

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Voltage-dependent T-type calcium channel subunit alpha-1G (Homo sapiens (Human)) | BDBM50157857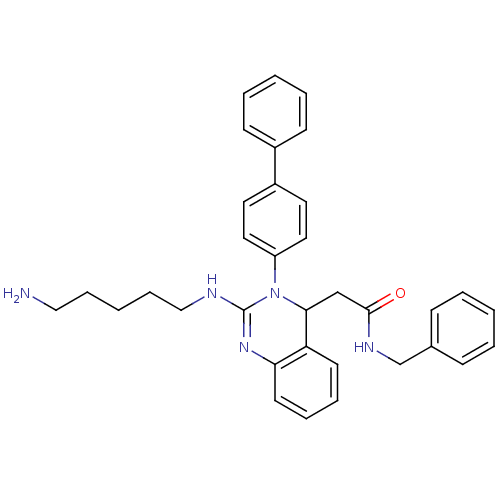 (2-(2-(5-aminopentylamino)-3-(biphenyl-4-yl)-3,4-di...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyung Hee University Curated by ChEMBL | Assay Description Inhibition of T-type calcium channel Cav3.1 expressed in HEK293 cells coexpressing alpha1G subunit by whole-cell patch clamp method | Bioorg Med Chem Lett 17: 471-5 (2007) Article DOI: 10.1016/j.bmcl.2006.10.024 BindingDB Entry DOI: 10.7270/Q24F1QBW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50029600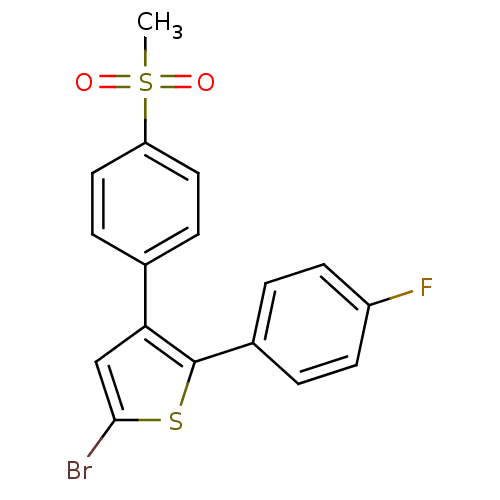 (5-Bromo-2-(4-fluoro-phenyl)-3-(4-methanesulfonyl-p...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyung Hee University Curated by ChEMBL | Assay Description Inhibition of COX2 assessed as PGF2alpha level by EIA | Bioorg Med Chem Lett 20: 734-7 (2010) Article DOI: 10.1016/j.bmcl.2009.11.067 BindingDB Entry DOI: 10.7270/Q2Z31ZQQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Voltage-dependent T-type calcium channel subunit alpha-1G (Homo sapiens (Human)) | BDBM50197240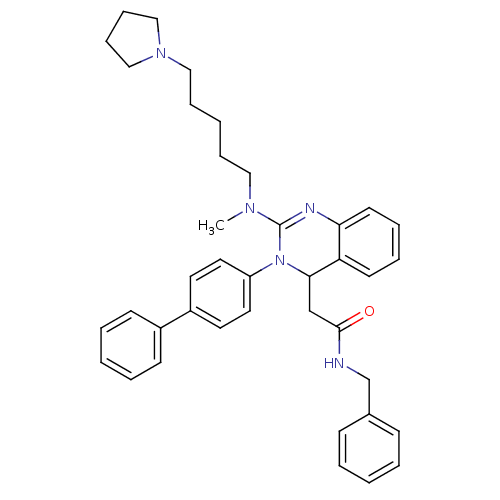 (CHEMBL248088 | KYS-05080 | N-Benzyl-2-{3-biphenyl-...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 260 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyung Hee University Curated by ChEMBL | Assay Description Inhibition of T-type calcium channel Cav3.1 expressed in HEK293 cells coexpressing alpha1G subunit by whole-cell patch clamp method | Bioorg Med Chem Lett 17: 471-5 (2007) Article DOI: 10.1016/j.bmcl.2006.10.024 BindingDB Entry DOI: 10.7270/Q24F1QBW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pancreatic triacylglycerol lipase (Sus scrofa (Pig)) | BDBM24567 ((2S)-1-[(2S,3S)-3-hexyl-4-oxooxetan-2-yl]tridecan-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungbuk National University Curated by ChEMBL | Assay Description Inhibition of porcine pancreatic lipase using p-nitrophenylbutyrate as substrate assessed as formation of p-nitrophenol preincubated for 15 mins foll... | Bioorg Med Chem Lett 25: 2269-74 (2015) Article DOI: 10.1016/j.bmcl.2015.04.045 BindingDB Entry DOI: 10.7270/Q2GF0W7B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Voltage-dependent T-type calcium channel subunit alpha-1G (Homo sapiens (Human)) | BDBM50197238 (CHEMBL239375 | methyl 2-(3-(biphenyl-4-yl)-2-(meth...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 340 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyung Hee University Curated by ChEMBL | Assay Description Inhibition of T-type calcium channel Cav3.1 expressed in HEK293 cells coexpressing alpha1G subunit by whole-cell patch clamp method | Bioorg Med Chem Lett 17: 471-5 (2007) Article DOI: 10.1016/j.bmcl.2006.10.024 BindingDB Entry DOI: 10.7270/Q24F1QBW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Voltage-dependent T-type calcium channel subunit alpha-1G (Homo sapiens (Human)) | BDBM50157859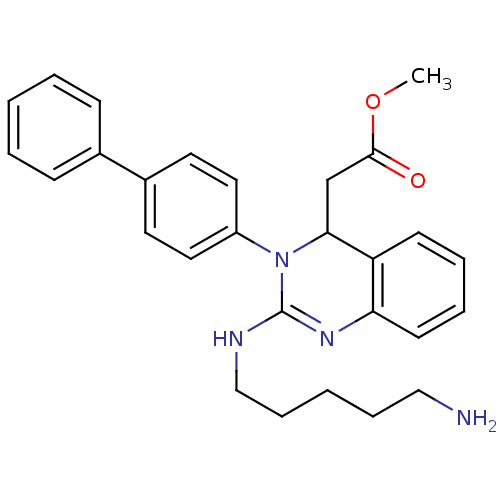 (CHEMBL360359 | [2-(5-amino-pentylamino)-3-biphenyl...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 560 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyung Hee University Curated by ChEMBL | Assay Description Inhibition of T-type calcium channel Cav3.1 expressed in HEK293 cells coexpressing alpha1G subunit by whole-cell patch clamp method | Bioorg Med Chem Lett 17: 471-5 (2007) Article DOI: 10.1016/j.bmcl.2006.10.024 BindingDB Entry DOI: 10.7270/Q24F1QBW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Voltage-dependent T-type calcium channel subunit alpha-1G (Homo sapiens (Human)) | BDBM50117922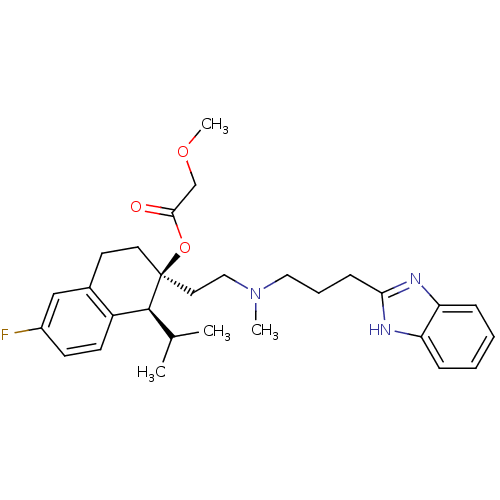 ((1S,2S)-2-(2-((3-(1H-benzo[d]imidazol-2-yl)propyl)...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 1.34E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyung Hee University Curated by ChEMBL | Assay Description Inhibition of T-type calcium channel Cav3.1 expressed in HEK293 cells coexpressing alpha1G subunit by whole-cell patch clamp method | Bioorg Med Chem Lett 17: 471-5 (2007) Article DOI: 10.1016/j.bmcl.2006.10.024 BindingDB Entry DOI: 10.7270/Q24F1QBW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Voltage-dependent T-type calcium channel subunit alpha-1G (Homo sapiens (Human)) | BDBM50197241 (CHEMBL393304 | KYS-05077 | N-benzyl-2-(2-(methyl(5...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyung Hee University Curated by ChEMBL | Assay Description Inhibition of T-type calcium channel Cav3.1 expressed in HEK293 cells coexpressing alpha1G subunit by whole-cell patch clamp method | Bioorg Med Chem Lett 17: 471-5 (2007) Article DOI: 10.1016/j.bmcl.2006.10.024 BindingDB Entry DOI: 10.7270/Q24F1QBW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Voltage-dependent T-type calcium channel subunit alpha-1G (Homo sapiens (Human)) | BDBM50197239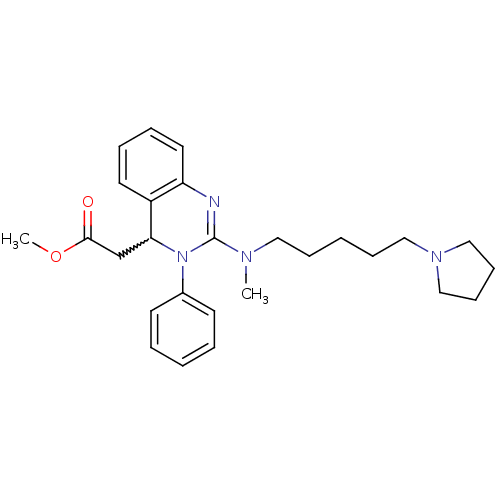 (CHEMBL245610 | methyl 2-(2-(methyl(5-(pyrrolidin-1...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.84E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyung Hee University Curated by ChEMBL | Assay Description Inhibition of T-type calcium channel Cav3.1 expressed in HEK293 cells coexpressing alpha1G subunit by whole-cell patch clamp method | Bioorg Med Chem Lett 17: 471-5 (2007) Article DOI: 10.1016/j.bmcl.2006.10.024 BindingDB Entry DOI: 10.7270/Q24F1QBW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pancreatic triacylglycerol lipase (Sus scrofa (Pig)) | BDBM50251013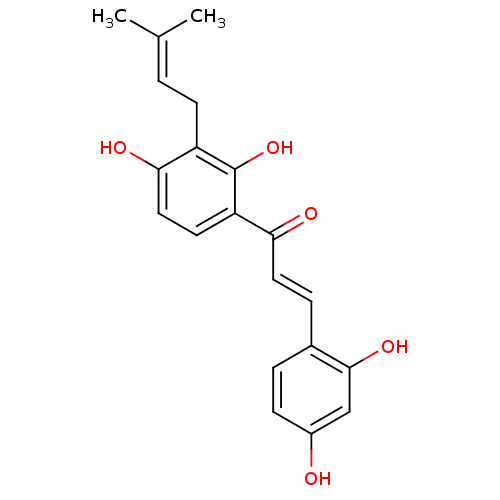 (2,4,2',4'-tetrahydroxy-3'-prenylchalcone | CHEMBL4...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 6.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungbuk National University Curated by ChEMBL | Assay Description Inhibition of porcine pancreatic lipase using p-nitrophenylbutyrate as substrate assessed as formation of p-nitrophenol preincubated for 15 mins foll... | Bioorg Med Chem Lett 25: 2269-74 (2015) Article DOI: 10.1016/j.bmcl.2015.04.045 BindingDB Entry DOI: 10.7270/Q2GF0W7B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50305810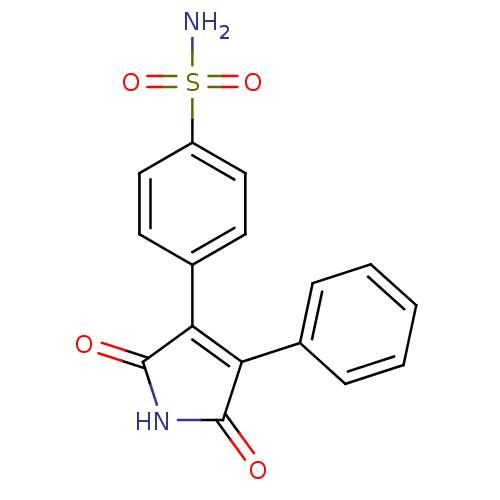 (1H-3-(4-sulfamoylphenyl)-4-phenyl-pyrrole-2,5-dion...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.56E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyung Hee University Curated by ChEMBL | Assay Description Inhibition of COX2 assessed as PGF2alpha level by EIA | Bioorg Med Chem Lett 20: 734-7 (2010) Article DOI: 10.1016/j.bmcl.2009.11.067 BindingDB Entry DOI: 10.7270/Q2Z31ZQQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50305806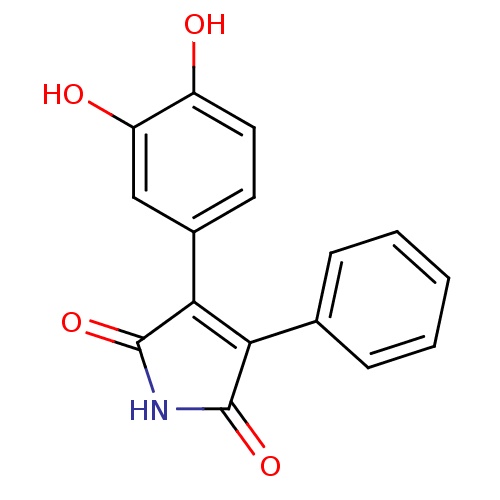 (3-(3,4-dihydroxyphenyl)-4-phenyl-1H-pyrrole-2,5-di...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyung Hee University Curated by ChEMBL | Assay Description Inhibition of COX2 assessed as PGF2alpha level by EIA | Bioorg Med Chem Lett 20: 734-7 (2010) Article DOI: 10.1016/j.bmcl.2009.11.067 BindingDB Entry DOI: 10.7270/Q2Z31ZQQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50305808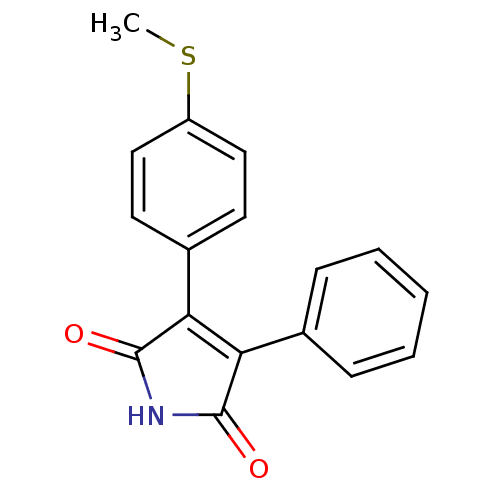 (3-(4-(methylthio)phenyl)-4-phenyl-1H-pyrrole-2,5-d...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.63E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyung Hee University Curated by ChEMBL | Assay Description Inhibition of COX2 assessed as PGF2alpha level by EIA | Bioorg Med Chem Lett 20: 734-7 (2010) Article DOI: 10.1016/j.bmcl.2009.11.067 BindingDB Entry DOI: 10.7270/Q2Z31ZQQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50305804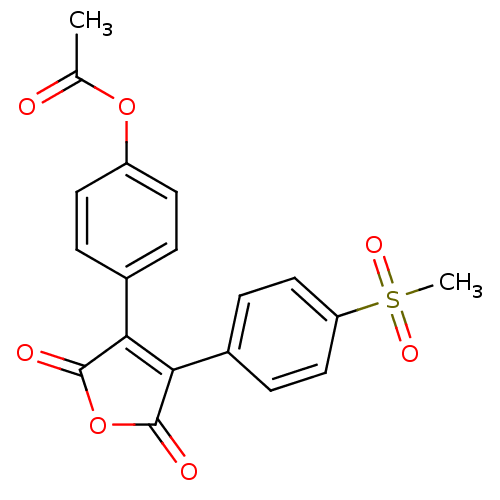 (4-(4-(4-(methylsulfonyl)phenyl)-2,5-dioxo-2,5-dihy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.66E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyung Hee University Curated by ChEMBL | Assay Description Inhibition of COX2 assessed as PGF2alpha level by EIA | Bioorg Med Chem Lett 20: 734-7 (2010) Article DOI: 10.1016/j.bmcl.2009.11.067 BindingDB Entry DOI: 10.7270/Q2Z31ZQQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50305809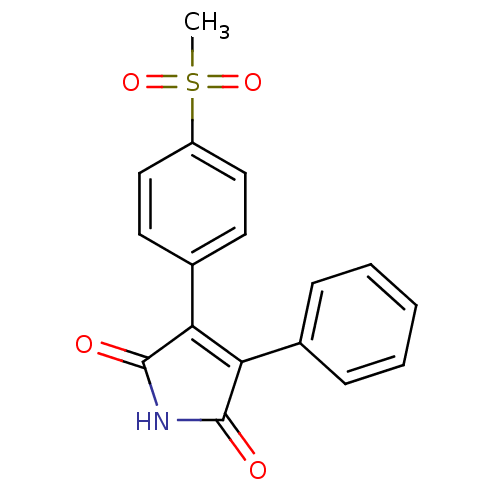 (3-(4-(methylsulfonyl)phenyl)-4-phenyl-1H-pyrrole-2...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.75E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyung Hee University Curated by ChEMBL | Assay Description Inhibition of COX2 assessed as PGF2alpha level by EIA | Bioorg Med Chem Lett 20: 734-7 (2010) Article DOI: 10.1016/j.bmcl.2009.11.067 BindingDB Entry DOI: 10.7270/Q2Z31ZQQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50305807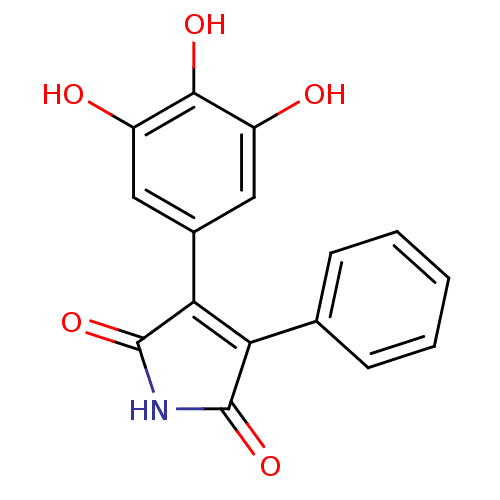 (3-phenyl-4-(3,4,5-trihydroxyphenyl)-1H-pyrrole-2,5...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.75E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyung Hee University Curated by ChEMBL | Assay Description Inhibition of COX2 assessed as PGF2alpha level by EIA | Bioorg Med Chem Lett 20: 734-7 (2010) Article DOI: 10.1016/j.bmcl.2009.11.067 BindingDB Entry DOI: 10.7270/Q2Z31ZQQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50305803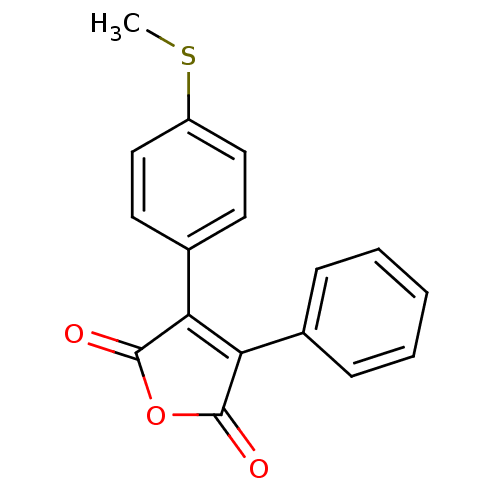 (3-(4-(methylthio)phenyl)-4-phenylfuran-2,5-dione |...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.85E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyung Hee University Curated by ChEMBL | Assay Description Inhibition of COX2 assessed as PGF2alpha level by EIA | Bioorg Med Chem Lett 20: 734-7 (2010) Article DOI: 10.1016/j.bmcl.2009.11.067 BindingDB Entry DOI: 10.7270/Q2Z31ZQQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50305802 (3-(benzo[d][1,3]dioxol-5-yl)-4-phenylfuran-2,5-dio...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.85E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyung Hee University Curated by ChEMBL | Assay Description Inhibition of COX2 assessed as PGF2alpha level by EIA | Bioorg Med Chem Lett 20: 734-7 (2010) Article DOI: 10.1016/j.bmcl.2009.11.067 BindingDB Entry DOI: 10.7270/Q2Z31ZQQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pancreatic triacylglycerol lipase (Sus scrofa (Pig)) | BDBM50242015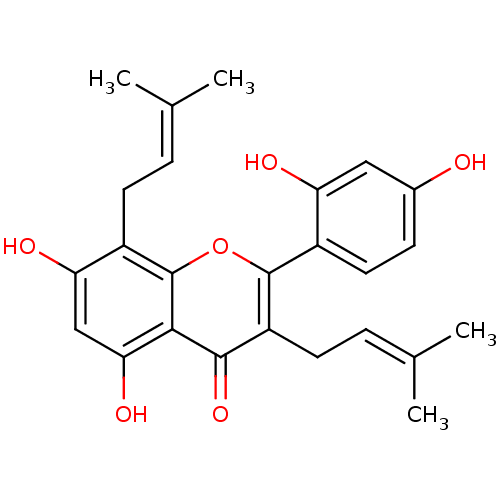 (CHEMBL518543 | Kuwanon C, 4 | kuwanon C) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.86E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungbuk National University Curated by ChEMBL | Assay Description Inhibition of porcine pancreatic lipase using p-nitrophenylbutyrate as substrate assessed as formation of p-nitrophenol preincubated for 15 mins foll... | Bioorg Med Chem Lett 25: 2269-74 (2015) Article DOI: 10.1016/j.bmcl.2015.04.045 BindingDB Entry DOI: 10.7270/Q2GF0W7B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50305805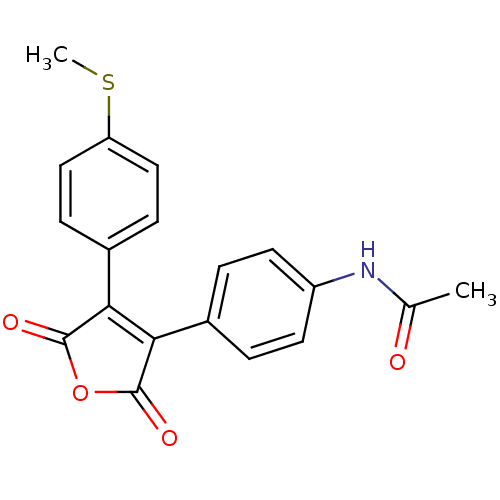 (CHEMBL595852 | N-(4-(4-(4-(methylthio)phenyl)-2,5-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.01E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyung Hee University Curated by ChEMBL | Assay Description Inhibition of COX2 assessed as PGF2alpha level by EIA | Bioorg Med Chem Lett 20: 734-7 (2010) Article DOI: 10.1016/j.bmcl.2009.11.067 BindingDB Entry DOI: 10.7270/Q2Z31ZQQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50305811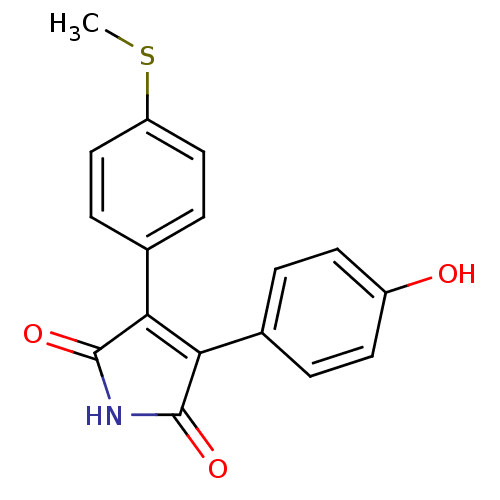 (3-(4-hydroxyphenyl)-4-(4-(methylthio)phenyl)-1H-py...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyung Hee University Curated by ChEMBL | Assay Description Inhibition of COX2 assessed as PGF2alpha level by EIA | Bioorg Med Chem Lett 20: 734-7 (2010) Article DOI: 10.1016/j.bmcl.2009.11.067 BindingDB Entry DOI: 10.7270/Q2Z31ZQQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pancreatic triacylglycerol lipase (Sus scrofa (Pig)) | BDBM50083074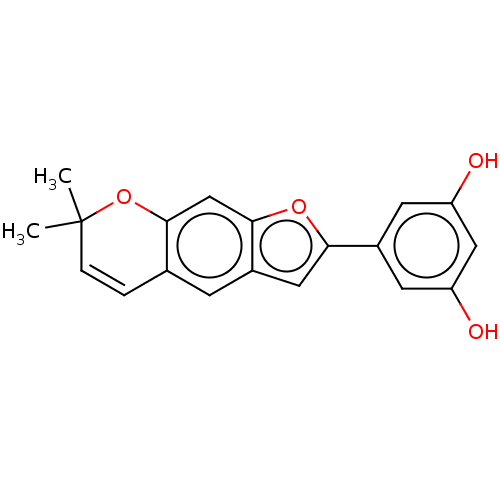 (CHEMBL3422851) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.92E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungbuk National University Curated by ChEMBL | Assay Description Inhibition of porcine pancreatic lipase using p-nitrophenylbutyrate as substrate assessed as formation of p-nitrophenol preincubated for 15 mins foll... | Bioorg Med Chem Lett 25: 2269-74 (2015) Article DOI: 10.1016/j.bmcl.2015.04.045 BindingDB Entry DOI: 10.7270/Q2GF0W7B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pancreatic triacylglycerol lipase (Sus scrofa (Pig)) | BDBM50251014 (CHEMBL465881 | moracin N) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.97E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungbuk National University Curated by ChEMBL | Assay Description Inhibition of porcine pancreatic lipase using p-nitrophenylbutyrate as substrate assessed as formation of p-nitrophenol preincubated for 15 mins foll... | Bioorg Med Chem Lett 25: 2269-74 (2015) Article DOI: 10.1016/j.bmcl.2015.04.045 BindingDB Entry DOI: 10.7270/Q2GF0W7B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pancreatic triacylglycerol lipase (Sus scrofa (Pig)) | BDBM50381284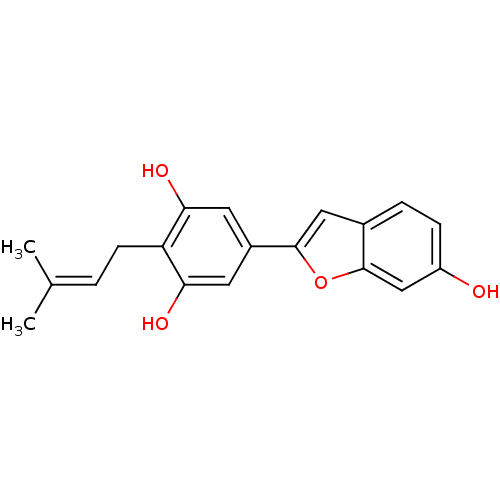 (CHEMBL2018876) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungbuk National University Curated by ChEMBL | Assay Description Inhibition of porcine pancreatic lipase using p-nitrophenylbutyrate as substrate assessed as formation of p-nitrophenol preincubated for 15 mins foll... | Bioorg Med Chem Lett 25: 2269-74 (2015) Article DOI: 10.1016/j.bmcl.2015.04.045 BindingDB Entry DOI: 10.7270/Q2GF0W7B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pancreatic triacylglycerol lipase (Sus scrofa (Pig)) | BDBM50083070 (CHEMBL3422848) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.64E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungbuk National University Curated by ChEMBL | Assay Description Inhibition of porcine pancreatic lipase using p-nitrophenylbutyrate as substrate assessed as formation of p-nitrophenol preincubated for 15 mins foll... | Bioorg Med Chem Lett 25: 2269-74 (2015) Article DOI: 10.1016/j.bmcl.2015.04.045 BindingDB Entry DOI: 10.7270/Q2GF0W7B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pancreatic triacylglycerol lipase (Sus scrofa (Pig)) | BDBM50083071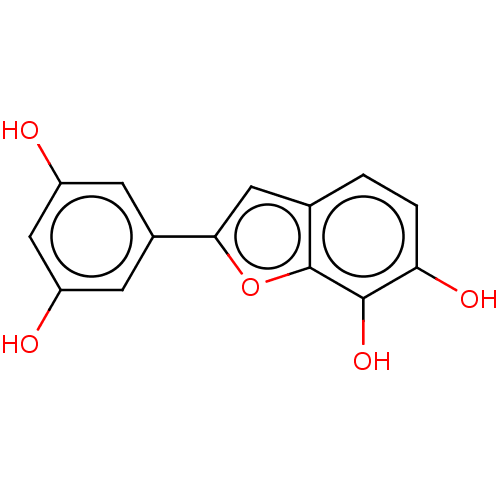 (CHEMBL3422849) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungbuk National University Curated by ChEMBL | Assay Description Inhibition of porcine pancreatic lipase using p-nitrophenylbutyrate as substrate assessed as formation of p-nitrophenol preincubated for 15 mins foll... | Bioorg Med Chem Lett 25: 2269-74 (2015) Article DOI: 10.1016/j.bmcl.2015.04.045 BindingDB Entry DOI: 10.7270/Q2GF0W7B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pancreatic triacylglycerol lipase (Sus scrofa (Pig)) | BDBM50083072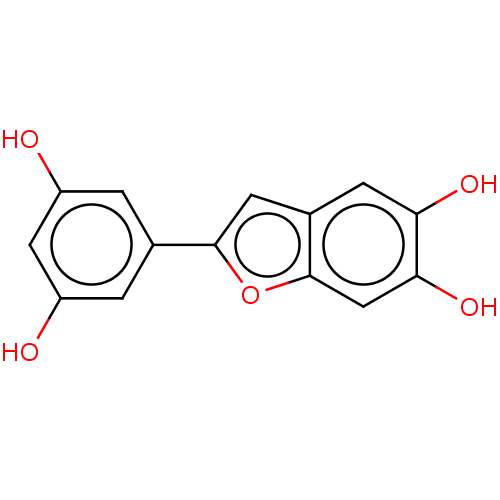 (CHEMBL3422850) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.48E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungbuk National University Curated by ChEMBL | Assay Description Inhibition of porcine pancreatic lipase using p-nitrophenylbutyrate as substrate assessed as formation of p-nitrophenol preincubated for 15 mins foll... | Bioorg Med Chem Lett 25: 2269-74 (2015) Article DOI: 10.1016/j.bmcl.2015.04.045 BindingDB Entry DOI: 10.7270/Q2GF0W7B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pancreatic triacylglycerol lipase (Sus scrofa (Pig)) | BDBM50442403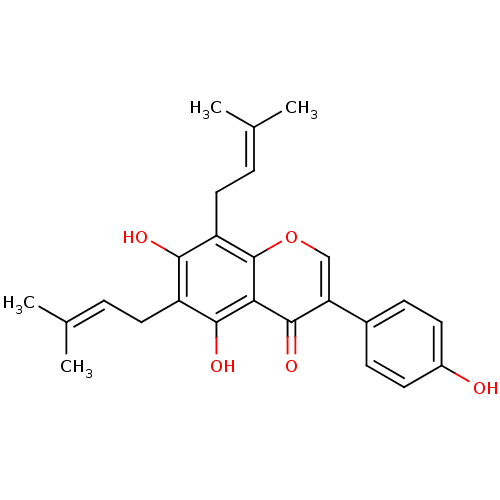 (CHEMBL494252) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungbuk National University Curated by ChEMBL | Assay Description Inhibition of porcine pancreatic lipase assessed as hydrolysis of p-NPB to p-nitrophenol by microplate reader | Bioorg Med Chem Lett 24: 2329-33 (2014) Article DOI: 10.1016/j.bmcl.2014.03.067 BindingDB Entry DOI: 10.7270/Q2V69M43 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pancreatic triacylglycerol lipase (Sus scrofa (Pig)) | BDBM50083073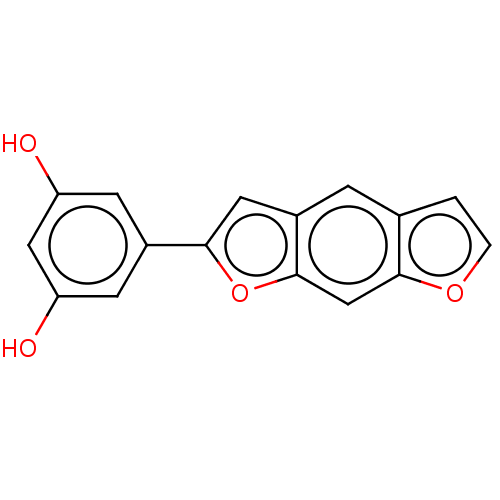 (CHEMBL3397404) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >7.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungbuk National University Curated by ChEMBL | Assay Description Inhibition of porcine pancreatic lipase using p-nitrophenylbutyrate as substrate assessed as formation of p-nitrophenol preincubated for 15 mins foll... | Bioorg Med Chem Lett 25: 2269-74 (2015) Article DOI: 10.1016/j.bmcl.2015.04.045 BindingDB Entry DOI: 10.7270/Q2GF0W7B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pancreatic triacylglycerol lipase (Sus scrofa (Pig)) | BDBM50303002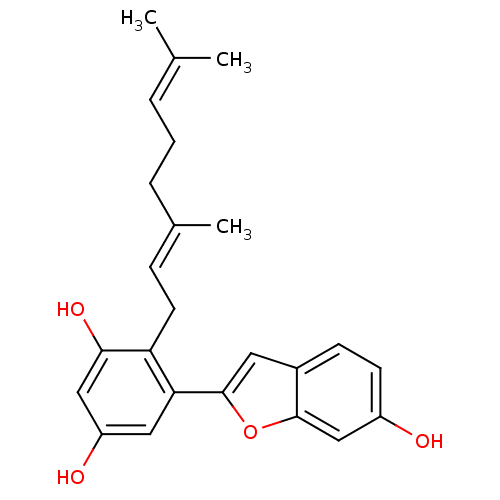 (CHEMBL564896 | albafuran A) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >7.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungbuk National University Curated by ChEMBL | Assay Description Inhibition of porcine pancreatic lipase using p-nitrophenylbutyrate as substrate assessed as formation of p-nitrophenol preincubated for 15 mins foll... | Bioorg Med Chem Lett 25: 2269-74 (2015) Article DOI: 10.1016/j.bmcl.2015.04.045 BindingDB Entry DOI: 10.7270/Q2GF0W7B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pancreatic triacylglycerol lipase (Sus scrofa (Pig)) | BDBM50250915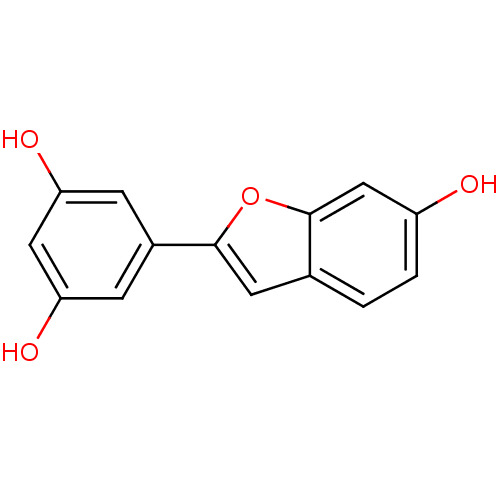 (CHEMBL512578 | moracin M) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >7.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungbuk National University Curated by ChEMBL | Assay Description Inhibition of porcine pancreatic lipase using p-nitrophenylbutyrate as substrate assessed as formation of p-nitrophenol preincubated for 15 mins foll... | Bioorg Med Chem Lett 25: 2269-74 (2015) Article DOI: 10.1016/j.bmcl.2015.04.045 BindingDB Entry DOI: 10.7270/Q2GF0W7B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pancreatic triacylglycerol lipase (Sus scrofa (Pig)) | BDBM50269605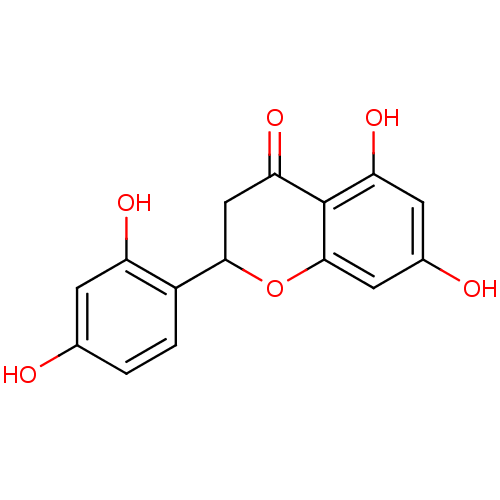 (CHEMBL465194 | steppogenin) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >7.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungbuk National University Curated by ChEMBL | Assay Description Inhibition of porcine pancreatic lipase using p-nitrophenylbutyrate as substrate assessed as formation of p-nitrophenol preincubated for 15 mins foll... | Bioorg Med Chem Lett 25: 2269-74 (2015) Article DOI: 10.1016/j.bmcl.2015.04.045 BindingDB Entry DOI: 10.7270/Q2GF0W7B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pancreatic triacylglycerol lipase (Sus scrofa (Pig)) | BDBM50083068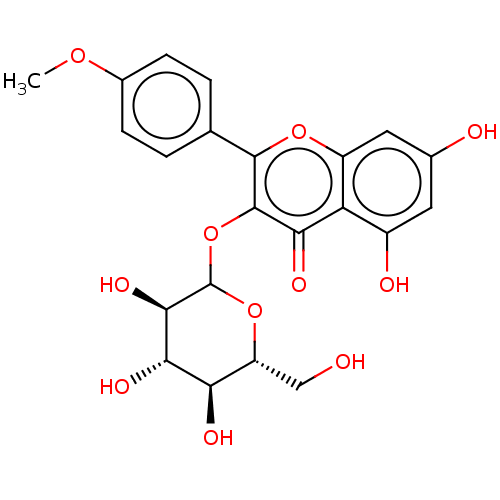 (CHEMBL3422847) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >7.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungbuk National University Curated by ChEMBL | Assay Description Inhibition of porcine pancreatic lipase using p-nitrophenylbutyrate as substrate assessed as formation of p-nitrophenol preincubated for 15 mins foll... | Bioorg Med Chem Lett 25: 2269-74 (2015) Article DOI: 10.1016/j.bmcl.2015.04.045 BindingDB Entry DOI: 10.7270/Q2GF0W7B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pancreatic triacylglycerol lipase (Sus scrofa (Pig)) | BDBM50241354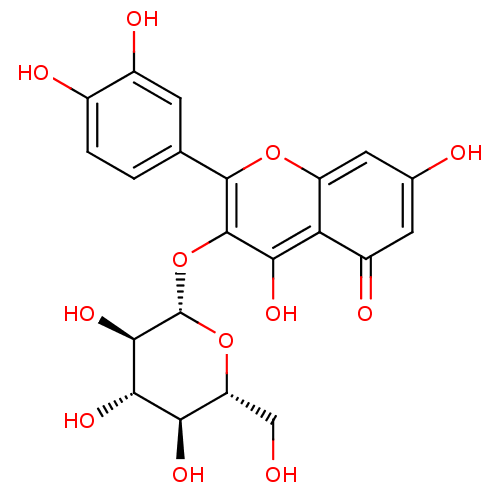 (2-(3,4-Dihydroxy-phenyl)-5,7-dihydroxy-3-(3,4,5-tr...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >7.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungbuk National University Curated by ChEMBL | Assay Description Inhibition of porcine pancreatic lipase using p-nitrophenylbutyrate as substrate assessed as formation of p-nitrophenol preincubated for 15 mins foll... | Bioorg Med Chem Lett 25: 2269-74 (2015) Article DOI: 10.1016/j.bmcl.2015.04.045 BindingDB Entry DOI: 10.7270/Q2GF0W7B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pancreatic triacylglycerol lipase (Sus scrofa (Pig)) | BDBM50241243 (3,4',5,7-Tetrahydroxyflavone-3-glucoside | 3-(beta...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >7.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungbuk National University Curated by ChEMBL | Assay Description Inhibition of porcine pancreatic lipase using p-nitrophenylbutyrate as substrate assessed as formation of p-nitrophenol preincubated for 15 mins foll... | Bioorg Med Chem Lett 25: 2269-74 (2015) Article DOI: 10.1016/j.bmcl.2015.04.045 BindingDB Entry DOI: 10.7270/Q2GF0W7B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pancreatic triacylglycerol lipase (Sus scrofa (Pig)) | BDBM50269559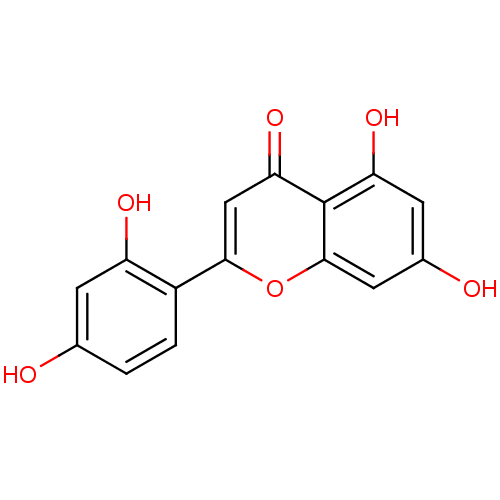 (2-(2,4-dihydroxyphenyl)-5,7-dihydroxy-4H-chromen-4...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >7.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungbuk National University Curated by ChEMBL | Assay Description Inhibition of porcine pancreatic lipase using p-nitrophenylbutyrate as substrate assessed as formation of p-nitrophenol preincubated for 15 mins foll... | Bioorg Med Chem Lett 25: 2269-74 (2015) Article DOI: 10.1016/j.bmcl.2015.04.045 BindingDB Entry DOI: 10.7270/Q2GF0W7B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pancreatic triacylglycerol lipase (Sus scrofa (Pig)) | BDBM23409 (3,5,7-trihydroxy-2-(4-hydroxy-3-methoxyphenyl)-4H-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | >7.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungbuk National University Curated by ChEMBL | Assay Description Inhibition of porcine pancreatic lipase using p-nitrophenylbutyrate as substrate assessed as formation of p-nitrophenol preincubated for 15 mins foll... | Bioorg Med Chem Lett 25: 2269-74 (2015) Article DOI: 10.1016/j.bmcl.2015.04.045 BindingDB Entry DOI: 10.7270/Q2GF0W7B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pancreatic triacylglycerol lipase (Sus scrofa (Pig)) | BDBM7462 (3,5,7-trihydroxy-2-(4-hydroxyphenyl)-4H-chromen-4-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | >7.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungbuk National University Curated by ChEMBL | Assay Description Inhibition of porcine pancreatic lipase using p-nitrophenylbutyrate as substrate assessed as formation of p-nitrophenol preincubated for 15 mins foll... | Bioorg Med Chem Lett 25: 2269-74 (2015) Article DOI: 10.1016/j.bmcl.2015.04.045 BindingDB Entry DOI: 10.7270/Q2GF0W7B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pancreatic triacylglycerol lipase (Sus scrofa (Pig)) | BDBM7460 (2-(3,4-dihydroxyphenyl)-3,5,7-trihydroxy-4H-chrome...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | >7.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungbuk National University Curated by ChEMBL | Assay Description Inhibition of porcine pancreatic lipase using p-nitrophenylbutyrate as substrate assessed as formation of p-nitrophenol preincubated for 15 mins foll... | Bioorg Med Chem Lett 25: 2269-74 (2015) Article DOI: 10.1016/j.bmcl.2015.04.045 BindingDB Entry DOI: 10.7270/Q2GF0W7B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pancreatic triacylglycerol lipase (Sus scrofa (Pig)) | BDBM50108046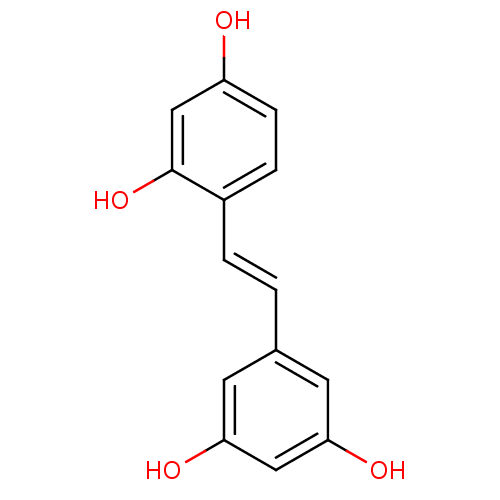 ((oxyresveratrol)4-[(E)-2-(3,5-dihydroxyphenyl)viny...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | >7.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungbuk National University Curated by ChEMBL | Assay Description Inhibition of porcine pancreatic lipase using p-nitrophenylbutyrate as substrate assessed as formation of p-nitrophenol preincubated for 15 mins foll... | Bioorg Med Chem Lett 25: 2269-74 (2015) Article DOI: 10.1016/j.bmcl.2015.04.045 BindingDB Entry DOI: 10.7270/Q2GF0W7B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||