

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Anthranilate synthase component 1 (Salmonella typhimurium) | BDBM50200013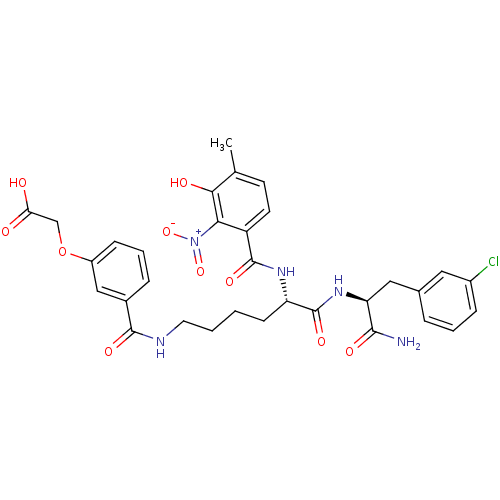 (2-(3-(((S)-6-((S)-1-amino-3-(3-chlorophenyl)-1-oxo...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of Salmonella typhimurium anthranilate synthase trpE:trpD expressed in Escherichia coli BL21(DE3) coexpressing stop codon in trpD gene for... | J Med Chem 53: 3718-29 (2010) Article DOI: 10.1021/jm100158v BindingDB Entry DOI: 10.7270/Q25T3KN4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Anthranilate synthase component 1 (Salmonella typhimurium) | BDBM50317145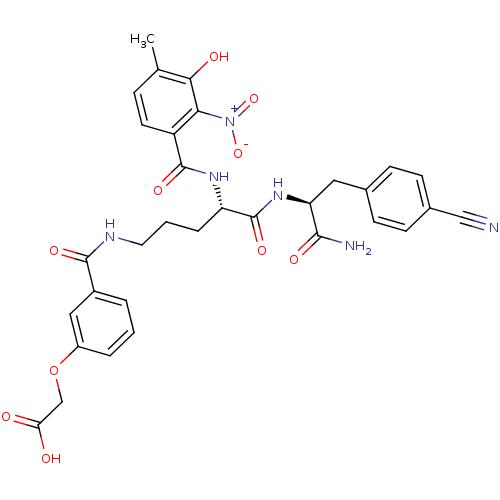 (2-(3-((S)-5-((S)-1-amino-3-(4-cyanophenyl)-1-oxopr...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of Salmonella typhimurium anthranilate synthase trpE:trpD expressed in Escherichia coli BL21(DE3) coexpressing stop codon in trpD gene for... | J Med Chem 53: 3718-29 (2010) Article DOI: 10.1021/jm100158v BindingDB Entry DOI: 10.7270/Q25T3KN4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Anthranilate synthase component 1 (Salmonella typhimurium) | BDBM50317147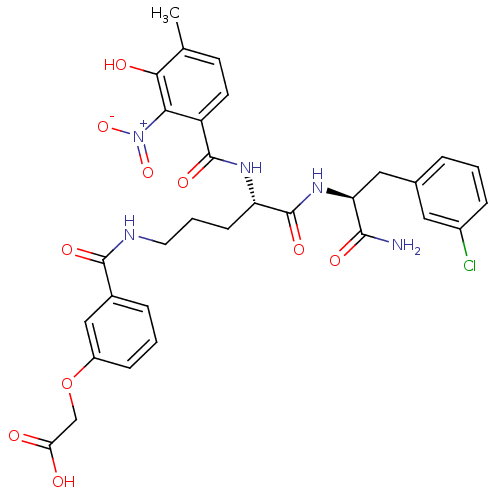 (2-(3-(-(S)-5-((S)-1-Amino-3-(3-chlorophenyl)-1-oxo...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of Salmonella typhimurium anthranilate synthase trpE:trpD expressed in Escherichia coli BL21(DE3) coexpressing stop codon in trpD gene for... | J Med Chem 53: 3718-29 (2010) Article DOI: 10.1021/jm100158v BindingDB Entry DOI: 10.7270/Q25T3KN4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Anthranilate synthase component 1 (Salmonella typhimurium) | BDBM50200013 (2-(3-(((S)-6-((S)-1-amino-3-(3-chlorophenyl)-1-oxo...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of Salmonella typhimurium anthranilate synthase trpE:trpD expressed in Escherichia coli BL21(DE3) coexpressing stop codon in trpD gene for... | J Med Chem 53: 3718-29 (2010) Article DOI: 10.1021/jm100158v BindingDB Entry DOI: 10.7270/Q25T3KN4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isochorismate synthase EntC (Escherichia coli (strain K12)) | BDBM50317142 (2-(3-((S)-5-((S)-1-amino-3-(3-hydroxy-4-nitropheny...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of Escherichia coli K12 IS expressed in Escherichia coli BL21(DE3) by spectrophotometry | J Med Chem 53: 3718-29 (2010) Article DOI: 10.1021/jm100158v BindingDB Entry DOI: 10.7270/Q25T3KN4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Anthranilate synthase component 1 (Salmonella typhimurium) | BDBM50317142 (2-(3-((S)-5-((S)-1-amino-3-(3-hydroxy-4-nitropheny...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 8.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of Salmonella typhimurium anthranilate synthase trpE:trpD expressed in Escherichia coli BL21(DE3) coexpressing stop codon in trpD gene for... | J Med Chem 53: 3718-29 (2010) Article DOI: 10.1021/jm100158v BindingDB Entry DOI: 10.7270/Q25T3KN4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Anthranilate synthase component 1 (Salmonella typhimurium) | BDBM50317145 (2-(3-((S)-5-((S)-1-amino-3-(4-cyanophenyl)-1-oxopr...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 8.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of Salmonella typhimurium anthranilate synthase trpE:trpD expressed in Escherichia coli BL21(DE3) coexpressing stop codon in trpD gene for... | J Med Chem 53: 3718-29 (2010) Article DOI: 10.1021/jm100158v BindingDB Entry DOI: 10.7270/Q25T3KN4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Anthranilate synthase component 1 (Salmonella typhimurium) | BDBM50317149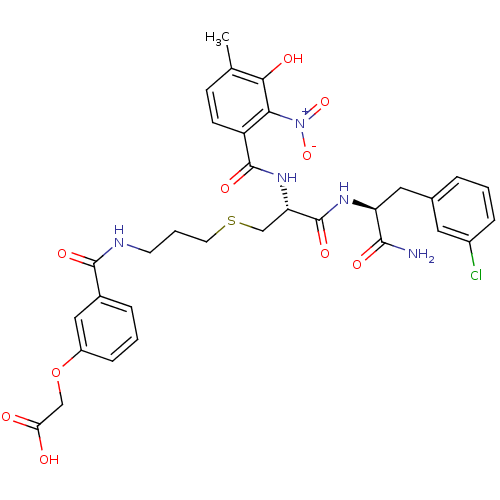 (2-(3-(3-((R)-3-((S)-1-Amino-3-(3-chlorophenyl)-1-o...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 9.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of Salmonella typhimurium anthranilate synthase trpE:trpD expressed in Escherichia coli BL21(DE3) coexpressing stop codon in trpD gene for... | J Med Chem 53: 3718-29 (2010) Article DOI: 10.1021/jm100158v BindingDB Entry DOI: 10.7270/Q25T3KN4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Anthranilate synthase component 1 (Salmonella typhimurium) | BDBM50317150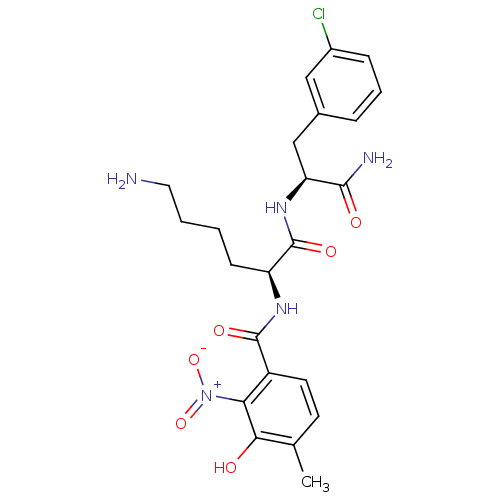 (CHEMBL1088356 | N-((S)-6-Amino-1-((S)-1-amino-3-(3...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.10E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of Salmonella typhimurium anthranilate synthase trpE:trpD expressed in Escherichia coli BL21(DE3) coexpressing stop codon in trpD gene for... | J Med Chem 53: 3718-29 (2010) Article DOI: 10.1021/jm100158v BindingDB Entry DOI: 10.7270/Q25T3KN4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Anthranilate synthase component 1 (Salmonella typhimurium) | BDBM50317141 (2-(3-((4S)-5-(2-(6-(3-amino-3-oxopropyl)dibenzo[b,...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.10E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of Salmonella typhimurium anthranilate synthase trpE:trpD expressed in Escherichia coli BL21(DE3) coexpressing stop codon in trpD gene for... | J Med Chem 53: 3718-29 (2010) Article DOI: 10.1021/jm100158v BindingDB Entry DOI: 10.7270/Q25T3KN4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isochorismate synthase EntC (Escherichia coli (strain K12)) | BDBM50317149 (2-(3-(3-((R)-3-((S)-1-Amino-3-(3-chlorophenyl)-1-o...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of Escherichia coli K12 IS expressed in Escherichia coli BL21(DE3) by spectrophotometry | J Med Chem 53: 3718-29 (2010) Article DOI: 10.1021/jm100158v BindingDB Entry DOI: 10.7270/Q25T3KN4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Anthranilate synthase component 1 (Salmonella typhimurium) | BDBM50317143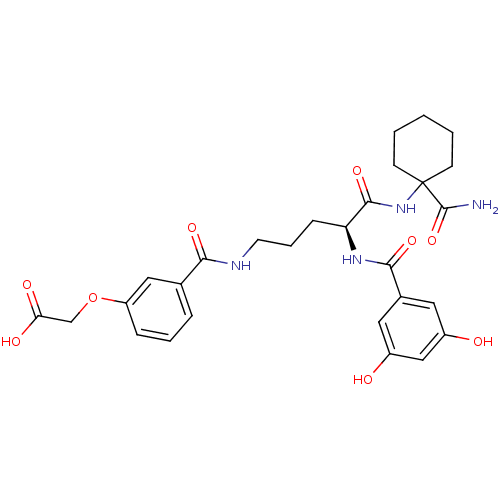 ((S)-2-(3-(5-(1-carbamoylcyclohexylamino)-4-(3,5-di...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.60E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of Salmonella typhimurium anthranilate synthase trpE:trpD expressed in Escherichia coli BL21(DE3) coexpressing stop codon in trpD gene for... | J Med Chem 53: 3718-29 (2010) Article DOI: 10.1021/jm100158v BindingDB Entry DOI: 10.7270/Q25T3KN4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isochorismate synthase EntC (Escherichia coli (strain K12)) | BDBM50317144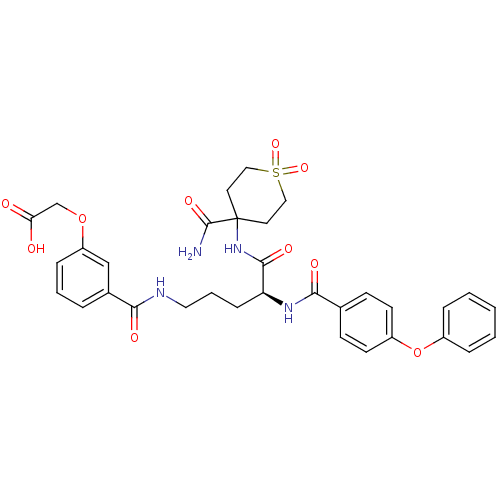 (CHEMBL1094071 | {3-[(S)-4-(4-Carbamoyl-1,1-dioxo-h...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.70E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of Escherichia coli K12 IS expressed in Escherichia coli BL21(DE3) by spectrophotometry | J Med Chem 53: 3718-29 (2010) Article DOI: 10.1021/jm100158v BindingDB Entry DOI: 10.7270/Q25T3KN4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isochorismate synthase EntC (Escherichia coli (strain K12)) | BDBM50317141 (2-(3-((4S)-5-(2-(6-(3-amino-3-oxopropyl)dibenzo[b,...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.90E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of Escherichia coli K12 IS expressed in Escherichia coli BL21(DE3) by spectrophotometry | J Med Chem 53: 3718-29 (2010) Article DOI: 10.1021/jm100158v BindingDB Entry DOI: 10.7270/Q25T3KN4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Anthranilate synthase component 1 (Salmonella typhimurium) | BDBM50317148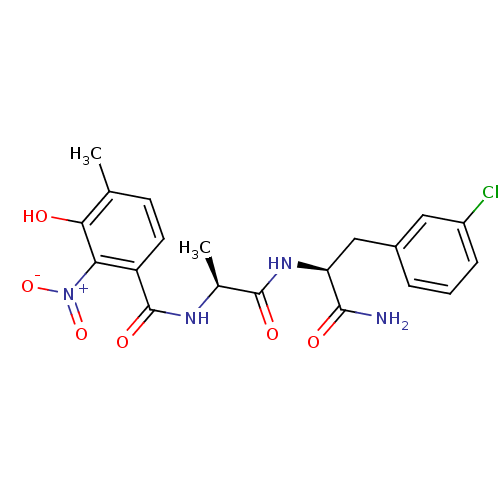 (CHEMBL1094717 | N-((S)-1-((S)-1-Amino-3-(3-chlorop...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of Salmonella typhimurium anthranilate synthase trpE:trpD expressed in Escherichia coli BL21(DE3) coexpressing stop codon in trpD gene for... | J Med Chem 53: 3718-29 (2010) Article DOI: 10.1021/jm100158v BindingDB Entry DOI: 10.7270/Q25T3KN4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Anthranilate synthase component 1 (Salmonella typhimurium) | BDBM50200003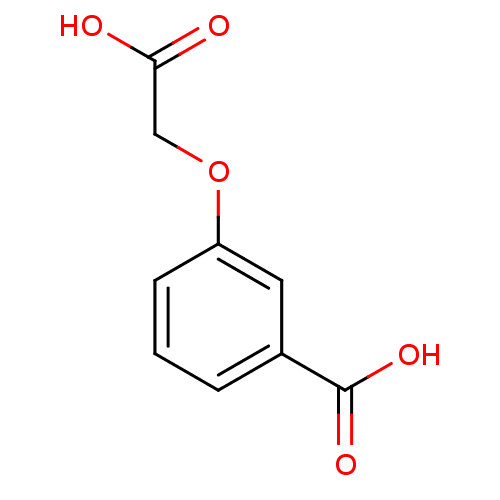 (3-(carboxymethoxy)benzoic acid | CHEMBL216545) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 2.10E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of Salmonella typhimurium anthranilate synthase trpE:trpD expressed in Escherichia coli BL21(DE3) coexpressing stop codon in trpD gene for... | J Med Chem 53: 3718-29 (2010) Article DOI: 10.1021/jm100158v BindingDB Entry DOI: 10.7270/Q25T3KN4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isochorismate synthase EntC (Escherichia coli (strain K12)) | BDBM50200013 (2-(3-(((S)-6-((S)-1-amino-3-(3-chlorophenyl)-1-oxo...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.10E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of Escherichia coli K12 IS expressed in Escherichia coli BL21(DE3) by spectrophotometry | J Med Chem 53: 3718-29 (2010) Article DOI: 10.1021/jm100158v BindingDB Entry DOI: 10.7270/Q25T3KN4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Anthranilate synthase component 1 (Salmonella typhimurium) | BDBM50317141 (2-(3-((4S)-5-(2-(6-(3-amino-3-oxopropyl)dibenzo[b,...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of Salmonella typhimurium anthranilate synthase trpE:trpD expressed in Escherichia coli BL21(DE3) coexpressing stop codon in trpD gene for... | J Med Chem 53: 3718-29 (2010) Article DOI: 10.1021/jm100158v BindingDB Entry DOI: 10.7270/Q25T3KN4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isochorismate synthase EntC (Escherichia coli (strain K12)) | BDBM50317145 (2-(3-((S)-5-((S)-1-amino-3-(4-cyanophenyl)-1-oxopr...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.60E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of Escherichia coli K12 IS expressed in Escherichia coli BL21(DE3) by spectrophotometry | J Med Chem 53: 3718-29 (2010) Article DOI: 10.1021/jm100158v BindingDB Entry DOI: 10.7270/Q25T3KN4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isochorismate synthase EntC (Escherichia coli (strain K12)) | BDBM50317147 (2-(3-(-(S)-5-((S)-1-Amino-3-(3-chlorophenyl)-1-oxo...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.60E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of Escherichia coli K12 IS expressed in Escherichia coli BL21(DE3) by spectrophotometry | J Med Chem 53: 3718-29 (2010) Article DOI: 10.1021/jm100158v BindingDB Entry DOI: 10.7270/Q25T3KN4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Anthranilate synthase component 1 (Salmonella typhimurium) | BDBM50317143 ((S)-2-(3-(5-(1-carbamoylcyclohexylamino)-4-(3,5-di...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.89E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of Salmonella typhimurium anthranilate synthase trpE:trpD expressed in Escherichia coli BL21(DE3) coexpressing stop codon in trpD gene for... | J Med Chem 53: 3718-29 (2010) Article DOI: 10.1021/jm100158v BindingDB Entry DOI: 10.7270/Q25T3KN4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isochorismate synthase EntC (Escherichia coli (strain K12)) | BDBM50317146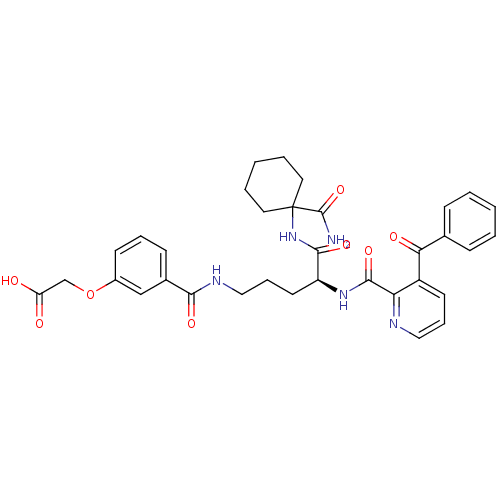 ((S)-2-(3-(4-(3-benzoylpicolinamido)-5-(1-carbamoyl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.30E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of Escherichia coli K12 IS expressed in Escherichia coli BL21(DE3) by spectrophotometry | J Med Chem 53: 3718-29 (2010) Article DOI: 10.1021/jm100158v BindingDB Entry DOI: 10.7270/Q25T3KN4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Anthranilate synthase component 1 (Salmonella typhimurium) | BDBM50317146 ((S)-2-(3-(4-(3-benzoylpicolinamido)-5-(1-carbamoyl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.90E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of Salmonella typhimurium anthranilate synthase trpE:trpD expressed in Escherichia coli BL21(DE3) coexpressing stop codon in trpD gene for... | J Med Chem 53: 3718-29 (2010) Article DOI: 10.1021/jm100158v BindingDB Entry DOI: 10.7270/Q25T3KN4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isochorismate synthase EntC (Escherichia coli (strain K12)) | BDBM50317148 (CHEMBL1094717 | N-((S)-1-((S)-1-Amino-3-(3-chlorop...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of Escherichia coli K12 IS expressed in Escherichia coli BL21(DE3) by spectrophotometry | J Med Chem 53: 3718-29 (2010) Article DOI: 10.1021/jm100158v BindingDB Entry DOI: 10.7270/Q25T3KN4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Anthranilate synthase component 1 (Salmonella typhimurium) | BDBM50200003 (3-(carboxymethoxy)benzoic acid | CHEMBL216545) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 5.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of Salmonella typhimurium anthranilate synthase trpE:trpD expressed in Escherichia coli BL21(DE3) coexpressing stop codon in trpD gene for... | J Med Chem 53: 3718-29 (2010) Article DOI: 10.1021/jm100158v BindingDB Entry DOI: 10.7270/Q25T3KN4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Anthranilate synthase component 1 (Salmonella typhimurium) | BDBM50317144 (CHEMBL1094071 | {3-[(S)-4-(4-Carbamoyl-1,1-dioxo-h...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.70E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of Salmonella typhimurium anthranilate synthase trpE:trpD expressed in Escherichia coli BL21(DE3) coexpressing stop codon in trpD gene for... | J Med Chem 53: 3718-29 (2010) Article DOI: 10.1021/jm100158v BindingDB Entry DOI: 10.7270/Q25T3KN4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Anthranilate synthase component 1 (Salmonella typhimurium) | BDBM50200006 (2-(3-carbamoylphenoxy)acetic acid | CHEMBL216601 |...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents | Article PubMed | 7.60E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of Salmonella typhimurium anthranilate synthase trpE:trpD expressed in Escherichia coli BL21(DE3) coexpressing stop codon in trpD gene for... | J Med Chem 53: 3718-29 (2010) Article DOI: 10.1021/jm100158v BindingDB Entry DOI: 10.7270/Q25T3KN4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aminodeoxychorismate lyase (Escherichia coli (strain K12)) | BDBM50200013 (2-(3-(((S)-6-((S)-1-amino-3-(3-chlorophenyl)-1-oxo...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7.70E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of Escherichia coli K12 ADCS by spectrophotometry | J Med Chem 53: 3718-29 (2010) Article DOI: 10.1021/jm100158v BindingDB Entry DOI: 10.7270/Q25T3KN4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isochorismate synthase EntC (Escherichia coli (strain K12)) | BDBM50317150 (CHEMBL1088356 | N-((S)-6-Amino-1-((S)-1-amino-3-(3...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7.90E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of Escherichia coli K12 IS expressed in Escherichia coli BL21(DE3) by spectrophotometry | J Med Chem 53: 3718-29 (2010) Article DOI: 10.1021/jm100158v BindingDB Entry DOI: 10.7270/Q25T3KN4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aminodeoxychorismate lyase (Escherichia coli (strain K12)) | BDBM50317147 (2-(3-(-(S)-5-((S)-1-Amino-3-(3-chlorophenyl)-1-oxo...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of Escherichia coli K12 ADCS by spectrophotometry | J Med Chem 53: 3718-29 (2010) Article DOI: 10.1021/jm100158v BindingDB Entry DOI: 10.7270/Q25T3KN4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Anthranilate synthase component 1 (Salmonella typhimurium) | BDBM50317144 (CHEMBL1094071 | {3-[(S)-4-(4-Carbamoyl-1,1-dioxo-h...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.02E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of Salmonella typhimurium anthranilate synthase trpE:trpD expressed in Escherichia coli BL21(DE3) coexpressing stop codon in trpD gene for... | J Med Chem 53: 3718-29 (2010) Article DOI: 10.1021/jm100158v BindingDB Entry DOI: 10.7270/Q25T3KN4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Anthranilate synthase component 1 (Salmonella typhimurium) | BDBM50317146 ((S)-2-(3-(4-(3-benzoylpicolinamido)-5-(1-carbamoyl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.08E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of Salmonella typhimurium anthranilate synthase trpE:trpD expressed in Escherichia coli BL21(DE3) coexpressing stop codon in trpD gene for... | J Med Chem 53: 3718-29 (2010) Article DOI: 10.1021/jm100158v BindingDB Entry DOI: 10.7270/Q25T3KN4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isochorismate synthase EntC (Escherichia coli (strain K12)) | BDBM50317143 ((S)-2-(3-(5-(1-carbamoylcyclohexylamino)-4-(3,5-di...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.20E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of Escherichia coli K12 IS expressed in Escherichia coli BL21(DE3) by spectrophotometry | J Med Chem 53: 3718-29 (2010) Article DOI: 10.1021/jm100158v BindingDB Entry DOI: 10.7270/Q25T3KN4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isochorismate synthase EntC (Escherichia coli (strain K12)) | BDBM50200003 (3-(carboxymethoxy)benzoic acid | CHEMBL216545) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 1.30E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of Escherichia coli K12 IS expressed in Escherichia coli BL21(DE3) by spectrophotometry | J Med Chem 53: 3718-29 (2010) Article DOI: 10.1021/jm100158v BindingDB Entry DOI: 10.7270/Q25T3KN4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aminodeoxychorismate lyase (Escherichia coli (strain K12)) | BDBM50317149 (2-(3-(3-((R)-3-((S)-1-Amino-3-(3-chlorophenyl)-1-o...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.50E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of Escherichia coli K12 ADCS by spectrophotometry | J Med Chem 53: 3718-29 (2010) Article DOI: 10.1021/jm100158v BindingDB Entry DOI: 10.7270/Q25T3KN4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Anthranilate synthase component 1 (Salmonella typhimurium) | BDBM50200006 (2-(3-carbamoylphenoxy)acetic acid | CHEMBL216601 |...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents | Article PubMed | 2.60E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of Salmonella typhimurium anthranilate synthase trpE:trpD expressed in Escherichia coli BL21(DE3) coexpressing stop codon in trpD gene for... | J Med Chem 53: 3718-29 (2010) Article DOI: 10.1021/jm100158v BindingDB Entry DOI: 10.7270/Q25T3KN4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isochorismate synthase EntC (Escherichia coli (strain K12)) | BDBM50200006 (2-(3-carbamoylphenoxy)acetic acid | CHEMBL216601 |...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents | Article PubMed | 2.60E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of Escherichia coli K12 IS expressed in Escherichia coli BL21(DE3) by spectrophotometry | J Med Chem 53: 3718-29 (2010) Article DOI: 10.1021/jm100158v BindingDB Entry DOI: 10.7270/Q25T3KN4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aminodeoxychorismate lyase (Escherichia coli (strain K12)) | BDBM50317150 (CHEMBL1088356 | N-((S)-6-Amino-1-((S)-1-amino-3-(3...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.70E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of Escherichia coli K12 ADCS by spectrophotometry | J Med Chem 53: 3718-29 (2010) Article DOI: 10.1021/jm100158v BindingDB Entry DOI: 10.7270/Q25T3KN4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aminodeoxychorismate lyase (Escherichia coli (strain K12)) | BDBM50317148 (CHEMBL1094717 | N-((S)-1-((S)-1-Amino-3-(3-chlorop...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.80E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of Escherichia coli K12 ADCS by spectrophotometry | J Med Chem 53: 3718-29 (2010) Article DOI: 10.1021/jm100158v BindingDB Entry DOI: 10.7270/Q25T3KN4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform (Homo sapiens (Human)) | BDBM25028 (4-[2-(1H-indazol-4-yl)-6-[(4-methanesulfonylpipera...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112904 BindingDB Entry DOI: 10.7270/Q2XP791Q | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50606478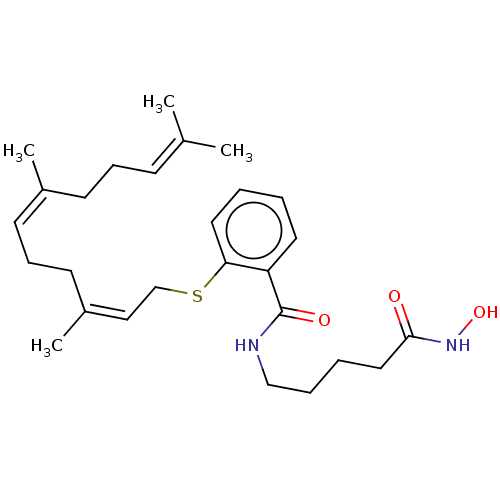 (CHEMBL5220511) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.580 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112904 BindingDB Entry DOI: 10.7270/Q2XP791Q | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 4 (Homo sapiens (Human)) | BDBM50606473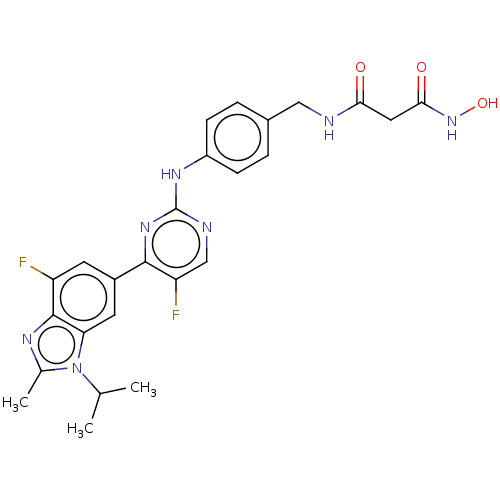 (CHEMBL5220945) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112904 BindingDB Entry DOI: 10.7270/Q2XP791Q | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50029668 (AZD-9291 | Osimertinib | US10085983, Compound AZD-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112904 BindingDB Entry DOI: 10.7270/Q2XP791Q | ||||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cyclin-dependent kinase 4 (Homo sapiens (Human)) | BDBM50110183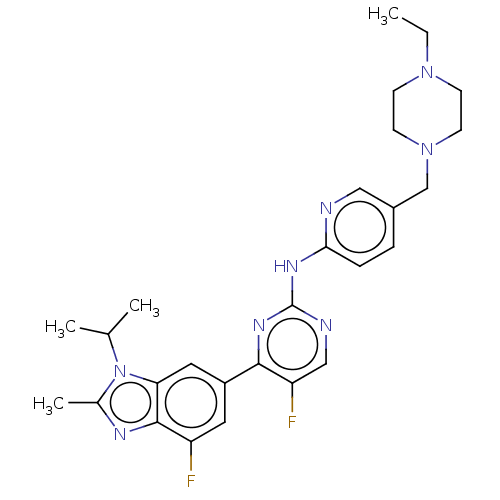 (Abemaciclib | LY-2835219 | US10626107, Example LY2...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112904 BindingDB Entry DOI: 10.7270/Q2XP791Q | ||||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50606482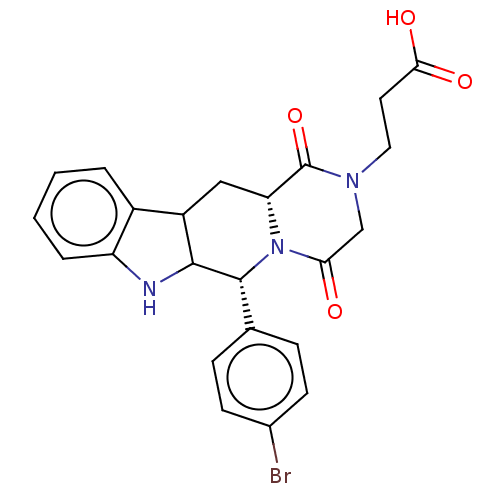 (CHEMBL5220907) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112904 BindingDB Entry DOI: 10.7270/Q2XP791Q | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50029668 (AZD-9291 | Osimertinib | US10085983, Compound AZD-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112904 BindingDB Entry DOI: 10.7270/Q2XP791Q | ||||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Histone deacetylase (Homo sapiens (Human)) | BDBM50142796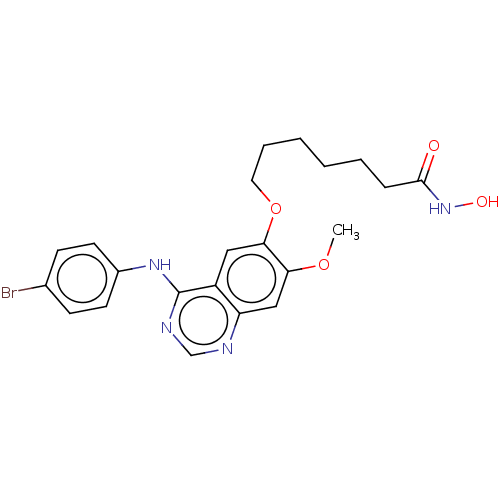 (CHEMBL3759186) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112904 BindingDB Entry DOI: 10.7270/Q2XP791Q | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM50591644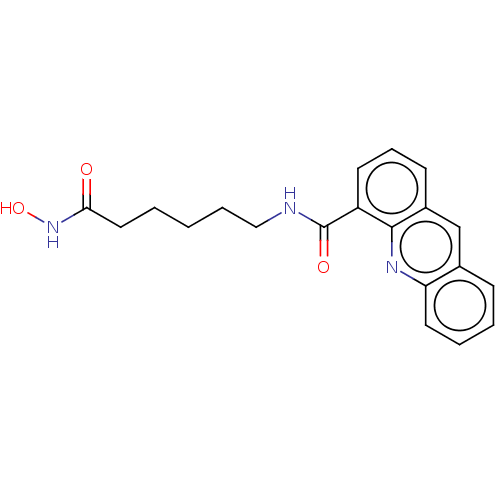 (CHEMBL5206589) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112904 BindingDB Entry DOI: 10.7270/Q2XP791Q | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50307768 (7-(4-(3-Ethynylphenylamino)-7-methoxyquinazolin-6-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112904 BindingDB Entry DOI: 10.7270/Q2XP791Q | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase (Homo sapiens (Human)) | BDBM50606484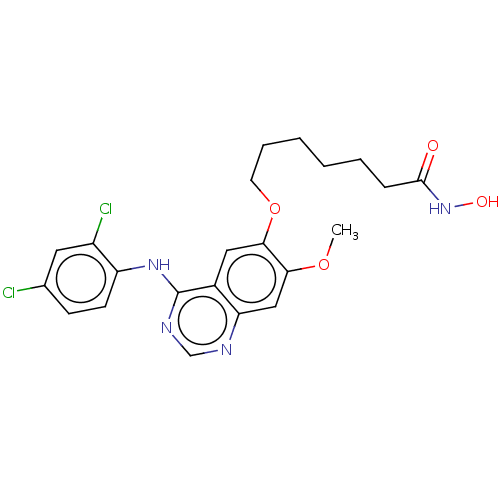 (CHEMBL5220848) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112904 BindingDB Entry DOI: 10.7270/Q2XP791Q | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 277 total ) | Next | Last >> |