 Found 51 hits with Last Name = 'chootip' and Initial = 'k'
Found 51 hits with Last Name = 'chootip' and Initial = 'k' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM14390

(5-[2-ethoxy-5-(4-methyl-1-piperazinylsulfonyl)phen...)Show SMILES CCCc1nn(C)c2c1nc([nH]c2=O)-c1cc(ccc1OCC)S(=O)(=O)N1CCN(C)CC1 Show InChI InChI=1S/C22H30N6O4S/c1-5-7-17-19-20(27(4)25-17)22(29)24-21(23-19)16-14-15(8-9-18(16)32-6-2)33(30,31)28-12-10-26(3)11-13-28/h8-9,14H,5-7,10-13H2,1-4H3,(H,23,24,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2022.117092
BindingDB Entry DOI: 10.7270/Q21N8568 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Rattus norvegicus) | BDBM14390

(5-[2-ethoxy-5-(4-methyl-1-piperazinylsulfonyl)phen...)Show SMILES CCCc1nn(C)c2c1nc([nH]c2=O)-c1cc(ccc1OCC)S(=O)(=O)N1CCN(C)CC1 Show InChI InChI=1S/C22H30N6O4S/c1-5-7-17-19-20(27(4)25-17)22(29)24-21(23-19)16-14-15(8-9-18(16)32-6-2)33(30,31)28-12-10-26(3)11-13-28/h8-9,14H,5-7,10-13H2,1-4H3,(H,23,24,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Kasetsart University
Curated by ChEMBL
| Assay Description
Inhibition of rat lung PDE5 using [3H]cGMP as substrate measured after 10 mins by scintillation counting method |
Bioorg Med Chem Lett 29: 267-270 (2019)
Article DOI: 10.1016/j.bmcl.2018.11.043
BindingDB Entry DOI: 10.7270/Q2RJ4NT3 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50033312
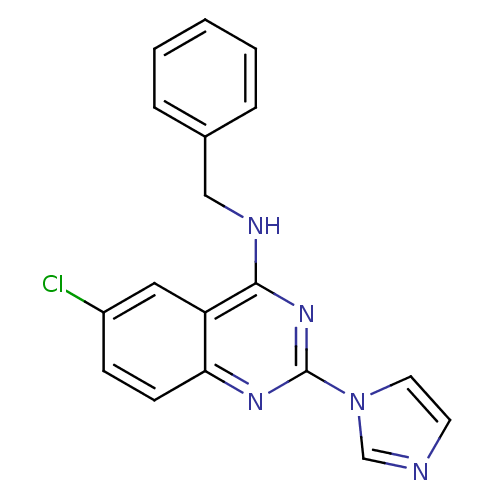
(Benzyl-(6-chloro-2-imidazol-1-yl-quinazolin-4-yl)-...)Show InChI InChI=1S/C18H14ClN5/c19-14-6-7-16-15(10-14)17(21-11-13-4-2-1-3-5-13)23-18(22-16)24-9-8-20-12-24/h1-10,12H,11H2,(H,21,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2022.117092
BindingDB Entry DOI: 10.7270/Q21N8568 |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50518237

(CHEMBL4552529)Show SMILES [Na;v0+].[#8-]-[#6](=O)-[#6]-1-[#6]-[#6]-[#7](-[#6]-[#6]-1)-c1ncc2c(-[#7]-[#6]-c3ccc4-[#8]-[#6]-[#8]-c4c3)c(Cl)ccc2n1 Show InChI InChI=1S/C22H21ClN4O4/c23-16-2-3-17-15(11-25-22(26-17)27-7-5-14(6-8-27)21(28)29)20(16)24-10-13-1-4-18-19(9-13)31-12-30-18/h1-4,9,11,14,24H,5-8,10,12H2,(H,28,29)/p-1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Kasetsart University
Curated by ChEMBL
| Assay Description
Inhibition of PDE5 (unknown origin) |
Bioorg Med Chem Lett 29: 267-270 (2019)
Article DOI: 10.1016/j.bmcl.2018.11.043
BindingDB Entry DOI: 10.7270/Q2RJ4NT3 |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Rattus norvegicus) | BDBM50518228

(CHEMBL4445920)Show SMILES NS(=O)(=O)c1cccc(Nc2nc(NCc3ccccc3)c3ccccc3n2)c1 Show InChI InChI=1S/C21H19N5O2S/c22-29(27,28)17-10-6-9-16(13-17)24-21-25-19-12-5-4-11-18(19)20(26-21)23-14-15-7-2-1-3-8-15/h1-13H,14H2,(H2,22,27,28)(H2,23,24,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 72 | n/a | n/a | n/a | n/a | n/a | n/a |
Kasetsart University
Curated by ChEMBL
| Assay Description
Inhibition of rat lung PDE5 using [3H]cGMP as substrate measured after 10 mins by scintillation counting method |
Bioorg Med Chem Lett 29: 267-270 (2019)
Article DOI: 10.1016/j.bmcl.2018.11.043
BindingDB Entry DOI: 10.7270/Q2RJ4NT3 |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Rattus norvegicus) | BDBM50518224

(CHEMBL4570769)Show SMILES NS(=O)(=O)c1cccc(Nc2nc(NCc3cccs3)c3ccccc3n2)c1 Show InChI InChI=1S/C19H17N5O2S2/c20-28(25,26)15-7-3-5-13(11-15)22-19-23-17-9-2-1-8-16(17)18(24-19)21-12-14-6-4-10-27-14/h1-11H,12H2,(H2,20,25,26)(H2,21,22,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 89 | n/a | n/a | n/a | n/a | n/a | n/a |
Kasetsart University
Curated by ChEMBL
| Assay Description
Inhibition of rat lung PDE5 using [3H]cGMP as substrate measured after 10 mins by scintillation counting method |
Bioorg Med Chem Lett 29: 267-270 (2019)
Article DOI: 10.1016/j.bmcl.2018.11.043
BindingDB Entry DOI: 10.7270/Q2RJ4NT3 |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50518228

(CHEMBL4445920)Show SMILES NS(=O)(=O)c1cccc(Nc2nc(NCc3ccccc3)c3ccccc3n2)c1 Show InChI InChI=1S/C21H19N5O2S/c22-29(27,28)17-10-6-9-16(13-17)24-21-25-19-12-5-4-11-18(19)20(26-21)23-14-15-7-2-1-3-8-15/h1-13H,14H2,(H2,22,27,28)(H2,23,24,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2022.117092
BindingDB Entry DOI: 10.7270/Q21N8568 |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Rattus norvegicus) | BDBM50518223

(CHEMBL4529354)Show SMILES NS(=O)(=O)c1cccc(Nc2nc(NCc3ccco3)c3ccccc3n2)c1 Show InChI InChI=1S/C19H17N5O3S/c20-28(25,26)15-7-3-5-13(11-15)22-19-23-17-9-2-1-8-16(17)18(24-19)21-12-14-6-4-10-27-14/h1-11H,12H2,(H2,20,25,26)(H2,21,22,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 116 | n/a | n/a | n/a | n/a | n/a | n/a |
Kasetsart University
Curated by ChEMBL
| Assay Description
Inhibition of rat lung PDE5 using [3H]cGMP as substrate measured after 10 mins by scintillation counting method |
Bioorg Med Chem Lett 29: 267-270 (2019)
Article DOI: 10.1016/j.bmcl.2018.11.043
BindingDB Entry DOI: 10.7270/Q2RJ4NT3 |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50602260

(CHEMBL5175706)Show SMILES CC(C)N1CCC(CC1)Nc1nc(Nc2cccc(c2)S(=O)(=O)N2CCN(C)CC2)nc2ccccc12 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2022.117092
BindingDB Entry DOI: 10.7270/Q21N8568 |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Rattus norvegicus) | BDBM50518225
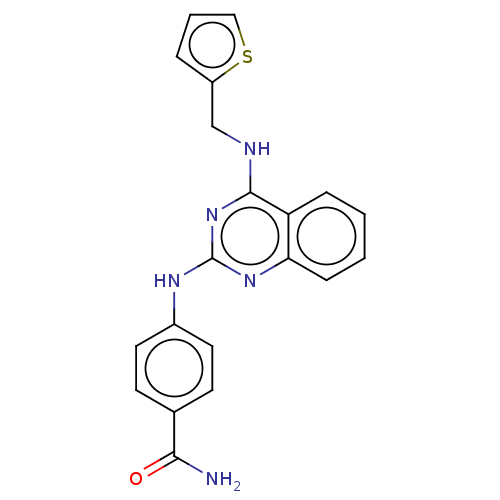
(CHEMBL4434898)Show InChI InChI=1S/C20H17N5OS/c21-18(26)13-7-9-14(10-8-13)23-20-24-17-6-2-1-5-16(17)19(25-20)22-12-15-4-3-11-27-15/h1-11H,12H2,(H2,21,26)(H2,22,23,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 177 | n/a | n/a | n/a | n/a | n/a | n/a |
Kasetsart University
Curated by ChEMBL
| Assay Description
Inhibition of rat lung PDE5 using [3H]cGMP as substrate measured after 10 mins by scintillation counting method |
Bioorg Med Chem Lett 29: 267-270 (2019)
Article DOI: 10.1016/j.bmcl.2018.11.043
BindingDB Entry DOI: 10.7270/Q2RJ4NT3 |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Rattus norvegicus) | BDBM50518217
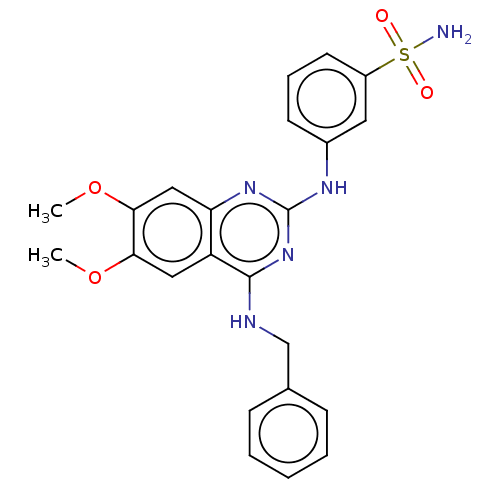
(CHEMBL4544782)Show SMILES COc1cc2nc(Nc3cccc(c3)S(N)(=O)=O)nc(NCc3ccccc3)c2cc1OC Show InChI InChI=1S/C23H23N5O4S/c1-31-20-12-18-19(13-21(20)32-2)27-23(26-16-9-6-10-17(11-16)33(24,29)30)28-22(18)25-14-15-7-4-3-5-8-15/h3-13H,14H2,1-2H3,(H2,24,29,30)(H2,25,26,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 218 | n/a | n/a | n/a | n/a | n/a | n/a |
Kasetsart University
Curated by ChEMBL
| Assay Description
Inhibition of rat lung PDE5 using [3H]cGMP as substrate measured after 10 mins by scintillation counting method |
Bioorg Med Chem Lett 29: 267-270 (2019)
Article DOI: 10.1016/j.bmcl.2018.11.043
BindingDB Entry DOI: 10.7270/Q2RJ4NT3 |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50602263
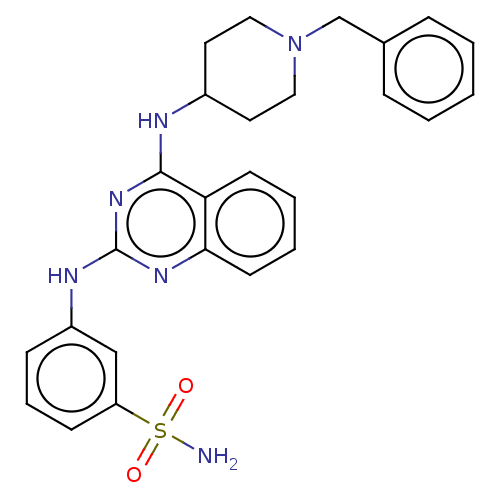
(CHEMBL5178863)Show SMILES NS(=O)(=O)c1cccc(Nc2nc(NC3CCN(Cc4ccccc4)CC3)c3ccccc3n2)c1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 220 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2022.117092
BindingDB Entry DOI: 10.7270/Q21N8568 |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Rattus norvegicus) | BDBM50518232

(CHEMBL4460740)Show SMILES NS(=O)(=O)c1ccc(Nc2nc(NCc3cccs3)c3ccccc3n2)cc1 Show InChI InChI=1S/C19H17N5O2S2/c20-28(25,26)15-9-7-13(8-10-15)22-19-23-17-6-2-1-5-16(17)18(24-19)21-12-14-4-3-11-27-14/h1-11H,12H2,(H2,20,25,26)(H2,21,22,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 224 | n/a | n/a | n/a | n/a | n/a | n/a |
Kasetsart University
Curated by ChEMBL
| Assay Description
Inhibition of rat lung PDE5 using [3H]cGMP as substrate measured after 10 mins by scintillation counting method |
Bioorg Med Chem Lett 29: 267-270 (2019)
Article DOI: 10.1016/j.bmcl.2018.11.043
BindingDB Entry DOI: 10.7270/Q2RJ4NT3 |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Rattus norvegicus) | BDBM50518220

(CHEMBL4540726)Show SMILES COc1cc2nc(Nc3cccc(c3)S(N)(=O)=O)nc(NCc3ccco3)c2cc1OC Show InChI InChI=1S/C21H21N5O5S/c1-29-18-10-16-17(11-19(18)30-2)25-21(26-20(16)23-12-14-6-4-8-31-14)24-13-5-3-7-15(9-13)32(22,27)28/h3-11H,12H2,1-2H3,(H2,22,27,28)(H2,23,24,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 284 | n/a | n/a | n/a | n/a | n/a | n/a |
Kasetsart University
Curated by ChEMBL
| Assay Description
Inhibition of rat lung PDE5 using [3H]cGMP as substrate measured after 10 mins by scintillation counting method |
Bioorg Med Chem Lett 29: 267-270 (2019)
Article DOI: 10.1016/j.bmcl.2018.11.043
BindingDB Entry DOI: 10.7270/Q2RJ4NT3 |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Rattus norvegicus) | BDBM50518234
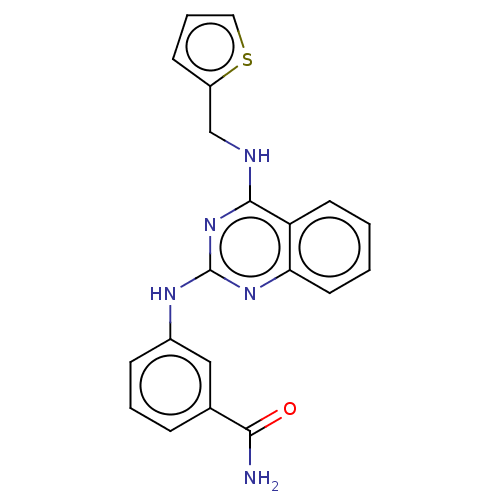
(CHEMBL4516390)Show InChI InChI=1S/C20H17N5OS/c21-18(26)13-5-3-6-14(11-13)23-20-24-17-9-2-1-8-16(17)19(25-20)22-12-15-7-4-10-27-15/h1-11H,12H2,(H2,21,26)(H2,22,23,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 290 | n/a | n/a | n/a | n/a | n/a | n/a |
Kasetsart University
Curated by ChEMBL
| Assay Description
Inhibition of rat lung PDE5 using [3H]cGMP as substrate measured after 10 mins by scintillation counting method |
Bioorg Med Chem Lett 29: 267-270 (2019)
Article DOI: 10.1016/j.bmcl.2018.11.043
BindingDB Entry DOI: 10.7270/Q2RJ4NT3 |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50602240

(CHEMBL5194921)Show SMILES NS(=O)(=O)c1cccc(Nc2nc(NC3CCN(Cc4ccccc4)CC3)c3nc[nH]c3n2)c1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 290 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2022.117092
BindingDB Entry DOI: 10.7270/Q21N8568 |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Rattus norvegicus) | BDBM50518227
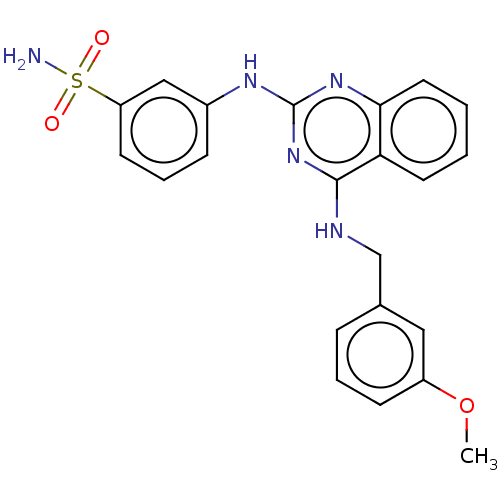
(CHEMBL4452377)Show SMILES COc1cccc(CNc2nc(Nc3cccc(c3)S(N)(=O)=O)nc3ccccc23)c1 Show InChI InChI=1S/C22H21N5O3S/c1-30-17-8-4-6-15(12-17)14-24-21-19-10-2-3-11-20(19)26-22(27-21)25-16-7-5-9-18(13-16)31(23,28)29/h2-13H,14H2,1H3,(H2,23,28,29)(H2,24,25,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 350 | n/a | n/a | n/a | n/a | n/a | n/a |
Kasetsart University
Curated by ChEMBL
| Assay Description
Inhibition of rat lung PDE5 using [3H]cGMP as substrate measured after 10 mins by scintillation counting method |
Bioorg Med Chem Lett 29: 267-270 (2019)
Article DOI: 10.1016/j.bmcl.2018.11.043
BindingDB Entry DOI: 10.7270/Q2RJ4NT3 |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50602247

(CHEMBL5187496)Show SMILES CC(C)CNc1nc(Nc2ccc(cc2)S(=O)(=O)N2CCN(C)CC2)nc2ccccc12 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 360 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2022.117092
BindingDB Entry DOI: 10.7270/Q21N8568 |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50602245
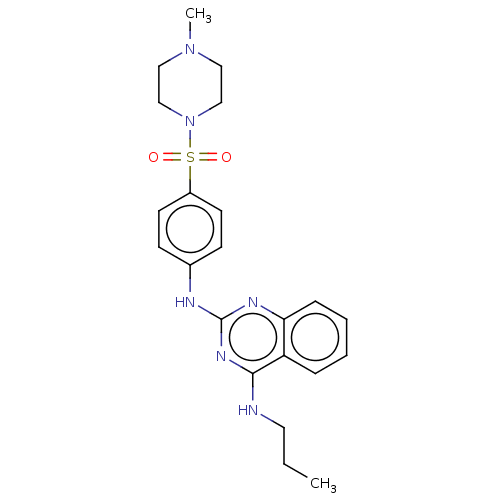
(CHEMBL5194783)Show SMILES CCCNc1nc(Nc2ccc(cc2)S(=O)(=O)N2CCN(C)CC2)nc2ccccc12 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 410 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2022.117092
BindingDB Entry DOI: 10.7270/Q21N8568 |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50602246
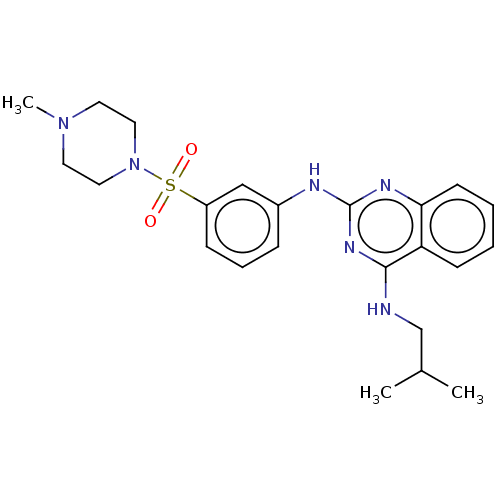
(CHEMBL5184989)Show SMILES CC(C)CNc1nc(Nc2cccc(c2)S(=O)(=O)N2CCN(C)CC2)nc2ccccc12 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 430 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2022.117092
BindingDB Entry DOI: 10.7270/Q21N8568 |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Rattus norvegicus) | BDBM50518233

(CHEMBL4526365)Show SMILES COc1cccc(CNc2nc(Nc3cccc(c3)S(N)(=O)=O)nc3cc(OC)c(OC)cc23)c1 Show InChI InChI=1S/C24H25N5O5S/c1-32-17-8-4-6-15(10-17)14-26-23-19-12-21(33-2)22(34-3)13-20(19)28-24(29-23)27-16-7-5-9-18(11-16)35(25,30)31/h4-13H,14H2,1-3H3,(H2,25,30,31)(H2,26,27,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 443 | n/a | n/a | n/a | n/a | n/a | n/a |
Kasetsart University
Curated by ChEMBL
| Assay Description
Inhibition of rat lung PDE5 using [3H]cGMP as substrate measured after 10 mins by scintillation counting method |
Bioorg Med Chem Lett 29: 267-270 (2019)
Article DOI: 10.1016/j.bmcl.2018.11.043
BindingDB Entry DOI: 10.7270/Q2RJ4NT3 |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50602243
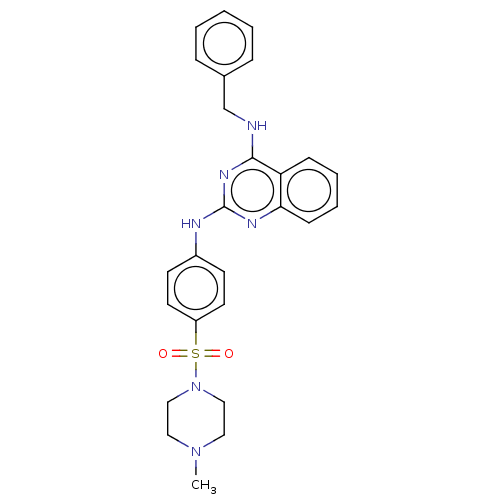
(CHEMBL5203939)Show SMILES CN1CCN(CC1)S(=O)(=O)c1ccc(Nc2nc(NCc3ccccc3)c3ccccc3n2)cc1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 490 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2022.117092
BindingDB Entry DOI: 10.7270/Q21N8568 |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50602241
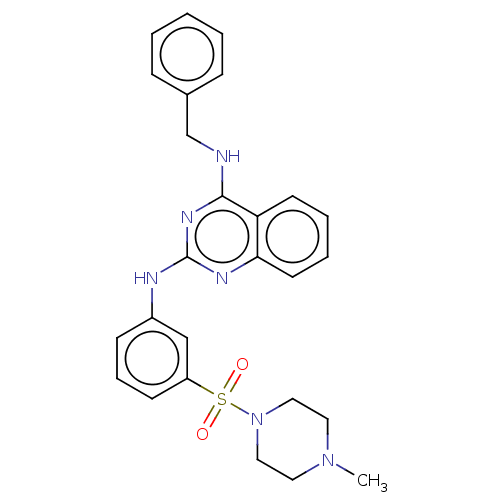
(CHEMBL5186856)Show SMILES CN1CCN(CC1)S(=O)(=O)c1cccc(Nc2nc(NCc3ccccc3)c3ccccc3n2)c1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2022.117092
BindingDB Entry DOI: 10.7270/Q21N8568 |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Rattus norvegicus) | BDBM50518226
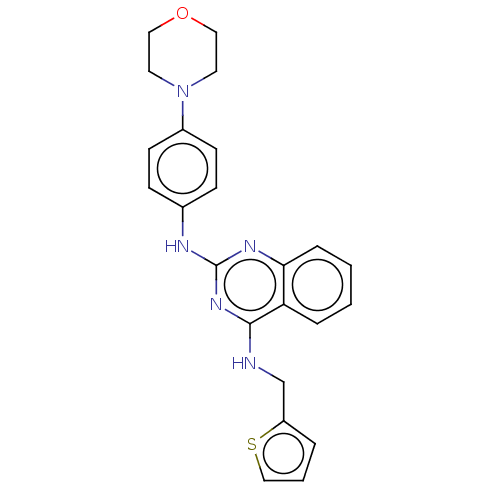
(CHEMBL4534674)Show SMILES C(Nc1nc(Nc2ccc(cc2)N2CCOCC2)nc2ccccc12)c1cccs1 Show InChI InChI=1S/C23H23N5OS/c1-2-6-21-20(5-1)22(24-16-19-4-3-15-30-19)27-23(26-21)25-17-7-9-18(10-8-17)28-11-13-29-14-12-28/h1-10,15H,11-14,16H2,(H2,24,25,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 514 | n/a | n/a | n/a | n/a | n/a | n/a |
Kasetsart University
Curated by ChEMBL
| Assay Description
Inhibition of rat lung PDE5 using [3H]cGMP as substrate measured after 10 mins by scintillation counting method |
Bioorg Med Chem Lett 29: 267-270 (2019)
Article DOI: 10.1016/j.bmcl.2018.11.043
BindingDB Entry DOI: 10.7270/Q2RJ4NT3 |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50602242
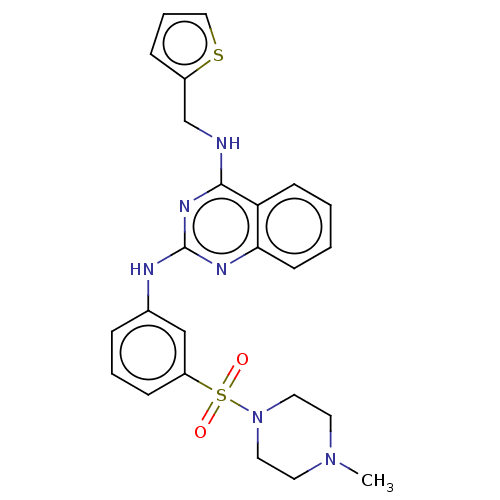
(CHEMBL5195499)Show SMILES CN1CCN(CC1)S(=O)(=O)c1cccc(Nc2nc(NCc3cccs3)c3ccccc3n2)c1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 520 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2022.117092
BindingDB Entry DOI: 10.7270/Q21N8568 |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Rattus norvegicus) | BDBM50518230
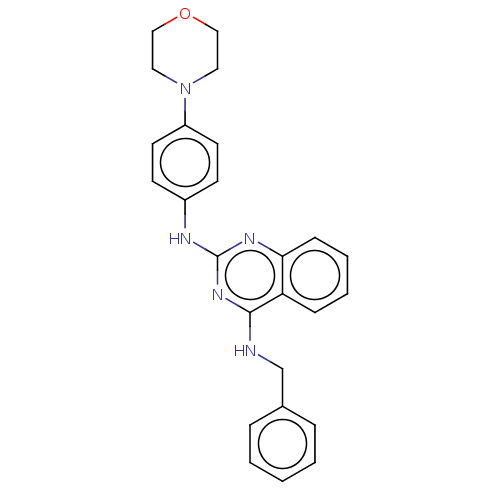
(CHEMBL4453311)Show SMILES C(Nc1nc(Nc2ccc(cc2)N2CCOCC2)nc2ccccc12)c1ccccc1 Show InChI InChI=1S/C25H25N5O/c1-2-6-19(7-3-1)18-26-24-22-8-4-5-9-23(22)28-25(29-24)27-20-10-12-21(13-11-20)30-14-16-31-17-15-30/h1-13H,14-18H2,(H2,26,27,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 525 | n/a | n/a | n/a | n/a | n/a | n/a |
Kasetsart University
Curated by ChEMBL
| Assay Description
Inhibition of rat lung PDE5 using [3H]cGMP as substrate measured after 10 mins by scintillation counting method |
Bioorg Med Chem Lett 29: 267-270 (2019)
Article DOI: 10.1016/j.bmcl.2018.11.043
BindingDB Entry DOI: 10.7270/Q2RJ4NT3 |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Rattus norvegicus) | BDBM50518221

(CHEMBL4450878)Show SMILES COc1cc2nc(Nc3cccc(c3)S(N)(=O)=O)nc(NCc3cccs3)c2cc1OC Show InChI InChI=1S/C21H21N5O4S2/c1-29-18-10-16-17(11-19(18)30-2)25-21(26-20(16)23-12-14-6-4-8-31-14)24-13-5-3-7-15(9-13)32(22,27)28/h3-11H,12H2,1-2H3,(H2,22,27,28)(H2,23,24,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 555 | n/a | n/a | n/a | n/a | n/a | n/a |
Kasetsart University
Curated by ChEMBL
| Assay Description
Inhibition of rat lung PDE5 using [3H]cGMP as substrate measured after 10 mins by scintillation counting method |
Bioorg Med Chem Lett 29: 267-270 (2019)
Article DOI: 10.1016/j.bmcl.2018.11.043
BindingDB Entry DOI: 10.7270/Q2RJ4NT3 |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50602244

(CHEMBL5191104)Show SMILES CCCNc1nc(Nc2cccc(c2)S(=O)(=O)N2CCN(C)CC2)nc2ccccc12 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2022.117092
BindingDB Entry DOI: 10.7270/Q21N8568 |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Rattus norvegicus) | BDBM50518218

(CHEMBL4567109)Show SMILES O=C(Nc1ccccc1)Nc1ccc(Nc2nc(NCc3cccs3)c3ccccc3n2)cc1 Show InChI InChI=1S/C26H22N6OS/c33-26(29-18-7-2-1-3-8-18)30-20-14-12-19(13-15-20)28-25-31-23-11-5-4-10-22(23)24(32-25)27-17-21-9-6-16-34-21/h1-16H,17H2,(H2,29,30,33)(H2,27,28,31,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 775 | n/a | n/a | n/a | n/a | n/a | n/a |
Kasetsart University
Curated by ChEMBL
| Assay Description
Inhibition of rat lung PDE5 using [3H]cGMP as substrate measured after 10 mins by scintillation counting method |
Bioorg Med Chem Lett 29: 267-270 (2019)
Article DOI: 10.1016/j.bmcl.2018.11.043
BindingDB Entry DOI: 10.7270/Q2RJ4NT3 |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Rattus norvegicus) | BDBM50518229

(CHEMBL4558761)Show SMILES NS(=O)(=O)c1ccc(Nc2nc(NCc3ccccc3)c3ccccc3n2)cc1 Show InChI InChI=1S/C21H19N5O2S/c22-29(27,28)17-12-10-16(11-13-17)24-21-25-19-9-5-4-8-18(19)20(26-21)23-14-15-6-2-1-3-7-15/h1-13H,14H2,(H2,22,27,28)(H2,23,24,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 946 | n/a | n/a | n/a | n/a | n/a | n/a |
Kasetsart University
Curated by ChEMBL
| Assay Description
Inhibition of rat lung PDE5 using [3H]cGMP as substrate measured after 10 mins by scintillation counting method |
Bioorg Med Chem Lett 29: 267-270 (2019)
Article DOI: 10.1016/j.bmcl.2018.11.043
BindingDB Entry DOI: 10.7270/Q2RJ4NT3 |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50602259

(CHEMBL5200202)Show SMILES CC(C)N1CCC(CC1)Nc1nc(Nc2cccc(c2)S(N)(=O)=O)nc2ccccc12 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.03E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2022.117092
BindingDB Entry DOI: 10.7270/Q21N8568 |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Rattus norvegicus) | BDBM50518236
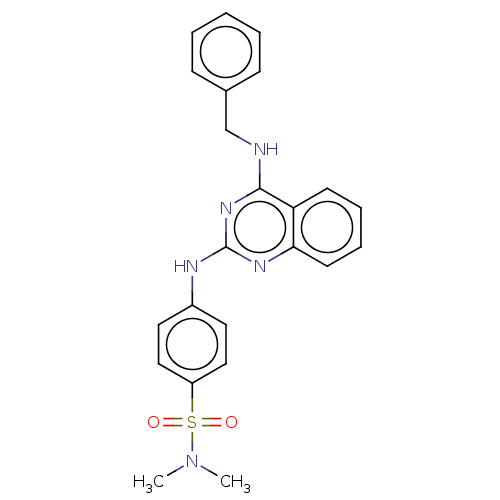
(CHEMBL4469064)Show SMILES CN(C)S(=O)(=O)c1ccc(Nc2nc(NCc3ccccc3)c3ccccc3n2)cc1 Show InChI InChI=1S/C23H23N5O2S/c1-28(2)31(29,30)19-14-12-18(13-15-19)25-23-26-21-11-7-6-10-20(21)22(27-23)24-16-17-8-4-3-5-9-17/h3-15H,16H2,1-2H3,(H2,24,25,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.26E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Kasetsart University
Curated by ChEMBL
| Assay Description
Inhibition of rat lung PDE5 using [3H]cGMP as substrate measured after 10 mins by scintillation counting method |
Bioorg Med Chem Lett 29: 267-270 (2019)
Article DOI: 10.1016/j.bmcl.2018.11.043
BindingDB Entry DOI: 10.7270/Q2RJ4NT3 |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Rattus norvegicus) | BDBM50518231
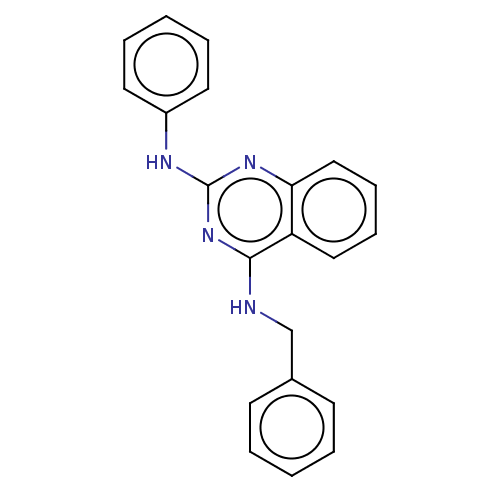
(CHEMBL597248 | GNF-Pf-4180 | MMV006169)Show InChI InChI=1S/C21H18N4/c1-3-9-16(10-4-1)15-22-20-18-13-7-8-14-19(18)24-21(25-20)23-17-11-5-2-6-12-17/h1-14H,15H2,(H2,22,23,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.38E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Kasetsart University
Curated by ChEMBL
| Assay Description
Inhibition of rat lung PDE5 using [3H]cGMP as substrate measured after 10 mins by scintillation counting method |
Bioorg Med Chem Lett 29: 267-270 (2019)
Article DOI: 10.1016/j.bmcl.2018.11.043
BindingDB Entry DOI: 10.7270/Q2RJ4NT3 |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Rattus norvegicus) | BDBM50518235
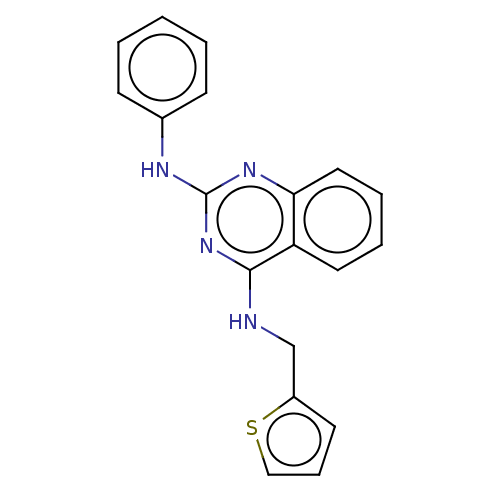
(CHEMBL4525521)Show InChI InChI=1S/C19H16N4S/c1-2-7-14(8-3-1)21-19-22-17-11-5-4-10-16(17)18(23-19)20-13-15-9-6-12-24-15/h1-12H,13H2,(H2,20,21,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.44E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Kasetsart University
Curated by ChEMBL
| Assay Description
Inhibition of rat lung PDE5 using [3H]cGMP as substrate measured after 10 mins by scintillation counting method |
Bioorg Med Chem Lett 29: 267-270 (2019)
Article DOI: 10.1016/j.bmcl.2018.11.043
BindingDB Entry DOI: 10.7270/Q2RJ4NT3 |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50602248
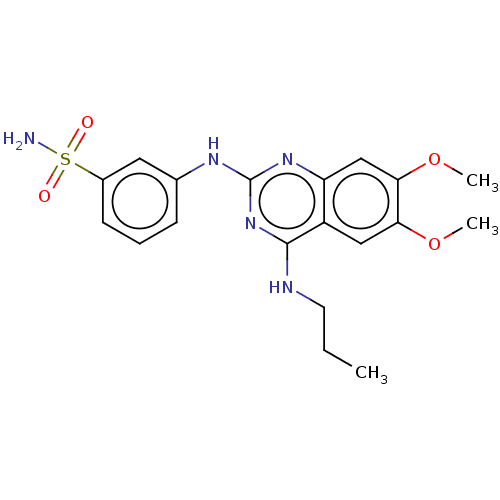
(CHEMBL5199387)Show SMILES CCCNc1nc(Nc2cccc(c2)S(N)(=O)=O)nc2cc(OC)c(OC)cc12 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.44E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2022.117092
BindingDB Entry DOI: 10.7270/Q21N8568 |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50602239
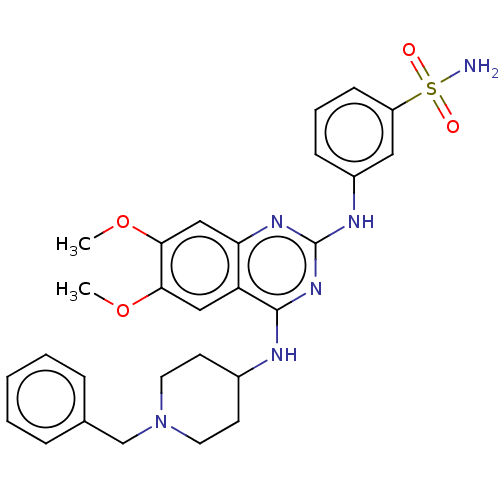
(CHEMBL5188731)Show SMILES COc1cc2nc(Nc3cccc(c3)S(N)(=O)=O)nc(NC3CCN(Cc4ccccc4)CC3)c2cc1OC | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.64E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2022.117092
BindingDB Entry DOI: 10.7270/Q21N8568 |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50602258
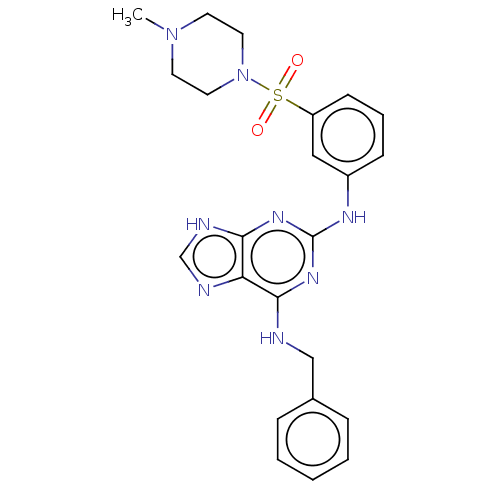
(CHEMBL5177893)Show SMILES CN1CCN(CC1)S(=O)(=O)c1cccc(Nc2nc(NCc3ccccc3)c3nc[nH]c3n2)c1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.02E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2022.117092
BindingDB Entry DOI: 10.7270/Q21N8568 |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50602264

(CHEMBL5195434)Show SMILES COc1cc2nc(Nc3cccc(c3)S(N)(=O)=O)nc(NC3CCN(CC3)C(C)C)c2cc1OC | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.41E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2022.117092
BindingDB Entry DOI: 10.7270/Q21N8568 |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Rattus norvegicus) | BDBM50518222

(CHEMBL4552918)Show SMILES Cc1cc(Nc2nc(Nc3cccc(c3)S(N)(=O)=O)nc3ccccc23)n[nH]1 Show InChI InChI=1S/C18H17N7O2S/c1-11-9-16(25-24-11)22-17-14-7-2-3-8-15(14)21-18(23-17)20-12-5-4-6-13(10-12)28(19,26)27/h2-10H,1H3,(H2,19,26,27)(H3,20,21,22,23,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.71E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Kasetsart University
Curated by ChEMBL
| Assay Description
Inhibition of rat lung PDE5 using [3H]cGMP as substrate measured after 10 mins by scintillation counting method |
Bioorg Med Chem Lett 29: 267-270 (2019)
Article DOI: 10.1016/j.bmcl.2018.11.043
BindingDB Entry DOI: 10.7270/Q2RJ4NT3 |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50602250

(CHEMBL5171693)Show SMILES COc1cc2nc(Nc3ccc(cc3)S(N)(=O)=O)nc(NCC(C)C)c2cc1OC | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.97E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2022.117092
BindingDB Entry DOI: 10.7270/Q21N8568 |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50602256
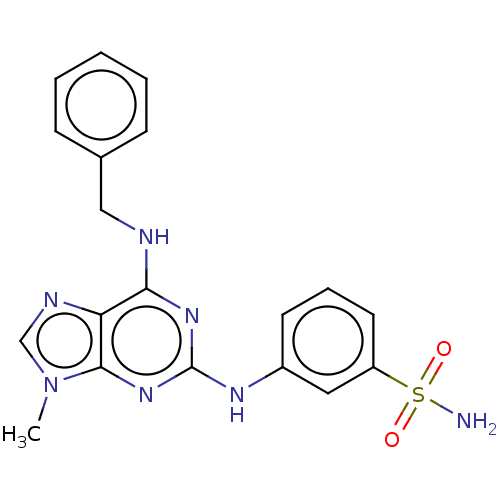
(CHEMBL5183230)Show SMILES Cn1cnc2c(NCc3ccccc3)nc(Nc3cccc(c3)S(N)(=O)=O)nc12 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.46E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2022.117092
BindingDB Entry DOI: 10.7270/Q21N8568 |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50602249
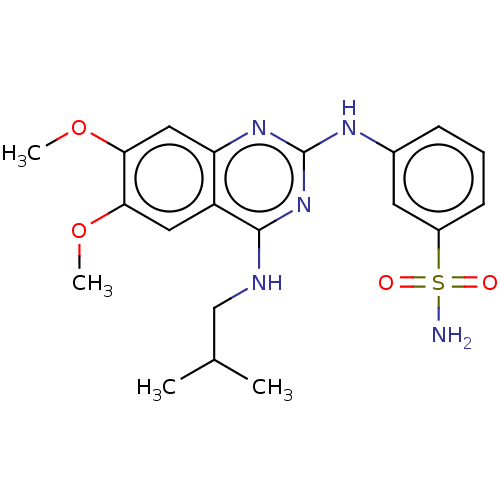
(CHEMBL5208363)Show SMILES COc1cc2nc(Nc3cccc(c3)S(N)(=O)=O)nc(NCC(C)C)c2cc1OC | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.23E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2022.117092
BindingDB Entry DOI: 10.7270/Q21N8568 |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50602262
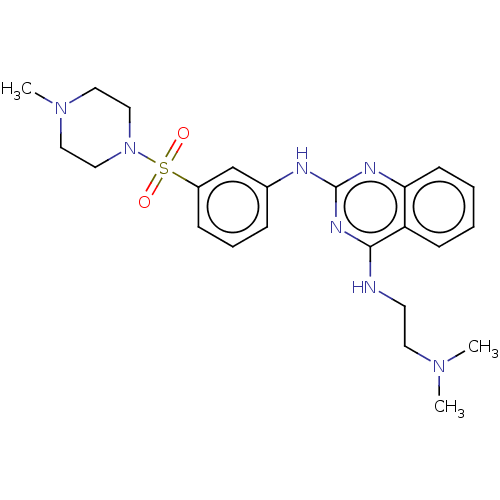
(CHEMBL5193660)Show SMILES CN(C)CCNc1nc(Nc2cccc(c2)S(=O)(=O)N2CCN(C)CC2)nc2ccccc12 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.28E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2022.117092
BindingDB Entry DOI: 10.7270/Q21N8568 |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50602261
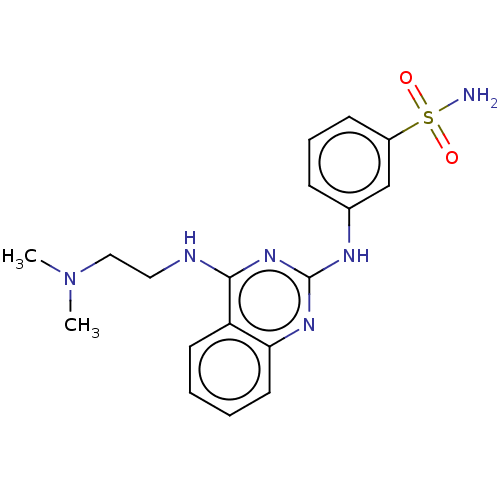
(CHEMBL5196307)Show SMILES CN(C)CCNc1nc(Nc2cccc(c2)S(N)(=O)=O)nc2ccccc12 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 9.39E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2022.117092
BindingDB Entry DOI: 10.7270/Q21N8568 |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Rattus norvegicus) | BDBM50518219
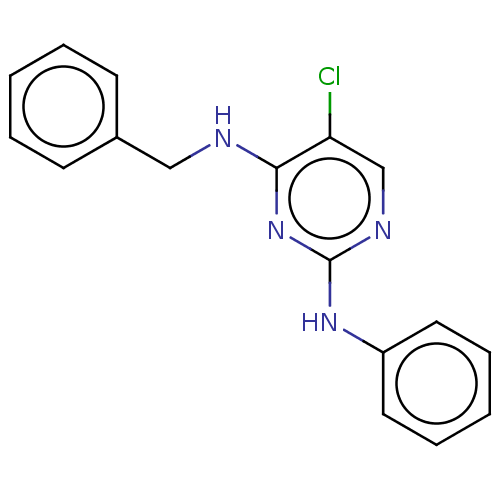
(CHEMBL4565213)Show InChI InChI=1S/C17H15ClN4/c18-15-12-20-17(21-14-9-5-2-6-10-14)22-16(15)19-11-13-7-3-1-4-8-13/h1-10,12H,11H2,(H2,19,20,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Kasetsart University
Curated by ChEMBL
| Assay Description
Inhibition of rat lung PDE5 using [3H]cGMP as substrate measured after 10 mins by scintillation counting method |
Bioorg Med Chem Lett 29: 267-270 (2019)
Article DOI: 10.1016/j.bmcl.2018.11.043
BindingDB Entry DOI: 10.7270/Q2RJ4NT3 |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50602253

(CHEMBL5183757)Show SMILES CCCNc1nc(Nc2ccc(cc2)S(=O)(=O)N2CCN(C)CC2)nc2n(C)cnc12 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.89E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2022.117092
BindingDB Entry DOI: 10.7270/Q21N8568 |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50602257
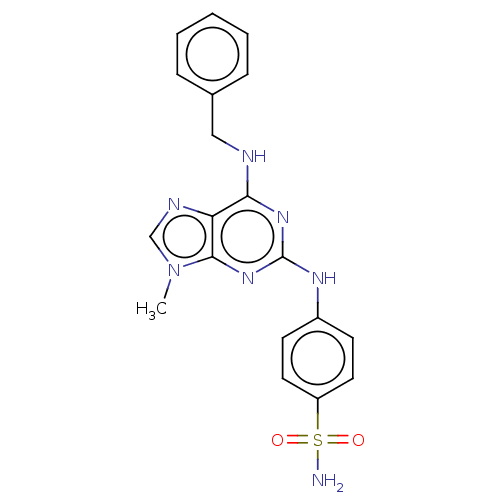
(CHEMBL5206939)Show SMILES Cn1cnc2c(NCc3ccccc3)nc(Nc3ccc(cc3)S(N)(=O)=O)nc12 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2022.117092
BindingDB Entry DOI: 10.7270/Q21N8568 |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50602255

(CHEMBL5195857)Show SMILES CC(C)CNc1nc(Nc2ccc(cc2)S(=O)(=O)N2CCN(C)CC2)nc2n(C)cnc12 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.85E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2022.117092
BindingDB Entry DOI: 10.7270/Q21N8568 |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50602254

(CHEMBL5193806)Show SMILES CC(C)CNc1nc(Nc2cccc(c2)S(=O)(=O)N2CCN(C)CC2)nc2n(C)cnc12 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.33E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2022.117092
BindingDB Entry DOI: 10.7270/Q21N8568 |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50602251

(CHEMBL5188011)Show SMILES CN1CCN(CC1)S(=O)(=O)c1ccc(Nc2nc(NCc3ccccc3)c3ncn(C)c3n2)cc1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.84E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2022.117092
BindingDB Entry DOI: 10.7270/Q21N8568 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data