

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50008834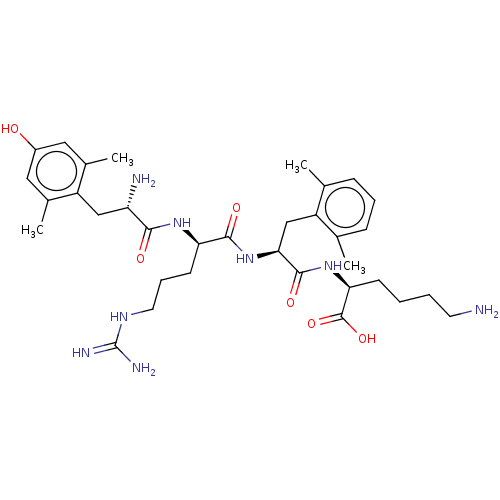 (CHEMBL3236671) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 0.00935 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing Medical University Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from mu opioid receptor in rat brain membranes after 2 hrs | Bioorg Med Chem 22: 2333-8 (2014) Article DOI: 10.1016/j.bmc.2014.02.011 BindingDB Entry DOI: 10.7270/Q20P11JG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50008837 (CHEMBL3236674) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 0.0958 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing Medical University Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from mu opioid receptor in rat brain membranes after 2 hrs | Bioorg Med Chem 22: 2333-8 (2014) Article DOI: 10.1016/j.bmc.2014.02.011 BindingDB Entry DOI: 10.7270/Q20P11JG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50008840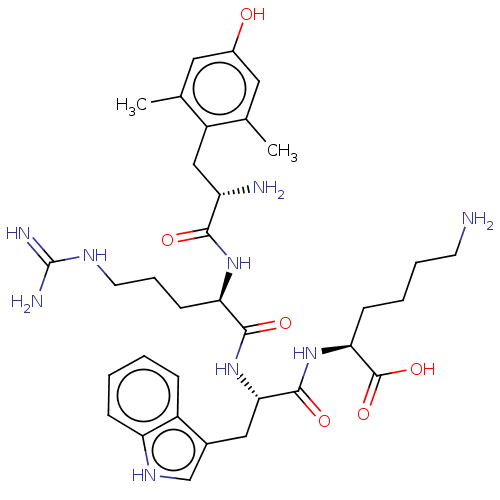 (CHEMBL3236677) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 0.0991 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing Medical University Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from mu opioid receptor in rat brain membranes after 2 hrs | Bioorg Med Chem 22: 2333-8 (2014) Article DOI: 10.1016/j.bmc.2014.02.011 BindingDB Entry DOI: 10.7270/Q20P11JG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50008838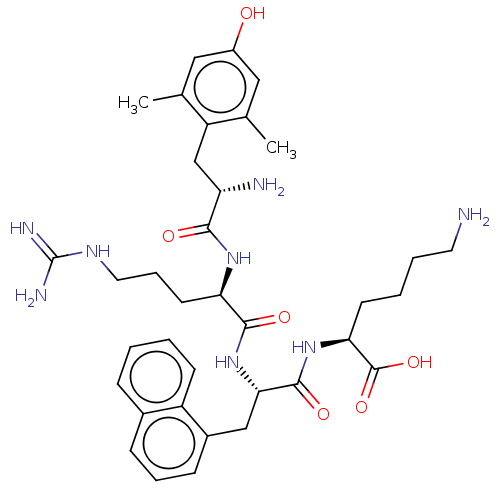 (CHEMBL3236675) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 0.109 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing Medical University Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from mu opioid receptor in rat brain membranes after 2 hrs | Bioorg Med Chem 22: 2333-8 (2014) Article DOI: 10.1016/j.bmc.2014.02.011 BindingDB Entry DOI: 10.7270/Q20P11JG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Apoptosis regulator Bcl-2 (Homo sapiens (Human)) | BDBM161571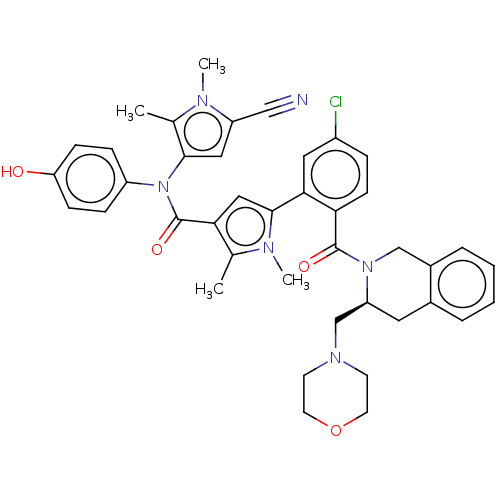 (US9108983, Example 386 | US9108983, Example 449) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1016/j.ejmech.2022.114184 BindingDB Entry DOI: 10.7270/Q2M049DT | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50008841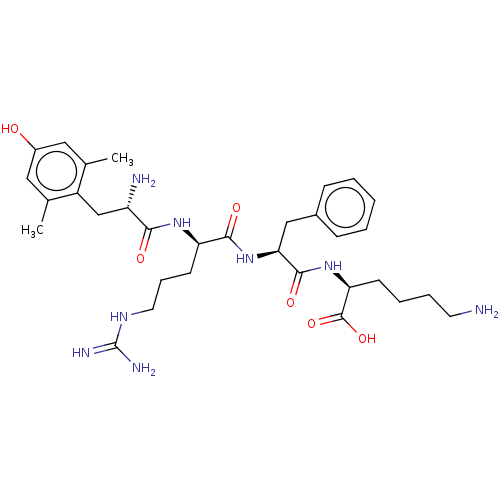 (CHEMBL3236678) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 0.143 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing Medical University Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from mu opioid receptor in rat brain membranes | Bioorg Med Chem 22: 2333-8 (2014) Article DOI: 10.1016/j.bmc.2014.02.011 BindingDB Entry DOI: 10.7270/Q20P11JG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50008836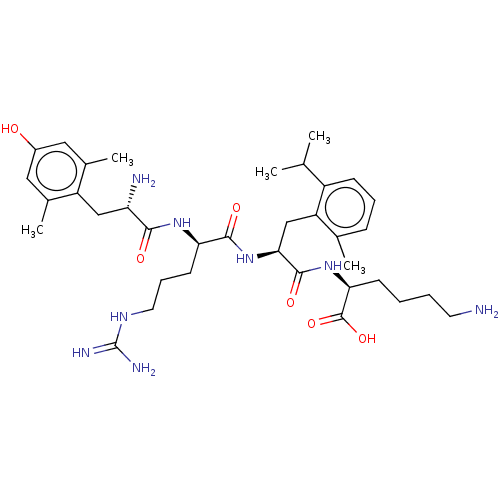 (CHEMBL3236673) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 0.155 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing Medical University Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from mu opioid receptor in rat brain membranes after 2 hrs | Bioorg Med Chem 22: 2333-8 (2014) Article DOI: 10.1016/j.bmc.2014.02.011 BindingDB Entry DOI: 10.7270/Q20P11JG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50008835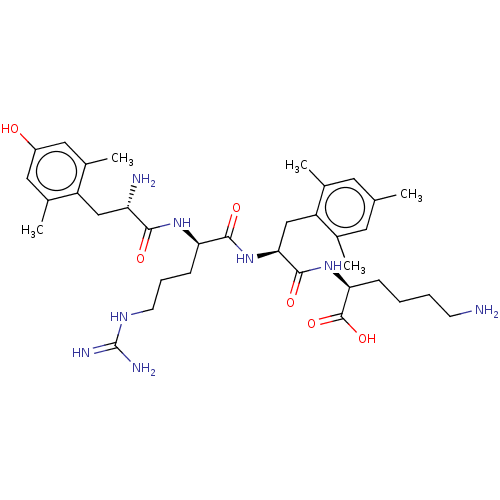 (CHEMBL3236672) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 0.158 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing Medical University Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from mu opioid receptor in rat brain membranes after 2 hrs | Bioorg Med Chem 22: 2333-8 (2014) Article DOI: 10.1016/j.bmc.2014.02.011 BindingDB Entry DOI: 10.7270/Q20P11JG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 3 (Homo sapiens (Human)) | BDBM50459819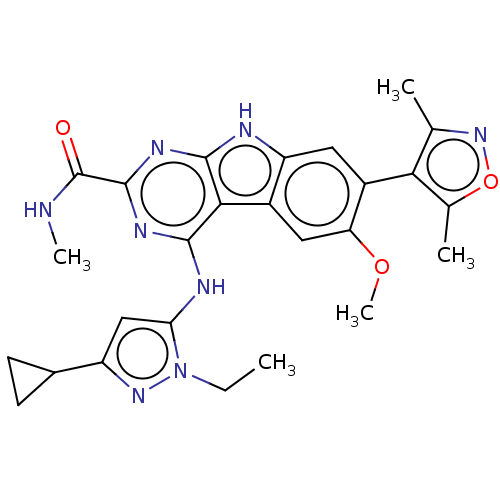 (CHEMBL4228445) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Comprehensive Cancer Center Curated by ChEMBL | Assay Description Inhibition of FAM-labeled ZBA248 binding to recombinant N-terminal His6-tagged BRD3 bromodomain 1 (24 to 144 residues) (unknown origin) expressed in ... | J Med Chem 61: 462-481 (2018) Article DOI: 10.1021/acs.jmedchem.6b01816 BindingDB Entry DOI: 10.7270/Q2CV4MD0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 3 (Homo sapiens (Human)) | BDBM50459819 (CHEMBL4228445) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 0.210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Comprehensive Cancer Center Curated by ChEMBL | Assay Description Inhibition of FAM-labeled ZBA248 binding to recombinant N-terminal His6-tagged BRD3 bromodomain 2 (306 to 417residues) (unknown origin) expressed in ... | J Med Chem 61: 462-481 (2018) Article DOI: 10.1021/acs.jmedchem.6b01816 BindingDB Entry DOI: 10.7270/Q2CV4MD0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50008839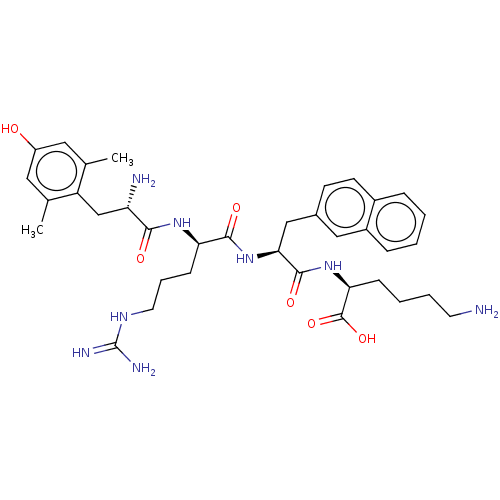 (CHEMBL3236676) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 0.235 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing Medical University Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from mu opioid receptor in rat brain membranes after 2 hrs | Bioorg Med Chem 22: 2333-8 (2014) Article DOI: 10.1016/j.bmc.2014.02.011 BindingDB Entry DOI: 10.7270/Q20P11JG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50008836 (CHEMBL3236673) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 0.236 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing Medical University Curated by ChEMBL | Assay Description Displacement of [3H]U-69,593 from kappa opioid receptor in guinea pig brain membranes after 2 hrs | Bioorg Med Chem 22: 2333-8 (2014) Article DOI: 10.1016/j.bmc.2014.02.011 BindingDB Entry DOI: 10.7270/Q20P11JG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Baculoviral IAP repeat-containing protein 3 (Homo sapiens (Human)) | BDBM50255183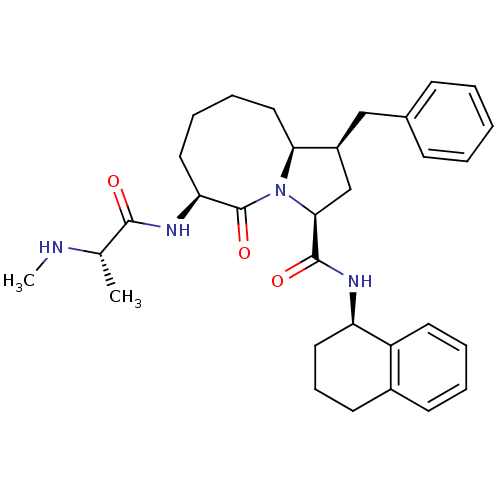 ((1S,3S,6S,10aS)-1-benzyl-6-((S)-2-(methylamino)pro...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Displacement of fluorescent SM5F peptide from His-tagged human cIAP2 BIR3 domain expressed in Escherichia coli BL21(DE3) cells by fluorescence polari... | J Med Chem 52: 593-6 (2009) Article DOI: 10.1021/jm801101z BindingDB Entry DOI: 10.7270/Q2Z03816 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 2 (Homo sapiens (Human)) | BDBM50459819 (CHEMBL4228445) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 0.270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Comprehensive Cancer Center Curated by ChEMBL | Assay Description Inhibition of FAM-labeled ZBA248 binding to recombinant N-terminal His6-tagged BRD2 bromodomain 2 (349 to 460 residues) (unknown origin) expressed in... | J Med Chem 61: 462-481 (2018) Article DOI: 10.1021/acs.jmedchem.6b01816 BindingDB Entry DOI: 10.7270/Q2CV4MD0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Apoptosis regulator Bcl-2 (Homo sapiens (Human)) | BDBM161571 (US9108983, Example 386 | US9108983, Example 449) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | 0.280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1016/j.ejmech.2022.114184 BindingDB Entry DOI: 10.7270/Q2M049DT | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Apoptosis regulator Bcl-2 (Homo sapiens (Human)) | BDBM161571 (US9108983, Example 386 | US9108983, Example 449) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | 0.280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1016/j.ejmech.2022.114184 BindingDB Entry DOI: 10.7270/Q2M049DT | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50075926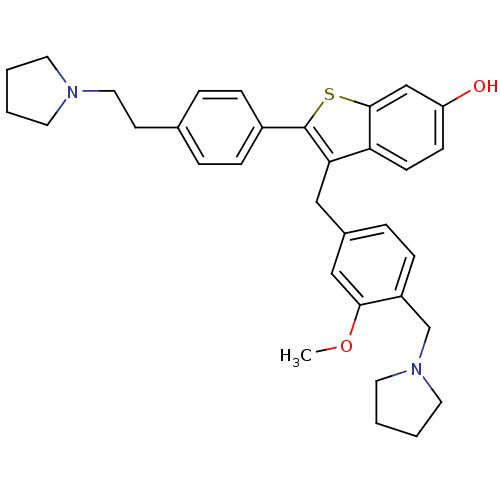 (3-(3-Methoxy-4-pyrrolidin-1-ylmethyl-benzyl)-2-[4-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Binding inhibition against thrombin was measured by nonlinear regression analysis using Morrison's equation for tight-binding inhibition | Bioorg Med Chem Lett 9: 775-80 (1999) BindingDB Entry DOI: 10.7270/Q2PR7V46 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Induced myeloid leukemia cell differentiation protein Mcl-1 (Homo sapiens (Human)) | BDBM50590323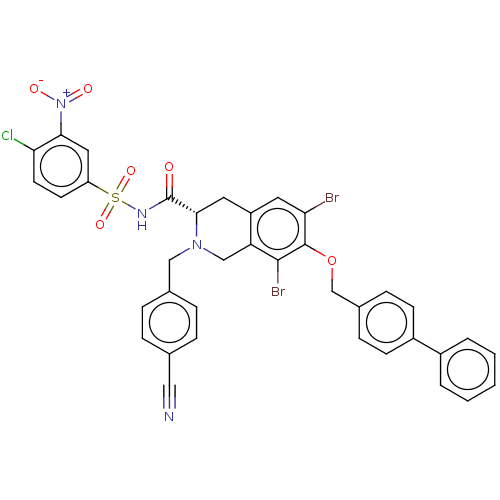 (CHEMBL5173522) | PDB MMDB NCI pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1016/j.ejmech.2022.114184 BindingDB Entry DOI: 10.7270/Q2M049DT | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Baculoviral IAP repeat-containing protein 2 (Homo sapiens (Human)) | BDBM50255183 ((1S,3S,6S,10aS)-1-benzyl-6-((S)-2-(methylamino)pro...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.370 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Displacement of fluorescent SM5F peptide from His-tagged human cIAP1 BIR3 domain expressed in Escherichia coli BL21(DE3) cells by fluorescence polari... | J Med Chem 52: 593-6 (2009) Article DOI: 10.1021/jm801101z BindingDB Entry DOI: 10.7270/Q2Z03816 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50075934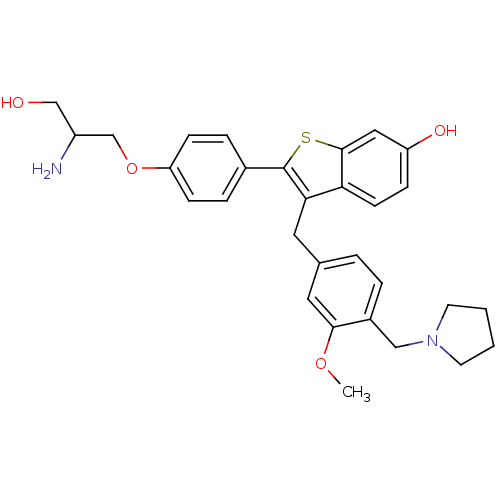 (2-[4-(2-Amino-3-hydroxy-propoxy)-phenyl]-3-(3-meth...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Binding inhibition against thrombin was measured by nonlinear regression analysis using Morrison's equation for tight-binding inhibition | Bioorg Med Chem Lett 9: 775-80 (1999) BindingDB Entry DOI: 10.7270/Q2PR7V46 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Apoptosis regulator Bcl-2 (Homo sapiens (Human)) | BDBM50590323 (CHEMBL5173522) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1016/j.ejmech.2022.114184 BindingDB Entry DOI: 10.7270/Q2M049DT | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50075937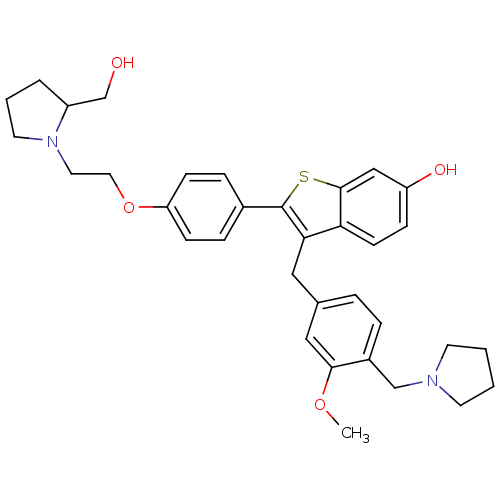 (2-{4-[2-(2-Hydroxymethyl-pyrrolidin-1-yl)-ethoxy]-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Binding inhibition against thrombin was measured by nonlinear regression analysis using Morrison's equation for tight-binding inhibition | Bioorg Med Chem Lett 9: 775-80 (1999) BindingDB Entry DOI: 10.7270/Q2PR7V46 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM50453769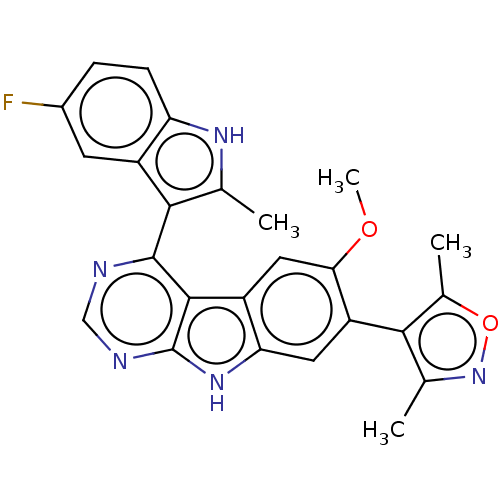 (CHEMBL4214978) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | <0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Inhibition of FAM-labeled ZBA248 binding to recombinant human N-terminal His6-tagged BRD4 bromodomain 1 (44 to 168 residues) expressed in Rosetta2 DE... | J Med Chem 60: 3887-3901 (2017) Article DOI: 10.1021/acs.jmedchem.7b00193 BindingDB Entry DOI: 10.7270/Q28W3GWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM50459819 (CHEMBL4228445) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Comprehensive Cancer Center Curated by ChEMBL | Assay Description Inhibition of FAM-labeled ZBA248 binding to recombinant N-terminal His6-tagged BRD4 bromodomain 1 (44 to 168 residues) (unknown origin) expressed in ... | J Med Chem 61: 462-481 (2018) Article DOI: 10.1021/acs.jmedchem.6b01816 BindingDB Entry DOI: 10.7270/Q2CV4MD0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM50366704 (CHEMBL4166630) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of FAM-labeled ZBA248 binding to recombinant human BRD4 bromodomain 1 (44 to 168 residues) expressed in Rosetta2 Escherichia coli DE3 cell... | J Med Chem 61: 6110-6120 (2018) Article DOI: 10.1021/acs.jmedchem.8b00483 BindingDB Entry DOI: 10.7270/Q26M39B1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM179480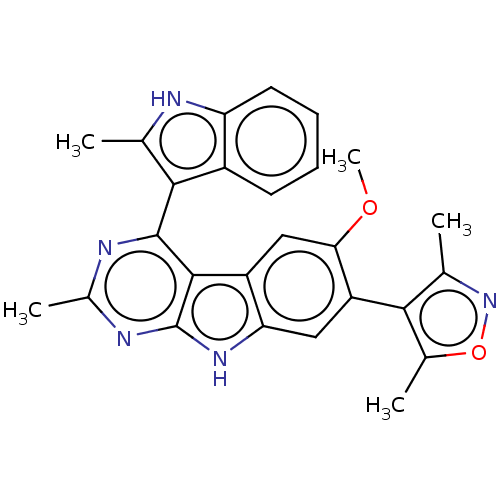 (US9675697, Cpd. No. 68) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | <0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Inhibition of FAM-labeled ZBA248 binding to recombinant human N-terminal His6-tagged BRD4 bromodomain 2 (333 to 460 residues) expressed in Rosetta2 D... | J Med Chem 60: 3887-3901 (2017) Article DOI: 10.1021/acs.jmedchem.7b00193 BindingDB Entry DOI: 10.7270/Q28W3GWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM50453810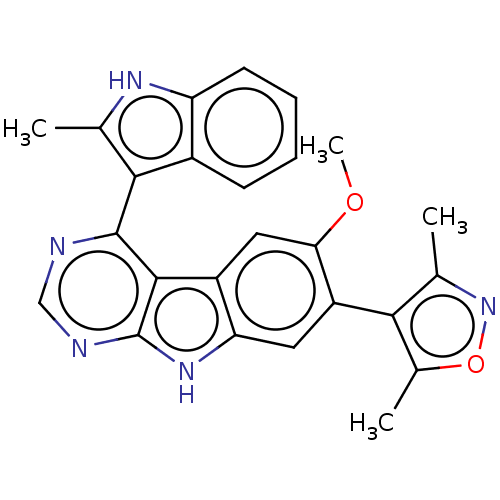 (CHEMBL4208405) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | <0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Inhibition of FAM-labeled ZBA248 binding to recombinant human N-terminal His6-tagged BRD4 bromodomain 1 (44 to 168 residues) expressed in Rosetta2 DE... | J Med Chem 60: 3887-3901 (2017) Article DOI: 10.1021/acs.jmedchem.7b00193 BindingDB Entry DOI: 10.7270/Q28W3GWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM50453807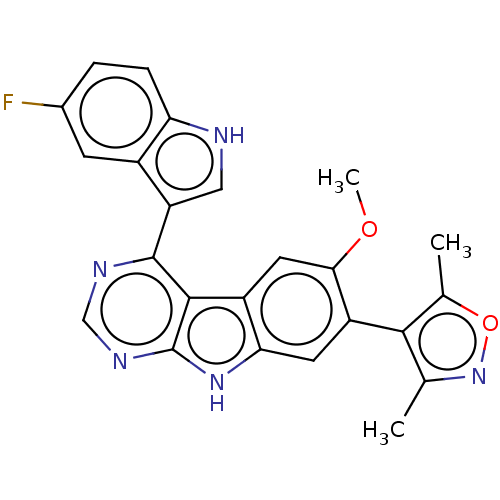 (CHEMBL4211497) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | <0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Inhibition of FAM-labeled ZBA248 binding to recombinant human N-terminal His6-tagged BRD4 bromodomain 1 (44 to 168 residues) expressed in Rosetta2 DE... | J Med Chem 60: 3887-3901 (2017) Article DOI: 10.1021/acs.jmedchem.7b00193 BindingDB Entry DOI: 10.7270/Q28W3GWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50075928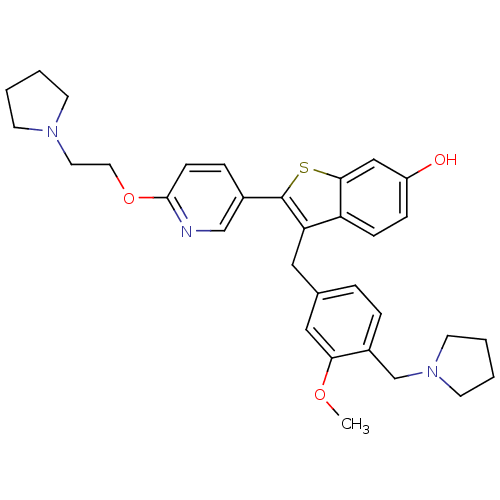 (3-(3-Methoxy-4-pyrrolidin-1-ylmethyl-benzyl)-2-[6-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Binding inhibition against thrombin was measured by nonlinear regression analysis using Morrison's equation for tight-binding inhibition | Bioorg Med Chem Lett 9: 775-80 (1999) BindingDB Entry DOI: 10.7270/Q2PR7V46 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Apoptosis regulator Bcl-2 (Homo sapiens (Human)) | BDBM161571 (US9108983, Example 386 | US9108983, Example 449) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | 0.530 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1016/j.ejmech.2022.114184 BindingDB Entry DOI: 10.7270/Q2M049DT | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 3 [326-398] (Homo sapiens (Human)) | BDBM179480 (US9675697, Cpd. No. 68) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.650 | -52.4 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
THE REGENTS OF THE UNIVERSITY OF MICHIGAN US Patent | Assay Description Fluorescence Polarization (FP) competitive binding studies (see above) were carried out using the FAM labeled fluorescent probe Cpd. No. 350 to deter... | US Patent US9675697 (2017) BindingDB Entry DOI: 10.7270/Q2CC0XV6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Baculoviral IAP repeat-containing protein 2 (Homo sapiens (Human)) | BDBM26223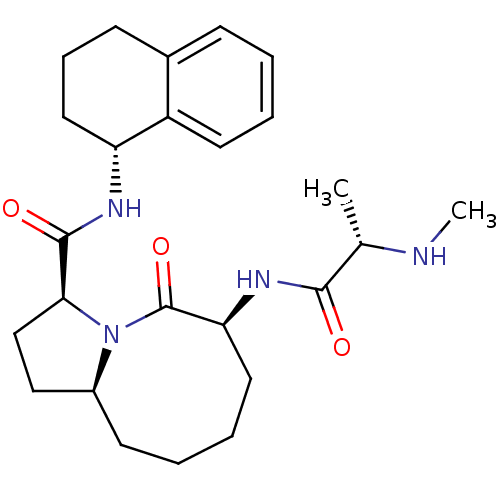 ((3S,6S,10aS)-6-[(2S)-2-(methylamino)propanamido]-5...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.680 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Displacement of fluorescent SM5F peptide from His-tagged human cIAP1 BIR3 domain expressed in Escherichia coli BL21(DE3) cells by fluorescence polari... | J Med Chem 52: 593-6 (2009) Article DOI: 10.1021/jm801101z BindingDB Entry DOI: 10.7270/Q2Z03816 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM50453810 (CHEMBL4208405) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Inhibition of FAM-labeled ZBA248 binding to recombinant human N-terminal His6-tagged BRD4 bromodomain 2 (333 to 460 residues) expressed in Rosetta2 D... | J Med Chem 60: 3887-3901 (2017) Article DOI: 10.1021/acs.jmedchem.7b00193 BindingDB Entry DOI: 10.7270/Q28W3GWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM50366716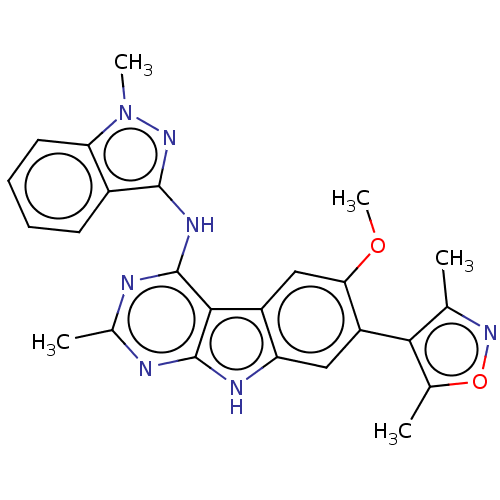 (CHEMBL4174669) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of FAM-labeled ZBA248 binding to recombinant human BRD4 bromodomain 1 (44 to 168 residues) expressed in Rosetta2 Escherichia coli DE3 cell... | J Med Chem 61: 6110-6120 (2018) Article DOI: 10.1021/acs.jmedchem.8b00483 BindingDB Entry DOI: 10.7270/Q26M39B1 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Apoptosis regulator Bcl-2 (Homo sapiens (Human)) | BDBM50266959 (CHEMBL4085804) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1016/j.ejmech.2022.114184 BindingDB Entry DOI: 10.7270/Q2M049DT | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM179480 (US9675697, Cpd. No. 68) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Inhibition of FAM-labeled ZBA248 binding to recombinant human N-terminal His6-tagged BRD4 bromodomain 1 (44 to 168 residues) expressed in Rosetta2 DE... | J Med Chem 60: 3887-3901 (2017) Article DOI: 10.1021/acs.jmedchem.7b00193 BindingDB Entry DOI: 10.7270/Q28W3GWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM179480 (US9675697, Cpd. No. 68) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of FAM-labeled ZBA248 binding to recombinant human BRD4 bromodomain 1 (44 to 168 residues) expressed in Rosetta2 Escherichia coli DE3 cell... | J Med Chem 61: 6110-6120 (2018) Article DOI: 10.1021/acs.jmedchem.8b00483 BindingDB Entry DOI: 10.7270/Q26M39B1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM50366724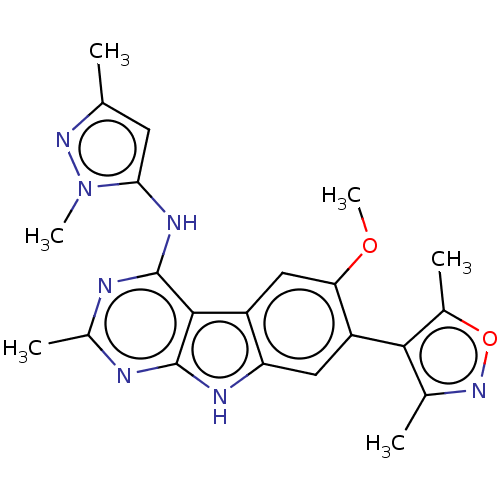 (CHEMBL4172277) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of FAM-labeled ZBA248 binding to recombinant human BRD4 bromodomain 1 (44 to 168 residues) expressed in Rosetta2 Escherichia coli DE3 cell... | J Med Chem 61: 6110-6120 (2018) Article DOI: 10.1021/acs.jmedchem.8b00483 BindingDB Entry DOI: 10.7270/Q26M39B1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Baculoviral IAP repeat-containing protein 2 (Homo sapiens (Human)) | BDBM26218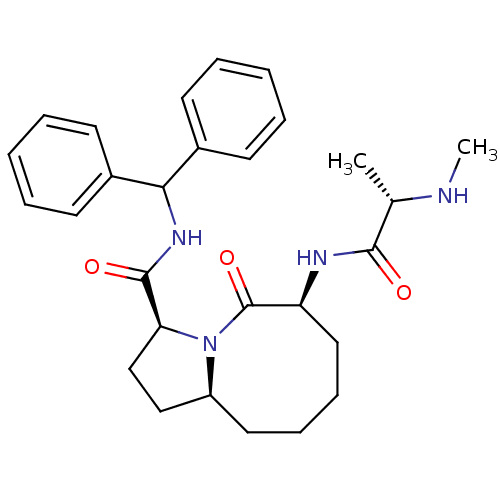 ((3S,6S,10aS)-N-(diphenylmethyl)-6-[(2S)-2-(methyla...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Displacement of fluorescent SM5F peptide from His-tagged human cIAP1 BIR3 domain expressed in Escherichia coli BL21(DE3) cells by fluorescence polari... | J Med Chem 52: 593-6 (2009) Article DOI: 10.1021/jm801101z BindingDB Entry DOI: 10.7270/Q2Z03816 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Apoptosis regulator Bcl-2 (Homo sapiens (Human)) | BDBM50388994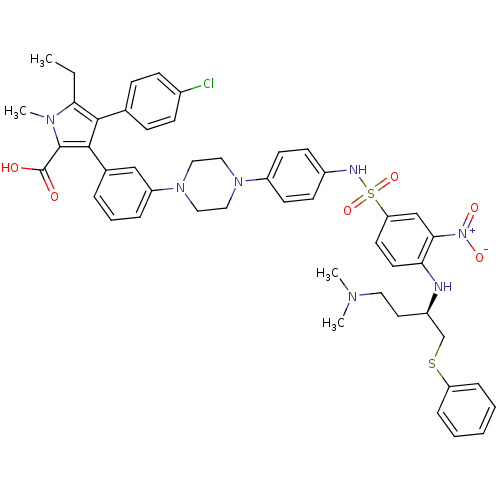 (CHEMBL2063897) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Binding affinity to N-terminus 6X His-tagged human Bcl2 expressed in Escherichia coli BL21 (DE3) cells after 2 hrs by fluorescence polarization assay | J Med Chem 55: 4664-82 (2012) Article DOI: 10.1021/jm300178u BindingDB Entry DOI: 10.7270/Q2862HHV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Apoptosis regulator Bcl-2 (Homo sapiens (Human)) | BDBM50397455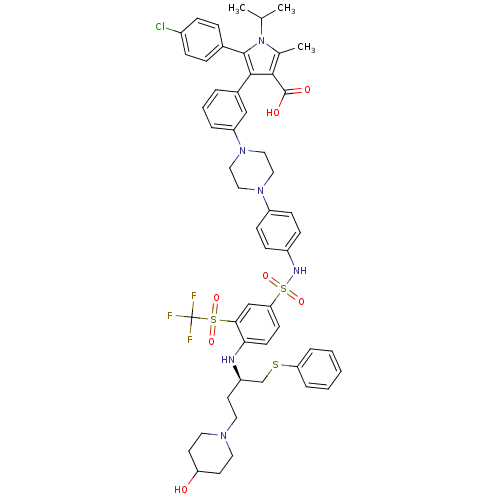 (CHEMBL2170838) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Binding affinity to N-terminal 6xHis-tagged human Bcl-2 expressed in Escherichia coli BL21 (DE3) after 2 hrs by fluorescence polarization assay | J Med Chem 55: 8502-14 (2012) Article DOI: 10.1021/jm3010306 BindingDB Entry DOI: 10.7270/Q2V69KQ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 3 [326-398] (Homo sapiens (Human)) | BDBM179633 (US9675697, Cpd. No. 316) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.820 | -51.9 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
THE REGENTS OF THE UNIVERSITY OF MICHIGAN US Patent | Assay Description Fluorescence Polarization (FP) competitive binding studies (see above) were carried out using the FAM labeled fluorescent probe Cpd. No. 350 to deter... | US Patent US9675697 (2017) BindingDB Entry DOI: 10.7270/Q2CC0XV6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 2 (Homo sapiens (Human)) | BDBM50459819 (CHEMBL4228445) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Comprehensive Cancer Center Curated by ChEMBL | Assay Description Inhibition of FAM-labeled ZBA248 binding to recombinant N-terminal His6-tagged BRD2 bromodomain 1 (72 to 205 residues) (unknown origin) expressed in ... | J Med Chem 61: 462-481 (2018) Article DOI: 10.1021/acs.jmedchem.6b01816 BindingDB Entry DOI: 10.7270/Q2CV4MD0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50075932 (2-(1,1-Dioxo-1lambda*6*-thiomorpholin-4-yl)-N-{4-[...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Binding inhibition against thrombin was measured by nonlinear regression analysis using Morrison's equation for tight-binding inhibition | Bioorg Med Chem Lett 9: 775-80 (1999) BindingDB Entry DOI: 10.7270/Q2PR7V46 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50075938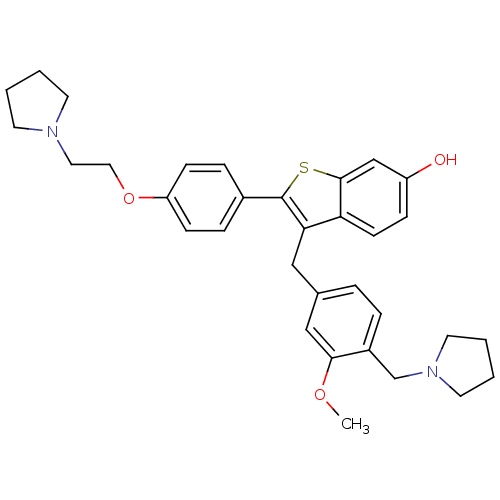 (3-(3-Methoxy-4-pyrrolidin-1-ylmethyl-benzyl)-2-[4-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Binding inhibition against thrombin was measured by nonlinear regression analysis using Morrison's equation for tight-binding inhibition | Bioorg Med Chem Lett 9: 775-80 (1999) BindingDB Entry DOI: 10.7270/Q2PR7V46 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM50453769 (CHEMBL4214978) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Inhibition of FAM-labeled ZBA248 binding to recombinant human N-terminal His6-tagged BRD4 bromodomain 2 (333 to 460 residues) expressed in Rosetta2 D... | J Med Chem 60: 3887-3901 (2017) Article DOI: 10.1021/acs.jmedchem.7b00193 BindingDB Entry DOI: 10.7270/Q28W3GWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50008837 (CHEMBL3236674) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 0.961 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing Medical University Curated by ChEMBL | Assay Description Displacement of [3H]U-69,593 from kappa opioid receptor in guinea pig brain membranes after 2 hrs | Bioorg Med Chem 22: 2333-8 (2014) Article DOI: 10.1016/j.bmc.2014.02.011 BindingDB Entry DOI: 10.7270/Q20P11JG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM50366699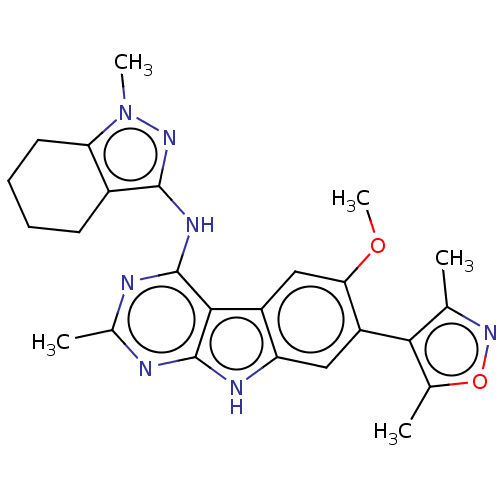 (CHEMBL4169068) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | <1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of FAM-labeled ZBA248 binding to recombinant human BRD4 bromodomain 1 (44 to 168 residues) expressed in Rosetta2 Escherichia coli DE3 cell... | J Med Chem 61: 6110-6120 (2018) Article DOI: 10.1021/acs.jmedchem.8b00483 BindingDB Entry DOI: 10.7270/Q26M39B1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM50366670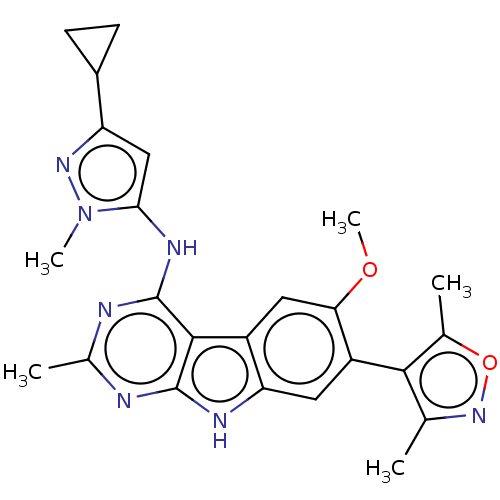 (CHEMBL4173488) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | <1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of FAM-labeled ZBA248 binding to recombinant human BRD4 bromodomain 1 (44 to 168 residues) expressed in Rosetta2 Escherichia coli DE3 cell... | J Med Chem 61: 6110-6120 (2018) Article DOI: 10.1021/acs.jmedchem.8b00483 BindingDB Entry DOI: 10.7270/Q26M39B1 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM50366710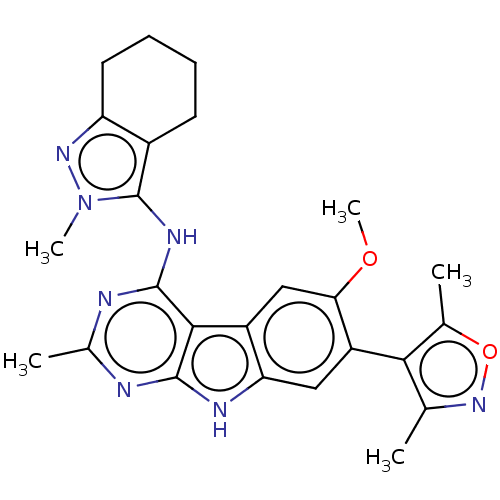 (CHEMBL4161145) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | <1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of FAM-labeled ZBA248 binding to recombinant human BRD4 bromodomain 1 (44 to 168 residues) expressed in Rosetta2 Escherichia coli DE3 cell... | J Med Chem 61: 6110-6120 (2018) Article DOI: 10.1021/acs.jmedchem.8b00483 BindingDB Entry DOI: 10.7270/Q26M39B1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 1914 total ) | Next | Last >> |