 Found 383 hits with Last Name = 'skorey' and Initial = 'k'
Found 383 hits with Last Name = 'skorey' and Initial = 'k' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50373455

(CHEMBL261107)Show InChI InChI=1S/C11H8BrF2O3P/c12-10-6-8-4-2-1-3-7(8)5-9(10)11(13,14)18(15,16)17/h1-6H,(H2,15,16,17) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.79E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B by fluorescein diphosphate assay |
Bioorg Med Chem Lett 18: 3200-5 (2008)
Article DOI: 10.1016/j.bmcl.2008.04.064
BindingDB Entry DOI: 10.7270/Q24M95DX |
More data for this
Ligand-Target Pair | |
Stearoyl-CoA desaturase
(Homo sapiens (Human)) | BDBM50362592
(CHEMBL1938870)Show SMILES OC(=O)Cn1nnc(n1)-c1cc(no1)N1CCC(CC1)Oc1cc(F)ccc1Br Show InChI InChI=1S/C17H16BrFN6O4/c18-12-2-1-10(19)7-13(12)28-11-3-5-24(6-4-11)15-8-14(29-22-15)17-20-23-25(21-17)9-16(26)27/h1-2,7-8,11H,3-6,9H2,(H,26,27) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibition of human SCD-1 |
J Med Chem 54: 5082-96 (2011)
Article DOI: 10.1021/jm200319u
BindingDB Entry DOI: 10.7270/Q2348MGP |
More data for this
Ligand-Target Pair | |
Acyl-CoA desaturase 1
(Rattus norvegicus (Rat)) | BDBM50305768
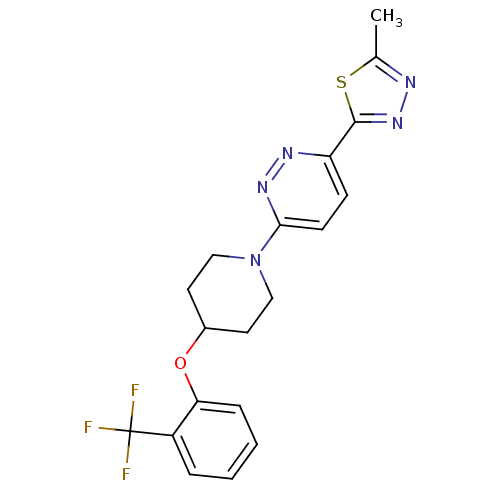
(2-methyl-5-(6-(4-(2-(trifluoromethyl)phenoxy)piper...)Show SMILES Cc1nnc(s1)-c1ccc(nn1)N1CCC(CC1)Oc1ccccc1C(F)(F)F Show InChI InChI=1S/C19H18F3N5OS/c1-12-23-26-18(29-12)15-6-7-17(25-24-15)27-10-8-13(9-11-27)28-16-5-3-2-4-14(16)19(20,21)22/h2-7,13H,8-11H2,1H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibition of rat SCD by rat microsomal assay |
Bioorg Med Chem Lett 21: 6505-9 (2011)
Article DOI: 10.1016/j.bmcl.2011.08.073
BindingDB Entry DOI: 10.7270/Q2VQ332W |
More data for this
Ligand-Target Pair | |
Acyl-CoA desaturase 1
(Rattus norvegicus (Rat)) | BDBM50305768

(2-methyl-5-(6-(4-(2-(trifluoromethyl)phenoxy)piper...)Show SMILES Cc1nnc(s1)-c1ccc(nn1)N1CCC(CC1)Oc1ccccc1C(F)(F)F Show InChI InChI=1S/C19H18F3N5OS/c1-12-23-26-18(29-12)15-6-7-17(25-24-15)27-10-8-13(9-11-27)28-16-5-3-2-4-14(16)19(20,21)22/h2-7,13H,8-11H2,1H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibition of SCD-1 activity in Sprague-Dawley rat microsome assessed as reduction in [I-14C] stearoyl CoA desaturation by scintillation counting |
J Med Chem 54: 5082-96 (2011)
Article DOI: 10.1021/jm200319u
BindingDB Entry DOI: 10.7270/Q2348MGP |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(RAT) | BDBM50289087
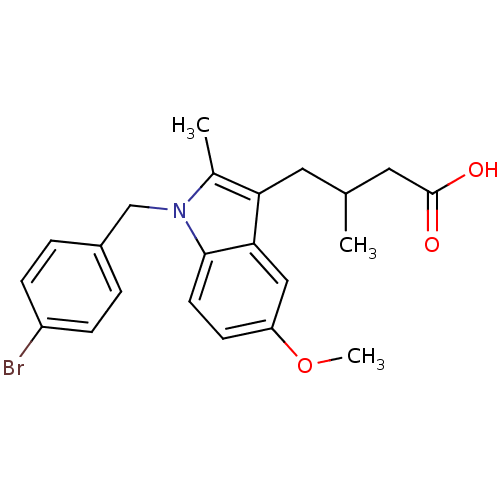
(4-[1-(4-Bromo-benzyl)-5-methoxy-2-methyl-1H-indol-...)Show SMILES COc1ccc2n(Cc3ccc(Br)cc3)c(C)c(CC(C)CC(O)=O)c2c1 Show InChI InChI=1S/C22H24BrNO3/c1-14(11-22(25)26)10-19-15(2)24(13-16-4-6-17(23)7-5-16)21-9-8-18(27-3)12-20(19)21/h4-9,12,14H,10-11,13H2,1-3H3,(H,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of Prostaglandin G/H synthase 2 |
Bioorg Med Chem Lett 6: 725-730 (1996)
Article DOI: 10.1016/0960-894X(96)00100-X
BindingDB Entry DOI: 10.7270/Q25B02GQ |
More data for this
Ligand-Target Pair | |
Acyl-CoA desaturase 1
(Rattus norvegicus (Rat)) | BDBM50362592

(CHEMBL1938870)Show SMILES OC(=O)Cn1nnc(n1)-c1cc(no1)N1CCC(CC1)Oc1cc(F)ccc1Br Show InChI InChI=1S/C17H16BrFN6O4/c18-12-2-1-10(19)7-13(12)28-11-3-5-24(6-4-11)15-8-14(29-22-15)17-20-23-25(21-17)9-16(26)27/h1-2,7-8,11H,3-6,9H2,(H,26,27) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibition of rat SCD-1 |
J Med Chem 54: 5082-96 (2011)
Article DOI: 10.1021/jm200319u
BindingDB Entry DOI: 10.7270/Q2348MGP |
More data for this
Ligand-Target Pair | |
Acyl-CoA desaturase 1
(Mus musculus) | BDBM50362592

(CHEMBL1938870)Show SMILES OC(=O)Cn1nnc(n1)-c1cc(no1)N1CCC(CC1)Oc1cc(F)ccc1Br Show InChI InChI=1S/C17H16BrFN6O4/c18-12-2-1-10(19)7-13(12)28-11-3-5-24(6-4-11)15-8-14(29-22-15)17-20-23-25(21-17)9-16(26)27/h1-2,7-8,11H,3-6,9H2,(H,26,27) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibition of mouse SCD-1 |
J Med Chem 54: 5082-96 (2011)
Article DOI: 10.1021/jm200319u
BindingDB Entry DOI: 10.7270/Q2348MGP |
More data for this
Ligand-Target Pair | |
Acyl-CoA desaturase 1
(Rattus norvegicus (Rat)) | BDBM50362592

(CHEMBL1938870)Show SMILES OC(=O)Cn1nnc(n1)-c1cc(no1)N1CCC(CC1)Oc1cc(F)ccc1Br Show InChI InChI=1S/C17H16BrFN6O4/c18-12-2-1-10(19)7-13(12)28-11-3-5-24(6-4-11)15-8-14(29-22-15)17-20-23-25(21-17)9-16(26)27/h1-2,7-8,11H,3-6,9H2,(H,26,27) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibition of SCD-1 activity in Sprague-Dawley rat microsome assessed as reduction in [I-14C] stearoyl CoA desaturation by scintillation counting |
J Med Chem 54: 5082-96 (2011)
Article DOI: 10.1021/jm200319u
BindingDB Entry DOI: 10.7270/Q2348MGP |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 2
(Homo sapiens (Human)) | BDBM13599
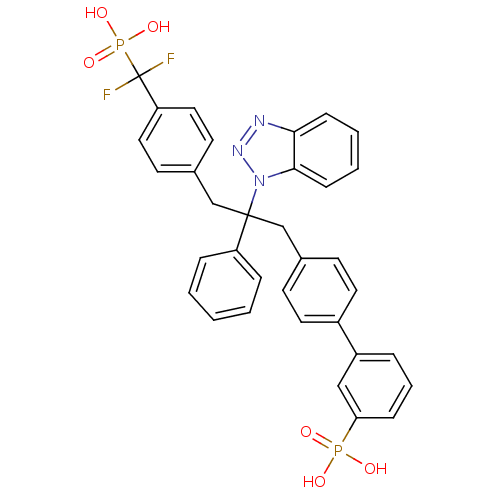
(3-{4-[2-(1H-1,2,3-benzotriazol-1-yl)-3-{4-[difluor...)Show SMILES OP(O)(=O)c1cccc(c1)-c1ccc(CC(Cc2ccc(cc2)C(F)(F)P(O)(O)=O)(c2ccccc2)n2nnc3ccccc23)cc1 Show InChI InChI=1S/C34H29F2N3O6P2/c35-34(36,47(43,44)45)29-19-15-25(16-20-29)23-33(28-8-2-1-3-9-28,39-32-12-5-4-11-31(32)37-38-39)22-24-13-17-26(18-14-24)27-7-6-10-30(21-27)46(40,41)42/h1-21H,22-23H2,(H2,40,41,42)(H2,43,44,45) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against T cell protein tyrosine phosphatase |
Bioorg Med Chem Lett 14: 1043-8 (2004)
Article DOI: 10.1016/j.bmcl.2003.11.076
BindingDB Entry DOI: 10.7270/Q24M9404 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(RAT) | BDBM50289082
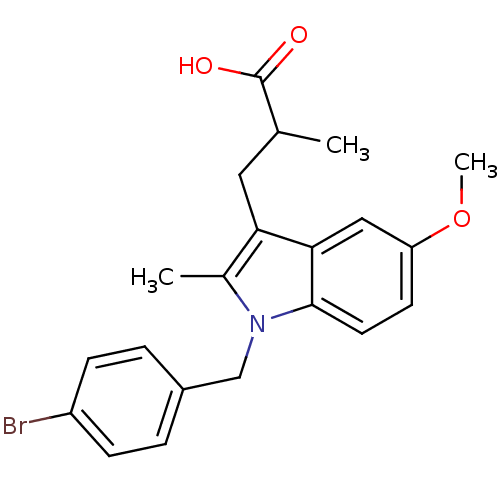
(3-[1-(4-Bromo-benzyl)-5-methoxy-2-methyl-1H-indol-...)Show SMILES COc1ccc2n(Cc3ccc(Br)cc3)c(C)c(CC(C)C(O)=O)c2c1 Show InChI InChI=1S/C21H22BrNO3/c1-13(21(24)25)10-18-14(2)23(12-15-4-6-16(22)7-5-15)20-9-8-17(26-3)11-19(18)20/h4-9,11,13H,10,12H2,1-3H3,(H,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of Prostaglandin G/H synthase 2 |
Bioorg Med Chem Lett 6: 725-730 (1996)
Article DOI: 10.1016/0960-894X(96)00100-X
BindingDB Entry DOI: 10.7270/Q25B02GQ |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(RAT) | BDBM50289084
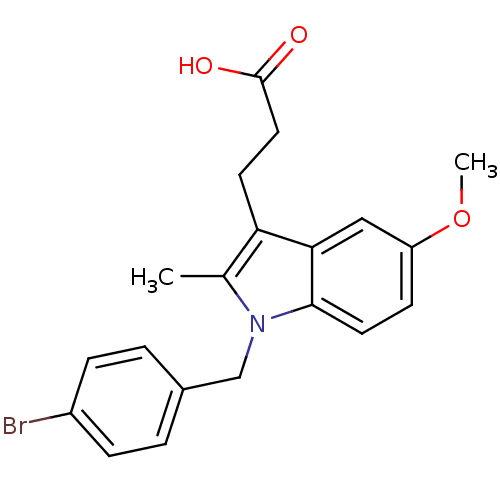
(3-[1-(4-Bromo-benzyl)-5-methoxy-2-methyl-1H-indol-...)Show SMILES COc1ccc2n(Cc3ccc(Br)cc3)c(C)c(CCC(O)=O)c2c1 Show InChI InChI=1S/C20H20BrNO3/c1-13-17(8-10-20(23)24)18-11-16(25-2)7-9-19(18)22(13)12-14-3-5-15(21)6-4-14/h3-7,9,11H,8,10,12H2,1-2H3,(H,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of Prostaglandin G/H synthase 2 |
Bioorg Med Chem Lett 6: 725-730 (1996)
Article DOI: 10.1016/0960-894X(96)00100-X
BindingDB Entry DOI: 10.7270/Q25B02GQ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1 [1-298]
(Homo sapiens (Human)) | BDBM13599

(3-{4-[2-(1H-1,2,3-benzotriazol-1-yl)-3-{4-[difluor...)Show SMILES OP(O)(=O)c1cccc(c1)-c1ccc(CC(Cc2ccc(cc2)C(F)(F)P(O)(O)=O)(c2ccccc2)n2nnc3ccccc23)cc1 Show InChI InChI=1S/C34H29F2N3O6P2/c35-34(36,47(43,44)45)29-19-15-25(16-20-29)23-33(28-8-2-1-3-9-28,39-32-12-5-4-11-31(32)37-38-39)22-24-13-17-26(18-14-24)27-7-6-10-30(21-27)46(40,41)42/h1-21H,22-23H2,(H2,40,41,42)(H2,43,44,45) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | 6.3 | 22 |
Merck Research Laboratories
| Assay Description
Hydrolysis of substrate FDP was monitored continuously on a Cytofluor microplate reader with excitation and emission wavelengths set at 440 and 515 ... |
Biochemistry 42: 11451-9 (2003)
Article DOI: 10.1021/bi035098j
BindingDB Entry DOI: 10.7270/Q2HX19XS |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein phosphatase non-receptor type 2
(Homo sapiens (Human)) | BDBM13599

(3-{4-[2-(1H-1,2,3-benzotriazol-1-yl)-3-{4-[difluor...)Show SMILES OP(O)(=O)c1cccc(c1)-c1ccc(CC(Cc2ccc(cc2)C(F)(F)P(O)(O)=O)(c2ccccc2)n2nnc3ccccc23)cc1 Show InChI InChI=1S/C34H29F2N3O6P2/c35-34(36,47(43,44)45)29-19-15-25(16-20-29)23-33(28-8-2-1-3-9-28,39-32-12-5-4-11-31(32)37-38-39)22-24-13-17-26(18-14-24)27-7-6-10-30(21-27)46(40,41)42/h1-21H,22-23H2,(H2,40,41,42)(H2,43,44,45) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | 6.3 | 22 |
Merck Research Laboratories
| Assay Description
Hydrolysis of substrate FDP was monitored continuously on a Cytofluor microplate reader with excitation and emission wavelengths set at 440 and 515 ... |
Biochemistry 42: 11451-9 (2003)
Article DOI: 10.1021/bi035098j
BindingDB Entry DOI: 10.7270/Q2HX19XS |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM13599

(3-{4-[2-(1H-1,2,3-benzotriazol-1-yl)-3-{4-[difluor...)Show SMILES OP(O)(=O)c1cccc(c1)-c1ccc(CC(Cc2ccc(cc2)C(F)(F)P(O)(O)=O)(c2ccccc2)n2nnc3ccccc23)cc1 Show InChI InChI=1S/C34H29F2N3O6P2/c35-34(36,47(43,44)45)29-19-15-25(16-20-29)23-33(28-8-2-1-3-9-28,39-32-12-5-4-11-31(32)37-38-39)22-24-13-17-26(18-14-24)27-7-6-10-30(21-27)46(40,41)42/h1-21H,22-23H2,(H2,40,41,42)(H2,43,44,45) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against protein tyrosine phosphatase 1B (PTP1B) was determined in fluorescein diphosphate (FDP) assay |
Bioorg Med Chem Lett 14: 1043-8 (2004)
Article DOI: 10.1016/j.bmcl.2003.11.076
BindingDB Entry DOI: 10.7270/Q24M9404 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein phosphatase non-receptor type 2
(Homo sapiens (Human)) | BDBM50142323
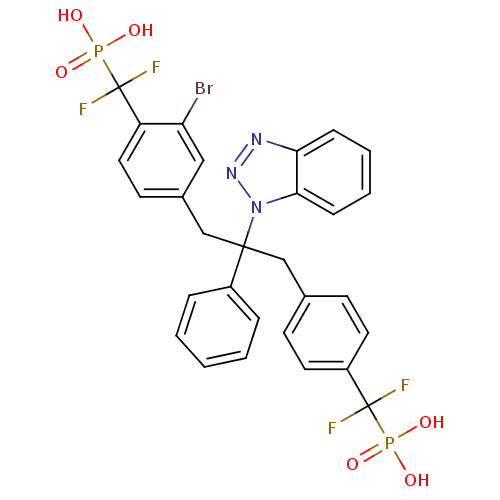
(CHEMBL267488 | [(4-{2-Benzotriazol-1-yl-3-[3-bromo...)Show SMILES OP(O)(=O)C(F)(F)c1ccc(CC(Cc2ccc(c(Br)c2)C(F)(F)P(O)(O)=O)(c2ccccc2)n2nnc3ccccc23)cc1 Show InChI InChI=1S/C29H24BrF4N3O6P2/c30-24-16-20(12-15-23(24)29(33,34)45(41,42)43)18-27(21-6-2-1-3-7-21,37-26-9-5-4-8-25(26)35-36-37)17-19-10-13-22(14-11-19)28(31,32)44(38,39)40/h1-16H,17-18H2,(H2,38,39,40)(H2,41,42,43) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against T cell protein tyrosine phosphatase |
Bioorg Med Chem Lett 14: 1043-8 (2004)
Article DOI: 10.1016/j.bmcl.2003.11.076
BindingDB Entry DOI: 10.7270/Q24M9404 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(RAT) | BDBM50289083

(3-[1-(4-Bromo-benzyl)-5-methoxy-2-methyl-1H-indol-...)Show SMILES COc1ccc2n(Cc3ccc(Br)cc3)c(C)c(C(C)CC(O)=O)c2c1 Show InChI InChI=1S/C21H22BrNO3/c1-13(10-20(24)25)21-14(2)23(12-15-4-6-16(22)7-5-15)19-9-8-17(26-3)11-18(19)21/h4-9,11,13H,10,12H2,1-3H3,(H,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of Prostaglandin G/H synthase 2 |
Bioorg Med Chem Lett 6: 725-730 (1996)
Article DOI: 10.1016/0960-894X(96)00100-X
BindingDB Entry DOI: 10.7270/Q25B02GQ |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(RAT) | BDBM50289088
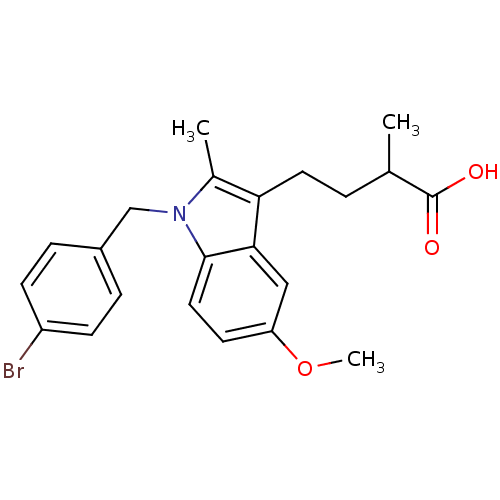
(4-[1-(4-Bromo-benzyl)-5-methoxy-2-methyl-1H-indol-...)Show SMILES COc1ccc2n(Cc3ccc(Br)cc3)c(C)c(CCC(C)C(O)=O)c2c1 Show InChI InChI=1S/C22H24BrNO3/c1-14(22(25)26)4-10-19-15(2)24(13-16-5-7-17(23)8-6-16)21-11-9-18(27-3)12-20(19)21/h5-9,11-12,14H,4,10,13H2,1-3H3,(H,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of Prostaglandin G/H synthase 2 |
Bioorg Med Chem Lett 6: 725-730 (1996)
Article DOI: 10.1016/0960-894X(96)00100-X
BindingDB Entry DOI: 10.7270/Q25B02GQ |
More data for this
Ligand-Target Pair | |
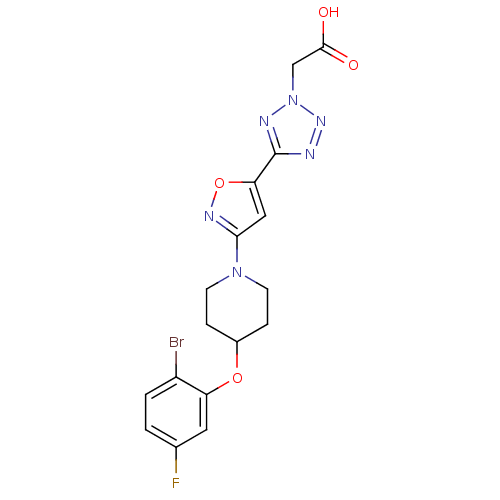
Stearoyl-CoA desaturase
(Homo sapiens (Human)) | BDBM50362592

(CHEMBL1938870)Show SMILES OC(=O)Cn1nnc(n1)-c1cc(no1)N1CCC(CC1)Oc1cc(F)ccc1Br Show InChI InChI=1S/C17H16BrFN6O4/c18-12-2-1-10(19)7-13(12)28-11-3-5-24(6-4-11)15-8-14(29-22-15)17-20-23-25(21-17)9-16(26)27/h1-2,7-8,11H,3-6,9H2,(H,26,27) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibition of SCD-1 activity in human Hepatocytes expressing organic anion transporting polypeptides assessed as reduction in [I-14C] stearoyl CoA de... |
J Med Chem 54: 5082-96 (2011)
Article DOI: 10.1021/jm200319u
BindingDB Entry DOI: 10.7270/Q2348MGP |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM13604

(6-{4-[2-(1H-1,2,3-benzotriazol-1-yl)-3-{4-[difluor...)Show SMILES COC(CC(C)C)c1ccc2cc(cc(c2n1)P(O)(O)=O)-c1ccc(CC(Cc2ccc(cc2)C(F)(F)P(O)(O)=O)(c2ccccc2)n2nnc3ccccc23)cc1 Show InChI InChI=1S/C43H42F2N4O7P2/c1-28(2)23-39(56-3)37-22-19-32-24-33(25-40(41(32)46-37)57(50,51)52)31-17-13-29(14-18-31)26-42(34-9-5-4-6-10-34,49-38-12-8-7-11-36(38)47-48-49)27-30-15-20-35(21-16-30)43(44,45)58(53,54)55/h4-22,24-25,28,39H,23,26-27H2,1-3H3,(H2,50,51,52)(H2,53,54,55) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against protein tyrosine phosphatase 1B (PTP1B) was determined in fluorescein diphosphate (FDP) assay |
Bioorg Med Chem Lett 14: 1043-8 (2004)
Article DOI: 10.1016/j.bmcl.2003.11.076
BindingDB Entry DOI: 10.7270/Q24M9404 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein phosphatase non-receptor type 1 [1-298]
(Homo sapiens (Human)) | BDBM13604

(6-{4-[2-(1H-1,2,3-benzotriazol-1-yl)-3-{4-[difluor...)Show SMILES COC(CC(C)C)c1ccc2cc(cc(c2n1)P(O)(O)=O)-c1ccc(CC(Cc2ccc(cc2)C(F)(F)P(O)(O)=O)(c2ccccc2)n2nnc3ccccc23)cc1 Show InChI InChI=1S/C43H42F2N4O7P2/c1-28(2)23-39(56-3)37-22-19-32-24-33(25-40(41(32)46-37)57(50,51)52)31-17-13-29(14-18-31)26-42(34-9-5-4-6-10-34,49-38-12-8-7-11-36(38)47-48-49)27-30-15-20-35(21-16-30)43(44,45)58(53,54)55/h4-22,24-25,28,39H,23,26-27H2,1-3H3,(H2,50,51,52)(H2,53,54,55) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | 6.3 | 22 |
Merck Research Laboratories
| Assay Description
Hydrolysis of substrate FDP was monitored continuously on a Cytofluor microplate reader with excitation and emission wavelengths set at 440 and 515 ... |
Biochemistry 42: 11451-9 (2003)
Article DOI: 10.1021/bi035098j
BindingDB Entry DOI: 10.7270/Q2HX19XS |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50142323

(CHEMBL267488 | [(4-{2-Benzotriazol-1-yl-3-[3-bromo...)Show SMILES OP(O)(=O)C(F)(F)c1ccc(CC(Cc2ccc(c(Br)c2)C(F)(F)P(O)(O)=O)(c2ccccc2)n2nnc3ccccc23)cc1 Show InChI InChI=1S/C29H24BrF4N3O6P2/c30-24-16-20(12-15-23(24)29(33,34)45(41,42)43)18-27(21-6-2-1-3-7-21,37-26-9-5-4-8-25(26)35-36-37)17-19-10-13-22(14-11-19)28(31,32)44(38,39)40/h1-16H,17-18H2,(H2,38,39,40)(H2,41,42,43) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against protein tyrosine phosphatase 1B (PTP1B) was determined in fluorescein diphosphate (FDP) assay |
Bioorg Med Chem Lett 14: 1043-8 (2004)
Article DOI: 10.1016/j.bmcl.2003.11.076
BindingDB Entry DOI: 10.7270/Q24M9404 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Acyl-CoA desaturase 1
(Rattus norvegicus (Rat)) | BDBM50356891
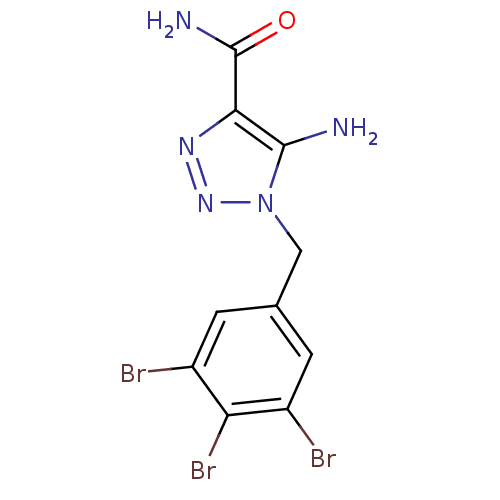
(CHEMBL1915541)Show InChI InChI=1S/C10H8Br3N5O/c11-5-1-4(2-6(12)7(5)13)3-18-9(14)8(10(15)19)16-17-18/h1-2H,3,14H2,(H2,15,19) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibition of rat SCD by rat microsomal assay |
Bioorg Med Chem Lett 21: 6505-9 (2011)
Article DOI: 10.1016/j.bmcl.2011.08.073
BindingDB Entry DOI: 10.7270/Q2VQ332W |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(RAT) | BDBM17638

(2-{1-[(4-chlorophenyl)carbonyl]-5-methoxy-2-methyl...)Show SMILES COc1ccc2n(C(=O)c3ccc(Cl)cc3)c(C)c(CC(O)=O)c2c1 Show InChI InChI=1S/C19H16ClNO4/c1-11-15(10-18(22)23)16-9-14(25-2)7-8-17(16)21(11)19(24)12-3-5-13(20)6-4-12/h3-9H,10H2,1-2H3,(H,22,23) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of Prostaglandin G/H synthase 1 using whole cell assay |
Bioorg Med Chem Lett 6: 725-730 (1996)
Article DOI: 10.1016/0960-894X(96)00100-X
BindingDB Entry DOI: 10.7270/Q25B02GQ |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(RAT) | BDBM50289081
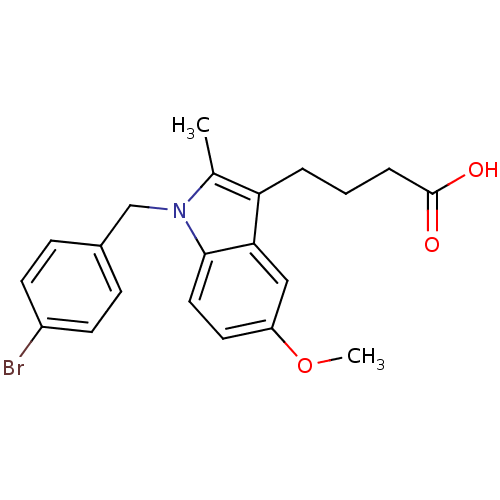
(4-[1-(4-Bromo-benzyl)-5-methoxy-2-methyl-1H-indol-...)Show SMILES COc1ccc2n(Cc3ccc(Br)cc3)c(C)c(CCCC(O)=O)c2c1 Show InChI InChI=1S/C21H22BrNO3/c1-14-18(4-3-5-21(24)25)19-12-17(26-2)10-11-20(19)23(14)13-15-6-8-16(22)9-7-15/h6-12H,3-5,13H2,1-2H3,(H,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of Prostaglandin G/H synthase 2 |
Bioorg Med Chem Lett 6: 725-730 (1996)
Article DOI: 10.1016/0960-894X(96)00100-X
BindingDB Entry DOI: 10.7270/Q25B02GQ |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(RAT) | BDBM17638

(2-{1-[(4-chlorophenyl)carbonyl]-5-methoxy-2-methyl...)Show SMILES COc1ccc2n(C(=O)c3ccc(Cl)cc3)c(C)c(CC(O)=O)c2c1 Show InChI InChI=1S/C19H16ClNO4/c1-11-15(10-18(22)23)16-9-14(25-2)7-8-17(16)21(11)19(24)12-3-5-13(20)6-4-12/h3-9H,10H2,1-2H3,(H,22,23) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was evaluated in vitro for inhibition of Prostaglandin G/H synthase 1 |
Bioorg Med Chem Lett 6: 731-736 (1996)
Article DOI: 10.1016/0960-894X(96)00101-1
BindingDB Entry DOI: 10.7270/Q21J99RF |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1 [1-298]
(Homo sapiens (Human)) | BDBM13601
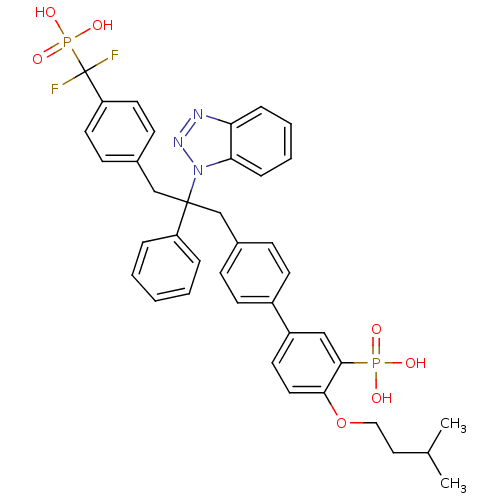
(5-{4-[2-(1H-1,2,3-benzotriazol-1-yl)-3-{4-[difluor...)Show SMILES CC(C)CCOc1ccc(cc1P(O)(O)=O)-c1ccc(CC(Cc2ccc(cc2)C(F)(F)P(O)(O)=O)(c2ccccc2)n2nnc3ccccc23)cc1 Show InChI InChI=1S/C39H39F2N3O7P2/c1-27(2)22-23-51-36-21-18-31(24-37(36)52(45,46)47)30-16-12-28(13-17-30)25-38(32-8-4-3-5-9-32,44-35-11-7-6-10-34(35)42-43-44)26-29-14-19-33(20-15-29)39(40,41)53(48,49)50/h3-21,24,27H,22-23,25-26H2,1-2H3,(H2,45,46,47)(H2,48,49,50) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | 6.3 | 22 |
Merck Research Laboratories
| Assay Description
Hydrolysis of substrate FDP was monitored continuously on a Cytofluor microplate reader with excitation and emission wavelengths set at 440 and 515 ... |
Biochemistry 42: 11451-9 (2003)
Article DOI: 10.1021/bi035098j
BindingDB Entry DOI: 10.7270/Q2HX19XS |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Tyrosine-protein phosphatase non-receptor type 1 [1-298]
(Homo sapiens (Human)) | BDBM13603
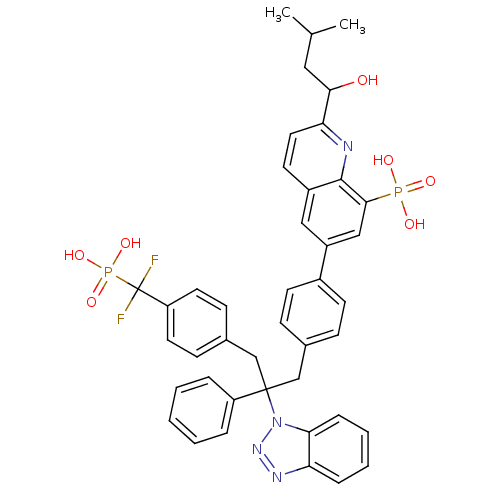
(6-{4-[2-(1H-1,2,3-benzotriazol-1-yl)-3-{4-[difluor...)Show SMILES CC(C)CC(O)c1ccc2cc(cc(c2n1)P(O)(O)=O)-c1ccc(CC(Cc2ccc(cc2)C(F)(F)P(O)(O)=O)(c2ccccc2)n2nnc3ccccc23)cc1 Show InChI InChI=1S/C42H40F2N4O7P2/c1-27(2)22-38(49)36-21-18-31-23-32(24-39(40(31)45-36)56(50,51)52)30-16-12-28(13-17-30)25-41(33-8-4-3-5-9-33,48-37-11-7-6-10-35(37)46-47-48)26-29-14-19-34(20-15-29)42(43,44)57(53,54)55/h3-21,23-24,27,38,49H,22,25-26H2,1-2H3,(H2,50,51,52)(H2,53,54,55) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | 6.3 | 22 |
Merck Research Laboratories
| Assay Description
Hydrolysis of substrate FDP was monitored continuously on a Cytofluor microplate reader with excitation and emission wavelengths set at 440 and 515 ... |
Biochemistry 42: 11451-9 (2003)
Article DOI: 10.1021/bi035098j
BindingDB Entry DOI: 10.7270/Q2HX19XS |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM13604

(6-{4-[2-(1H-1,2,3-benzotriazol-1-yl)-3-{4-[difluor...)Show SMILES COC(CC(C)C)c1ccc2cc(cc(c2n1)P(O)(O)=O)-c1ccc(CC(Cc2ccc(cc2)C(F)(F)P(O)(O)=O)(c2ccccc2)n2nnc3ccccc23)cc1 Show InChI InChI=1S/C43H42F2N4O7P2/c1-28(2)23-39(56-3)37-22-19-32-24-33(25-40(41(32)46-37)57(50,51)52)31-17-13-29(14-18-31)26-42(34-9-5-4-6-10-34,49-38-12-8-7-11-36(38)47-48-49)27-30-15-20-35(21-16-30)43(44,45)58(53,54)55/h4-22,24-25,28,39H,23,26-27H2,1-3H3,(H2,50,51,52)(H2,53,54,55) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against protein tyrosine phosphatase 1B (PTP1B) was determined in fluorescein diphosphate (FDP) assay |
Bioorg Med Chem Lett 14: 1043-8 (2004)
Article DOI: 10.1016/j.bmcl.2003.11.076
BindingDB Entry DOI: 10.7270/Q24M9404 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50373451
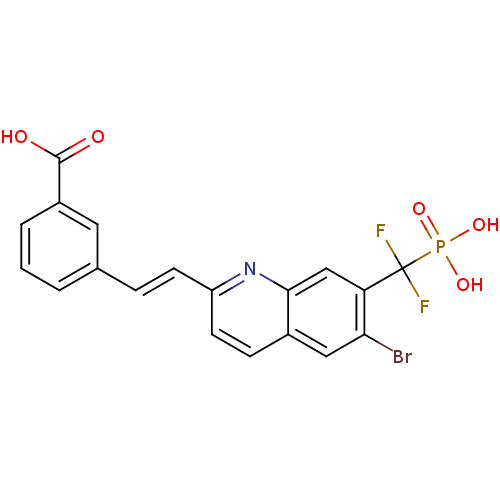
(CHEMBL411295)Show SMILES OC(=O)c1cccc(\C=C\c2ccc3cc(Br)c(cc3n2)C(F)(F)P(O)(O)=O)c1 Show InChI InChI=1S/C19H13BrF2NO5P/c20-16-9-12-5-7-14(6-4-11-2-1-3-13(8-11)18(24)25)23-17(12)10-15(16)19(21,22)29(26,27)28/h1-10H,(H,24,25)(H2,26,27,28)/b6-4+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B by fluorescein diphosphate assay |
Bioorg Med Chem Lett 18: 3200-5 (2008)
Article DOI: 10.1016/j.bmcl.2008.04.064
BindingDB Entry DOI: 10.7270/Q24M95DX |
More data for this
Ligand-Target Pair | |
Acyl-CoA desaturase 1
(Rattus norvegicus (Rat)) | BDBM50393166

(CHEMBL2153601)Show SMILES OC(=O)Cn1nnc(n1)-c1nnc(s1)N1CCC(CC1)Oc1cc(F)ccc1Br Show InChI InChI=1S/C16H15BrFN7O3S/c17-11-2-1-9(18)7-12(11)28-10-3-5-24(6-4-10)16-21-20-15(29-16)14-19-23-25(22-14)8-13(26)27/h1-2,7,10H,3-6,8H2,(H,26,27) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibition of SCD-1 activity in Sprague-Dawley rat microsome assessed as reduction in [I-14C] stearoyl CoA desaturation by scintillation counting |
J Med Chem 54: 5082-96 (2011)
Article DOI: 10.1021/jm200319u
BindingDB Entry DOI: 10.7270/Q2348MGP |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 2
(Homo sapiens (Human)) | BDBM13596

(({4-[2-(1H-1,2,3-benzotriazol-1-yl)-3-{4-[difluoro...)Show SMILES OP(O)(=O)C(F)(F)c1ccc(CC(Cc2ccc(cc2)C(F)(F)P(O)(O)=O)(c2ccc(F)c(F)c2)n2nnc3ccccc23)cc1 Show InChI InChI=1S/C29H23F6N3O6P2/c30-23-14-13-22(15-24(23)31)27(38-26-4-2-1-3-25(26)36-37-38,16-18-5-9-20(10-6-18)28(32,33)45(39,40)41)17-19-7-11-21(12-8-19)29(34,35)46(42,43)44/h1-15H,16-17H2,(H2,39,40,41)(H2,42,43,44) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against T cell protein tyrosine phosphatase |
Bioorg Med Chem Lett 14: 1043-8 (2004)
Article DOI: 10.1016/j.bmcl.2003.11.076
BindingDB Entry DOI: 10.7270/Q24M9404 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(RAT) | BDBM22970
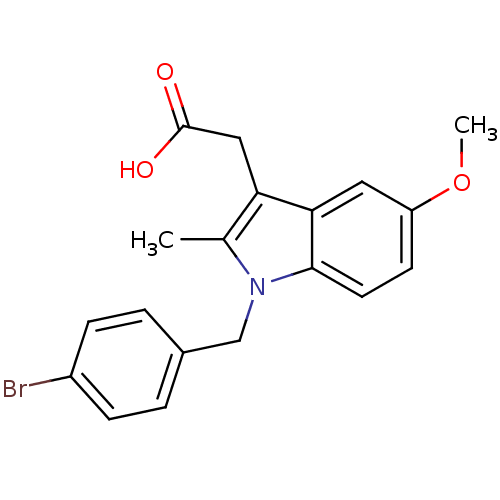
(2-{1-[(4-bromophenyl)methyl]-5-methoxy-2-methyl-1H...)Show InChI InChI=1S/C19H18BrNO3/c1-12-16(10-19(22)23)17-9-15(24-2)7-8-18(17)21(12)11-13-3-5-14(20)6-4-13/h3-9H,10-11H2,1-2H3,(H,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of Prostaglandin G/H synthase 2 |
Bioorg Med Chem Lett 6: 725-730 (1996)
Article DOI: 10.1016/0960-894X(96)00100-X
BindingDB Entry DOI: 10.7270/Q25B02GQ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 2
(Homo sapiens (Human)) | BDBM13595
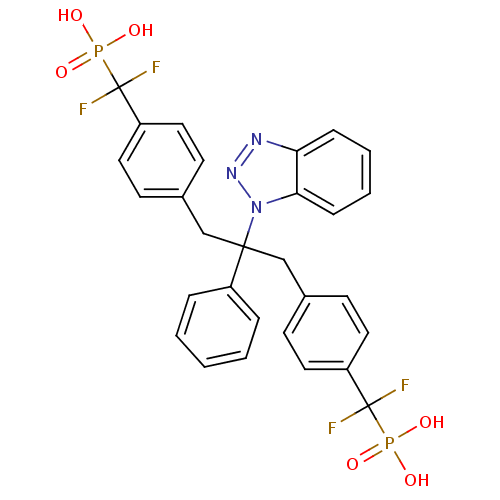
(({4-[2-(1H-1,2,3-benzotriazol-1-yl)-3-{4-[difluoro...)Show SMILES OP(O)(=O)C(F)(F)c1ccc(CC(Cc2ccc(cc2)C(F)(F)P(O)(O)=O)(c2ccccc2)n2nnc3ccccc23)cc1 Show InChI InChI=1S/C29H25F4N3O6P2/c30-28(31,43(37,38)39)23-14-10-20(11-15-23)18-27(22-6-2-1-3-7-22,36-26-9-5-4-8-25(26)34-35-36)19-21-12-16-24(17-13-21)29(32,33)44(40,41)42/h1-17H,18-19H2,(H2,37,38,39)(H2,40,41,42) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against T cell protein tyrosine phosphatase |
Bioorg Med Chem Lett 14: 1043-8 (2004)
Article DOI: 10.1016/j.bmcl.2003.11.076
BindingDB Entry DOI: 10.7270/Q24M9404 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 2
(Homo sapiens (Human)) | BDBM50142335
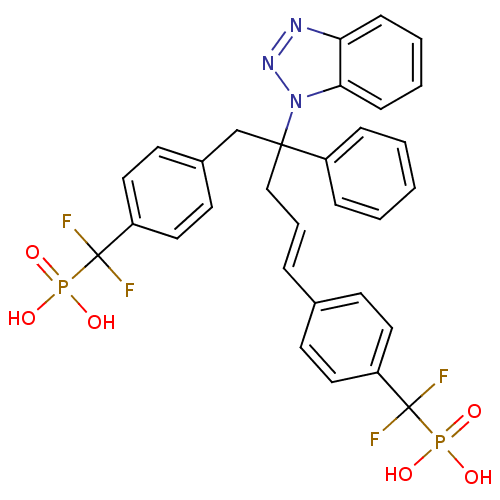
(CHEMBL274435 | [(4-{4-Benzotriazol-1-yl-5-[4-(difl...)Show SMILES OP(O)(=O)C(F)(F)c1ccc(CC(C\C=C\c2ccc(cc2)C(F)(F)P(O)(O)=O)(c2ccccc2)n2nnc3ccccc23)cc1 Show InChI InChI=1S/C31H27F4N3O6P2/c32-30(33,45(39,40)41)25-16-12-22(13-17-25)7-6-20-29(24-8-2-1-3-9-24,38-28-11-5-4-10-27(28)36-37-38)21-23-14-18-26(19-15-23)31(34,35)46(42,43)44/h1-19H,20-21H2,(H2,39,40,41)(H2,42,43,44)/b7-6+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against T cell protein tyrosine phosphatase |
Bioorg Med Chem Lett 14: 1043-8 (2004)
Article DOI: 10.1016/j.bmcl.2003.11.076
BindingDB Entry DOI: 10.7270/Q24M9404 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(RAT) | BDBM17638

(2-{1-[(4-chlorophenyl)carbonyl]-5-methoxy-2-methyl...)Show SMILES COc1ccc2n(C(=O)c3ccc(Cl)cc3)c(C)c(CC(O)=O)c2c1 Show InChI InChI=1S/C19H16ClNO4/c1-11-15(10-18(22)23)16-9-14(25-2)7-8-17(16)21(11)19(24)12-3-5-13(20)6-4-12/h3-9H,10H2,1-2H3,(H,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of Prostaglandin G/H synthase 2 |
Bioorg Med Chem Lett 6: 725-730 (1996)
Article DOI: 10.1016/0960-894X(96)00100-X
BindingDB Entry DOI: 10.7270/Q25B02GQ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Prostaglandin G/H synthase 2
(RAT) | BDBM17638

(2-{1-[(4-chlorophenyl)carbonyl]-5-methoxy-2-methyl...)Show SMILES COc1ccc2n(C(=O)c3ccc(Cl)cc3)c(C)c(CC(O)=O)c2c1 Show InChI InChI=1S/C19H16ClNO4/c1-11-15(10-18(22)23)16-9-14(25-2)7-8-17(16)21(11)19(24)12-3-5-13(20)6-4-12/h3-9H,10H2,1-2H3,(H,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was evaluated in vitro for inhibition of Prostaglandin G/H synthase 2 |
Bioorg Med Chem Lett 6: 731-736 (1996)
Article DOI: 10.1016/0960-894X(96)00101-1
BindingDB Entry DOI: 10.7270/Q21J99RF |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein phosphatase non-receptor type 1 [1-298]
(Homo sapiens (Human)) | BDBM13600
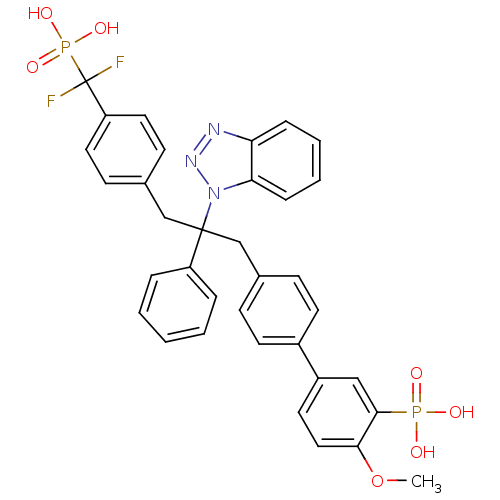
(5-{4-[2-(1H-1,2,3-benzotriazol-1-yl)-3-{4-[difluor...)Show SMILES COc1ccc(cc1P(O)(O)=O)-c1ccc(CC(Cc2ccc(cc2)C(F)(F)P(O)(O)=O)(c2ccccc2)n2nnc3ccccc23)cc1 Show InChI InChI=1S/C35H31F2N3O7P2/c1-47-32-20-17-27(21-33(32)48(41,42)43)26-15-11-24(12-16-26)22-34(28-7-3-2-4-8-28,40-31-10-6-5-9-30(31)38-39-40)23-25-13-18-29(19-14-25)35(36,37)49(44,45)46/h2-21H,22-23H2,1H3,(H2,41,42,43)(H2,44,45,46) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | 6.3 | 22 |
Merck Research Laboratories
| Assay Description
Hydrolysis of substrate FDP was monitored continuously on a Cytofluor microplate reader with excitation and emission wavelengths set at 440 and 515 ... |
Biochemistry 42: 11451-9 (2003)
Article DOI: 10.1021/bi035098j
BindingDB Entry DOI: 10.7270/Q2HX19XS |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50142328
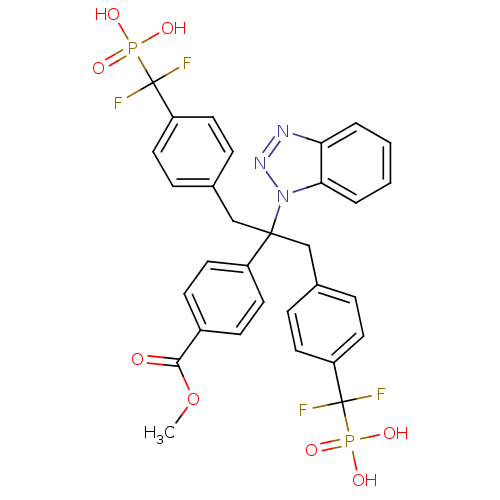
(4-{1-Benzotriazol-1-yl-1-[4-(difluoro-phosphono-me...)Show SMILES COC(=O)c1ccc(cc1)C(Cc1ccc(cc1)C(F)(F)P(O)(O)=O)(Cc1ccc(cc1)C(F)(F)P(O)(O)=O)n1nnc2ccccc12 Show InChI InChI=1S/C31H27F4N3O8P2/c1-46-28(39)22-10-16-23(17-11-22)29(38-27-5-3-2-4-26(27)36-37-38,18-20-6-12-24(13-7-20)30(32,33)47(40,41)42)19-21-8-14-25(15-9-21)31(34,35)48(43,44)45/h2-17H,18-19H2,1H3,(H2,40,41,42)(H2,43,44,45) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against Protein tyrosine phosphatase 1B (PTP1B) overexpressed in intact Sf9 cell assay |
Bioorg Med Chem Lett 14: 1043-8 (2004)
Article DOI: 10.1016/j.bmcl.2003.11.076
BindingDB Entry DOI: 10.7270/Q24M9404 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Tyrosine-protein phosphatase non-receptor type 1 [1-298]
(Homo sapiens (Human)) | BDBM13605
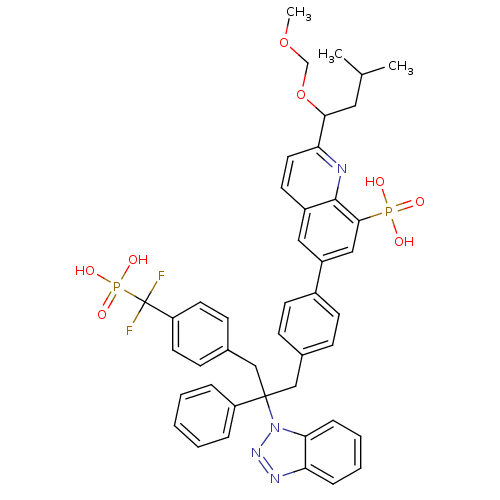
(6-{4-[2-(1H-1,2,3-benzotriazol-1-yl)-3-{4-[difluor...)Show SMILES COCOC(CC(C)C)c1ccc2cc(cc(c2n1)P(O)(O)=O)-c1ccc(CC(Cc2ccc(cc2)C(F)(F)P(O)(O)=O)(c2ccccc2)n2nnc3ccccc23)cc1 Show InChI InChI=1S/C44H44F2N4O8P2/c1-29(2)23-40(58-28-57-3)38-22-19-33-24-34(25-41(42(33)47-38)59(51,52)53)32-17-13-30(14-18-32)26-43(35-9-5-4-6-10-35,50-39-12-8-7-11-37(39)48-49-50)27-31-15-20-36(21-16-31)44(45,46)60(54,55)56/h4-22,24-25,29,40H,23,26-28H2,1-3H3,(H2,51,52,53)(H2,54,55,56) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | 6.3 | 22 |
Merck Research Laboratories
| Assay Description
Hydrolysis of substrate FDP was monitored continuously on a Cytofluor microplate reader with excitation and emission wavelengths set at 440 and 515 ... |
Biochemistry 42: 11451-9 (2003)
Article DOI: 10.1021/bi035098j
BindingDB Entry DOI: 10.7270/Q2HX19XS |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Tyrosine-protein phosphatase non-receptor type 2
(Homo sapiens (Human)) | BDBM13595

(({4-[2-(1H-1,2,3-benzotriazol-1-yl)-3-{4-[difluoro...)Show SMILES OP(O)(=O)C(F)(F)c1ccc(CC(Cc2ccc(cc2)C(F)(F)P(O)(O)=O)(c2ccccc2)n2nnc3ccccc23)cc1 Show InChI InChI=1S/C29H25F4N3O6P2/c30-28(31,43(37,38)39)23-14-10-20(11-15-23)18-27(22-6-2-1-3-7-22,36-26-9-5-4-8-25(26)34-35-36)19-21-12-16-24(17-13-21)29(32,33)44(40,41)42/h1-17H,18-19H2,(H2,37,38,39)(H2,40,41,42) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | 6.3 | 22 |
Merck Research Laboratories
| Assay Description
Hydrolysis of substrate FDP was monitored continuously on a Cytofluor microplate reader with excitation and emission wavelengths set at 440 and 515 ... |
Biochemistry 42: 11451-9 (2003)
Article DOI: 10.1021/bi035098j
BindingDB Entry DOI: 10.7270/Q2HX19XS |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM13602

(6-{4-[2-(1H-1,2,3-benzotriazol-1-yl)-3-{4-[difluor...)Show SMILES Cc1ccc2cc(cc(c2n1)P(O)(O)=O)-c1ccc(CC(Cc2ccc(cc2)C(F)(F)P(O)(O)=O)(c2ccccc2)n2nnc3ccccc23)cc1 Show InChI InChI=1S/C38H32F2N4O6P2/c1-25-11-16-29-21-30(22-35(36(29)41-25)51(45,46)47)28-17-12-26(13-18-28)23-37(31-7-3-2-4-8-31,44-34-10-6-5-9-33(34)42-43-44)24-27-14-19-32(20-15-27)38(39,40)52(48,49)50/h2-22H,23-24H2,1H3,(H2,45,46,47)(H2,48,49,50) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against Protein tyrosine phosphatase 1B (PTP1B) overexpressed in intact Sf9 cell assay |
Bioorg Med Chem Lett 14: 1043-8 (2004)
Article DOI: 10.1016/j.bmcl.2003.11.076
BindingDB Entry DOI: 10.7270/Q24M9404 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Acyl-CoA desaturase 1
(Rattus norvegicus (Rat)) | BDBM50356892
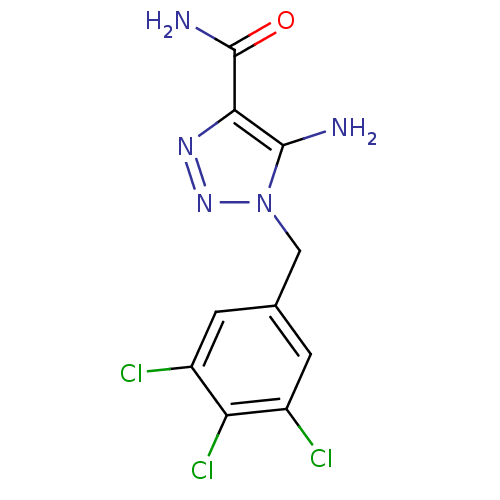
(CHEMBL1915542)Show InChI InChI=1S/C10H8Cl3N5O/c11-5-1-4(2-6(12)7(5)13)3-18-9(14)8(10(15)19)16-17-18/h1-2H,3,14H2,(H2,15,19) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibition of rat SCD by rat microsomal assay |
Bioorg Med Chem Lett 21: 6505-9 (2011)
Article DOI: 10.1016/j.bmcl.2011.08.073
BindingDB Entry DOI: 10.7270/Q2VQ332W |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1 [1-298]
(Homo sapiens (Human)) | BDBM13602

(6-{4-[2-(1H-1,2,3-benzotriazol-1-yl)-3-{4-[difluor...)Show SMILES Cc1ccc2cc(cc(c2n1)P(O)(O)=O)-c1ccc(CC(Cc2ccc(cc2)C(F)(F)P(O)(O)=O)(c2ccccc2)n2nnc3ccccc23)cc1 Show InChI InChI=1S/C38H32F2N4O6P2/c1-25-11-16-29-21-30(22-35(36(29)41-25)51(45,46)47)28-17-12-26(13-18-28)23-37(31-7-3-2-4-8-31,44-34-10-6-5-9-33(34)42-43-44)24-27-14-19-32(20-15-27)38(39,40)52(48,49)50/h2-22H,23-24H2,1H3,(H2,45,46,47)(H2,48,49,50) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | 6.3 | 22 |
Merck Research Laboratories
| Assay Description
Hydrolysis of substrate FDP was monitored continuously on a Cytofluor microplate reader with excitation and emission wavelengths set at 440 and 515 ... |
Biochemistry 42: 11451-9 (2003)
Article DOI: 10.1021/bi035098j
BindingDB Entry DOI: 10.7270/Q2HX19XS |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM13596

(({4-[2-(1H-1,2,3-benzotriazol-1-yl)-3-{4-[difluoro...)Show SMILES OP(O)(=O)C(F)(F)c1ccc(CC(Cc2ccc(cc2)C(F)(F)P(O)(O)=O)(c2ccc(F)c(F)c2)n2nnc3ccccc23)cc1 Show InChI InChI=1S/C29H23F6N3O6P2/c30-23-14-13-22(15-24(23)31)27(38-26-4-2-1-3-25(26)36-37-38,16-18-5-9-20(10-6-18)28(32,33)45(39,40)41)17-19-7-11-21(12-8-19)29(34,35)46(42,43)44/h1-15H,16-17H2,(H2,39,40,41)(H2,42,43,44) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against protein tyrosine phosphatase 1B (PTP1B) was determined in fluorescein diphosphate (FDP) assay |
Bioorg Med Chem Lett 14: 1043-8 (2004)
Article DOI: 10.1016/j.bmcl.2003.11.076
BindingDB Entry DOI: 10.7270/Q24M9404 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein phosphatase non-receptor type 1 [1-298]
(Homo sapiens (Human)) | BDBM13596

(({4-[2-(1H-1,2,3-benzotriazol-1-yl)-3-{4-[difluoro...)Show SMILES OP(O)(=O)C(F)(F)c1ccc(CC(Cc2ccc(cc2)C(F)(F)P(O)(O)=O)(c2ccc(F)c(F)c2)n2nnc3ccccc23)cc1 Show InChI InChI=1S/C29H23F6N3O6P2/c30-23-14-13-22(15-24(23)31)27(38-26-4-2-1-3-25(26)36-37-38,16-18-5-9-20(10-6-18)28(32,33)45(39,40)41)17-19-7-11-21(12-8-19)29(34,35)46(42,43)44/h1-15H,16-17H2,(H2,39,40,41)(H2,42,43,44) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | 6.3 | 22 |
Merck Research Laboratories
| Assay Description
Hydrolysis of substrate FDP was monitored continuously on a Cytofluor microplate reader with excitation and emission wavelengths set at 440 and 515 ... |
Biochemistry 42: 11451-9 (2003)
Article DOI: 10.1021/bi035098j
BindingDB Entry DOI: 10.7270/Q2HX19XS |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Stearoyl-CoA desaturase
(Homo sapiens (Human)) | BDBM50329985
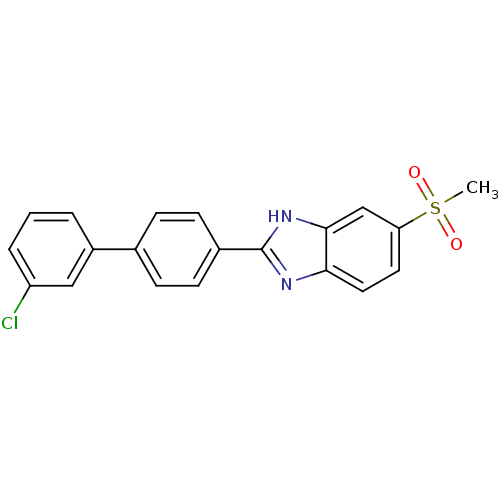
(2-(3'-chlorobiphenyl-4-yl)-6-(methylsulfonyl)-1H-b...)Show SMILES CS(=O)(=O)c1ccc2nc([nH]c2c1)-c1ccc(cc1)-c1cccc(Cl)c1 Show InChI InChI=1S/C20H15ClN2O2S/c1-26(24,25)17-9-10-18-19(12-17)23-20(22-18)14-7-5-13(6-8-14)15-3-2-4-16(21)11-15/h2-12H,1H3,(H,22,23) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibition of human SCD1 |
Bioorg Med Chem Lett 20: 6366-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.094
BindingDB Entry DOI: 10.7270/Q20V8D1P |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50142335

(CHEMBL274435 | [(4-{4-Benzotriazol-1-yl-5-[4-(difl...)Show SMILES OP(O)(=O)C(F)(F)c1ccc(CC(C\C=C\c2ccc(cc2)C(F)(F)P(O)(O)=O)(c2ccccc2)n2nnc3ccccc23)cc1 Show InChI InChI=1S/C31H27F4N3O6P2/c32-30(33,45(39,40)41)25-16-12-22(13-17-25)7-6-20-29(24-8-2-1-3-9-24,38-28-11-5-4-10-27(28)36-37-38)21-23-14-18-26(19-15-23)31(34,35)46(42,43)44/h1-19H,20-21H2,(H2,39,40,41)(H2,42,43,44)/b7-6+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against protein tyrosine phosphatase 1B (PTP1B) was determined in fluorescein diphosphate (FDP) assay |
Bioorg Med Chem Lett 14: 1043-8 (2004)
Article DOI: 10.1016/j.bmcl.2003.11.076
BindingDB Entry DOI: 10.7270/Q24M9404 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50373441
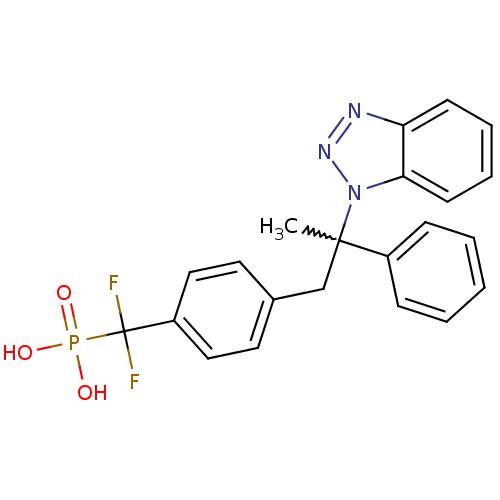
(CHEMBL410646)Show SMILES CC(Cc1ccc(cc1)C(F)(F)P(O)(O)=O)(c1ccccc1)n1nnc2ccccc12 |w:1.0| Show InChI InChI=1S/C22H20F2N3O3P/c1-21(17-7-3-2-4-8-17,27-20-10-6-5-9-19(20)25-26-27)15-16-11-13-18(14-12-16)22(23,24)31(28,29)30/h2-14H,15H2,1H3,(H2,28,29,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B by fluorescein diphosphate assay |
Bioorg Med Chem Lett 18: 3200-5 (2008)
Article DOI: 10.1016/j.bmcl.2008.04.064
BindingDB Entry DOI: 10.7270/Q24M95DX |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 2
(Homo sapiens (Human)) | BDBM50142324
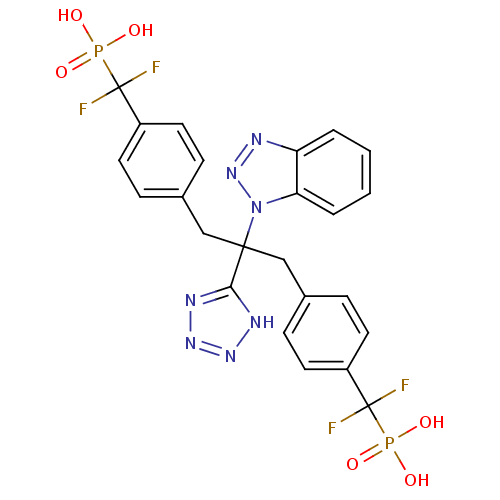
(({4-[2-Benzotriazol-1-yl-3-[4-(difluoro-phosphono-...)Show SMILES OP(O)(=O)C(F)(F)c1ccc(CC(Cc2ccc(cc2)C(F)(F)P(O)(O)=O)(c2nnn[nH]2)n2nnc3ccccc23)cc1 Show InChI InChI=1S/C24H21F4N7O6P2/c25-23(26,42(36,37)38)17-9-5-15(6-10-17)13-22(21-30-32-33-31-21,35-20-4-2-1-3-19(20)29-34-35)14-16-7-11-18(12-8-16)24(27,28)43(39,40)41/h1-12H,13-14H2,(H2,36,37,38)(H2,39,40,41)(H,30,31,32,33) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against T cell protein tyrosine phosphatase |
Bioorg Med Chem Lett 14: 1043-8 (2004)
Article DOI: 10.1016/j.bmcl.2003.11.076
BindingDB Entry DOI: 10.7270/Q24M9404 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(RAT) | BDBM50289090
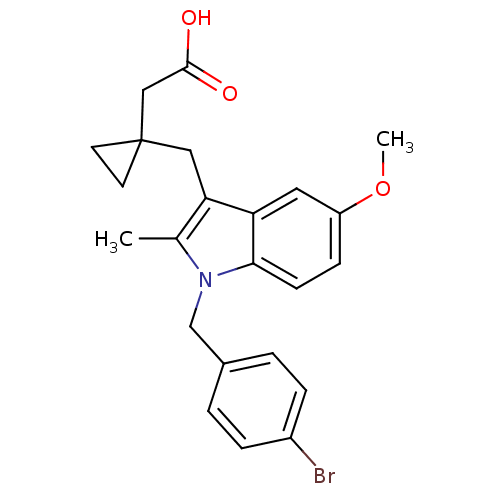
(CHEMBL162776 | {1-[1-(4-Bromo-benzyl)-5-methoxy-2-...)Show SMILES COc1ccc2n(Cc3ccc(Br)cc3)c(C)c(CC3(CC(O)=O)CC3)c2c1 Show InChI InChI=1S/C23H24BrNO3/c1-15-20(12-23(9-10-23)13-22(26)27)19-11-18(28-2)7-8-21(19)25(15)14-16-3-5-17(24)6-4-16/h3-8,11H,9-10,12-14H2,1-2H3,(H,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of Prostaglandin G/H synthase 2 |
Bioorg Med Chem Lett 6: 725-730 (1996)
Article DOI: 10.1016/0960-894X(96)00100-X
BindingDB Entry DOI: 10.7270/Q25B02GQ |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data