 Found 322 hits with Last Name = 'glick' and Initial = 'm'
Found 322 hits with Last Name = 'glick' and Initial = 'm' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Disintegrin and metalloproteinase domain-containing protein 17
(Homo sapiens (Human)) | BDBM50182403

((2R,3S)-N1-((S)-1-(3-phenoxybenzyl)-2-oxoazepan-3-...)Show SMILES CCC[C@@H]([C@@H](CC(C)C)C(=O)N[C@H]1CCCCN(Cc2cccc(Oc3ccccc3)c2)C1=O)C(=O)NO Show InChI InChI=1S/C30H41N3O5/c1-4-11-25(29(35)32-37)26(18-21(2)3)28(34)31-27-16-8-9-17-33(30(27)36)20-22-12-10-15-24(19-22)38-23-13-6-5-7-14-23/h5-7,10,12-15,19,21,25-27,37H,4,8-9,11,16-18,20H2,1-3H3,(H,31,34)(H,32,35)/t25-,26+,27-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of TACE |
Bioorg Med Chem Lett 16: 2357-63 (2006)
Article DOI: 10.1016/j.bmcl.2006.01.055
BindingDB Entry DOI: 10.7270/Q2WD405K |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50182403

((2R,3S)-N1-((S)-1-(3-phenoxybenzyl)-2-oxoazepan-3-...)Show SMILES CCC[C@@H]([C@@H](CC(C)C)C(=O)N[C@H]1CCCCN(Cc2cccc(Oc3ccccc3)c2)C1=O)C(=O)NO Show InChI InChI=1S/C30H41N3O5/c1-4-11-25(29(35)32-37)26(18-21(2)3)28(34)31-27-16-8-9-17-33(30(27)36)20-22-12-10-15-24(19-22)38-23-13-6-5-7-14-23/h5-7,10,12-15,19,21,25-27,37H,4,8-9,11,16-18,20H2,1-3H3,(H,31,34)(H,32,35)/t25-,26+,27-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of MMP9 |
Bioorg Med Chem Lett 16: 2357-63 (2006)
Article DOI: 10.1016/j.bmcl.2006.01.055
BindingDB Entry DOI: 10.7270/Q2WD405K |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50182403

((2R,3S)-N1-((S)-1-(3-phenoxybenzyl)-2-oxoazepan-3-...)Show SMILES CCC[C@@H]([C@@H](CC(C)C)C(=O)N[C@H]1CCCCN(Cc2cccc(Oc3ccccc3)c2)C1=O)C(=O)NO Show InChI InChI=1S/C30H41N3O5/c1-4-11-25(29(35)32-37)26(18-21(2)3)28(34)31-27-16-8-9-17-33(30(27)36)20-22-12-10-15-24(19-22)38-23-13-6-5-7-14-23/h5-7,10,12-15,19,21,25-27,37H,4,8-9,11,16-18,20H2,1-3H3,(H,31,34)(H,32,35)/t25-,26+,27-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of MMP2 |
Bioorg Med Chem Lett 16: 2357-63 (2006)
Article DOI: 10.1016/j.bmcl.2006.01.055
BindingDB Entry DOI: 10.7270/Q2WD405K |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50182403

((2R,3S)-N1-((S)-1-(3-phenoxybenzyl)-2-oxoazepan-3-...)Show SMILES CCC[C@@H]([C@@H](CC(C)C)C(=O)N[C@H]1CCCCN(Cc2cccc(Oc3ccccc3)c2)C1=O)C(=O)NO Show InChI InChI=1S/C30H41N3O5/c1-4-11-25(29(35)32-37)26(18-21(2)3)28(34)31-27-16-8-9-17-33(30(27)36)20-22-12-10-15-24(19-22)38-23-13-6-5-7-14-23/h5-7,10,12-15,19,21,25-27,37H,4,8-9,11,16-18,20H2,1-3H3,(H,31,34)(H,32,35)/t25-,26+,27-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of MMP13 |
Bioorg Med Chem Lett 16: 2357-63 (2006)
Article DOI: 10.1016/j.bmcl.2006.01.055
BindingDB Entry DOI: 10.7270/Q2WD405K |
More data for this
Ligand-Target Pair | |
Interstitial collagenase
(Homo sapiens (Human)) | BDBM50182403

((2R,3S)-N1-((S)-1-(3-phenoxybenzyl)-2-oxoazepan-3-...)Show SMILES CCC[C@@H]([C@@H](CC(C)C)C(=O)N[C@H]1CCCCN(Cc2cccc(Oc3ccccc3)c2)C1=O)C(=O)NO Show InChI InChI=1S/C30H41N3O5/c1-4-11-25(29(35)32-37)26(18-21(2)3)28(34)31-27-16-8-9-17-33(30(27)36)20-22-12-10-15-24(19-22)38-23-13-6-5-7-14-23/h5-7,10,12-15,19,21,25-27,37H,4,8-9,11,16-18,20H2,1-3H3,(H,31,34)(H,32,35)/t25-,26+,27-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of MMP1 |
Bioorg Med Chem Lett 16: 2357-63 (2006)
Article DOI: 10.1016/j.bmcl.2006.01.055
BindingDB Entry DOI: 10.7270/Q2WD405K |
More data for this
Ligand-Target Pair | |
Stromelysin-2
(Homo sapiens (Human)) | BDBM50182403

((2R,3S)-N1-((S)-1-(3-phenoxybenzyl)-2-oxoazepan-3-...)Show SMILES CCC[C@@H]([C@@H](CC(C)C)C(=O)N[C@H]1CCCCN(Cc2cccc(Oc3ccccc3)c2)C1=O)C(=O)NO Show InChI InChI=1S/C30H41N3O5/c1-4-11-25(29(35)32-37)26(18-21(2)3)28(34)31-27-16-8-9-17-33(30(27)36)20-22-12-10-15-24(19-22)38-23-13-6-5-7-14-23/h5-7,10,12-15,19,21,25-27,37H,4,8-9,11,16-18,20H2,1-3H3,(H,31,34)(H,32,35)/t25-,26+,27-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of MMP10 |
Bioorg Med Chem Lett 16: 2357-63 (2006)
Article DOI: 10.1016/j.bmcl.2006.01.055
BindingDB Entry DOI: 10.7270/Q2WD405K |
More data for this
Ligand-Target Pair | |
Matrilysin
(Homo sapiens (Human)) | BDBM50182403

((2R,3S)-N1-((S)-1-(3-phenoxybenzyl)-2-oxoazepan-3-...)Show SMILES CCC[C@@H]([C@@H](CC(C)C)C(=O)N[C@H]1CCCCN(Cc2cccc(Oc3ccccc3)c2)C1=O)C(=O)NO Show InChI InChI=1S/C30H41N3O5/c1-4-11-25(29(35)32-37)26(18-21(2)3)28(34)31-27-16-8-9-17-33(30(27)36)20-22-12-10-15-24(19-22)38-23-13-6-5-7-14-23/h5-7,10,12-15,19,21,25-27,37H,4,8-9,11,16-18,20H2,1-3H3,(H,31,34)(H,32,35)/t25-,26+,27-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 660 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of MMP7 |
Bioorg Med Chem Lett 16: 2357-63 (2006)
Article DOI: 10.1016/j.bmcl.2006.01.055
BindingDB Entry DOI: 10.7270/Q2WD405K |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-14
(Homo sapiens (Human)) | BDBM50182403

((2R,3S)-N1-((S)-1-(3-phenoxybenzyl)-2-oxoazepan-3-...)Show SMILES CCC[C@@H]([C@@H](CC(C)C)C(=O)N[C@H]1CCCCN(Cc2cccc(Oc3ccccc3)c2)C1=O)C(=O)NO Show InChI InChI=1S/C30H41N3O5/c1-4-11-25(29(35)32-37)26(18-21(2)3)28(34)31-27-16-8-9-17-33(30(27)36)20-22-12-10-15-24(19-22)38-23-13-6-5-7-14-23/h5-7,10,12-15,19,21,25-27,37H,4,8-9,11,16-18,20H2,1-3H3,(H,31,34)(H,32,35)/t25-,26+,27-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of MMP14 |
Bioorg Med Chem Lett 16: 2357-63 (2006)
Article DOI: 10.1016/j.bmcl.2006.01.055
BindingDB Entry DOI: 10.7270/Q2WD405K |
More data for this
Ligand-Target Pair | |
cAMP-dependent protein kinase
(Oryctolagus cuniculus (Rabbit)) | BDBM6760

((+)-K-252a | CHEMBL281948 | K-252a | methyl (15S,1...)Show SMILES COC(=O)[C@@]1(O)C[C@H]2O[C@]1(C)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C27H21N3O5/c1-26-27(33,25(32)34-2)11-18(35-26)29-16-9-5-3-7-13(16)20-21-15(12-28-24(21)31)19-14-8-4-6-10-17(14)30(26)23(19)22(20)29/h3-10,18,33H,11-12H2,1-2H3,(H,28,31)/t18?,26-,27-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.0160 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyowa Hakko Kogyo Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of cAMP dependent Protein kinase A of rabbit Skeletal Muscle. |
J Med Chem 40: 1863-9 (1997)
Article DOI: 10.1021/jm970031d
BindingDB Entry DOI: 10.7270/Q2W95891 |
More data for this
Ligand-Target Pair | |
Myosin light chain kinase, smooth muscle
(Gallus gallus (chicken)) | BDBM6760

((+)-K-252a | CHEMBL281948 | K-252a | methyl (15S,1...)Show SMILES COC(=O)[C@@]1(O)C[C@H]2O[C@]1(C)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C27H21N3O5/c1-26-27(33,25(32)34-2)11-18(35-26)29-16-9-5-3-7-13(16)20-21-15(12-28-24(21)31)19-14-8-4-6-10-17(14)30(26)23(19)22(20)29/h3-10,18,33H,11-12H2,1-2H3,(H,28,31)/t18?,26-,27-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyowa Hakko Kogyo Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of Smooth muscle myosin light chain kinase of chicken gizzard |
J Med Chem 40: 1863-9 (1997)
Article DOI: 10.1021/jm970031d
BindingDB Entry DOI: 10.7270/Q2W95891 |
More data for this
Ligand-Target Pair | |
Estrogen receptor beta
(Homo sapiens (Human)) | BDBM50154078
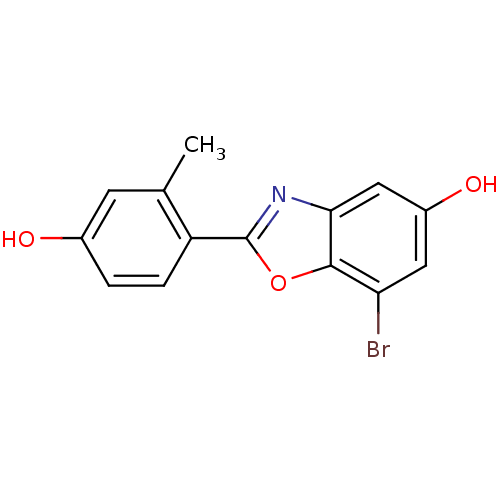
(7-Bromo-2-(4-hydroxy-2-methyl-phenyl)-benzooxazol-...)Show InChI InChI=1S/C14H10BrNO3/c1-7-4-8(17)2-3-10(7)14-16-12-6-9(18)5-11(15)13(12)19-14/h2-6,17-18H,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of estrogen receptor beta |
J Med Chem 51: 2481-91 (2008)
Article DOI: 10.1021/jm701314u
BindingDB Entry DOI: 10.7270/Q2GX4CF5 |
More data for this
Ligand-Target Pair | |
Estrogen receptor beta
(Homo sapiens (Human)) | BDBM50154137
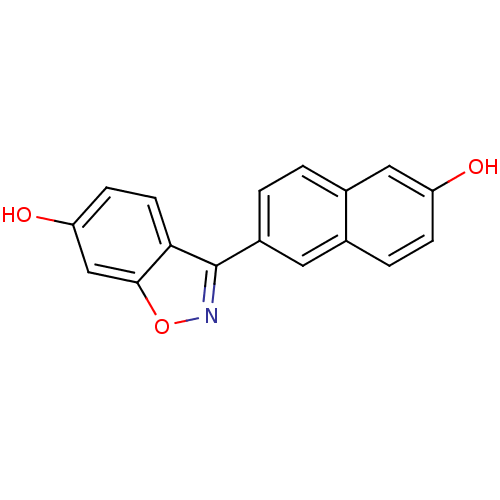
(3-(6-HYDROXY-NAPHTHALEN-2-YL)-BENZO[D]ISOOXAZOL-6-...)Show InChI InChI=1S/C17H11NO3/c19-13-4-3-10-7-12(2-1-11(10)8-13)17-15-6-5-14(20)9-16(15)21-18-17/h1-9,19-20H | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of estrogen receptor beta |
J Med Chem 51: 2481-91 (2008)
Article DOI: 10.1021/jm701314u
BindingDB Entry DOI: 10.7270/Q2GX4CF5 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Pseudomonas aeruginosa) | BDBM50469565
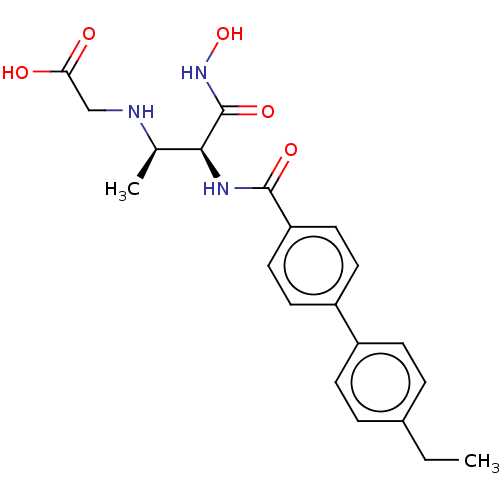
(CHEMBL4082918)Show SMILES CCc1ccc(cc1)-c1ccc(cc1)C(=O)N[C@@H]([C@@H](C)NCC(O)=O)C(=O)NO |r| Show InChI InChI=1S/C21H25N3O5/c1-3-14-4-6-15(7-5-14)16-8-10-17(11-9-16)20(27)23-19(21(28)24-29)13(2)22-12-18(25)26/h4-11,13,19,22,29H,3,12H2,1-2H3,(H,23,27)(H,24,28)(H,25,26)/t13-,19+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of Pseudomonas aeruginosa LpxC using UDP-3-O-(R-hydroxydecanoyl)-N-acetylglucosamine as substrate preincubated for 30 mins followed by sub... |
J Med Chem 60: 5002-5014 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00377
BindingDB Entry DOI: 10.7270/Q2FN18MZ |
More data for this
Ligand-Target Pair | |
Dual specificity mitogen-activated protein kinase kinase 1/2
(Homo sapiens (Human)) | BDBM50531540
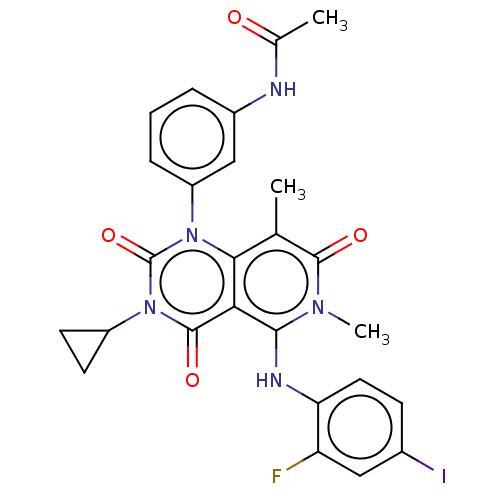
(CHEBI:75998 | GSK-1120212 | GSK1120212 | JTP 74057...)Show SMILES CC(=O)Nc1cccc(c1)-n1c2c(C)c(=O)n(C)c(Nc3ccc(I)cc3F)c2c(=O)n(C2CC2)c1=O Show InChI InChI=1S/C26H23FIN5O4/c1-13-22-21(23(31(3)24(13)35)30-20-10-7-15(28)11-19(20)27)25(36)33(17-8-9-17)26(37)32(22)18-6-4-5-16(12-18)29-14(2)34/h4-7,10-12,17,30H,8-9H2,1-3H3,(H,29,34) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of MEK in human KYSE-520 cells assessed as reduction in p-ERK levels |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01170
BindingDB Entry DOI: 10.7270/Q2CC14BB |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Pseudomonas aeruginosa) | BDBM50469558
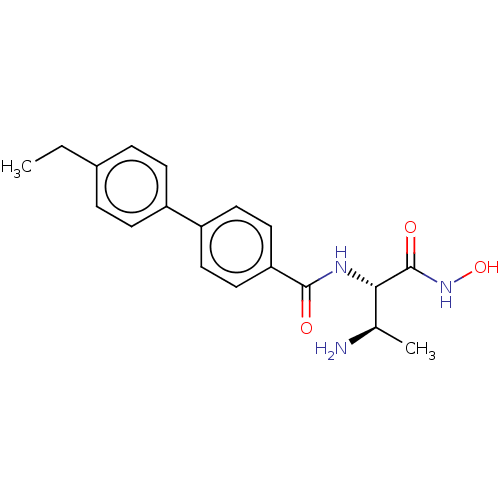
(CHEMBL4061041)Show SMILES CCc1ccc(cc1)-c1ccc(cc1)C(=O)N[C@@H]([C@@H](C)N)C(=O)NO |r| Show InChI InChI=1S/C19H23N3O3/c1-3-13-4-6-14(7-5-13)15-8-10-16(11-9-15)18(23)21-17(12(2)20)19(24)22-25/h4-12,17,25H,3,20H2,1-2H3,(H,21,23)(H,22,24)/t12-,17+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.46 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of Pseudomonas aeruginosa LpxC using UDP-3-O-(R-hydroxydecanoyl)-N-acetylglucosamine as substrate preincubated for 30 mins followed by sub... |
J Med Chem 60: 5002-5014 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00377
BindingDB Entry DOI: 10.7270/Q2FN18MZ |
More data for this
Ligand-Target Pair | |
High affinity nerve growth factor receptor
(Homo sapiens (Human)) | BDBM6760

((+)-K-252a | CHEMBL281948 | K-252a | methyl (15S,1...)Show SMILES COC(=O)[C@@]1(O)C[C@H]2O[C@]1(C)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C27H21N3O5/c1-26-27(33,25(32)34-2)11-18(35-26)29-16-9-5-3-7-13(16)20-21-15(12-28-24(21)31)19-14-8-4-6-10-17(14)30(26)23(19)22(20)29/h3-10,18,33H,11-12H2,1-2H3,(H,28,31)/t18?,26-,27-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyowa Hakko Kogyo Co., Ltd.
Curated by ChEMBL
| Assay Description
Ability to inhibit human trk A tyrosine kinase expressed in baculovirus using ELISA based enzyme assay was determined |
J Med Chem 40: 1863-9 (1997)
Article DOI: 10.1021/jm970031d
BindingDB Entry DOI: 10.7270/Q2W95891 |
More data for this
Ligand-Target Pair | |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Pseudomonas aeruginosa) | BDBM50249583

(CHEMBL4097399)Show SMILES CC#CCOc1ccc(cc1)C(=O)N[C@H](C(=O)NO)C(C)(C)N |r| Show InChI InChI=1S/C16H21N3O4/c1-4-5-10-23-12-8-6-11(7-9-12)14(20)18-13(15(21)19-22)16(2,3)17/h6-9,13,22H,10,17H2,1-3H3,(H,18,20)(H,19,21)/t13-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 3.13 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of Pseudomonas aeruginosa LpxC using UDP-3-O-(R-hydroxydecanoyl)-N-acetylglucosamine as substrate preincubated for 30 mins followed by sub... |
J Med Chem 60: 5002-5014 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00377
BindingDB Entry DOI: 10.7270/Q2FN18MZ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Pseudomonas aeruginosa) | BDBM50469562
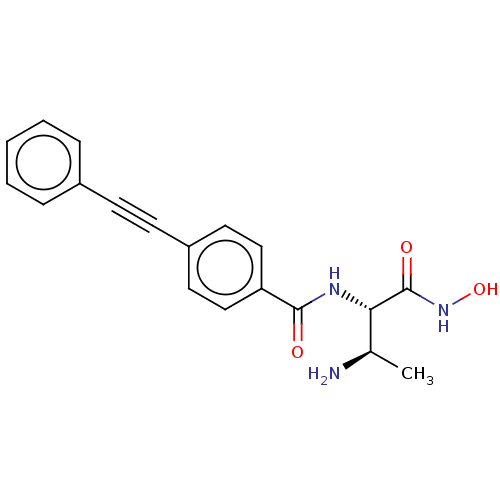
(CHEMBL4069725)Show SMILES C[C@@H](N)[C@H](NC(=O)c1ccc(cc1)C#Cc1ccccc1)C(=O)NO |r| Show InChI InChI=1S/C19H19N3O3/c1-13(20)17(19(24)22-25)21-18(23)16-11-9-15(10-12-16)8-7-14-5-3-2-4-6-14/h2-6,9-13,17,25H,20H2,1H3,(H,21,23)(H,22,24)/t13-,17+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.56 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of Pseudomonas aeruginosa LpxC using UDP-3-O-(R-hydroxydecanoyl)-N-acetylglucosamine as substrate preincubated for 30 mins followed by sub... |
J Med Chem 60: 5002-5014 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00377
BindingDB Entry DOI: 10.7270/Q2FN18MZ |
More data for this
Ligand-Target Pair | |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Pseudomonas aeruginosa) | BDBM50469559
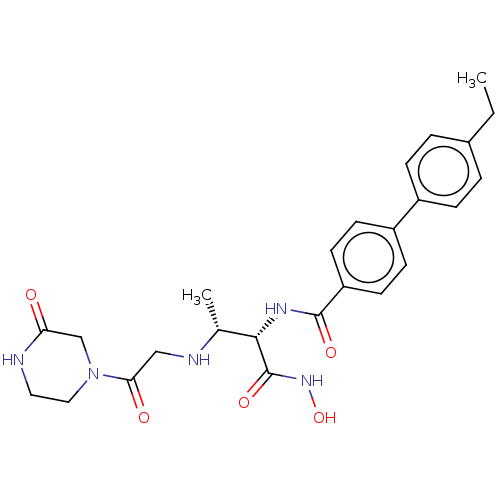
(CHEMBL4063087)Show SMILES CCc1ccc(cc1)-c1ccc(cc1)C(=O)N[C@@H]([C@@H](C)NCC(=O)N1CCNC(=O)C1)C(=O)NO |r| Show InChI InChI=1S/C25H31N5O5/c1-3-17-4-6-18(7-5-17)19-8-10-20(11-9-19)24(33)28-23(25(34)29-35)16(2)27-14-22(32)30-13-12-26-21(31)15-30/h4-11,16,23,27,35H,3,12-15H2,1-2H3,(H,26,31)(H,28,33)(H,29,34)/t16-,23+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.15 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of Pseudomonas aeruginosa LpxC using UDP-3-O-(R-hydroxydecanoyl)-N-acetylglucosamine as substrate preincubated for 30 mins followed by sub... |
J Med Chem 60: 5002-5014 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00377
BindingDB Entry DOI: 10.7270/Q2FN18MZ |
More data for this
Ligand-Target Pair | |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Pseudomonas aeruginosa) | BDBM50469555

(CHEMBL4090716)Show SMILES C[C@@H](NCC(=O)N1CCNC(=O)C1)[C@H](NC(=O)c1ccc(cc1)C#Cc1ccccc1)C(=O)NO |r| Show InChI InChI=1S/C25H27N5O5/c1-17(27-15-22(32)30-14-13-26-21(31)16-30)23(25(34)29-35)28-24(33)20-11-9-19(10-12-20)8-7-18-5-3-2-4-6-18/h2-6,9-12,17,23,27,35H,13-16H2,1H3,(H,26,31)(H,28,33)(H,29,34)/t17-,23+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.19 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of Pseudomonas aeruginosa LpxC using UDP-3-O-(R-hydroxydecanoyl)-N-acetylglucosamine as substrate preincubated for 30 mins followed by sub... |
J Med Chem 60: 5002-5014 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00377
BindingDB Entry DOI: 10.7270/Q2FN18MZ |
More data for this
Ligand-Target Pair | |
Estrogen receptor beta
(Homo sapiens (Human)) | BDBM50154062

(2-(5-HYDROXY-NAPHTHALEN-1-YL)-1,3-BENZOOXAZOL-6-OL...)Show InChI InChI=1S/C17H11NO3/c19-10-7-8-14-16(9-10)21-17(18-14)13-5-1-4-12-11(13)3-2-6-15(12)20/h1-9,19-20H | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of estrogen receptor beta |
J Med Chem 51: 2481-91 (2008)
Article DOI: 10.1021/jm701314u
BindingDB Entry DOI: 10.7270/Q2GX4CF5 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein phosphatase non-receptor type 11
(Homo sapiens (Human)) | BDBM408067
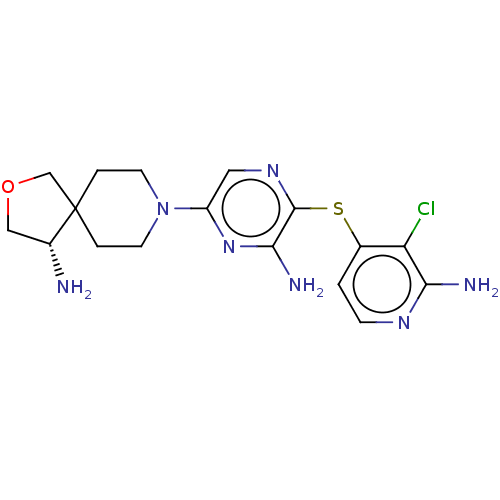
(US10336774, Example 52)Show SMILES N[C@@H]1COCC11CCN(CC1)c1cnc(Sc2ccnc(N)c2Cl)c(N)n1 |r| Show InChI InChI=1S/C17H22ClN7OS/c18-13-10(1-4-22-14(13)20)27-16-15(21)24-12(7-23-16)25-5-2-17(3-6-25)9-26-8-11(17)19/h1,4,7,11H,2-3,5-6,8-9,19H2,(H2,20,22)(H2,21,24)/t11-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Allosteric inhibition of 6x-histidine tagged human SHP2 (Met1-L525 residues) expressed in Escherichia coli BL21 Star (DE3) using IRS1_pY1172(dPEG8)pY... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01170
BindingDB Entry DOI: 10.7270/Q2CC14BB |
More data for this
Ligand-Target Pair | |
cAMP-dependent protein kinase
(Oryctolagus cuniculus (Rabbit)) | BDBM50058332
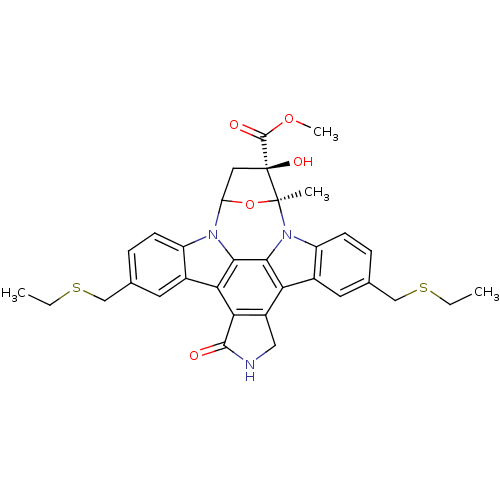
(CHEMBL299496 | methyl 10,23-di(ethylsulfanylmethyl...)Show SMILES CCSCc1ccc2n3C4C[C@](O)(C(=O)OC)[C@](C)(O4)n4c5ccc(CSCC)cc5c5c6CNC(=O)c6c(c2c1)c3c45 Show InChI InChI=1S/C33H33N3O5S2/c1-5-42-15-17-7-9-22-19(11-17)26-27-21(14-34-30(27)37)25-20-12-18(16-43-6-2)8-10-23(20)36-29(25)28(26)35(22)24-13-33(39,31(38)40-4)32(36,3)41-24/h7-12,24,39H,5-6,13-16H2,1-4H3,(H,34,37)/t24?,32-,33-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >10 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyowa Hakko Kogyo Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of cAMP dependent Protein kinase A of rabbit Skeletal Muscle. |
J Med Chem 40: 1863-9 (1997)
Article DOI: 10.1021/jm970031d
BindingDB Entry DOI: 10.7270/Q2W95891 |
More data for this
Ligand-Target Pair | |
GTPase KRas
(Homo sapiens (Human)) | BDBM50539763
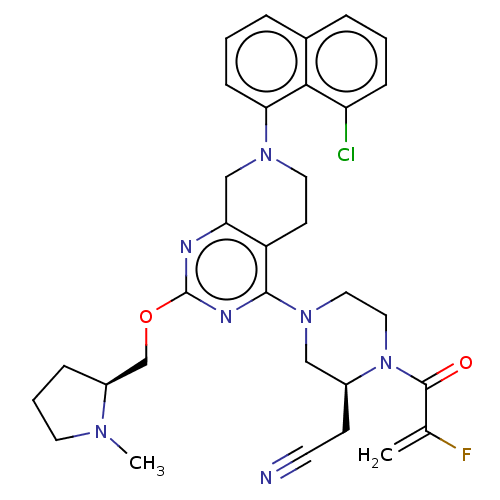
(Adagrasib | Mrtx-849 | Mrtx849)Show SMILES CN1CCC[C@H]1COc1nc2CN(CCc2c(n1)N1CCN([C@@H](CC#N)C1)C(=O)C(F)=C)c1cccc2cccc(Cl)c12 Show InChI InChI=1S/C32H35ClFN7O2/c1-21(34)31(42)41-17-16-40(18-23(41)11-13-35)30-25-12-15-39(28-10-4-7-22-6-3-9-26(33)29(22)28)19-27(25)36-32(37-30)43-20-24-8-5-14-38(24)2/h3-4,6-7,9-10,23-24H,1,5,8,11-12,14-20H2,2H3/t23-,24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of KRAS G12C mutant in human MIA PaCa-2 cells assessed as reduction in p-ERK levels |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01170
BindingDB Entry DOI: 10.7270/Q2CC14BB |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 11
(Homo sapiens (Human)) | BDBM50553790

(CHEMBL4763213)Show SMILES N[C@@H]1CCCC11CCN(CC1)c1cnc(Sc2ccnc(N)c2Cl)cn1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Allosteric inhibition of 6x-histidine tagged human SHP2 (Met1-L525 residues) expressed in Escherichia coli BL21 Star (DE3) using IRS1_pY1172(dPEG8)pY... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01170
BindingDB Entry DOI: 10.7270/Q2CC14BB |
More data for this
Ligand-Target Pair | |
Myosin light chain kinase, smooth muscle
(Gallus gallus (chicken)) | BDBM50058332

(CHEMBL299496 | methyl 10,23-di(ethylsulfanylmethyl...)Show SMILES CCSCc1ccc2n3C4C[C@](O)(C(=O)OC)[C@](C)(O4)n4c5ccc(CSCC)cc5c5c6CNC(=O)c6c(c2c1)c3c45 Show InChI InChI=1S/C33H33N3O5S2/c1-5-42-15-17-7-9-22-19(11-17)26-27-21(14-34-30(27)37)25-20-12-18(16-43-6-2)8-10-23(20)36-29(25)28(26)35(22)24-13-33(39,31(38)40-4)32(36,3)41-24/h7-12,24,39H,5-6,13-16H2,1-4H3,(H,34,37)/t24?,32-,33-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >10 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyowa Hakko Kogyo Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of Smooth muscle myosin light chain kinase of chicken gizzard |
J Med Chem 40: 1863-9 (1997)
Article DOI: 10.1021/jm970031d
BindingDB Entry DOI: 10.7270/Q2W95891 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 11
(Homo sapiens (Human)) | BDBM50553783

(Ptpn11 inhibitor tno155 | Shp2 inhibitor tno155 | ...)Show SMILES C[C@@H]1OCC2(CCN(CC2)c2cnc(Sc3ccnc(N)c3Cl)c(N)n2)[C@@H]1N |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Allosteric inhibition of 6x-histidine tagged human SHP2 (Met1-L525 residues) expressed in Escherichia coli BL21 Star (DE3) using IRS1_pY1172(dPEG8)pY... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01170
BindingDB Entry DOI: 10.7270/Q2CC14BB |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein phosphatase non-receptor type 11
(Homo sapiens (Human)) | BDBM50553783

(Ptpn11 inhibitor tno155 | Shp2 inhibitor tno155 | ...)Show SMILES C[C@@H]1OCC2(CCN(CC2)c2cnc(Sc3ccnc(N)c3Cl)c(N)n2)[C@@H]1N |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Allosteric inhibition of human SHP2 in human KYSE-520 cells assessed as reduction in ERK1/2 phosphorylation by fluorescence based assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01170
BindingDB Entry DOI: 10.7270/Q2CC14BB |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Phosphatidylinositol 3-kinase catalytic subunit type 3
(Homo sapiens (Human)) | BDBM50156294

(CHEMBL3781415)Show InChI InChI=1S/C17H17N7/c18-16-21-10-13(15(23-16)9-11-1-2-11)14-5-8-20-17(24-14)22-12-3-6-19-7-4-12/h3-8,10-11H,1-2,9H2,(H2,18,21,23)(H,19,20,22,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of VPS34 in human U2OS cells incubated for 2 hrs by GFP-FYVE reporter gene assay |
ACS Med Chem Lett 7: 72-6 (2016)
Article DOI: 10.1021/acsmedchemlett.5b00335
BindingDB Entry DOI: 10.7270/Q2K35WK6 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein phosphatase non-receptor type 11
(Homo sapiens (Human)) | BDBM50553787

(CHEMBL4755819)Show SMILES N[C@@H]1CCCC11CCN(CC1)c1cnc(Sc2cccnc2C(F)(F)F)c(N)n1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Allosteric inhibition of 6x-histidine tagged human SHP2 (Met1-L525 residues) expressed in Escherichia coli BL21 Star (DE3) using IRS1_pY1172(dPEG8)pY... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01170
BindingDB Entry DOI: 10.7270/Q2CC14BB |
More data for this
Ligand-Target Pair | |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Pseudomonas aeruginosa) | BDBM50469560
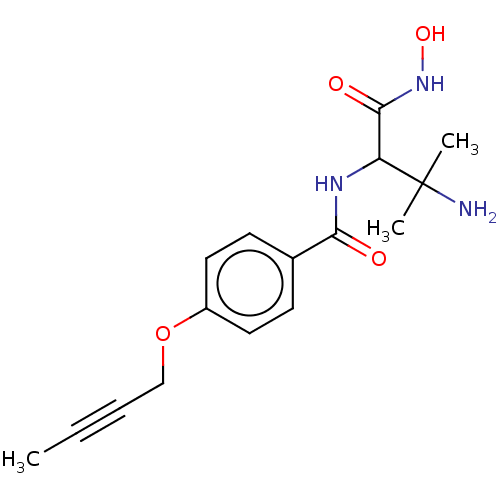
(CHEMBL4083624)Show InChI InChI=1S/C16H21N3O4/c1-4-5-10-23-12-8-6-11(7-9-12)14(20)18-13(15(21)19-22)16(2,3)17/h6-9,13,22H,10,17H2,1-3H3,(H,18,20)(H,19,21) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 12.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of Pseudomonas aeruginosa LpxC using UDP-3-O-(R-hydroxydecanoyl)-N-acetylglucosamine as substrate preincubated for 30 mins followed by sub... |
J Med Chem 60: 5002-5014 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00377
BindingDB Entry DOI: 10.7270/Q2FN18MZ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 11
(Homo sapiens (Human)) | BDBM50553790

(CHEMBL4763213)Show SMILES N[C@@H]1CCCC11CCN(CC1)c1cnc(Sc2ccnc(N)c2Cl)cn1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Allosteric inhibition of human SHP2 in human KYSE-520 cells assessed as reduction in ERK1/2 phosphorylation by fluorescence based assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01170
BindingDB Entry DOI: 10.7270/Q2CC14BB |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase catalytic subunit type 3
(Homo sapiens (Human)) | BDBM50156302
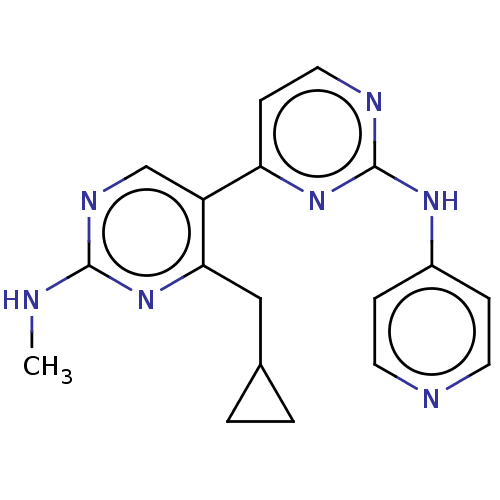
(CHEMBL3780029)Show InChI InChI=1S/C18H19N7/c1-19-17-22-11-14(16(25-17)10-12-2-3-12)15-6-9-21-18(24-15)23-13-4-7-20-8-5-13/h4-9,11-12H,2-3,10H2,1H3,(H,19,22,25)(H,20,21,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of VPS34 in human U2OS cells incubated for 2 hrs by GFP-FYVE reporter gene assay |
ACS Med Chem Lett 7: 72-6 (2016)
Article DOI: 10.1021/acsmedchemlett.5b00335
BindingDB Entry DOI: 10.7270/Q2K35WK6 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 11
(Homo sapiens (Human)) | BDBM50553789

(CHEMBL4747789)Show SMILES N[C@@H]1CCCC11CCN(CC1)c1cnc(Sc2ccnc(N)c2Cl)c(N)n1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Allosteric inhibition of 6x-histidine tagged human SHP2 (Met1-L525 residues) expressed in Escherichia coli BL21 Star (DE3) using IRS1_pY1172(dPEG8)pY... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01170
BindingDB Entry DOI: 10.7270/Q2CC14BB |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM5446

(CHEMBL553 | ERLOTINIB HYDROCHLORIDE | Erlotinib | ...)Show InChI InChI=1S/C22H23N3O4/c1-4-16-6-5-7-17(12-16)25-22-18-13-20(28-10-8-26-2)21(29-11-9-27-3)14-19(18)23-15-24-22/h1,5-7,12-15H,8-11H2,2-3H3,(H,23,24,25) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of EGFR in human KYSE-520 cells assessed as reduction in p-ERK levels |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01170
BindingDB Entry DOI: 10.7270/Q2CC14BB |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Amyloid-beta precursor protein
(Homo sapiens (Human)) | BDBM50182409
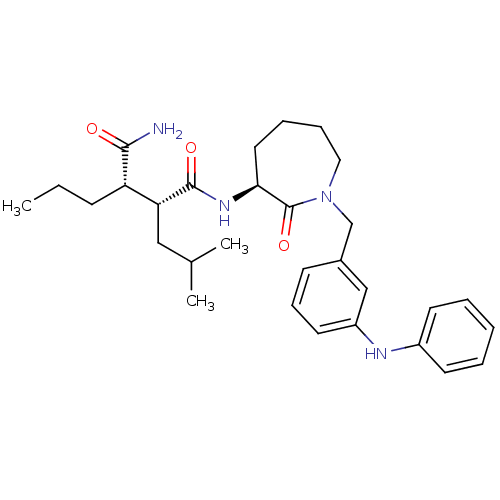
((2R,3S)-N1-((S)-1-(3-(phenylamino)benzyl)-2-oxoaze...)Show SMILES CCC[C@@H]([C@@H](CC(C)C)C(=O)N[C@H]1CCCCN(Cc2cccc(Nc3ccccc3)c2)C1=O)C(N)=O Show InChI InChI=1S/C30H42N4O3/c1-4-11-25(28(31)35)26(18-21(2)3)29(36)33-27-16-8-9-17-34(30(27)37)20-22-12-10-15-24(19-22)32-23-13-6-5-7-14-23/h5-7,10,12-15,19,21,25-27,32H,4,8-9,11,16-18,20H2,1-3H3,(H2,31,35)(H,33,36)/t25-,26+,27-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of beta-amyloid peptide production in CHO N9 cell line |
Bioorg Med Chem Lett 16: 2357-63 (2006)
Article DOI: 10.1016/j.bmcl.2006.01.055
BindingDB Entry DOI: 10.7270/Q2WD405K |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase catalytic subunit type 3
(Homo sapiens (Human)) | BDBM50156296

(CHEMBL3781466)Show SMILES CC(C)(O)CNc1ncc(c(CC2CC2)n1)-c1ccnc(Nc2ccncc2)n1 Show InChI InChI=1S/C21H25N7O/c1-21(2,29)13-25-19-24-12-16(18(28-19)11-14-3-4-14)17-7-10-23-20(27-17)26-15-5-8-22-9-6-15/h5-10,12,14,29H,3-4,11,13H2,1-2H3,(H,24,25,28)(H,22,23,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human VPS34 using L-alpha-phosphatidylinositol as substrate incubated for 10 mins by luminescence based ATP detection assay |
ACS Med Chem Lett 7: 72-6 (2016)
Article DOI: 10.1021/acsmedchemlett.5b00335
BindingDB Entry DOI: 10.7270/Q2K35WK6 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Pseudomonas aeruginosa) | BDBM50469563
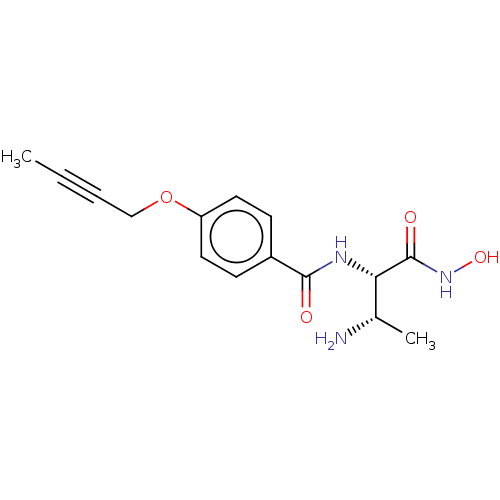
(CHEMBL4079368)Show SMILES CC#CCOc1ccc(cc1)C(=O)N[C@@H]([C@H](C)N)C(=O)NO |r| Show InChI InChI=1S/C15H19N3O4/c1-3-4-9-22-12-7-5-11(6-8-12)14(19)17-13(10(2)16)15(20)18-21/h5-8,10,13,21H,9,16H2,1-2H3,(H,17,19)(H,18,20)/t10-,13-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 16.4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of Pseudomonas aeruginosa LpxC using UDP-3-O-(R-hydroxydecanoyl)-N-acetylglucosamine as substrate preincubated for 30 mins followed by sub... |
J Med Chem 60: 5002-5014 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00377
BindingDB Entry DOI: 10.7270/Q2FN18MZ |
More data for this
Ligand-Target Pair | |
Amyloid-beta precursor protein
(Homo sapiens (Human)) | BDBM50410864
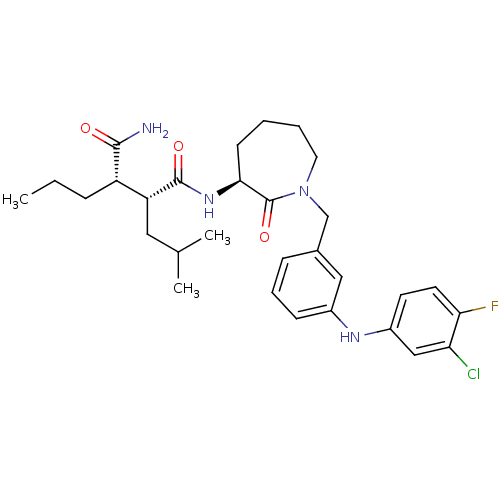
(CHEMBL208504)Show SMILES CCC[C@@H]([C@@H](CC(C)C)C(=O)N[C@H]1CCCCN(Cc2cccc(Nc3ccc(F)c(Cl)c3)c2)C1=O)C(N)=O Show InChI InChI=1S/C30H40ClFN4O3/c1-4-8-23(28(33)37)24(15-19(2)3)29(38)35-27-11-5-6-14-36(30(27)39)18-20-9-7-10-21(16-20)34-22-12-13-26(32)25(31)17-22/h7,9-10,12-13,16-17,19,23-24,27,34H,4-6,8,11,14-15,18H2,1-3H3,(H2,33,37)(H,35,38)/t23-,24+,27-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of beta-amyloid peptide production in CHO N9 cell line |
Bioorg Med Chem Lett 16: 2357-63 (2006)
Article DOI: 10.1016/j.bmcl.2006.01.055
BindingDB Entry DOI: 10.7270/Q2WD405K |
More data for this
Ligand-Target Pair | |
Amyloid-beta precursor protein
(Homo sapiens (Human)) | BDBM50410871
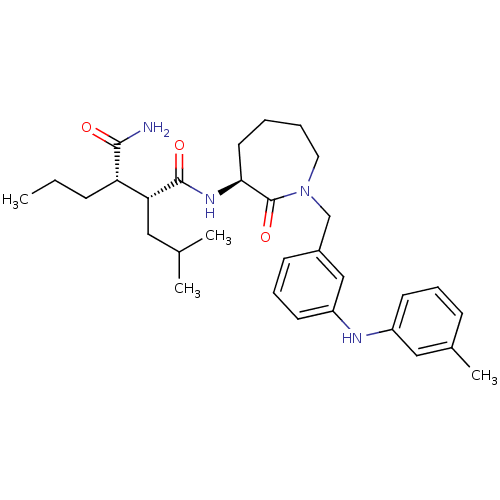
(CHEMBL208350)Show SMILES CCC[C@@H]([C@@H](CC(C)C)C(=O)N[C@H]1CCCCN(Cc2cccc(Nc3cccc(C)c3)c2)C1=O)C(N)=O Show InChI InChI=1S/C31H44N4O3/c1-5-10-26(29(32)36)27(17-21(2)3)30(37)34-28-15-6-7-16-35(31(28)38)20-23-12-9-14-25(19-23)33-24-13-8-11-22(4)18-24/h8-9,11-14,18-19,21,26-28,33H,5-7,10,15-17,20H2,1-4H3,(H2,32,36)(H,34,37)/t26-,27+,28-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of beta-amyloid peptide production in CHO N9 cell line |
Bioorg Med Chem Lett 16: 2357-63 (2006)
Article DOI: 10.1016/j.bmcl.2006.01.055
BindingDB Entry DOI: 10.7270/Q2WD405K |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 11
(Homo sapiens (Human)) | BDBM50553786

(CHEMBL4789106)Show SMILES CC1(CN)CCN(CC1)c1cnc(Sc2cccc(Cl)c2Cl)c(N)n1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Allosteric inhibition of 6x-histidine tagged human SHP2 (Met1-L525 residues) expressed in Escherichia coli BL21 Star (DE3) using IRS1_pY1172(dPEG8)pY... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01170
BindingDB Entry DOI: 10.7270/Q2CC14BB |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein phosphatase non-receptor type 11
(Homo sapiens (Human)) | BDBM50553791
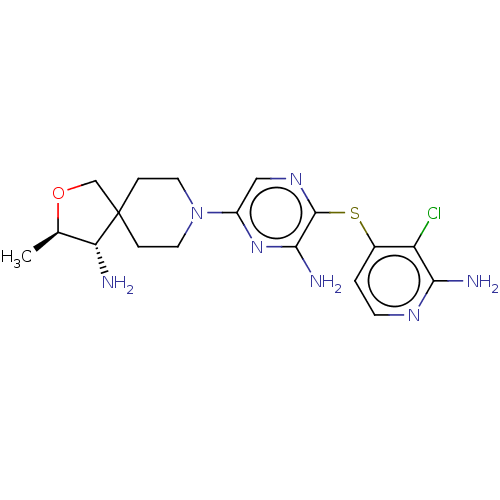
(CHEMBL4743002)Show SMILES C[C@H]1OCC2(CCN(CC2)c2cnc(Sc3ccnc(N)c3Cl)c(N)n2)[C@@H]1N |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Allosteric inhibition of 6x-histidine tagged human SHP2 (Met1-L525 residues) expressed in Escherichia coli BL21 Star (DE3) using IRS1_pY1172(dPEG8)pY... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01170
BindingDB Entry DOI: 10.7270/Q2CC14BB |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase catalytic subunit type 3
(Homo sapiens (Human)) | BDBM50156294

(CHEMBL3781415)Show InChI InChI=1S/C17H17N7/c18-16-21-10-13(15(23-16)9-11-1-2-11)14-5-8-20-17(24-14)22-12-3-6-19-7-4-12/h3-8,10-11H,1-2,9H2,(H2,18,21,23)(H,19,20,22,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human VPS34 using L-alpha-phosphatidylinositol as substrate incubated for 10 mins by luminescence based ATP detection assay |
ACS Med Chem Lett 7: 72-6 (2016)
Article DOI: 10.1021/acsmedchemlett.5b00335
BindingDB Entry DOI: 10.7270/Q2K35WK6 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Pseudomonas aeruginosa) | BDBM50469550
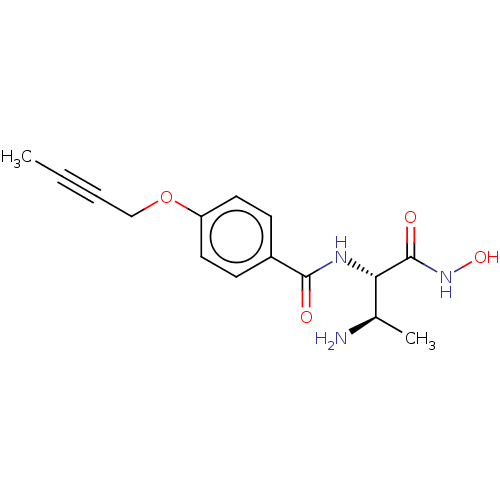
(CHEMBL4070478)Show SMILES CC#CCOc1ccc(cc1)C(=O)N[C@@H]([C@@H](C)N)C(=O)NO |r| Show InChI InChI=1S/C15H19N3O4/c1-3-4-9-22-12-7-5-11(6-8-12)14(19)17-13(10(2)16)15(20)18-21/h5-8,10,13,21H,9,16H2,1-2H3,(H,17,19)(H,18,20)/t10-,13+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 19.7 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of Pseudomonas aeruginosa LpxC using UDP-3-O-(R-hydroxydecanoyl)-N-acetylglucosamine as substrate preincubated for 30 mins followed by sub... |
J Med Chem 60: 5002-5014 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00377
BindingDB Entry DOI: 10.7270/Q2FN18MZ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 11
(Homo sapiens (Human)) | BDBM392338

(US10301278, Example 20)Show InChI InChI=1S/C17H23ClN6S/c1-17(10-19)5-7-24(8-6-17)13-9-22-16(15(21)23-13)25-12-4-2-3-11(20)14(12)18/h2-4,9H,5-8,10,19-20H2,1H3,(H2,21,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Allosteric inhibition of 6x-histidine tagged human SHP2 (Met1-L525 residues) expressed in Escherichia coli BL21 Star (DE3) using IRS1_pY1172(dPEG8)pY... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01170
BindingDB Entry DOI: 10.7270/Q2CC14BB |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 11
(Homo sapiens (Human)) | BDBM392323
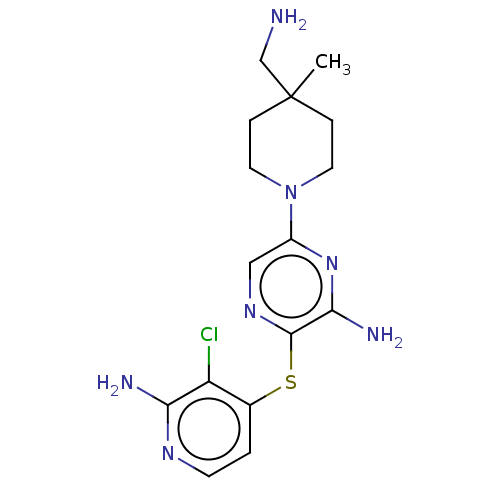
(3-((2-amino-3-chloropyridin-4-yl)thio)-6-(4-(amino...)Show InChI InChI=1S/C16H22ClN7S/c1-16(9-18)3-6-24(7-4-16)11-8-22-15(14(20)23-11)25-10-2-5-21-13(19)12(10)17/h2,5,8H,3-4,6-7,9,18H2,1H3,(H2,19,21)(H2,20,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Allosteric inhibition of 6x-histidine tagged human SHP2 (Met1-L525 residues) expressed in Escherichia coli BL21 Star (DE3) using IRS1_pY1172(dPEG8)pY... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01170
BindingDB Entry DOI: 10.7270/Q2CC14BB |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 11
(Homo sapiens (Human)) | BDBM50553789

(CHEMBL4747789)Show SMILES N[C@@H]1CCCC11CCN(CC1)c1cnc(Sc2ccnc(N)c2Cl)c(N)n1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Allosteric inhibition of human SHP2 in human KYSE-520 cells assessed as reduction in ERK1/2 phosphorylation by fluorescence based assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01170
BindingDB Entry DOI: 10.7270/Q2CC14BB |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase catalytic subunit type 3
(Homo sapiens (Human)) | BDBM50156296

(CHEMBL3781466)Show SMILES CC(C)(O)CNc1ncc(c(CC2CC2)n1)-c1ccnc(Nc2ccncc2)n1 Show InChI InChI=1S/C21H25N7O/c1-21(2,29)13-25-19-24-12-16(18(28-19)11-14-3-4-14)17-7-10-23-20(27-17)26-15-5-8-22-9-6-15/h5-10,12,14,29H,3-4,11,13H2,1-2H3,(H,24,25,28)(H,22,23,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of VPS34 in human U2OS cells incubated for 2 hrs by GFP-FYVE reporter gene assay |
ACS Med Chem Lett 7: 72-6 (2016)
Article DOI: 10.1021/acsmedchemlett.5b00335
BindingDB Entry DOI: 10.7270/Q2K35WK6 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein phosphatase non-receptor type 11
(Homo sapiens (Human)) | BDBM50553791

(CHEMBL4743002)Show SMILES C[C@H]1OCC2(CCN(CC2)c2cnc(Sc3ccnc(N)c3Cl)c(N)n2)[C@@H]1N |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Allosteric inhibition of human SHP2 in human KYSE-520 cells assessed as reduction in ERK1/2 phosphorylation by fluorescence based assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01170
BindingDB Entry DOI: 10.7270/Q2CC14BB |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 11
(Homo sapiens (Human)) | BDBM50553784
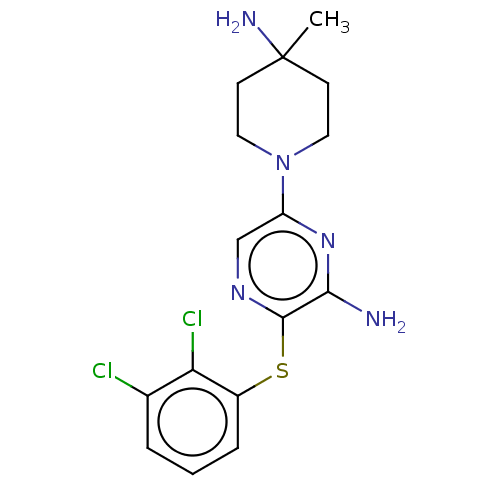
(CHEMBL4762625) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Allosteric inhibition of 6x-histidine tagged human SHP2 (Met1-L525 residues) expressed in Escherichia coli BL21 Star (DE3) using IRS1_pY1172(dPEG8)pY... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01170
BindingDB Entry DOI: 10.7270/Q2CC14BB |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data