

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Alpha-1A adrenergic receptor (Homo sapiens (Human)) | BDBM50082842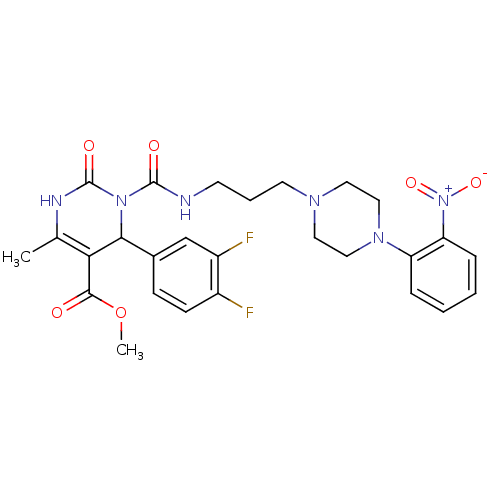 (4-(3,4-Difluoro-phenyl)-6-methyl-3-{3-[4-(2-nitro-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Synaptic Pharmaceutical Corporation Curated by ChEMBL | Assay Description In vitro binding affinity against Alpha-1A adrenergic receptor of human liver microsomes. | J Med Chem 42: 4794-803 (1999) BindingDB Entry DOI: 10.7270/Q25B01P0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (Homo sapiens (Human)) | BDBM50082839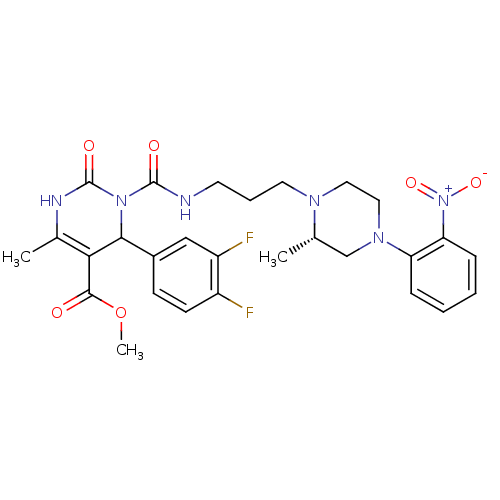 (4-(3,4-Difluoro-phenyl)-6-methyl-3-{3-[(S)-2-methy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Synaptic Pharmaceutical Corporation Curated by ChEMBL | Assay Description In vitro binding affinity against Alpha-1A adrenergic receptor of human liver microsomes. | J Med Chem 42: 4794-803 (1999) BindingDB Entry DOI: 10.7270/Q25B01P0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-4/beta-2 (Homo sapiens (Human)) | BDBM50049750 ((S)-3-(azetidin-2-ylmethoxy)pyridine | 3-((S)-1-Az...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago Curated by ChEMBL | Assay Description Binding affinity to alpha4beta2 nAChR in human brain | J Med Chem 55: 9998-10009 (2012) Article DOI: 10.1021/jm301177j BindingDB Entry DOI: 10.7270/Q2CJ8FM1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (Homo sapiens (Human)) | BDBM50082851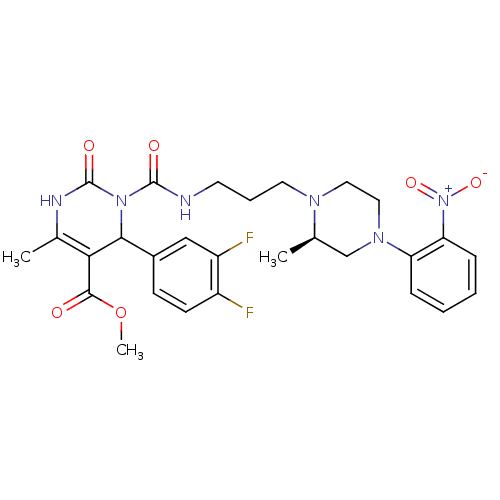 (4-(3,4-Difluoro-phenyl)-6-methyl-3-{3-[(R)-2-methy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Synaptic Pharmaceutical Corporation Curated by ChEMBL | Assay Description In vitro binding affinity against Alpha-1A adrenergic receptor of human liver microsomes. | J Med Chem 42: 4794-803 (1999) BindingDB Entry DOI: 10.7270/Q25B01P0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Norepinephrine transporter (RAT) | BDBM35229 (3-(10,11-dihydro-5H-dibenzo[b,f]azepin-5-yl)-N-met...) | UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard University Curated by PDSP Ki Database | J Pharmacol Exp Ther 301: 1097-102 (2002) Article DOI: 10.1124/jpet.301.3.1097 BindingDB Entry DOI: 10.7270/Q2ZW1JH0 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (Homo sapiens (Human)) | BDBM50082857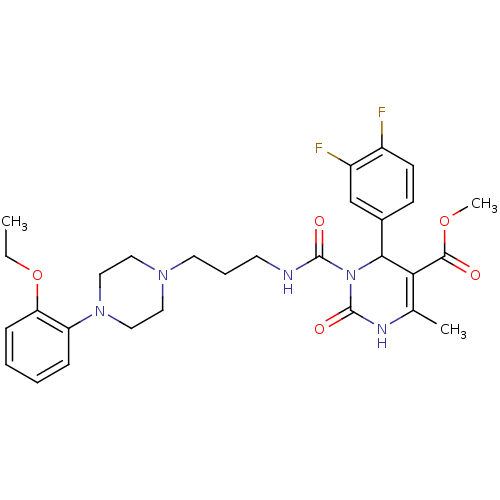 (4-(3,4-Difluoro-phenyl)-3-{3-[4-(2-ethoxy-phenyl)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Synaptic Pharmaceutical Corporation Curated by ChEMBL | Assay Description In vitro binding affinity against Alpha-1A adrenergic receptor of human liver microsomes. | J Med Chem 42: 4794-803 (1999) BindingDB Entry DOI: 10.7270/Q25B01P0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (Homo sapiens (Human)) | BDBM50082837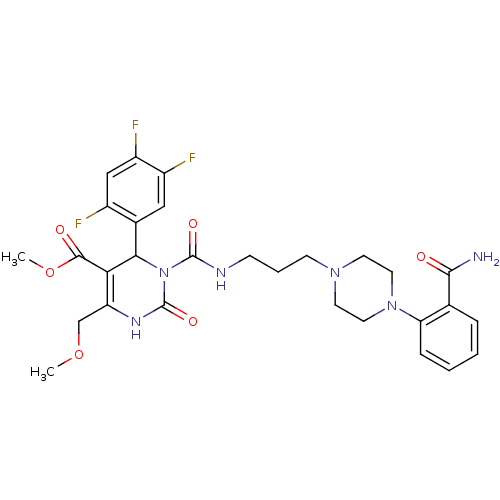 (3-{3-[4-(2-Carbamoyl-phenyl)-piperazin-1-yl]-propy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Synaptic Pharmaceutical Corporation Curated by ChEMBL | Assay Description In vitro binding affinity against Alpha-1A adrenergic receptor of human liver microsomes. | J Med Chem 42: 4794-803 (1999) BindingDB Entry DOI: 10.7270/Q25B01P0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (Homo sapiens (Human)) | BDBM50082856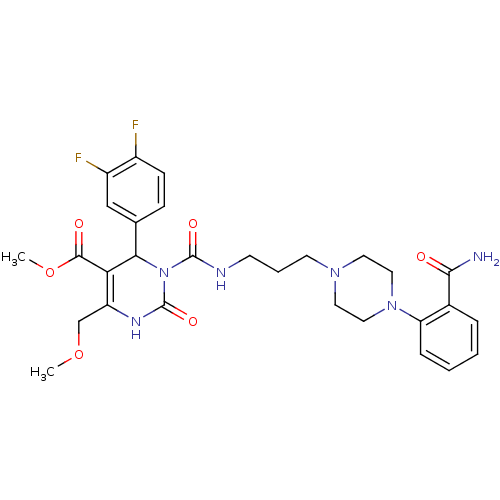 (3-{3-[4-(2-Carbamoyl-phenyl)-piperazin-1-yl]-propy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Synaptic Pharmaceutical Corporation Curated by ChEMBL | Assay Description In vitro binding affinity against Alpha-1A adrenergic receptor of human liver microsomes. | J Med Chem 42: 4794-803 (1999) BindingDB Entry DOI: 10.7270/Q25B01P0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (Homo sapiens (Human)) | BDBM50082880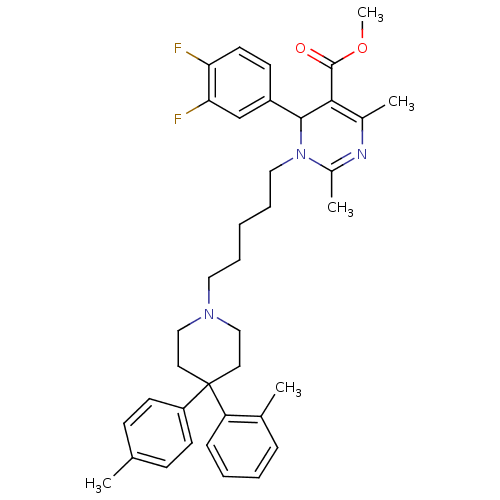 (6-(3,4-Difluoro-phenyl)-2,4-dimethyl-1-[5-(4-o-tol...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Synaptic Pharmaceutical Corporation Curated by ChEMBL | Assay Description Binding affinity against Alpha-1A adrenergic receptor (recombinant human receptor) using [3H]prazosin. | J Med Chem 42: 4804-13 (1999) BindingDB Entry DOI: 10.7270/Q2RJ4K5R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (Homo sapiens (Human)) | BDBM50082858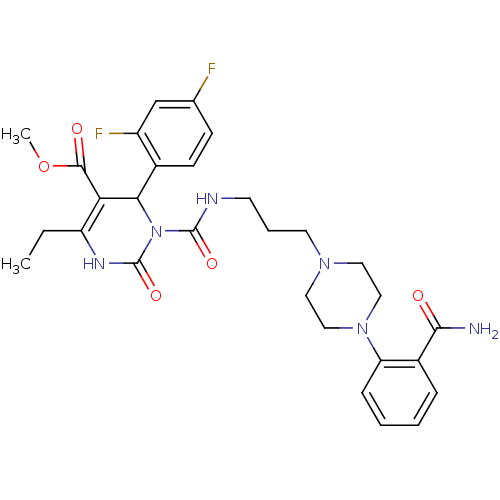 (3-{3-[4-(2-Carbamoyl-phenyl)-piperazin-1-yl]-propy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Synaptic Pharmaceutical Corporation Curated by ChEMBL | Assay Description In vitro binding affinity against Alpha-1A adrenergic receptor of human liver microsomes. | J Med Chem 42: 4794-803 (1999) BindingDB Entry DOI: 10.7270/Q25B01P0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (Homo sapiens (Human)) | BDBM50082825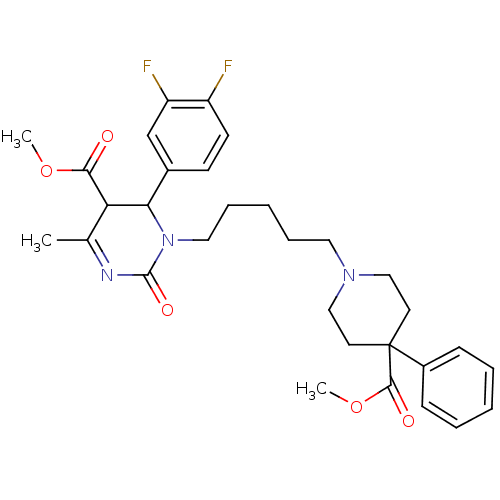 (4-(3,4-Difluoro-phenyl)-3-[5-(4-methoxycarbonyl-4-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Synaptic Pharmaceutical Corporation Curated by ChEMBL | Assay Description Binding affinity against Alpha-1A adrenergic receptor (recombinant human receptor) using [3H]prazosin. | J Med Chem 42: 4804-13 (1999) BindingDB Entry DOI: 10.7270/Q2RJ4K5R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase kinase kinase kinase 1 (Homo sapiens (Human)) | BDBM466723 (US10800761, Example 42 | US10800761, Example 55 | ...) | PDB NCI pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | <0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description In vitro inhibition of human carbonic anhydrase II (0.1 nM). | Citation and Details | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (Homo sapiens (Human)) | BDBM50082845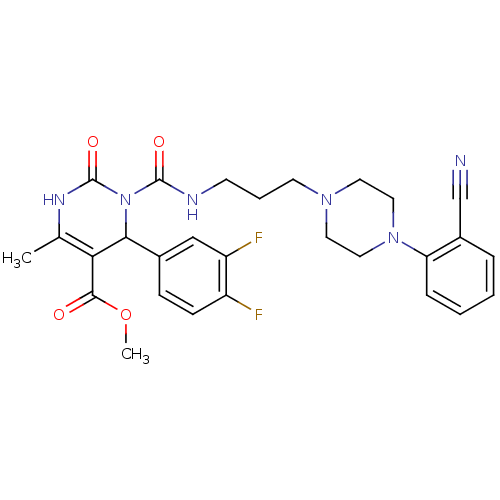 (3-{3-[4-(2-Cyano-phenyl)-piperazin-1-yl]-propylcar...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Synaptic Pharmaceutical Corporation Curated by ChEMBL | Assay Description In vitro binding affinity against Alpha-1A adrenergic receptor of human liver microsomes. | J Med Chem 42: 4794-803 (1999) BindingDB Entry DOI: 10.7270/Q25B01P0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (Homo sapiens (Human)) | BDBM50082845 (3-{3-[4-(2-Cyano-phenyl)-piperazin-1-yl]-propylcar...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Synaptic Pharmaceutical Corporation Curated by ChEMBL | Assay Description In vitro binding affinity against Alpha-1A adrenergic receptor of human liver microsomes. | J Med Chem 42: 4794-803 (1999) BindingDB Entry DOI: 10.7270/Q25B01P0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (Homo sapiens (Human)) | BDBM50082852 (4-(3,4-Difluoro-phenyl)-3-{3-[4-(2-methoxy-phenyl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Synaptic Pharmaceutical Corporation Curated by ChEMBL | Assay Description In vitro binding affinity against Alpha-1A adrenergic receptor of human liver microsomes. | J Med Chem 42: 4794-803 (1999) BindingDB Entry DOI: 10.7270/Q25B01P0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (Homo sapiens (Human)) | BDBM50082841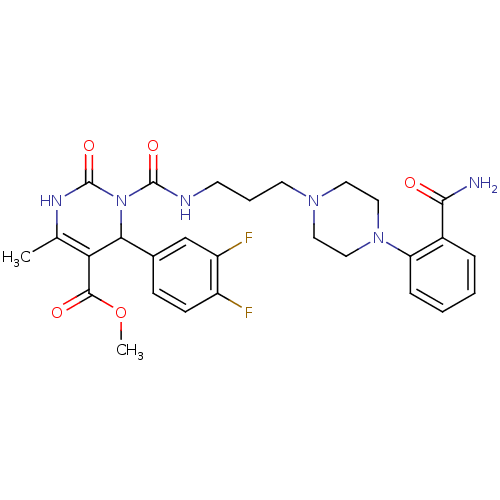 (3-{3-[4-(2-Carbamoyl-phenyl)-piperazin-1-yl]-propy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Synaptic Pharmaceutical Corporation Curated by ChEMBL | Assay Description In vitro binding affinity against Alpha-1A adrenergic receptor of human liver microsomes. | J Med Chem 42: 4794-803 (1999) BindingDB Entry DOI: 10.7270/Q25B01P0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (Homo sapiens (Human)) | BDBM50082841 (3-{3-[4-(2-Carbamoyl-phenyl)-piperazin-1-yl]-propy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Synaptic Pharmaceutical Corporation Curated by ChEMBL | Assay Description In vitro binding affinity against Alpha-1A adrenergic receptor of human liver microsomes. | J Med Chem 42: 4794-803 (1999) BindingDB Entry DOI: 10.7270/Q25B01P0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM194780 (7-(4-(4-(1-benzothiophen-4-yl)piperazin-1-yl)butox...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid UniChem | Article PubMed | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H](+)8-OH-DPAT from human 5HT1A receptor expressed in human HeLa cells measured after 60 mins | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112709 BindingDB Entry DOI: 10.7270/Q2XK8K6M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent dopamine transporter (Rattus norvegicus (rat)) | BDBM22165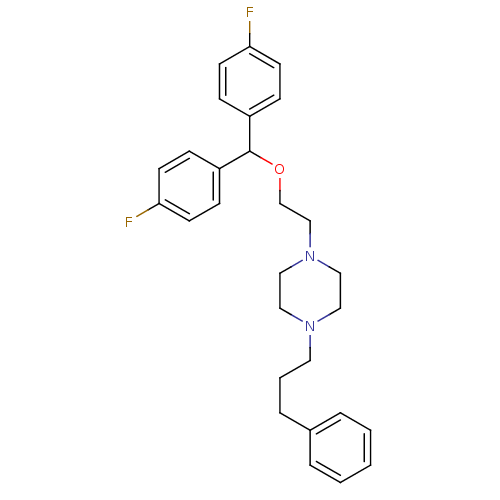 (1-{2-[bis(4-fluorophenyl)methoxy]ethyl}-4-(3-pheny...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard University Curated by PDSP Ki Database | J Pharmacol Exp Ther 301: 1097-102 (2002) Article DOI: 10.1124/jpet.301.3.1097 BindingDB Entry DOI: 10.7270/Q2ZW1JH0 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Rattus norvegicus (rat)) | BDBM50001885 ((risperidone)3-{2-[4-(6-Fluoro-benzo[d]isoxazol-3-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]ketanserin from rat cerebral cortex 5HT2A receptor measured after 15 mins by liquid scintillation counting method | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112709 BindingDB Entry DOI: 10.7270/Q2XK8K6M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (Homo sapiens (Human)) | BDBM50082840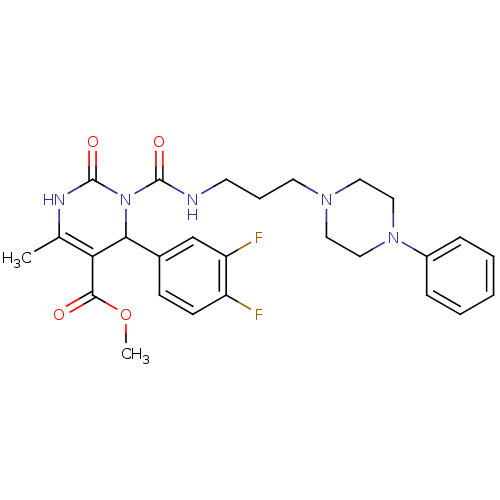 (4-(3,4-Difluoro-phenyl)-6-methyl-2-oxo-3-[3-(4-phe...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Synaptic Pharmaceutical Corporation Curated by ChEMBL | Assay Description In vitro binding affinity against Alpha-1A adrenergic receptor of human liver microsomes. | J Med Chem 42: 4794-803 (1999) BindingDB Entry DOI: 10.7270/Q25B01P0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (Homo sapiens (Human)) | BDBM50082861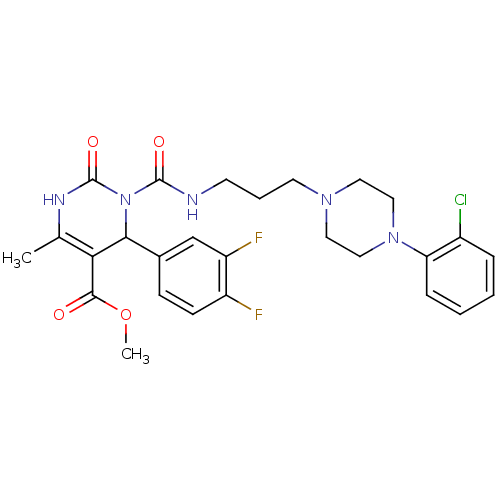 (3-{3-[4-(2-Chloro-phenyl)-piperazin-1-yl]-propylca...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Synaptic Pharmaceutical Corporation Curated by ChEMBL | Assay Description In vitro binding affinity against Alpha-1A adrenergic receptor of human liver microsomes. | J Med Chem 42: 4794-803 (1999) BindingDB Entry DOI: 10.7270/Q25B01P0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (Homo sapiens (Human)) | BDBM50082847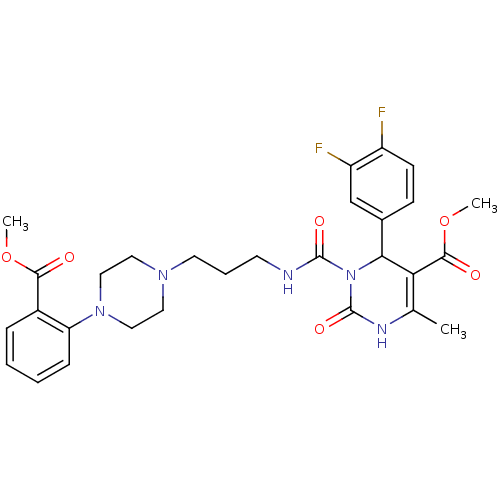 (4-(3,4-Difluoro-phenyl)-3-{3-[4-(2-methoxycarbonyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Synaptic Pharmaceutical Corporation Curated by ChEMBL | Assay Description In vitro binding affinity against Alpha-1A adrenergic receptor of human liver microsomes. | J Med Chem 42: 4794-803 (1999) BindingDB Entry DOI: 10.7270/Q25B01P0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (Homo sapiens (Human)) | BDBM50082784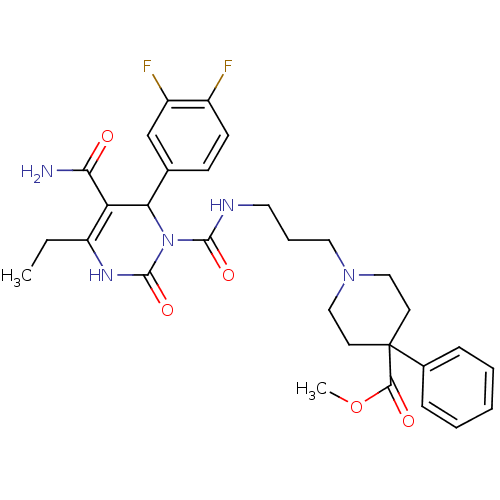 (1-(3-{[5-Carbamoyl-6-(3,4-difluoro-phenyl)-4-ethyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Synaptic Pharmaceutical Corporation Curated by ChEMBL | Assay Description In vitro binding affinity against Alpha-1A adrenergic receptor of human liver microsomes. | J Med Chem 42: 4794-803 (1999) BindingDB Entry DOI: 10.7270/Q25B01P0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (Homo sapiens (Human)) | BDBM50366618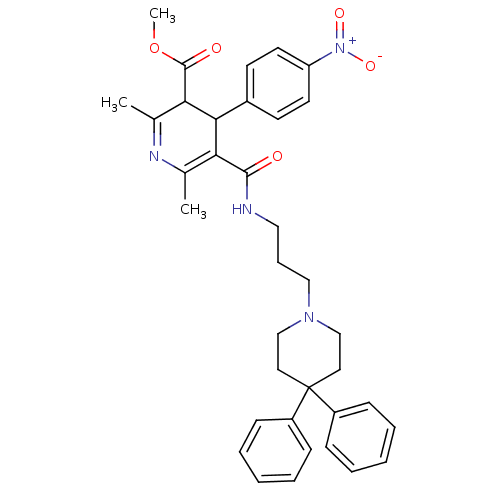 (CHEMBL448620 | SNAP-5089) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Synaptic Pharmaceutical Corporation Curated by ChEMBL | Assay Description Binding affinity against Alpha-1A adrenergic receptor (recombinant human receptor) using [3H]prazosin. | J Med Chem 42: 4804-13 (1999) BindingDB Entry DOI: 10.7270/Q2RJ4K5R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin D2 receptor 2 (Homo sapiens (Human)) | BDBM210759 (US9290454, 4.4) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Curated by ChEMBL | Assay Description Antagonist activity at CRTh2 (unknown origin) assessed as inhibition of CD11b activation | Bioorg Med Chem Lett 27: 5344-5348 (2017) Article DOI: 10.1016/j.bmcl.2017.07.064 BindingDB Entry DOI: 10.7270/Q2HX1G7W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-2/beta-2 (Rattus norvegicus (Rat)) | BDBM50210334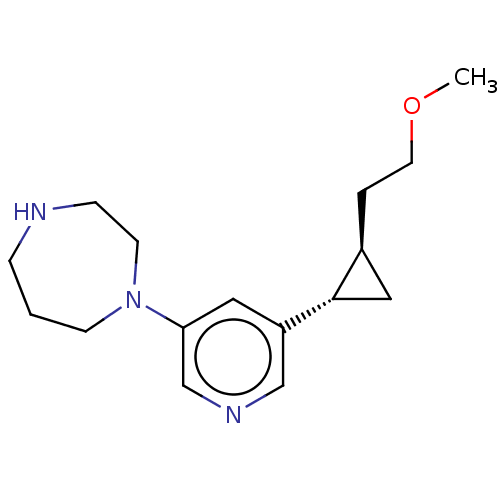 (CHEMBL3948977) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago Curated by ChEMBL | Assay Description Displacement of [3H]epibatidine from rat alpha2beta2 nAChR by liquid scintillation counting | Eur J Med Chem 124: 689-697 (2016) Article DOI: 10.1016/j.ejmech.2016.09.016 BindingDB Entry DOI: 10.7270/Q2H41TDB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-2/beta-2 (Rattus norvegicus (Rat)) | BDBM50210335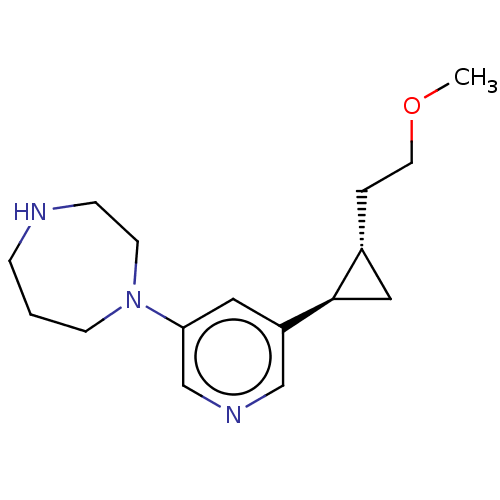 (CHEMBL3898434) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago Curated by ChEMBL | Assay Description Displacement of [3H]epibatidine from rat alpha2beta2 nAChR by liquid scintillation counting | Eur J Med Chem 124: 689-697 (2016) Article DOI: 10.1016/j.ejmech.2016.09.016 BindingDB Entry DOI: 10.7270/Q2H41TDB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (Homo sapiens (Human)) | BDBM50082859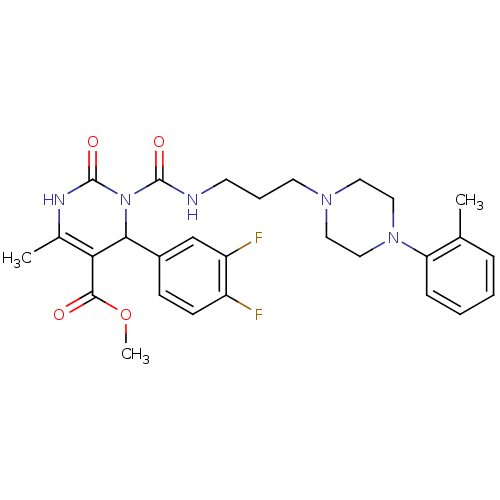 (4-(3,4-Difluoro-phenyl)-6-methyl-2-oxo-3-[3-(4-o-t...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Synaptic Pharmaceutical Corporation Curated by ChEMBL | Assay Description In vitro binding affinity against Alpha-1A adrenergic receptor of human liver microsomes. | J Med Chem 42: 4794-803 (1999) BindingDB Entry DOI: 10.7270/Q25B01P0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-2/beta-2 (Rattus norvegicus (Rat)) | BDBM50216347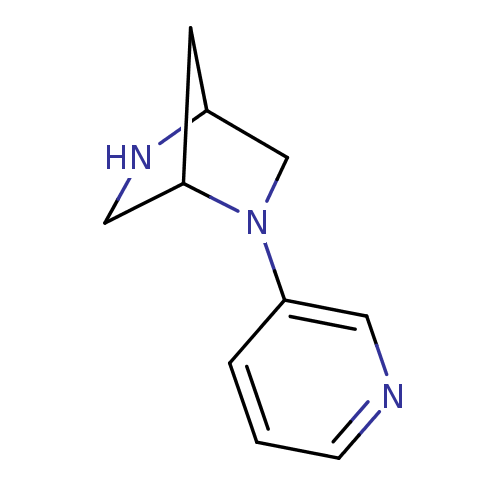 ((1R,4R)-2-(3-pyridinyl)-2,5-diazabicyclo[2.2.1]hep...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago Curated by ChEMBL | Assay Description Displacement of [3H]epibatidine from rat alpha2beta2 nAChR by liquid scintillation counting | Eur J Med Chem 124: 689-697 (2016) Article DOI: 10.1016/j.ejmech.2016.09.016 BindingDB Entry DOI: 10.7270/Q2H41TDB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (Homo sapiens (Human)) | BDBM50082872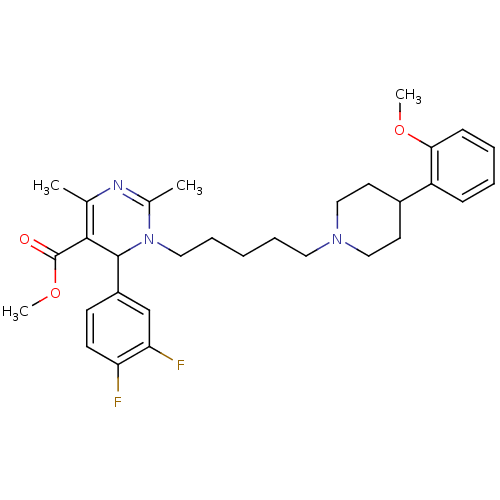 (6-(3,4-Difluoro-phenyl)-1-{5-[4-(2-methoxy-phenyl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Synaptic Pharmaceutical Corporation Curated by ChEMBL | Assay Description Binding affinity against Alpha-1A adrenergic receptor (recombinant human receptor) using [3H]prazosin. | J Med Chem 42: 4804-13 (1999) BindingDB Entry DOI: 10.7270/Q2RJ4K5R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM194780 (7-(4-(4-(1-benzothiophen-4-yl)piperazin-1-yl)butox...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid UniChem | DrugBank Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]raclopride from human D2 long receptor expressed in CHO cells measured after 60 mins | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112709 BindingDB Entry DOI: 10.7270/Q2XK8K6M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (Homo sapiens (Human)) | BDBM50082860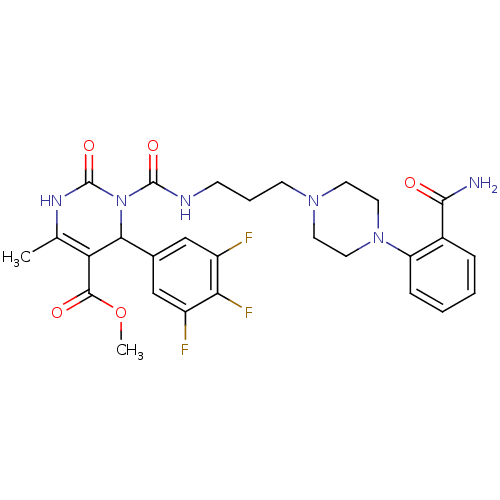 (3-{3-[4-(2-Carbamoyl-phenyl)-piperazin-1-yl]-propy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Synaptic Pharmaceutical Corporation Curated by ChEMBL | Assay Description In vitro binding affinity against Alpha-1A adrenergic receptor of human liver microsomes. | J Med Chem 42: 4794-803 (1999) BindingDB Entry DOI: 10.7270/Q25B01P0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (Homo sapiens (Human)) | BDBM50082865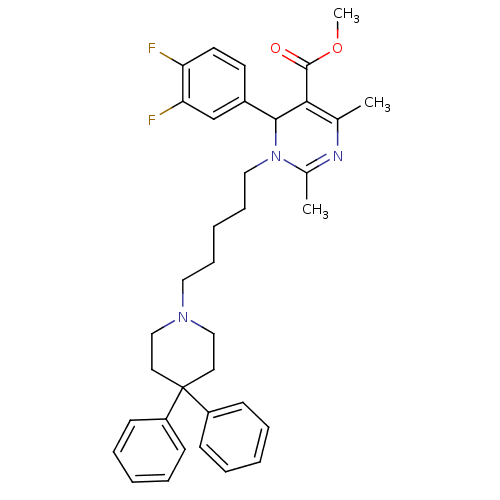 (6-(3,4-Difluoro-phenyl)-1-[5-(4,4-diphenyl-piperid...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Synaptic Pharmaceutical Corporation Curated by ChEMBL | Assay Description Binding affinity against isolated human prostate using [3H]- prazosin. | J Med Chem 42: 4804-13 (1999) BindingDB Entry DOI: 10.7270/Q2RJ4K5R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (Homo sapiens (Human)) | BDBM50082873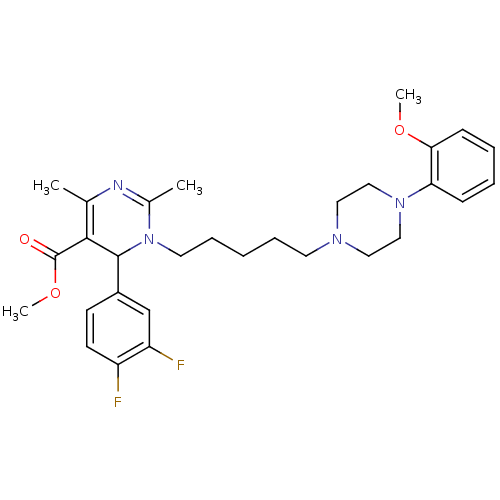 (6-(3,4-Difluoro-phenyl)-1-{5-[4-(2-methoxy-phenyl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Synaptic Pharmaceutical Corporation Curated by ChEMBL | Assay Description Binding affinity against Alpha-1A adrenergic receptor (recombinant human receptor) using [3H]prazosin. | J Med Chem 42: 4804-13 (1999) BindingDB Entry DOI: 10.7270/Q2RJ4K5R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (Homo sapiens (Human)) | BDBM50082875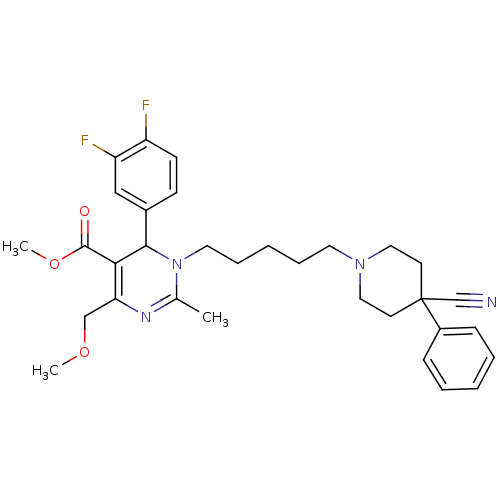 (1-[5-(4-Cyano-4-phenyl-piperidin-1-yl)-pentyl]-6-(...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Synaptic Pharmaceutical Corporation Curated by ChEMBL | Assay Description Binding affinity against Alpha-1A adrenergic receptor (recombinant human receptor) using [3H]prazosin. | J Med Chem 42: 4804-13 (1999) BindingDB Entry DOI: 10.7270/Q2RJ4K5R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (Homo sapiens (Human)) | BDBM50082865 (6-(3,4-Difluoro-phenyl)-1-[5-(4,4-diphenyl-piperid...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Synaptic Pharmaceutical Corporation Curated by ChEMBL | Assay Description Binding affinity against Alpha-1A adrenergic receptor (recombinant human receptor) using [3H]prazosin. | J Med Chem 42: 4804-13 (1999) BindingDB Entry DOI: 10.7270/Q2RJ4K5R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-4/beta-2 (Homo sapiens (Human)) | BDBM50166908 (5,8,14-triazatetracyclo[10.3.1.02,11.04,9]hexadeca...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago Curated by ChEMBL | Assay Description Binding affinity to alpha4beta2 nAChR | J Med Chem 55: 9998-10009 (2012) Article DOI: 10.1021/jm301177j BindingDB Entry DOI: 10.7270/Q2CJ8FM1 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Alpha-1A adrenergic receptor (Homo sapiens (Human)) | BDBM50082879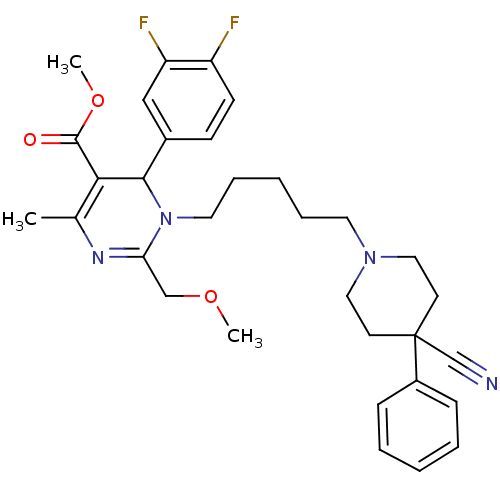 (1-[5-(4-Cyano-4-phenyl-piperidin-1-yl)-pentyl]-6-(...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Synaptic Pharmaceutical Corporation Curated by ChEMBL | Assay Description Binding affinity against Alpha-1A adrenergic receptor (recombinant human receptor) using [3H]prazosin. | J Med Chem 42: 4804-13 (1999) BindingDB Entry DOI: 10.7270/Q2RJ4K5R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin D2 receptor 2 (Homo sapiens (Human)) | BDBM210749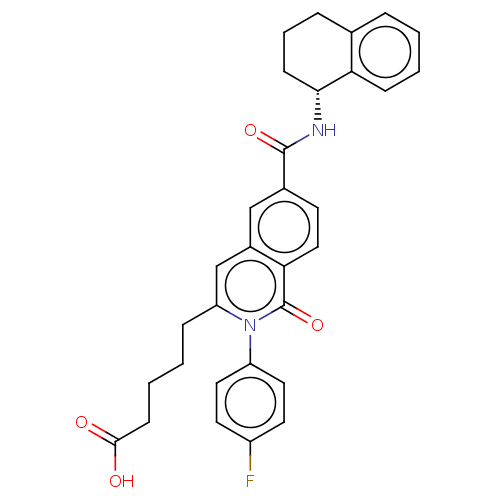 (US9290454, 3.1) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Curated by ChEMBL | Assay Description Antagonist activity at CRTh2 (unknown origin) assessed as inhibition of CD11b activation | Bioorg Med Chem Lett 27: 5344-5348 (2017) Article DOI: 10.1016/j.bmcl.2017.07.064 BindingDB Entry DOI: 10.7270/Q2HX1G7W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (Homo sapiens (Human)) | BDBM50082870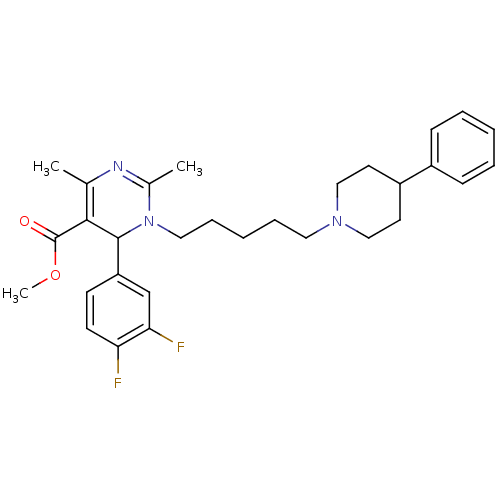 (6-(3,4-Difluoro-phenyl)-2,4-dimethyl-1-[5-(4-pheny...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Synaptic Pharmaceutical Corporation Curated by ChEMBL | Assay Description Binding affinity against Alpha-1A adrenergic receptor (recombinant human receptor) using [3H]prazosin. | J Med Chem 42: 4804-13 (1999) BindingDB Entry DOI: 10.7270/Q2RJ4K5R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (Homo sapiens (Human)) | BDBM50082862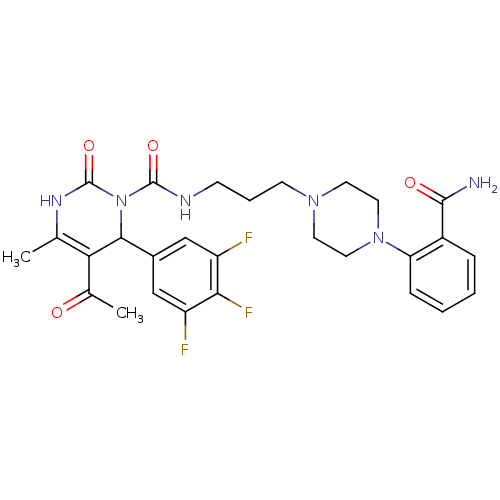 (5-Acetyl-4-methyl-2-oxo-6-(3,4,5-trifluoro-phenyl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Synaptic Pharmaceutical Corporation Curated by ChEMBL | Assay Description In vitro binding affinity against Alpha-1A adrenergic receptor of human liver microsomes. | J Med Chem 42: 4794-803 (1999) BindingDB Entry DOI: 10.7270/Q25B01P0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Norepinephrine transporter (RAT) | BDBM22417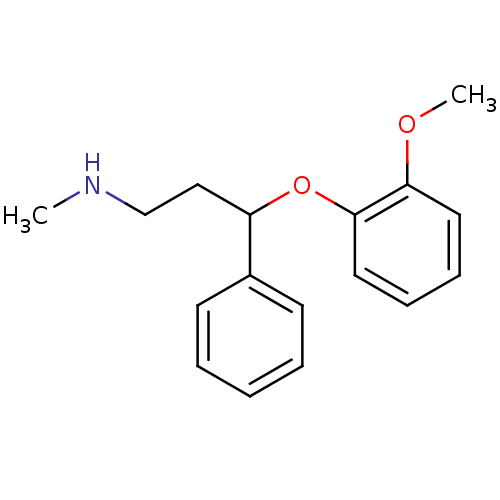 (3-(2-methoxyphenoxy)-N-methyl-3-phenylpropan-1-ami...) | UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.460 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard University Curated by PDSP Ki Database | J Pharmacol Exp Ther 301: 1097-102 (2002) Article DOI: 10.1124/jpet.301.3.1097 BindingDB Entry DOI: 10.7270/Q2ZW1JH0 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM194780 (7-(4-(4-(1-benzothiophen-4-yl)piperazin-1-yl)butox...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid UniChem | Article PubMed | 0.470 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]ketanserin from human 5HT2A receptor expressed in CHO-K1 cells measured after 20 mins | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112709 BindingDB Entry DOI: 10.7270/Q2XK8K6M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-4/beta-2 (Rattus norvegicus (Rat)) | BDBM50210335 (CHEMBL3898434) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago Curated by ChEMBL | Assay Description Displacement of [3H]epibatidine from rat alpha4beta2 nAChR by liquid scintillation counting | Eur J Med Chem 124: 689-697 (2016) Article DOI: 10.1016/j.ejmech.2016.09.016 BindingDB Entry DOI: 10.7270/Q2H41TDB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (Homo sapiens (Human)) | BDBM50082871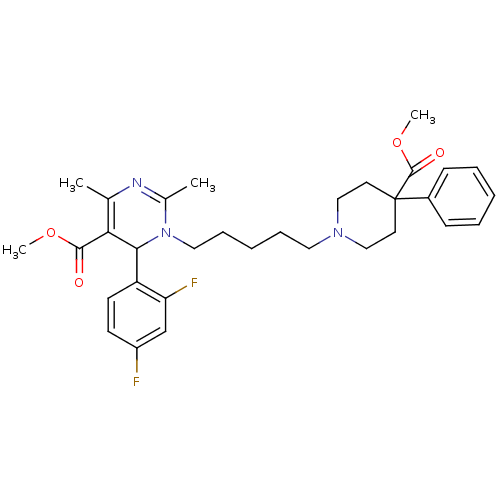 (6-(2,4-Difluoro-phenyl)-1-[5-(4-methoxycarbonyl-4-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Synaptic Pharmaceutical Corporation Curated by ChEMBL | Assay Description Binding affinity against Alpha-1A adrenergic receptor (recombinant human receptor) using [3H]prazosin. | J Med Chem 42: 4804-13 (1999) BindingDB Entry DOI: 10.7270/Q2RJ4K5R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (Homo sapiens (Human)) | BDBM50082863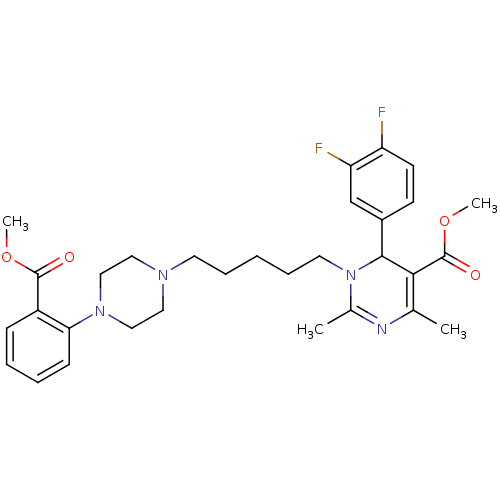 (6-(3,4-Difluoro-phenyl)-1-{5-[4-(2-methoxycarbonyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Synaptic Pharmaceutical Corporation Curated by ChEMBL | Assay Description Binding affinity against Alpha-1A adrenergic receptor (recombinant human receptor) using [3H]prazosin. | J Med Chem 42: 4804-13 (1999) BindingDB Entry DOI: 10.7270/Q2RJ4K5R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (Homo sapiens (Human)) | BDBM50084999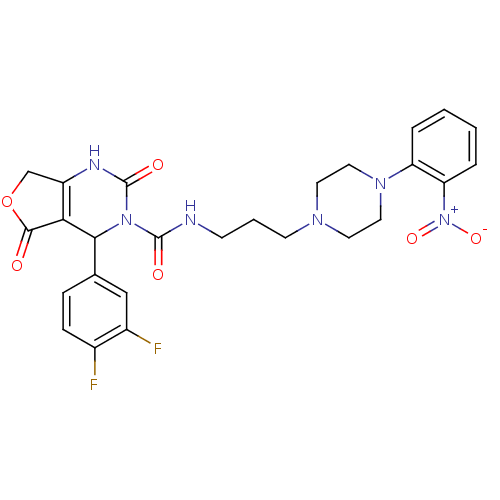 (4-(3,4-Difluoro-phenyl)-2,5-dioxo-1,2,5,7-tetrahyd...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Synaptic Pharmaceutical Corporation Curated by ChEMBL | Assay Description Displacement of [3H]- prazosin from human recombinant Alpha-1A adrenergic receptor | Bioorg Med Chem Lett 10: 175-8 (2000) BindingDB Entry DOI: 10.7270/Q2N29W5M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (Homo sapiens (Human)) | BDBM50082874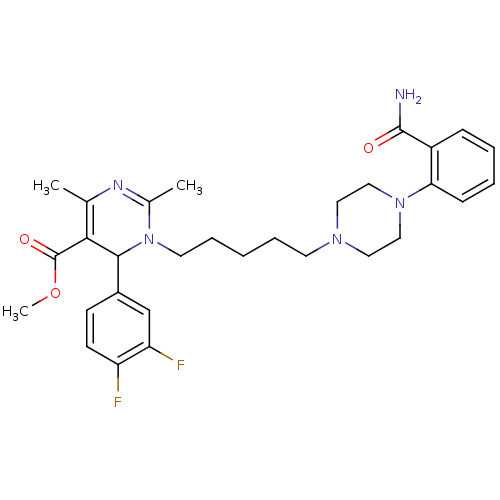 (1-{5-[4-(2-Carbamoyl-phenyl)-piperazin-1-yl]-penty...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Synaptic Pharmaceutical Corporation Curated by ChEMBL | Assay Description Binding affinity against Alpha-1A adrenergic receptor (recombinant human receptor) using [3H]prazosin. | J Med Chem 42: 4804-13 (1999) BindingDB Entry DOI: 10.7270/Q2RJ4K5R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (Homo sapiens (Human)) | BDBM50082843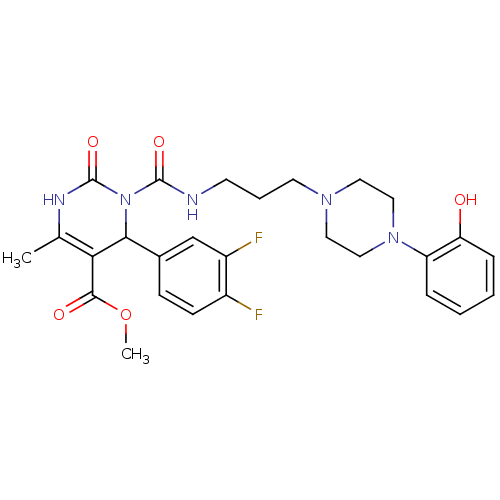 (4-(3,4-Difluoro-phenyl)-3-{3-[4-(2-hydroxy-phenyl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Synaptic Pharmaceutical Corporation Curated by ChEMBL | Assay Description In vitro binding affinity against Alpha-1A adrenergic receptor of human liver microsomes. | J Med Chem 42: 4794-803 (1999) BindingDB Entry DOI: 10.7270/Q25B01P0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 5313 total ) | Next | Last >> |