 Found 1004 hits with Last Name = 'ontoria' and Initial = 'jm'
Found 1004 hits with Last Name = 'ontoria' and Initial = 'jm' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Genome polyprotein/Non-structural protein 4A
(Hepatitis C virus) | BDBM50150997

(4-{2-[(S)-4,4-Difluoro-2-({(2S,4R)-1-((S)-2-isobut...)Show SMILES COc1ccc2c(O[C@@H]3C[C@H](N(C3)C(=O)[C@@H](NC(=O)OCC(C)C)C(C)C)C(=O)N[C@@H](CC(F)F)C(=O)NCCc3c(F)cc(cc3F)C(O)=O)cc(nc2c1)-c1csc(NC(C)C)n1 Show InChI InChI=1S/C44H53F4N7O9S/c1-21(2)19-63-44(61)54-38(22(3)4)41(58)55-18-26(64-36-16-32(34-20-65-43(53-34)50-23(5)6)51-31-14-25(62-7)8-9-28(31)36)15-35(55)40(57)52-33(17-37(47)48)39(56)49-11-10-27-29(45)12-24(42(59)60)13-30(27)46/h8-9,12-14,16,20-23,26,33,35,37-38H,10-11,15,17-19H2,1-7H3,(H,49,56)(H,50,53)(H,52,57)(H,54,61)(H,59,60)/t26-,33+,35+,38+/m1/s1 | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against Hepatitis C virus Non structural protein 3 serine protease/Non structural protein 4A serine protease |
Bioorg Med Chem Lett 14: 4575-9 (2004)
Article DOI: 10.1016/j.bmcl.2004.05.093
BindingDB Entry DOI: 10.7270/Q27S7PHC |
More data for this
Ligand-Target Pair | |
Genome polyprotein/Non-structural protein 4A
(Hepatitis C virus) | BDBM50150993

(4-[2-(4,4-Difluoro-2-{[(S)-(R)-1-(2-isobutoxycarbo...)Show SMILES COc1ccc2c(O[C@@H]3C[C@H](N(C3)C(=O)C(NC(=O)OCC(C)C)C(C)(C)C)C(=O)N[C@@H](CC(F)F)C(=O)NCCc3c(F)cc(cc3F)C(O)=O)cc(nc2c1)-c1ccccc1 Show InChI InChI=1S/C45H51F4N5O9/c1-24(2)23-62-44(60)53-39(45(3,4)5)42(57)54-22-28(63-37-20-33(25-10-8-7-9-11-25)51-34-18-27(61-6)12-13-30(34)37)19-36(54)41(56)52-35(21-38(48)49)40(55)50-15-14-29-31(46)16-26(43(58)59)17-32(29)47/h7-13,16-18,20,24,28,35-36,38-39H,14-15,19,21-23H2,1-6H3,(H,50,55)(H,52,56)(H,53,60)(H,58,59)/t28-,35+,36+,39?/m1/s1 | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against Hepatitis C virus Non structural protein 3 serine protease/Non structural protein 4A serine protease |
Bioorg Med Chem Lett 14: 4575-9 (2004)
Article DOI: 10.1016/j.bmcl.2004.05.093
BindingDB Entry DOI: 10.7270/Q27S7PHC |
More data for this
Ligand-Target Pair | |
Genome polyprotein/Non-structural protein 4A
(Hepatitis C virus) | BDBM50150992
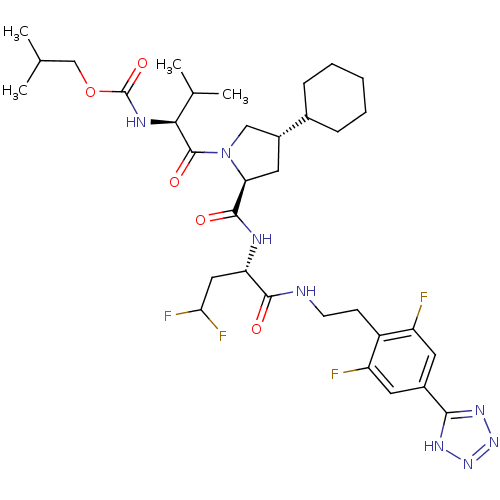
(CHEMBL366279 | {(S)-1-[(2S,4S)-4-Cyclohexyl-2-((S)...)Show SMILES CC(C)COC(=O)N[C@@H](C(C)C)C(=O)N1C[C@@H](C[C@H]1C(=O)N[C@@H](CC(F)F)C(=O)NCCc1c(F)cc(cc1F)-c1nnn[nH]1)C1CCCCC1 Show InChI InChI=1S/C34H48F4N8O5/c1-18(2)17-51-34(50)41-29(19(3)4)33(49)46-16-22(20-8-6-5-7-9-20)14-27(46)32(48)40-26(15-28(37)38)31(47)39-11-10-23-24(35)12-21(13-25(23)36)30-42-44-45-43-30/h12-13,18-20,22,26-29H,5-11,14-17H2,1-4H3,(H,39,47)(H,40,48)(H,41,50)(H,42,43,44,45)/t22-,26+,27+,29+/m1/s1 | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against Hepatitis C virus Non structural protein 3 serine protease/Non structural protein 4A serine protease |
Bioorg Med Chem Lett 14: 4575-9 (2004)
Article DOI: 10.1016/j.bmcl.2004.05.093
BindingDB Entry DOI: 10.7270/Q27S7PHC |
More data for this
Ligand-Target Pair | |
Genome polyprotein/Non-structural protein 4A
(Hepatitis C virus) | BDBM50150991
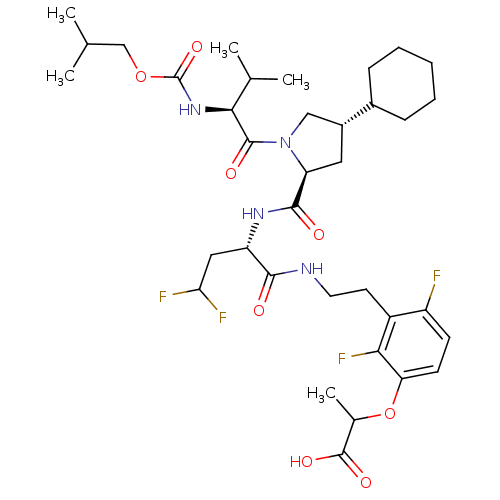
(2-{3-[2-((S)-2-{[(2S,4S)-4-Cyclohexyl-1-((S)-2-iso...)Show SMILES CC(C)COC(=O)N[C@@H](C(C)C)C(=O)N1C[C@@H](C[C@H]1C(=O)N[C@@H](CC(F)F)C(=O)NCCc1c(F)ccc(OC(C)C(O)=O)c1F)C1CCCCC1 Show InChI InChI=1S/C36H52F4N4O8/c1-19(2)18-51-36(50)43-31(20(3)4)34(47)44-17-23(22-9-7-6-8-10-22)15-27(44)33(46)42-26(16-29(38)39)32(45)41-14-13-24-25(37)11-12-28(30(24)40)52-21(5)35(48)49/h11-12,19-23,26-27,29,31H,6-10,13-18H2,1-5H3,(H,41,45)(H,42,46)(H,43,50)(H,48,49)/t21?,23-,26+,27+,31+/m1/s1 | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against Hepatitis C virus Non structural protein 3 serine protease/Non structural protein 4A serine protease |
Bioorg Med Chem Lett 14: 4575-9 (2004)
Article DOI: 10.1016/j.bmcl.2004.05.093
BindingDB Entry DOI: 10.7270/Q27S7PHC |
More data for this
Ligand-Target Pair | |
Genome polyprotein/Non-structural protein 4A
(Hepatitis C virus) | BDBM50150989

(CHEMBL362406 | {3-[2-((S)-2-{[(2S,4S)-4-Cyclohexyl...)Show SMILES CC(C)COC(=O)N[C@@H](C(C)C)C(=O)N1C[C@@H](C[C@H]1C(=O)N[C@@H](CC(F)F)C(=O)NCCc1c(F)ccc(OCC(O)=O)c1F)C1CCCCC1 Show InChI InChI=1S/C35H50F4N4O8/c1-19(2)17-51-35(49)42-31(20(3)4)34(48)43-16-22(21-8-6-5-7-9-21)14-26(43)33(47)41-25(15-28(37)38)32(46)40-13-12-23-24(36)10-11-27(30(23)39)50-18-29(44)45/h10-11,19-22,25-26,28,31H,5-9,12-18H2,1-4H3,(H,40,46)(H,41,47)(H,42,49)(H,44,45)/t22-,25+,26+,31+/m1/s1 | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against Hepatitis C virus Non structural protein 3 serine protease/Non structural protein 4A serine protease |
Bioorg Med Chem Lett 14: 4575-9 (2004)
Article DOI: 10.1016/j.bmcl.2004.05.093
BindingDB Entry DOI: 10.7270/Q27S7PHC |
More data for this
Ligand-Target Pair | |
Genome polyprotein/Non-structural protein 4A
(Hepatitis C virus) | BDBM50150990
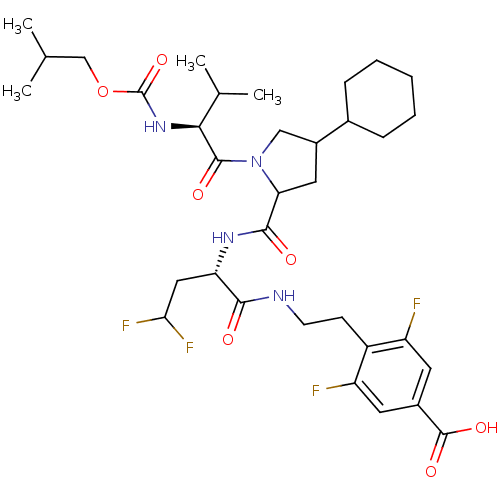
(4-[2-((S)-2-{[(R)-4-Cyclohexyl-1-((S)-2-isobutoxyc...)Show SMILES CC(C)COC(=O)N[C@@H](C(C)C)C(=O)N1CC(CC1C(=O)N[C@@H](CC(F)F)C(=O)NCCc1c(F)cc(cc1F)C(O)=O)C1CCCCC1 Show InChI InChI=1S/C34H48F4N4O7/c1-18(2)17-49-34(48)41-29(19(3)4)32(45)42-16-22(20-8-6-5-7-9-20)14-27(42)31(44)40-26(15-28(37)38)30(43)39-11-10-23-24(35)12-21(33(46)47)13-25(23)36/h12-13,18-20,22,26-29H,5-11,14-17H2,1-4H3,(H,39,43)(H,40,44)(H,41,48)(H,46,47)/t22?,26-,27?,29-/m0/s1 | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against Hepatitis C virus Non structural protein 3 serine protease/Non structural protein 4A serine protease |
Bioorg Med Chem Lett 14: 4575-9 (2004)
Article DOI: 10.1016/j.bmcl.2004.05.093
BindingDB Entry DOI: 10.7270/Q27S7PHC |
More data for this
Ligand-Target Pair | |
Genome polyprotein/Non-structural protein 4A
(Hepatitis C virus) | BDBM50150986

((E)-3-{3-[2-((S)-4,4-Difluoro-2-{[(2S,4S)-1-((S)-2...)Show SMILES CC(C)COC(=O)N[C@@H](C(C)C)C(=O)N1C[C@@H](C[C@H]1C(=O)N[C@@H](CC(F)F)C(=O)NCCc1cc(C=CC(O)=O)ccc1F)c1ccccc1 |w:36.37| Show InChI InChI=1S/C36H45F3N4O7/c1-21(2)20-50-36(49)42-32(22(3)4)35(48)43-19-26(24-8-6-5-7-9-24)17-29(43)34(47)41-28(18-30(38)39)33(46)40-15-14-25-16-23(10-12-27(25)37)11-13-31(44)45/h5-13,16,21-22,26,28-30,32H,14-15,17-20H2,1-4H3,(H,40,46)(H,41,47)(H,42,49)(H,44,45)/t26-,28+,29+,32+/m1/s1 | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against Hepatitis C virus Non structural protein 3 serine protease/Non structural protein 4A serine protease |
Bioorg Med Chem Lett 14: 4575-9 (2004)
Article DOI: 10.1016/j.bmcl.2004.05.093
BindingDB Entry DOI: 10.7270/Q27S7PHC |
More data for this
Ligand-Target Pair | |
Genome polyprotein/Non-structural protein 4A
(Hepatitis C virus) | BDBM50150994

((E)-3-{3-[2-((S)-4,4-Difluoro-2-{[(2S,4S)-1-((S)-2...)Show SMILES CC(C)COC(=O)N[C@@H](C(C)C)C(=O)N1C[C@@H](C[C@H]1C(=O)N[C@@H](CC(F)F)C(=O)NCCc1cccc(C=CC(O)=O)c1F)c1ccccc1 |w:38.39| Show InChI InChI=1S/C36H45F3N4O7/c1-21(2)20-50-36(49)42-32(22(3)4)35(48)43-19-26(23-9-6-5-7-10-23)17-28(43)34(47)41-27(18-29(37)38)33(46)40-16-15-25-12-8-11-24(31(25)39)13-14-30(44)45/h5-14,21-22,26-29,32H,15-20H2,1-4H3,(H,40,46)(H,41,47)(H,42,49)(H,44,45)/t26-,27+,28+,32+/m1/s1 | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against Hepatitis C virus Non structural protein 3 serine protease/Non structural protein 4A serine protease |
Bioorg Med Chem Lett 14: 4575-9 (2004)
Article DOI: 10.1016/j.bmcl.2004.05.093
BindingDB Entry DOI: 10.7270/Q27S7PHC |
More data for this
Ligand-Target Pair | |
Genome polyprotein/Non-structural protein 4A
(Hepatitis C virus) | BDBM50150984
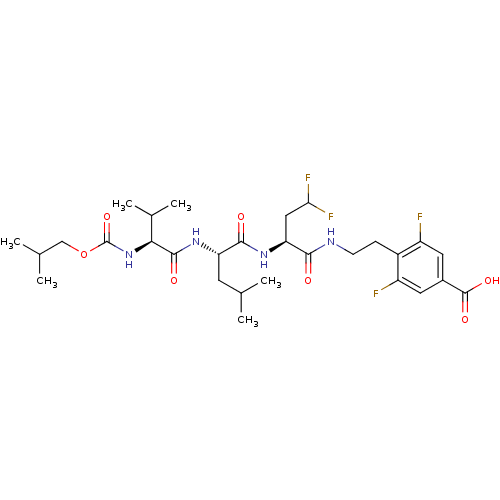
(4-(2-{(S)-4,4-Difluoro-2-[(S)-2-((S)-2-isobutoxyca...)Show SMILES CC(C)COC(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CC(F)F)C(=O)NCCc1c(F)cc(cc1F)C(O)=O Show InChI InChI=1S/C29H42F4N4O7/c1-14(2)9-21(36-27(40)24(16(5)6)37-29(43)44-13-15(3)4)26(39)35-22(12-23(32)33)25(38)34-8-7-18-19(30)10-17(28(41)42)11-20(18)31/h10-11,14-16,21-24H,7-9,12-13H2,1-6H3,(H,34,38)(H,35,39)(H,36,40)(H,37,43)(H,41,42)/t21-,22-,24-/m0/s1 | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against Hepatitis C virus Non structural protein 3 serine protease/Non structural protein 4A serine protease |
Bioorg Med Chem Lett 14: 4575-9 (2004)
Article DOI: 10.1016/j.bmcl.2004.05.093
BindingDB Entry DOI: 10.7270/Q27S7PHC |
More data for this
Ligand-Target Pair | |
Genome polyprotein/Non-structural protein 4A
(Hepatitis C virus) | BDBM50144349
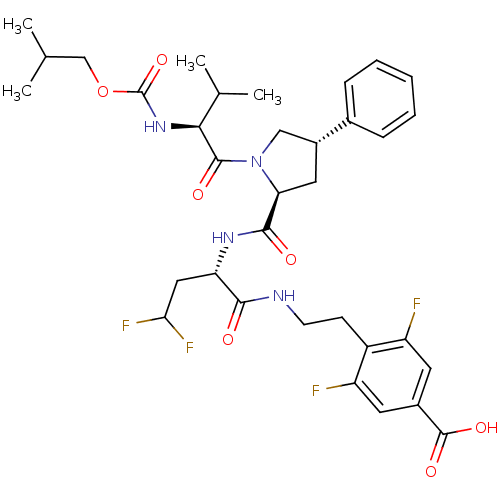
(4-[2-((S)-4,4-Difluoro-2-{[(2S,4S)-1-(2-isobutoxyc...)Show SMILES CC(C)COC(=O)N[C@@H](C(C)C)C(=O)N1C[C@@H](C[C@H]1C(=O)N[C@@H](CC(F)F)C(=O)NCCc1c(F)cc(cc1F)C(O)=O)c1ccccc1 Show InChI InChI=1S/C34H42F4N4O7/c1-18(2)17-49-34(48)41-29(19(3)4)32(45)42-16-22(20-8-6-5-7-9-20)14-27(42)31(44)40-26(15-28(37)38)30(43)39-11-10-23-24(35)12-21(33(46)47)13-25(23)36/h5-9,12-13,18-19,22,26-29H,10-11,14-17H2,1-4H3,(H,39,43)(H,40,44)(H,41,48)(H,46,47)/t22-,26+,27+,29+/m1/s1 | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against Hepatitis C virus Non structural protein 3 serine protease/Non structural protein 4A serine protease |
Bioorg Med Chem Lett 14: 4575-9 (2004)
Article DOI: 10.1016/j.bmcl.2004.05.093
BindingDB Entry DOI: 10.7270/Q27S7PHC |
More data for this
Ligand-Target Pair | |
Genome polyprotein/Non-structural protein 4A
(Hepatitis C virus) | BDBM50150995
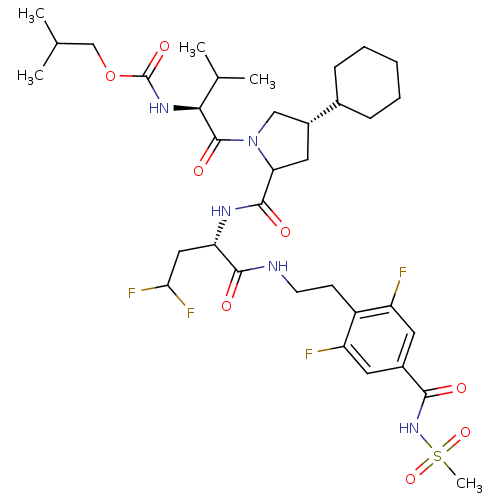
(CHEMBL263183 | [(S)-1-((2S,4S)-4-Cyclohexyl-2-{(S)...)Show SMILES CC(C)COC(=O)N[C@@H](C(C)C)C(=O)N1C[C@@H](CC1C(=O)N[C@@H](CC(F)F)C(=O)NCCc1c(F)cc(cc1F)C(=O)NS(C)(=O)=O)C1CCCCC1 Show InChI InChI=1S/C35H51F4N5O8S/c1-19(2)18-52-35(49)42-30(20(3)4)34(48)44-17-23(21-9-7-6-8-10-21)15-28(44)33(47)41-27(16-29(38)39)32(46)40-12-11-24-25(36)13-22(14-26(24)37)31(45)43-53(5,50)51/h13-14,19-21,23,27-30H,6-12,15-18H2,1-5H3,(H,40,46)(H,41,47)(H,42,49)(H,43,45)/t23-,27+,28?,30+/m1/s1 | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against Hepatitis C virus Non structural protein 3 serine protease/Non structural protein 4A serine protease |
Bioorg Med Chem Lett 14: 4575-9 (2004)
Article DOI: 10.1016/j.bmcl.2004.05.093
BindingDB Entry DOI: 10.7270/Q27S7PHC |
More data for this
Ligand-Target Pair | |
Genome polyprotein/Non-structural protein 4A
(Hepatitis C virus) | BDBM50150983
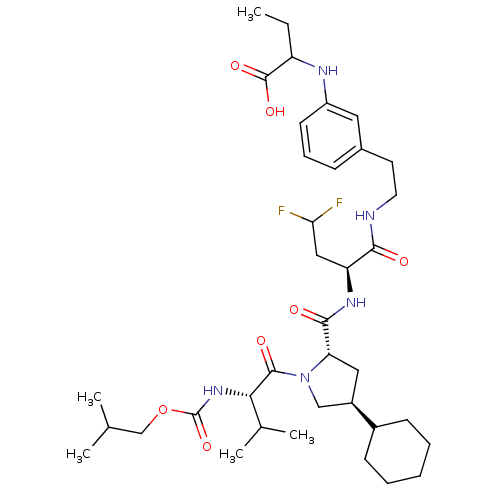
(2-{3-[2-((S)-2-{[(2S,4S)-4-Cyclohexyl-1-((S)-2-iso...)Show SMILES CCC(Nc1cccc(CCNC(=O)[C@H](CC(F)F)NC(=O)[C@@H]2C[C@H](CN2C(=O)[C@@H](NC(=O)OCC(C)C)C(C)C)C2CCCCC2)c1)C(O)=O Show InChI InChI=1S/C37H57F2N5O7/c1-6-28(36(48)49)41-27-14-10-11-24(17-27)15-16-40-33(45)29(19-31(38)39)42-34(46)30-18-26(25-12-8-7-9-13-25)20-44(30)35(47)32(23(4)5)43-37(50)51-21-22(2)3/h10-11,14,17,22-23,25-26,28-32,41H,6-9,12-13,15-16,18-21H2,1-5H3,(H,40,45)(H,42,46)(H,43,50)(H,48,49)/t26-,28?,29+,30+,32+/m1/s1 | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against Hepatitis C virus Non structural protein 3 serine protease/Non structural protein 4A serine protease |
Bioorg Med Chem Lett 14: 4575-9 (2004)
Article DOI: 10.1016/j.bmcl.2004.05.093
BindingDB Entry DOI: 10.7270/Q27S7PHC |
More data for this
Ligand-Target Pair | |
Genome polyprotein/Non-structural protein 4A
(Hepatitis C virus) | BDBM50150996

(CHEMBL181465 | {3-[2-((S)-2-{[(2S,4S)-4-Cyclohexyl...)Show SMILES CC(C)COC(=O)N[C@@H](C(C)C)C(=O)N1C[C@@H](C[C@H]1C(=O)N[C@@H](CC(F)F)C(=O)NCCc1cccc(NC(C(O)=O)c2ccccc2)c1)C1CCCCC1 Show InChI InChI=1S/C41H57F2N5O7/c1-25(2)24-55-41(54)47-35(26(3)4)39(51)48-23-30(28-13-7-5-8-14-28)21-33(48)38(50)46-32(22-34(42)43)37(49)44-19-18-27-12-11-17-31(20-27)45-36(40(52)53)29-15-9-6-10-16-29/h6,9-12,15-17,20,25-26,28,30,32-36,45H,5,7-8,13-14,18-19,21-24H2,1-4H3,(H,44,49)(H,46,50)(H,47,54)(H,52,53)/t30-,32+,33+,35+,36?/m1/s1 | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 650 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against Hepatitis C virus Non structural protein 3 serine protease/Non structural protein 4A serine protease |
Bioorg Med Chem Lett 14: 4575-9 (2004)
Article DOI: 10.1016/j.bmcl.2004.05.093
BindingDB Entry DOI: 10.7270/Q27S7PHC |
More data for this
Ligand-Target Pair | |
Genome polyprotein/Non-structural protein 4A
(Hepatitis C virus) | BDBM50150985

(CHEMBL362404 | {3-[2-((S)-4,4-Difluoro-2-{[(2S,4S)...)Show SMILES CC(C)COC(=O)N[C@@H](C(C)C)C(=O)N1C[C@@H](C[C@H]1C(=O)N[C@@H](CC(F)F)C(=O)NCCc1cccc(OC(C(O)=O)c2ccccc2)c1)c1ccccc1 Show InChI InChI=1S/C41H50F2N4O8/c1-25(2)24-54-41(53)46-35(26(3)4)39(50)47-23-30(28-13-7-5-8-14-28)21-33(47)38(49)45-32(22-34(42)43)37(48)44-19-18-27-12-11-17-31(20-27)55-36(40(51)52)29-15-9-6-10-16-29/h5-17,20,25-26,30,32-36H,18-19,21-24H2,1-4H3,(H,44,48)(H,45,49)(H,46,53)(H,51,52)/t30-,32+,33+,35+,36?/m1/s1 | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against Hepatitis C virus Non structural protein 3 serine protease/Non structural protein 4A serine protease |
Bioorg Med Chem Lett 14: 4575-9 (2004)
Article DOI: 10.1016/j.bmcl.2004.05.093
BindingDB Entry DOI: 10.7270/Q27S7PHC |
More data for this
Ligand-Target Pair | |
Genome polyprotein/Non-structural protein 4A
(Hepatitis C virus) | BDBM50144357

(3-Chloro-4-(2-{(S)-4,4-difluoro-2-[(S)-2-((S)-2-is...)Show SMILES CC(C)COC(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CC(F)F)C(=O)NCCc1ccc(cc1Cl)C(O)=O Show InChI InChI=1S/C29H43ClF2N4O7/c1-15(2)11-21(35-27(39)24(17(5)6)36-29(42)43-14-16(3)4)26(38)34-22(13-23(31)32)25(37)33-10-9-18-7-8-19(28(40)41)12-20(18)30/h7-8,12,15-17,21-24H,9-11,13-14H2,1-6H3,(H,33,37)(H,34,38)(H,35,39)(H,36,42)(H,40,41)/t21-,22-,24-/m0/s1 | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against Hepatitis C virus Non structural protein 3 serine protease/Non structural protein 4A serine protease |
Bioorg Med Chem Lett 14: 4575-9 (2004)
Article DOI: 10.1016/j.bmcl.2004.05.093
BindingDB Entry DOI: 10.7270/Q27S7PHC |
More data for this
Ligand-Target Pair | |
Genome polyprotein/Non-structural protein 4A
(Hepatitis C virus) | BDBM50150988

((E)-3-{3-[2-((S)-4,4-Difluoro-2-{[(2S,4S)-1-((S)-2...)Show SMILES CC(C)COC(=O)N[C@@H](C(C)C)C(=O)N1C[C@@H](C[C@H]1C(=O)N[C@@H](CC(F)F)C(=O)NCCc1cccc(C=CC(O)=O)c1)c1ccccc1 |w:38.39| Show InChI InChI=1S/C36H46F2N4O7/c1-22(2)21-49-36(48)41-32(23(3)4)35(47)42-20-27(26-11-6-5-7-12-26)18-29(42)34(46)40-28(19-30(37)38)33(45)39-16-15-25-10-8-9-24(17-25)13-14-31(43)44/h5-14,17,22-23,27-30,32H,15-16,18-21H2,1-4H3,(H,39,45)(H,40,46)(H,41,48)(H,43,44)/t27-,28+,29+,32+/m1/s1 | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against Hepatitis C virus Non structural protein 3 serine protease/Non structural protein 4A serine protease |
Bioorg Med Chem Lett 14: 4575-9 (2004)
Article DOI: 10.1016/j.bmcl.2004.05.093
BindingDB Entry DOI: 10.7270/Q27S7PHC |
More data for this
Ligand-Target Pair | |
Genome polyprotein/Non-structural protein 4A
(Hepatitis C virus) | BDBM50150987
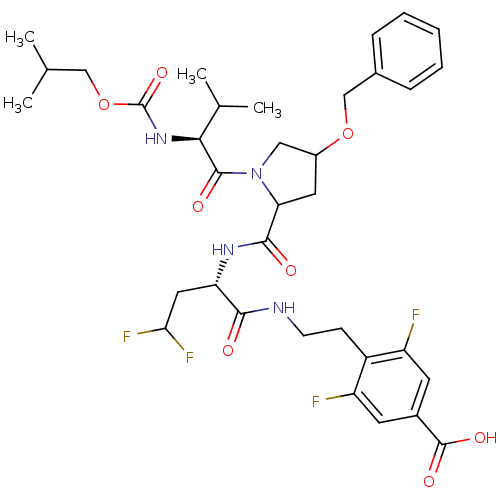
(4-[2-((S)-2-{[(R)-4-Benzyloxy-1-((S)-2-isobutoxyca...)Show SMILES CC(C)COC(=O)N[C@@H](C(C)C)C(=O)N1CC(CC1C(=O)N[C@@H](CC(F)F)C(=O)NCCc1c(F)cc(cc1F)C(O)=O)OCc1ccccc1 Show InChI InChI=1S/C35H44F4N4O8/c1-19(2)17-51-35(49)42-30(20(3)4)33(46)43-16-23(50-18-21-8-6-5-7-9-21)14-28(43)32(45)41-27(15-29(38)39)31(44)40-11-10-24-25(36)12-22(34(47)48)13-26(24)37/h5-9,12-13,19-20,23,27-30H,10-11,14-18H2,1-4H3,(H,40,44)(H,41,45)(H,42,49)(H,47,48)/t23?,27-,28?,30-/m0/s1 | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against Hepatitis C virus Non structural protein 3 serine protease/Non structural protein 4A serine protease |
Bioorg Med Chem Lett 14: 4575-9 (2004)
Article DOI: 10.1016/j.bmcl.2004.05.093
BindingDB Entry DOI: 10.7270/Q27S7PHC |
More data for this
Ligand-Target Pair | |
Genome polyprotein/Non-structural protein 4A
(Hepatitis C virus) | BDBM50150987

(4-[2-((S)-2-{[(R)-4-Benzyloxy-1-((S)-2-isobutoxyca...)Show SMILES CC(C)COC(=O)N[C@@H](C(C)C)C(=O)N1CC(CC1C(=O)N[C@@H](CC(F)F)C(=O)NCCc1c(F)cc(cc1F)C(O)=O)OCc1ccccc1 Show InChI InChI=1S/C35H44F4N4O8/c1-19(2)17-51-35(49)42-30(20(3)4)33(46)43-16-23(50-18-21-8-6-5-7-9-21)14-28(43)32(45)41-27(15-29(38)39)31(44)40-11-10-24-25(36)12-22(34(47)48)13-26(24)37/h5-9,12-13,19-20,23,27-30H,10-11,14-18H2,1-4H3,(H,40,44)(H,41,45)(H,42,49)(H,47,48)/t23?,27-,28?,30-/m0/s1 | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against Hepatitis C virus Non structural protein 3 serine protease/Non structural protein 4A serine protease |
Bioorg Med Chem Lett 14: 4575-9 (2004)
Article DOI: 10.1016/j.bmcl.2004.05.093
BindingDB Entry DOI: 10.7270/Q27S7PHC |
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase 1
(Homo sapiens (Human)) | BDBM50306267
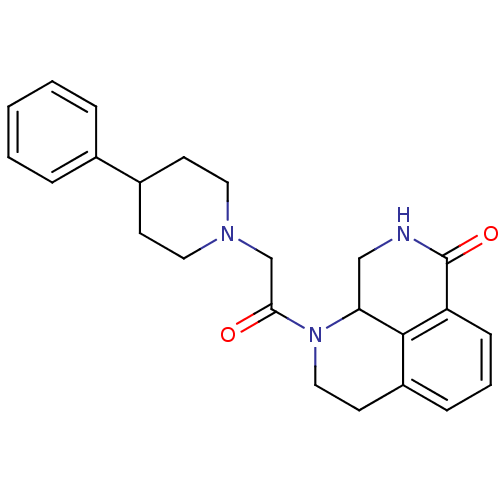
(1-(2-(4-phenylpiperidin-1-yl)acetyl)-2,3,9,9a-tetr...)Show SMILES O=C(CN1CCC(CC1)c1ccccc1)N1CCc2cccc3C(=O)NCC1c23 Show InChI InChI=1S/C24H27N3O2/c28-22(16-26-12-9-18(10-13-26)17-5-2-1-3-6-17)27-14-11-19-7-4-8-20-23(19)21(27)15-25-24(20)29/h1-8,18,21H,9-16H2,(H,25,29) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM-Merck Research Laboratories Rome
Curated by ChEMBL
| Assay Description
Inhibition of human PARP1 by scintillation proximity assay |
Bioorg Med Chem Lett 20: 448-52 (2010)
Article DOI: 10.1016/j.bmcl.2009.12.002
BindingDB Entry DOI: 10.7270/Q2GT5N91 |
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase 1
(Homo sapiens (Human)) | BDBM50306257
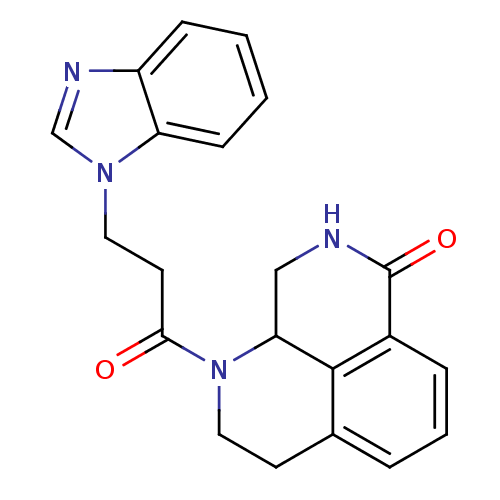
(1-(3-(1H-benzo[d]imidazol-1-yl)propanoyl)-2,3,9,9a...)Show SMILES O=C(CCn1cnc2ccccc12)N1CCc2cccc3C(=O)NCC1c23 Show InChI InChI=1S/C21H20N4O2/c26-19(9-10-24-13-23-16-6-1-2-7-17(16)24)25-11-8-14-4-3-5-15-20(14)18(25)12-22-21(15)27/h1-7,13,18H,8-12H2,(H,22,27) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM-Merck Research Laboratories Rome
Curated by ChEMBL
| Assay Description
Inhibition of human PARP1 by scintillation proximity assay |
Bioorg Med Chem Lett 20: 448-52 (2010)
Article DOI: 10.1016/j.bmcl.2009.12.002
BindingDB Entry DOI: 10.7270/Q2GT5N91 |
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase 1
(Homo sapiens (Human)) | BDBM50306253

(1-(2-(pyridin-3-yl)acetyl)-2,3,9,9a-tetrahydro-1H-...)Show InChI InChI=1S/C18H17N3O2/c22-16(9-12-3-2-7-19-10-12)21-8-6-13-4-1-5-14-17(13)15(21)11-20-18(14)23/h1-5,7,10,15H,6,8-9,11H2,(H,20,23) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM-Merck Research Laboratories Rome
Curated by ChEMBL
| Assay Description
Inhibition of human PARP1 by scintillation proximity assay |
Bioorg Med Chem Lett 20: 448-52 (2010)
Article DOI: 10.1016/j.bmcl.2009.12.002
BindingDB Entry DOI: 10.7270/Q2GT5N91 |
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase 1
(Homo sapiens (Human)) | BDBM50306263
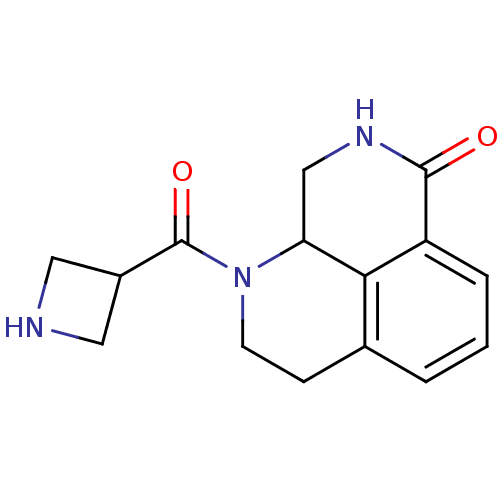
(1-(azetidine-3-carbonyl)-2,3,9,9a-tetrahydro-1H-be...)Show InChI InChI=1S/C15H17N3O2/c19-14-11-3-1-2-9-4-5-18(12(8-17-14)13(9)11)15(20)10-6-16-7-10/h1-3,10,12,16H,4-8H2,(H,17,19) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM-Merck Research Laboratories Rome
Curated by ChEMBL
| Assay Description
Inhibition of human PARP1 by scintillation proximity assay |
Bioorg Med Chem Lett 20: 448-52 (2010)
Article DOI: 10.1016/j.bmcl.2009.12.002
BindingDB Entry DOI: 10.7270/Q2GT5N91 |
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase 2
(Homo sapiens (Human)) | BDBM50306253

(1-(2-(pyridin-3-yl)acetyl)-2,3,9,9a-tetrahydro-1H-...)Show InChI InChI=1S/C18H17N3O2/c22-16(9-12-3-2-7-19-10-12)21-8-6-13-4-1-5-14-17(13)15(21)11-20-18(14)23/h1-5,7,10,15H,6,8-9,11H2,(H,20,23) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM-Merck Research Laboratories Rome
Curated by ChEMBL
| Assay Description
Inhibition of PARP2 |
Bioorg Med Chem Lett 20: 448-52 (2010)
Article DOI: 10.1016/j.bmcl.2009.12.002
BindingDB Entry DOI: 10.7270/Q2GT5N91 |
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase 2
(Homo sapiens (Human)) | BDBM50306256
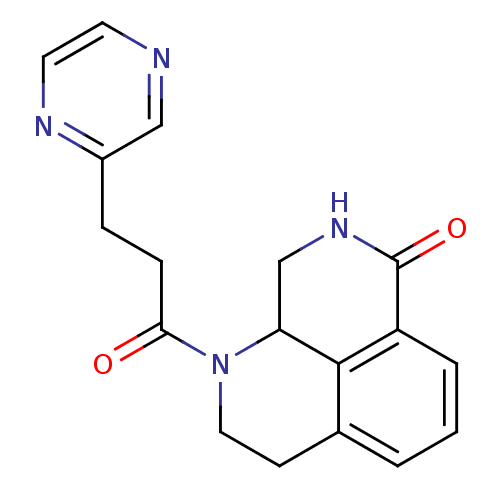
(1-(3-(pyrazin-2-yl)propanoyl)-2,3,9,9a-tetrahydro-...)Show InChI InChI=1S/C18H18N4O2/c23-16(5-4-13-10-19-7-8-20-13)22-9-6-12-2-1-3-14-17(12)15(22)11-21-18(14)24/h1-3,7-8,10,15H,4-6,9,11H2,(H,21,24) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM-Merck Research Laboratories Rome
Curated by ChEMBL
| Assay Description
Inhibition of PARP2 |
Bioorg Med Chem Lett 20: 448-52 (2010)
Article DOI: 10.1016/j.bmcl.2009.12.002
BindingDB Entry DOI: 10.7270/Q2GT5N91 |
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase 1
(Homo sapiens (Human)) | BDBM50306262

(1-(pyrrolidine-3-carbonyl)-2,3,9,9a-tetrahydro-1H-...)Show InChI InChI=1S/C16H19N3O2/c20-15-12-3-1-2-10-5-7-19(13(9-18-15)14(10)12)16(21)11-4-6-17-8-11/h1-3,11,13,17H,4-9H2,(H,18,20) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM-Merck Research Laboratories Rome
Curated by ChEMBL
| Assay Description
Inhibition of human PARP1 by scintillation proximity assay |
Bioorg Med Chem Lett 20: 448-52 (2010)
Article DOI: 10.1016/j.bmcl.2009.12.002
BindingDB Entry DOI: 10.7270/Q2GT5N91 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 3
(Homo sapiens (Human)) | BDBM50612449

(CHEMBL5271471)Show SMILES CCC(=O)CCCCC[C@H](NC(=O)C1CN(C)C1)c1ncc(o1)-c1cc2ccccc2nc1OC |r| | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50175036
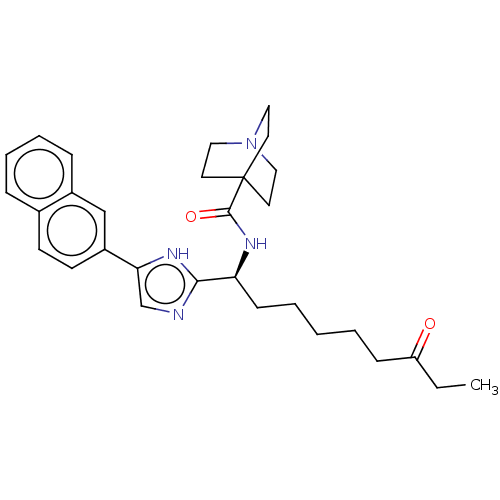
(CHEMBL3809599)Show SMILES CCC(=O)CCCCC[C@H](NC(=O)C12CCN(CC1)CC2)c1ncc([nH]1)-c1ccc2ccccc2c1 |r| Show InChI InChI=1S/C18H11Cl2F3N2OS/c19-13-2-1-3-14(20)12(13)8-16-24-9-15(27-16)17(26)25-11-6-4-10(5-7-11)18(21,22)23/h1-7,9H,8H2,(H,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM Science Park
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human His-tagged HDAC1 expressed in insect cells preincubated for 10 mins followed by addition of FLUOR DE LYS as fluoresce... |
ACS Med Chem Lett 7: 454-9 (2016)
Article DOI: 10.1021/acsmedchemlett.5b00468
BindingDB Entry DOI: 10.7270/Q2S184FM |
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase 1
(Homo sapiens (Human)) | BDBM50306179
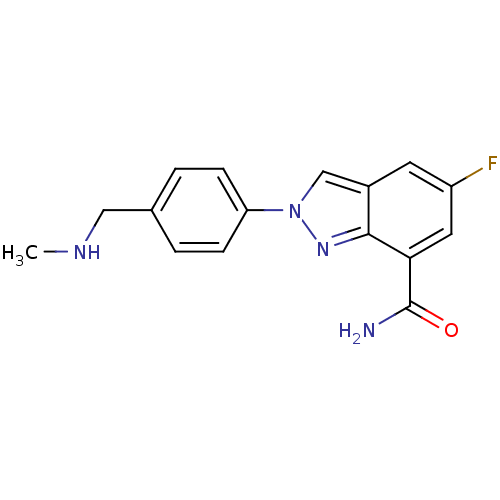
(5-fluoro-2-(4-((methylamino)methyl)phenyl)-2H-inda...)Show InChI InChI=1S/C16H15FN4O/c1-19-8-10-2-4-13(5-3-10)21-9-11-6-12(17)7-14(16(18)22)15(11)20-21/h2-7,9,19H,8H2,1H3,(H2,18,22) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM-Merck Research Laboratories Rome
Curated by ChEMBL
| Assay Description
Inhibition of human PARP1 after 3 hrs using [3H]NAD+ by scintillation proximity assay |
Bioorg Med Chem Lett 20: 488-92 (2010)
Article DOI: 10.1016/j.bmcl.2009.11.127
BindingDB Entry DOI: 10.7270/Q2W37WFZ |
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase 1
(Homo sapiens (Human)) | BDBM50316229
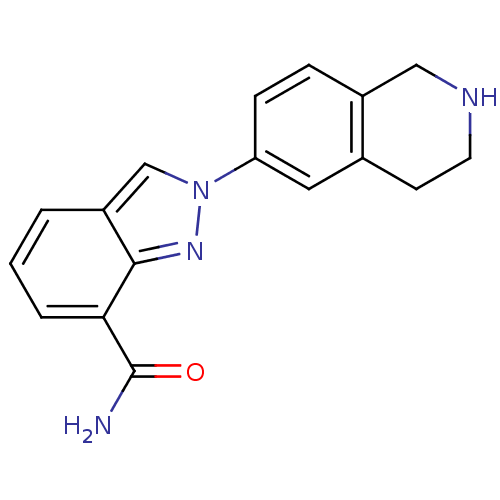
(2-(1,2,3,4-tetrahydroisoquinolin-6-yl)-2H-indazole...)Show InChI InChI=1S/C17H16N4O/c18-17(22)15-3-1-2-13-10-21(20-16(13)15)14-5-4-12-9-19-7-6-11(12)8-14/h1-5,8,10,19H,6-7,9H2,(H2,18,22) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM/Merck Research Labs Rome
Curated by ChEMBL
| Assay Description
Inhibition of human PARP1 by SPA |
J Med Chem 52: 7170-85 (2009)
Article DOI: 10.1021/jm901188v
BindingDB Entry DOI: 10.7270/Q2DN457M |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50612447

(CHEMBL5285293)Show SMILES CCC(=O)CCCCC[C@H](NC(=O)C1CN(C)C1)c1ncc([nH]1)-c1ccc2ccccc2c1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50612445

(CHEMBL5282110)Show SMILES CCC(=O)CCCCC[C@H](NC(=O)C12CCN(CC1)CC2)c1ncc(o1)-c1cc2ccccc2nc1OC |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50612449

(CHEMBL5271471)Show SMILES CCC(=O)CCCCC[C@H](NC(=O)C1CN(C)C1)c1ncc(o1)-c1cc2ccccc2nc1OC |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase 1
(Homo sapiens (Human)) | BDBM50316242
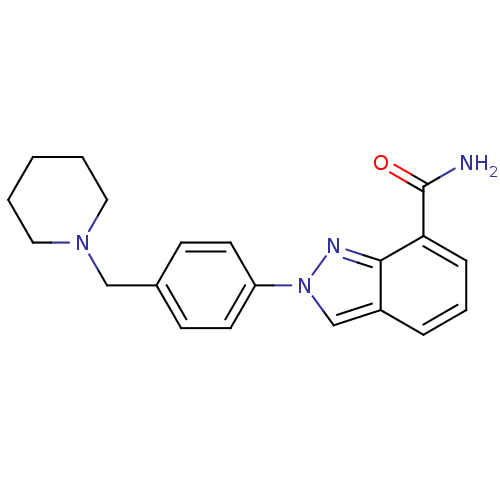
(2-(4-(piperidin-1-ylmethyl)phenyl)-2H-indazole-7-c...)Show InChI InChI=1S/C20H22N4O/c21-20(25)18-6-4-5-16-14-24(22-19(16)18)17-9-7-15(8-10-17)13-23-11-2-1-3-12-23/h4-10,14H,1-3,11-13H2,(H2,21,25) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM/Merck Research Labs Rome
Curated by ChEMBL
| Assay Description
Inhibition of human PARP1 by SPA |
J Med Chem 52: 7170-85 (2009)
Article DOI: 10.1021/jm901188v
BindingDB Entry DOI: 10.7270/Q2DN457M |
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase 1
(Homo sapiens (Human)) | BDBM50306173
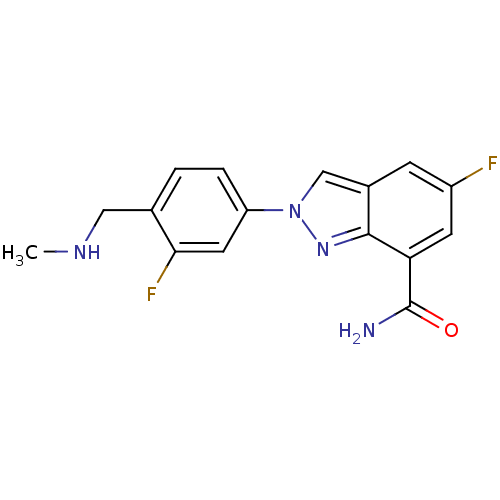
(5-fluoro-2-(3-fluoro-4-((methylamino)methyl)phenyl...)Show InChI InChI=1S/C16H14F2N4O/c1-20-7-9-2-3-12(6-14(9)18)22-8-10-4-11(17)5-13(16(19)23)15(10)21-22/h2-6,8,20H,7H2,1H3,(H2,19,23) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM-Merck Research Laboratories Rome
Curated by ChEMBL
| Assay Description
Inhibition of human PARP1 after 3 hrs using [3H]NAD+ by scintillation proximity assay |
Bioorg Med Chem Lett 20: 488-92 (2010)
Article DOI: 10.1016/j.bmcl.2009.11.127
BindingDB Entry DOI: 10.7270/Q2W37WFZ |
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase 2
(Homo sapiens (Human)) | BDBM50316226
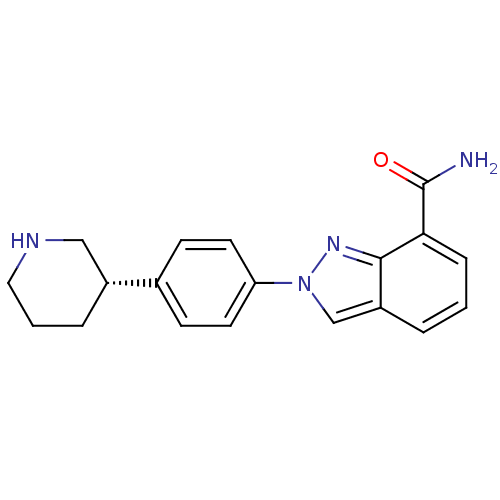
((S)-2-(4-(piperidin-3-yl)phenyl)-2H-indazole-7-car...)Show SMILES NC(=O)c1cccc2cn(nc12)-c1ccc(cc1)[C@@H]1CCCNC1 |r| Show InChI InChI=1S/C19H20N4O/c20-19(24)17-5-1-3-15-12-23(22-18(15)17)16-8-6-13(7-9-16)14-4-2-10-21-11-14/h1,3,5-9,12,14,21H,2,4,10-11H2,(H2,20,24)/t14-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM/Merck Research Labs Rome
Curated by ChEMBL
| Assay Description
Inhibition of human PARP2 by trichloroacetic acid precipitation assay |
J Med Chem 52: 7170-85 (2009)
Article DOI: 10.1021/jm901188v
BindingDB Entry DOI: 10.7270/Q2DN457M |
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase 1
(Homo sapiens (Human)) | BDBM50316237
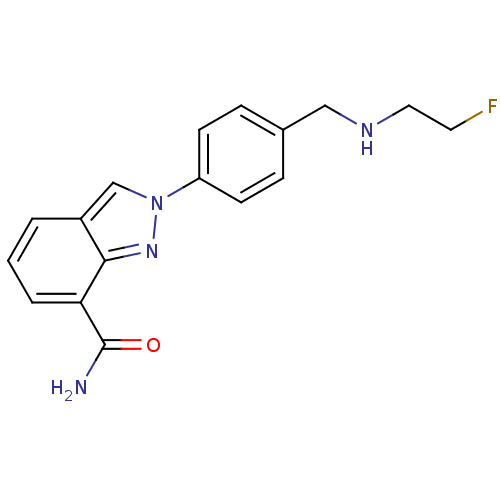
(2-(4-{[(2-Fluoroethyl)amino]methyl}phenyl)-2H-inda...)Show InChI InChI=1S/C17H17FN4O/c18-8-9-20-10-12-4-6-14(7-5-12)22-11-13-2-1-3-15(17(19)23)16(13)21-22/h1-7,11,20H,8-10H2,(H2,19,23) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM/Merck Research Labs Rome
Curated by ChEMBL
| Assay Description
Inhibition of human PARP1 by SPA |
J Med Chem 52: 7170-85 (2009)
Article DOI: 10.1021/jm901188v
BindingDB Entry DOI: 10.7270/Q2DN457M |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50612450
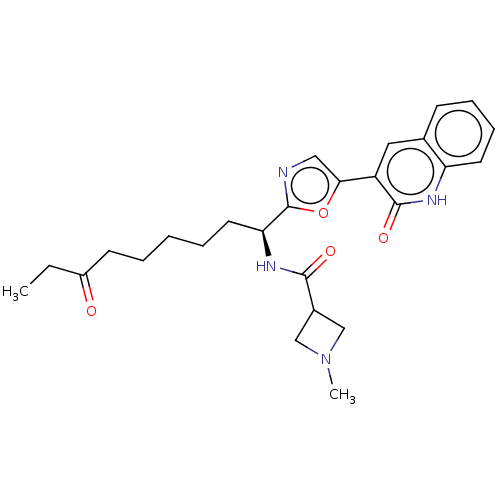
(CHEMBL5279520)Show SMILES CCC(=O)CCCCC[C@H](NC(=O)C1CN(C)C1)c1ncc(o1)-c1cc2ccccc2[nH]c1=O |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase 1
(Homo sapiens (Human)) | BDBM50316230
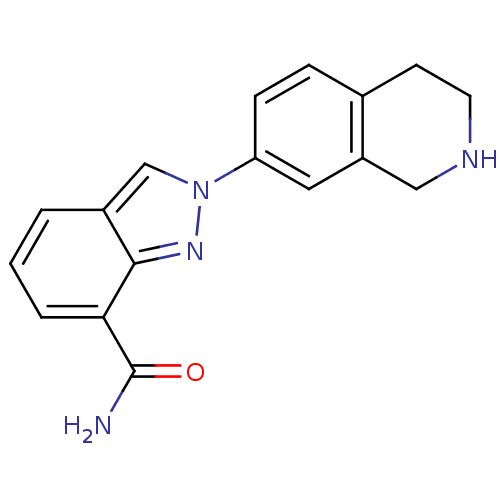
(2-(1,2,3,4-tetrahydroisoquinolin-7-yl)-2H-indazole...)Show InChI InChI=1S/C17H16N4O/c18-17(22)15-3-1-2-12-10-21(20-16(12)15)14-5-4-11-6-7-19-9-13(11)8-14/h1-5,8,10,19H,6-7,9H2,(H2,18,22) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM/Merck Research Labs Rome
Curated by ChEMBL
| Assay Description
Inhibition of human PARP1 by SPA |
J Med Chem 52: 7170-85 (2009)
Article DOI: 10.1021/jm901188v
BindingDB Entry DOI: 10.7270/Q2DN457M |
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase 1
(Homo sapiens (Human)) | BDBM50316225
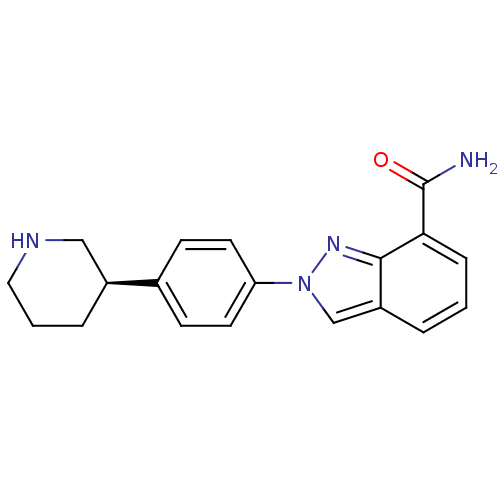
((R)-2-(4-(piperidin-3-yl)phenyl)-2H-indazole-7-car...)Show SMILES NC(=O)c1cccc2cn(nc12)-c1ccc(cc1)[C@H]1CCCNC1 |r| Show InChI InChI=1S/C19H20N4O/c20-19(24)17-5-1-3-15-12-23(22-18(15)17)16-8-6-13(7-9-16)14-4-2-10-21-11-14/h1,3,5-9,12,14,21H,2,4,10-11H2,(H2,20,24)/t14-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM/Merck Research Labs Rome
Curated by ChEMBL
| Assay Description
Inhibition of human PARP1 by SPA |
J Med Chem 52: 7170-85 (2009)
Article DOI: 10.1021/jm901188v
BindingDB Entry DOI: 10.7270/Q2DN457M |
More data for this
Ligand-Target Pair | |
Histone deacetylase 2
(Homo sapiens (Human)) | BDBM50612449

(CHEMBL5271471)Show SMILES CCC(=O)CCCCC[C@H](NC(=O)C1CN(C)C1)c1ncc(o1)-c1cc2ccccc2nc1OC |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Smoothened homolog
(Homo sapiens (Human)) | BDBM50352418
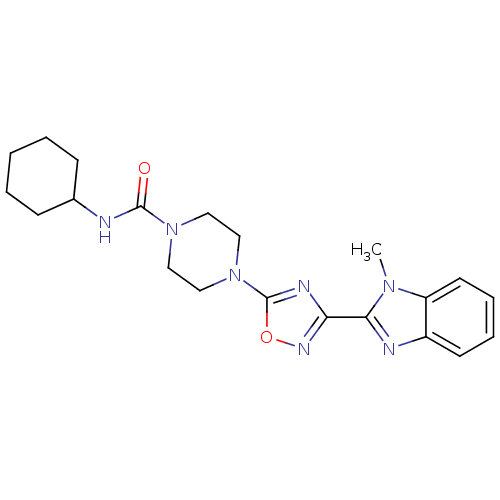
(CHEMBL1823863)Show SMILES Cn1c(nc2ccccc12)-c1noc(n1)N1CCN(CC1)C(=O)NC1CCCCC1 Show InChI InChI=1S/C21H27N7O2/c1-26-17-10-6-5-9-16(17)23-19(26)18-24-21(30-25-18)28-13-11-27(12-14-28)20(29)22-15-7-3-2-4-8-15/h5-6,9-10,15H,2-4,7-8,11-14H2,1H3,(H,22,29) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Rome
Curated by ChEMBL
| Assay Description
Displacement of Bodipy-labelled cyclopamine from Smo expressed in COS-1 cells in presence of 2% FBS after 4 to 6 hrs by FACS flow cytometric analysis |
Bioorg Med Chem Lett 21: 5274-82 (2011)
Article DOI: 10.1016/j.bmcl.2011.07.031
BindingDB Entry DOI: 10.7270/Q2MC90DS |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50258551
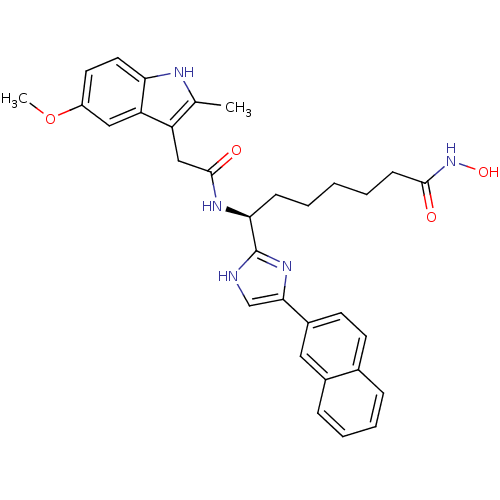
((S)-N-hydroxy-7-(2-(5-methoxy-2-methyl-1H-indol-3-...)Show SMILES COc1ccc2[nH]c(C)c(CC(=O)N[C@@H](CCCCCC(=O)NO)c3nc(c[nH]3)-c3ccc4ccccc4c3)c2c1 |r| Show InChI InChI=1S/C32H35N5O4/c1-20-25(26-17-24(41-2)14-15-27(26)34-20)18-31(39)35-28(10-4-3-5-11-30(38)37-40)32-33-19-29(36-32)23-13-12-21-8-6-7-9-22(21)16-23/h6-9,12-17,19,28,34,40H,3-5,10-11,18H2,1-2H3,(H,33,36)(H,35,39)(H,37,38)/t28-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM/Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of C-terminal FLAG tagged HDAC1 (unknown origin) |
Bioorg Med Chem Lett 19: 3081-4 (2009)
Article DOI: 10.1016/j.bmcl.2009.04.011
BindingDB Entry DOI: 10.7270/Q2P84BSJ |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50612452
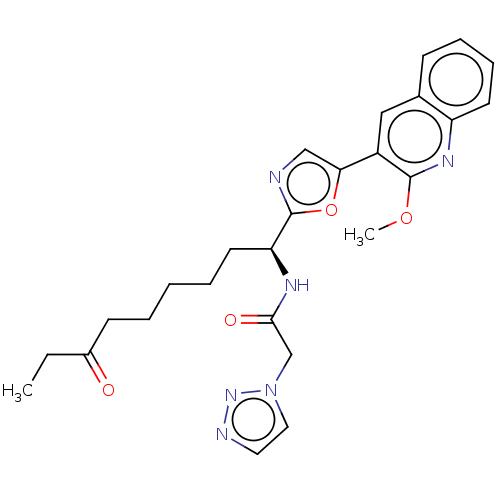
(CHEMBL5285418)Show SMILES CCC(=O)CCCCC[C@H](NC(=O)Cn1ccnn1)c1ncc(o1)-c1cc2ccccc2nc1OC |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 11
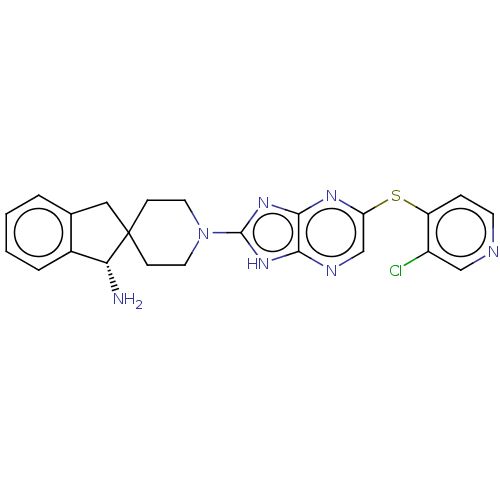
(Homo sapiens (Human)) | BDBM50406432
(CHEMBL5288562)Show InChI InChI=1S/C11H17NO5S2/c1-11(2,13)7-8-18(14,15)9-3-5-10(6-4-9)19(12,16)17/h3-6,13H,7-8H2,1-2H3,(H2,12,16,17) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
In vitro inhibition of human dihydrofolate reductase |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase 1
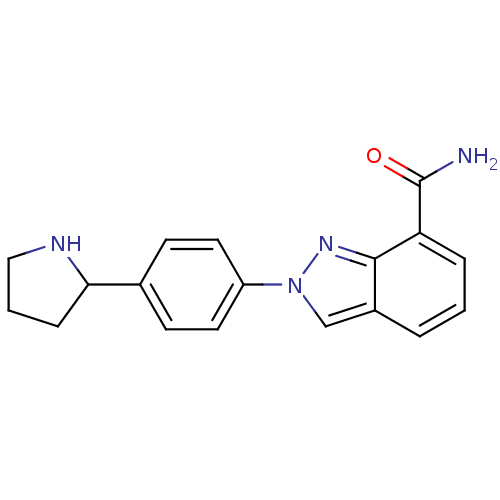
(Homo sapiens (Human)) | BDBM50316228
(2-(4-(pyrrolidin-2-yl)phenyl)-2H-indazole-7-carbox...)Show InChI InChI=1S/C18H18N4O/c19-18(23)15-4-1-3-13-11-22(21-17(13)15)14-8-6-12(7-9-14)16-5-2-10-20-16/h1,3-4,6-9,11,16,20H,2,5,10H2,(H2,19,23) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM/Merck Research Labs Rome
Curated by ChEMBL
| Assay Description
Inhibition of human PARP1 by SPA |
J Med Chem 52: 7170-85 (2009)
Article DOI: 10.1021/jm901188v
BindingDB Entry DOI: 10.7270/Q2DN457M |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
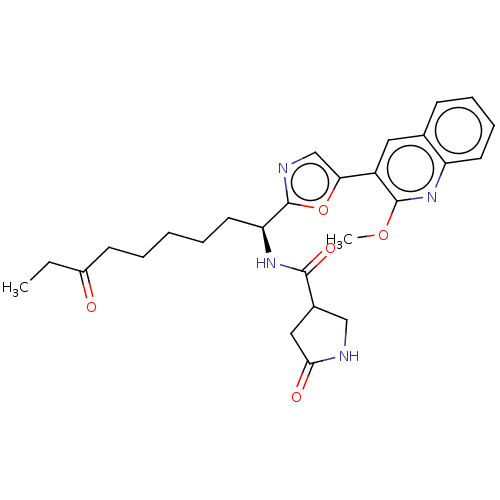
(Homo sapiens (Human)) | BDBM50612453
(CHEMBL5285433)Show SMILES CCC(=O)CCCCC[C@H](NC(=O)C1CNC(=O)C1)c1ncc(o1)-c1cc2ccccc2nc1OC |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase 1
(Homo sapiens (Human)) | BDBM50316225

((R)-2-(4-(piperidin-3-yl)phenyl)-2H-indazole-7-car...)Show SMILES NC(=O)c1cccc2cn(nc12)-c1ccc(cc1)[C@H]1CCCNC1 |r| Show InChI InChI=1S/C19H20N4O/c20-19(24)17-5-1-3-15-12-23(22-18(15)17)16-8-6-13(7-9-16)14-4-2-10-21-11-14/h1,3,5-9,12,14,21H,2,4,10-11H2,(H2,20,24)/t14-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM/Merck Research Labs Rome
Curated by ChEMBL
| Assay Description
Inhibition of human PARP1 by SPA |
J Med Chem 52: 7170-85 (2009)
Article DOI: 10.1021/jm901188v
BindingDB Entry DOI: 10.7270/Q2DN457M |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50258549
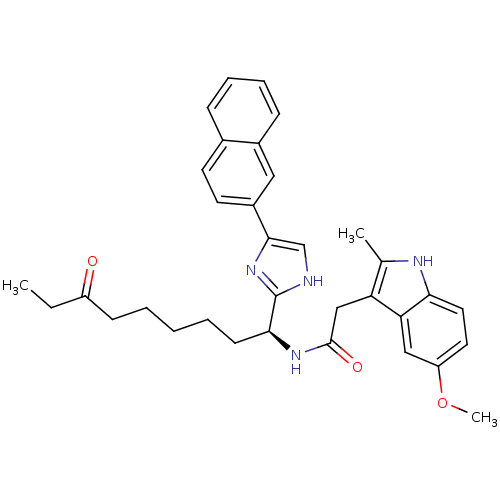
((S)-2-(5-methoxy-2-methyl-1H-indol-3-yl)-N-(1-(5-(...)Show SMILES CCC(=O)CCCCC[C@H](NC(=O)Cc1c(C)[nH]c2ccc(OC)cc12)c1nc(c[nH]1)-c1ccc2ccccc2c1 |r| Show InChI InChI=1S/C34H38N4O3/c1-4-26(39)12-6-5-7-13-31(34-35-21-32(38-34)25-15-14-23-10-8-9-11-24(23)18-25)37-33(40)20-28-22(2)36-30-17-16-27(41-3)19-29(28)30/h8-11,14-19,21,31,36H,4-7,12-13,20H2,1-3H3,(H,35,38)(H,37,40)/t31-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase 1
(Homo sapiens (Human)) | BDBM50316226

((S)-2-(4-(piperidin-3-yl)phenyl)-2H-indazole-7-car...)Show SMILES NC(=O)c1cccc2cn(nc12)-c1ccc(cc1)[C@@H]1CCCNC1 |r| Show InChI InChI=1S/C19H20N4O/c20-19(24)17-5-1-3-15-12-23(22-18(15)17)16-8-6-13(7-9-16)14-4-2-10-21-11-14/h1,3,5-9,12,14,21H,2,4,10-11H2,(H2,20,24)/t14-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM/Merck Research Labs Rome
Curated by ChEMBL
| Assay Description
Inhibition of human PARP1 by SPA |
J Med Chem 52: 7170-85 (2009)
Article DOI: 10.1021/jm901188v
BindingDB Entry DOI: 10.7270/Q2DN457M |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Histone deacetylase 3
(Homo sapiens (Human)) | BDBM50612445

(CHEMBL5282110)Show SMILES CCC(=O)CCCCC[C@H](NC(=O)C12CCN(CC1)CC2)c1ncc(o1)-c1cc2ccccc2nc1OC |r| | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | <3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data