 Found 94 hits with Last Name = 'silverman' and Initial = 'kc'
Found 94 hits with Last Name = 'silverman' and Initial = 'kc' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50287709

((E)-3-Carboxy-2-(16-sulfooxy-hexadecyl)-pent-2-ene...)Show SMILES OC(=O)C\C(C(O)=O)=C(\CCCCCCCCCCCCCCCCOS(O)(=O)=O)C(O)=O Show InChI InChI=1S/C22H38O10S/c23-20(24)17-19(22(27)28)18(21(25)26)15-13-11-9-7-5-3-1-2-4-6-8-10-12-14-16-32-33(29,30)31/h1-17H2,(H,23,24)(H,25,26)(H,27,28)(H,29,30,31)/b19-18+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
| Article
| 4.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibition of human recombinant Protein farnesyltransferase with respect to FPP |
Bioorg Med Chem Lett 6: 2081-2084 (1996)
Article DOI: 10.1016/0960-894X(96)00372-1
BindingDB Entry DOI: 10.7270/Q2222TQ1 |
More data for this
Ligand-Target Pair | |
Squalene synthase
(Rattus norvegicus) | BDBM50292415

(CHEMBL504845 | Zaragozic Acid B)Show SMILES C\C=C\CCCC\C=C\CCCCC(=O)O[C@@H]1[C@@H](O)[C@]2(CCC(C)C(O)C(C)C\C=C\c3ccccc3)O[C@@]1(C(O)=O)[C@@](O)([C@H](O2)C(O)=O)C(O)=O |r| Show InChI InChI=1S/C39H54O13/c1-4-5-6-7-8-9-10-11-12-13-17-23-29(40)50-32-31(42)37(51-33(34(43)44)38(49,35(45)46)39(32,52-37)36(47)48)25-24-27(3)30(41)26(2)19-18-22-28-20-15-14-16-21-28/h4-5,10-11,14-16,18,20-22,26-27,30-33,41-42,49H,6-9,12-13,17,19,23-25H2,1-3H3,(H,43,44)(H,45,46)(H,47,48)/b5-4+,11-10+,22-18+/t26?,27?,30?,31-,32-,33-,37+,38-,39+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of rat liver squalene synthase by liqiud scintillation counting |
J Nat Prod 56: 1923-1929 (1993)
Article DOI: 10.1021/np50101a009
BindingDB Entry DOI: 10.7270/Q2M908Q1 |
More data for this
Ligand-Target Pair | |
Squalene synthase
(Rattus norvegicus) | BDBM50292333
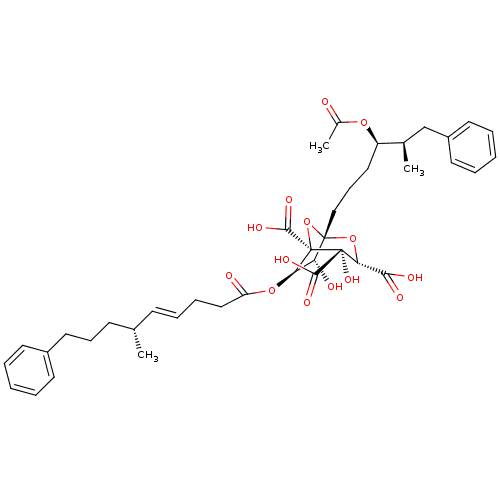
(CHEMBL505374 | zaragozic acid C)Show SMILES C[C@H](CCCc1ccccc1)\C=C\CCC(=O)O[C@@H]1[C@@H](O)[C@]2(CCC[C@@H](OC(C)=O)[C@H](C)Cc3ccccc3)O[C@@]1(C(O)=O)[C@@](O)([C@H](O2)C(O)=O)C(O)=O |r| Show InChI InChI=1S/C40H50O14/c1-25(15-12-20-28-16-6-4-7-17-28)14-10-11-22-31(42)52-33-32(43)38(53-34(35(44)45)39(50,36(46)47)40(33,54-38)37(48)49)23-13-21-30(51-27(3)41)26(2)24-29-18-8-5-9-19-29/h4-10,14,16-19,25-26,30,32-34,43,50H,11-13,15,20-24H2,1-3H3,(H,44,45)(H,46,47)(H,48,49)/b14-10+/t25-,26+,30+,32+,33+,34+,38-,39+,40-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of rat liver squalene synthase by liqiud scintillation counting |
J Nat Prod 56: 1923-1929 (1993)
Article DOI: 10.1021/np50101a009
BindingDB Entry DOI: 10.7270/Q2M908Q1 |
More data for this
Ligand-Target Pair | |
Squalene synthase
(Rattus norvegicus) | BDBM50051873
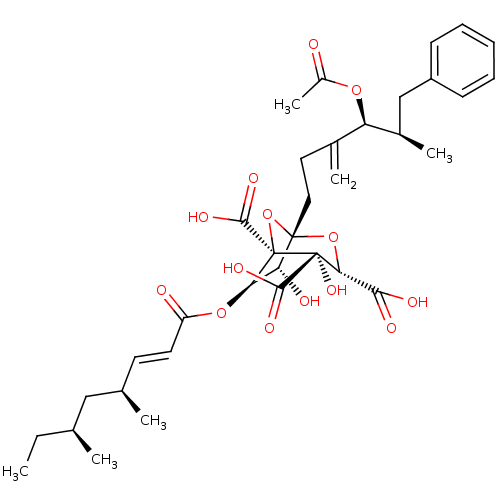
((1S,3S,4S,5R,6R,7R)-1-((4S,5R)-4-Acetoxy-5-methyl-...)Show SMILES CC[C@H](C)C[C@H](C)\C=C\C(=O)O[C@@H]1[C@@H](O)[C@]2(CCC(=C)[C@@H](OC(C)=O)[C@H](C)Cc3ccccc3)O[C@@]1(C(O)=O)[C@@](O)([C@H](O2)C(O)=O)C(O)=O |r| Show InChI InChI=1S/C35H46O14/c1-7-19(2)17-20(3)13-14-25(37)47-28-27(38)33(48-29(30(39)40)34(45,31(41)42)35(28,49-33)32(43)44)16-15-21(4)26(46-23(6)36)22(5)18-24-11-9-8-10-12-24/h8-14,19-20,22,26-29,38,45H,4,7,15-18H2,1-3,5-6H3,(H,39,40)(H,41,42)(H,43,44)/b14-13+/t19-,20+,22+,26+,27+,28+,29+,33-,34+,35-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of rat liver squalene synthase by liqiud scintillation counting |
J Nat Prod 56: 1923-1929 (1993)
Article DOI: 10.1021/np50101a009
BindingDB Entry DOI: 10.7270/Q2M908Q1 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Squalene synthase
(Rattus norvegicus) | BDBM50292413
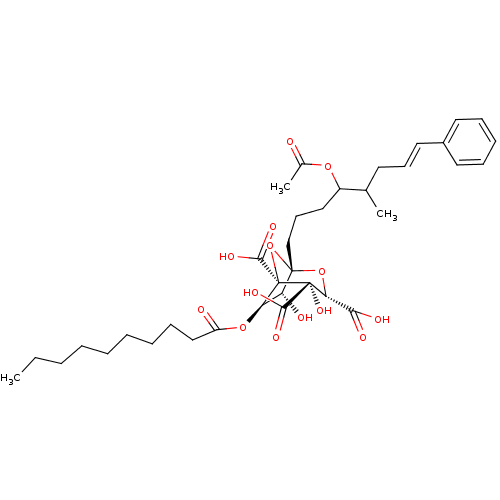
(CHEMBL502210 | Zaragozic Acid D2)Show SMILES CCCCCCCCCC(=O)O[C@@H]1[C@@H](O)[C@]2(CCCC(OC(C)=O)C(C)C\C=C\c3ccccc3)O[C@@]1(C(O)=O)[C@@](O)([C@H](O2)C(O)=O)C(O)=O |r| Show InChI InChI=1S/C36H50O14/c1-4-5-6-7-8-9-13-21-27(38)48-29-28(39)34(49-30(31(40)41)35(46,32(42)43)36(29,50-34)33(44)45)22-15-20-26(47-24(3)37)23(2)16-14-19-25-17-11-10-12-18-25/h10-12,14,17-19,23,26,28-30,39,46H,4-9,13,15-16,20-22H2,1-3H3,(H,40,41)(H,42,43)(H,44,45)/b19-14+/t23?,26?,28-,29-,30-,34+,35-,36+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of rat liver squalene synthase by liqiud scintillation counting |
J Nat Prod 56: 1923-1929 (1993)
Article DOI: 10.1021/np50101a009
BindingDB Entry DOI: 10.7270/Q2M908Q1 |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50287707
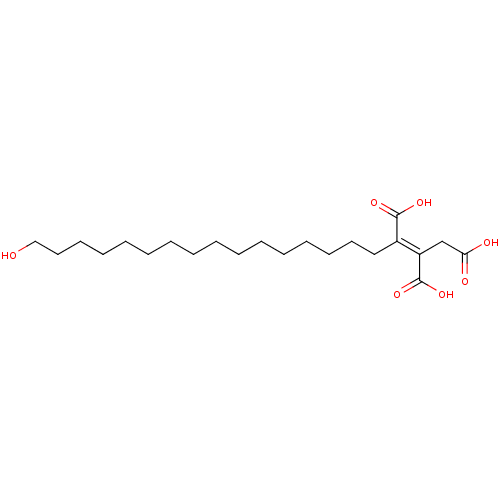
((E)-3-Carboxy-2-(16-hydroxy-hexadecyl)-pent-2-ened...)Show SMILES OCCCCCCCCCCCCCCCC\C(C(O)=O)=C(\CC(O)=O)C(O)=O Show InChI InChI=1S/C22H38O7/c23-16-14-12-10-8-6-4-2-1-3-5-7-9-11-13-15-18(21(26)27)19(22(28)29)17-20(24)25/h23H,1-17H2,(H,24,25)(H,26,27)(H,28,29)/b19-18+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
| n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibition of human recombinant Protein farnesyltransferase |
Bioorg Med Chem Lett 6: 2081-2084 (1996)
Article DOI: 10.1016/0960-894X(96)00372-1
BindingDB Entry DOI: 10.7270/Q2222TQ1 |
More data for this
Ligand-Target Pair | |
Squalene synthase
(Rattus norvegicus) | BDBM50292414
(CHEMBL502872 | Zaragozic Acid D)Show SMILES CCCCCCCC(=O)O[C@@H]1[C@@H](O)[C@]2(CCCC(OC(C)=O)C(C)C\C=C\c3ccccc3)O[C@@]1(C(O)=O)[C@@](O)([C@H](O2)C(O)=O)C(O)=O |r| Show InChI InChI=1S/C34H46O14/c1-4-5-6-7-11-19-25(36)46-27-26(37)32(47-28(29(38)39)33(44,30(40)41)34(27,48-32)31(42)43)20-13-18-24(45-22(3)35)21(2)14-12-17-23-15-9-8-10-16-23/h8-10,12,15-17,21,24,26-28,37,44H,4-7,11,13-14,18-20H2,1-3H3,(H,38,39)(H,40,41)(H,42,43)/b17-12+/t21?,24?,26-,27-,28-,32+,33-,34+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of rat liver squalene synthase by liqiud scintillation counting |
J Nat Prod 56: 1923-1929 (1993)
Article DOI: 10.1021/np50101a009
BindingDB Entry DOI: 10.7270/Q2M908Q1 |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50287708
((E)-3-Methoxycarbonyl-2-(16-sulfooxy-hexadecyl)-pe...)Show SMILES COC(=O)C\C(C(=O)OC)=C(\CCCCCCCCCCCCCCCCOS(O)(=O)=O)C(=O)OC Show InChI InChI=1S/C25H44O10S/c1-32-23(26)20-22(25(28)34-3)21(24(27)33-2)18-16-14-12-10-8-6-4-5-7-9-11-13-15-17-19-35-36(29,30)31/h4-20H2,1-3H3,(H,29,30,31)/b22-21+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
| Article
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibition of human recombinant Protein farnesyltransferase |
Bioorg Med Chem Lett 6: 2081-2084 (1996)
Article DOI: 10.1016/0960-894X(96)00372-1
BindingDB Entry DOI: 10.7270/Q2222TQ1 |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50287709

((E)-3-Carboxy-2-(16-sulfooxy-hexadecyl)-pent-2-ene...)Show SMILES OC(=O)C\C(C(O)=O)=C(\CCCCCCCCCCCCCCCCOS(O)(=O)=O)C(O)=O Show InChI InChI=1S/C22H38O10S/c23-20(24)17-19(22(27)28)18(21(25)26)15-13-11-9-7-5-3-1-2-4-6-8-10-12-14-16-32-33(29,30)31/h1-17H2,(H,23,24)(H,25,26)(H,27,28)(H,29,30,31)/b19-18+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
| Article
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibition of human recombinant Protein farnesyltransferase |
Bioorg Med Chem Lett 6: 2081-2084 (1996)
Article DOI: 10.1016/0960-894X(96)00372-1
BindingDB Entry DOI: 10.7270/Q2222TQ1 |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50067848
((S)-2-(((3aR,6R,9S)-3a,6,9-trimethyl-3-(6-methylhe...)Show SMILES CC(C)CCCC(C)C1CCC2C3[C@@H](C)CC(=O)[C@](C)(C[C@@H](CC(O)=O)C(O)=O)C3CC[C@]12C Show InChI InChI=1S/C29H48O5/c1-17(2)8-7-9-18(3)21-10-11-22-26-19(4)14-24(30)29(6,23(26)12-13-28(21,22)5)16-20(27(33)34)15-25(31)32/h17-23,26H,7-16H2,1-6H3,(H,31,32)(H,33,34)/t18?,19-,20+,21?,22?,23?,26?,28+,29+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against human Farnesyltransferase |
J Med Chem 41: 4492-501 (1998)
Article DOI: 10.1021/jm980356+
BindingDB Entry DOI: 10.7270/Q23779DQ |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50067843
((S)-2-[(3aR,6R)-3-(1,5-Dimethyl-hexyl)-3a,6-dimeth...)Show SMILES CC(C)CCCC(C)C1CCC2C3CCC(=O)[C@](C)(C[C@@H](CC(O)=O)C(O)=O)C3CC[C@]12C Show InChI InChI=1S/C28H46O5/c1-17(2)7-6-8-18(3)21-10-11-22-20-9-12-24(29)28(5,23(20)13-14-27(21,22)4)16-19(26(32)33)15-25(30)31/h17-23H,6-16H2,1-5H3,(H,30,31)(H,32,33)/t18?,19-,20?,21?,22?,23?,27-,28-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human Farnesyltransferase |
J Med Chem 41: 4492-501 (1998)
Article DOI: 10.1021/jm980356+
BindingDB Entry DOI: 10.7270/Q23779DQ |
More data for this
Ligand-Target Pair | |
Integrase
(Human immunodeficiency virus 1) | BDBM50123449
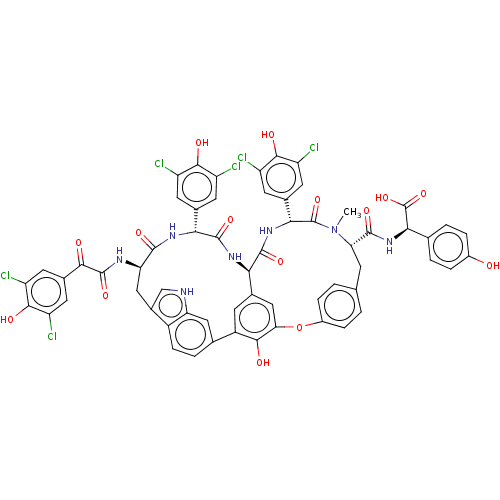
(CHEMBL437996 | Chloropeptin II | ISOCOMPLESTATIN)Show SMILES [H][C@@]12NC(=O)[C@H](NC(=O)[C@@H](Cc3c[nH]c4cc(ccc34)-c3cc1cc(Oc1ccc(C[C@H](N(C)C(=O)[C@H](NC2=O)c2cc(Cl)c(O)c(Cl)c2)C(=O)N[C@@H](C(O)=O)c2ccc(O)cc2)cc1)c3O)NC(=O)C(=O)c1cc(Cl)c(O)c(Cl)c1)c1cc(Cl)c(O)c(Cl)c1 |r| Show InChI InChI=1S/C61H45Cl6N7O15/c1-74-44(56(82)73-49(61(87)88)25-4-7-32(75)8-5-25)12-24-2-9-33(10-3-24)89-45-22-27-13-35(51(45)77)26-6-11-34-31(23-68-42(34)20-26)21-43(69-59(85)50(76)30-18-40(66)54(80)41(67)19-30)55(81)70-47(28-14-36(62)52(78)37(63)15-28)57(83)71-46(27)58(84)72-48(60(74)86)29-16-38(64)53(79)39(65)17-29/h2-11,13-20,22-23,43-44,46-49,68,75,77-80H,12,21H2,1H3,(H,69,85)(H,70,81)(H,71,83)(H,72,84)(H,73,82)(H,87,88) | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 integrase 3' processing/strand transfer coupled activity |
J Nat Prod 64: 874-82 (2001)
Article DOI: 10.1021/np000632z
BindingDB Entry DOI: 10.7270/Q22N552N |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50250718

(2-alpha-(3'-hydroxy-3'-methylglutaroyl)-24,25-dihy...)Show SMILES C[C@H](CCC(O)C(C)(C)O)[C@H]1CC[C@@]2(C)C3=C(CC[C@]12C)[C@@]1(C)C[C@@H](OC(=O)CC(C)(O)CC(O)=O)C(=O)C(C)(C)[C@@H]1CC3 |r,c:15| Show InChI InChI=1S/C36H58O8/c1-21(10-13-27(37)32(4,5)42)22-14-16-36(9)24-11-12-26-31(2,3)30(41)25(44-29(40)20-33(6,43)19-28(38)39)18-34(26,7)23(24)15-17-35(22,36)8/h21-22,25-27,37,42-43H,10-20H2,1-9H3,(H,38,39)/t21-,22-,25-,26+,27?,33?,34-,35-,36+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 350 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human Farnesyltransferase using Ras-CVLS |
J Med Chem 41: 4492-501 (1998)
Article DOI: 10.1021/jm980356+
BindingDB Entry DOI: 10.7270/Q23779DQ |
More data for this
Ligand-Target Pair | |
Integrase
(Human immunodeficiency virus 1) | BDBM50366855
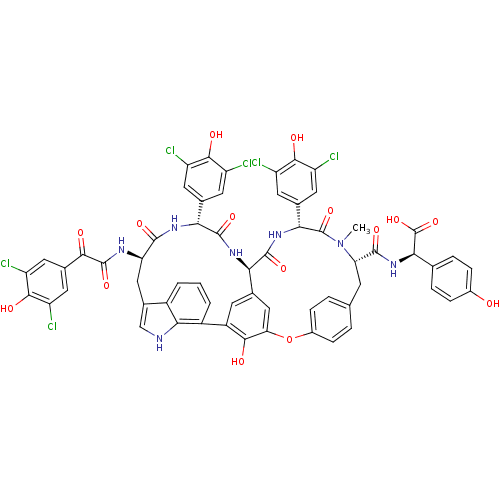
(CHEMBL525803 | Chloropeptin)Show SMILES CN1[C@@H](Cc2ccc(Oc3cc4cc(c3O)-c3cccc5c(C[C@@H](NC(=O)C(=O)c6cc(Cl)c(O)c(Cl)c6)C(=O)N[C@H](c6cc(Cl)c(O)c(Cl)c6)C(=O)N[C@H]4C(=O)N[C@H](c4cc(Cl)c(O)c(Cl)c4)C1=O)c[nH]c35)cc2)C(=O)N[C@@H](C(O)=O)c1ccc(O)cc1 |r| Show InChI InChI=1S/C61H45Cl6N7O15/c1-74-43(56(82)73-48(61(87)88)25-7-9-31(75)10-8-25)13-24-5-11-32(12-6-24)89-44-22-26-14-35(51(44)77)34-4-2-3-33-30(23-68-49(33)34)21-42(69-59(85)50(76)29-19-40(66)54(80)41(67)20-29)55(81)70-46(27-15-36(62)52(78)37(63)16-27)57(83)71-45(26)58(84)72-47(60(74)86)28-17-38(64)53(79)39(65)18-28/h2-12,14-20,22-23,42-43,45-48,68,75,77-80H,13,21H2,1H3,(H,69,85)(H,70,81)(H,71,83)(H,72,84)(H,73,82)(H,87,88)/t42-,43+,45-,46-,47-,48-/m1/s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 integrase 3' processing/strand transfer coupled activity |
J Nat Prod 64: 874-82 (2001)
Article DOI: 10.1021/np000632z
BindingDB Entry DOI: 10.7270/Q22N552N |
More data for this
Ligand-Target Pair | |
Integrase
(Human immunodeficiency virus 1) | BDBM50123449

(CHEMBL437996 | Chloropeptin II | ISOCOMPLESTATIN)Show SMILES [H][C@@]12NC(=O)[C@H](NC(=O)[C@@H](Cc3c[nH]c4cc(ccc34)-c3cc1cc(Oc1ccc(C[C@H](N(C)C(=O)[C@H](NC2=O)c2cc(Cl)c(O)c(Cl)c2)C(=O)N[C@@H](C(O)=O)c2ccc(O)cc2)cc1)c3O)NC(=O)C(=O)c1cc(Cl)c(O)c(Cl)c1)c1cc(Cl)c(O)c(Cl)c1 |r| Show InChI InChI=1S/C61H45Cl6N7O15/c1-74-44(56(82)73-49(61(87)88)25-4-7-32(75)8-5-25)12-24-2-9-33(10-3-24)89-45-22-27-13-35(51(45)77)26-6-11-34-31(23-68-42(34)20-26)21-43(69-59(85)50(76)30-18-40(66)54(80)41(67)19-30)55(81)70-47(28-14-36(62)52(78)37(63)15-28)57(83)71-46(27)58(84)72-48(60(74)86)29-16-38(64)53(79)39(65)17-29/h2-11,13-20,22-23,43-44,46-49,68,75,77-80H,12,21H2,1H3,(H,69,85)(H,70,81)(H,71,83)(H,72,84)(H,73,82)(H,87,88) | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of disintegration activity of HIV1 integrase catalytic core domain (50 to 212) |
J Nat Prod 64: 874-82 (2001)
Article DOI: 10.1021/np000632z
BindingDB Entry DOI: 10.7270/Q22N552N |
More data for this
Ligand-Target Pair | |
Integrase
(Human immunodeficiency virus 1) | BDBM50123449

(CHEMBL437996 | Chloropeptin II | ISOCOMPLESTATIN)Show SMILES [H][C@@]12NC(=O)[C@H](NC(=O)[C@@H](Cc3c[nH]c4cc(ccc34)-c3cc1cc(Oc1ccc(C[C@H](N(C)C(=O)[C@H](NC2=O)c2cc(Cl)c(O)c(Cl)c2)C(=O)N[C@@H](C(O)=O)c2ccc(O)cc2)cc1)c3O)NC(=O)C(=O)c1cc(Cl)c(O)c(Cl)c1)c1cc(Cl)c(O)c(Cl)c1 |r| Show InChI InChI=1S/C61H45Cl6N7O15/c1-74-44(56(82)73-49(61(87)88)25-4-7-32(75)8-5-25)12-24-2-9-33(10-3-24)89-45-22-27-13-35(51(45)77)26-6-11-34-31(23-68-42(34)20-26)21-43(69-59(85)50(76)30-18-40(66)54(80)41(67)19-30)55(81)70-47(28-14-36(62)52(78)37(63)15-28)57(83)71-46(27)58(84)72-48(60(74)86)29-16-38(64)53(79)39(65)17-29/h2-11,13-20,22-23,43-44,46-49,68,75,77-80H,12,21H2,1H3,(H,69,85)(H,70,81)(H,71,83)(H,72,84)(H,73,82)(H,87,88) | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of disintegration activity of HIV1 intact integrase |
J Nat Prod 64: 874-82 (2001)
Article DOI: 10.1021/np000632z
BindingDB Entry DOI: 10.7270/Q22N552N |
More data for this
Ligand-Target Pair | |
Integrase
(Human immunodeficiency virus 1) | BDBM50478734
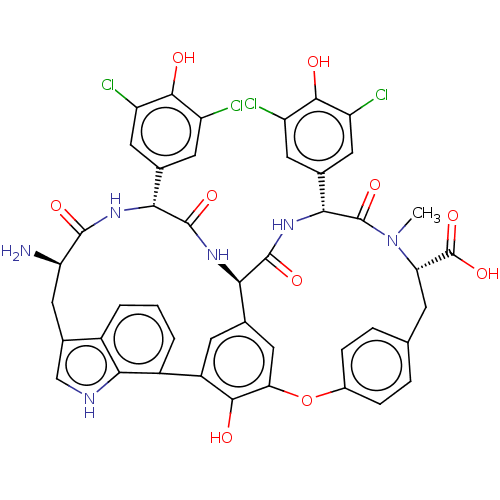
(CHEMBL448564)Show SMILES [H][C@@]12NC(=O)[C@H](NC(=O)[C@H](N)Cc3c[nH]c4c(cccc34)-c3cc1cc(Oc1ccc(C[C@H](N(C)C(=O)[C@H](NC2=O)c2cc(Cl)c(O)c(Cl)c2)C(O)=O)cc1)c3O)c1cc(Cl)c(O)c(Cl)c1 |r| Show InChI InChI=1S/C45H36Cl4N6O10/c1-55-32(45(63)64)9-18-5-7-23(8-6-18)65-33-16-19-10-26(38(33)56)25-4-2-3-24-22(17-51-37(24)25)15-31(50)41(59)52-35(20-11-27(46)39(57)28(47)12-20)42(60)53-34(19)43(61)54-36(44(55)62)21-13-29(48)40(58)30(49)14-21/h2-8,10-14,16-17,31-32,34-36,51,56-58H,9,15,50H2,1H3,(H,52,59)(H,53,60)(H,54,61)(H,63,64)/t31-,32+,34-,35-,36-/m1/s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 integrase 3' processing/strand transfer coupled activity |
J Nat Prod 64: 874-82 (2001)
Article DOI: 10.1021/np000632z
BindingDB Entry DOI: 10.7270/Q22N552N |
More data for this
Ligand-Target Pair | |
Integrase
(Human immunodeficiency virus 1) | BDBM50478738
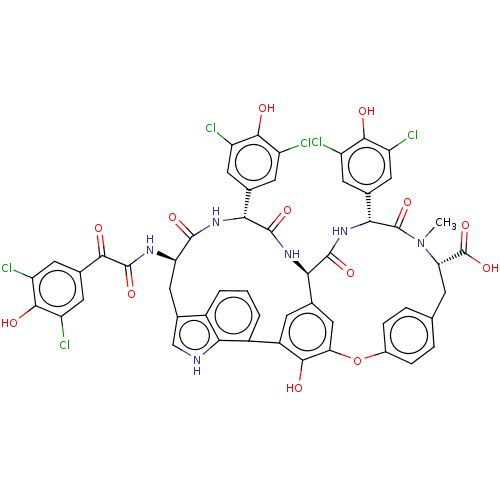
(CHEMBL507121)Show SMILES [H][C@@]12NC(=O)[C@H](NC(=O)[C@@H](Cc3c[nH]c4c(cccc34)-c3cc1cc(Oc1ccc(C[C@H](N(C)C(=O)[C@H](NC2=O)c2cc(Cl)c(O)c(Cl)c2)C(O)=O)cc1)c3O)NC(=O)C(=O)c1cc(Cl)c(O)c(Cl)c1)c1cc(Cl)c(O)c(Cl)c1 |r| Show InChI InChI=1S/C53H38Cl6N6O13/c1-65-37(53(76)77)9-20-5-7-26(8-6-20)78-38-18-21-10-29(44(38)67)28-4-2-3-27-25(19-60-42(27)28)17-36(61-51(74)43(66)24-15-34(58)47(70)35(59)16-24)48(71)62-40(22-11-30(54)45(68)31(55)12-22)49(72)63-39(21)50(73)64-41(52(65)75)23-13-32(56)46(69)33(57)14-23/h2-8,10-16,18-19,36-37,39-41,60,67-70H,9,17H2,1H3,(H,61,74)(H,62,71)(H,63,72)(H,64,73)(H,76,77)/t36-,37+,39-,40-,41-/m1/s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 integrase 3' processing/strand transfer coupled activity |
J Nat Prod 64: 874-82 (2001)
Article DOI: 10.1021/np000632z
BindingDB Entry DOI: 10.7270/Q22N552N |
More data for this
Ligand-Target Pair | |
Integrase
(Human immunodeficiency virus 1) | BDBM50478735
(CHEBI:65655 | COMPLESTATINS A)Show SMILES [H][C@@]12NC(=O)[C@H](NC(=O)[C@@H](CC3C(=O)Nc4cc(ccc34)-c3cc1cc(Oc1ccc(C[C@H](N(C)C(=O)[C@H](NC2=O)c2cc(Cl)c(O)c(Cl)c2)C(=O)N[C@@H](C(O)=O)c2ccc(O)cc2)cc1)c3O)NC(=O)C(=O)c1cc(Cl)c(O)c(Cl)c1)c1cc(Cl)c(O)c(Cl)c1 |r| Show InChI InChI=1S/C61H45Cl6N7O16/c1-74-43(56(83)73-48(61(88)89)24-4-7-30(75)8-5-24)12-23-2-9-31(10-3-23)90-44-21-26-13-33(50(44)77)25-6-11-32-34(54(81)68-41(32)20-25)22-42(69-59(86)49(76)29-18-39(66)53(80)40(67)19-29)55(82)70-46(27-14-35(62)51(78)36(63)15-27)57(84)71-45(26)58(85)72-47(60(74)87)28-16-37(64)52(79)38(65)17-28/h2-11,13-21,34,42-43,45-48,75,77-80H,12,22H2,1H3,(H,68,81)(H,69,86)(H,70,82)(H,71,84)(H,72,85)(H,73,83)(H,88,89)/t34?,42-,43+,45-,46-,47-,48-/m1/s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 integrase 3' processing/strand transfer coupled activity |
J Nat Prod 64: 874-82 (2001)
Article DOI: 10.1021/np000632z
BindingDB Entry DOI: 10.7270/Q22N552N |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50067852
(3-[(3aR,6R,9S)-3-(1,5-Dimethyl-hexyl)-3a,6,9-trime...)Show SMILES CC(C)CCCC(C)C1CCC2C3[C@@H](C)CC(=O)[C@](C)(CCC(O)=O)C3CC[C@]12C Show InChI InChI=1S/C27H46O3/c1-17(2)8-7-9-18(3)20-10-11-21-25-19(4)16-23(28)27(6,15-13-24(29)30)22(25)12-14-26(20,21)5/h17-22,25H,7-16H2,1-6H3,(H,29,30)/t18?,19-,20?,21?,22?,25?,26+,27+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 900 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against human Farnesyltransferase |
J Med Chem 41: 4492-501 (1998)
Article DOI: 10.1021/jm980356+
BindingDB Entry DOI: 10.7270/Q23779DQ |
More data for this
Ligand-Target Pair | |
Integrase
(Human immunodeficiency virus 1) | BDBM50124971

((2R,3S)-5,6,8,5',6',8'-Hexahydroxy-2,3,2',3'-tetra...)Show SMILES C[C@H]1Oc2cc3c(c(O)cc(O)c3c(O)c2C(=O)[C@H]1C)-c1c(O)cc(O)c2c(O)c3c(cc12)oc(C)c(C)c3=O |wU:18.21,1.0,(7.61,-9.78,;6.28,-9.01,;4.93,-9.81,;3.6,-9.04,;2.27,-9.81,;.93,-9.04,;-.41,-9.83,;-1.74,-9.06,;-3.07,-9.81,;-1.74,-7.52,;-.41,-6.73,;-.41,-5.21,;.93,-7.5,;2.25,-6.73,;2.25,-5.19,;3.59,-7.5,;4.92,-6.7,;4.91,-5.16,;6.26,-7.47,;7.59,-6.68,;-.4,-11.35,;-1.74,-12.12,;-3.07,-11.37,;-1.73,-13.66,;-.39,-14.43,;-.39,-15.97,;.94,-13.66,;2.27,-14.4,;2.27,-15.94,;3.58,-13.63,;3.58,-12.09,;2.25,-11.35,;.93,-12.12,;4.88,-11.32,;6.23,-12.07,;7.54,-11.3,;6.24,-13.61,;7.58,-14.38,;4.91,-14.4,;4.91,-15.94,)| Show InChI InChI=1S/C30H24O10/c1-9-11(3)39-19-5-13-21(15(31)7-17(33)23(13)29(37)25(19)27(9)35)22-14-6-20-26(28(36)10(2)12(4)40-20)30(38)24(14)18(34)8-16(22)32/h5-9,11,31-34,37-38H,1-4H3/t9-,11+/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of HIV-1 integrase in coupled transfer assay |
Bioorg Med Chem Lett 13: 713-7 (2003)
BindingDB Entry DOI: 10.7270/Q22R3R1H |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50067842
(2'',15''-dimethyl-5'',17''-dioxo-(2''R,5'R,15''S)-...)Show SMILES C[C@]12CC(=O)C3C(CCC4=CC(=O)C(C=O)=C[C@]34C)C1CC[C@@]21OCOC11COCO1 |c:15,t:9| Show InChI InChI=1S/C24H28O7/c1-21-8-14(10-25)18(26)7-15(21)3-4-16-17-5-6-23(22(17,2)9-19(27)20(16)21)24(31-13-29-23)11-28-12-30-24/h7-8,10,16-17,20H,3-6,9,11-13H2,1-2H3/t16?,17?,20?,21-,22-,23+,24?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
inhibitory activity against human Farnesyltransferase |
J Med Chem 41: 4492-501 (1998)
Article DOI: 10.1021/jm980356+
BindingDB Entry DOI: 10.7270/Q23779DQ |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50067825
(3-[(R)-3-(1,5-Dimethyl-hexyl)-3a-methyl-7-oxo-dode...)Show SMILES CC(C)CCCC(C)C1CCC2C3CCC(=O)C(CCC(O)=O)C3CC[C@]12C Show InChI InChI=1S/C25H42O3/c1-16(2)6-5-7-17(3)21-10-11-22-19-8-12-23(26)20(9-13-24(27)28)18(19)14-15-25(21,22)4/h16-22H,5-15H2,1-4H3,(H,27,28)/t17?,18?,19?,20?,21?,22?,25-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against human Farnesyltransferase |
J Med Chem 41: 4492-501 (1998)
Article DOI: 10.1021/jm980356+
BindingDB Entry DOI: 10.7270/Q23779DQ |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50250718

(2-alpha-(3'-hydroxy-3'-methylglutaroyl)-24,25-dihy...)Show SMILES C[C@H](CCC(O)C(C)(C)O)[C@H]1CC[C@@]2(C)C3=C(CC[C@]12C)[C@@]1(C)C[C@@H](OC(=O)CC(C)(O)CC(O)=O)C(=O)C(C)(C)[C@@H]1CC3 |r,c:15| Show InChI InChI=1S/C36H58O8/c1-21(10-13-27(37)32(4,5)42)22-14-16-36(9)24-11-12-26-31(2,3)30(41)25(44-29(40)20-33(6,43)19-28(38)39)18-34(26,7)23(24)15-17-35(22,36)8/h21-22,25-27,37,42-43H,10-20H2,1-9H3,(H,38,39)/t21-,22-,25-,26+,27?,33?,34-,35-,36+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human Farnesyltransferase using Ras-CVIM |
J Med Chem 41: 4492-501 (1998)
Article DOI: 10.1021/jm980356+
BindingDB Entry DOI: 10.7270/Q23779DQ |
More data for this
Ligand-Target Pair | |
Integrase
(Human immunodeficiency virus 1) | BDBM50478740
(CHEBI:65656 | COMPLESTATIN B)Show SMILES [H][C@@]12NC(=O)[C@H](NC(=O)[C@@H](CC3(O)C(=O)Nc4cc(ccc34)-c3cc1cc(Oc1ccc(C[C@H](N(C)C(=O)[C@H](NC2=O)c2cc(Cl)c(O)c(Cl)c2)C(=O)N[C@@H](C(O)=O)c2ccc(O)cc2)cc1)c3O)NC(=O)C(=O)c1cc(Cl)c(O)c(Cl)c1)c1cc(Cl)c(O)c(Cl)c1 |r| Show InChI InChI=1S/C61H45Cl6N7O17/c1-74-42(54(82)73-47(59(87)88)24-4-7-30(75)8-5-24)12-23-2-9-31(10-3-23)91-43-21-26-13-32(49(43)77)25-6-11-33-40(20-25)69-60(89)61(33,90)22-41(68-57(85)48(76)29-18-38(66)52(80)39(67)19-29)53(81)70-45(27-14-34(62)50(78)35(63)15-27)55(83)71-44(26)56(84)72-46(58(74)86)28-16-36(64)51(79)37(65)17-28/h2-11,13-21,41-42,44-47,75,77-80,90H,12,22H2,1H3,(H,68,85)(H,69,89)(H,70,81)(H,71,83)(H,72,84)(H,73,82)(H,87,88)/t41-,42+,44-,45-,46-,47-,61?/m1/s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 integrase 3' processing/strand transfer coupled activity |
J Nat Prod 64: 874-82 (2001)
Article DOI: 10.1021/np000632z
BindingDB Entry DOI: 10.7270/Q22N552N |
More data for this
Ligand-Target Pair | |
Integrase
(Human immunodeficiency virus 1) | BDBM50124967

(5,6,8,5',6',8'-Hexahydroxy-2,3,2',3'-tetramethyl-2...)Show SMILES CC1Oc2cc3c(c(O)cc(O)c3c(O)c2C(=O)C1C)-c1c(O)cc(O)c2c(O)c3C(=O)C(C)C(C)Oc3cc12 |(7.61,-9.78,;6.28,-9.01,;4.93,-9.81,;3.6,-9.04,;2.27,-9.81,;.93,-9.04,;-.41,-9.83,;-1.74,-9.06,;-3.07,-9.81,;-1.74,-7.52,;-.41,-6.73,;-.41,-5.21,;.93,-7.5,;2.25,-6.73,;2.25,-5.19,;3.59,-7.5,;4.92,-6.7,;4.91,-5.16,;6.26,-7.47,;7.59,-6.68,;-.4,-11.35,;-1.74,-12.12,;-3.07,-11.37,;-1.73,-13.66,;-.39,-14.43,;-.39,-15.97,;.94,-13.65,;2.27,-14.4,;2.27,-15.94,;3.58,-13.63,;4.91,-14.4,;4.91,-15.94,;6.24,-13.61,;7.58,-14.38,;6.23,-12.07,;7.54,-11.3,;4.88,-11.32,;3.58,-12.09,;2.25,-11.35,;.93,-12.12,)| Show InChI InChI=1S/C30H26O10/c1-9-11(3)39-19-5-13-21(15(31)7-17(33)23(13)29(37)25(19)27(9)35)22-14-6-20-26(28(36)10(2)12(4)40-20)30(38)24(14)18(34)8-16(22)32/h5-12,31-34,37-38H,1-4H3 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of HIV-1 integrase in strand transfer assay |
Bioorg Med Chem Lett 13: 713-7 (2003)
BindingDB Entry DOI: 10.7270/Q22R3R1H |
More data for this
Ligand-Target Pair | |
Integrase
(Human immunodeficiency virus 1) | BDBM50124967

(5,6,8,5',6',8'-Hexahydroxy-2,3,2',3'-tetramethyl-2...)Show SMILES CC1Oc2cc3c(c(O)cc(O)c3c(O)c2C(=O)C1C)-c1c(O)cc(O)c2c(O)c3C(=O)C(C)C(C)Oc3cc12 |(7.61,-9.78,;6.28,-9.01,;4.93,-9.81,;3.6,-9.04,;2.27,-9.81,;.93,-9.04,;-.41,-9.83,;-1.74,-9.06,;-3.07,-9.81,;-1.74,-7.52,;-.41,-6.73,;-.41,-5.21,;.93,-7.5,;2.25,-6.73,;2.25,-5.19,;3.59,-7.5,;4.92,-6.7,;4.91,-5.16,;6.26,-7.47,;7.59,-6.68,;-.4,-11.35,;-1.74,-12.12,;-3.07,-11.37,;-1.73,-13.66,;-.39,-14.43,;-.39,-15.97,;.94,-13.65,;2.27,-14.4,;2.27,-15.94,;3.58,-13.63,;4.91,-14.4,;4.91,-15.94,;6.24,-13.61,;7.58,-14.38,;6.23,-12.07,;7.54,-11.3,;4.88,-11.32,;3.58,-12.09,;2.25,-11.35,;.93,-12.12,)| Show InChI InChI=1S/C30H26O10/c1-9-11(3)39-19-5-13-21(15(31)7-17(33)23(13)29(37)25(19)27(9)35)22-14-6-20-26(28(36)10(2)12(4)40-20)30(38)24(14)18(34)8-16(22)32/h5-12,31-34,37-38H,1-4H3 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of HIV-1 integrase in coupled transfer assay |
Bioorg Med Chem Lett 13: 713-7 (2003)
BindingDB Entry DOI: 10.7270/Q22R3R1H |
More data for this
Ligand-Target Pair | |
Integrase
(Human immunodeficiency virus 1) | BDBM50123449

(CHEMBL437996 | Chloropeptin II | ISOCOMPLESTATIN)Show SMILES [H][C@@]12NC(=O)[C@H](NC(=O)[C@@H](Cc3c[nH]c4cc(ccc34)-c3cc1cc(Oc1ccc(C[C@H](N(C)C(=O)[C@H](NC2=O)c2cc(Cl)c(O)c(Cl)c2)C(=O)N[C@@H](C(O)=O)c2ccc(O)cc2)cc1)c3O)NC(=O)C(=O)c1cc(Cl)c(O)c(Cl)c1)c1cc(Cl)c(O)c(Cl)c1 |r| Show InChI InChI=1S/C61H45Cl6N7O15/c1-74-44(56(82)73-49(61(87)88)25-4-7-32(75)8-5-25)12-24-2-9-33(10-3-24)89-45-22-27-13-35(51(45)77)26-6-11-34-31(23-68-42(34)20-26)21-43(69-59(85)50(76)30-18-40(66)54(80)41(67)19-30)55(81)70-47(28-14-36(62)52(78)37(63)15-28)57(83)71-46(27)58(84)72-48(60(74)86)29-16-38(64)53(79)39(65)17-29/h2-11,13-20,22-23,43-44,46-49,68,75,77-80H,12,21H2,1H3,(H,69,85)(H,70,81)(H,71,83)(H,72,84)(H,73,82)(H,87,88) | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of integration activity of HIV1 intact integrase |
J Nat Prod 64: 874-82 (2001)
Article DOI: 10.1021/np000632z
BindingDB Entry DOI: 10.7270/Q22N552N |
More data for this
Ligand-Target Pair | |
Integrase
(Human immunodeficiency virus 1) | BDBM50124966

((8R,9R)-2,4,5-Trihydroxy-8,9-dimethyl-1-((2S,3R)-5...)Show SMILES C[C@@H]1Oc2cc3c(c(O)cc(O)c3c(O)c2C(=O)[C@@H]1C)-c1c(O)cc(O)c2c(O)c3OC(=O)[C@H](C)[C@@H](C)Oc3cc12 |wU:18.21,35.38,1.0,wD:33.36,(12.23,-.68,;10.88,-1.45,;9.55,-.68,;8.22,-1.45,;6.88,-.68,;5.55,-1.45,;4.21,-.68,;2.88,-1.45,;1.54,-.68,;2.88,-3.01,;4.21,-3.78,;4.21,-5.32,;5.55,-3.01,;6.88,-3.78,;6.88,-5.32,;8.22,-3.01,;9.55,-3.78,;9.55,-5.32,;10.88,-3.01,;12.23,-3.79,;4.21,.86,;2.88,1.63,;1.54,.84,;2.88,3.17,;4.21,3.94,;4.21,5.48,;5.55,3.17,;6.88,3.94,;6.88,5.48,;8.22,3.17,;9.41,4.12,;10.9,3.78,;11.86,4.99,;11.58,2.4,;13.12,2.42,;10.9,1.02,;11.88,-.22,;9.41,.67,;8.22,1.63,;6.88,.86,;5.55,1.63,)| Show InChI InChI=1S/C30H26O11/c1-9-11(3)39-19-5-13-21(15(31)7-17(33)23(13)27(36)25(19)26(9)35)22-14-6-20-29(41-30(38)10(2)12(4)40-20)28(37)24(14)18(34)8-16(22)32/h5-12,31-34,36-37H,1-4H3/t9-,10-,11+,12-/m1/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of HIV-1 integrase in coupled transfer assay |
Bioorg Med Chem Lett 13: 713-7 (2003)
BindingDB Entry DOI: 10.7270/Q22R3R1H |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50067849
((S)-2-[(3aR,6R,9S)-3-(1,5-Dimethyl-hexyl)-9-ethyl-...)Show SMILES CC[C@H]1CC(=O)[C@](C)(C[C@@H](CC(O)=O)C(O)=O)C2CC[C@]3(C)C(CCC3C12)C(C)CCCC(C)C Show InChI InChI=1S/C30H50O5/c1-7-20-15-25(31)30(6,17-21(28(34)35)16-26(32)33)24-13-14-29(5)22(11-12-23(29)27(20)24)19(4)10-8-9-18(2)3/h18-24,27H,7-17H2,1-6H3,(H,32,33)(H,34,35)/t19?,20-,21+,22?,23?,24?,27?,29+,30+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against human Farnesyltransferase |
J Med Chem 41: 4492-501 (1998)
Article DOI: 10.1021/jm980356+
BindingDB Entry DOI: 10.7270/Q23779DQ |
More data for this
Ligand-Target Pair | |
Integrase
(Human immunodeficiency virus 1) | BDBM50478736
(CHEMBL505739)Show SMILES CN[C@@H](Cc1ccc(Oc2cc3cc(c2O)-c2cccc4c(C[C@@H](N)C(=O)N[C@H](c5cc(Cl)c(O)c(Cl)c5)C(=O)N[C@H]3C(=O)N[C@@H](C(O)=O)c3cc(Cl)c(O)c(Cl)c3)c[nH]c24)cc1)C(O)=O |r| Show InChI InChI=1S/C45H38Cl4N6O11/c1-51-32(44(62)63)9-18-5-7-23(8-6-18)66-33-16-19-10-26(38(33)56)25-4-2-3-24-22(17-52-37(24)25)15-31(50)41(59)53-35(20-11-27(46)39(57)28(47)12-20)42(60)54-34(19)43(61)55-36(45(64)65)21-13-29(48)40(58)30(49)14-21/h2-8,10-14,16-17,31-32,34-36,51-52,56-58H,9,15,50H2,1H3,(H,53,59)(H,54,60)(H,55,61)(H,62,63)(H,64,65)/t31-,32+,34-,35-,36-/m1/s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 integrase 3' processing/strand transfer coupled activity |
J Nat Prod 64: 874-82 (2001)
Article DOI: 10.1021/np000632z
BindingDB Entry DOI: 10.7270/Q22N552N |
More data for this
Ligand-Target Pair | |
Integrase
(Human immunodeficiency virus 1) | BDBM50123449

(CHEMBL437996 | Chloropeptin II | ISOCOMPLESTATIN)Show SMILES [H][C@@]12NC(=O)[C@H](NC(=O)[C@@H](Cc3c[nH]c4cc(ccc34)-c3cc1cc(Oc1ccc(C[C@H](N(C)C(=O)[C@H](NC2=O)c2cc(Cl)c(O)c(Cl)c2)C(=O)N[C@@H](C(O)=O)c2ccc(O)cc2)cc1)c3O)NC(=O)C(=O)c1cc(Cl)c(O)c(Cl)c1)c1cc(Cl)c(O)c(Cl)c1 |r| Show InChI InChI=1S/C61H45Cl6N7O15/c1-74-44(56(82)73-49(61(87)88)25-4-7-32(75)8-5-25)12-24-2-9-33(10-3-24)89-45-22-27-13-35(51(45)77)26-6-11-34-31(23-68-42(34)20-26)21-43(69-59(85)50(76)30-18-40(66)54(80)41(67)19-30)55(81)70-47(28-14-36(62)52(78)37(63)15-28)57(83)71-46(27)58(84)72-48(60(74)86)29-16-38(64)53(79)39(65)17-29/h2-11,13-20,22-23,43-44,46-49,68,75,77-80H,12,21H2,1H3,(H,69,85)(H,70,81)(H,71,83)(H,72,84)(H,73,82)(H,87,88) | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 integrase strand transfer activity |
J Nat Prod 64: 874-82 (2001)
Article DOI: 10.1021/np000632z
BindingDB Entry DOI: 10.7270/Q22N552N |
More data for this
Ligand-Target Pair | |
Integrase
(Human immunodeficiency virus 1) | BDBM50124971

((2R,3S)-5,6,8,5',6',8'-Hexahydroxy-2,3,2',3'-tetra...)Show SMILES C[C@H]1Oc2cc3c(c(O)cc(O)c3c(O)c2C(=O)[C@H]1C)-c1c(O)cc(O)c2c(O)c3c(cc12)oc(C)c(C)c3=O |wU:18.21,1.0,(7.61,-9.78,;6.28,-9.01,;4.93,-9.81,;3.6,-9.04,;2.27,-9.81,;.93,-9.04,;-.41,-9.83,;-1.74,-9.06,;-3.07,-9.81,;-1.74,-7.52,;-.41,-6.73,;-.41,-5.21,;.93,-7.5,;2.25,-6.73,;2.25,-5.19,;3.59,-7.5,;4.92,-6.7,;4.91,-5.16,;6.26,-7.47,;7.59,-6.68,;-.4,-11.35,;-1.74,-12.12,;-3.07,-11.37,;-1.73,-13.66,;-.39,-14.43,;-.39,-15.97,;.94,-13.66,;2.27,-14.4,;2.27,-15.94,;3.58,-13.63,;3.58,-12.09,;2.25,-11.35,;.93,-12.12,;4.88,-11.32,;6.23,-12.07,;7.54,-11.3,;6.24,-13.61,;7.58,-14.38,;4.91,-14.4,;4.91,-15.94,)| Show InChI InChI=1S/C30H24O10/c1-9-11(3)39-19-5-13-21(15(31)7-17(33)23(13)29(37)25(19)27(9)35)22-14-6-20-26(28(36)10(2)12(4)40-20)30(38)24(14)18(34)8-16(22)32/h5-9,11,31-34,37-38H,1-4H3/t9-,11+/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of HIV-1 integrase in strand transfer assay |
Bioorg Med Chem Lett 13: 713-7 (2003)
BindingDB Entry DOI: 10.7270/Q22R3R1H |
More data for this
Ligand-Target Pair | |
Integrase
(Human immunodeficiency virus 1) | BDBM50366855

(CHEMBL525803 | Chloropeptin)Show SMILES CN1[C@@H](Cc2ccc(Oc3cc4cc(c3O)-c3cccc5c(C[C@@H](NC(=O)C(=O)c6cc(Cl)c(O)c(Cl)c6)C(=O)N[C@H](c6cc(Cl)c(O)c(Cl)c6)C(=O)N[C@H]4C(=O)N[C@H](c4cc(Cl)c(O)c(Cl)c4)C1=O)c[nH]c35)cc2)C(=O)N[C@@H](C(O)=O)c1ccc(O)cc1 |r| Show InChI InChI=1S/C61H45Cl6N7O15/c1-74-43(56(82)73-48(61(87)88)25-7-9-31(75)10-8-25)13-24-5-11-32(12-6-24)89-44-22-26-14-35(51(44)77)34-4-2-3-33-30(23-68-49(33)34)21-42(69-59(85)50(76)29-19-40(66)54(80)41(67)20-29)55(81)70-46(27-15-36(62)52(78)37(63)16-27)57(83)71-45(26)58(84)72-47(60(74)86)28-17-38(64)53(79)39(65)18-28/h2-12,14-20,22-23,42-43,45-48,68,75,77-80H,13,21H2,1H3,(H,69,85)(H,70,81)(H,71,83)(H,72,84)(H,73,82)(H,87,88)/t42-,43+,45-,46-,47-,48-/m1/s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 integrase strand transfer activity |
J Nat Prod 64: 874-82 (2001)
Article DOI: 10.1021/np000632z
BindingDB Entry DOI: 10.7270/Q22N552N |
More data for this
Ligand-Target Pair | |
Integrase
(Human immunodeficiency virus 1) | BDBM50478734

(CHEMBL448564)Show SMILES [H][C@@]12NC(=O)[C@H](NC(=O)[C@H](N)Cc3c[nH]c4c(cccc34)-c3cc1cc(Oc1ccc(C[C@H](N(C)C(=O)[C@H](NC2=O)c2cc(Cl)c(O)c(Cl)c2)C(O)=O)cc1)c3O)c1cc(Cl)c(O)c(Cl)c1 |r| Show InChI InChI=1S/C45H36Cl4N6O10/c1-55-32(45(63)64)9-18-5-7-23(8-6-18)65-33-16-19-10-26(38(33)56)25-4-2-3-24-22(17-51-37(24)25)15-31(50)41(59)52-35(20-11-27(46)39(57)28(47)12-20)42(60)53-34(19)43(61)54-36(44(55)62)21-13-29(48)40(58)30(49)14-21/h2-8,10-14,16-17,31-32,34-36,51,56-58H,9,15,50H2,1H3,(H,52,59)(H,53,60)(H,54,61)(H,63,64)/t31-,32+,34-,35-,36-/m1/s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 integrase strand transfer activity |
J Nat Prod 64: 874-82 (2001)
Article DOI: 10.1021/np000632z
BindingDB Entry DOI: 10.7270/Q22N552N |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50067854
(CHEMBL344638 | Isobutyric acid 2-((10R,13S)-10,13-...)Show SMILES CC(C)C(=O)OCC(=O)C1CCC2C3CCC4=CC(=O)CC[C@]4(C)C3C(=O)C[C@]12C |t:16| Show InChI InChI=1S/C25H34O5/c1-14(2)23(29)30-13-21(28)19-8-7-18-17-6-5-15-11-16(26)9-10-24(15,3)22(17)20(27)12-25(18,19)4/h11,14,17-19,22H,5-10,12-13H2,1-4H3/t17?,18?,19?,22?,24-,25-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
inhibitory activity against human Farnesyltransferase |
J Med Chem 41: 4492-501 (1998)
Article DOI: 10.1021/jm980356+
BindingDB Entry DOI: 10.7270/Q23779DQ |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50071242
(Acetic acid (6aR,6bS,9S,10aR,12aS)-5-hydroxy-1,3-d...)Show SMILES COC1OC(OC)c2c1cc(O)c1C[C@H]3[C@](C)(CC[C@H]4C(C)(C)[C@H](CC[C@]34C)OC(C)=O)Oc21 Show InChI InChI=1S/C27H38O7/c1-14(28)32-20-9-10-26(4)18(25(20,2)3)8-11-27(5)19(26)13-15-17(29)12-16-21(22(15)34-27)24(31-7)33-23(16)30-6/h12,18-20,23-24,29H,8-11,13H2,1-7H3/t18-,19+,20-,23?,24?,26-,27-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 7.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant farnesyltransferase |
Bioorg Med Chem Lett 8: 2071-6 (1999)
BindingDB Entry DOI: 10.7270/Q2W958BG |
More data for this
Ligand-Target Pair | |
Integrase
(Human immunodeficiency virus 1) | BDBM50478739
(CHEMBL448271)Show SMILES CN([C@@H](Cc1ccc(Oc2cc3cc(c2O)-c2cccc4c(C[C@@H](N)C(=O)N[C@H](c5cc(Cl)c(O)c(Cl)c5)C(=O)N[C@H]3C(O)=O)c[nH]c24)cc1)C(O)=O)C(=O)[C@H](N)c1cc(Cl)c(O)c(Cl)c1 |r| Show InChI InChI=1S/C45H38Cl4N6O11/c1-55(43(61)34(51)19-11-27(46)39(57)28(47)12-19)32(44(62)63)9-18-5-7-23(8-6-18)66-33-16-20-10-26(38(33)56)25-4-2-3-24-22(17-52-37(24)25)15-31(50)41(59)53-35(42(60)54-36(20)45(64)65)21-13-29(48)40(58)30(49)14-21/h2-8,10-14,16-17,31-32,34-36,52,56-58H,9,15,50-51H2,1H3,(H,53,59)(H,54,60)(H,62,63)(H,64,65)/t31-,32+,34-,35-,36-/m1/s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 integrase 3' processing/strand transfer coupled activity |
J Nat Prod 64: 874-82 (2001)
Article DOI: 10.1021/np000632z
BindingDB Entry DOI: 10.7270/Q22N552N |
More data for this
Ligand-Target Pair | |
Integrase
(Human immunodeficiency virus 1) | BDBM50124964

(6,8'-Dihydroxy-5,8,5',6'-tetramethoxy-2,3,2',3'-te...)Show SMILES COc1cc(O)c2c(OC)c3C(=O)C(C)C(C)Oc3cc2c1-c1c(O)cc(OC)c2c(OC)c3C(=O)C(C)C(C)Oc3cc12 |(-4.08,-12.58,;-2.99,-11.49,;-1.66,-12.26,;-1.65,-13.8,;-.32,-14.57,;-.3,-16.08,;1.01,-13.77,;2.34,-14.54,;2.36,-16.06,;1.26,-17.16,;3.66,-13.75,;4.99,-14.52,;4.98,-16.06,;6.31,-13.75,;7.66,-14.5,;6.31,-12.21,;7.63,-11.42,;4.97,-11.44,;3.65,-12.23,;2.32,-11.46,;1.01,-12.23,;-.32,-11.49,;-.33,-9.95,;-1.66,-9.18,;-3,-9.95,;-1.66,-7.64,;-.33,-6.87,;-.33,-5.33,;-1.66,-4.56,;1.01,-7.64,;2.33,-6.84,;2.33,-5.3,;1.24,-4.21,;3.67,-7.61,;5,-6.84,;4.99,-5.3,;6.33,-7.59,;7.66,-6.8,;6.35,-9.15,;7.7,-9.9,;5.02,-9.92,;3.67,-9.15,;2.34,-9.95,;1.01,-9.18,)| Show InChI InChI=1S/C34H34O10/c1-13-15(3)44-24-10-18-26(33(41-7)29(24)31(13)37)20(36)12-21(39-5)27(18)25-17-9-23-30(32(38)14(2)16(4)43-23)34(42-8)28(17)22(40-6)11-19(25)35/h9-16,35-36H,1-8H3 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of HIV-1 integrase in coupled transfer assay |
Bioorg Med Chem Lett 13: 713-7 (2003)
BindingDB Entry DOI: 10.7270/Q22R3R1H |
More data for this
Ligand-Target Pair | |
Integrase
(Human immunodeficiency virus 1) | BDBM50478738

(CHEMBL507121)Show SMILES [H][C@@]12NC(=O)[C@H](NC(=O)[C@@H](Cc3c[nH]c4c(cccc34)-c3cc1cc(Oc1ccc(C[C@H](N(C)C(=O)[C@H](NC2=O)c2cc(Cl)c(O)c(Cl)c2)C(O)=O)cc1)c3O)NC(=O)C(=O)c1cc(Cl)c(O)c(Cl)c1)c1cc(Cl)c(O)c(Cl)c1 |r| Show InChI InChI=1S/C53H38Cl6N6O13/c1-65-37(53(76)77)9-20-5-7-26(8-6-20)78-38-18-21-10-29(44(38)67)28-4-2-3-27-25(19-60-42(27)28)17-36(61-51(74)43(66)24-15-34(58)47(70)35(59)16-24)48(71)62-40(22-11-30(54)45(68)31(55)12-22)49(72)63-39(21)50(73)64-41(52(65)75)23-13-32(56)46(69)33(57)14-23/h2-8,10-16,18-19,36-37,39-41,60,67-70H,9,17H2,1H3,(H,61,74)(H,62,71)(H,63,72)(H,64,73)(H,76,77)/t36-,37+,39-,40-,41-/m1/s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 integrase strand transfer activity |
J Nat Prod 64: 874-82 (2001)
Article DOI: 10.1021/np000632z
BindingDB Entry DOI: 10.7270/Q22N552N |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50067834
((3aR,6R)-6-(2-Carboxy-ethyl)-3-(1,5-dimethyl-hexyl...)Show SMILES CC(C)CCCC(C)C1CCC2C3CC=C(C(O)=O)[C@](C)(CCC(O)=O)C3CC[C@]12C |t:14| Show InChI InChI=1S/C27H44O4/c1-17(2)7-6-8-18(3)20-11-12-21-19-9-10-23(25(30)31)27(5,16-14-24(28)29)22(19)13-15-26(20,21)4/h10,17-22H,6-9,11-16H2,1-5H3,(H,28,29)(H,30,31)/t18?,19?,20?,21?,22?,26-,27-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against human Farnesyltransferase |
J Med Chem 41: 4492-501 (1998)
Article DOI: 10.1021/jm980356+
BindingDB Entry DOI: 10.7270/Q23779DQ |
More data for this
Ligand-Target Pair | |
Integrase
(Human immunodeficiency virus 1) | BDBM50124966

((8R,9R)-2,4,5-Trihydroxy-8,9-dimethyl-1-((2S,3R)-5...)Show SMILES C[C@@H]1Oc2cc3c(c(O)cc(O)c3c(O)c2C(=O)[C@@H]1C)-c1c(O)cc(O)c2c(O)c3OC(=O)[C@H](C)[C@@H](C)Oc3cc12 |wU:18.21,35.38,1.0,wD:33.36,(12.23,-.68,;10.88,-1.45,;9.55,-.68,;8.22,-1.45,;6.88,-.68,;5.55,-1.45,;4.21,-.68,;2.88,-1.45,;1.54,-.68,;2.88,-3.01,;4.21,-3.78,;4.21,-5.32,;5.55,-3.01,;6.88,-3.78,;6.88,-5.32,;8.22,-3.01,;9.55,-3.78,;9.55,-5.32,;10.88,-3.01,;12.23,-3.79,;4.21,.86,;2.88,1.63,;1.54,.84,;2.88,3.17,;4.21,3.94,;4.21,5.48,;5.55,3.17,;6.88,3.94,;6.88,5.48,;8.22,3.17,;9.41,4.12,;10.9,3.78,;11.86,4.99,;11.58,2.4,;13.12,2.42,;10.9,1.02,;11.88,-.22,;9.41,.67,;8.22,1.63,;6.88,.86,;5.55,1.63,)| Show InChI InChI=1S/C30H26O11/c1-9-11(3)39-19-5-13-21(15(31)7-17(33)23(13)27(36)25(19)26(9)35)22-14-6-20-29(41-30(38)10(2)12(4)40-20)28(37)24(14)18(34)8-16(22)32/h5-12,31-34,36-37H,1-4H3/t9-,10-,11+,12-/m1/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 9.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of HIV-1 integrase in strand transfer assay |
Bioorg Med Chem Lett 13: 713-7 (2003)
BindingDB Entry DOI: 10.7270/Q22R3R1H |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50067835

((10S,13R,14R)-17-(1,5-Dimethyl-hex-4-enyl)-4,4,10,...)Show SMILES [#6]-[#6](-[#6]-[#6]\[#6]=[#6](/[#6])-[#6])-[#6]1-[#6]-[#6][C@@]2([#6])[#6]-3=[#6](-[#6]-[#6][C@]12[#6])[C@@]1([#6])[#6]-[#6]-[#6](=O)C([#6])([#6])[#6]1-[#6]-[#6]-3 |c:13| Show InChI InChI=1S/C30H48O/c1-20(2)10-9-11-21(3)22-14-18-30(8)24-12-13-25-27(4,5)26(31)16-17-28(25,6)23(24)15-19-29(22,30)7/h10,21-22,25H,9,11-19H2,1-8H3/t21?,22?,25?,28-,29-,30+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
inhibitory activity against human Farnesyltransferase |
J Med Chem 41: 4492-501 (1998)
Article DOI: 10.1021/jm980356+
BindingDB Entry DOI: 10.7270/Q23779DQ |
More data for this
Ligand-Target Pair | |
Integrase
(Human immunodeficiency virus 1) | BDBM50124967

(5,6,8,5',6',8'-Hexahydroxy-2,3,2',3'-tetramethyl-2...)Show SMILES CC1Oc2cc3c(c(O)cc(O)c3c(O)c2C(=O)C1C)-c1c(O)cc(O)c2c(O)c3C(=O)C(C)C(C)Oc3cc12 |(7.61,-9.78,;6.28,-9.01,;4.93,-9.81,;3.6,-9.04,;2.27,-9.81,;.93,-9.04,;-.41,-9.83,;-1.74,-9.06,;-3.07,-9.81,;-1.74,-7.52,;-.41,-6.73,;-.41,-5.21,;.93,-7.5,;2.25,-6.73,;2.25,-5.19,;3.59,-7.5,;4.92,-6.7,;4.91,-5.16,;6.26,-7.47,;7.59,-6.68,;-.4,-11.35,;-1.74,-12.12,;-3.07,-11.37,;-1.73,-13.66,;-.39,-14.43,;-.39,-15.97,;.94,-13.65,;2.27,-14.4,;2.27,-15.94,;3.58,-13.63,;4.91,-14.4,;4.91,-15.94,;6.24,-13.61,;7.58,-14.38,;6.23,-12.07,;7.54,-11.3,;4.88,-11.32,;3.58,-12.09,;2.25,-11.35,;.93,-12.12,)| Show InChI InChI=1S/C30H26O10/c1-9-11(3)39-19-5-13-21(15(31)7-17(33)23(13)29(37)25(19)27(9)35)22-14-6-20-26(28(36)10(2)12(4)40-20)30(38)24(14)18(34)8-16(22)32/h5-12,31-34,37-38H,1-4H3 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of HIV-1 integrase in strand transfer assay |
Bioorg Med Chem Lett 13: 713-7 (2003)
BindingDB Entry DOI: 10.7270/Q22R3R1H |
More data for this
Ligand-Target Pair | |
Integrase
(Human immunodeficiency virus 1) | BDBM50478735

(CHEBI:65655 | COMPLESTATINS A)Show SMILES [H][C@@]12NC(=O)[C@H](NC(=O)[C@@H](CC3C(=O)Nc4cc(ccc34)-c3cc1cc(Oc1ccc(C[C@H](N(C)C(=O)[C@H](NC2=O)c2cc(Cl)c(O)c(Cl)c2)C(=O)N[C@@H](C(O)=O)c2ccc(O)cc2)cc1)c3O)NC(=O)C(=O)c1cc(Cl)c(O)c(Cl)c1)c1cc(Cl)c(O)c(Cl)c1 |r| Show InChI InChI=1S/C61H45Cl6N7O16/c1-74-43(56(83)73-48(61(88)89)24-4-7-30(75)8-5-24)12-23-2-9-31(10-3-23)90-44-21-26-13-33(50(44)77)25-6-11-32-34(54(81)68-41(32)20-25)22-42(69-59(86)49(76)29-18-39(66)53(80)40(67)19-29)55(82)70-46(27-14-35(62)51(78)36(63)15-27)57(84)71-45(26)58(85)72-47(60(74)87)28-16-37(64)52(79)38(65)17-28/h2-11,13-21,34,42-43,45-48,75,77-80H,12,22H2,1H3,(H,68,81)(H,69,86)(H,70,82)(H,71,84)(H,72,85)(H,73,83)(H,88,89)/t34?,42-,43+,45-,46-,47-,48-/m1/s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.25E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 integrase strand transfer activity |
J Nat Prod 64: 874-82 (2001)
Article DOI: 10.1021/np000632z
BindingDB Entry DOI: 10.7270/Q22N552N |
More data for this
Ligand-Target Pair | |
Integrase
(Human immunodeficiency virus 1) | BDBM50478740

(CHEBI:65656 | COMPLESTATIN B)Show SMILES [H][C@@]12NC(=O)[C@H](NC(=O)[C@@H](CC3(O)C(=O)Nc4cc(ccc34)-c3cc1cc(Oc1ccc(C[C@H](N(C)C(=O)[C@H](NC2=O)c2cc(Cl)c(O)c(Cl)c2)C(=O)N[C@@H](C(O)=O)c2ccc(O)cc2)cc1)c3O)NC(=O)C(=O)c1cc(Cl)c(O)c(Cl)c1)c1cc(Cl)c(O)c(Cl)c1 |r| Show InChI InChI=1S/C61H45Cl6N7O17/c1-74-42(54(82)73-47(59(87)88)24-4-7-30(75)8-5-24)12-23-2-9-31(10-3-23)91-43-21-26-13-32(49(43)77)25-6-11-33-40(20-25)69-60(89)61(33,90)22-41(68-57(85)48(76)29-18-38(66)52(80)39(67)19-29)53(81)70-45(27-14-34(62)50(78)35(63)15-27)55(83)71-44(26)56(84)72-46(58(74)86)28-16-36(64)51(79)37(65)17-28/h2-11,13-21,41-42,44-47,75,77-80,90H,12,22H2,1H3,(H,68,85)(H,69,89)(H,70,81)(H,71,83)(H,72,84)(H,73,82)(H,87,88)/t41-,42+,44-,45-,46-,47-,61?/m1/s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.25E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 integrase strand transfer activity |
J Nat Prod 64: 874-82 (2001)
Article DOI: 10.1021/np000632z
BindingDB Entry DOI: 10.7270/Q22N552N |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50071241
(Acetic acid (6aR,6bS,9S,10aR,12aS)-5-hydroxy-6b,10...)Show SMILES CC(=O)O[C@H]1CC[C@@]2(C)[C@@H](CC[C@]3(C)Oc4c5COC(=O)c5cc(O)c4C[C@H]23)C1(C)C Show InChI InChI=1S/C25H32O6/c1-13(26)30-20-7-8-24(4)18(23(20,2)3)6-9-25(5)19(24)11-15-17(27)10-14-16(21(15)31-25)12-29-22(14)28/h10,18-20,27H,6-9,11-12H2,1-5H3/t18-,19+,20-,24-,25-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant farnesyltransferase |
Bioorg Med Chem Lett 8: 2071-6 (1999)
BindingDB Entry DOI: 10.7270/Q2W958BG |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50455157
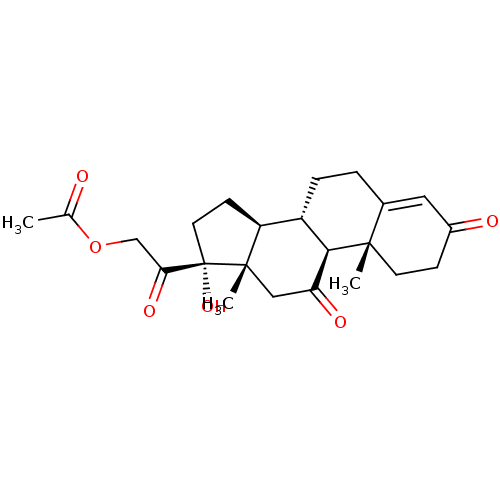
(Cortisone | Cortisone acetate | Cortone)Show SMILES [H][C@@]12CC[C@](O)(C(=O)COC(C)=O)[C@@]1(C)CC(=O)[C@@]1([H])[C@@]2([H])CCC2=CC(=O)CC[C@]12C |r,t:26| Show InChI InChI=1S/C23H30O6/c1-13(24)29-12-19(27)23(28)9-7-17-16-5-4-14-10-15(25)6-8-21(14,2)20(16)18(26)11-22(17,23)3/h10,16-17,20,28H,4-9,11-12H2,1-3H3/t16-,17-,20+,21-,22-,23-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
inhibitory activity against human Farnesyltransferase |
J Med Chem 41: 4492-501 (1998)
Article DOI: 10.1021/jm980356+
BindingDB Entry DOI: 10.7270/Q23779DQ |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50067846
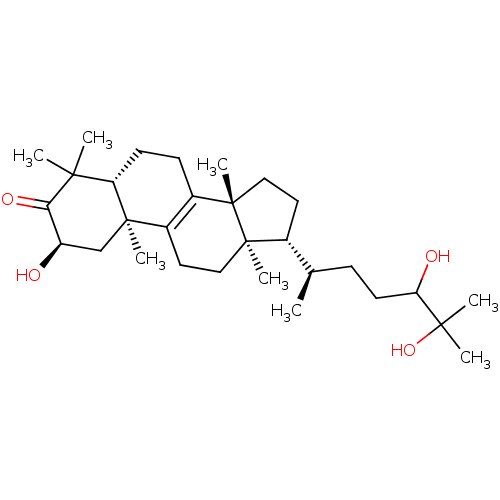
((2R,10S,13R,14R,17R)-17-((R)-4,5-Dihydroxy-1,5-dim...)Show SMILES [H][C@@]1(CC[C@@]2(C)C3=C(CC[C@]12C)[C@@]1(C)C[C@@H](O)C(=O)C(C)(C)[C@]1([H])CC3)[C@H](C)CCC(O)C(C)(C)O |r,c:6| Show InChI InChI=1S/C30H50O4/c1-18(9-12-24(32)27(4,5)34)19-13-15-30(8)21-10-11-23-26(2,3)25(33)22(31)17-28(23,6)20(21)14-16-29(19,30)7/h18-19,22-24,31-32,34H,9-17H2,1-8H3/t18-,19-,22-,23+,24?,28-,29-,30+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
inhibitory activity against human Farnesyltransferase |
J Med Chem 41: 4492-501 (1998)
Article DOI: 10.1021/jm980356+
BindingDB Entry DOI: 10.7270/Q23779DQ |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50067841
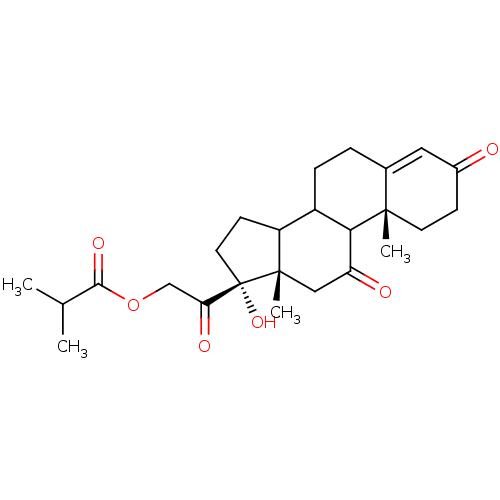
(CHEMBL138291 | Isobutyric acid 2-((10R,13S,17R)-17...)Show SMILES CC(C)C(=O)OCC(=O)[C@@]1(O)CCC2C3CCC4=CC(=O)CC[C@]4(C)C3C(=O)C[C@]12C |t:17| Show InChI InChI=1S/C25H34O6/c1-14(2)22(29)31-13-20(28)25(30)10-8-18-17-6-5-15-11-16(26)7-9-23(15,3)21(17)19(27)12-24(18,25)4/h11,14,17-18,21,30H,5-10,12-13H2,1-4H3/t17?,18?,21?,23-,24-,25-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
inhibitory activity against human Farnesyltransferase |
J Med Chem 41: 4492-501 (1998)
Article DOI: 10.1021/jm980356+
BindingDB Entry DOI: 10.7270/Q23779DQ |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data