 Found 123 hits with Last Name = 'meguro' and Initial = 'm'
Found 123 hits with Last Name = 'meguro' and Initial = 'm' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Cytochrome P450 11B2, mitochondrial
(Homo sapiens (Human)) | BDBM50238110
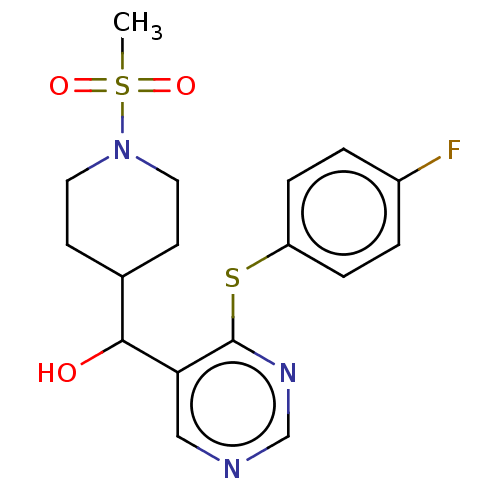
(CHEMBL4088766)Show SMILES CS(=O)(=O)N1CCC(CC1)C(O)c1cncnc1Sc1ccc(F)cc1 Show InChI InChI=1S/C17H20FN3O3S2/c1-26(23,24)21-8-6-12(7-9-21)16(22)15-10-19-11-20-17(15)25-14-4-2-13(18)3-5-14/h2-5,10-12,16,22H,6-9H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi-Sankyo Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human CYP11B2 expressed in HEK293A cells using deoxycorticosterone as substrate pretreated for 1 hr followed by substrate addition meas... |
Bioorg Med Chem Lett 27: 1902-1906 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.034
BindingDB Entry DOI: 10.7270/Q2KS6TTS |
More data for this
Ligand-Target Pair | |
Cytochrome P450 11B1, mitochondrial
(Homo sapiens (Human)) | BDBM50238110

(CHEMBL4088766)Show SMILES CS(=O)(=O)N1CCC(CC1)C(O)c1cncnc1Sc1ccc(F)cc1 Show InChI InChI=1S/C17H20FN3O3S2/c1-26(23,24)21-8-6-12(7-9-21)16(22)15-10-19-11-20-17(15)25-14-4-2-13(18)3-5-14/h2-5,10-12,16,22H,6-9H2,1H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi-Sankyo Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human CYP11B1 expressed in HEK293A cells |
Bioorg Med Chem Lett 27: 1902-1906 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.034
BindingDB Entry DOI: 10.7270/Q2KS6TTS |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50387262
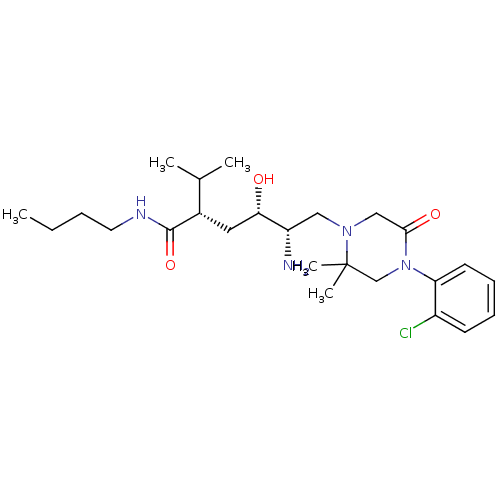
(CHEMBL2048702)Show SMILES CCCCNC(=O)[C@@H](C[C@H](O)[C@@H](N)CN1CC(=O)N(CC1(C)C)c1ccccc1Cl)C(C)C |r| Show InChI InChI=1S/C25H41ClN4O3/c1-6-7-12-28-24(33)18(17(2)3)13-22(31)20(27)14-29-15-23(32)30(16-25(29,4)5)21-11-9-8-10-19(21)26/h8-11,17-18,20,22,31H,6-7,12-16,27H2,1-5H3,(H,28,33)/t18-,20-,22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human purified renin |
Bioorg Med Chem Lett 22: 4561-6 (2012)
Article DOI: 10.1016/j.bmcl.2012.05.092
BindingDB Entry DOI: 10.7270/Q2BK1DD9 |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50387263
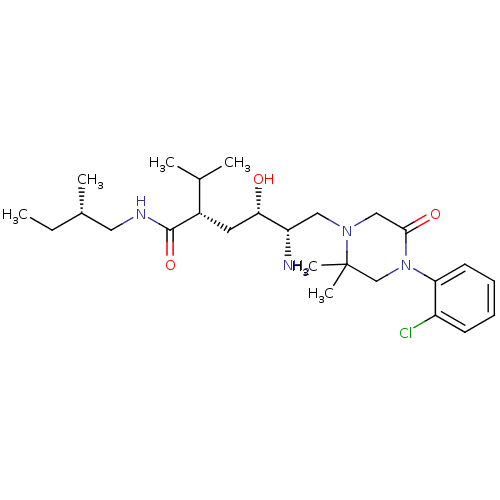
(CHEMBL2048703)Show SMILES CC[C@H](C)CNC(=O)[C@@H](C[C@H](O)[C@@H](N)CN1CC(=O)N(CC1(C)C)c1ccccc1Cl)C(C)C |r| Show InChI InChI=1S/C26H43ClN4O3/c1-7-18(4)13-29-25(34)19(17(2)3)12-23(32)21(28)14-30-15-24(33)31(16-26(30,5)6)22-11-9-8-10-20(22)27/h8-11,17-19,21,23,32H,7,12-16,28H2,1-6H3,(H,29,34)/t18-,19-,21-,23-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human purified renin |
Bioorg Med Chem Lett 22: 4561-6 (2012)
Article DOI: 10.1016/j.bmcl.2012.05.092
BindingDB Entry DOI: 10.7270/Q2BK1DD9 |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50387264
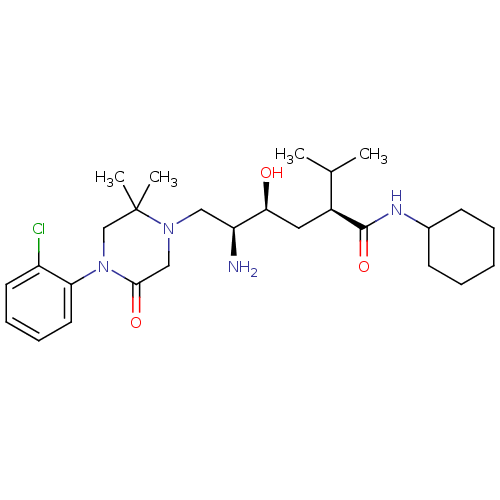
(CHEMBL2048704)Show SMILES CC(C)[C@H](C[C@H](O)[C@@H](N)CN1CC(=O)N(CC1(C)C)c1ccccc1Cl)C(=O)NC1CCCCC1 |r| Show InChI InChI=1S/C27H43ClN4O3/c1-18(2)20(26(35)30-19-10-6-5-7-11-19)14-24(33)22(29)15-31-16-25(34)32(17-27(31,3)4)23-13-9-8-12-21(23)28/h8-9,12-13,18-20,22,24,33H,5-7,10-11,14-17,29H2,1-4H3,(H,30,35)/t20-,22-,24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human purified renin |
Bioorg Med Chem Lett 22: 4561-6 (2012)
Article DOI: 10.1016/j.bmcl.2012.05.092
BindingDB Entry DOI: 10.7270/Q2BK1DD9 |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50387274
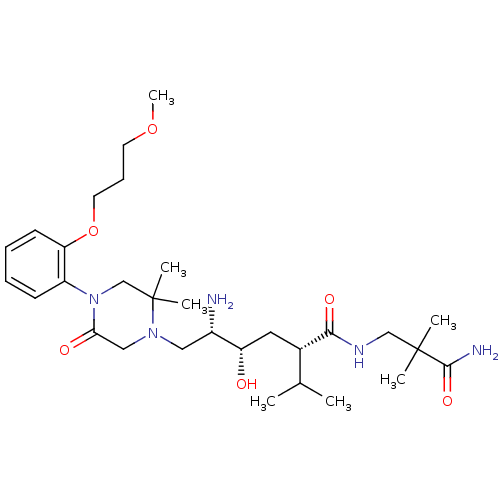
(CHEMBL2048564)Show SMILES COCCCOc1ccccc1N1CC(C)(C)N(C[C@H](N)[C@@H](O)C[C@@H](C(C)C)C(=O)NCC(C)(C)C(N)=O)CC1=O |r| Show InChI InChI=1S/C30H51N5O6/c1-20(2)21(27(38)33-18-29(3,4)28(32)39)15-24(36)22(31)16-34-17-26(37)35(19-30(34,5)6)23-11-8-9-12-25(23)41-14-10-13-40-7/h8-9,11-12,20-22,24,36H,10,13-19,31H2,1-7H3,(H2,32,39)(H,33,38)/t21-,22-,24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human purified renin |
Bioorg Med Chem Lett 22: 4561-6 (2012)
Article DOI: 10.1016/j.bmcl.2012.05.092
BindingDB Entry DOI: 10.7270/Q2BK1DD9 |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50387280
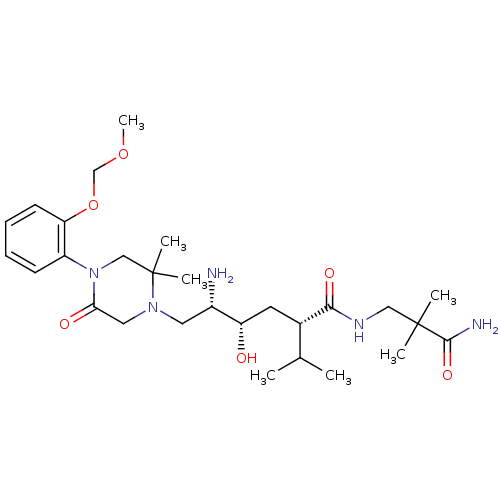
(CHEMBL2046465)Show SMILES COCOc1ccccc1N1CC(C)(C)N(C[C@H](N)[C@@H](O)C[C@@H](C(C)C)C(=O)NCC(C)(C)C(N)=O)CC1=O |r| Show InChI InChI=1S/C28H47N5O6/c1-18(2)19(25(36)31-15-27(3,4)26(30)37)12-22(34)20(29)13-32-14-24(35)33(16-28(32,5)6)21-10-8-9-11-23(21)39-17-38-7/h8-11,18-20,22,34H,12-17,29H2,1-7H3,(H2,30,37)(H,31,36)/t19-,20-,22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human purified renin |
Bioorg Med Chem Lett 22: 4561-6 (2012)
Article DOI: 10.1016/j.bmcl.2012.05.092
BindingDB Entry DOI: 10.7270/Q2BK1DD9 |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50387257
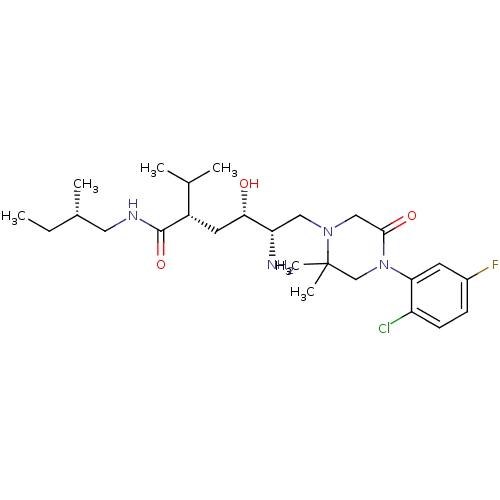
(CHEMBL2048706)Show SMILES CC[C@H](C)CNC(=O)[C@@H](C[C@H](O)[C@@H](N)CN1CC(=O)N(CC1(C)C)c1cc(F)ccc1Cl)C(C)C |r| Show InChI InChI=1S/C26H42ClFN4O3/c1-7-17(4)12-30-25(35)19(16(2)3)11-23(33)21(29)13-31-14-24(34)32(15-26(31,5)6)22-10-18(28)8-9-20(22)27/h8-10,16-17,19,21,23,33H,7,11-15,29H2,1-6H3,(H,30,35)/t17-,19-,21-,23-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human purified renin |
Bioorg Med Chem Lett 22: 4561-6 (2012)
Article DOI: 10.1016/j.bmcl.2012.05.092
BindingDB Entry DOI: 10.7270/Q2BK1DD9 |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50387268
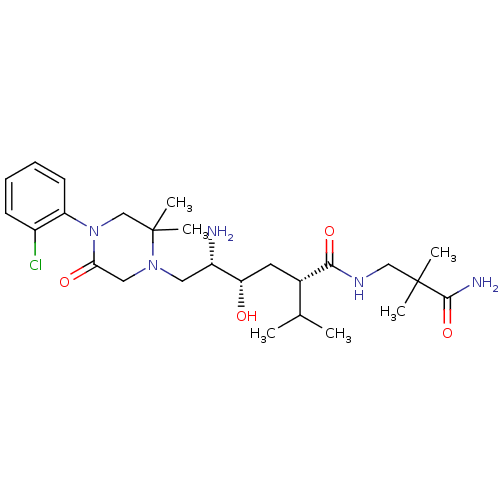
(CHEMBL2048571)Show SMILES CC(C)[C@H](C[C@H](O)[C@@H](N)CN1CC(=O)N(CC1(C)C)c1ccccc1Cl)C(=O)NCC(C)(C)C(N)=O |r| Show InChI InChI=1S/C26H42ClN5O4/c1-16(2)17(23(35)30-14-25(3,4)24(29)36)11-21(33)19(28)12-31-13-22(34)32(15-26(31,5)6)20-10-8-7-9-18(20)27/h7-10,16-17,19,21,33H,11-15,28H2,1-6H3,(H2,29,36)(H,30,35)/t17-,19-,21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human purified renin |
Bioorg Med Chem Lett 22: 4561-6 (2012)
Article DOI: 10.1016/j.bmcl.2012.05.092
BindingDB Entry DOI: 10.7270/Q2BK1DD9 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Renin
(Homo sapiens (Human)) | BDBM50387279

(CHEMBL2048566)Show SMILES COCCOc1ccccc1N1CC(C)(C)N(C[C@H](N)[C@@H](O)C[C@@H](C(C)C)C(=O)NCC(C)(C)C(N)=O)CC1=O |r| Show InChI InChI=1S/C29H49N5O6/c1-19(2)20(26(37)32-17-28(3,4)27(31)38)14-23(35)21(30)15-33-16-25(36)34(18-29(33,5)6)22-10-8-9-11-24(22)40-13-12-39-7/h8-11,19-21,23,35H,12-18,30H2,1-7H3,(H2,31,38)(H,32,37)/t20-,21-,23-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human purified renin |
Bioorg Med Chem Lett 22: 4561-6 (2012)
Article DOI: 10.1016/j.bmcl.2012.05.092
BindingDB Entry DOI: 10.7270/Q2BK1DD9 |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50387284
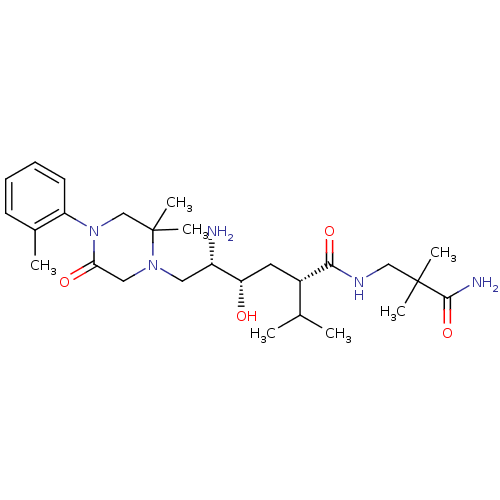
(CHEMBL2048570)Show SMILES CC(C)[C@H](C[C@H](O)[C@@H](N)CN1CC(=O)N(CC1(C)C)c1ccccc1C)C(=O)NCC(C)(C)C(N)=O |r| Show InChI InChI=1S/C27H45N5O4/c1-17(2)19(24(35)30-15-26(4,5)25(29)36)12-22(33)20(28)13-31-14-23(34)32(16-27(31,6)7)21-11-9-8-10-18(21)3/h8-11,17,19-20,22,33H,12-16,28H2,1-7H3,(H2,29,36)(H,30,35)/t19-,20-,22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human purified renin |
Bioorg Med Chem Lett 22: 4561-6 (2012)
Article DOI: 10.1016/j.bmcl.2012.05.092
BindingDB Entry DOI: 10.7270/Q2BK1DD9 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Renin
(Homo sapiens (Human)) | BDBM50387281

(CHEMBL2048567)Show SMILES COc1ccccc1N1CC(C)(C)N(C[C@H](N)[C@@H](O)C[C@@H](C(C)C)C(=O)NCC(C)(C)C(N)=O)CC1=O |r| Show InChI InChI=1S/C27H45N5O5/c1-17(2)18(24(35)30-15-26(3,4)25(29)36)12-21(33)19(28)13-31-14-23(34)32(16-27(31,5)6)20-10-8-9-11-22(20)37-7/h8-11,17-19,21,33H,12-16,28H2,1-7H3,(H2,29,36)(H,30,35)/t18-,19-,21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human purified renin |
Bioorg Med Chem Lett 22: 4561-6 (2012)
Article DOI: 10.1016/j.bmcl.2012.05.092
BindingDB Entry DOI: 10.7270/Q2BK1DD9 |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50387266
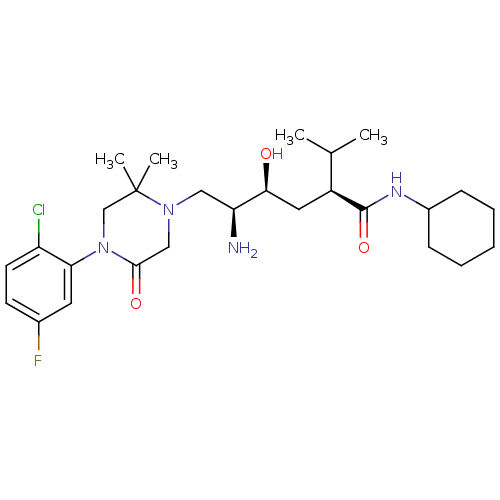
(CHEMBL2048707)Show SMILES CC(C)[C@H](C[C@H](O)[C@@H](N)CN1CC(=O)N(CC1(C)C)c1cc(F)ccc1Cl)C(=O)NC1CCCCC1 |r| Show InChI InChI=1S/C27H42ClFN4O3/c1-17(2)20(26(36)31-19-8-6-5-7-9-19)13-24(34)22(30)14-32-15-25(35)33(16-27(32,3)4)23-12-18(29)10-11-21(23)28/h10-12,17,19-20,22,24,34H,5-9,13-16,30H2,1-4H3,(H,31,36)/t20-,22-,24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human purified renin |
Bioorg Med Chem Lett 22: 4561-6 (2012)
Article DOI: 10.1016/j.bmcl.2012.05.092
BindingDB Entry DOI: 10.7270/Q2BK1DD9 |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50387272
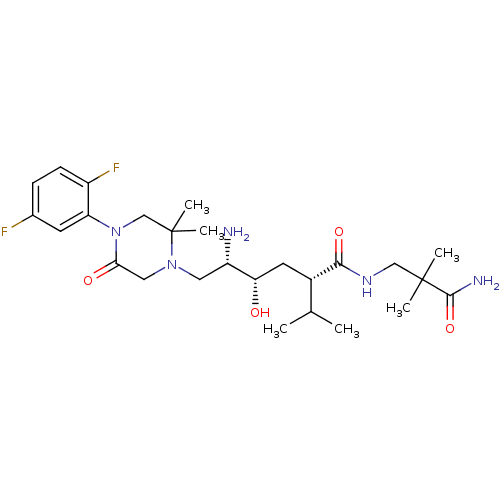
(CHEMBL2048576)Show SMILES CC(C)[C@H](C[C@H](O)[C@@H](N)CN1CC(=O)N(CC1(C)C)c1cc(F)ccc1F)C(=O)NCC(C)(C)C(N)=O |r| Show InChI InChI=1S/C26H41F2N5O4/c1-15(2)17(23(36)31-13-25(3,4)24(30)37)10-21(34)19(29)11-32-12-22(35)33(14-26(32,5)6)20-9-16(27)7-8-18(20)28/h7-9,15,17,19,21,34H,10-14,29H2,1-6H3,(H2,30,37)(H,31,36)/t17-,19-,21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human purified renin |
Bioorg Med Chem Lett 22: 4561-6 (2012)
Article DOI: 10.1016/j.bmcl.2012.05.092
BindingDB Entry DOI: 10.7270/Q2BK1DD9 |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50387267
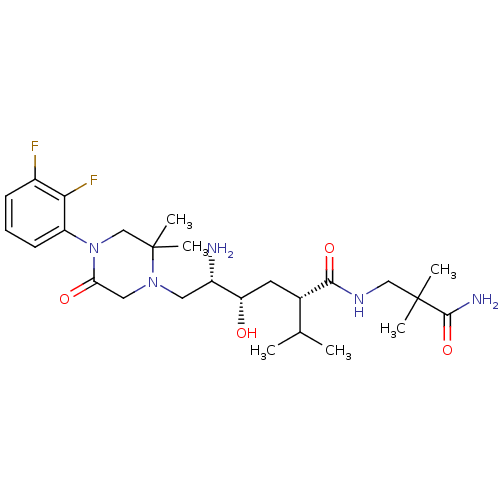
(CHEMBL2048575)Show SMILES CC(C)[C@H](C[C@H](O)[C@@H](N)CN1CC(=O)N(CC1(C)C)c1cccc(F)c1F)C(=O)NCC(C)(C)C(N)=O |r| Show InChI InChI=1S/C26H41F2N5O4/c1-15(2)16(23(36)31-13-25(3,4)24(30)37)10-20(34)18(29)11-32-12-21(35)33(14-26(32,5)6)19-9-7-8-17(27)22(19)28/h7-9,15-16,18,20,34H,10-14,29H2,1-6H3,(H2,30,37)(H,31,36)/t16-,18-,20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human purified renin |
Bioorg Med Chem Lett 22: 4561-6 (2012)
Article DOI: 10.1016/j.bmcl.2012.05.092
BindingDB Entry DOI: 10.7270/Q2BK1DD9 |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50387265

(CHEMBL2048705)Show SMILES CCCCNC(=O)[C@@H](C[C@H](O)[C@@H](N)CN1CC(=O)N(CC1(C)C)c1cc(F)ccc1Cl)C(C)C |r| Show InChI InChI=1S/C25H40ClFN4O3/c1-6-7-10-29-24(34)18(16(2)3)12-22(32)20(28)13-30-14-23(33)31(15-25(30,4)5)21-11-17(27)8-9-19(21)26/h8-9,11,16,18,20,22,32H,6-7,10,12-15,28H2,1-5H3,(H,29,34)/t18-,20-,22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human purified renin |
Bioorg Med Chem Lett 22: 4561-6 (2012)
Article DOI: 10.1016/j.bmcl.2012.05.092
BindingDB Entry DOI: 10.7270/Q2BK1DD9 |
More data for this
Ligand-Target Pair | |
Renin
(Macaca fascicularis) | BDBM50387263

(CHEMBL2048703)Show SMILES CC[C@H](C)CNC(=O)[C@@H](C[C@H](O)[C@@H](N)CN1CC(=O)N(CC1(C)C)c1ccccc1Cl)C(C)C |r| Show InChI InChI=1S/C26H43ClN4O3/c1-7-18(4)13-29-25(34)19(17(2)3)12-23(32)21(28)14-30-15-24(33)31(16-26(30,5)6)22-11-9-8-10-20(22)27/h8-11,17-19,21,23,32H,7,12-16,28H2,1-6H3,(H,29,34)/t18-,19-,21-,23-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of renin in cynomolgus monkey plasma |
Bioorg Med Chem Lett 22: 4561-6 (2012)
Article DOI: 10.1016/j.bmcl.2012.05.092
BindingDB Entry DOI: 10.7270/Q2BK1DD9 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 11B2, mitochondrial
(Homo sapiens (Human)) | BDBM50238108
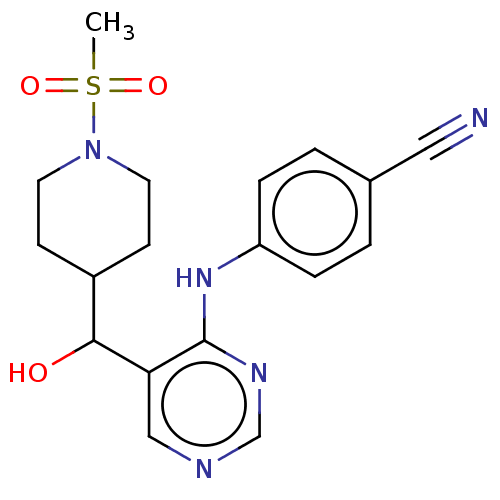
(CHEMBL4070230)Show SMILES CS(=O)(=O)N1CCC(CC1)C(O)c1cncnc1Nc1ccc(cc1)C#N Show InChI InChI=1S/C18H21N5O3S/c1-27(25,26)23-8-6-14(7-9-23)17(24)16-11-20-12-21-18(16)22-15-4-2-13(10-19)3-5-15/h2-5,11-12,14,17,24H,6-9H2,1H3,(H,20,21,22) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi-Sankyo Co., Ltd.
Curated by ChEMBL
| Assay Description
Concentration required to inhibit the activity of Angiotensin I converting enzyme by 50% |
Bioorg Med Chem Lett 27: 1902-1906 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.034
BindingDB Entry DOI: 10.7270/Q2KS6TTS |
More data for this
Ligand-Target Pair | |
Cytochrome P450 11B1, mitochondrial
(Homo sapiens (Human)) | BDBM50238115
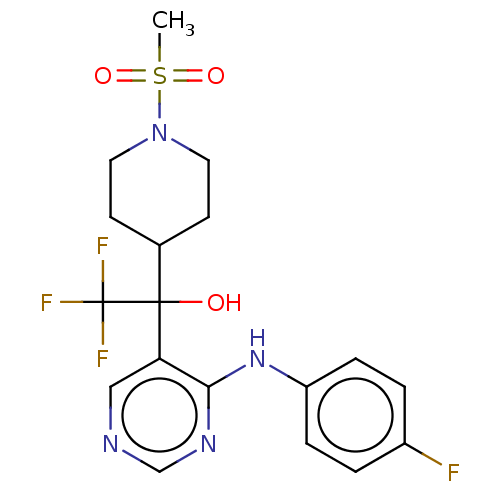
(CHEMBL4082403)Show SMILES CS(=O)(=O)N1CCC(CC1)C(O)(c1cncnc1Nc1ccc(F)cc1)C(F)(F)F Show InChI InChI=1S/C18H20F4N4O3S/c1-30(28,29)26-8-6-12(7-9-26)17(27,18(20,21)22)15-10-23-11-24-16(15)25-14-4-2-13(19)3-5-14/h2-5,10-12,27H,6-9H2,1H3,(H,23,24,25) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi-Sankyo Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human CYP11B1 expressed in HEK293A cells |
Bioorg Med Chem Lett 27: 1902-1906 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.034
BindingDB Entry DOI: 10.7270/Q2KS6TTS |
More data for this
Ligand-Target Pair | |
Cytochrome P450 11B2, mitochondrial
(Homo sapiens (Human)) | BDBM50238103

(CHEMBL4070323)Show SMILES CC(O)(C1CCN(CC1)S(C)(=O)=O)c1cncnc1Nc1ccc(F)cc1 Show InChI InChI=1S/C18H23FN4O3S/c1-18(24,13-7-9-23(10-8-13)27(2,25)26)16-11-20-12-21-17(16)22-15-5-3-14(19)4-6-15/h3-6,11-13,24H,7-10H2,1-2H3,(H,20,21,22) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi-Sankyo Co., Ltd.
Curated by ChEMBL
| Assay Description
Concentration required to inhibit the activity of Angiotensin I converting enzyme by 50% |
Bioorg Med Chem Lett 27: 1902-1906 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.034
BindingDB Entry DOI: 10.7270/Q2KS6TTS |
More data for this
Ligand-Target Pair | |
Cytochrome P450 11B1, mitochondrial
(Homo sapiens (Human)) | BDBM50238115

(CHEMBL4082403)Show SMILES CS(=O)(=O)N1CCC(CC1)C(O)(c1cncnc1Nc1ccc(F)cc1)C(F)(F)F Show InChI InChI=1S/C18H20F4N4O3S/c1-30(28,29)26-8-6-12(7-9-26)17(27,18(20,21)22)15-10-23-11-24-16(15)25-14-4-2-13(19)3-5-14/h2-5,10-12,27H,6-9H2,1H3,(H,23,24,25) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi-Sankyo Co., Ltd.
Curated by ChEMBL
| Assay Description
Concentration required to inhibit the activity of Angiotensin I converting enzyme by 50% |
Bioorg Med Chem Lett 27: 1902-1906 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.034
BindingDB Entry DOI: 10.7270/Q2KS6TTS |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50387259
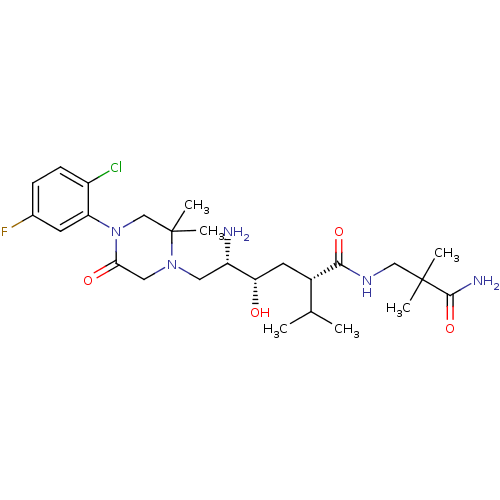
(CHEMBL2048579)Show SMILES CC(C)[C@H](C[C@H](O)[C@@H](N)CN1CC(=O)N(CC1(C)C)c1cc(F)ccc1Cl)C(=O)NCC(C)(C)C(N)=O |r| Show InChI InChI=1S/C26H41ClFN5O4/c1-15(2)17(23(36)31-13-25(3,4)24(30)37)10-21(34)19(29)11-32-12-22(35)33(14-26(32,5)6)20-9-16(28)7-8-18(20)27/h7-9,15,17,19,21,34H,10-14,29H2,1-6H3,(H2,30,37)(H,31,36)/t17-,19-,21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human purified renin |
Bioorg Med Chem Lett 22: 4561-6 (2012)
Article DOI: 10.1016/j.bmcl.2012.05.092
BindingDB Entry DOI: 10.7270/Q2BK1DD9 |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50387260
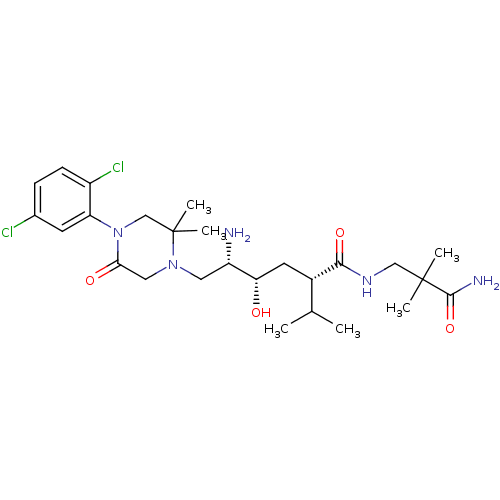
(CHEMBL2048580)Show SMILES CC(C)[C@H](C[C@H](O)[C@@H](N)CN1CC(=O)N(CC1(C)C)c1cc(Cl)ccc1Cl)C(=O)NCC(C)(C)C(N)=O |r| Show InChI InChI=1S/C26H41Cl2N5O4/c1-15(2)17(23(36)31-13-25(3,4)24(30)37)10-21(34)19(29)11-32-12-22(35)33(14-26(32,5)6)20-9-16(27)7-8-18(20)28/h7-9,15,17,19,21,34H,10-14,29H2,1-6H3,(H2,30,37)(H,31,36)/t17-,19-,21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human purified renin |
Bioorg Med Chem Lett 22: 4561-6 (2012)
Article DOI: 10.1016/j.bmcl.2012.05.092
BindingDB Entry DOI: 10.7270/Q2BK1DD9 |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50387271

(CHEMBL2048574)Show SMILES CC(C)[C@H](C[C@H](O)[C@@H](N)CN1CC(=O)N(CC1(C)C)c1cccc(Cl)c1)C(=O)NCC(C)(C)C(N)=O |r| Show InChI InChI=1S/C26H42ClN5O4/c1-16(2)19(23(35)30-14-25(3,4)24(29)36)11-21(33)20(28)12-31-13-22(34)32(15-26(31,5)6)18-9-7-8-17(27)10-18/h7-10,16,19-21,33H,11-15,28H2,1-6H3,(H2,29,36)(H,30,35)/t19-,20-,21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human purified renin |
Bioorg Med Chem Lett 22: 4561-6 (2012)
Article DOI: 10.1016/j.bmcl.2012.05.092
BindingDB Entry DOI: 10.7270/Q2BK1DD9 |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50387283
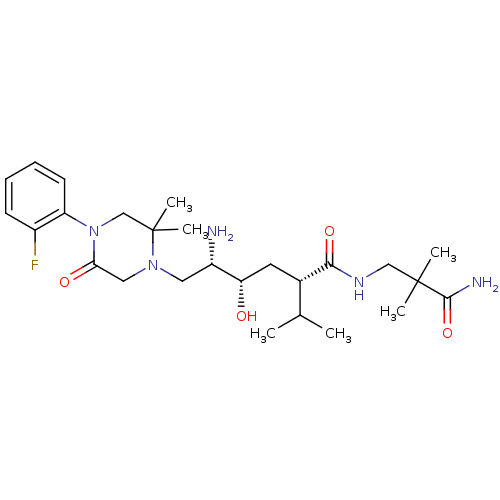
(CHEMBL2048569)Show SMILES CC(C)[C@H](C[C@H](O)[C@@H](N)CN1CC(=O)N(CC1(C)C)c1ccccc1F)C(=O)NCC(C)(C)C(N)=O |r| Show InChI InChI=1S/C26H42FN5O4/c1-16(2)17(23(35)30-14-25(3,4)24(29)36)11-21(33)19(28)12-31-13-22(34)32(15-26(31,5)6)20-10-8-7-9-18(20)27/h7-10,16-17,19,21,33H,11-15,28H2,1-6H3,(H2,29,36)(H,30,35)/t17-,19-,21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human purified renin |
Bioorg Med Chem Lett 22: 4561-6 (2012)
Article DOI: 10.1016/j.bmcl.2012.05.092
BindingDB Entry DOI: 10.7270/Q2BK1DD9 |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50387261

(CHEMBL2048701)Show SMILES COc1ccc(Cl)c(c1)N1CC(C)(C)N(C[C@H](N)[C@@H](O)C[C@@H](C(C)C)C(=O)NCC(C)(C)C(N)=O)CC1=O |r| Show InChI InChI=1S/C27H44ClN5O5/c1-16(2)18(24(36)31-14-26(3,4)25(30)37)11-22(34)20(29)12-32-13-23(35)33(15-27(32,5)6)21-10-17(38-7)8-9-19(21)28/h8-10,16,18,20,22,34H,11-15,29H2,1-7H3,(H2,30,37)(H,31,36)/t18-,20-,22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human purified renin |
Bioorg Med Chem Lett 22: 4561-6 (2012)
Article DOI: 10.1016/j.bmcl.2012.05.092
BindingDB Entry DOI: 10.7270/Q2BK1DD9 |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50387273
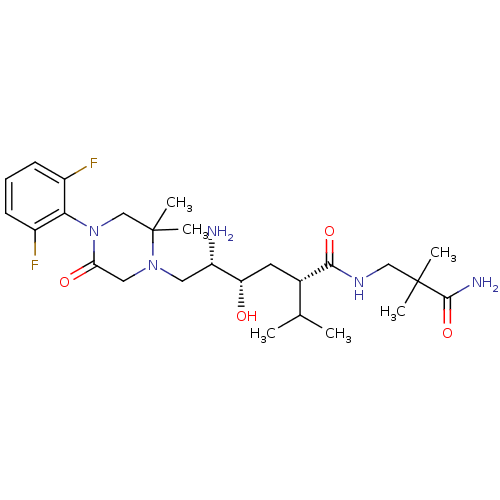
(CHEMBL2048577)Show SMILES CC(C)[C@H](C[C@H](O)[C@@H](N)CN1CC(=O)N(CC1(C)C)c1c(F)cccc1F)C(=O)NCC(C)(C)C(N)=O |r| Show InChI InChI=1S/C26H41F2N5O4/c1-15(2)16(23(36)31-13-25(3,4)24(30)37)10-20(34)19(29)11-32-12-21(35)33(14-26(32,5)6)22-17(27)8-7-9-18(22)28/h7-9,15-16,19-20,34H,10-14,29H2,1-6H3,(H2,30,37)(H,31,36)/t16-,19-,20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human purified renin |
Bioorg Med Chem Lett 22: 4561-6 (2012)
Article DOI: 10.1016/j.bmcl.2012.05.092
BindingDB Entry DOI: 10.7270/Q2BK1DD9 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 11B2, mitochondrial
(Homo sapiens (Human)) | BDBM50238109
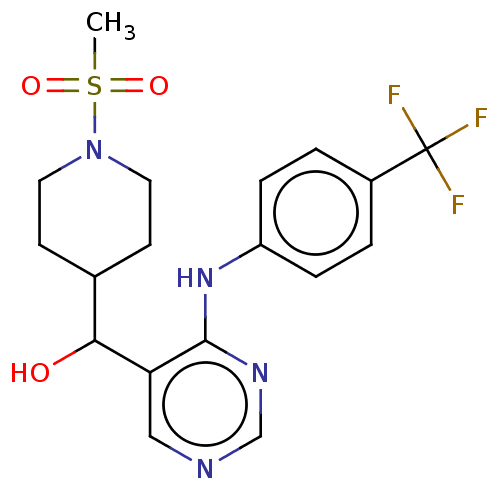
(CHEMBL4091167)Show SMILES CS(=O)(=O)N1CCC(CC1)C(O)c1cncnc1Nc1ccc(cc1)C(F)(F)F Show InChI InChI=1S/C18H21F3N4O3S/c1-29(27,28)25-8-6-12(7-9-25)16(26)15-10-22-11-23-17(15)24-14-4-2-13(3-5-14)18(19,20)21/h2-5,10-12,16,26H,6-9H2,1H3,(H,22,23,24) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi-Sankyo Co., Ltd.
Curated by ChEMBL
| Assay Description
Concentration required to inhibit the activity of Angiotensin I converting enzyme by 50% |
Bioorg Med Chem Lett 27: 1902-1906 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.034
BindingDB Entry DOI: 10.7270/Q2KS6TTS |
More data for this
Ligand-Target Pair | |
Cytochrome P450 11B2, mitochondrial
(Homo sapiens (Human)) | BDBM50238105

(CHEMBL4099824)Show SMILES CS(=O)(=O)N1CCC(CC1)C(O)c1cncnc1Nc1ccc(F)cc1 Show InChI InChI=1S/C17H21FN4O3S/c1-26(24,25)22-8-6-12(7-9-22)16(23)15-10-19-11-20-17(15)21-14-4-2-13(18)3-5-14/h2-5,10-12,16,23H,6-9H2,1H3,(H,19,20,21) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi-Sankyo Co., Ltd.
Curated by ChEMBL
| Assay Description
Concentration required to inhibit the activity of Angiotensin I converting enzyme by 50% |
Bioorg Med Chem Lett 27: 1902-1906 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.034
BindingDB Entry DOI: 10.7270/Q2KS6TTS |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50387270

(CHEMBL2048573)Show SMILES CC(C)[C@H](C[C@H](O)[C@@H](N)CN1CC(=O)N(CC1(C)C)c1cccc(F)c1)C(=O)NCC(C)(C)C(N)=O |r| Show InChI InChI=1S/C26H42FN5O4/c1-16(2)19(23(35)30-14-25(3,4)24(29)36)11-21(33)20(28)12-31-13-22(34)32(15-26(31,5)6)18-9-7-8-17(27)10-18/h7-10,16,19-21,33H,11-15,28H2,1-6H3,(H2,29,36)(H,30,35)/t19-,20-,21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human purified renin |
Bioorg Med Chem Lett 22: 4561-6 (2012)
Article DOI: 10.1016/j.bmcl.2012.05.092
BindingDB Entry DOI: 10.7270/Q2BK1DD9 |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50387282
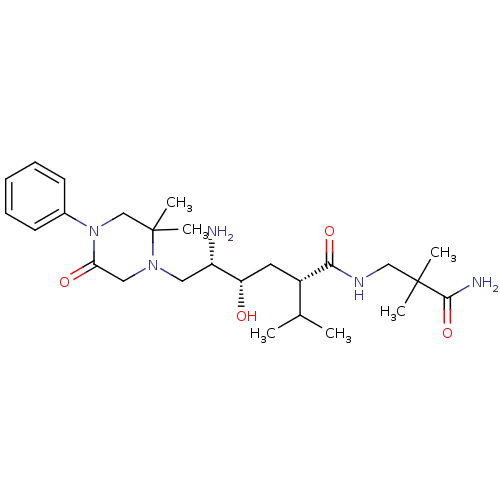
(CHEMBL2048568)Show SMILES CC(C)[C@H](C[C@H](O)[C@@H](N)CN1CC(=O)N(CC1(C)C)c1ccccc1)C(=O)NCC(C)(C)C(N)=O |r| Show InChI InChI=1S/C26H43N5O4/c1-17(2)19(23(34)29-15-25(3,4)24(28)35)12-21(32)20(27)13-30-14-22(33)31(16-26(30,5)6)18-10-8-7-9-11-18/h7-11,17,19-21,32H,12-16,27H2,1-6H3,(H2,28,35)(H,29,34)/t19-,20-,21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human purified renin |
Bioorg Med Chem Lett 22: 4561-6 (2012)
Article DOI: 10.1016/j.bmcl.2012.05.092
BindingDB Entry DOI: 10.7270/Q2BK1DD9 |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50387274

(CHEMBL2048564)Show SMILES COCCCOc1ccccc1N1CC(C)(C)N(C[C@H](N)[C@@H](O)C[C@@H](C(C)C)C(=O)NCC(C)(C)C(N)=O)CC1=O |r| Show InChI InChI=1S/C30H51N5O6/c1-20(2)21(27(38)33-18-29(3,4)28(32)39)15-24(36)22(31)16-34-17-26(37)35(19-30(34,5)6)23-11-8-9-12-25(23)41-14-10-13-40-7/h8-9,11-12,20-22,24,36H,10,13-19,31H2,1-7H3,(H2,32,39)(H,33,38)/t21-,22-,24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human purified renin |
Bioorg Med Chem Lett 22: 4561-6 (2012)
Article DOI: 10.1016/j.bmcl.2012.05.092
BindingDB Entry DOI: 10.7270/Q2BK1DD9 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 11B2, mitochondrial
(Homo sapiens (Human)) | BDBM50047262
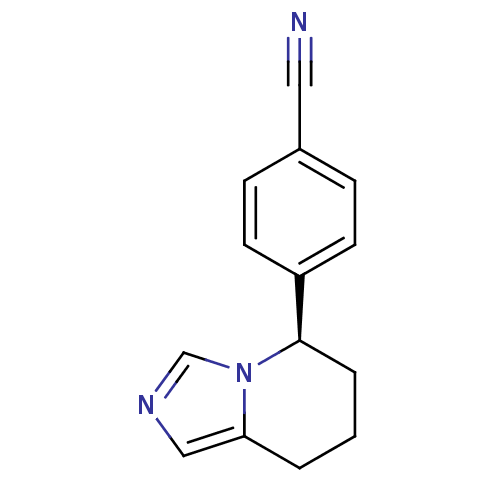
((R)-4-(5,6,7,8-tetrahydroimidazo[1,5-a]pyridin-5-y...)Show InChI InChI=1S/C14H13N3/c15-8-11-4-6-12(7-5-11)14-3-1-2-13-9-16-10-17(13)14/h4-7,9-10,14H,1-3H2/t14-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi-Sankyo Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human CYP11B2 expressed in HEK293A cells using deoxycorticosterone as substrate pretreated for 1 hr followed by substrate addition meas... |
Bioorg Med Chem Lett 27: 1902-1906 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.034
BindingDB Entry DOI: 10.7270/Q2KS6TTS |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cytochrome P450 11B2, mitochondrial
(Homo sapiens (Human)) | BDBM50238115

(CHEMBL4082403)Show SMILES CS(=O)(=O)N1CCC(CC1)C(O)(c1cncnc1Nc1ccc(F)cc1)C(F)(F)F Show InChI InChI=1S/C18H20F4N4O3S/c1-30(28,29)26-8-6-12(7-9-26)17(27,18(20,21)22)15-10-23-11-24-16(15)25-14-4-2-13(19)3-5-14/h2-5,10-12,27H,6-9H2,1H3,(H,23,24,25) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi-Sankyo Co., Ltd.
Curated by ChEMBL
| Assay Description
Concentration required to inhibit the activity of Angiotensin I converting enzyme by 50% |
Bioorg Med Chem Lett 27: 1902-1906 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.034
BindingDB Entry DOI: 10.7270/Q2KS6TTS |
More data for this
Ligand-Target Pair | |
Cytochrome P450 11B2, mitochondrial
(Homo sapiens (Human)) | BDBM50238115

(CHEMBL4082403)Show SMILES CS(=O)(=O)N1CCC(CC1)C(O)(c1cncnc1Nc1ccc(F)cc1)C(F)(F)F Show InChI InChI=1S/C18H20F4N4O3S/c1-30(28,29)26-8-6-12(7-9-26)17(27,18(20,21)22)15-10-23-11-24-16(15)25-14-4-2-13(19)3-5-14/h2-5,10-12,27H,6-9H2,1H3,(H,23,24,25) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi-Sankyo Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human CYP11B2 expressed in HEK293A cells using deoxycorticosterone as substrate pretreated for 1 hr followed by substrate addition meas... |
Bioorg Med Chem Lett 27: 1902-1906 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.034
BindingDB Entry DOI: 10.7270/Q2KS6TTS |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50387269

(CHEMBL2048572)Show SMILES COc1cccc(c1)N1CC(C)(C)N(C[C@H](N)[C@@H](O)C[C@@H](C(C)C)C(=O)NCC(C)(C)C(N)=O)CC1=O |r| Show InChI InChI=1S/C27H45N5O5/c1-17(2)20(24(35)30-15-26(3,4)25(29)36)12-22(33)21(28)13-31-14-23(34)32(16-27(31,5)6)18-9-8-10-19(11-18)37-7/h8-11,17,20-22,33H,12-16,28H2,1-7H3,(H2,29,36)(H,30,35)/t20-,21-,22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human purified renin |
Bioorg Med Chem Lett 22: 4561-6 (2012)
Article DOI: 10.1016/j.bmcl.2012.05.092
BindingDB Entry DOI: 10.7270/Q2BK1DD9 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 11B2, mitochondrial
(Homo sapiens (Human)) | BDBM50238114

(CHEMBL4093575)Show SMILES CC(C)C(O)(C1CCN(CC1)S(C)(=O)=O)c1cncnc1Nc1ccc(F)cc1 Show InChI InChI=1S/C20H27FN4O3S/c1-14(2)20(26,15-8-10-25(11-9-15)29(3,27)28)18-12-22-13-23-19(18)24-17-6-4-16(21)5-7-17/h4-7,12-15,26H,8-11H2,1-3H3,(H,22,23,24) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi-Sankyo Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human CYP11B2 expressed in HEK293A cells using deoxycorticosterone as substrate pretreated for 1 hr followed by substrate addition meas... |
Bioorg Med Chem Lett 27: 1902-1906 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.034
BindingDB Entry DOI: 10.7270/Q2KS6TTS |
More data for this
Ligand-Target Pair | |
Renin
(Macaca fascicularis) | BDBM50387266

(CHEMBL2048707)Show SMILES CC(C)[C@H](C[C@H](O)[C@@H](N)CN1CC(=O)N(CC1(C)C)c1cc(F)ccc1Cl)C(=O)NC1CCCCC1 |r| Show InChI InChI=1S/C27H42ClFN4O3/c1-17(2)20(26(36)31-19-8-6-5-7-9-19)13-24(34)22(30)14-32-15-25(35)33(16-27(32,3)4)23-12-18(29)10-11-21(23)28/h10-12,17,19-20,22,24,34H,5-9,13-16,30H2,1-4H3,(H,31,36)/t20-,22-,24-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of renin in cynomolgus monkey plasma |
Bioorg Med Chem Lett 22: 4561-6 (2012)
Article DOI: 10.1016/j.bmcl.2012.05.092
BindingDB Entry DOI: 10.7270/Q2BK1DD9 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 11B2, mitochondrial
(Homo sapiens (Human)) | BDBM50238106
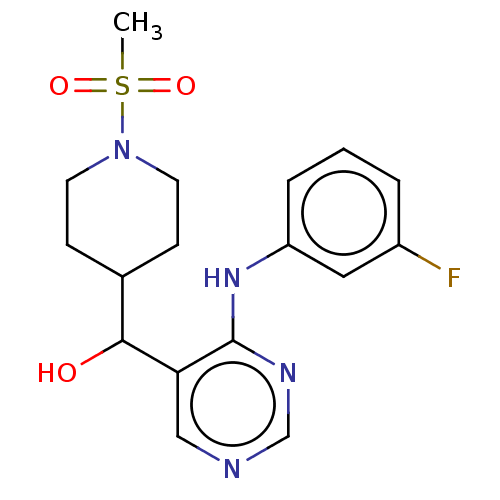
(CHEMBL4072943)Show SMILES CS(=O)(=O)N1CCC(CC1)C(O)c1cncnc1Nc1cccc(F)c1 Show InChI InChI=1S/C17H21FN4O3S/c1-26(24,25)22-7-5-12(6-8-22)16(23)15-10-19-11-20-17(15)21-14-4-2-3-13(18)9-14/h2-4,9-12,16,23H,5-8H2,1H3,(H,19,20,21) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi-Sankyo Co., Ltd.
Curated by ChEMBL
| Assay Description
Concentration required to inhibit the activity of Angiotensin I converting enzyme by 50% |
Bioorg Med Chem Lett 27: 1902-1906 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.034
BindingDB Entry DOI: 10.7270/Q2KS6TTS |
More data for this
Ligand-Target Pair | |
Renin
(Macaca fascicularis) | BDBM50387274

(CHEMBL2048564)Show SMILES COCCCOc1ccccc1N1CC(C)(C)N(C[C@H](N)[C@@H](O)C[C@@H](C(C)C)C(=O)NCC(C)(C)C(N)=O)CC1=O |r| Show InChI InChI=1S/C30H51N5O6/c1-20(2)21(27(38)33-18-29(3,4)28(32)39)15-24(36)22(31)16-34-17-26(37)35(19-30(34,5)6)23-11-8-9-12-25(23)41-14-10-13-40-7/h8-9,11-12,20-22,24,36H,10,13-19,31H2,1-7H3,(H2,32,39)(H,33,38)/t21-,22-,24-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of renin in cynomolgus monkey plasma |
Bioorg Med Chem Lett 22: 4561-6 (2012)
Article DOI: 10.1016/j.bmcl.2012.05.092
BindingDB Entry DOI: 10.7270/Q2BK1DD9 |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50387258
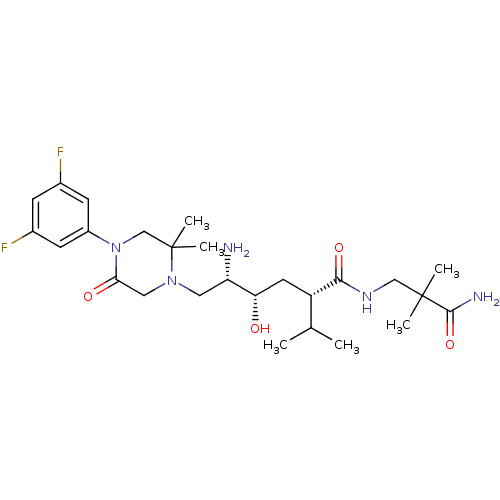
(CHEMBL2048578)Show SMILES CC(C)[C@H](C[C@H](O)[C@@H](N)CN1CC(=O)N(CC1(C)C)c1cc(F)cc(F)c1)C(=O)NCC(C)(C)C(N)=O |r| Show InChI InChI=1S/C26H41F2N5O4/c1-15(2)19(23(36)31-13-25(3,4)24(30)37)10-21(34)20(29)11-32-12-22(35)33(14-26(32,5)6)18-8-16(27)7-17(28)9-18/h7-9,15,19-21,34H,10-14,29H2,1-6H3,(H2,30,37)(H,31,36)/t19-,20-,21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human purified renin |
Bioorg Med Chem Lett 22: 4561-6 (2012)
Article DOI: 10.1016/j.bmcl.2012.05.092
BindingDB Entry DOI: 10.7270/Q2BK1DD9 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 11B1, mitochondrial
(Homo sapiens (Human)) | BDBM50238103

(CHEMBL4070323)Show SMILES CC(O)(C1CCN(CC1)S(C)(=O)=O)c1cncnc1Nc1ccc(F)cc1 Show InChI InChI=1S/C18H23FN4O3S/c1-18(24,13-7-9-23(10-8-13)27(2,25)26)16-11-20-12-21-17(16)22-15-5-3-14(19)4-6-15/h3-6,11-13,24H,7-10H2,1-2H3,(H,20,21,22) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi-Sankyo Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human CYP11B1 expressed in HEK293A cells |
Bioorg Med Chem Lett 27: 1902-1906 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.034
BindingDB Entry DOI: 10.7270/Q2KS6TTS |
More data for this
Ligand-Target Pair | |
Renin
(Macaca fascicularis) | BDBM50387280

(CHEMBL2046465)Show SMILES COCOc1ccccc1N1CC(C)(C)N(C[C@H](N)[C@@H](O)C[C@@H](C(C)C)C(=O)NCC(C)(C)C(N)=O)CC1=O |r| Show InChI InChI=1S/C28H47N5O6/c1-18(2)19(25(36)31-15-27(3,4)26(30)37)12-22(34)20(29)13-32-14-24(35)33(16-28(32,5)6)21-10-8-9-11-23(21)39-17-38-7/h8-11,18-20,22,34H,12-17,29H2,1-7H3,(H2,30,37)(H,31,36)/t19-,20-,22-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of renin in cynomolgus monkey plasma |
Bioorg Med Chem Lett 22: 4561-6 (2012)
Article DOI: 10.1016/j.bmcl.2012.05.092
BindingDB Entry DOI: 10.7270/Q2BK1DD9 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 11B2, mitochondrial
(Homo sapiens (Human)) | BDBM50238120
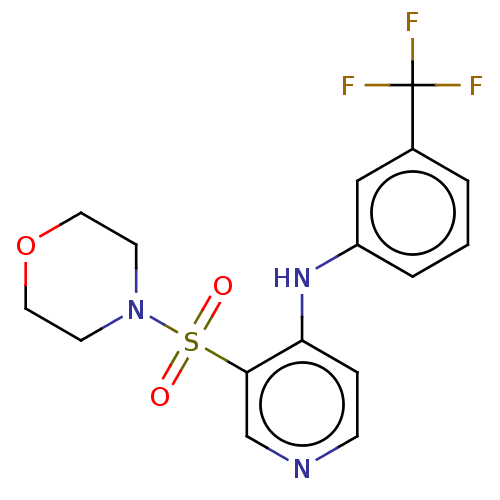
(CHEMBL4097020)Show SMILES FC(F)(F)c1cccc(Nc2ccncc2S(=O)(=O)N2CCOCC2)c1 Show InChI InChI=1S/C16H16F3N3O3S/c17-16(18,19)12-2-1-3-13(10-12)21-14-4-5-20-11-15(14)26(23,24)22-6-8-25-9-7-22/h1-5,10-11H,6-9H2,(H,20,21) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi-Sankyo Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human CYP11B2 expressed in HEK293A cells using deoxycorticosterone as substrate pretreated for 1 hr followed by substrate addition meas... |
Bioorg Med Chem Lett 27: 1902-1906 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.034
BindingDB Entry DOI: 10.7270/Q2KS6TTS |
More data for this
Ligand-Target Pair | |
Renin
(Macaca fascicularis) | BDBM50387268

(CHEMBL2048571)Show SMILES CC(C)[C@H](C[C@H](O)[C@@H](N)CN1CC(=O)N(CC1(C)C)c1ccccc1Cl)C(=O)NCC(C)(C)C(N)=O |r| Show InChI InChI=1S/C26H42ClN5O4/c1-16(2)17(23(35)30-14-25(3,4)24(29)36)11-21(33)19(28)12-31-13-22(34)32(15-26(31,5)6)20-10-8-7-9-18(20)27/h7-10,16-17,19,21,33H,11-15,28H2,1-6H3,(H2,29,36)(H,30,35)/t17-,19-,21-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of renin in cynomolgus monkey plasma |
Bioorg Med Chem Lett 22: 4561-6 (2012)
Article DOI: 10.1016/j.bmcl.2012.05.092
BindingDB Entry DOI: 10.7270/Q2BK1DD9 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Renin
(Macaca fascicularis) | BDBM50387259

(CHEMBL2048579)Show SMILES CC(C)[C@H](C[C@H](O)[C@@H](N)CN1CC(=O)N(CC1(C)C)c1cc(F)ccc1Cl)C(=O)NCC(C)(C)C(N)=O |r| Show InChI InChI=1S/C26H41ClFN5O4/c1-15(2)17(23(36)31-13-25(3,4)24(30)37)10-21(34)19(29)11-32-12-22(35)33(14-26(32,5)6)20-9-16(28)7-8-18(20)27/h7-9,15,17,19,21,34H,10-14,29H2,1-6H3,(H2,30,37)(H,31,36)/t17-,19-,21-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of renin in cynomolgus monkey plasma |
Bioorg Med Chem Lett 22: 4561-6 (2012)
Article DOI: 10.1016/j.bmcl.2012.05.092
BindingDB Entry DOI: 10.7270/Q2BK1DD9 |
More data for this
Ligand-Target Pair | |
Renin
(Macaca fascicularis) | BDBM50387257

(CHEMBL2048706)Show SMILES CC[C@H](C)CNC(=O)[C@@H](C[C@H](O)[C@@H](N)CN1CC(=O)N(CC1(C)C)c1cc(F)ccc1Cl)C(C)C |r| Show InChI InChI=1S/C26H42ClFN4O3/c1-7-17(4)12-30-25(35)19(16(2)3)11-23(33)21(29)13-31-14-24(34)32(15-26(31,5)6)22-10-18(28)8-9-20(22)27/h8-10,16-17,19,21,23,33H,7,11-15,29H2,1-6H3,(H,30,35)/t17-,19-,21-,23-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of renin in cynomolgus monkey plasma |
Bioorg Med Chem Lett 22: 4561-6 (2012)
Article DOI: 10.1016/j.bmcl.2012.05.092
BindingDB Entry DOI: 10.7270/Q2BK1DD9 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 11B2, mitochondrial
(Homo sapiens (Human)) | BDBM50238117
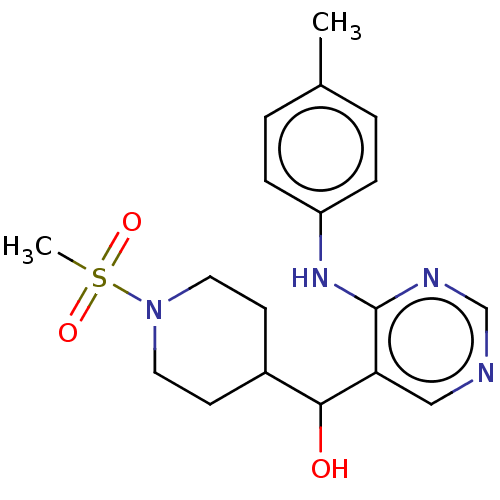
(CHEMBL4092081)Show SMILES Cc1ccc(Nc2ncncc2C(O)C2CCN(CC2)S(C)(=O)=O)cc1 Show InChI InChI=1S/C18H24N4O3S/c1-13-3-5-15(6-4-13)21-18-16(11-19-12-20-18)17(23)14-7-9-22(10-8-14)26(2,24)25/h3-6,11-12,14,17,23H,7-10H2,1-2H3,(H,19,20,21) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi-Sankyo Co., Ltd.
Curated by ChEMBL
| Assay Description
Concentration required to inhibit the activity of Angiotensin I converting enzyme by 50% |
Bioorg Med Chem Lett 27: 1902-1906 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.034
BindingDB Entry DOI: 10.7270/Q2KS6TTS |
More data for this
Ligand-Target Pair | |
Cytochrome P450 11B2, mitochondrial
(Homo sapiens (Human)) | BDBM50238123

(CHEMBL4061565)Show SMILES CS(=O)(=O)N1CCC(CC1)C(O)c1cncnc1Nc1cccc2ccccc12 Show InChI InChI=1S/C21H24N4O3S/c1-29(27,28)25-11-9-16(10-12-25)20(26)18-13-22-14-23-21(18)24-19-8-4-6-15-5-2-3-7-17(15)19/h2-8,13-14,16,20,26H,9-12H2,1H3,(H,22,23,24) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi-Sankyo Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human CYP11B2 expressed in HEK293A cells using deoxycorticosterone as substrate pretreated for 1 hr followed by substrate addition meas... |
Bioorg Med Chem Lett 27: 1902-1906 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.034
BindingDB Entry DOI: 10.7270/Q2KS6TTS |
More data for this
Ligand-Target Pair | |
Renin
(Macaca fascicularis) | BDBM50387262

(CHEMBL2048702)Show SMILES CCCCNC(=O)[C@@H](C[C@H](O)[C@@H](N)CN1CC(=O)N(CC1(C)C)c1ccccc1Cl)C(C)C |r| Show InChI InChI=1S/C25H41ClN4O3/c1-6-7-12-28-24(33)18(17(2)3)13-22(31)20(27)14-29-15-23(32)30(16-25(29,4)5)21-11-9-8-10-19(21)26/h8-11,17-18,20,22,31H,6-7,12-16,27H2,1-5H3,(H,28,33)/t18-,20-,22-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of renin in cynomolgus monkey plasma |
Bioorg Med Chem Lett 22: 4561-6 (2012)
Article DOI: 10.1016/j.bmcl.2012.05.092
BindingDB Entry DOI: 10.7270/Q2BK1DD9 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data