

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Alpha-1A adrenergic receptor (Homo sapiens (Human)) | BDBM50090023 (4-(3,4-Difluoro-phenyl)-2-oxo-1,2,3,4-tetrahydro-p...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Indo-Soviet Friendship College of Pharmacy (ISFCP) Curated by ChEMBL | Assay Description Displacement of [125I]L-762,459 from recombinant human alpha1a adrenergic receptor expressed in mammalian cells measured after 1 hr | Eur J Med Chem 132: 108-134 (2017) Article DOI: 10.1016/j.ejmech.2017.03.025 BindingDB Entry DOI: 10.7270/Q2X350V4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (Homo sapiens (Human)) | BDBM50090018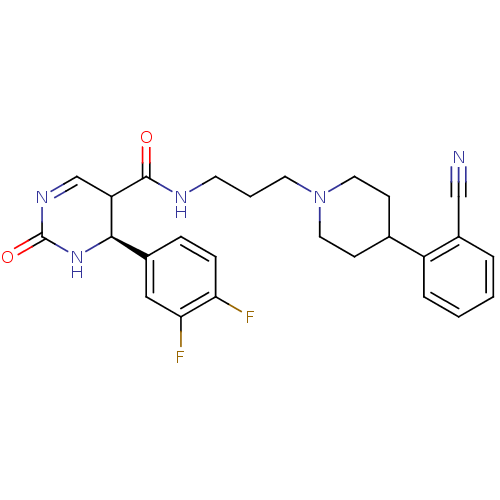 (4-(3,4-Difluoro-phenyl)-2-oxo-1,2,3,4-tetrahydro-p...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Indo-Soviet Friendship College of Pharmacy (ISFCP) Curated by ChEMBL | Assay Description Displacement of [125I]L-762,459 from recombinant human alpha1a adrenergic receptor expressed in mammalian cells measured after 1 hr | Eur J Med Chem 132: 108-134 (2017) Article DOI: 10.1016/j.ejmech.2017.03.025 BindingDB Entry DOI: 10.7270/Q2X350V4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (Homo sapiens (Human)) | BDBM50090027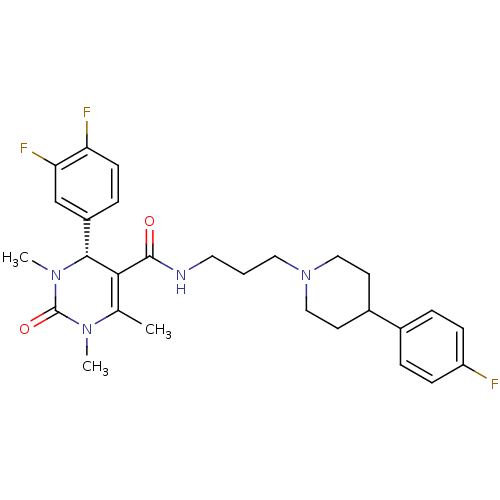 ((R)-4-(3,4-Difluoro-phenyl)-1,3,6-trimethyl-2-oxo-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Indo-Soviet Friendship College of Pharmacy (ISFCP) Curated by ChEMBL | Assay Description Displacement of [125I]L-762,459 from recombinant human alpha1a adrenergic receptor expressed in mammalian cells measured after 1 hr | Eur J Med Chem 132: 108-134 (2017) Article DOI: 10.1016/j.ejmech.2017.03.025 BindingDB Entry DOI: 10.7270/Q2X350V4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (Homo sapiens (Human)) | BDBM50245653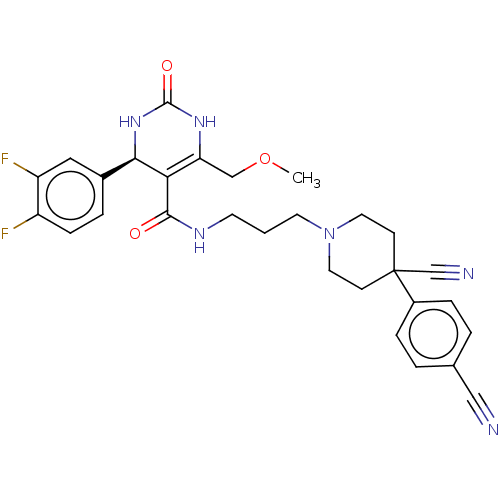 (CHEMBL4100620) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Indo-Soviet Friendship College of Pharmacy (ISFCP) Curated by ChEMBL | Assay Description Displacement of [125I]L-762,459 from recombinant human alpha1a adrenergic receptor expressed in mammalian cells measured after 1 hr | Eur J Med Chem 132: 108-134 (2017) Article DOI: 10.1016/j.ejmech.2017.03.025 BindingDB Entry DOI: 10.7270/Q2X350V4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (Homo sapiens (Human)) | BDBM50090031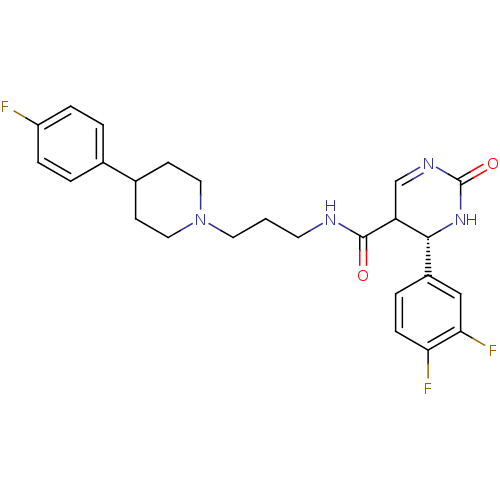 (4-(3,4-Difluoro-phenyl)-2-oxo-1,2,3,4-tetrahydro-p...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Indo-Soviet Friendship College of Pharmacy (ISFCP) Curated by ChEMBL | Assay Description Displacement of [125I]L-762,459 from recombinant human alpha1a adrenergic receptor expressed in mammalian cells measured after 1 hr | Eur J Med Chem 132: 108-134 (2017) Article DOI: 10.1016/j.ejmech.2017.03.025 BindingDB Entry DOI: 10.7270/Q2X350V4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (Homo sapiens (Human)) | BDBM50245650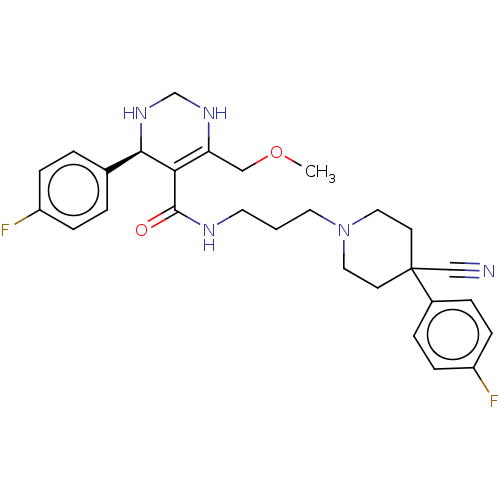 (CHEMBL4062896) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Indo-Soviet Friendship College of Pharmacy (ISFCP) Curated by ChEMBL | Assay Description Displacement of [125I]L-762,459 from recombinant human alpha1a adrenergic receptor expressed in mammalian cells measured after 1 hr | Eur J Med Chem 132: 108-134 (2017) Article DOI: 10.1016/j.ejmech.2017.03.025 BindingDB Entry DOI: 10.7270/Q2X350V4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (Homo sapiens (Human)) | BDBM50245652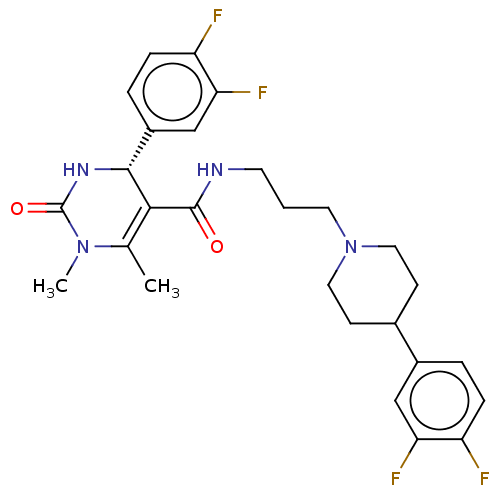 (CHEMBL4081581) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Indo-Soviet Friendship College of Pharmacy (ISFCP) Curated by ChEMBL | Assay Description Displacement of [125I]L-762,459 from recombinant human alpha1a adrenergic receptor expressed in mammalian cells measured after 1 hr | Eur J Med Chem 132: 108-134 (2017) Article DOI: 10.1016/j.ejmech.2017.03.025 BindingDB Entry DOI: 10.7270/Q2X350V4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (Homo sapiens (Human)) | BDBM50306442 ((6aS,13bS)-11-chloro-7-methyl-4-phenyl-6,6a,7,8,9,...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]SCh23390 from dopamine D1 receptor expressed in mouse LTK cells by scintillation counting | Bioorg Med Chem Lett 20: 832-5 (2010) Article DOI: 10.1016/j.bmcl.2009.12.094 BindingDB Entry DOI: 10.7270/Q2FX79JH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (Homo sapiens (Human)) | BDBM50306314 ((R)-N-(4-(7-chloro-8-hydroxy-3-methyl-2,3,4,5-tetr...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity to dopamine D1 receptor | Bioorg Med Chem Lett 20: 836-40 (2010) Article DOI: 10.1016/j.bmcl.2009.12.100 BindingDB Entry DOI: 10.7270/Q23N23HT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 1 (RAT) | BDBM50345941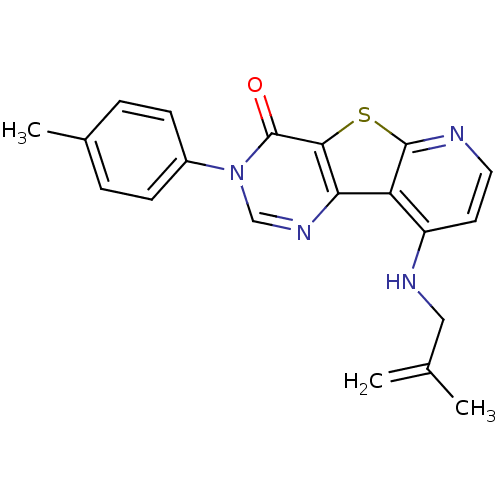 (9-(2-Methyl-allylamino)-3-p-tolyl-3H-pyrido[3',2':...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Antagonist activity at rat mGluR1 | Bioorg Med Chem Lett 19: 3199-203 (2009) Article DOI: 10.1016/j.bmcl.2009.04.104 BindingDB Entry DOI: 10.7270/Q27081SJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (Homo sapiens (Human)) | BDBM50306440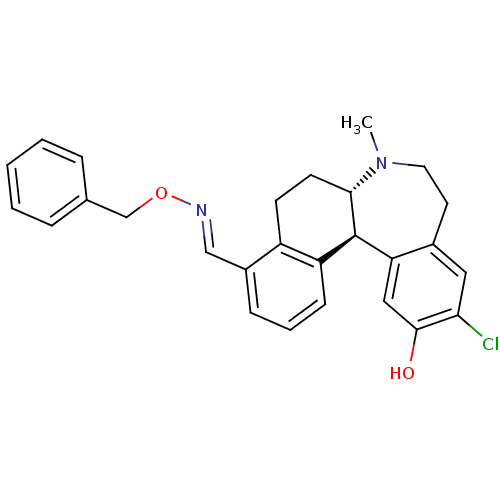 (11-chloro-12-hydroxy-7-methyl-6,6a,7,8,9,13b-hexah...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]SCh23390 from dopamine D1 receptor expressed in mouse LTK cells by scintillation counting | Bioorg Med Chem Lett 20: 832-5 (2010) Article DOI: 10.1016/j.bmcl.2009.12.094 BindingDB Entry DOI: 10.7270/Q2FX79JH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (Homo sapiens (Human)) | BDBM50090043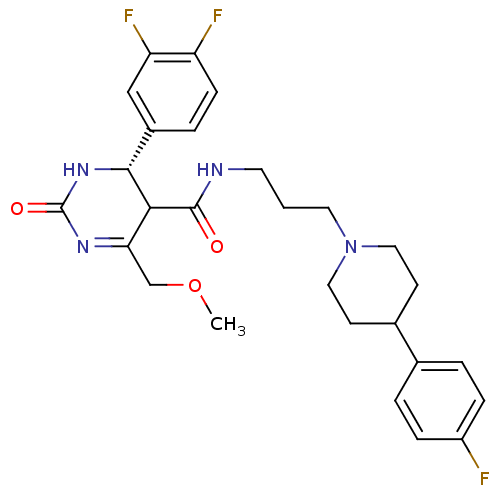 (4-(3,4-Difluoro-phenyl)-6-methoxymethyl-2-oxo-1,2,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Indo-Soviet Friendship College of Pharmacy (ISFCP) Curated by ChEMBL | Assay Description Displacement of [125I]L-762,459 from recombinant human alpha1a adrenergic receptor expressed in mammalian cells measured after 1 hr | Eur J Med Chem 132: 108-134 (2017) Article DOI: 10.1016/j.ejmech.2017.03.025 BindingDB Entry DOI: 10.7270/Q2X350V4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 2 (Homo sapiens (Human)) | BDBM190708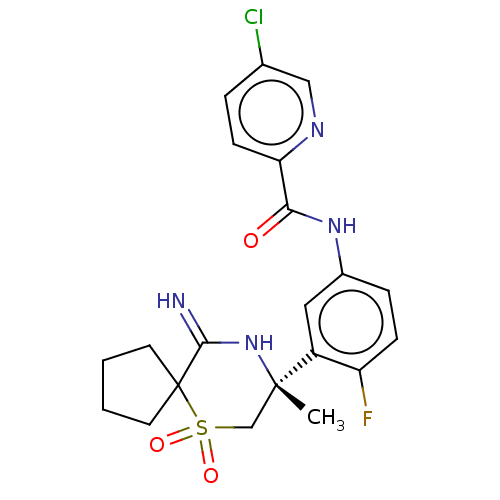 (US9181236, 12) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.300 | -55.3 | n/a | n/a | n/a | n/a | n/a | 5.0 | 30 |
Merck Sharp & Dohme Corp. US Patent | Assay Description Inhibitor IC50s at purified human autoBACE-2 are determined in a time-resolved endpoint proteolysis assay that measures hydrolysis of the QSY7-EISE... | US Patent US9181236 (2015) BindingDB Entry DOI: 10.7270/Q2NC5ZZB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (Homo sapiens (Human)) | BDBM50306452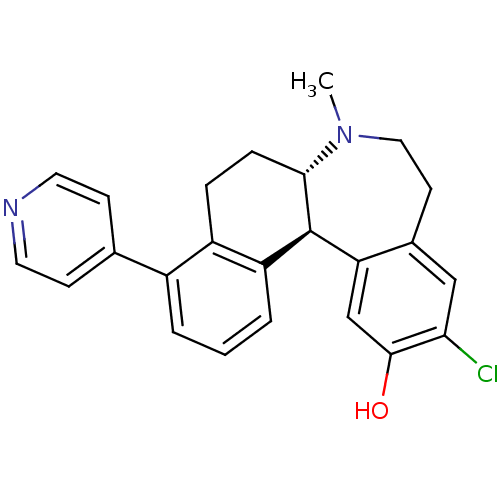 ((6aS,13bS)-11-chloro-7-methyl-4-(pyridin-4-yl)-6,6...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]SCh23390 from dopamine D1 receptor expressed in mouse LTK cells by scintillation counting | Bioorg Med Chem Lett 20: 832-5 (2010) Article DOI: 10.1016/j.bmcl.2009.12.094 BindingDB Entry DOI: 10.7270/Q2FX79JH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 2 (Homo sapiens (Human)) | BDBM190709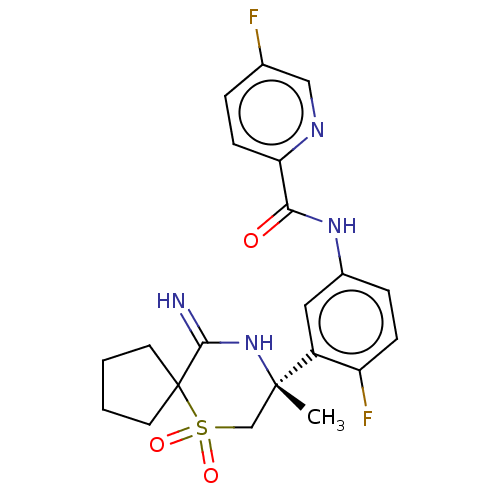 (US9181236, 13) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.300 | -55.3 | n/a | n/a | n/a | n/a | n/a | 5.0 | 30 |
Merck Sharp & Dohme Corp. US Patent | Assay Description Inhibitor IC50s at purified human autoBACE-2 are determined in a time-resolved endpoint proteolysis assay that measures hydrolysis of the QSY7-EISE... | US Patent US9181236 (2015) BindingDB Entry DOI: 10.7270/Q2NC5ZZB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (Homo sapiens (Human)) | BDBM50306443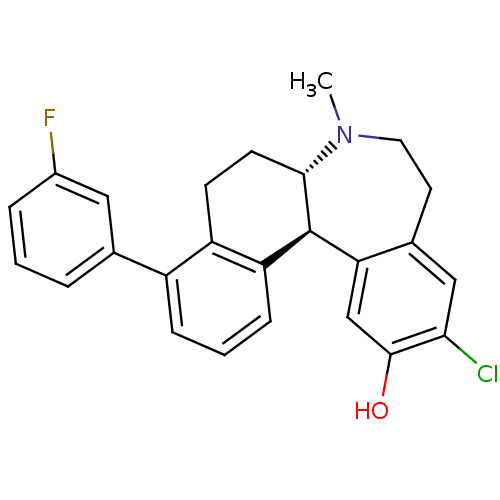 ((6aS,13bS)-11-chloro-4-(3-fluorophenyl)-7-methyl-6...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]SCh23390 from dopamine D1 receptor expressed in mouse LTK cells by scintillation counting | Bioorg Med Chem Lett 20: 832-5 (2010) Article DOI: 10.1016/j.bmcl.2009.12.094 BindingDB Entry DOI: 10.7270/Q2FX79JH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 1 (RAT) | BDBM50345942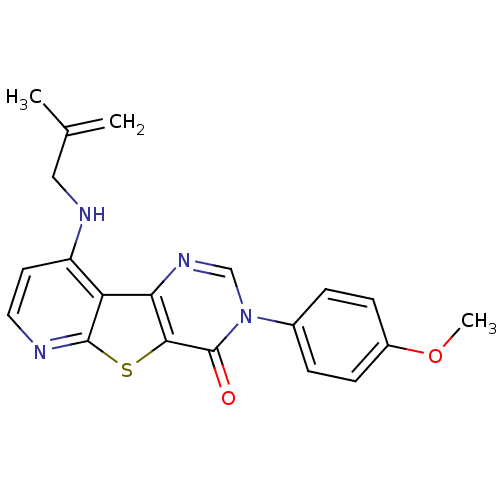 (3-(4-Methoxy-phenyl)-9-(2-methyl-allylamino)-3H-py...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Antagonist activity at rat mGluR1 | Bioorg Med Chem Lett 19: 3199-203 (2009) Article DOI: 10.1016/j.bmcl.2009.04.104 BindingDB Entry DOI: 10.7270/Q27081SJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 2 (Homo sapiens (Human)) | BDBM190697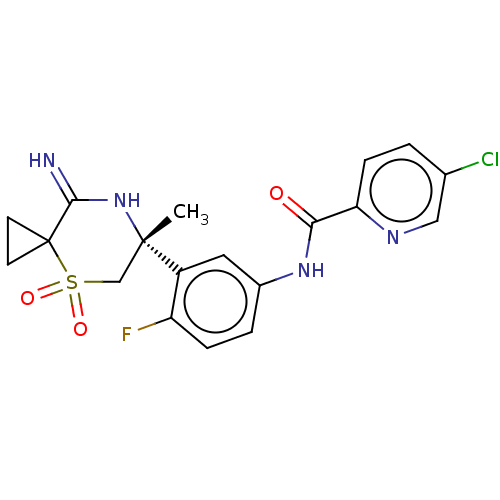 (US9181236, 1) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.400 | -54.5 | n/a | n/a | n/a | n/a | n/a | 5.0 | 30 |
Merck Sharp & Dohme Corp. US Patent | Assay Description Inhibitor IC50s at purified human autoBACE-2 are determined in a time-resolved endpoint proteolysis assay that measures hydrolysis of the QSY7-EISE... | US Patent US9181236 (2015) BindingDB Entry DOI: 10.7270/Q2NC5ZZB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (Homo sapiens (Human)) | BDBM50090013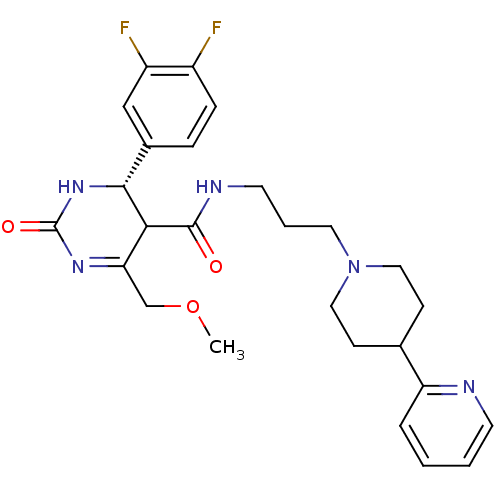 (4-(3,4-Difluoro-phenyl)-6-methoxymethyl-2-oxo-1,2,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Indo-Soviet Friendship College of Pharmacy (ISFCP) Curated by ChEMBL | Assay Description Displacement of [125I]L-762,459 from recombinant human alpha1a adrenergic receptor expressed in mammalian cells measured after 1 hr | Eur J Med Chem 132: 108-134 (2017) Article DOI: 10.1016/j.ejmech.2017.03.025 BindingDB Entry DOI: 10.7270/Q2X350V4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (Homo sapiens (Human)) | BDBM50306316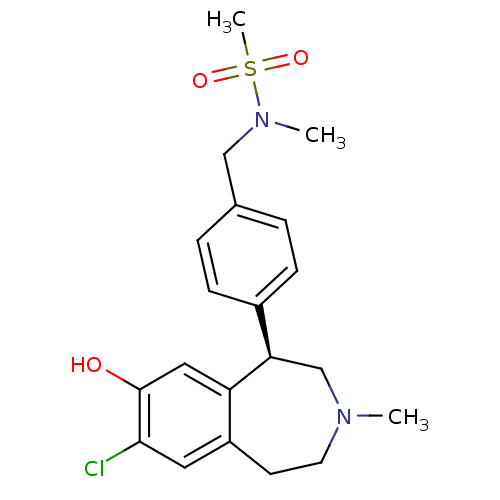 ((R)-N-(4-(7-chloro-8-hydroxy-3-methyl-2,3,4,5-tetr...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity to dopamine D1 receptor | Bioorg Med Chem Lett 20: 836-40 (2010) Article DOI: 10.1016/j.bmcl.2009.12.100 BindingDB Entry DOI: 10.7270/Q23N23HT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (Homo sapiens (Human)) | BDBM50306315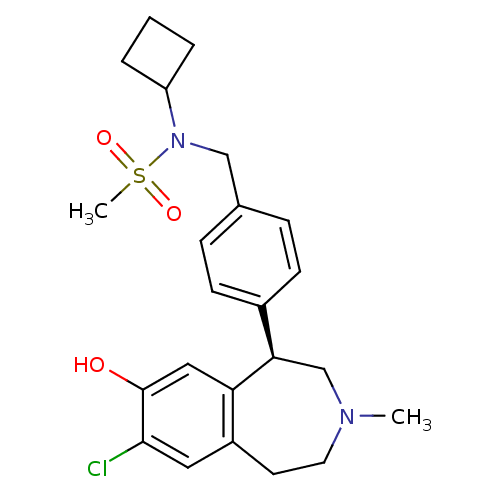 ((R)-N-(4-(7-chloro-8-hydroxy-3-methyl-2,3,4,5-tetr...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity to dopamine D1 receptor | Bioorg Med Chem Lett 20: 836-40 (2010) Article DOI: 10.1016/j.bmcl.2009.12.100 BindingDB Entry DOI: 10.7270/Q23N23HT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 2 (Homo sapiens (Human)) | BDBM190700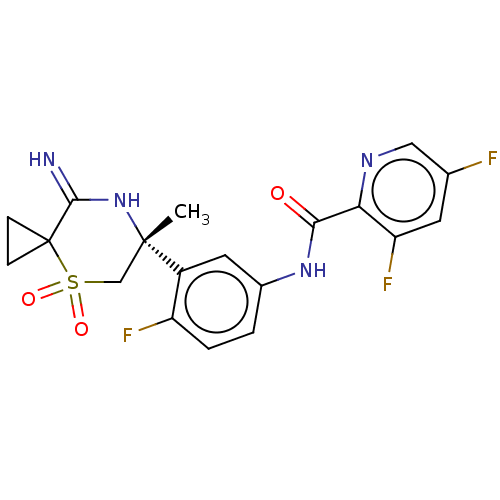 (US9181236, 4) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.5 | -54.0 | n/a | n/a | n/a | n/a | n/a | 5.0 | 30 |
Merck Sharp & Dohme Corp. US Patent | Assay Description Inhibitor IC50s at purified human autoBACE-2 are determined in a time-resolved endpoint proteolysis assay that measures hydrolysis of the QSY7-EISE... | US Patent US9181236 (2015) BindingDB Entry DOI: 10.7270/Q2NC5ZZB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (Homo sapiens (Human)) | BDBM50306322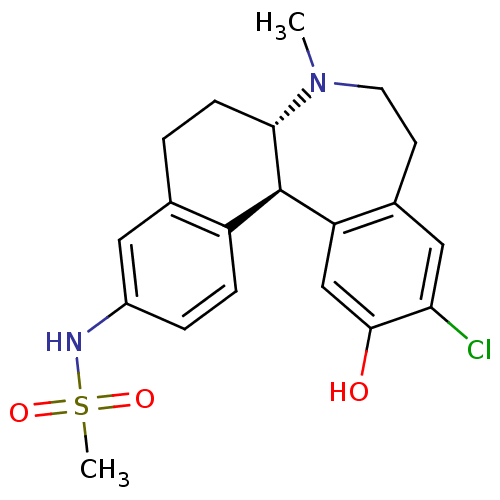 (CHEMBL597909 | N-((6aS,13bR)-11-chloro-12-hydroxy-...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity to dopamine D1 receptor | Bioorg Med Chem Lett 20: 836-40 (2010) Article DOI: 10.1016/j.bmcl.2009.12.100 BindingDB Entry DOI: 10.7270/Q23N23HT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (Homo sapiens (Human)) | BDBM50306453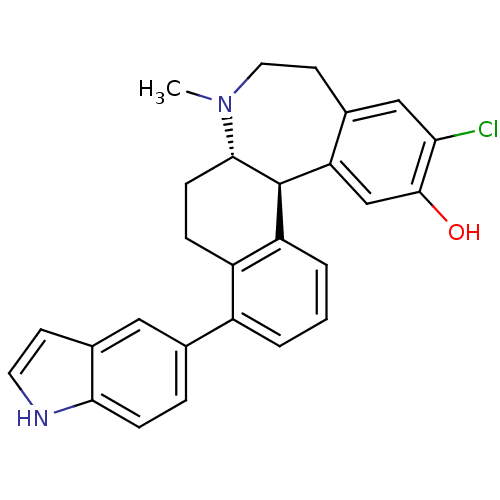 ((6aS,13bS)-11-chloro-4-(1H-indol-5-yl)-7-methyl-6,...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]SCh23390 from dopamine D1 receptor expressed in mouse LTK cells by scintillation counting | Bioorg Med Chem Lett 20: 832-5 (2010) Article DOI: 10.1016/j.bmcl.2009.12.094 BindingDB Entry DOI: 10.7270/Q2FX79JH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (Homo sapiens (Human)) | BDBM50306444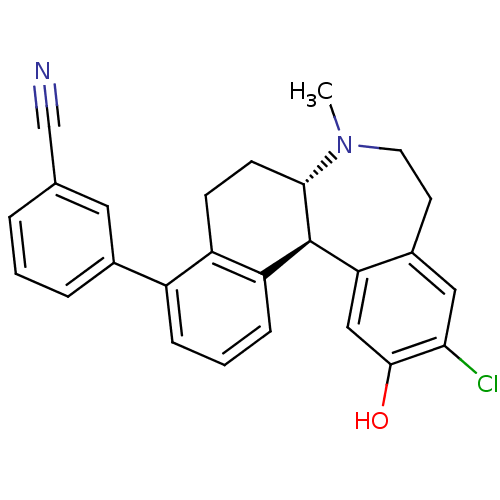 (3-((6aS,13bS)-11-chloro-12-hydroxy-7-methyl-6,6a,7...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]SCh23390 from dopamine D1 receptor expressed in mouse LTK cells by scintillation counting | Bioorg Med Chem Lett 20: 832-5 (2010) Article DOI: 10.1016/j.bmcl.2009.12.094 BindingDB Entry DOI: 10.7270/Q2FX79JH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 1 (RAT) | BDBM50345947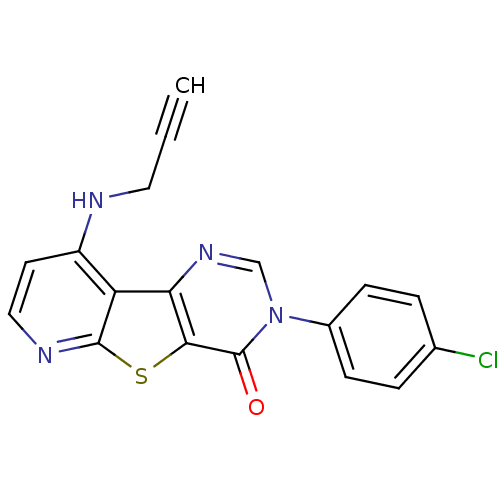 (3-(4-Chloro-phenyl)-9-prop-2-ynylamino-3H-pyrido[3...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Antagonist activity at rat mGluR1 | Bioorg Med Chem Lett 19: 3199-203 (2009) Article DOI: 10.1016/j.bmcl.2009.04.104 BindingDB Entry DOI: 10.7270/Q27081SJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (Homo sapiens (Human)) | BDBM50306445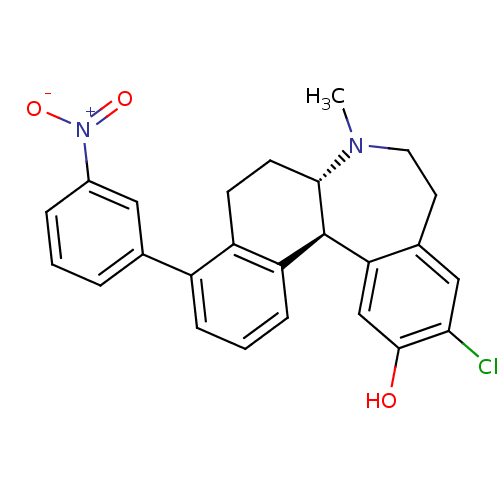 ((6aS,13bS)-11-chloro-7-methyl-4-(3-nitrophenyl)-6,...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]SCh23390 from dopamine D1 receptor expressed in mouse LTK cells by scintillation counting | Bioorg Med Chem Lett 20: 832-5 (2010) Article DOI: 10.1016/j.bmcl.2009.12.094 BindingDB Entry DOI: 10.7270/Q2FX79JH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 2 (Homo sapiens (Human)) | BDBM190711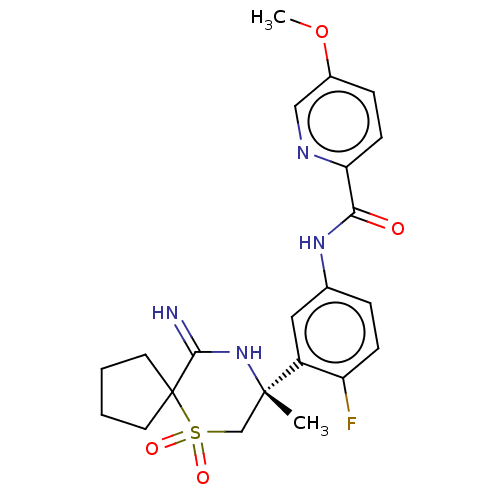 (US9181236, 15) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.600 | -53.5 | n/a | n/a | n/a | n/a | n/a | 5.0 | 30 |
Merck Sharp & Dohme Corp. US Patent | Assay Description Inhibitor IC50s at purified human autoBACE-2 are determined in a time-resolved endpoint proteolysis assay that measures hydrolysis of the QSY7-EISE... | US Patent US9181236 (2015) BindingDB Entry DOI: 10.7270/Q2NC5ZZB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (Homo sapiens (Human)) | BDBM50306317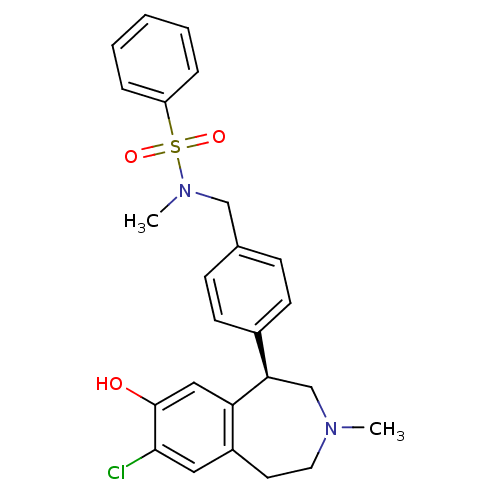 ((R)-N-(4-(7-chloro-8-hydroxy-3-methyl-2,3,4,5-tetr...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity to dopamine D1 receptor | Bioorg Med Chem Lett 20: 836-40 (2010) Article DOI: 10.1016/j.bmcl.2009.12.100 BindingDB Entry DOI: 10.7270/Q23N23HT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (Homo sapiens (Human)) | BDBM50306435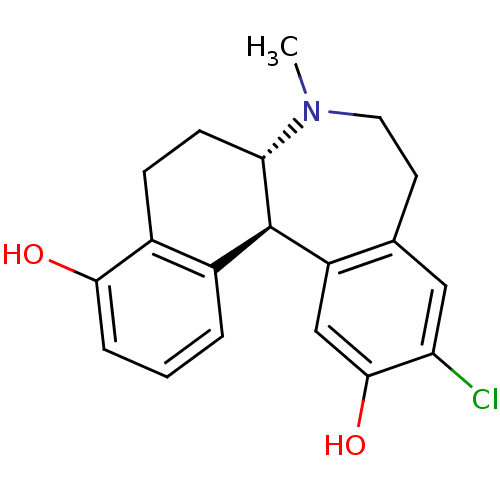 ((6aS,13bS)-11-chloro-7-methyl-6,6a,7,8,9,13b-hexah...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]SCh23390 from dopamine D1 receptor expressed in mouse LTK cells by scintillation counting | Bioorg Med Chem Lett 20: 832-5 (2010) Article DOI: 10.1016/j.bmcl.2009.12.094 BindingDB Entry DOI: 10.7270/Q2FX79JH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (Homo sapiens (Human)) | BDBM50306450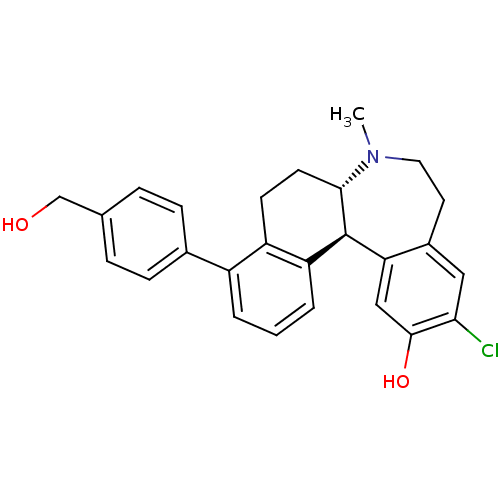 ((6aS,13bS)-11-chloro-4-(4-(hydroxymethyl)phenyl)-7...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]SCh23390 from dopamine D1 receptor expressed in mouse LTK cells by scintillation counting | Bioorg Med Chem Lett 20: 832-5 (2010) Article DOI: 10.1016/j.bmcl.2009.12.094 BindingDB Entry DOI: 10.7270/Q2FX79JH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (Homo sapiens (Human)) | BDBM50306449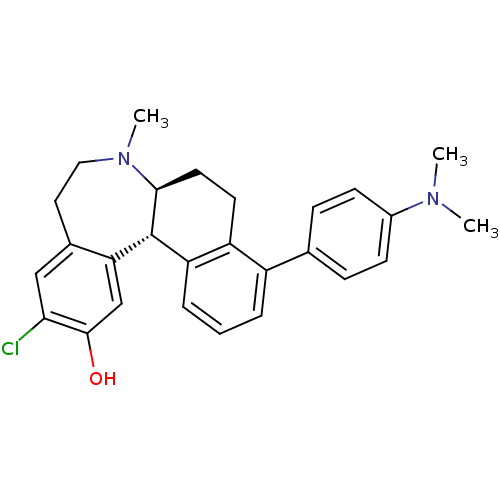 ((6aS,13bS)-11-chloro-4-(4-(dimethylamino)phenyl)-7...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]SCh23390 from dopamine D1 receptor expressed in mouse LTK cells by scintillation counting | Bioorg Med Chem Lett 20: 832-5 (2010) Article DOI: 10.1016/j.bmcl.2009.12.094 BindingDB Entry DOI: 10.7270/Q2FX79JH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (Homo sapiens (Human)) | BDBM50306448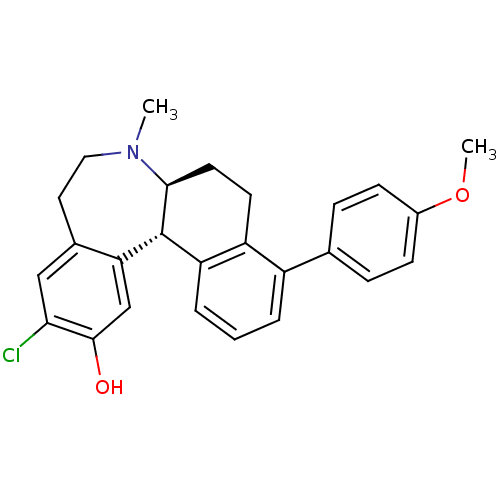 ((6aS,13bS)-11-chloro-4-(4-methoxyphenyl)-7-methyl-...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]SCh23390 from dopamine D1 receptor expressed in mouse LTK cells by scintillation counting | Bioorg Med Chem Lett 20: 832-5 (2010) Article DOI: 10.1016/j.bmcl.2009.12.094 BindingDB Entry DOI: 10.7270/Q2FX79JH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (Homo sapiens (Human)) | BDBM50306325 (1-((6aS,13bR)-11-chloro-12-hydroxy-7-methyl-6,6a,7...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity to dopamine D1 receptor | Bioorg Med Chem Lett 20: 836-40 (2010) Article DOI: 10.1016/j.bmcl.2009.12.100 BindingDB Entry DOI: 10.7270/Q23N23HT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (Homo sapiens (Human)) | BDBM50306319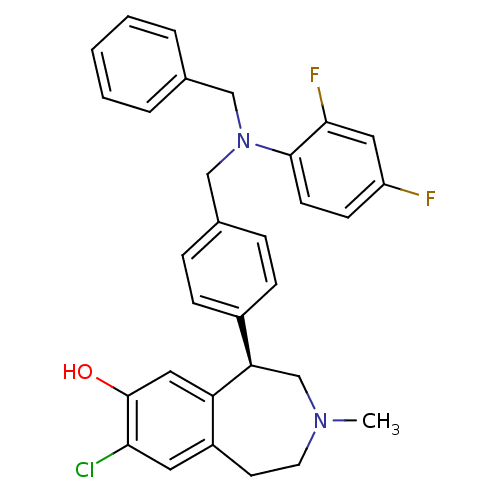 ((R)-5-(4-((benzyl(2,4-difluorophenyl)amino)methyl)...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity to dopamine D1 receptor | Bioorg Med Chem Lett 20: 836-40 (2010) Article DOI: 10.1016/j.bmcl.2009.12.100 BindingDB Entry DOI: 10.7270/Q23N23HT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 1 (RAT) | BDBM50345934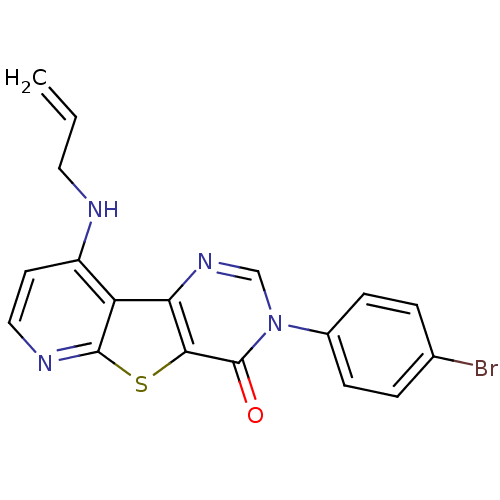 (9-Allylamino-3-(4-bromo-phenyl)-3H-pyrido[3',2':4,...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Antagonist activity at rat mGluR1 | Bioorg Med Chem Lett 19: 3199-203 (2009) Article DOI: 10.1016/j.bmcl.2009.04.104 BindingDB Entry DOI: 10.7270/Q27081SJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 1 (RAT) | BDBM50345950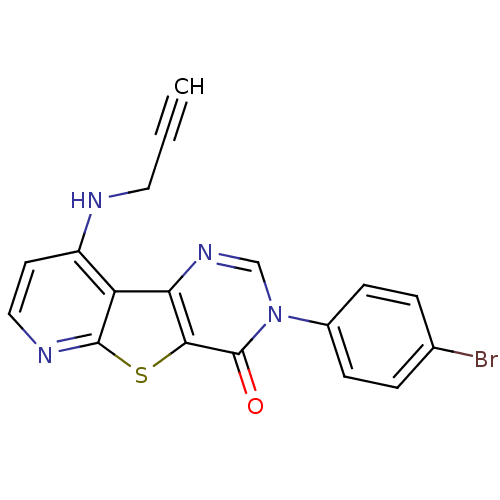 (3-(4-Bromo-phenyl)-9-prop-2-ynylamino-3H-pyrido[3'...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Antagonist activity at rat mGluR1 | Bioorg Med Chem Lett 19: 3199-203 (2009) Article DOI: 10.1016/j.bmcl.2009.04.104 BindingDB Entry DOI: 10.7270/Q27081SJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (Homo sapiens (Human)) | BDBM50306323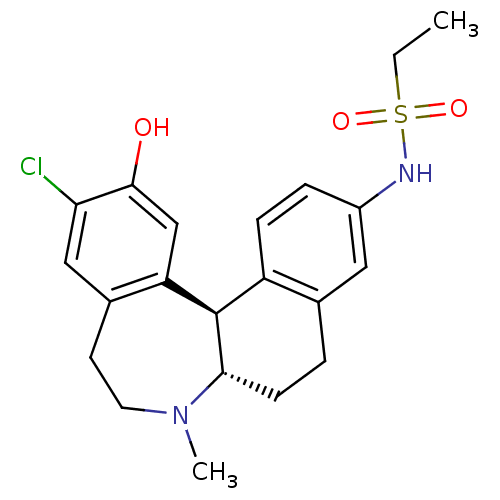 (CHEMBL600986 | N-((6aS,13bR)-11-chloro-12-hydroxy-...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity to dopamine D1 receptor | Bioorg Med Chem Lett 20: 836-40 (2010) Article DOI: 10.1016/j.bmcl.2009.12.100 BindingDB Entry DOI: 10.7270/Q23N23HT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (Homo sapiens (Human)) | BDBM50090041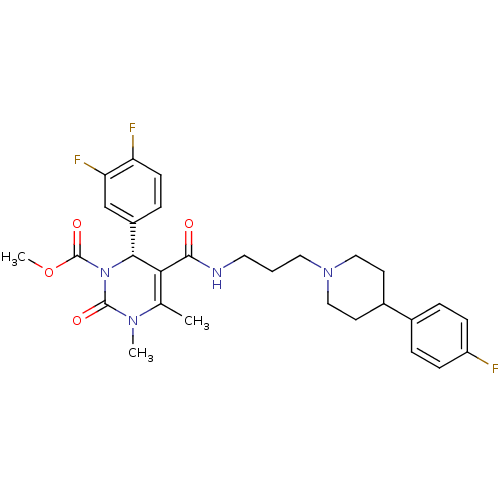 (6-(3,4-Difluoro-phenyl)-5-{3-[4-(4-fluoro-phenyl)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.75 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Indo-Soviet Friendship College of Pharmacy (ISFCP) Curated by ChEMBL | Assay Description Displacement of [125I]L-762,459 from recombinant human alpha1a adrenergic receptor expressed in mammalian cells measured after 1 hr | Eur J Med Chem 132: 108-134 (2017) Article DOI: 10.1016/j.ejmech.2017.03.025 BindingDB Entry DOI: 10.7270/Q2X350V4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 2 (Homo sapiens (Human)) | BDBM190714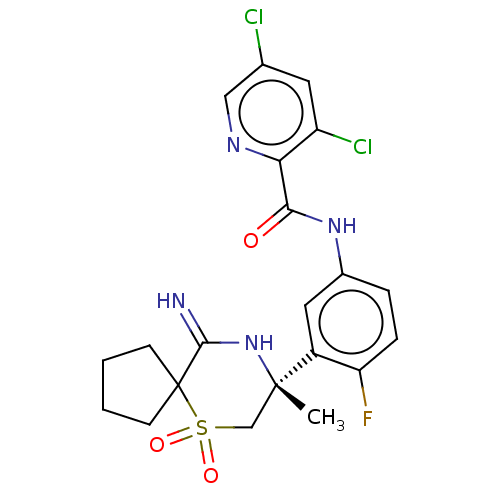 (US9181236, 18) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.800 | -52.8 | n/a | n/a | n/a | n/a | n/a | 5.0 | 30 |
Merck Sharp & Dohme Corp. US Patent | Assay Description Inhibitor IC50s at purified human autoBACE-2 are determined in a time-resolved endpoint proteolysis assay that measures hydrolysis of the QSY7-EISE... | US Patent US9181236 (2015) BindingDB Entry DOI: 10.7270/Q2NC5ZZB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (Homo sapiens (Human)) | BDBM50306451 ((6aS,13bS)-11-chloro-7-methyl-4-(thiophen-2-yl)-6,...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]SCh23390 from dopamine D1 receptor expressed in mouse LTK cells by scintillation counting | Bioorg Med Chem Lett 20: 832-5 (2010) Article DOI: 10.1016/j.bmcl.2009.12.094 BindingDB Entry DOI: 10.7270/Q2FX79JH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (Homo sapiens (Human)) | BDBM50306447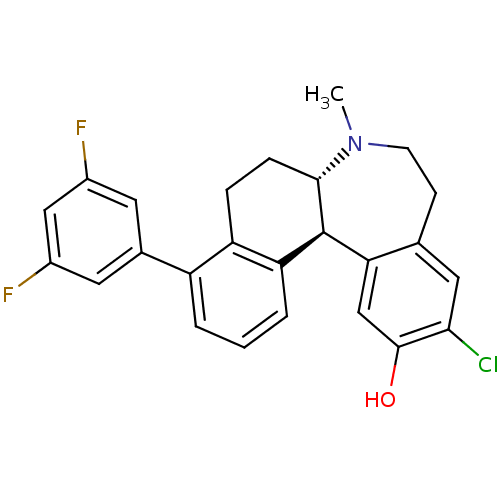 ((6aS,13bS)-11-chloro-4-(3,5-difluorophenyl)-7-meth...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]SCh23390 from dopamine D1 receptor expressed in mouse LTK cells by scintillation counting | Bioorg Med Chem Lett 20: 832-5 (2010) Article DOI: 10.1016/j.bmcl.2009.12.094 BindingDB Entry DOI: 10.7270/Q2FX79JH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (Homo sapiens (Human)) | BDBM50306313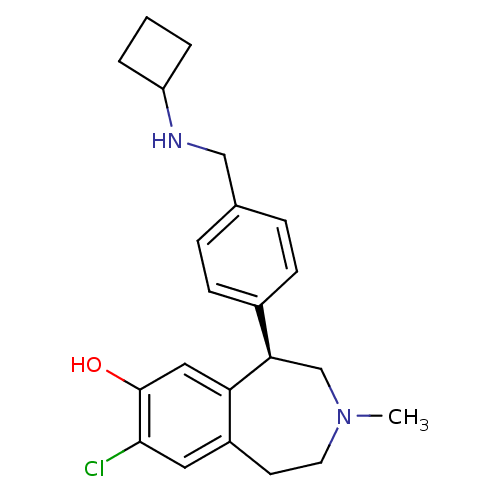 ((R)-8-chloro-5-(4-((cyclobutylamino)methyl)phenyl)...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity to dopamine D1 receptor | Bioorg Med Chem Lett 20: 836-40 (2010) Article DOI: 10.1016/j.bmcl.2009.12.100 BindingDB Entry DOI: 10.7270/Q23N23HT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 2 (Homo sapiens (Human)) | BDBM190773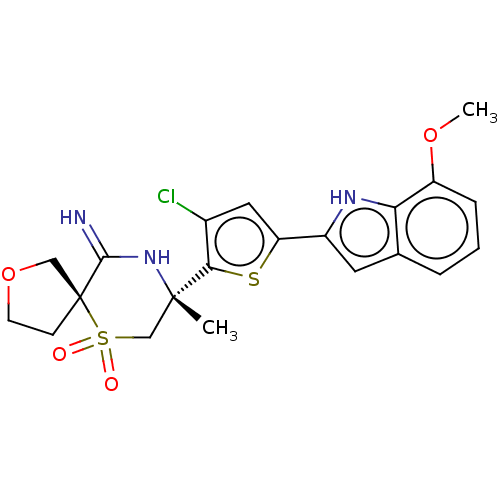 (US9181236, 62g) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.900 | -52.5 | n/a | n/a | n/a | n/a | n/a | 5.0 | 30 |
Merck Sharp & Dohme Corp. US Patent | Assay Description Inhibitor IC50s at purified human autoBACE-2 are determined in a time-resolved endpoint proteolysis assay that measures hydrolysis of the QSY7-EISE... | US Patent US9181236 (2015) BindingDB Entry DOI: 10.7270/Q2NC5ZZB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 2 (Homo sapiens (Human)) | BDBM190699 (US9181236, 3) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.900 | -52.5 | n/a | n/a | n/a | n/a | n/a | 5.0 | 30 |
Merck Sharp & Dohme Corp. US Patent | Assay Description Inhibitor IC50s at purified human autoBACE-2 are determined in a time-resolved endpoint proteolysis assay that measures hydrolysis of the QSY7-EISE... | US Patent US9181236 (2015) BindingDB Entry DOI: 10.7270/Q2NC5ZZB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM50190203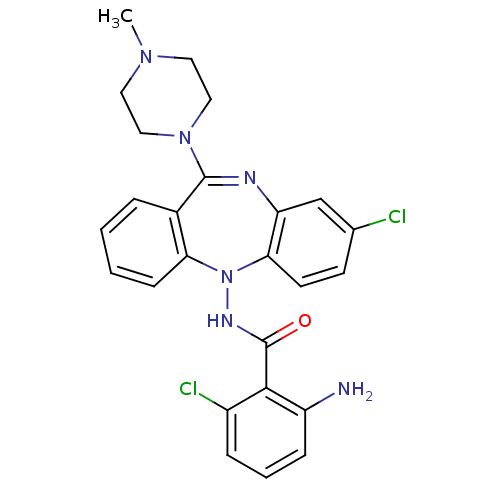 (2-amino-6-chloro-N-[8-chloro-11-(4-methyl-piperazi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Binding affinity at 5HT2A receptor | Bioorg Med Chem Lett 16: 4543-7 (2006) Article DOI: 10.1016/j.bmcl.2006.06.022 BindingDB Entry DOI: 10.7270/Q2GT5MSW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanin-concentrating hormone receptor 1 (Homo sapiens (Human)) | BDBM50186790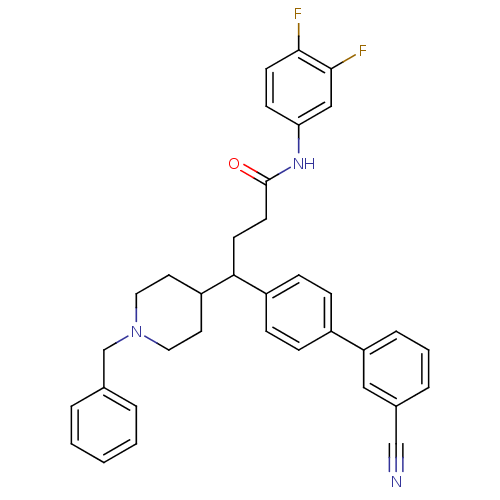 (4-(1-benzyl-piperidin-4-yl)-4-(3'-cyano-biphenyl-4...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering Plough Research Institute Curated by ChEMBL | Assay Description Binding affinity at human MCHR1 assessed as inhibition of MCH-mediated calcium ion influx by FLIPR assay | Bioorg Med Chem Lett 16: 3668-73 (2006) Article DOI: 10.1016/j.bmcl.2006.04.061 BindingDB Entry DOI: 10.7270/Q2251HT9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM50190193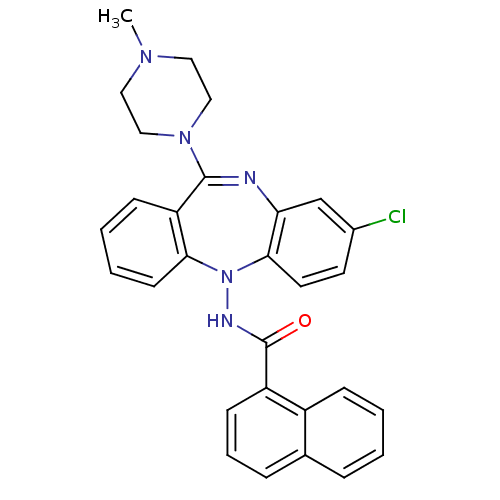 (CHEMBL213834 | naphthalene-1-carboxylic acid [8-ch...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Binding affinity at 5HT2A receptor | Bioorg Med Chem Lett 16: 4543-7 (2006) Article DOI: 10.1016/j.bmcl.2006.06.022 BindingDB Entry DOI: 10.7270/Q2GT5MSW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 2 (Homo sapiens (Human)) | BDBM190796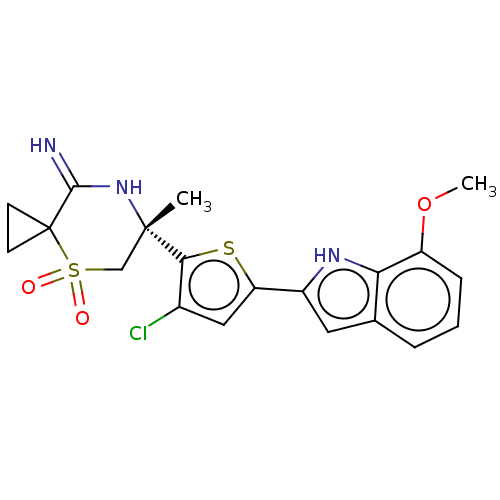 (US9181236, 64) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 1 | -52.2 | n/a | n/a | n/a | n/a | n/a | 5.0 | 30 |
Merck Sharp & Dohme Corp. US Patent | Assay Description Inhibitor IC50s at purified human autoBACE-2 are determined in a time-resolved endpoint proteolysis assay that measures hydrolysis of the QSY7-EISE... | US Patent US9181236 (2015) BindingDB Entry DOI: 10.7270/Q2NC5ZZB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (Homo sapiens (Human)) | BDBM50306441 (11-chloro-12-hydroxy-7-methyl-6,6a,7,8,9,13b-hexah...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]SCh23390 from dopamine D1 receptor expressed in mouse LTK cells by scintillation counting | Bioorg Med Chem Lett 20: 832-5 (2010) Article DOI: 10.1016/j.bmcl.2009.12.094 BindingDB Entry DOI: 10.7270/Q2FX79JH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 2549 total ) | Next | Last >> |