 Found 210 hits with Last Name = 'sacco' and Initial = 'm'
Found 210 hits with Last Name = 'sacco' and Initial = 'm' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Translocator protein
(Rattus norvegicus (rat)) | BDBM22032
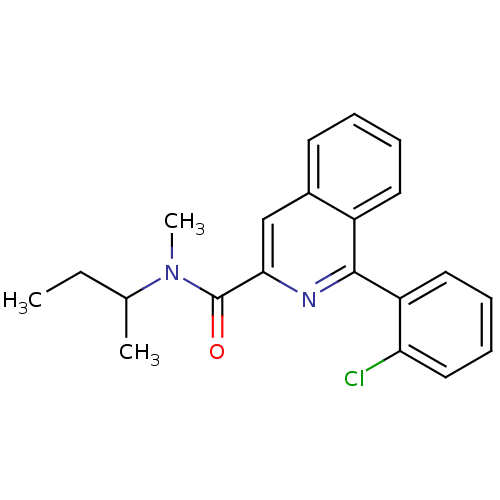
(1-(2-chlorophenyl)-N-methyl-N-(1-methylpropyl)isoq...)Show InChI InChI=1S/C21H21ClN2O/c1-4-14(2)24(3)21(25)19-13-15-9-5-6-10-16(15)20(23-19)17-11-7-8-12-18(17)22/h5-14H,4H2,1-3H3 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Bari"A. Moro"
Curated by ChEMBL
| Assay Description
Displacement of [3H]PK11195 from TSPO in rat C6 cell membrane after 90 mins by radioligand binding assay |
ACS Med Chem Lett 5: 685-9 (2014)
Article DOI: 10.1021/ml5000788
BindingDB Entry DOI: 10.7270/Q26D5VKG |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Translocator protein
(Rattus norvegicus (rat)) | BDBM50408728

(CHEMBL339816)Show SMILES CCCN(CCC)C(=O)Cc1c(nc2c(N)cccn12)-c1ccc(Cl)cc1 Show InChI InChI=1S/C21H25ClN4O/c1-3-11-25(12-4-2)19(27)14-18-20(15-7-9-16(22)10-8-15)24-21-17(23)6-5-13-26(18)21/h5-10,13H,3-4,11-12,14,23H2,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Bari"A. Moro"
Curated by ChEMBL
| Assay Description
Displacement of [3H]PK11195 from TSPO in rat C6 cell membrane after 90 mins by radioligand binding assay |
ACS Med Chem Lett 5: 685-9 (2014)
Article DOI: 10.1021/ml5000788
BindingDB Entry DOI: 10.7270/Q26D5VKG |
More data for this
Ligand-Target Pair | |
Replicase polyprotein 1ab
(2019-nCoV) | BDBM429328
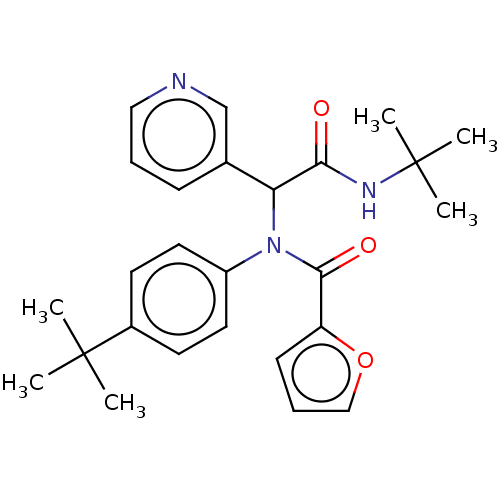
(ALP-POS-c59291d4-6 | CVD-0006355 | jm5b01461, Comp...)Show SMILES CC(C)(C)NC(=O)C(N(C(=O)c1ccco1)c1ccc(cc1)C(C)(C)C)c1cccnc1 Show InChI InChI=1S/C26H31N3O3/c1-25(2,3)19-11-13-20(14-12-19)29(24(31)21-10-8-16-32-21)22(18-9-7-15-27-17-18)23(30)28-26(4,5)6/h7-17,22H,1-6H3,(H,28,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00509
BindingDB Entry DOI: 10.7270/Q2765KD5 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Translocator protein
(Rattus norvegicus (rat)) | BDBM50021054

(CHEMBL3287610)Show SMILES CCCN(CCC)C(=O)Cc1c(nc2c(NC(=O)CN(Cc3ccccn3)Cc3ccccn3)cccn12)-c1ccc(Cl)cc1 Show InChI InChI=1S/C35H38ClN7O2/c1-3-19-42(20-4-2)33(45)22-31-34(26-13-15-27(36)16-14-26)40-35-30(12-9-21-43(31)35)39-32(44)25-41(23-28-10-5-7-17-37-28)24-29-11-6-8-18-38-29/h5-18,21H,3-4,19-20,22-25H2,1-2H3,(H,39,44) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 239 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Bari"A. Moro"
Curated by ChEMBL
| Assay Description
Displacement of [3H]PK11195 from TSPO in rat C6 cell membrane after 90 mins by radioligand binding assay |
ACS Med Chem Lett 5: 685-9 (2014)
Article DOI: 10.1021/ml5000788
BindingDB Entry DOI: 10.7270/Q26D5VKG |
More data for this
Ligand-Target Pair | |
Replicase polyprotein 1ab
(2019-nCoV) | BDBM50602444
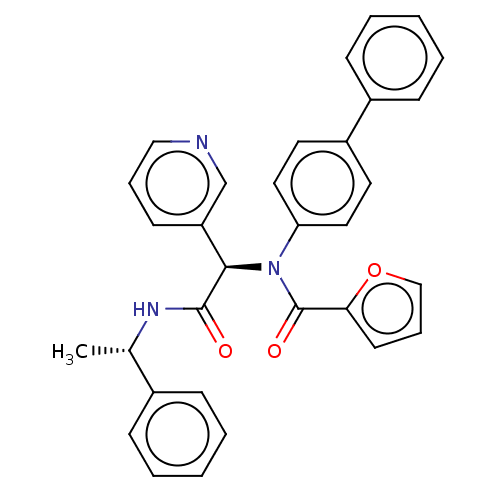
(CHEMBL5206501)Show SMILES C[C@H](NC(=O)[C@H](N(C(=O)c1ccco1)c1ccc(cc1)-c1ccccc1)c1cccnc1)c1ccccc1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 2.29E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00509
BindingDB Entry DOI: 10.7270/Q2765KD5 |
More data for this
Ligand-Target Pair | |
Cathepsin B
(Homo sapiens (Human)) | BDBM420296
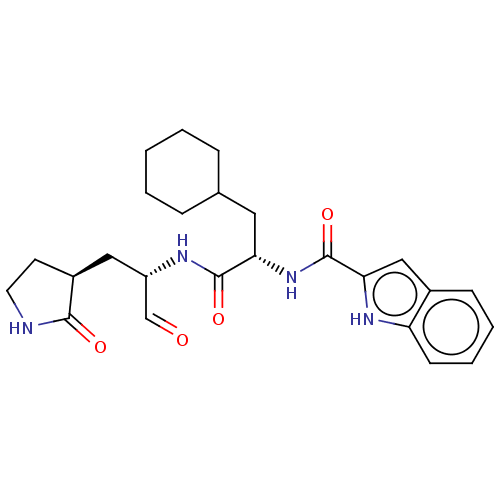
(Advanced SARS-CoV-2 Inhibitor 11a | MPI10 | acs.jm...)Show SMILES O=C[C@H](C[C@@H]1CCNC1=O)NC(=O)[C@H](CC1CCCCC1)NC(=O)c1cc2ccccc2[nH]1 Show InChI InChI=1S/C25H32N4O4/c30-15-19(13-18-10-11-26-23(18)31)27-24(32)21(12-16-6-2-1-3-7-16)29-25(33)22-14-17-8-4-5-9-20(17)28-22/h4-5,8-9,14-16,18-19,21,28H,1-3,6-7,10-13H2,(H,26,31)(H,27,32)(H,29,33)/t18-,19-,21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PDB
UniChem
| Article
PubMed
| n/a | n/a | 0.210 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
FRET-based enzymatic assay. |
Citation and Details
Article DOI: 10.1021/jacs.1c08060
BindingDB Entry DOI: 10.7270/Q2BK1GHG |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM448319
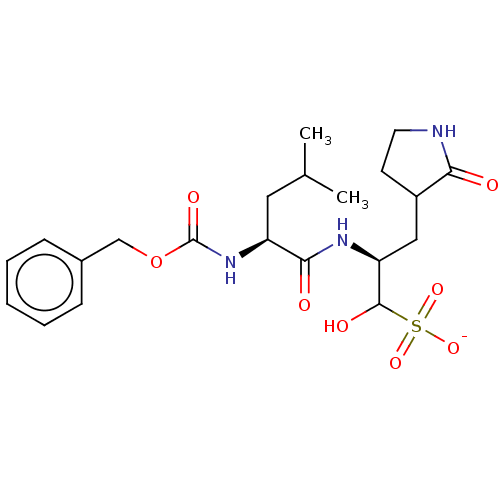
(GC-376 | GC376)Show SMILES CC(C)C[C@H](NC(=O)OCc1ccccc1)C(=O)N[C@@H](CC1CCNC1=O)C(O)S([O-])(=O)=O Show InChI InChI=1S/C21H31N3O8S/c1-13(2)10-16(24-21(28)32-12-14-6-4-3-5-7-14)19(26)23-17(20(27)33(29,30)31)11-15-8-9-22-18(15)25/h3-7,13,15-17,20,27H,8-12H2,1-2H3,(H,22,25)(H,23,26)(H,24,28)(H,29,30,31)/p-1/t15?,16-,17-,20?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.260 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Arizona
| Assay Description
Enzymatic inhibition of GC-376, UAWJ9-36-1, and UAWJ9-36-3 against Mpro's from all seven human coronaviruses. Data fittings of the proteolytic progre... |
ACS Pharmacol Transl Sci 4: 1408-1421 (2021)
Article DOI: 10.1021/acsptsci.1c00099
BindingDB Entry DOI: 10.7270/Q20V8GWQ |
More data for this
Ligand-Target Pair | |
Cathepsin B
(Homo sapiens (Human)) | BDBM509973
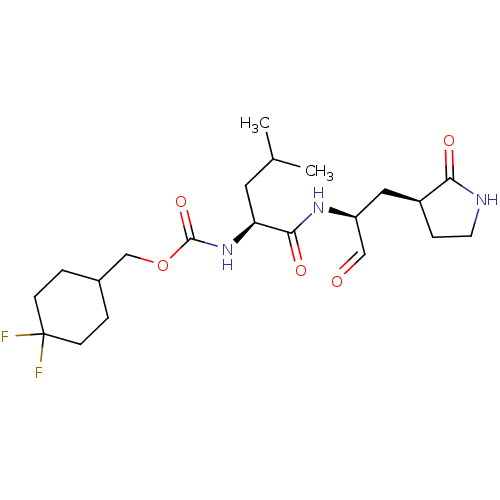
(Advanced SARS-CoV-2 Inhibitor 6j | acs.jmedchem.1c...)Show SMILES CC(C)C[C@H](NC(=O)OCC1CCC(F)(F)CC1)C(=O)N[C@@H](C[C@@H]1CCNC1=O)C=O | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | <0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
FRET-based enzymatic assay. |
Citation and Details
Article DOI: 10.1021/jacs.1c08060
BindingDB Entry DOI: 10.7270/Q2BK1GHG |
More data for this
Ligand-Target Pair | |
Cathepsin B
(Homo sapiens (Human)) | BDBM509972

(Advanced SARS-CoV-2 Inhibitor 6e | acs.jmedchem.1c...)Show SMILES CCCCC1CCC(CC1)OC(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](C[C@@H]1CCNC1=O)C=O |wU:22.22,24.24,wD:14.14,(-2.6,-5.27,;-1.26,-6.04,;.07,-5.27,;1.41,-6.04,;2.74,-5.27,;2.74,-3.73,;4.07,-2.96,;5.41,-3.73,;5.41,-5.27,;4.07,-6.04,;6.73,-2.93,;8.07,-3.67,;8.1,-5.21,;9.39,-2.88,;10.74,-3.62,;10.77,-5.16,;12.12,-5.91,;12.15,-7.45,;13.44,-5.11,;12.06,-2.83,;12.03,-1.29,;13.41,-3.57,;14.73,-2.78,;14.7,-1.24,;16.01,-.44,;17.41,-1.09,;18.46,.03,;17.72,1.38,;16.2,1.09,;15.08,2.14,;16.07,-3.52,;16.1,-5.06,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | <0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
FRET-based enzymatic assay. |
Citation and Details
Article DOI: 10.1021/jacs.1c08060
BindingDB Entry DOI: 10.7270/Q2BK1GHG |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM448319

(GC-376 | GC376)Show SMILES CC(C)C[C@H](NC(=O)OCc1ccccc1)C(=O)N[C@@H](CC1CCNC1=O)C(O)S([O-])(=O)=O Show InChI InChI=1S/C21H31N3O8S/c1-13(2)10-16(24-21(28)32-12-14-6-4-3-5-7-14)19(26)23-17(20(27)33(29,30)31)11-15-8-9-22-18(15)25/h3-7,13,15-17,20,27H,8-12H2,1-2H3,(H,22,25)(H,23,26)(H,24,28)(H,29,30,31)/p-1/t15?,16-,17-,20?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.560 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
FRET-based enzymatic assay. |
Citation and Details
Article DOI: 10.1021/jacs.1c08060
BindingDB Entry DOI: 10.7270/Q2BK1GHG |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM448319

(GC-376 | GC376)Show SMILES CC(C)C[C@H](NC(=O)OCc1ccccc1)C(=O)N[C@@H](CC1CCNC1=O)C(O)S([O-])(=O)=O Show InChI InChI=1S/C21H31N3O8S/c1-13(2)10-16(24-21(28)32-12-14-6-4-3-5-7-14)19(26)23-17(20(27)33(29,30)31)11-15-8-9-22-18(15)25/h3-7,13,15-17,20,27H,8-12H2,1-2H3,(H,22,25)(H,23,26)(H,24,28)(H,29,30,31)/p-1/t15?,16-,17-,20?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.990 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
FRET-based enzymatic assay. |
Citation and Details
Article DOI: 10.1021/jacs.1c08060
BindingDB Entry DOI: 10.7270/Q2BK1GHG |
More data for this
Ligand-Target Pair | |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Pseudomonas aeruginosa) | BDBM50200120

(CHEMBL260091 | CHIR-090 | US10875832, Compound ChI...)Show SMILES C[C@@H](O)[C@H](NC(=O)c1ccc(cc1)C#Cc1ccc(CN2CCOCC2)cc1)C(=O)NO |r| Show InChI InChI=1S/C24H27N3O5/c1-17(28)22(24(30)26-31)25-23(29)21-10-8-19(9-11-21)3-2-18-4-6-20(7-5-18)16-27-12-14-32-15-13-27/h4-11,17,22,28,31H,12-16H2,1H3,(H,25,29)(H,26,30)/t17-,22+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of fluorescent ligand from Pseudomonas aeruginosa LpxC measured after 30 mins by fluorescence anisotrophy assay |
Citation and Details
Article DOI: 10.1016/j.bmc.2020.115826
BindingDB Entry DOI: 10.7270/Q2C2513C |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Procathepsin L
(Homo sapiens (Human)) | BDBM429100
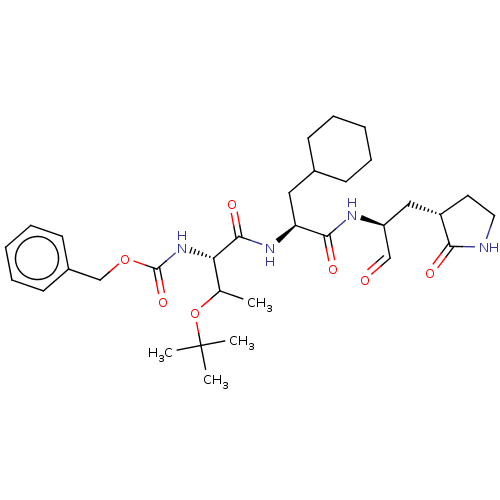
(MPI8 | jm5b01461, Compound 45)Show SMILES CC(OC(C)(C)C)[C@H](NC(=O)OCc1ccccc1)C(=O)N[C@@H](CC1CCCCC1)C(=O)N[C@@H](C[C@@H]1CCNC1=O)C=O Show InChI InChI=1S/C32H48N4O7/c1-21(43-32(2,3)4)27(36-31(41)42-20-23-13-9-6-10-14-23)30(40)35-26(17-22-11-7-5-8-12-22)29(39)34-25(19-37)18-24-15-16-33-28(24)38/h6,9-10,13-14,19,21-22,24-27H,5,7-8,11-12,15-18,20H2,1-4H3,(H,33,38)(H,34,39)(H,35,40)(H,36,41)/t21?,24-,25-,26-,27-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
FRET-based enzymatic assay. |
Citation and Details
Article DOI: 10.1021/jacs.1c08060
BindingDB Entry DOI: 10.7270/Q2BK1GHG |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM448319

(GC-376 | GC376)Show SMILES CC(C)C[C@H](NC(=O)OCc1ccccc1)C(=O)N[C@@H](CC1CCNC1=O)C(O)S([O-])(=O)=O Show InChI InChI=1S/C21H31N3O8S/c1-13(2)10-16(24-21(28)32-12-14-6-4-3-5-7-14)19(26)23-17(20(27)33(29,30)31)11-15-8-9-22-18(15)25/h3-7,13,15-17,20,27H,8-12H2,1-2H3,(H,22,25)(H,23,26)(H,24,28)(H,29,30,31)/p-1/t15?,16-,17-,20?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.40 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Arizona
| Assay Description
Enzymatic inhibition of GC-376, UAWJ9-36-1, and UAWJ9-36-3 against Mpro's from all seven human coronaviruses. Data fittings of the proteolytic progre... |
ACS Pharmacol Transl Sci 4: 1408-1421 (2021)
Article DOI: 10.1021/acsptsci.1c00099
BindingDB Entry DOI: 10.7270/Q20V8GWQ |
More data for this
Ligand-Target Pair | |
Replicase polyprotein 1ab
(2019-nCoV) | BDBM420298

(CVD-0006356 | PF-00835231 | PF-0835231 | US1152494...)Show SMILES COc1cccc2[nH]c(cc12)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](C[C@@H]1CCNC1=O)C(=O)CO Show InChI InChI=1S/C24H32N4O6/c1-13(2)9-18(23(32)27-17(20(30)12-29)10-14-7-8-25-22(14)31)28-24(33)19-11-15-16(26-19)5-4-6-21(15)34-3/h4-6,11,13-14,17-18,26,29H,7-10,12H2,1-3H3,(H,25,31)(H,27,32)(H,28,33)/t14-,17-,18-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
MCE
PDB
UniChem
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
FRET-based enzymatic assay. |
Citation and Details
Article DOI: 10.1021/jacs.1c08060
BindingDB Entry DOI: 10.7270/Q2BK1GHG |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM484194

(UAWJ9-36-1)Show SMILES O=C[C@H](C[C@@H]1CCNC1=O)NC(=O)[C@@H]1[C@H]2CCC[C@H]2CN1C(=O)OCc1ccccc1 Show InChI InChI=1S/C23H29N3O5/c27-13-18(11-16-9-10-24-21(16)28)25-22(29)20-19-8-4-7-17(19)12-26(20)23(30)31-14-15-5-2-1-3-6-15/h1-3,5-6,13,16-20H,4,7-12,14H2,(H,24,28)(H,25,29)/t16-,17-,18-,19-,20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.5 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Arizona
| Assay Description
Enzymatic inhibition of GC-376, UAWJ9-36-1, and UAWJ9-36-3 against Mpro's from all seven human coronaviruses. Data fittings of the proteolytic progre... |
ACS Pharmacol Transl Sci 4: 1408-1421 (2021)
Article DOI: 10.1021/acsptsci.1c00099
BindingDB Entry DOI: 10.7270/Q20V8GWQ |
More data for this
Ligand-Target Pair | |
Replicase polyprotein 1ab
(2019-nCoV) | BDBM509973

(Advanced SARS-CoV-2 Inhibitor 6j | acs.jmedchem.1c...)Show SMILES CC(C)C[C@H](NC(=O)OCC1CCC(F)(F)CC1)C(=O)N[C@@H](C[C@@H]1CCNC1=O)C=O | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
FRET-based enzymatic assay. |
Citation and Details
Article DOI: 10.1021/jacs.1c08060
BindingDB Entry DOI: 10.7270/Q2BK1GHG |
More data for this
Ligand-Target Pair | |
Replicase polyprotein 1ab
(2019-nCoV) | BDBM420296

(Advanced SARS-CoV-2 Inhibitor 11a | MPI10 | acs.jm...)Show SMILES O=C[C@H](C[C@@H]1CCNC1=O)NC(=O)[C@H](CC1CCCCC1)NC(=O)c1cc2ccccc2[nH]1 Show InChI InChI=1S/C25H32N4O4/c30-15-19(13-18-10-11-26-23(18)31)27-24(32)21(12-16-6-2-1-3-7-16)29-25(33)22-14-17-8-4-5-9-20(17)28-22/h4-5,8-9,14-16,18-19,21,28H,1-3,6-7,10-13H2,(H,26,31)(H,27,32)(H,29,33)/t18-,19-,21-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
MCE
PDB
UniChem
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
FRET-based enzymatic assay. |
Citation and Details
Article DOI: 10.1021/jacs.1c08060
BindingDB Entry DOI: 10.7270/Q2BK1GHG |
More data for this
Ligand-Target Pair | |
Enoyl-[acyl-carrier-protein] reductase [NADPH] FabI
(Staphylococcus aureus) | BDBM50399411

(CHEMBL2178304 | PT405)Show InChI InChI=1S/C14H12F2O2/c1-2-9-7-12(17)14(8-11(9)16)18-13-6-4-3-5-10(13)15/h3-8,17H,2H2,1H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Mutabilis
Curated by ChEMBL
| Assay Description
Inhibition of Staphylococcus aureus recombinant FabI using trans-2-octenoyl N-acetylcysteamine thioester as substrate preincubated for 60 mins |
J Med Chem 55: 9914-28 (2012)
Article DOI: 10.1021/jm301113w
BindingDB Entry DOI: 10.7270/Q2H99699 |
More data for this
Ligand-Target Pair | |
Replicase polyprotein 1ab
(2019-nCoV) | BDBM509972

(Advanced SARS-CoV-2 Inhibitor 6e | acs.jmedchem.1c...)Show SMILES CCCCC1CCC(CC1)OC(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](C[C@@H]1CCNC1=O)C=O |wU:22.22,24.24,wD:14.14,(-2.6,-5.27,;-1.26,-6.04,;.07,-5.27,;1.41,-6.04,;2.74,-5.27,;2.74,-3.73,;4.07,-2.96,;5.41,-3.73,;5.41,-5.27,;4.07,-6.04,;6.73,-2.93,;8.07,-3.67,;8.1,-5.21,;9.39,-2.88,;10.74,-3.62,;10.77,-5.16,;12.12,-5.91,;12.15,-7.45,;13.44,-5.11,;12.06,-2.83,;12.03,-1.29,;13.41,-3.57,;14.73,-2.78,;14.7,-1.24,;16.01,-.44,;17.41,-1.09,;18.46,.03,;17.72,1.38,;16.2,1.09,;15.08,2.14,;16.07,-3.52,;16.1,-5.06,)| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
FRET-based enzymatic assay. |
Citation and Details
Article DOI: 10.1021/jacs.1c08060
BindingDB Entry DOI: 10.7270/Q2BK1GHG |
More data for this
Ligand-Target Pair | |
Replicase polyprotein 1ab
(HCoV-HKU1) | BDBM448319

(GC-376 | GC376)Show SMILES CC(C)C[C@H](NC(=O)OCc1ccccc1)C(=O)N[C@@H](CC1CCNC1=O)C(O)S([O-])(=O)=O Show InChI InChI=1S/C21H31N3O8S/c1-13(2)10-16(24-21(28)32-12-14-6-4-3-5-7-14)19(26)23-17(20(27)33(29,30)31)11-15-8-9-22-18(15)25/h3-7,13,15-17,20,27H,8-12H2,1-2H3,(H,22,25)(H,23,26)(H,24,28)(H,29,30,31)/p-1/t15?,16-,17-,20?/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Arizona
| Assay Description
Enzymatic inhibition of GC-376, UAWJ9-36-1, and UAWJ9-36-3 against Mpro's from all seven human coronaviruses. Data fittings of the proteolytic progre... |
ACS Pharmacol Transl Sci 4: 1408-1421 (2021)
Article DOI: 10.1021/acsptsci.1c00099
BindingDB Entry DOI: 10.7270/Q20V8GWQ |
More data for this
Ligand-Target Pair | |
Enoyl-[acyl-carrier-protein] reductase [NADPH] FabI
(Staphylococcus aureus) | BDBM50399407
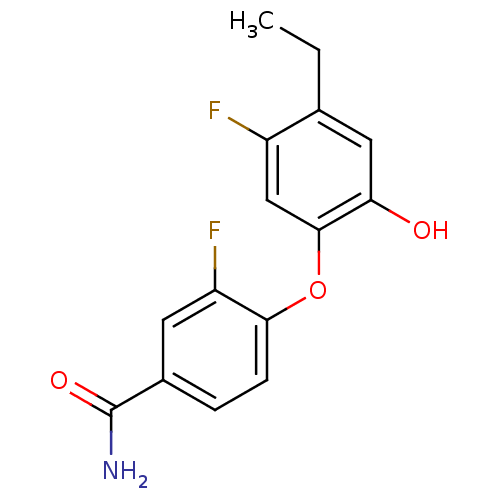
(CHEMBL2178284 | MUT056399)Show InChI InChI=1S/C15H13F2NO3/c1-2-8-6-12(19)14(7-10(8)16)21-13-4-3-9(15(18)20)5-11(13)17/h3-7,19H,2H2,1H3,(H2,18,20) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Mutabilis
Curated by ChEMBL
| Assay Description
Inhibition of Staphylococcus aureus recombinant FabI using trans-2-octenoyl N-acetylcysteamine thioester as substrate preincubated for 60 mins |
J Med Chem 55: 9914-28 (2012)
Article DOI: 10.1021/jm301113w
BindingDB Entry DOI: 10.7270/Q2H99699 |
More data for this
Ligand-Target Pair | |
Enoyl-[acyl-carrier-protein] reductase [NADPH] FabI
(Staphylococcus aureus) | BDBM50399400

(CHEMBL2178291)Show InChI InChI=1S/C13H11F2NO2/c1-2-8-6-10(17)12(7-9(8)14)18-11-4-3-5-16-13(11)15/h3-7,17H,2H2,1H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Mutabilis
Curated by ChEMBL
| Assay Description
Inhibition of Staphylococcus aureus recombinant FabI using trans-2-octenoyl N-acetylcysteamine thioester as substrate preincubated for 60 mins |
J Med Chem 55: 9914-28 (2012)
Article DOI: 10.1021/jm301113w
BindingDB Entry DOI: 10.7270/Q2H99699 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Enoyl-[acyl-carrier-protein] reductase [NADPH] FabI
(Staphylococcus aureus) | BDBM50399404
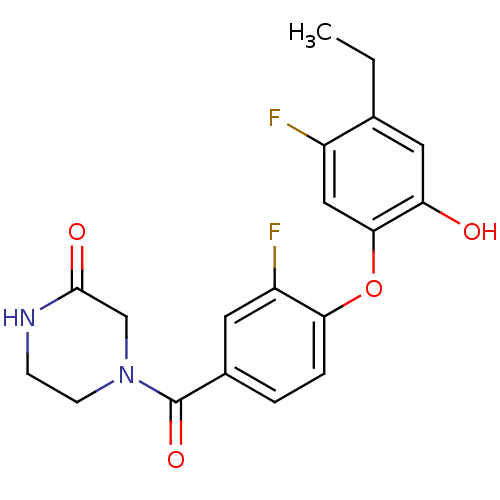
(CHEMBL2178287 | US8623865, 1)Show SMILES CCc1cc(O)c(Oc2ccc(cc2F)C(=O)N2CCNC(=O)C2)cc1F Show InChI InChI=1S/C19H18F2N2O4/c1-2-11-8-15(24)17(9-13(11)20)27-16-4-3-12(7-14(16)21)19(26)23-6-5-22-18(25)10-23/h3-4,7-9,24H,2,5-6,10H2,1H3,(H,22,25) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Mutabilis
Curated by ChEMBL
| Assay Description
Inhibition of Staphylococcus aureus recombinant FabI using trans-2-octenoyl N-acetylcysteamine thioester as substrate preincubated for 60 mins |
J Med Chem 55: 9914-28 (2012)
Article DOI: 10.1021/jm301113w
BindingDB Entry DOI: 10.7270/Q2H99699 |
More data for this
Ligand-Target Pair | |
Replicase polyprotein 1ab
(HCoV-OC43) | BDBM448319

(GC-376 | GC376)Show SMILES CC(C)C[C@H](NC(=O)OCc1ccccc1)C(=O)N[C@@H](CC1CCNC1=O)C(O)S([O-])(=O)=O Show InChI InChI=1S/C21H31N3O8S/c1-13(2)10-16(24-21(28)32-12-14-6-4-3-5-7-14)19(26)23-17(20(27)33(29,30)31)11-15-8-9-22-18(15)25/h3-7,13,15-17,20,27H,8-12H2,1-2H3,(H,22,25)(H,23,26)(H,24,28)(H,29,30,31)/p-1/t15?,16-,17-,20?/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Arizona
| Assay Description
Enzymatic inhibition of GC-376, UAWJ9-36-1, and UAWJ9-36-3 against Mpro's from all seven human coronaviruses. Data fittings of the proteolytic progre... |
ACS Pharmacol Transl Sci 4: 1408-1421 (2021)
Article DOI: 10.1021/acsptsci.1c00099
BindingDB Entry DOI: 10.7270/Q20V8GWQ |
More data for this
Ligand-Target Pair | |
Enoyl-[acyl-carrier-protein] reductase [NADPH] FabI
(Staphylococcus aureus) | BDBM50399405

(CHEMBL2178286 | US8623865, 2)Show SMILES CCc1ccc(Oc2ccc(cc2F)C(=O)N2CCNC(=O)C2)c(O)c1 Show InChI InChI=1S/C19H19FN2O4/c1-2-12-3-5-17(15(23)9-12)26-16-6-4-13(10-14(16)20)19(25)22-8-7-21-18(24)11-22/h3-6,9-10,23H,2,7-8,11H2,1H3,(H,21,24) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
Mutabilis
Curated by ChEMBL
| Assay Description
Inhibition of Staphylococcus aureus recombinant FabI using trans-2-octenoyl N-acetylcysteamine thioester as substrate preincubated for 60 mins |
J Med Chem 55: 9914-28 (2012)
Article DOI: 10.1021/jm301113w
BindingDB Entry DOI: 10.7270/Q2H99699 |
More data for this
Ligand-Target Pair | |
Orf1a protein
(MERS-CoV) | BDBM448319

(GC-376 | GC376)Show SMILES CC(C)C[C@H](NC(=O)OCc1ccccc1)C(=O)N[C@@H](CC1CCNC1=O)C(O)S([O-])(=O)=O Show InChI InChI=1S/C21H31N3O8S/c1-13(2)10-16(24-21(28)32-12-14-6-4-3-5-7-14)19(26)23-17(20(27)33(29,30)31)11-15-8-9-22-18(15)25/h3-7,13,15-17,20,27H,8-12H2,1-2H3,(H,22,25)(H,23,26)(H,24,28)(H,29,30,31)/p-1/t15?,16-,17-,20?/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Arizona
| Assay Description
Enzymatic inhibition of GC-376, UAWJ9-36-1, and UAWJ9-36-3 against Mpro's from all seven human coronaviruses. Data fittings of the proteolytic progre... |
ACS Pharmacol Transl Sci 4: 1408-1421 (2021)
Article DOI: 10.1021/acsptsci.1c00099
BindingDB Entry DOI: 10.7270/Q20V8GWQ |
More data for this
Ligand-Target Pair | |
Enoyl-[acyl-carrier-protein] reductase [NADPH] FabI
(Staphylococcus aureus) | BDBM50399410
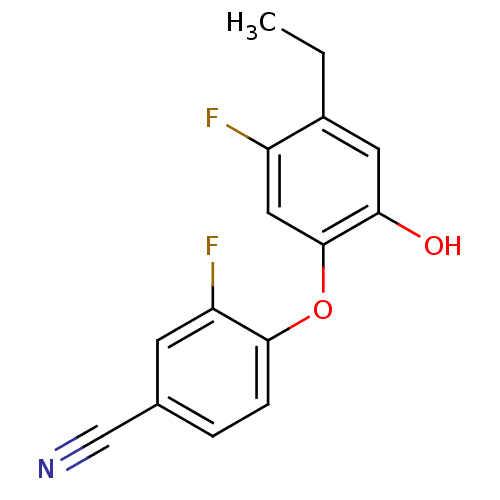
(CHEMBL2178305)Show InChI InChI=1S/C15H11F2NO2/c1-2-10-6-13(19)15(7-11(10)16)20-14-4-3-9(8-18)5-12(14)17/h3-7,19H,2H2,1H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
Mutabilis
Curated by ChEMBL
| Assay Description
Inhibition of Staphylococcus aureus recombinant FabI using trans-2-octenoyl N-acetylcysteamine thioester as substrate preincubated for 60 mins |
J Med Chem 55: 9914-28 (2012)
Article DOI: 10.1021/jm301113w
BindingDB Entry DOI: 10.7270/Q2H99699 |
More data for this
Ligand-Target Pair | |
Replicase polyprotein 1ab
(2019-nCoV) | BDBM448319

(GC-376 | GC376)Show SMILES CC(C)C[C@H](NC(=O)OCc1ccccc1)C(=O)N[C@@H](CC1CCNC1=O)C(O)S([O-])(=O)=O Show InChI InChI=1S/C21H31N3O8S/c1-13(2)10-16(24-21(28)32-12-14-6-4-3-5-7-14)19(26)23-17(20(27)33(29,30)31)11-15-8-9-22-18(15)25/h3-7,13,15-17,20,27H,8-12H2,1-2H3,(H,22,25)(H,23,26)(H,24,28)(H,29,30,31)/p-1/t15?,16-,17-,20?/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
FRET-based enzymatic assay. |
Citation and Details
Article DOI: 10.1021/jacs.1c08060
BindingDB Entry DOI: 10.7270/Q2BK1GHG |
More data for this
Ligand-Target Pair | |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Pseudomonas aeruginosa) | BDBM50554708
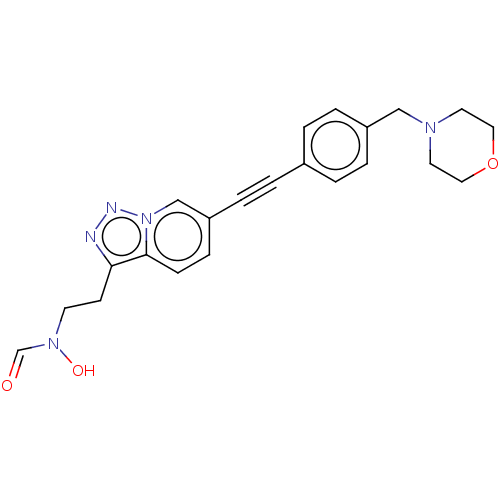
(CHEMBL4746493)Show SMILES ON(CCc1nnn2cc(ccc12)C#Cc1ccc(CN2CCOCC2)cc1)C=O | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of fluorescent ligand from Pseudomonas aeruginosa LpxC measured after 30 mins by fluorescence anisotrophy assay |
Citation and Details
Article DOI: 10.1016/j.bmc.2020.115826
BindingDB Entry DOI: 10.7270/Q2C2513C |
More data for this
Ligand-Target Pair | |
Enoyl-[acyl-carrier-protein] reductase [NADPH] FabI
(Staphylococcus aureus) | BDBM50399412
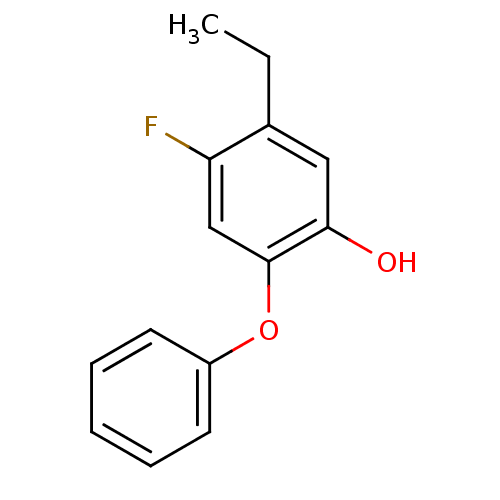
(CHEMBL2178303 | PT411)Show InChI InChI=1S/C14H13FO2/c1-2-10-8-13(16)14(9-12(10)15)17-11-6-4-3-5-7-11/h3-9,16H,2H2,1H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
Mutabilis
Curated by ChEMBL
| Assay Description
Inhibition of Staphylococcus aureus recombinant FabI using trans-2-octenoyl N-acetylcysteamine thioester as substrate preincubated for 60 mins |
J Med Chem 55: 9914-28 (2012)
Article DOI: 10.1021/jm301113w
BindingDB Entry DOI: 10.7270/Q2H99699 |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM484195

(UAWJ9-36-3)Show SMILES CC1(C)[C@H]2CN([C@@H]([C@@H]12)C(=O)N[C@@H](C[C@@H]1CCNC1=O)C=O)C(=O)OCc1ccccc1 Show InChI InChI=1S/C23H29N3O5/c1-23(2)17-11-26(22(30)31-13-14-6-4-3-5-7-14)19(18(17)23)21(29)25-16(12-27)10-15-8-9-24-20(15)28/h3-7,12,15-19H,8-11,13H2,1-2H3,(H,24,28)(H,25,29)/t15-,16-,17-,18-,19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 42 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Arizona
| Assay Description
Enzymatic inhibition of GC-376, UAWJ9-36-1, and UAWJ9-36-3 against Mpro's from all seven human coronaviruses. Data fittings of the proteolytic progre... |
ACS Pharmacol Transl Sci 4: 1408-1421 (2021)
Article DOI: 10.1021/acsptsci.1c00099
BindingDB Entry DOI: 10.7270/Q20V8GWQ |
More data for this
Ligand-Target Pair | |
Replicase polyprotein 1ab
(2019-nCoV) | BDBM448319

(GC-376 | GC376)Show SMILES CC(C)C[C@H](NC(=O)OCc1ccccc1)C(=O)N[C@@H](CC1CCNC1=O)C(O)S([O-])(=O)=O Show InChI InChI=1S/C21H31N3O8S/c1-13(2)10-16(24-21(28)32-12-14-6-4-3-5-7-14)19(26)23-17(20(27)33(29,30)31)11-15-8-9-22-18(15)25/h3-7,13,15-17,20,27H,8-12H2,1-2H3,(H,22,25)(H,23,26)(H,24,28)(H,29,30,31)/p-1/t15?,16-,17-,20?/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 43 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Arizona
| Assay Description
Enzymatic inhibition of GC-376, UAWJ9-36-1, and UAWJ9-36-3 against Mpro's from all seven human coronaviruses. Data fittings of the proteolytic progre... |
ACS Pharmacol Transl Sci 4: 1408-1421 (2021)
Article DOI: 10.1021/acsptsci.1c00099
BindingDB Entry DOI: 10.7270/Q20V8GWQ |
More data for this
Ligand-Target Pair | |
Replicase polyprotein 1ab
(Human SARS coronavirus (SARS-CoV) (Severe acute re...) | BDBM448319

(GC-376 | GC376)Show SMILES CC(C)C[C@H](NC(=O)OCc1ccccc1)C(=O)N[C@@H](CC1CCNC1=O)C(O)S([O-])(=O)=O Show InChI InChI=1S/C21H31N3O8S/c1-13(2)10-16(24-21(28)32-12-14-6-4-3-5-7-14)19(26)23-17(20(27)33(29,30)31)11-15-8-9-22-18(15)25/h3-7,13,15-17,20,27H,8-12H2,1-2H3,(H,22,25)(H,23,26)(H,24,28)(H,29,30,31)/p-1/t15?,16-,17-,20?/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 44 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Arizona
| Assay Description
Enzymatic inhibition of GC-376, UAWJ9-36-1, and UAWJ9-36-3 against Mpro's from all seven human coronaviruses. Data fittings of the proteolytic progre... |
ACS Pharmacol Transl Sci 4: 1408-1421 (2021)
Article DOI: 10.1021/acsptsci.1c00099
BindingDB Entry DOI: 10.7270/Q20V8GWQ |
More data for this
Ligand-Target Pair | |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Pseudomonas aeruginosa) | BDBM50554707

(CHEMBL4751248)Show SMILES ON(CCn1nnc2cc(ccc12)C#Cc1ccc(CN2CCOCC2)cc1)C=O | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 44 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of fluorescent ligand from Pseudomonas aeruginosa LpxC measured after 30 mins by fluorescence anisotrophy assay |
Citation and Details
Article DOI: 10.1016/j.bmc.2020.115826
BindingDB Entry DOI: 10.7270/Q2C2513C |
More data for this
Ligand-Target Pair | |
Replicase polyprotein 1ab
(2019-nCoV) | BDBM448319

(GC-376 | GC376)Show SMILES CC(C)C[C@H](NC(=O)OCc1ccccc1)C(=O)N[C@@H](CC1CCNC1=O)C(O)S([O-])(=O)=O Show InChI InChI=1S/C21H31N3O8S/c1-13(2)10-16(24-21(28)32-12-14-6-4-3-5-7-14)19(26)23-17(20(27)33(29,30)31)11-15-8-9-22-18(15)25/h3-7,13,15-17,20,27H,8-12H2,1-2H3,(H,22,25)(H,23,26)(H,24,28)(H,29,30,31)/p-1/t15?,16-,17-,20?/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 44 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Arizona
| Assay Description
Enzymatic inhibition of GC-376, UAWJ9-36-1, and UAWJ9-36-3 against Mpro's from all seven human coronaviruses. Data fittings of the proteolytic progre... |
ACS Pharmacol Transl Sci 4: 1408-1421 (2021)
Article DOI: 10.1021/acsptsci.1c00099
BindingDB Entry DOI: 10.7270/Q20V8GWQ |
More data for this
Ligand-Target Pair | |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Pseudomonas aeruginosa) | BDBM50554702
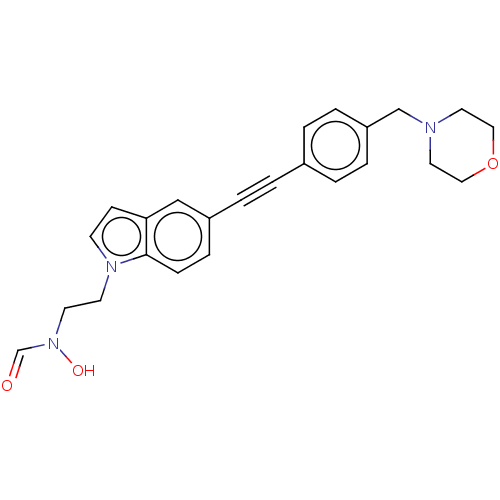
(CHEMBL4776055)Show SMILES ON(CCn1ccc2cc(ccc12)C#Cc1ccc(CN2CCOCC2)cc1)C=O | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 45 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of fluorescent ligand from Pseudomonas aeruginosa LpxC measured after 30 mins by fluorescence anisotrophy assay |
Citation and Details
Article DOI: 10.1016/j.bmc.2020.115826
BindingDB Entry DOI: 10.7270/Q2C2513C |
More data for this
Ligand-Target Pair | |
Enoyl-[acyl-carrier-protein] reductase [NADPH] FabI
(Staphylococcus aureus) | BDBM50399409
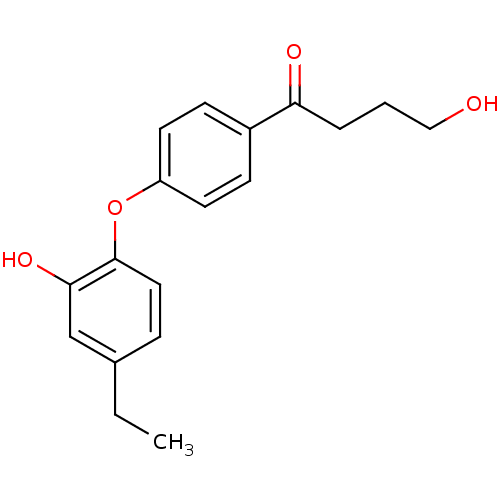
(CHEMBL2178282)Show InChI InChI=1S/C18H20O4/c1-2-13-5-10-18(17(21)12-13)22-15-8-6-14(7-9-15)16(20)4-3-11-19/h5-10,12,19,21H,2-4,11H2,1H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 48 | n/a | n/a | n/a | n/a | n/a | n/a |
Mutabilis
Curated by ChEMBL
| Assay Description
Inhibition of Staphylococcus aureus recombinant FabI using trans-2-octenoyl N-acetylcysteamine thioester as substrate preincubated for 60 mins |
J Med Chem 55: 9914-28 (2012)
Article DOI: 10.1021/jm301113w
BindingDB Entry DOI: 10.7270/Q2H99699 |
More data for this
Ligand-Target Pair | |
Replicase polyprotein 1ab
(2019-nCoV) | BDBM509983
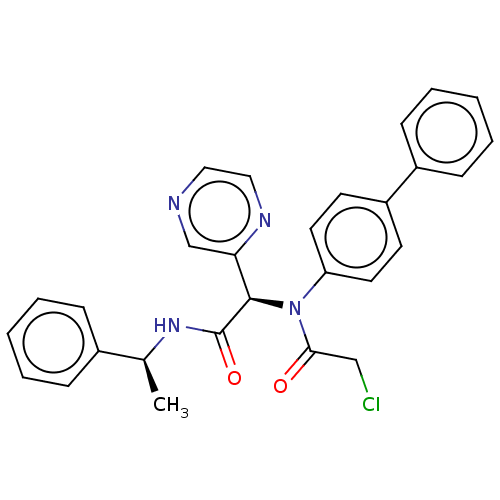
(Jun9-57-3R)Show SMILES C[C@H](NC(=O)[C@H](N(C(=O)CCl)c1ccc(cc1)-c1ccccc1)c1cnccn1)c1ccccc1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
FRET-based enzymatic assay. |
Citation and Details
Article DOI: 10.1021/jacs.1c08060
BindingDB Entry DOI: 10.7270/Q2BK1GHG |
More data for this
Ligand-Target Pair | |
Replicase polyprotein 1ab
(2019-nCoV) | BDBM510003
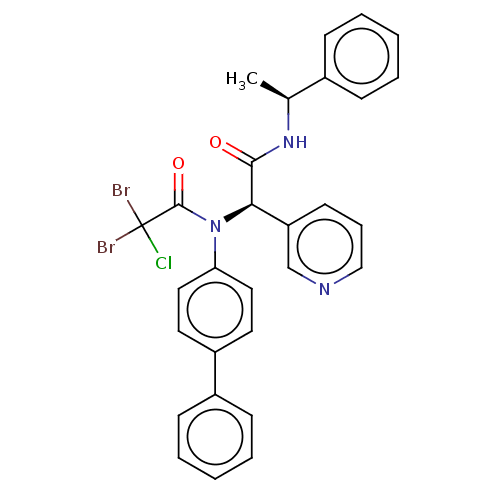
(Jun9-89-4R)Show SMILES C[C@H](NC(=O)[C@H](N(C(=O)C(Cl)(Br)Br)c1ccc(cc1)-c1ccccc1)c1cccnc1)c1ccccc1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
FRET-based enzymatic assay. |
Citation and Details
Article DOI: 10.1021/jacs.1c08060
BindingDB Entry DOI: 10.7270/Q2BK1GHG |
More data for this
Ligand-Target Pair | |
Replicase polyprotein 1ab
(2019-nCoV) | BDBM484194

(UAWJ9-36-1)Show SMILES O=C[C@H](C[C@@H]1CCNC1=O)NC(=O)[C@@H]1[C@H]2CCC[C@H]2CN1C(=O)OCc1ccccc1 Show InChI InChI=1S/C23H29N3O5/c27-13-18(11-16-9-10-24-21(16)28)25-22(29)20-19-8-4-7-17(19)12-26(20)23(30)31-14-15-5-2-1-3-6-15/h1-3,5-6,13,16-20H,4,7-12,14H2,(H,24,28)(H,25,29)/t16-,17-,18-,19-,20-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 51 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Arizona
| Assay Description
Enzymatic inhibition of GC-376, UAWJ9-36-1, and UAWJ9-36-3 against Mpro's from all seven human coronaviruses. Data fittings of the proteolytic progre... |
ACS Pharmacol Transl Sci 4: 1408-1421 (2021)
Article DOI: 10.1021/acsptsci.1c00099
BindingDB Entry DOI: 10.7270/Q20V8GWQ |
More data for this
Ligand-Target Pair | |
Replicase polyprotein 1ab
(2019-nCoV) | BDBM484194

(UAWJ9-36-1)Show SMILES O=C[C@H](C[C@@H]1CCNC1=O)NC(=O)[C@@H]1[C@H]2CCC[C@H]2CN1C(=O)OCc1ccccc1 Show InChI InChI=1S/C23H29N3O5/c27-13-18(11-16-9-10-24-21(16)28)25-22(29)20-19-8-4-7-17(19)12-26(20)23(30)31-14-15-5-2-1-3-6-15/h1-3,5-6,13,16-20H,4,7-12,14H2,(H,24,28)(H,25,29)/t16-,17-,18-,19-,20-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 51 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Arizona
| Assay Description
Enzymatic inhibition of GC-376, UAWJ9-36-1, and UAWJ9-36-3 against Mpro's from all seven human coronaviruses. Data fittings of the proteolytic progre... |
ACS Pharmacol Transl Sci 4: 1408-1421 (2021)
Article DOI: 10.1021/acsptsci.1c00099
BindingDB Entry DOI: 10.7270/Q20V8GWQ |
More data for this
Ligand-Target Pair | |
Replicase polyprotein 1ab
(2019-nCoV) | BDBM484195

(UAWJ9-36-3)Show SMILES CC1(C)[C@H]2CN([C@@H]([C@@H]12)C(=O)N[C@@H](C[C@@H]1CCNC1=O)C=O)C(=O)OCc1ccccc1 Show InChI InChI=1S/C23H29N3O5/c1-23(2)17-11-26(22(30)31-13-14-6-4-3-5-7-14)19(18(17)23)21(29)25-16(12-27)10-15-8-9-24-20(15)28/h3-7,12,15-19H,8-11,13H2,1-2H3,(H,24,28)(H,25,29)/t15-,16-,17-,18-,19-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 54 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Arizona
| Assay Description
Enzymatic inhibition of GC-376, UAWJ9-36-1, and UAWJ9-36-3 against Mpro's from all seven human coronaviruses. Data fittings of the proteolytic progre... |
ACS Pharmacol Transl Sci 4: 1408-1421 (2021)
Article DOI: 10.1021/acsptsci.1c00099
BindingDB Entry DOI: 10.7270/Q20V8GWQ |
More data for this
Ligand-Target Pair | |
Replicase polyprotein 1ab
(2019-nCoV) | BDBM484195

(UAWJ9-36-3)Show SMILES CC1(C)[C@H]2CN([C@@H]([C@@H]12)C(=O)N[C@@H](C[C@@H]1CCNC1=O)C=O)C(=O)OCc1ccccc1 Show InChI InChI=1S/C23H29N3O5/c1-23(2)17-11-26(22(30)31-13-14-6-4-3-5-7-14)19(18(17)23)21(29)25-16(12-27)10-15-8-9-24-20(15)28/h3-7,12,15-19H,8-11,13H2,1-2H3,(H,24,28)(H,25,29)/t15-,16-,17-,18-,19-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 54 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Arizona
| Assay Description
Enzymatic inhibition of GC-376, UAWJ9-36-1, and UAWJ9-36-3 against Mpro's from all seven human coronaviruses. Data fittings of the proteolytic progre... |
ACS Pharmacol Transl Sci 4: 1408-1421 (2021)
Article DOI: 10.1021/acsptsci.1c00099
BindingDB Entry DOI: 10.7270/Q20V8GWQ |
More data for this
Ligand-Target Pair | |
Enoyl-[acyl-carrier-protein] reductase [NADPH] FabI
(Staphylococcus aureus) | BDBM50399402
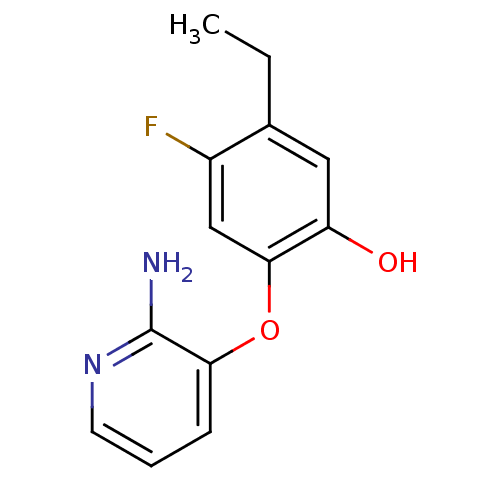
(CHEMBL2178289)Show InChI InChI=1S/C13H13FN2O2/c1-2-8-6-10(17)12(7-9(8)14)18-11-4-3-5-16-13(11)15/h3-7,17H,2H2,1H3,(H2,15,16) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 56 | n/a | n/a | n/a | n/a | n/a | n/a |
Mutabilis
Curated by ChEMBL
| Assay Description
Inhibition of Staphylococcus aureus recombinant FabI using trans-2-octenoyl N-acetylcysteamine thioester as substrate preincubated for 60 mins |
J Med Chem 55: 9914-28 (2012)
Article DOI: 10.1021/jm301113w
BindingDB Entry DOI: 10.7270/Q2H99699 |
More data for this
Ligand-Target Pair | |
Enoyl-[acyl-carrier-protein] reductase [NADPH] FabI
(Staphylococcus aureus) | BDBM8726

(5-chloro-2-(2,4-dichlorophenoxy)phenol | CHEMBL849...)Show InChI InChI=1S/C12H7Cl3O2/c13-7-1-3-11(9(15)5-7)17-12-4-2-8(14)6-10(12)16/h1-6,16H | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 59 | n/a | n/a | n/a | n/a | n/a | n/a |
Mutabilis
Curated by ChEMBL
| Assay Description
Inhibition of Staphylococcus aureus recombinant FabI using trans-2-octenoyl N-acetylcysteamine thioester as substrate preincubated for 60 mins |
J Med Chem 55: 9914-28 (2012)
Article DOI: 10.1021/jm301113w
BindingDB Entry DOI: 10.7270/Q2H99699 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Pseudomonas aeruginosa) | BDBM50554703
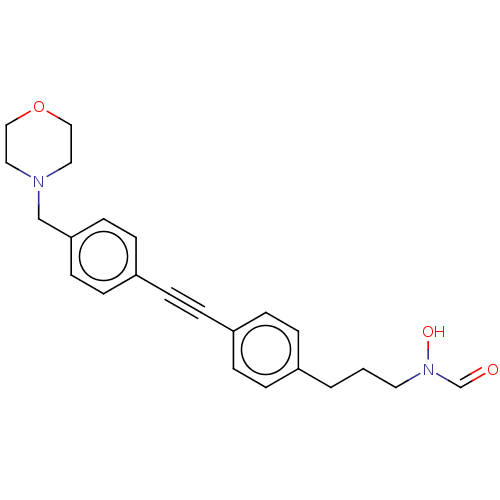
(CHEMBL4798753) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 63 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of fluorescent ligand from Pseudomonas aeruginosa LpxC measured after 30 mins by fluorescence anisotrophy assay |
Citation and Details
Article DOI: 10.1016/j.bmc.2020.115826
BindingDB Entry DOI: 10.7270/Q2C2513C |
More data for this
Ligand-Target Pair | |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Pseudomonas aeruginosa) | BDBM50554700
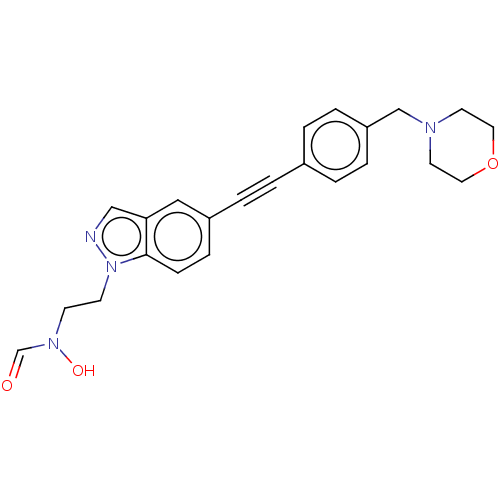
(CHEMBL4786538)Show SMILES ON(CCn1ncc2cc(ccc12)C#Cc1ccc(CN2CCOCC2)cc1)C=O | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 64 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of fluorescent ligand from Pseudomonas aeruginosa LpxC measured after 30 mins by fluorescence anisotrophy assay |
Citation and Details
Article DOI: 10.1016/j.bmc.2020.115826
BindingDB Entry DOI: 10.7270/Q2C2513C |
More data for this
Ligand-Target Pair | |
Replicase polyprotein 1ab
(HCoV-229E) | BDBM448319

(GC-376 | GC376)Show SMILES CC(C)C[C@H](NC(=O)OCc1ccccc1)C(=O)N[C@@H](CC1CCNC1=O)C(O)S([O-])(=O)=O Show InChI InChI=1S/C21H31N3O8S/c1-13(2)10-16(24-21(28)32-12-14-6-4-3-5-7-14)19(26)23-17(20(27)33(29,30)31)11-15-8-9-22-18(15)25/h3-7,13,15-17,20,27H,8-12H2,1-2H3,(H,22,25)(H,23,26)(H,24,28)(H,29,30,31)/p-1/t15?,16-,17-,20?/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 69 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Arizona
| Assay Description
Enzymatic inhibition of GC-376, UAWJ9-36-1, and UAWJ9-36-3 against Mpro's from all seven human coronaviruses. Data fittings of the proteolytic progre... |
ACS Pharmacol Transl Sci 4: 1408-1421 (2021)
Article DOI: 10.1021/acsptsci.1c00099
BindingDB Entry DOI: 10.7270/Q20V8GWQ |
More data for this
Ligand-Target Pair | |
Calpain-1 catalytic subunit
(Homo sapiens (Human)) | BDBM448319

(GC-376 | GC376)Show SMILES CC(C)C[C@H](NC(=O)OCc1ccccc1)C(=O)N[C@@H](CC1CCNC1=O)C(O)S([O-])(=O)=O Show InChI InChI=1S/C21H31N3O8S/c1-13(2)10-16(24-21(28)32-12-14-6-4-3-5-7-14)19(26)23-17(20(27)33(29,30)31)11-15-8-9-22-18(15)25/h3-7,13,15-17,20,27H,8-12H2,1-2H3,(H,22,25)(H,23,26)(H,24,28)(H,29,30,31)/p-1/t15?,16-,17-,20?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 74 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
FRET-based enzymatic assay. |
Citation and Details
Article DOI: 10.1021/jacs.1c08060
BindingDB Entry DOI: 10.7270/Q2BK1GHG |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data