

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Muscarinic acetylcholine receptor M1/M2/M3/M4/M5 (RAT) | BDBM50471498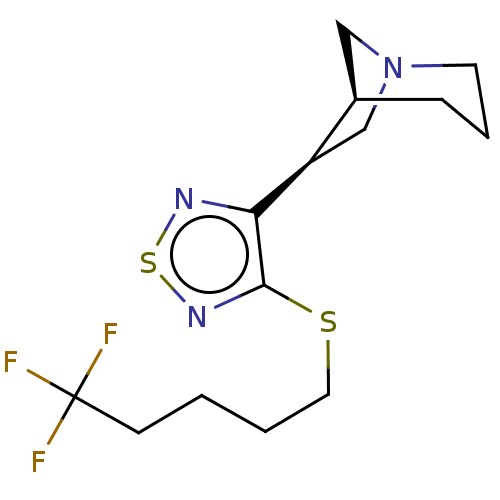 (CHEMBL150450) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Binding affinity against Muscarinic acetylcholine receptors using [3H]oxotremorine-M as radioligand in rat cortex | J Med Chem 40: 538-46 (1997) Article DOI: 10.1021/jm9602470 BindingDB Entry DOI: 10.7270/Q2SQ934F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1/M2/M3/M4/M5 (RAT) | BDBM50471487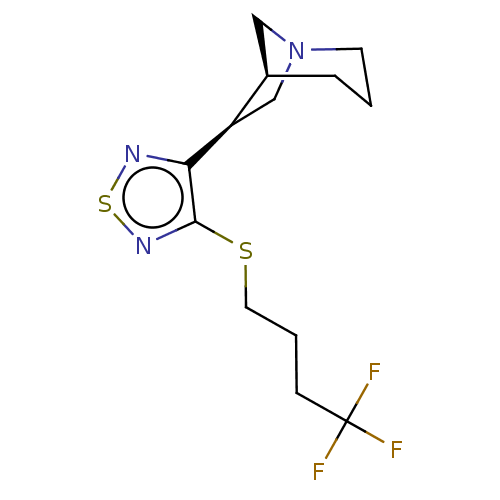 (CHEMBL436075) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Binding affinity against Muscarinic acetylcholine receptors using [3H]oxotremorine-M as radioligand in rat cortex | J Med Chem 40: 538-46 (1997) Article DOI: 10.1021/jm9602470 BindingDB Entry DOI: 10.7270/Q2SQ934F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1/M2/M3/M4/M5 (RAT) | BDBM50471494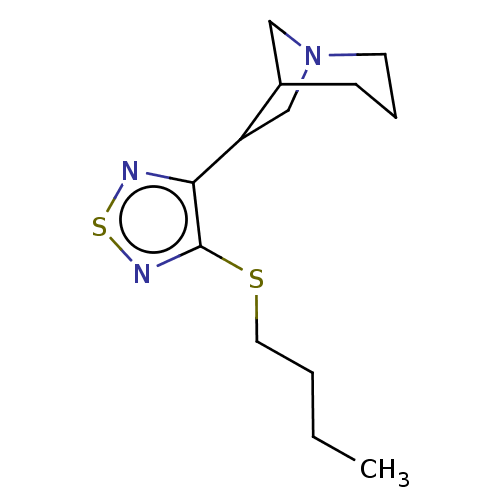 (CHEMBL153723) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Binding affinity against Muscarinic acetylcholine receptors using [3H]oxotremorine-M as radioligand in rat cortex | J Med Chem 40: 538-46 (1997) Article DOI: 10.1021/jm9602470 BindingDB Entry DOI: 10.7270/Q2SQ934F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1/M2/M3/M4/M5 (RAT) | BDBM50471485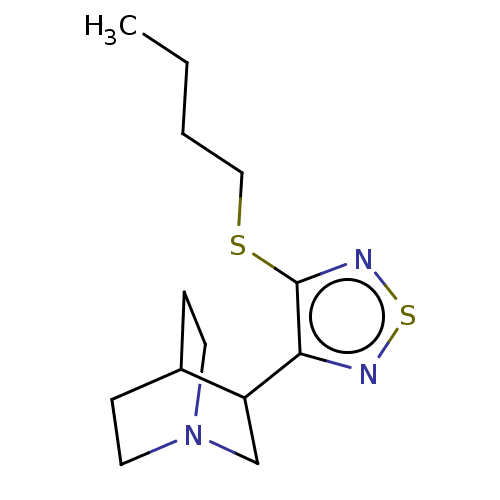 (Vedaclidine) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Binding affinity against Muscarinic acetylcholine receptors using [3H]oxotremorine-M as radioligand in rat cortex | J Med Chem 40: 538-46 (1997) Article DOI: 10.1021/jm9602470 BindingDB Entry DOI: 10.7270/Q2SQ934F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1/M2/M3/M4/M5 (RAT) | BDBM50471499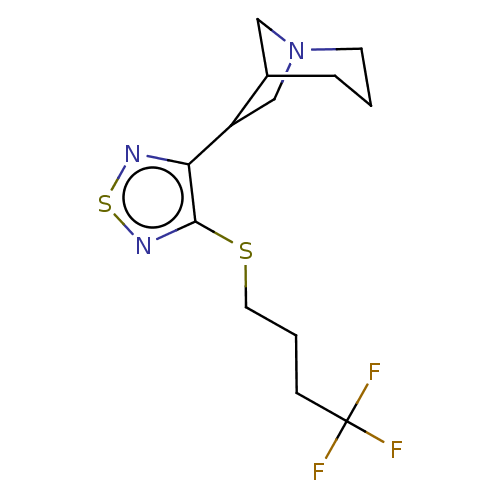 (CHEMBL345774) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Binding affinity against Muscarinic acetylcholine receptor using [3H]oxotremorine-M as radioligand in rat cortex | J Med Chem 40: 538-46 (1997) Article DOI: 10.1021/jm9602470 BindingDB Entry DOI: 10.7270/Q2SQ934F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1/M2/M3/M4/M5 (RAT) | BDBM50471492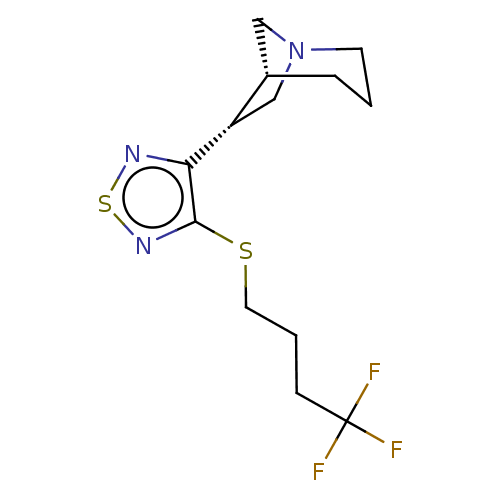 (CHEMBL150293) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Binding affinity against Muscarinic acetylcholine receptors using [3H]oxotremorine-M as radioligand in rat cortex | J Med Chem 40: 538-46 (1997) Article DOI: 10.1021/jm9602470 BindingDB Entry DOI: 10.7270/Q2SQ934F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1/M2/M3/M4/M5 (RAT) | BDBM50471495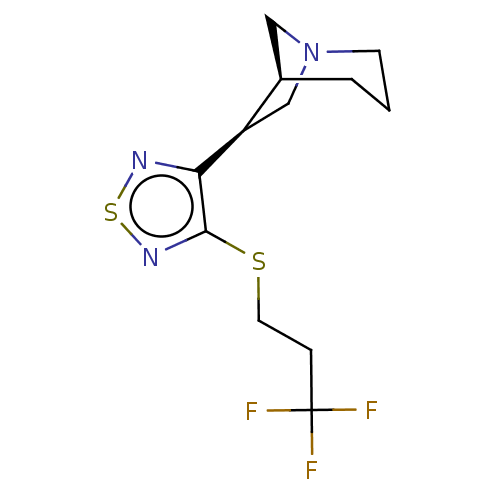 (CHEMBL153478) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.440 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Binding affinity against Muscarinic acetylcholine receptors using [3H]oxotremorine-M as radioligand in rat cortex | J Med Chem 40: 538-46 (1997) Article DOI: 10.1021/jm9602470 BindingDB Entry DOI: 10.7270/Q2SQ934F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1/M2/M3/M4/M5 (RAT) | BDBM50471486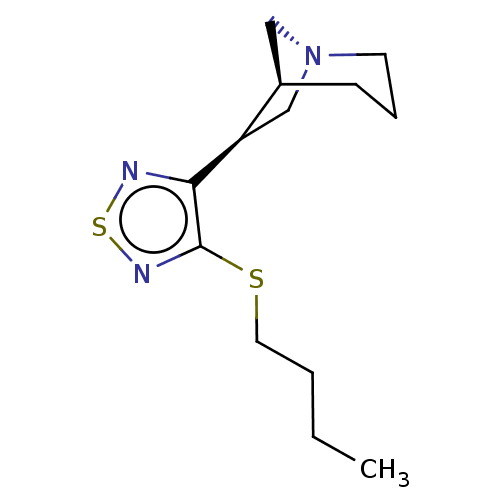 (CHEMBL343731) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Binding affinity against Muscarinic acetylcholine receptors using [3H]oxotremorine-M as radioligand in rat cortex | J Med Chem 40: 538-46 (1997) Article DOI: 10.1021/jm9602470 BindingDB Entry DOI: 10.7270/Q2SQ934F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1/M2/M3/M4/M5 (RAT) | BDBM50033155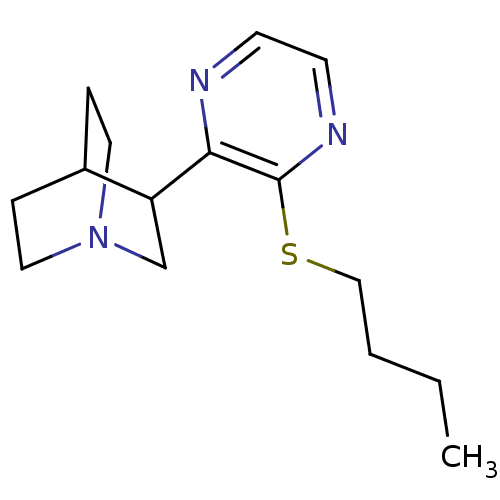 (3-(3-Butylsulfanyl-pyrazin-2-yl)-1-aza-bicyclo[2.2...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Binding affinity against Muscarinic acetylcholine receptors using [3H]oxotremorine-M as radioligand in rat cortex | J Med Chem 40: 538-46 (1997) Article DOI: 10.1021/jm9602470 BindingDB Entry DOI: 10.7270/Q2SQ934F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1/M2/M3/M4/M5 (RAT) | BDBM50403547 (ATROPEN | ATROPINE) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Binding affinity against Muscarinic acetylcholine receptors using [3H]oxotremorine-M as radioligand in rat cortex | J Med Chem 40: 538-46 (1997) Article DOI: 10.1021/jm9602470 BindingDB Entry DOI: 10.7270/Q2SQ934F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1/M2/M3/M4/M5 (RAT) | BDBM50471491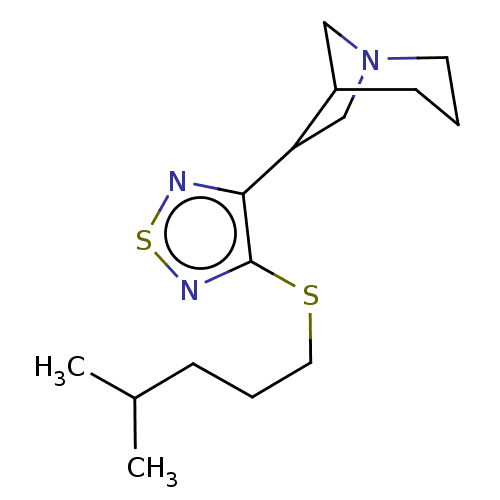 (CHEMBL357279) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.910 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Binding affinity against Muscarinic acetylcholine receptors using [3H]oxotremorine-M as radioligand in rat cortex | J Med Chem 40: 538-46 (1997) Article DOI: 10.1021/jm9602470 BindingDB Entry DOI: 10.7270/Q2SQ934F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1/M2/M3/M4/M5 (RAT) | BDBM50070725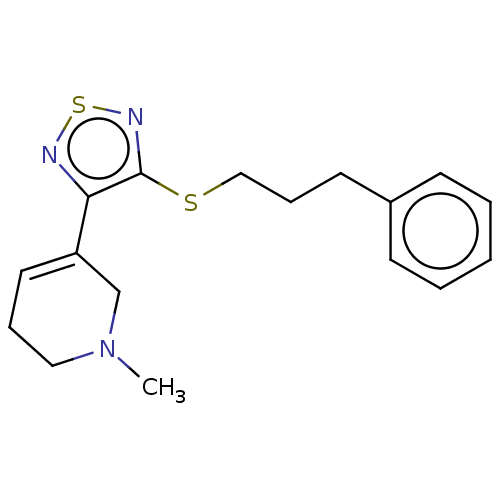 (CHEMBL318403) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Binding affinity against Muscarinic acetylcholine receptors using [3H]oxotremorine-M as radioligand in rat cortex | J Med Chem 40: 538-46 (1997) Article DOI: 10.1021/jm9602470 BindingDB Entry DOI: 10.7270/Q2SQ934F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1/M2/M3/M4/M5 (RAT) | BDBM50003351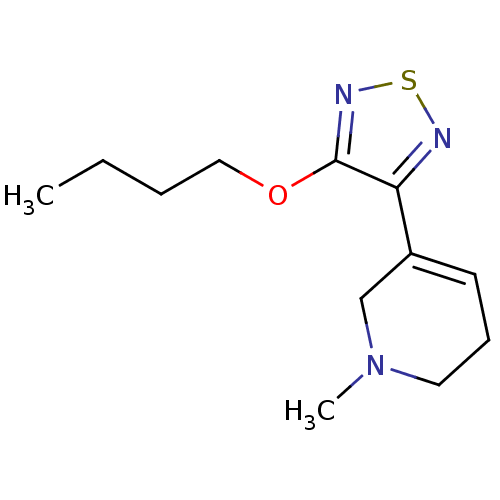 (3-(4-butoxy-1,2,5-thiadiazol-3-yl)-1-methyl-1,2,5,...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Binding affinity against Muscarinic acetylcholine receptors using [3H]oxotremorine-M as radioligand in rat cortex | J Med Chem 40: 538-46 (1997) Article DOI: 10.1021/jm9602470 BindingDB Entry DOI: 10.7270/Q2SQ934F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1/M2/M3/M4/M5 (RAT) | BDBM50471489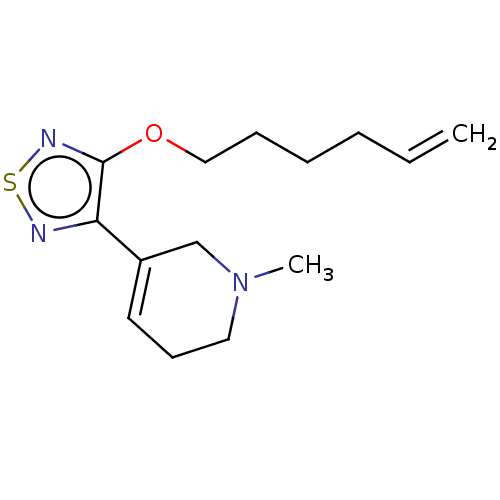 (CHEMBL153150) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Binding affinity against Muscarinic acetylcholine receptors using [3H]oxotremorine-M as radioligand in rat cortex | J Med Chem 40: 538-46 (1997) Article DOI: 10.1021/jm9602470 BindingDB Entry DOI: 10.7270/Q2SQ934F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1/M2/M3/M4/M5 (RAT) | BDBM50471496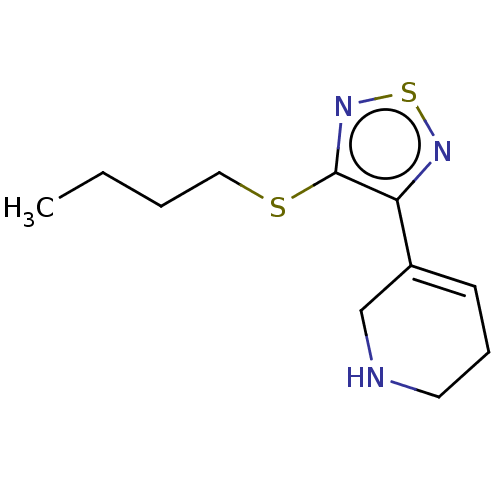 (CHEMBL153031) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Binding affinity against Muscarinic acetylcholine receptors using [3H]oxotremorine-M as radioligand in rat cortex | J Med Chem 40: 538-46 (1997) Article DOI: 10.1021/jm9602470 BindingDB Entry DOI: 10.7270/Q2SQ934F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1/M2/M3/M4/M5 (RAT) | BDBM50033151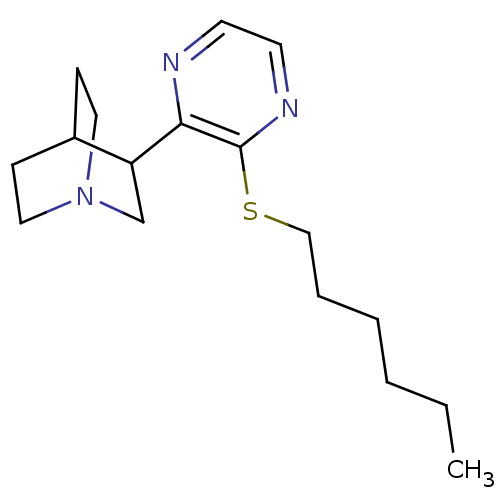 (3-(3-Hexylsulfanyl-pyrazin-2-yl)-1-aza-bicyclo[2.2...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Binding affinity against Muscarinic acetylcholine receptors using [3H]oxotremorine-M as radioligand in rat cortex | J Med Chem 40: 538-46 (1997) Article DOI: 10.1021/jm9602470 BindingDB Entry DOI: 10.7270/Q2SQ934F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1/M2/M3/M4/M5 (RAT) | BDBM50471497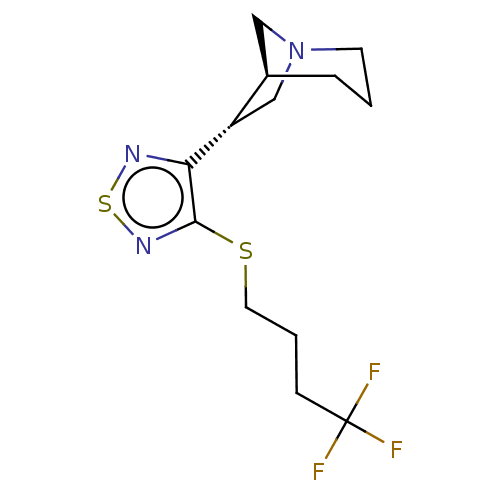 (CHEMBL150501) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 9.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Binding affinity against Muscarinic acetylcholine receptors using [3H]oxotremorine-M as radioligand in rat cortex | J Med Chem 40: 538-46 (1997) Article DOI: 10.1021/jm9602470 BindingDB Entry DOI: 10.7270/Q2SQ934F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1/M2/M3/M4/M5 (RAT) | BDBM50471488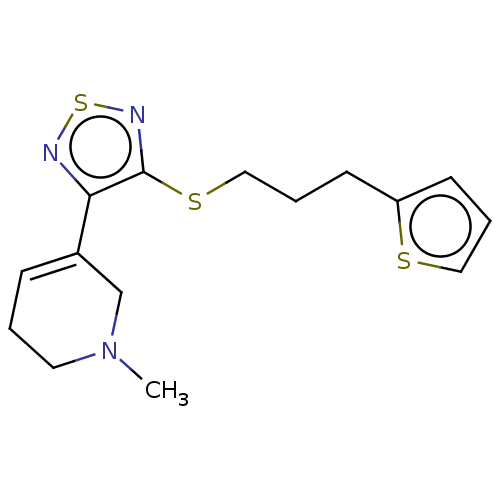 (CHEMBL152892) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Binding affinity against Muscarinic acetylcholine receptors using [3H]oxotremorine-M as radioligand in rat cortex | J Med Chem 40: 538-46 (1997) Article DOI: 10.1021/jm9602470 BindingDB Entry DOI: 10.7270/Q2SQ934F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1/M2/M3/M4/M5 (RAT) | BDBM50471493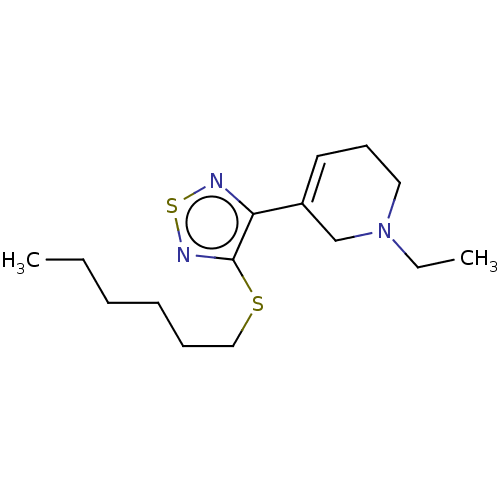 (CHEMBL152919) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Binding affinity against Muscarinic acetylcholine receptors using [3H]oxotremorine-M as radioligand in rat cortex | J Med Chem 40: 538-46 (1997) Article DOI: 10.1021/jm9602470 BindingDB Entry DOI: 10.7270/Q2SQ934F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1/M2/M3/M4/M5 (RAT) | BDBM50471490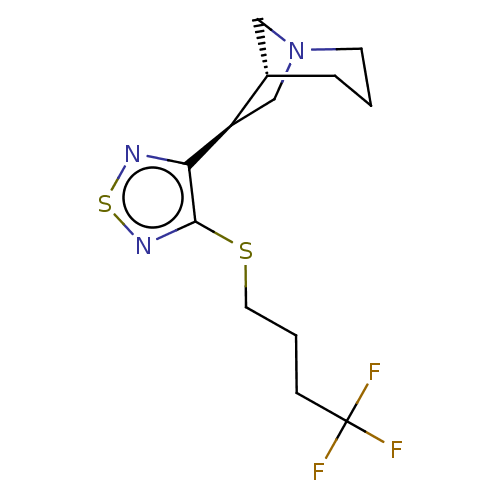 (CHEMBL154923) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Binding affinity against Muscarinic acetylcholine receptors using [3H]oxotremorine-M as radioligand in rat cortex | J Med Chem 40: 538-46 (1997) Article DOI: 10.1021/jm9602470 BindingDB Entry DOI: 10.7270/Q2SQ934F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (Homo sapiens (Human)) | BDBM50003355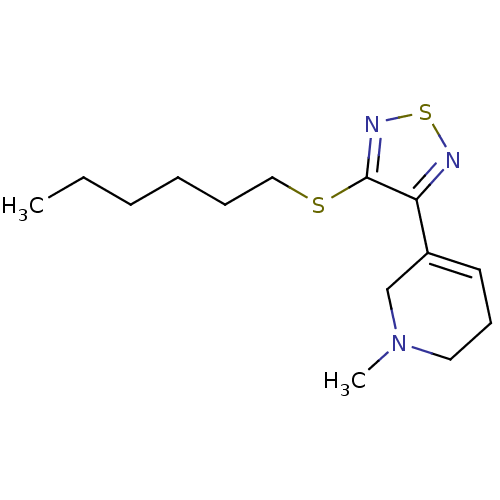 (5-(4-Hexylsulfanyl-[1,2,5]thiadiazol-3-yl)-1-methy...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.00100 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Efficacy at muscarinic acetylcholine receptor M1 measured by the ability to inhibit the electrically stimulated twitch of the rabbit vas deferens | J Med Chem 35: 4011-9 (1992) BindingDB Entry DOI: 10.7270/Q2KW5F1K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (Homo sapiens (Human)) | BDBM50003363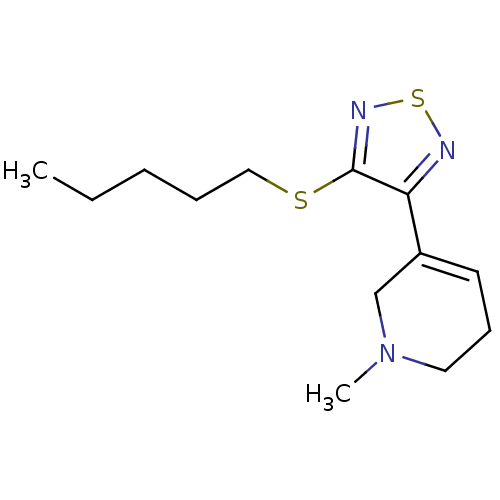 (1-Methyl-5-(4-pentylsulfanyl-[1,2,5]thiadiazol-3-y...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.00200 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Efficacy at muscarinic acetylcholine receptor M1 measured by the ability to inhibit the electrically stimulated twitch of the rabbit vas deferens | J Med Chem 35: 4011-9 (1992) BindingDB Entry DOI: 10.7270/Q2KW5F1K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (Homo sapiens (Human)) | BDBM50003366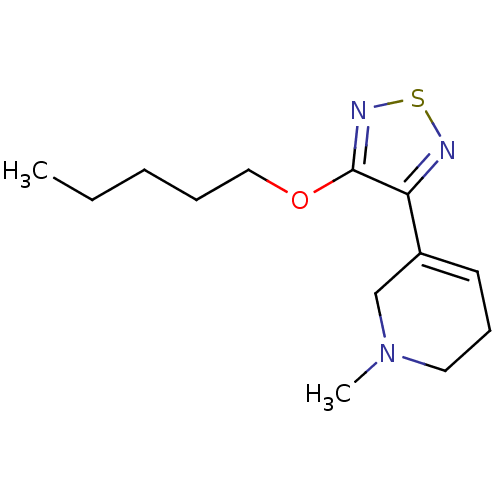 (1-Methyl-5-(4-pentyloxy-[1,2,5]thiadiazol-3-yl)-1,...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.00400 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Efficacy at muscarinic acetylcholine receptor M1 measured by the ability to inhibit the electrically stimulated twitch of the rabbit vas deferens | J Med Chem 35: 4011-9 (1992) BindingDB Entry DOI: 10.7270/Q2KW5F1K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (Homo sapiens (Human)) | BDBM50003359 (5-(4-Hexyloxy-[1,2,5]thiadiazol-3-yl)-1-methyl-1,2...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 0.00800 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Efficacy at muscarinic acetylcholine receptor M1 measured by the ability to inhibit the electrically stimulated twitch of the rabbit vas deferens | J Med Chem 35: 4011-9 (1992) BindingDB Entry DOI: 10.7270/Q2KW5F1K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (RAT) | BDBM50062577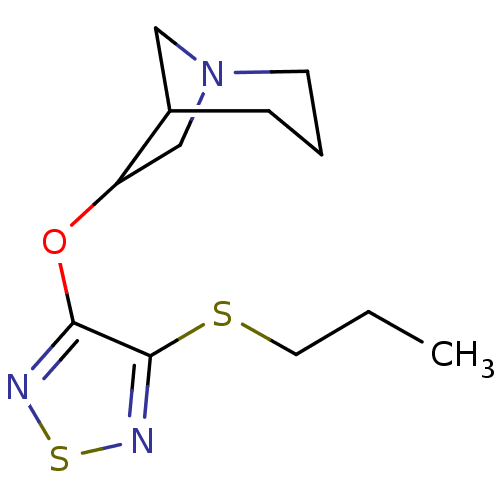 (6-(4-Propylsulfanyl-[1,2,5]thiadiazol-3-yloxy)-1-a...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.380 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Binding affinity for muscarinic acetylcholine receptor M1 using [3H]-oxotremorine-M (Oxo-M) radioligand in rat hippocampus membranes | J Med Chem 41: 379-92 (1998) Article DOI: 10.1021/jm970125n BindingDB Entry DOI: 10.7270/Q2SX6CBC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (RAT) | BDBM50033155 (3-(3-Butylsulfanyl-pyrazin-2-yl)-1-aza-bicyclo[2.2...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Binding activity against muscarinic acetylcholine receptor M1 in rat brain, using [3H]-Pz as the radioligand. | J Med Chem 38: 3469-81 (1995) BindingDB Entry DOI: 10.7270/Q2TB17JZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (RAT) | BDBM50033155 (3-(3-Butylsulfanyl-pyrazin-2-yl)-1-aza-bicyclo[2.2...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Binding activity against muscarinic acetylcholine receptor M1 in rat brain, using [3H]OXO-M as the radioligand. | J Med Chem 38: 3469-81 (1995) BindingDB Entry DOI: 10.7270/Q2TB17JZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (RAT) | BDBM50062577 (6-(4-Propylsulfanyl-[1,2,5]thiadiazol-3-yloxy)-1-a...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.550 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Binding affinity for muscarinic acetylcholine receptor M1 using [3H]-Pirenzepine (Pz) radioligand in rat hippocampus membranes. | J Med Chem 41: 379-92 (1998) Article DOI: 10.1021/jm970125n BindingDB Entry DOI: 10.7270/Q2SX6CBC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (RAT) | BDBM50062577 (6-(4-Propylsulfanyl-[1,2,5]thiadiazol-3-yloxy)-1-a...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.550 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Binding affinity for muscarinic acetylcholine receptor M1 using [3H]-Pirenzepine (Pz) radioligand in rat hippocampus membranes. | J Med Chem 41: 379-92 (1998) Article DOI: 10.1021/jm970125n BindingDB Entry DOI: 10.7270/Q2SX6CBC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (RAT) | BDBM50062595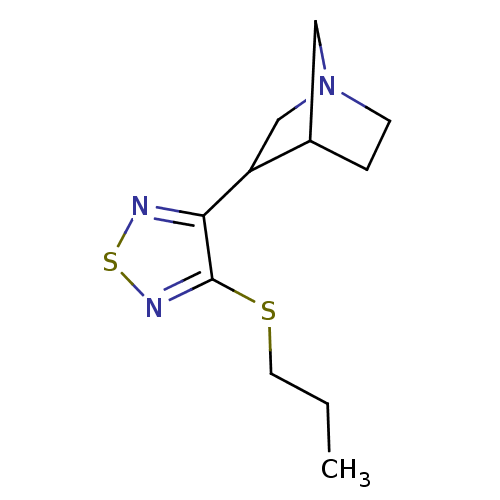 (3-(4-Propylsulfanyl-[1,2,5]thiadiazol-3-yl)-1-aza-...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Binding affinity for muscarinic acetylcholine receptor M1 using [3H]-oxotremorine-M (Oxo-M) radioligand in rat hippocampus membranes | J Med Chem 41: 379-92 (1998) Article DOI: 10.1021/jm970125n BindingDB Entry DOI: 10.7270/Q2SX6CBC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (RAT) | BDBM50062595 (3-(4-Propylsulfanyl-[1,2,5]thiadiazol-3-yl)-1-aza-...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Binding affinity for muscarinic acetylcholine receptor M1 using [3H]-oxotremorine-M (Oxo-M) radioligand in rat hippocampus membranes | J Med Chem 41: 379-92 (1998) Article DOI: 10.1021/jm970125n BindingDB Entry DOI: 10.7270/Q2SX6CBC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (RAT) | BDBM50033162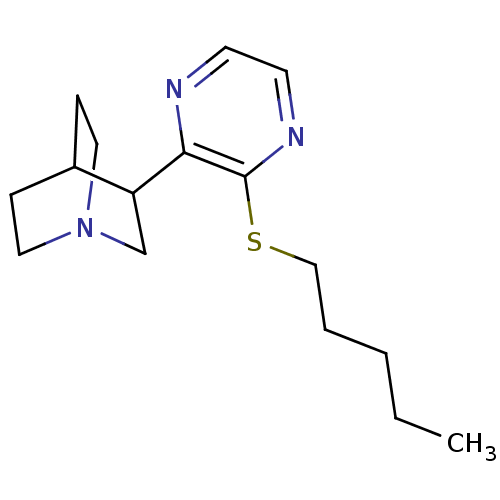 (3-(3-Pentylsulfanyl-pyrazin-2-yl)-1-aza-bicyclo[2....) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Binding activity against muscarinic acetylcholine receptor M1 in rat brain, using [3H]OXO-M as the radioligand. | J Med Chem 38: 3469-81 (1995) BindingDB Entry DOI: 10.7270/Q2TB17JZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1/M2/M3/M4/M5 (RAT) | BDBM50070692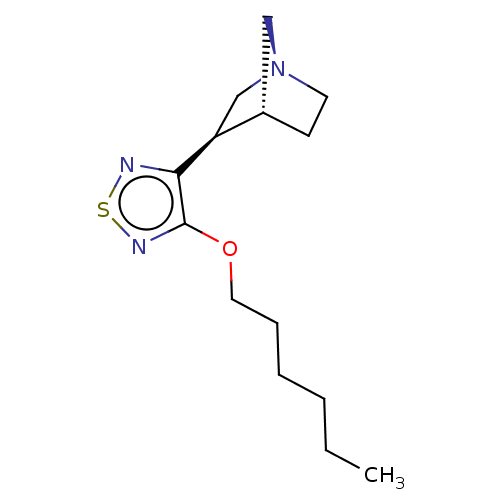 (CHEMBL99240) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.730 | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S Curated by ChEMBL | Assay Description Binding affinity against muscarinic receptor in rat brain membranes using oxotremorine-M as ligand | Bioorg Med Chem Lett 8: 2897-902 (1999) BindingDB Entry DOI: 10.7270/Q27M08GK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (RAT) | BDBM50062570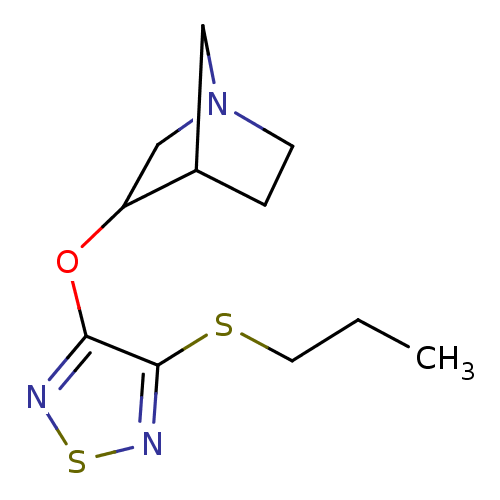 (3-(4-Propylsulfanyl-[1,2,5]thiadiazol-3-yloxy)-1-a...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.760 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Binding affinity for muscarinic acetylcholine receptor M1 using [3H]-oxotremorine-M (Oxo-M) radioligand in rat hippocampus membranes | J Med Chem 41: 379-92 (1998) Article DOI: 10.1021/jm970125n BindingDB Entry DOI: 10.7270/Q2SX6CBC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1/M2/M3/M4/M5 (RAT) | BDBM50072214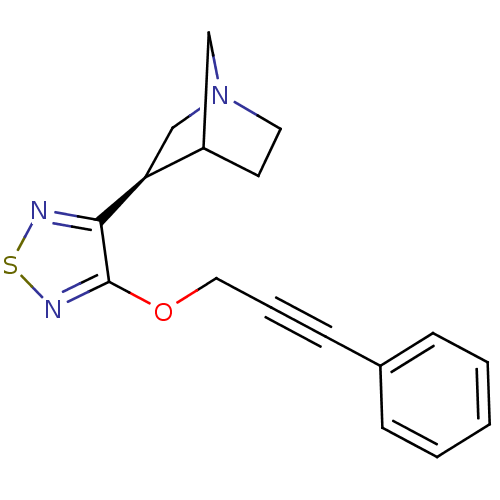 ((R)-3-[4-(3-Phenyl-prop-2-ynyloxy)-[1,2,5]thiadiaz...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S Curated by ChEMBL | Assay Description Binding affinity against muscarinic receptor in rat brain membranes using oxotremorine-M as ligand | Bioorg Med Chem Lett 8: 2897-902 (1999) BindingDB Entry DOI: 10.7270/Q27M08GK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (RAT) | BDBM50006582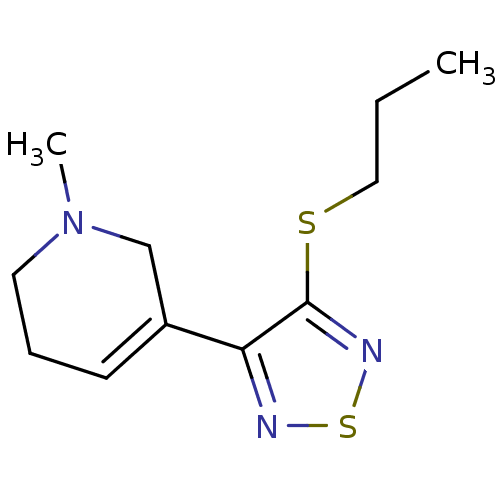 (1-Methyl-5-(4-propylsulfanyl-[1,2,5]thiadiazol-3-y...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk CNS Division Curated by ChEMBL | Assay Description In vitro binding affinity against muscarinic acetylcholine receptor M1 from rat hippocampus, using [3H]-oxotremorine-M (Oxo-M) as radioligand | J Med Chem 35: 2274-83 (1992) BindingDB Entry DOI: 10.7270/Q22J69TH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (RAT) | BDBM50033162 (3-(3-Pentylsulfanyl-pyrazin-2-yl)-1-aza-bicyclo[2....) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Binding activity against muscarinic acetylcholine receptor M1 in rat brain, using [3H]-Pz as the radioligand. | J Med Chem 38: 3469-81 (1995) BindingDB Entry DOI: 10.7270/Q2TB17JZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1/M2/M3/M4/M5 (RAT) | BDBM50072227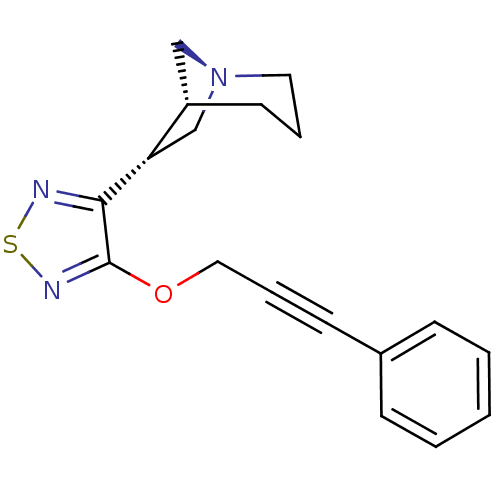 ((1R,5R,6R)-6-[4-(3-Phenyl-prop-2-ynyloxy)-[1,2,5]t...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S Curated by ChEMBL | Assay Description Binding affinity against muscarinic receptor in rat brain membranes using oxotremorine-M as ligand | Bioorg Med Chem Lett 8: 2897-902 (1999) BindingDB Entry DOI: 10.7270/Q27M08GK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1/M2/M3/M4/M5 (RAT) | BDBM50070735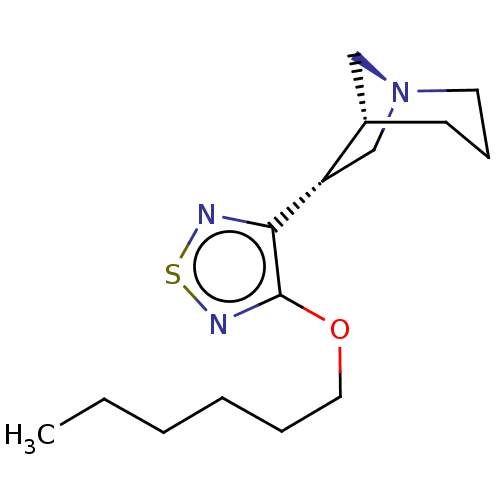 (CHEMBL317324) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S Curated by ChEMBL | Assay Description Binding affinity against muscarinic receptor in rat brain membranes using oxotremorine-M as ligand | Bioorg Med Chem Lett 8: 2897-902 (1999) BindingDB Entry DOI: 10.7270/Q27M08GK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1/M2/M3/M4/M5 (RAT) | BDBM50070647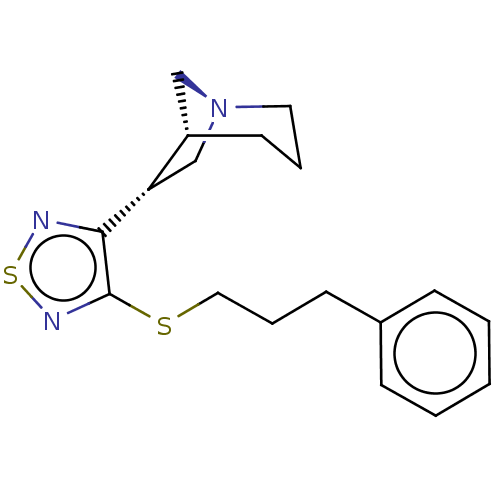 (CHEMBL329924) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S Curated by ChEMBL | Assay Description Binding affinity against muscarinic receptor in rat brain membranes using oxotremorine-M as ligand | Bioorg Med Chem Lett 8: 2897-902 (1999) BindingDB Entry DOI: 10.7270/Q27M08GK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (RAT) | BDBM50006572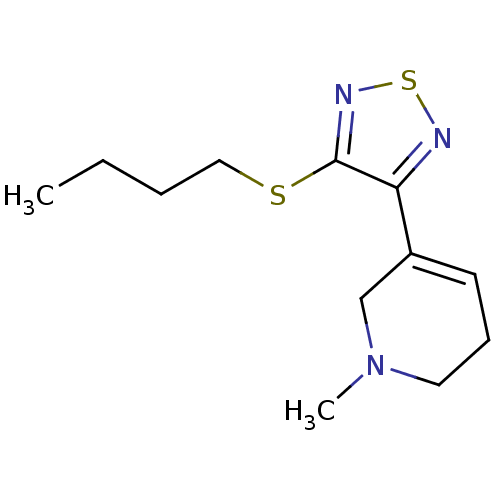 (5-(4-Butylsulfanyl-[1,2,5]thiadiazol-3-yl)-1-methy...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk CNS Division Curated by ChEMBL | Assay Description In vitro binding affinity against muscarinic acetylcholine receptor M1 from rat hippocampus, using [3H]-pirenzepine (Pz) as radioligand | J Med Chem 35: 2274-83 (1992) BindingDB Entry DOI: 10.7270/Q22J69TH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (RAT) | BDBM50006572 (5-(4-Butylsulfanyl-[1,2,5]thiadiazol-3-yl)-1-methy...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk CNS Division Curated by ChEMBL | Assay Description In vitro binding affinity against muscarinic acetylcholine receptor M1 from rat hippocampus, using [3H]-pirenzepine (Pz) as radioligand | J Med Chem 35: 2274-83 (1992) BindingDB Entry DOI: 10.7270/Q22J69TH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1/M2/M3/M4/M5 (RAT) | BDBM50070725 (CHEMBL318403) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S Curated by ChEMBL | Assay Description Binding affinity against muscarinic receptor in rat brain membranes using oxotremorine-M as ligand | Bioorg Med Chem Lett 8: 2897-902 (1999) BindingDB Entry DOI: 10.7270/Q27M08GK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (RAT) | BDBM50003351 (3-(4-butoxy-1,2,5-thiadiazol-3-yl)-1-methyl-1,2,5,...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]-oxotremorine-M (Oxo-M) from rat hippocampus muscarinic acetylcholine receptor M1 | J Med Chem 35: 4011-9 (1992) BindingDB Entry DOI: 10.7270/Q2KW5F1K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (RAT) | BDBM50003351 (3-(4-butoxy-1,2,5-thiadiazol-3-yl)-1-methyl-1,2,5,...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk CNS Division Curated by ChEMBL | Assay Description In vitro binding affinity against muscarinic acetylcholine receptor M1 from rat hippocampus, using [3H]-oxotremorine-M (Oxo-M) as radioligand | J Med Chem 35: 2274-83 (1992) BindingDB Entry DOI: 10.7270/Q22J69TH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1/M2/M3/M4/M5 (RAT) | BDBM50003351 (3-(4-butoxy-1,2,5-thiadiazol-3-yl)-1-methyl-1,2,5,...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S Curated by ChEMBL | Assay Description Binding affinity against muscarinic receptor in rat brain membranes using oxotremorine-M as ligand | Bioorg Med Chem Lett 8: 2897-902 (1999) BindingDB Entry DOI: 10.7270/Q27M08GK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (RAT) | BDBM50003351 (3-(4-butoxy-1,2,5-thiadiazol-3-yl)-1-methyl-1,2,5,...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk CNS Division Curated by ChEMBL | Assay Description In vitro binding affinity against muscarinic acetylcholine receptor M1 from rat hippocampus, using [3H]-oxotremorine-M (Oxo-M) as radioligand | J Med Chem 35: 2274-83 (1992) BindingDB Entry DOI: 10.7270/Q22J69TH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1/M2/M3/M4/M5 (RAT) | BDBM50003369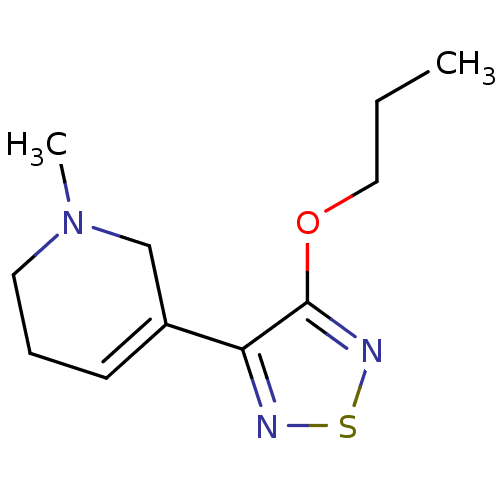 (1-Methyl-5-(4-propoxy-[1,2,5]thiadiazol-3-yl)-1,2,...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S Curated by ChEMBL | Assay Description Binding affinity against muscarinic receptor in rat brain membranes using oxotremorine-M as ligand | Bioorg Med Chem Lett 8: 2897-902 (1999) BindingDB Entry DOI: 10.7270/Q27M08GK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (RAT) | BDBM50003369 (1-Methyl-5-(4-propoxy-[1,2,5]thiadiazol-3-yl)-1,2,...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description In vitro binding affinity against rat hippocampus Muscarinic acetylcholine receptor M1 using [3H]-oxotremorine-M (Oxo-M) as radioligand | J Med Chem 35: 4011-9 (1992) BindingDB Entry DOI: 10.7270/Q2KW5F1K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (RAT) | BDBM50003369 (1-Methyl-5-(4-propoxy-[1,2,5]thiadiazol-3-yl)-1,2,...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk CNS Division Curated by ChEMBL | Assay Description In vitro binding affinity against muscarinic acetylcholine receptor M1 from rat hippocampus, using [3H]-pirenzepine (Pz) as radioligand | J Med Chem 35: 2274-83 (1992) BindingDB Entry DOI: 10.7270/Q22J69TH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 515 total ) | Next | Last >> |