 10.7270/Q218377V |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, endothelial
(Homo sapiens (Human)) | BDBM50062133

(4-Methyl-piperidin-(2E)-ylideneamine | 4-Methyl-pi...)Show InChI InChI=1S/C6H12N2/c1-5-2-3-8-6(7)4-5/h5H,2-4H2,1H3,(H2,7,8) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 220 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Rahway
Curated by ChEMBL
| Assay Description
Inhibitory activity against endothelial nitric oxide synthase (eNOS) |
Bioorg Med Chem Lett 10: 1975-8 (2001)
BindingDB Entry DOI: 10.7270/Q2319V30 |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50091813
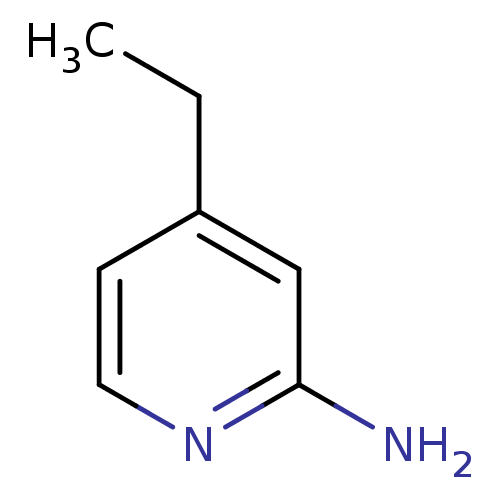
(4-Ethyl-pyridin-2-ylamine | 4-ethylpyridin-2-amine...)Show InChI InChI=1S/C7H10N2/c1-2-6-3-4-9-7(8)5-6/h3-5H,2H2,1H3,(H2,8,9) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 230 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Rahway
Curated by ChEMBL
| Assay Description
Inhibitory activity against inducible nitric oxide synthase (iNOS) |
Bioorg Med Chem Lett 10: 1975-8 (2001)
BindingDB Entry DOI: 10.7270/Q2319V30 |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, endothelial
(Homo sapiens (Human)) | BDBM50091813

(4-Ethyl-pyridin-2-ylamine | 4-ethylpyridin-2-amine...)Show InChI InChI=1S/C7H10N2/c1-2-6-3-4-9-7(8)5-6/h3-5H,2H2,1H3,(H2,8,9) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 230 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Rahway
Curated by ChEMBL
| Assay Description
Inhibitory activity against endothelial nitric oxide synthase (eNOS) |
Bioorg Med Chem Lett 10: 1975-8 (2001)
BindingDB Entry DOI: 10.7270/Q2319V30 |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM50049255
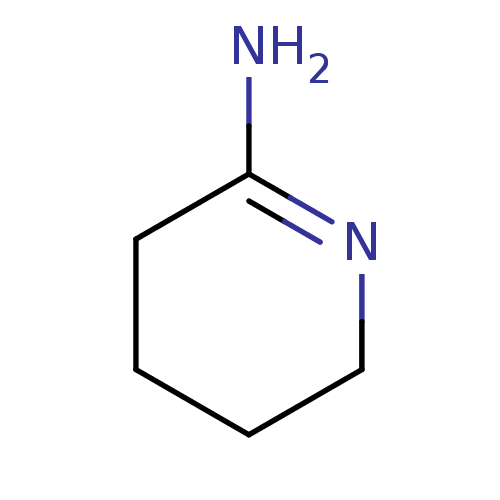
(CHEMBL269058 | PIPERIDIN-2-IMINE | Piperidin-(2E)-...)Show InChI InChI=1S/C5H10N2/c6-5-3-1-2-4-7-5/h1-4H2,(H2,6,7) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 240 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against human Neuronal nitric oxide synthase (nNOS) |
Bioorg Med Chem Lett 15: 1997-2001 (2005)
Article DOI: 10.1016/j.bmcl.2005.02.067
BindingDB Entry DOI: 10.7270/Q2XK8F3M |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM50164780
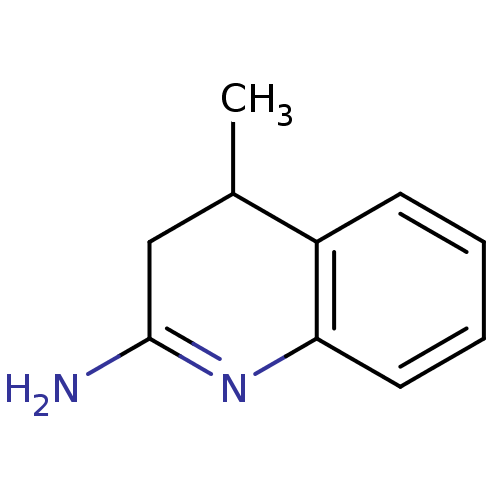
(4-Methyl-3,4-dihydro-1H-quinolin-(2E)-ylideneamine...)Show InChI InChI=1S/C10H12N2/c1-7-6-10(11)12-9-5-3-2-4-8(7)9/h2-5,7H,6H2,1H3,(H2,11,12) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 240 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against human Neuronal nitric oxide synthase (nNOS) |
Bioorg Med Chem Lett 15: 1997-2001 (2005)
Article DOI: 10.1016/j.bmcl.2005.02.067
BindingDB Entry DOI: 10.7270/Q2XK8F3M |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM50164781

((5S,6S)-[Octahydro-quinolin-(2E)-ylidene]amine | C...)Show InChI InChI=1S/C9H16N2/c10-9-6-5-7-3-1-2-4-8(7)11-9/h7-8H,1-6H2,(H2,10,11)/t7-,8-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 246 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against human Neuronal nitric oxide synthase (nNOS) |
Bioorg Med Chem Lett 15: 1997-2001 (2005)
Article DOI: 10.1016/j.bmcl.2005.02.067
BindingDB Entry DOI: 10.7270/Q2XK8F3M |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50155788
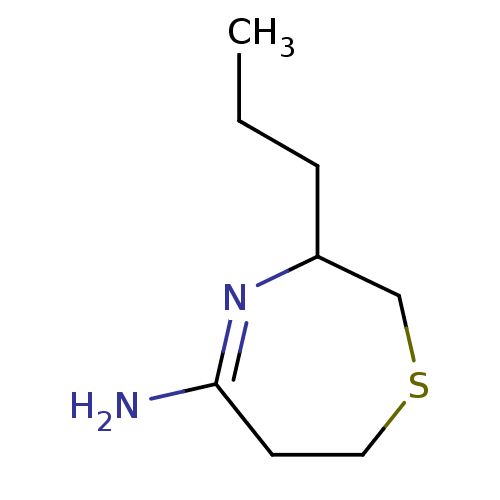
(3-Propyl-[1,4]thiazepan-(5E)-ylideneamine | CHEMBL...)Show InChI InChI=1S/C8H16N2S/c1-2-3-7-6-11-5-4-8(9)10-7/h7H,2-6H2,1H3,(H2,9,10) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 290 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory concentration against Inducible nitric oxide synthase |
Bioorg Med Chem Lett 14: 5907-11 (2004)
Article DOI: 10.1016/j.bmcl.2004.09.019
BindingDB Entry DOI: 10.7270/Q2JQ10HX |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data