

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50126474 (2-[4-(4-Naphthalen-1-yl-piperazin-1-yl)-butyl]-tet...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Complutense Curated by ChEMBL | Assay Description Binding affinity of the compound towards 5-HT 1A receptor in rat cerebral cortex membranes using [3H]-8-OH-DPAT as radioligand | Bioorg Med Chem Lett 13: 1429-32 (2003) BindingDB Entry DOI: 10.7270/Q2BV7FZX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50126481 (2-[4-(1H-Benzoimidazol-4-yl)-piperazin-1-ylmethyl]...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Complutense Curated by ChEMBL | Assay Description Binding affinity of the compound towards 5-HT 1A receptor in rat cerebral cortex membranes using [3H]-8-OH-DPAT as radioligand | Bioorg Med Chem Lett 13: 1429-32 (2003) BindingDB Entry DOI: 10.7270/Q2BV7FZX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50126475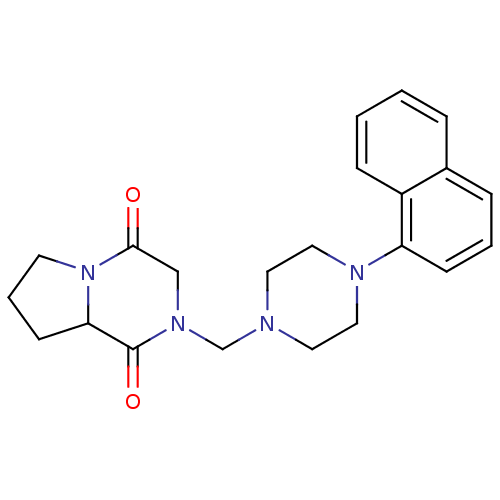 (2-(4-Naphthalen-1-yl-piperazin-1-ylmethyl)-hexahyd...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Complutense Curated by ChEMBL | Assay Description Binding affinity of the compound towards 5-HT 1A receptor in rat cerebral cortex membranes using [3H]-8-OH-DPAT as radioligand | Bioorg Med Chem Lett 13: 1429-32 (2003) BindingDB Entry DOI: 10.7270/Q2BV7FZX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50126477 (2-(4-Naphthalen-1-yl-piperazin-1-ylmethyl)-tetrahy...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 5.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Complutense Curated by ChEMBL | Assay Description Binding affinity of the compound towards 5-HT 1A receptor in rat cerebral cortex membranes using [3H]-8-OH-DPAT as radioligand | Bioorg Med Chem Lett 13: 1429-32 (2003) BindingDB Entry DOI: 10.7270/Q2BV7FZX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50451842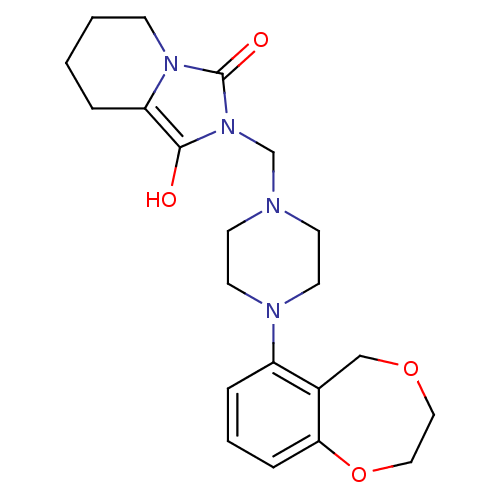 (CHEMBL2113339) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 6.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Complutense Curated by ChEMBL | Assay Description Binding affinity of the compound towards 5-HT 1A receptor in rat cerebral cortex membranes using [3H]-8-OH-DPAT as radioligand | Bioorg Med Chem Lett 13: 1429-32 (2003) BindingDB Entry DOI: 10.7270/Q2BV7FZX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 9 (Homo sapiens (Human)) | BDBM50364378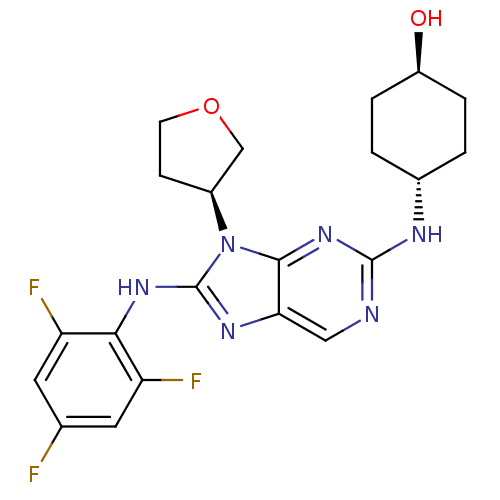 (CHEMBL1950289) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 6.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celgene Corporation Curated by ChEMBL | Assay Description Inhibition of hexa-His-tagged JNK2 expressed in baculoviral system using GST-tagged cJun as substrate preincubated for 15 mins prior ATP addition mea... | Bioorg Med Chem Lett 22: 1433-8 (2012) Article DOI: 10.1016/j.bmcl.2011.12.027 BindingDB Entry DOI: 10.7270/Q2C829SK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50126480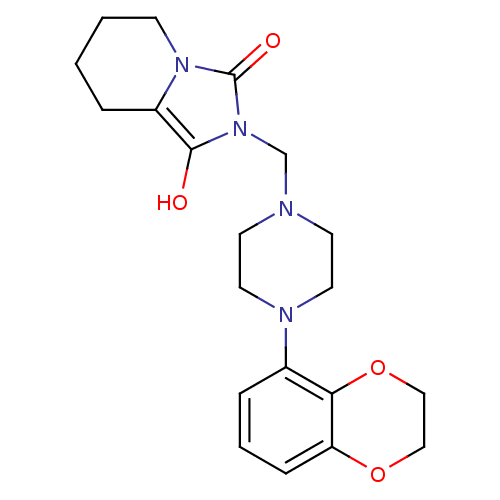 (2-[4-(2,3-Dihydro-benzo[1,4]dioxin-5-yl)-piperazin...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 9.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Complutense Curated by ChEMBL | Assay Description Binding affinity of the compound towards 5-HT 1A receptor in rat cerebral cortex membranes using [3H]-8-OH-DPAT as radioligand | Bioorg Med Chem Lett 13: 1429-32 (2003) BindingDB Entry DOI: 10.7270/Q2BV7FZX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50126476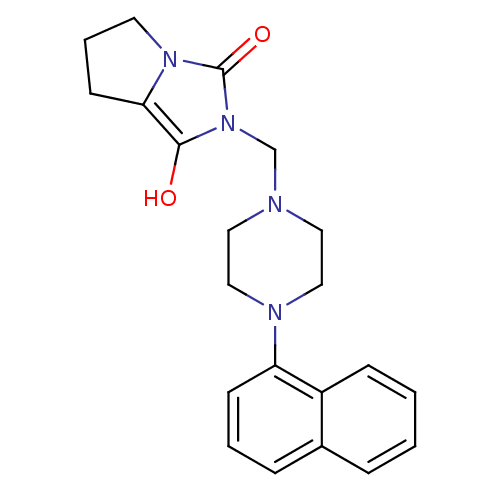 (2-(4-Naphthalen-1-yl-piperazin-1-ylmethyl)-tetrahy...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Complutense Curated by ChEMBL | Assay Description Binding affinity of the compound towards 5-HT 1A receptor in rat cerebral cortex membranes using [3H]-8-OH-DPAT as radioligand | Bioorg Med Chem Lett 13: 1429-32 (2003) BindingDB Entry DOI: 10.7270/Q2BV7FZX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50451843 (CHEMBL2113340) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Complutense Curated by ChEMBL | Assay Description Binding affinity of the compound towards 5-HT 1A receptor in rat cerebral cortex membranes using [3H]-8-OH-DPAT as radioligand | Bioorg Med Chem Lett 13: 1429-32 (2003) BindingDB Entry DOI: 10.7270/Q2BV7FZX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Rattus norvegicus (rat)) | BDBM50126475 (2-(4-Naphthalen-1-yl-piperazin-1-ylmethyl)-hexahyd...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Complutense Curated by ChEMBL | Assay Description Binding affinity of the compound towards 5-HT 2A receptor in rat cerebral frontal cortex membranes using [3H]-ketanserin as radioligand | Bioorg Med Chem Lett 13: 1429-32 (2003) BindingDB Entry DOI: 10.7270/Q2BV7FZX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 8 (Homo sapiens (Human)) | BDBM50364378 (CHEMBL1950289) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 44 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celgene Corporation Curated by ChEMBL | Assay Description Inhibition of hexa-His-tagged JNK1 expressed in baculoviral system using GST-tagged c-Jun as substrate preincubated for 15 mins prior ATP addition me... | Bioorg Med Chem Lett 22: 1433-8 (2012) Article DOI: 10.1016/j.bmcl.2011.12.027 BindingDB Entry DOI: 10.7270/Q2C829SK | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Alpha-1A/Alpha-1B/Alpha-1D adrenergic receptor (Rattus norvegicus (rat)-Rattus norvegicus (Rat)) | BDBM50126474 (2-[4-(4-Naphthalen-1-yl-piperazin-1-yl)-butyl]-tet...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 65 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Complutense Curated by ChEMBL | Assay Description Binding affinity towards Alpha-1 adrenergic receptor in rat cerebral cortex membranes using [3H]prazosin as radioligand | Bioorg Med Chem Lett 13: 1429-32 (2003) BindingDB Entry DOI: 10.7270/Q2BV7FZX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 7 (Rattus norvegicus (rat)) | BDBM50126475 (2-(4-Naphthalen-1-yl-piperazin-1-ylmethyl)-hexahyd...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 101 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Complutense Curated by ChEMBL | Assay Description Binding affinity of the compound towards 5-HT 7 receptor in rat hypothalamus membranes using [3H]-5-CT as radioligand | Bioorg Med Chem Lett 13: 1429-32 (2003) BindingDB Entry DOI: 10.7270/Q2BV7FZX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50126475 (2-(4-Naphthalen-1-yl-piperazin-1-ylmethyl)-hexahyd...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 192 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Complutense Curated by ChEMBL | Assay Description Binding affinity of the compound towards dopamine D2 receptor in rat striatum membranes using [3H]-raclopride as radioligand | Bioorg Med Chem Lett 13: 1429-32 (2003) BindingDB Entry DOI: 10.7270/Q2BV7FZX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50126475 (2-(4-Naphthalen-1-yl-piperazin-1-ylmethyl)-hexahyd...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 976 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Complutense Curated by ChEMBL | Assay Description Binding affinity of the compound towards 5-HT transporter in rat cerebral cortex membranes using [3H]-paroxetine as radioligand | Bioorg Med Chem Lett 13: 1429-32 (2003) BindingDB Entry DOI: 10.7270/Q2BV7FZX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A/Alpha-1B/Alpha-1D adrenergic receptor (Rattus norvegicus (rat)-Rattus norvegicus (Rat)) | BDBM50451843 (CHEMBL2113340) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Complutense Curated by ChEMBL | Assay Description Binding affinity towards Alpha-1 adrenergic receptor in rat cerebral cortex membranes using [3H]prazosin as radioligand | Bioorg Med Chem Lett 13: 1429-32 (2003) BindingDB Entry DOI: 10.7270/Q2BV7FZX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A/Alpha-1B/Alpha-1D adrenergic receptor (Rattus norvegicus (rat)-Rattus norvegicus (Rat)) | BDBM50451842 (CHEMBL2113339) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Complutense Curated by ChEMBL | Assay Description Binding affinity towards Alpha-1 adrenergic receptor in rat cerebral cortex membranes using [3H]prazosin as radioligand | Bioorg Med Chem Lett 13: 1429-32 (2003) BindingDB Entry DOI: 10.7270/Q2BV7FZX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A/Alpha-1B/Alpha-1D adrenergic receptor (Rattus norvegicus (rat)-Rattus norvegicus (Rat)) | BDBM50126477 (2-(4-Naphthalen-1-yl-piperazin-1-ylmethyl)-tetrahy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Complutense Curated by ChEMBL | Assay Description Binding affinity towards Alpha-1 adrenergic receptor in rat cerebral cortex membranes using [3H]prazosin as radioligand | Bioorg Med Chem Lett 13: 1429-32 (2003) BindingDB Entry DOI: 10.7270/Q2BV7FZX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A/Alpha-1B/Alpha-1D adrenergic receptor (Rattus norvegicus (rat)-Rattus norvegicus (Rat)) | BDBM50126480 (2-[4-(2,3-Dihydro-benzo[1,4]dioxin-5-yl)-piperazin...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Complutense Curated by ChEMBL | Assay Description Binding affinity towards Alpha-1 adrenergic receptor in rat cerebral cortex membranes using [3H]prazosin as radioligand | Bioorg Med Chem Lett 13: 1429-32 (2003) BindingDB Entry DOI: 10.7270/Q2BV7FZX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A/Alpha-1B/Alpha-1D adrenergic receptor (Rattus norvegicus (rat)-Rattus norvegicus (Rat)) | BDBM50126476 (2-(4-Naphthalen-1-yl-piperazin-1-ylmethyl)-tetrahy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Complutense Curated by ChEMBL | Assay Description Binding affinity towards Alpha-1 adrenergic receptor in rat cerebral cortex membranes using [3H]prazosin as radioligand | Bioorg Med Chem Lett 13: 1429-32 (2003) BindingDB Entry DOI: 10.7270/Q2BV7FZX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A/Alpha-1B/Alpha-1D adrenergic receptor (Rattus norvegicus (rat)-Rattus norvegicus (Rat)) | BDBM50126475 (2-(4-Naphthalen-1-yl-piperazin-1-ylmethyl)-hexahyd...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Complutense Curated by ChEMBL | Assay Description Binding affinity towards Alpha-1 adrenergic receptor in rat cerebral cortex membranes using [3H]prazosin as radioligand | Bioorg Med Chem Lett 13: 1429-32 (2003) BindingDB Entry DOI: 10.7270/Q2BV7FZX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A/Alpha-1B/Alpha-1D adrenergic receptor (Rattus norvegicus (rat)-Rattus norvegicus (Rat)) | BDBM50126481 (2-[4-(1H-Benzoimidazol-4-yl)-piperazin-1-ylmethyl]...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Complutense Curated by ChEMBL | Assay Description Binding affinity towards Alpha-1 adrenergic receptor in rat cerebral cortex membranes using [3H]prazosin as radioligand | Bioorg Med Chem Lett 13: 1429-32 (2003) BindingDB Entry DOI: 10.7270/Q2BV7FZX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (RAT) | BDBM50126475 (2-(4-Naphthalen-1-yl-piperazin-1-ylmethyl)-hexahyd...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Complutense Curated by ChEMBL | Assay Description Binding affinity of the compound towards 5-HT 4 receptor in rat striatum membranes using [3H]-GR-113,808 as radioligand | Bioorg Med Chem Lett 13: 1429-32 (2003) BindingDB Entry DOI: 10.7270/Q2BV7FZX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C theta type (Homo sapiens (Human)) | BDBM251460 (US9452998, 9) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of PKA-theta (unknown origin) using Fam-labelled S6-derived peptide incubated for 2 hrs by TR-FRET assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00388 BindingDB Entry DOI: 10.7270/Q22J6GPS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 8 (Homo sapiens (Human)) | BDBM50578360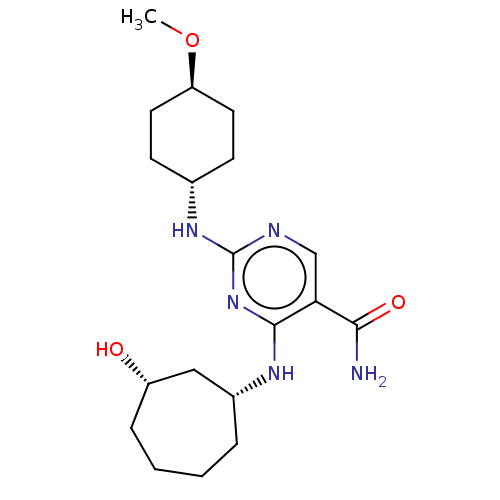 (CHEMBL4849353) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of JNK1 (unknown origin) assessed as reduction in phosphorylated ULight-labeled 4EBP1 peptide measured after 1 hr by time-resolved fluores... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01716 BindingDB Entry DOI: 10.7270/Q22Z19CX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 8 (Homo sapiens (Human)) | BDBM50578357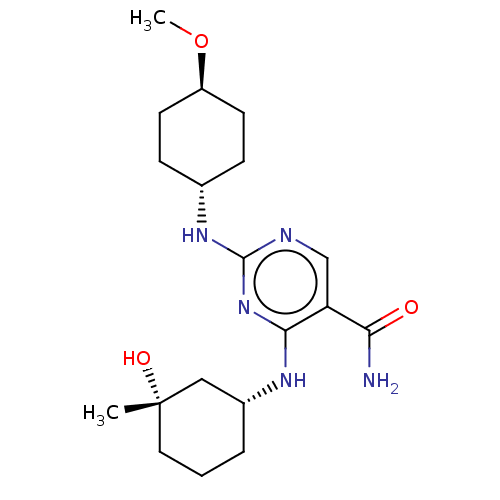 (CHEMBL4856984) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of JNK1 (unknown origin) assessed as reduction in phosphorylated ULight-labeled 4EBP1 peptide measured after 1 hr by time-resolved fluores... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01716 BindingDB Entry DOI: 10.7270/Q22Z19CX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C theta type (Homo sapiens (Human)) | BDBM50573043 (CHEMBL4849510) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of PKA-theta (unknown origin) using Fam-labelled S6-derived peptide incubated for 2 hrs by TR-FRET assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00388 BindingDB Entry DOI: 10.7270/Q22J6GPS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C theta type (Homo sapiens (Human)) | BDBM50573038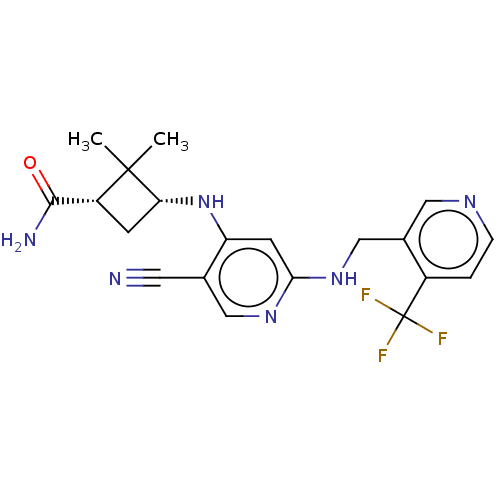 (CHEMBL4853353) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of PKA-theta (unknown origin) using Fam-labelled S6-derived peptide incubated for 2 hrs by TR-FRET assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00388 BindingDB Entry DOI: 10.7270/Q22J6GPS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclic AMP-dependent transcription factor ATF-4 (Human) | BDBM524158 (US11166942, Compound 60) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The ATF4 reporter was prepared by fusing the human full length 5′UTR of ATF4 (NCBI Accession No. BC022088.2) upstream of the firefly luciferase... | Citation and Details BindingDB Entry DOI: 10.7270/Q2QJ7MG1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclic AMP-dependent transcription factor ATF-4 (Human) | BDBM524152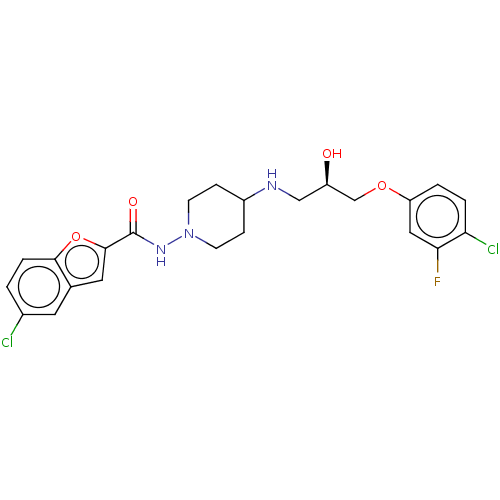 (US11166942, Compound 54) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The ATF4 reporter was prepared by fusing the human full length 5′UTR of ATF4 (NCBI Accession No. BC022088.2) upstream of the firefly luciferase... | Citation and Details BindingDB Entry DOI: 10.7270/Q2QJ7MG1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C alpha type (Homo sapiens (Human)) | BDBM251460 (US9452998, 9) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of PKC-alpha (unknown origin) | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00388 BindingDB Entry DOI: 10.7270/Q22J6GPS | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cyclic AMP-dependent transcription factor ATF-4 (Human) | BDBM524148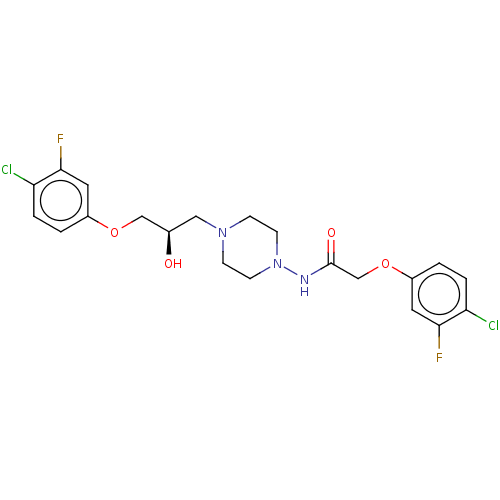 (US11166942, Compound 50) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.93 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The ATF4 reporter was prepared by fusing the human full length 5′UTR of ATF4 (NCBI Accession No. BC022088.2) upstream of the firefly luciferase... | Citation and Details BindingDB Entry DOI: 10.7270/Q2QJ7MG1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclic AMP-dependent transcription factor ATF-4 (Human) | BDBM524138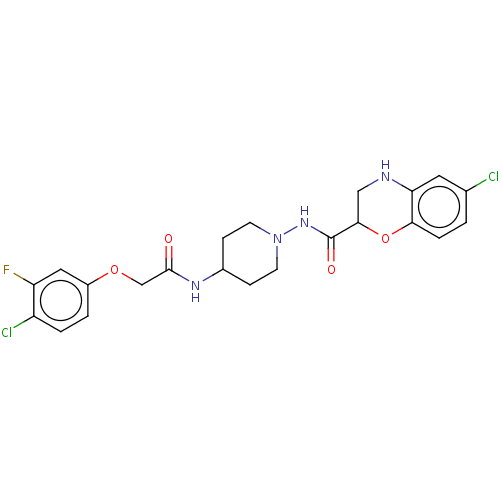 (US11166942, Compound 31 | US11166942, Compound 47) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.98 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The ATF4 reporter was prepared by fusing the human full length 5′UTR of ATF4 (NCBI Accession No. BC022088.2) upstream of the firefly luciferase... | Citation and Details BindingDB Entry DOI: 10.7270/Q2QJ7MG1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 8 (Homo sapiens (Human)) | BDBM50578347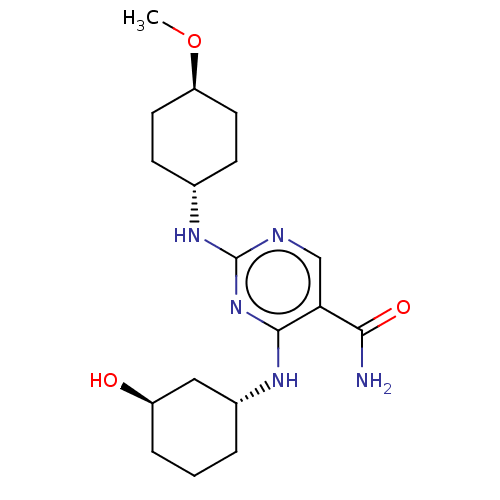 (CHEMBL4853125) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of JNK1 (unknown origin) assessed as reduction in phosphorylated ULight-labeled 4EBP1 peptide measured after 1 hr by time-resolved fluores... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01716 BindingDB Entry DOI: 10.7270/Q22Z19CX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 8 (Homo sapiens (Human)) | BDBM50578358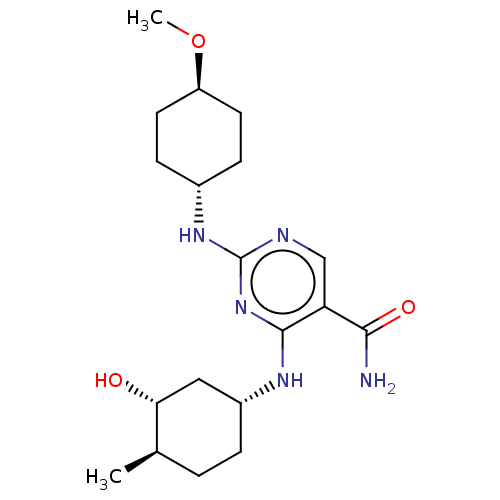 (CHEMBL4878370) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of JNK1 (unknown origin) assessed as reduction in phosphorylated ULight-labeled 4EBP1 peptide measured after 1 hr by time-resolved fluores... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01716 BindingDB Entry DOI: 10.7270/Q22Z19CX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 8 (Homo sapiens (Human)) | BDBM50578367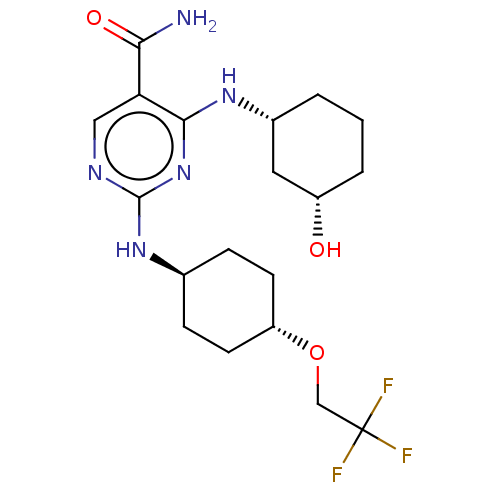 (CHEMBL4864618) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of JNK1 (unknown origin) assessed as reduction in phosphorylated ULight-labeled 4EBP1 peptide measured after 1 hr by time-resolved fluores... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01716 BindingDB Entry DOI: 10.7270/Q22Z19CX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C theta type (Homo sapiens (Human)) | BDBM50573042 (CHEMBL4854104) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of PKA-theta (unknown origin) using Fam-labelled S6-derived peptide incubated for 2 hrs by TR-FRET assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00388 BindingDB Entry DOI: 10.7270/Q22J6GPS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclic AMP-dependent transcription factor ATF-4 (Human) | BDBM524143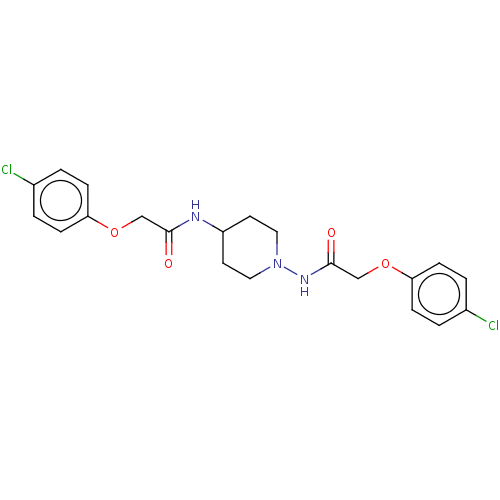 (US11166942, Compound 45) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The ATF4 reporter was prepared by fusing the human full length 5′UTR of ATF4 (NCBI Accession No. BC022088.2) upstream of the firefly luciferase... | Citation and Details BindingDB Entry DOI: 10.7270/Q2QJ7MG1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 8 (Homo sapiens (Human)) | BDBM50578366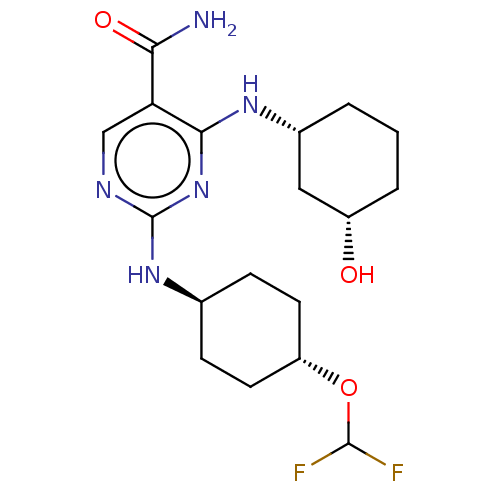 (CHEMBL4845965) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of JNK1 (unknown origin) assessed as reduction in phosphorylated ULight-labeled 4EBP1 peptide measured after 1 hr by time-resolved fluores... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01716 BindingDB Entry DOI: 10.7270/Q22Z19CX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 8 (Homo sapiens (Human)) | BDBM50578346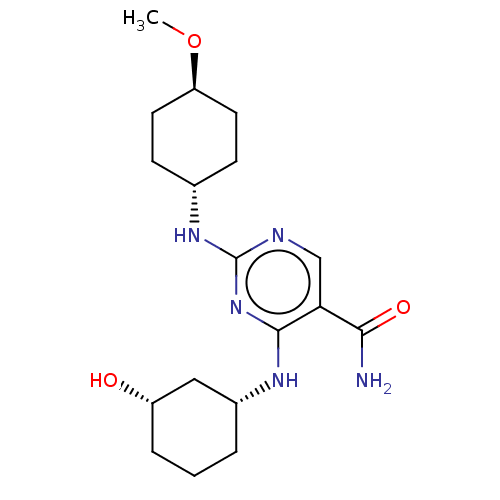 (CHEMBL4847078) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of JNK1 (unknown origin) assessed as reduction in phosphorylated ULight-labeled 4EBP1 peptide measured after 1 hr by time-resolved fluores... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01716 BindingDB Entry DOI: 10.7270/Q22Z19CX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 8 (Homo sapiens (Human)) | BDBM50578365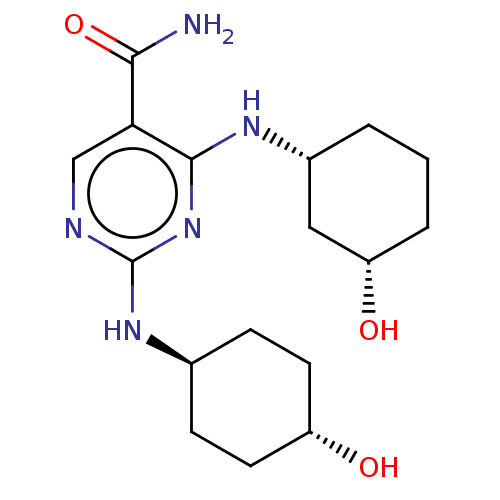 (CHEMBL4853525) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of JNK1 (unknown origin) assessed as reduction in phosphorylated ULight-labeled 4EBP1 peptide measured after 1 hr by time-resolved fluores... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01716 BindingDB Entry DOI: 10.7270/Q22Z19CX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 8 (Homo sapiens (Human)) | BDBM50578346 (CHEMBL4847078) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of JNK1 (unknown origin) assessed as reduction in phosphorylated ULight-labeled 4EBP1 peptide measured after 1 hr by time-resolved fluores... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01716 BindingDB Entry DOI: 10.7270/Q22Z19CX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclic AMP-dependent transcription factor ATF-4 (Human) | BDBM524115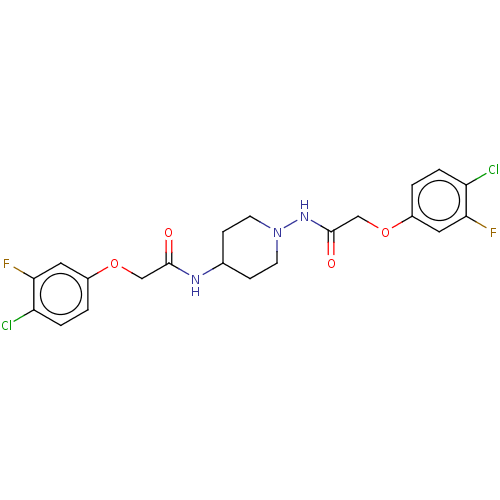 (US11166942, Compound 4) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The ATF4 reporter was prepared by fusing the human full length 5′UTR of ATF4 (NCBI Accession No. BC022088.2) upstream of the firefly luciferase... | Citation and Details BindingDB Entry DOI: 10.7270/Q2QJ7MG1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclic AMP-dependent transcription factor ATF-4 (Human) | BDBM524146 (US11166942, Compound 48) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.36 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The ATF4 reporter was prepared by fusing the human full length 5′UTR of ATF4 (NCBI Accession No. BC022088.2) upstream of the firefly luciferase... | Citation and Details BindingDB Entry DOI: 10.7270/Q2QJ7MG1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclic AMP-dependent transcription factor ATF-4 (Human) | BDBM524144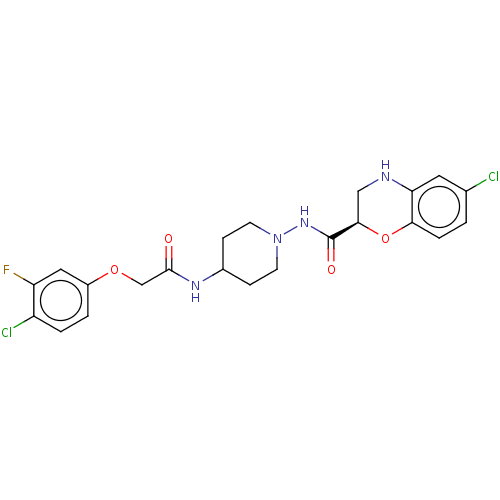 (US11166942, Compound 46) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The ATF4 reporter was prepared by fusing the human full length 5′UTR of ATF4 (NCBI Accession No. BC022088.2) upstream of the firefly luciferase... | Citation and Details BindingDB Entry DOI: 10.7270/Q2QJ7MG1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclic AMP-dependent transcription factor ATF-4 (Human) | BDBM524106 (US11166942, Compound 1) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The ATF4 reporter was prepared by fusing the human full length 5′UTR of ATF4 (NCBI Accession No. BC022088.2) upstream of the firefly luciferase... | Citation and Details BindingDB Entry DOI: 10.7270/Q2QJ7MG1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C theta type (Homo sapiens (Human)) | BDBM50573034 (CHEMBL4859091) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of PKA-theta (unknown origin) using Fam-labelled S6-derived peptide incubated for 2 hrs by TR-FRET assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00388 BindingDB Entry DOI: 10.7270/Q22J6GPS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C theta type (Homo sapiens (Human)) | BDBM50572994 (CHEMBL4854326) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of PKA-theta (unknown origin) using Fam-labelled S6-derived peptide incubated for 2 hrs by TR-FRET assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00388 BindingDB Entry DOI: 10.7270/Q22J6GPS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclic AMP-dependent transcription factor ATF-4 (Human) | BDBM524138 (US11166942, Compound 31 | US11166942, Compound 47) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4.16 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The ATF4 reporter was prepared by fusing the human full length 5′UTR of ATF4 (NCBI Accession No. BC022088.2) upstream of the firefly luciferase... | Citation and Details BindingDB Entry DOI: 10.7270/Q2QJ7MG1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 9 (Homo sapiens (Human)) | BDBM50578361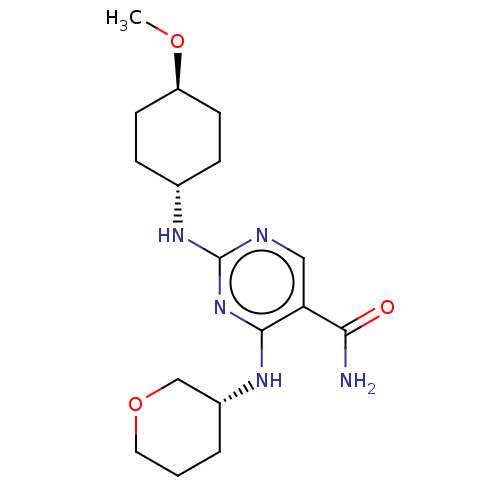 (CHEMBL4877560) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of JNK2 (unknown origin) assessed as reduction in phosphorylated ULight-labeled 4EBP1 peptide measured after 1 hr by time-resolved fluores... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01716 BindingDB Entry DOI: 10.7270/Q22Z19CX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 388 total ) | Next | Last >> |