

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Growth hormone secretagogue receptor type 1 (Homo sapiens (Human)) | BDBM50051783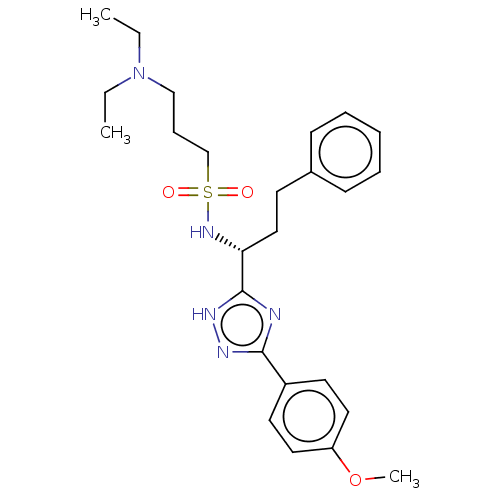 (CHEMBL3358450) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Binding affinity to human ghrelin receptor by receptor binding assay | J Med Chem 57: 8671-91 (2014) Article DOI: 10.1021/jm5003183 BindingDB Entry DOI: 10.7270/Q2542Q74 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM50439646 (CHEMBL2419600 | US8993586, 110) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human ACC2 using acetyl-CoA as substrate assessed as [14C]malonyl-CoA synthesis preincubated for 10 mins prior to substrate addition me... | J Med Chem 56: 7110-9 (2013) Article DOI: 10.1021/jm401033t BindingDB Entry DOI: 10.7270/Q2JW8G9D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 1 (Homo sapiens (Human)) | BDBM50439646 (CHEMBL2419600 | US8993586, 110) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human ACC1 using acetyl-CoA as substrate assessed as [14C]malonyl-CoA synthesis preincubated for 10 mins prior to substrate addition me... | J Med Chem 56: 7110-9 (2013) Article DOI: 10.1021/jm401033t BindingDB Entry DOI: 10.7270/Q2JW8G9D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM50439642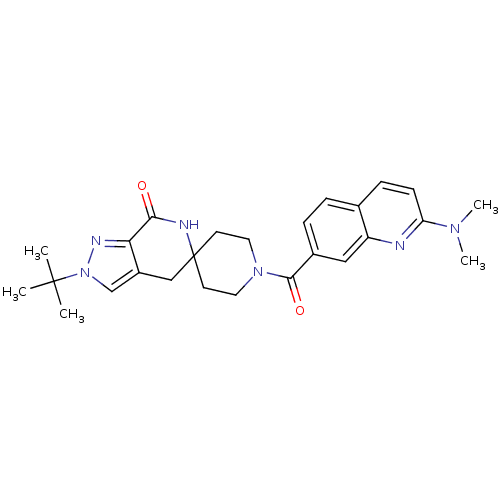 (CHEMBL2419589 | US8993586, 105) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human ACC2 using acetyl-CoA as substrate assessed as [14C]malonyl-CoA synthesis preincubated for 10 mins prior to substrate addition me... | J Med Chem 56: 7110-9 (2013) Article DOI: 10.1021/jm401033t BindingDB Entry DOI: 10.7270/Q2JW8G9D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM50439644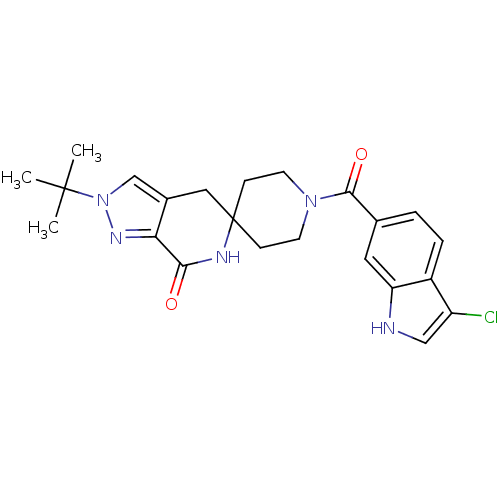 (CHEMBL2419593 | US8993586, 86) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human ACC2 using acetyl-CoA as substrate assessed as [14C]malonyl-CoA synthesis preincubated for 10 mins prior to substrate addition me... | J Med Chem 56: 7110-9 (2013) Article DOI: 10.1021/jm401033t BindingDB Entry DOI: 10.7270/Q2JW8G9D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM50439634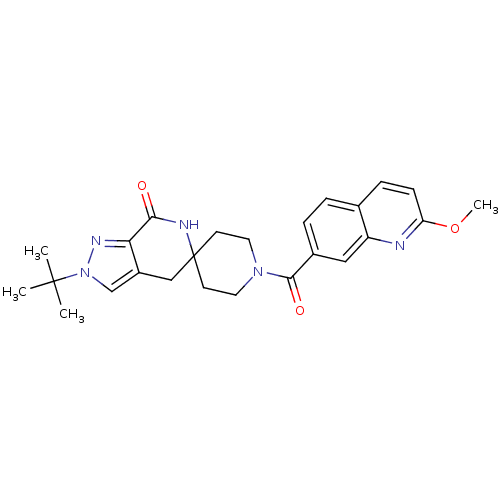 (CHEMBL2419596 | US8993586, 71) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human ACC2 using acetyl-CoA as substrate assessed as [14C]malonyl-CoA synthesis preincubated for 10 mins prior to substrate addition me... | J Med Chem 56: 7110-9 (2013) Article DOI: 10.1021/jm401033t BindingDB Entry DOI: 10.7270/Q2JW8G9D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Growth hormone secretagogue receptor type 1 (Homo sapiens (Human)) | BDBM50051718 (CHEMBL3358452) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Antagonist activity against human ghrelin receptor by FLIPR assay | J Med Chem 57: 8671-91 (2014) Article DOI: 10.1021/jm5003183 BindingDB Entry DOI: 10.7270/Q2542Q74 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM50439643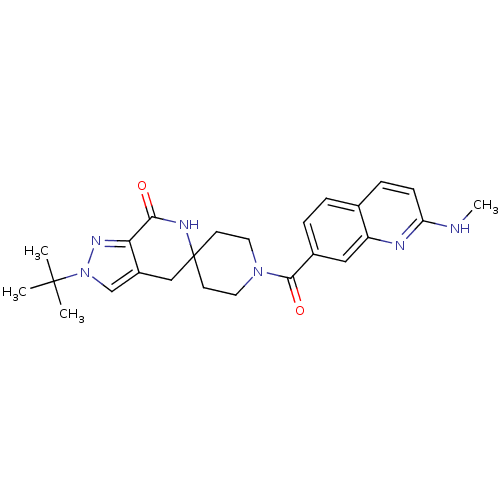 (CHEMBL2419598 | US8993586, 76) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human ACC2 using acetyl-CoA as substrate assessed as [14C]malonyl-CoA synthesis preincubated for 10 mins prior to substrate addition me... | J Med Chem 56: 7110-9 (2013) Article DOI: 10.1021/jm401033t BindingDB Entry DOI: 10.7270/Q2JW8G9D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM50439641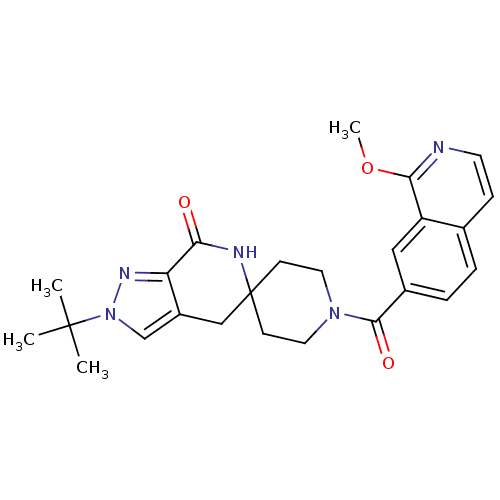 (CHEMBL2419597 | US8993586, 55) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human ACC2 using acetyl-CoA as substrate assessed as [14C]malonyl-CoA synthesis preincubated for 10 mins prior to substrate addition me... | J Med Chem 56: 7110-9 (2013) Article DOI: 10.1021/jm401033t BindingDB Entry DOI: 10.7270/Q2JW8G9D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM50439645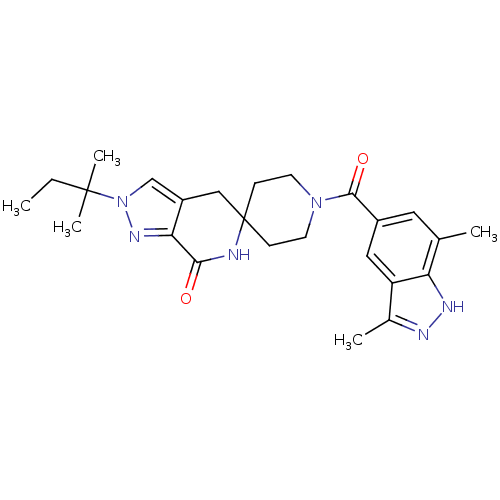 (CHEMBL2419607) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human ACC2 using acetyl-CoA as substrate assessed as [14C]malonyl-CoA synthesis preincubated for 10 mins prior to substrate addition me... | J Med Chem 56: 7110-9 (2013) Article DOI: 10.1021/jm401033t BindingDB Entry DOI: 10.7270/Q2JW8G9D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 1 (Homo sapiens (Human)) | BDBM50439642 (CHEMBL2419589 | US8993586, 105) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human ACC1 using acetyl-CoA as substrate assessed as [14C]malonyl-CoA synthesis preincubated for 10 mins prior to substrate addition me... | J Med Chem 56: 7110-9 (2013) Article DOI: 10.1021/jm401033t BindingDB Entry DOI: 10.7270/Q2JW8G9D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM50439635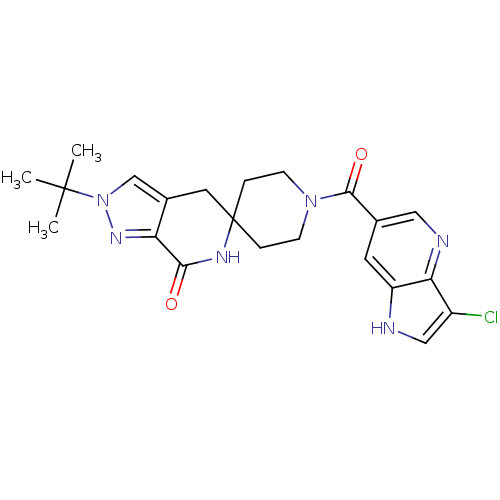 (CHEMBL2419594 | US8993586, 88) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human ACC2 using acetyl-CoA as substrate assessed as [14C]malonyl-CoA synthesis preincubated for 10 mins prior to substrate addition me... | J Med Chem 56: 7110-9 (2013) Article DOI: 10.1021/jm401033t BindingDB Entry DOI: 10.7270/Q2JW8G9D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 1 (Homo sapiens (Human)) | BDBM50439643 (CHEMBL2419598 | US8993586, 76) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human ACC1 using acetyl-CoA as substrate assessed as [14C]malonyl-CoA synthesis preincubated for 10 mins prior to substrate addition me... | J Med Chem 56: 7110-9 (2013) Article DOI: 10.1021/jm401033t BindingDB Entry DOI: 10.7270/Q2JW8G9D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM50439638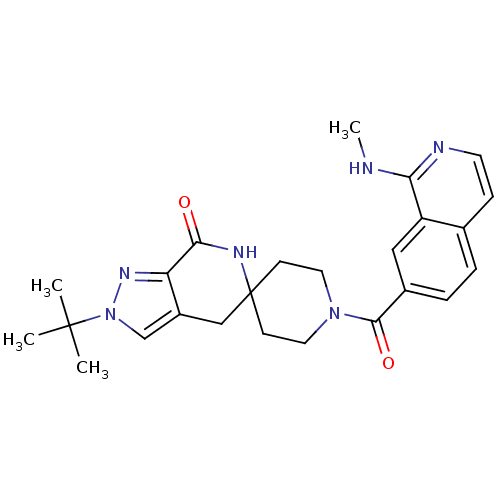 (CHEMBL2419599 | US8993586, 82) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human ACC2 using acetyl-CoA as substrate assessed as [14C]malonyl-CoA synthesis preincubated for 10 mins prior to substrate addition me... | J Med Chem 56: 7110-9 (2013) Article DOI: 10.1021/jm401033t BindingDB Entry DOI: 10.7270/Q2JW8G9D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM50439633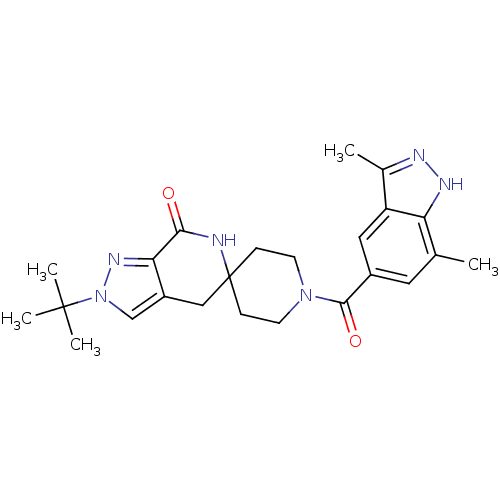 (CHEMBL2419604) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human ACC2 using acetyl-CoA as substrate assessed as [14C]malonyl-CoA synthesis preincubated for 10 mins prior to substrate addition me... | J Med Chem 56: 7110-9 (2013) Article DOI: 10.1021/jm401033t BindingDB Entry DOI: 10.7270/Q2JW8G9D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Growth hormone secretagogue receptor type 1 (Homo sapiens (Human)) | BDBM50051748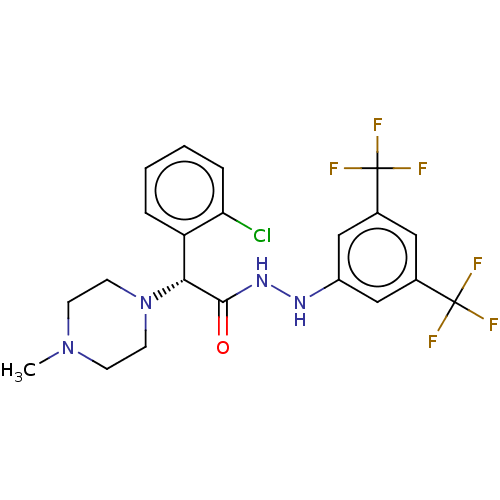 (CHEMBL3358451) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Antagonist activity against human ghrelin receptor by FLIPR assay | J Med Chem 57: 8671-91 (2014) Article DOI: 10.1021/jm5003183 BindingDB Entry DOI: 10.7270/Q2542Q74 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM50365293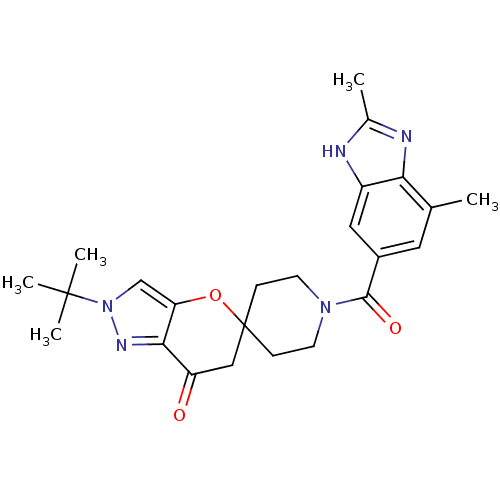 (CHEMBL1958374) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human Acetyl-CoA carboxylase 2 expressed in CHO cells after 1 hr by fluorescence assay | J Med Chem 55: 935-42 (2012) Article DOI: 10.1021/jm201503u BindingDB Entry DOI: 10.7270/Q2CZ37MS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM50439639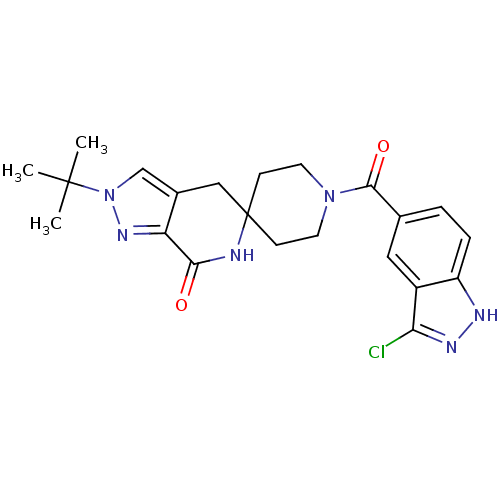 (CHEMBL2419591 | US8993586, 64) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human ACC2 using acetyl-CoA as substrate assessed as [14C]malonyl-CoA synthesis preincubated for 10 mins prior to substrate addition me... | J Med Chem 56: 7110-9 (2013) Article DOI: 10.1021/jm401033t BindingDB Entry DOI: 10.7270/Q2JW8G9D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM50439636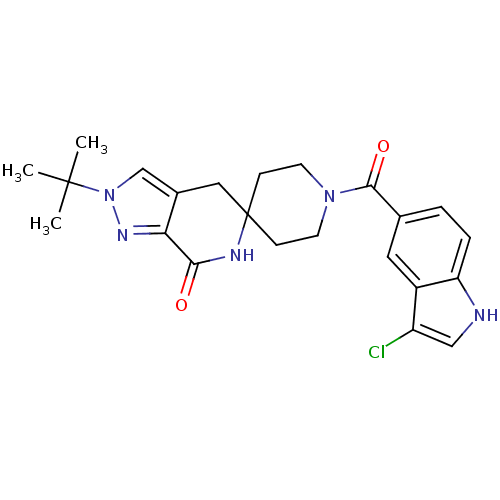 (CHEMBL2419592 | US8993586, 85) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human ACC2 using acetyl-CoA as substrate assessed as [14C]malonyl-CoA synthesis preincubated for 10 mins prior to substrate addition me... | J Med Chem 56: 7110-9 (2013) Article DOI: 10.1021/jm401033t BindingDB Entry DOI: 10.7270/Q2JW8G9D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM50365279 (CHEMBL1958360) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human Acetyl-CoA carboxylase 2 expressed in CHO cells after 1 hr by fluorescence assay | J Med Chem 55: 935-42 (2012) Article DOI: 10.1021/jm201503u BindingDB Entry DOI: 10.7270/Q2CZ37MS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 1 (Rattus norvegicus (Rat)) | BDBM50365279 (CHEMBL1958360) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of rat liver Acetyl-CoA carboxylase 1 using acetyl-CoA as substrate preincubated for 10 mins prior substrate addition measured after 20 mi... | J Med Chem 55: 935-42 (2012) Article DOI: 10.1021/jm201503u BindingDB Entry DOI: 10.7270/Q2CZ37MS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 1 (Homo sapiens (Human)) | BDBM50439644 (CHEMBL2419593 | US8993586, 86) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human ACC1 using acetyl-CoA as substrate assessed as [14C]malonyl-CoA synthesis preincubated for 10 mins prior to substrate addition me... | J Med Chem 56: 7110-9 (2013) Article DOI: 10.1021/jm401033t BindingDB Entry DOI: 10.7270/Q2JW8G9D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM50365292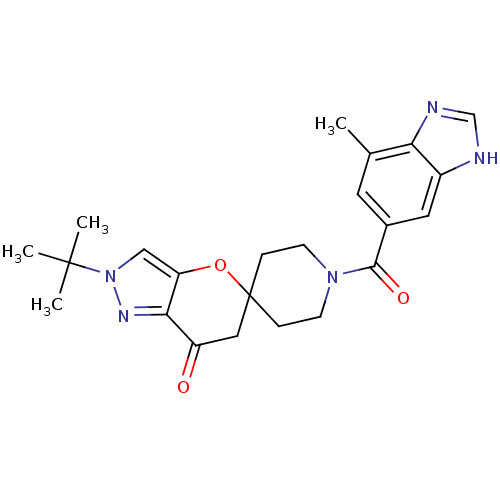 (CHEMBL1958373) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human Acetyl-CoA carboxylase 2 expressed in CHO cells after 1 hr by fluorescence assay | J Med Chem 55: 935-42 (2012) Article DOI: 10.1021/jm201503u BindingDB Entry DOI: 10.7270/Q2CZ37MS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 1 (Rattus norvegicus (Rat)) | BDBM50365287 (CHEMBL1958368) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of rat liver Acetyl-CoA carboxylase 1 using acetyl-CoA as substrate preincubated for 10 mins prior substrate addition measured after 20 mi... | J Med Chem 55: 935-42 (2012) Article DOI: 10.1021/jm201503u BindingDB Entry DOI: 10.7270/Q2CZ37MS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM50365277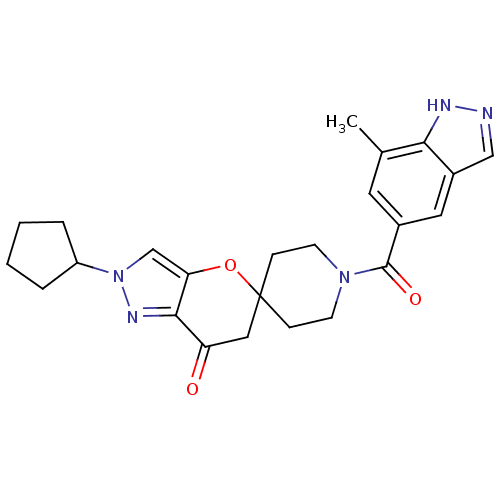 (CHEMBL1958359) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human Acetyl-CoA carboxylase 2 expressed in CHO cells after 1 hr by fluorescence assay | J Med Chem 55: 935-42 (2012) Article DOI: 10.1021/jm201503u BindingDB Entry DOI: 10.7270/Q2CZ37MS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 1 (Rattus norvegicus (Rat)) | BDBM50365292 (CHEMBL1958373) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of rat liver Acetyl-CoA carboxylase 1 using acetyl-CoA as substrate preincubated for 10 mins prior substrate addition measured after 20 mi... | J Med Chem 55: 935-42 (2012) Article DOI: 10.1021/jm201503u BindingDB Entry DOI: 10.7270/Q2CZ37MS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 1 (Rattus norvegicus (Rat)) | BDBM50365293 (CHEMBL1958374) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of rat liver Acetyl-CoA carboxylase 1 using acetyl-CoA as substrate preincubated for 10 mins prior substrate addition measured after 20 mi... | J Med Chem 55: 935-42 (2012) Article DOI: 10.1021/jm201503u BindingDB Entry DOI: 10.7270/Q2CZ37MS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM50439637 (CHEMBL2419601) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human ACC2 using acetyl-CoA as substrate assessed as [14C]malonyl-CoA synthesis preincubated for 10 mins prior to substrate addition me... | J Med Chem 56: 7110-9 (2013) Article DOI: 10.1021/jm401033t BindingDB Entry DOI: 10.7270/Q2JW8G9D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 1 (Homo sapiens (Human)) | BDBM50439634 (CHEMBL2419596 | US8993586, 71) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human ACC1 using acetyl-CoA as substrate assessed as [14C]malonyl-CoA synthesis preincubated for 10 mins prior to substrate addition me... | J Med Chem 56: 7110-9 (2013) Article DOI: 10.1021/jm401033t BindingDB Entry DOI: 10.7270/Q2JW8G9D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM50439626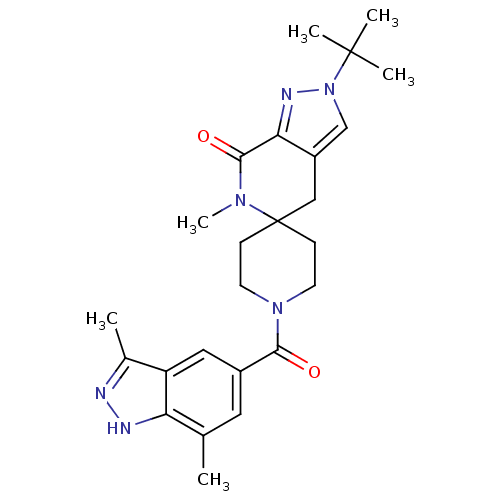 (CHEMBL2419610) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human ACC2 using acetyl-CoA as substrate assessed as [14C]malonyl-CoA synthesis preincubated for 10 mins prior to substrate addition me... | J Med Chem 56: 7110-9 (2013) Article DOI: 10.1021/jm401033t BindingDB Entry DOI: 10.7270/Q2JW8G9D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 1 (Homo sapiens (Human)) | BDBM50439641 (CHEMBL2419597 | US8993586, 55) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human ACC1 using acetyl-CoA as substrate assessed as [14C]malonyl-CoA synthesis preincubated for 10 mins prior to substrate addition me... | J Med Chem 56: 7110-9 (2013) Article DOI: 10.1021/jm401033t BindingDB Entry DOI: 10.7270/Q2JW8G9D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 1 (Homo sapiens (Human)) | BDBM50439635 (CHEMBL2419594 | US8993586, 88) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human ACC1 using acetyl-CoA as substrate assessed as [14C]malonyl-CoA synthesis preincubated for 10 mins prior to substrate addition me... | J Med Chem 56: 7110-9 (2013) Article DOI: 10.1021/jm401033t BindingDB Entry DOI: 10.7270/Q2JW8G9D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM50365295 (CHEMBL1955896) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human Acetyl-CoA carboxylase 2 expressed in CHO cells after 1 hr by fluorescence assay | J Med Chem 55: 935-42 (2012) Article DOI: 10.1021/jm201503u BindingDB Entry DOI: 10.7270/Q2CZ37MS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 1 (Rattus norvegicus (Rat)) | BDBM50365284 (CHEMBL1958365) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 11.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of rat liver Acetyl-CoA carboxylase 1 using acetyl-CoA as substrate preincubated for 10 mins prior substrate addition measured after 20 mi... | J Med Chem 55: 935-42 (2012) Article DOI: 10.1021/jm201503u BindingDB Entry DOI: 10.7270/Q2CZ37MS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 1 (Rattus norvegicus (Rat)) | BDBM50365281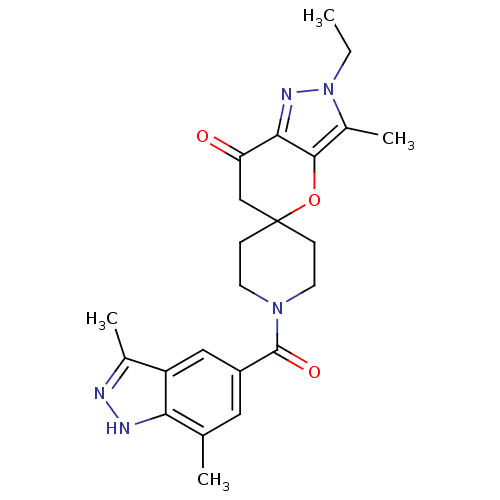 (CHEMBL1958362) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of rat liver Acetyl-CoA carboxylase 1 using acetyl-CoA as substrate preincubated for 10 mins prior substrate addition measured after 20 mi... | J Med Chem 55: 935-42 (2012) Article DOI: 10.1021/jm201503u BindingDB Entry DOI: 10.7270/Q2CZ37MS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 1 (Rattus norvegicus (Rat)) | BDBM50365290 (CHEMBL1958371) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of rat liver Acetyl-CoA carboxylase 1 using acetyl-CoA as substrate preincubated for 10 mins prior substrate addition measured after 20 mi... | J Med Chem 55: 935-42 (2012) Article DOI: 10.1021/jm201503u BindingDB Entry DOI: 10.7270/Q2CZ37MS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM50365281 (CHEMBL1958362) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human Acetyl-CoA carboxylase 2 expressed in CHO cells after 1 hr by fluorescence assay | J Med Chem 55: 935-42 (2012) Article DOI: 10.1021/jm201503u BindingDB Entry DOI: 10.7270/Q2CZ37MS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 1 (Rattus norvegicus (Rat)) | BDBM50365294 (CHEMBL1955895) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of rat liver Acetyl-CoA carboxylase 1 using acetyl-CoA as substrate preincubated for 10 mins prior substrate addition measured after 20 mi... | J Med Chem 55: 935-42 (2012) Article DOI: 10.1021/jm201503u BindingDB Entry DOI: 10.7270/Q2CZ37MS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 1 (Rattus norvegicus (Rat)) | BDBM50365285 (CHEMBL1958366) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of rat liver Acetyl-CoA carboxylase 1 using acetyl-CoA as substrate preincubated for 10 mins prior substrate addition measured after 20 mi... | J Med Chem 55: 935-42 (2012) Article DOI: 10.1021/jm201503u BindingDB Entry DOI: 10.7270/Q2CZ37MS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 1 (Homo sapiens (Human)) | BDBM50439636 (CHEMBL2419592 | US8993586, 85) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human ACC1 using acetyl-CoA as substrate assessed as [14C]malonyl-CoA synthesis preincubated for 10 mins prior to substrate addition me... | J Med Chem 56: 7110-9 (2013) Article DOI: 10.1021/jm401033t BindingDB Entry DOI: 10.7270/Q2JW8G9D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 1 (Rattus norvegicus (Rat)) | BDBM50365277 (CHEMBL1958359) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of rat liver Acetyl-CoA carboxylase 1 using acetyl-CoA as substrate preincubated for 10 mins prior substrate addition measured after 20 mi... | J Med Chem 55: 935-42 (2012) Article DOI: 10.1021/jm201503u BindingDB Entry DOI: 10.7270/Q2CZ37MS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM50365290 (CHEMBL1958371) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human Acetyl-CoA carboxylase 2 expressed in CHO cells after 1 hr by fluorescence assay | J Med Chem 55: 935-42 (2012) Article DOI: 10.1021/jm201503u BindingDB Entry DOI: 10.7270/Q2CZ37MS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM50365276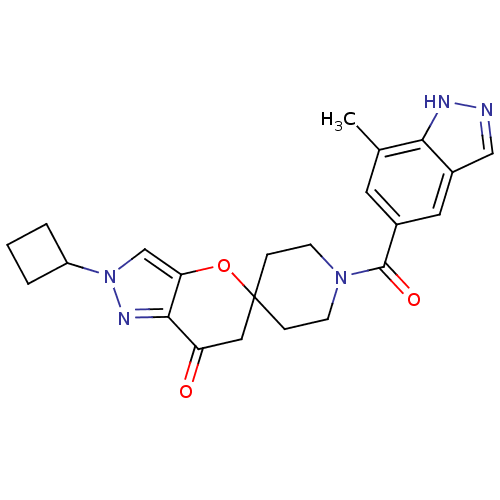 (CHEMBL1958358) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human Acetyl-CoA carboxylase 2 expressed in CHO cells after 1 hr by fluorescence assay | J Med Chem 55: 935-42 (2012) Article DOI: 10.1021/jm201503u BindingDB Entry DOI: 10.7270/Q2CZ37MS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 1 (Rattus norvegicus (Rat)) | BDBM50365276 (CHEMBL1958358) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of rat liver Acetyl-CoA carboxylase 1 using acetyl-CoA as substrate preincubated for 10 mins prior substrate addition measured after 20 mi... | J Med Chem 55: 935-42 (2012) Article DOI: 10.1021/jm201503u BindingDB Entry DOI: 10.7270/Q2CZ37MS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 1 (Rattus norvegicus (Rat)) | BDBM50127711 (CHEMBL3629719) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Patents | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Curated by ChEMBL | Assay Description Inhibition of rat liver ACC1 preincubated for 10 mins followed by acetyl-CoA/KHCO3/[14C]-NaHCO3/ATP addition measured after 20 mins by liquid scintil... | Bioorg Med Chem Lett 25: 5352-6 (2015) Article DOI: 10.1016/j.bmcl.2015.09.035 BindingDB Entry DOI: 10.7270/Q2W37Z4R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM50439640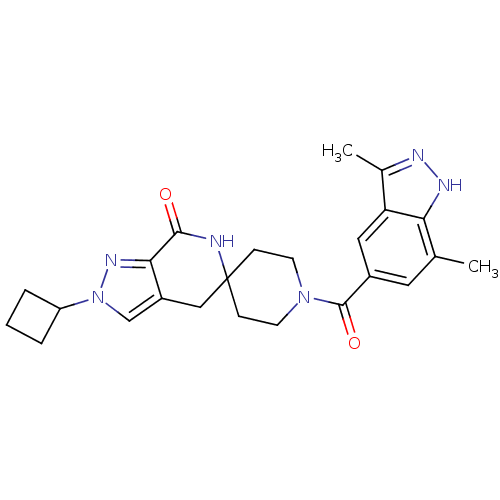 (CHEMBL2419606 | US8993586, 57) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human ACC2 using acetyl-CoA as substrate assessed as [14C]malonyl-CoA synthesis preincubated for 10 mins prior to substrate addition me... | J Med Chem 56: 7110-9 (2013) Article DOI: 10.1021/jm401033t BindingDB Entry DOI: 10.7270/Q2JW8G9D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 1 (Rattus norvegicus (Rat)) | BDBM50365295 (CHEMBL1955896) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of rat liver Acetyl-CoA carboxylase 1 using acetyl-CoA as substrate preincubated for 10 mins prior substrate addition measured after 20 mi... | J Med Chem 55: 935-42 (2012) Article DOI: 10.1021/jm201503u BindingDB Entry DOI: 10.7270/Q2CZ37MS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 1 (Homo sapiens (Human)) | BDBM50439639 (CHEMBL2419591 | US8993586, 64) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human ACC1 using acetyl-CoA as substrate assessed as [14C]malonyl-CoA synthesis preincubated for 10 mins prior to substrate addition me... | J Med Chem 56: 7110-9 (2013) Article DOI: 10.1021/jm401033t BindingDB Entry DOI: 10.7270/Q2JW8G9D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM50365285 (CHEMBL1958366) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human Acetyl-CoA carboxylase 2 expressed in CHO cells after 1 hr by fluorescence assay | J Med Chem 55: 935-42 (2012) Article DOI: 10.1021/jm201503u BindingDB Entry DOI: 10.7270/Q2CZ37MS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 1 (Homo sapiens (Human)) | BDBM50439638 (CHEMBL2419599 | US8993586, 82) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human ACC1 using acetyl-CoA as substrate assessed as [14C]malonyl-CoA synthesis preincubated for 10 mins prior to substrate addition me... | J Med Chem 56: 7110-9 (2013) Article DOI: 10.1021/jm401033t BindingDB Entry DOI: 10.7270/Q2JW8G9D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 141 total ) | Next | Last >> |