 Found 372 hits with Last Name = 'soural' and Initial = 'm'
Found 372 hits with Last Name = 'soural' and Initial = 'm' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
D(4) dopamine receptor
(Homo sapiens (Human)) | BDBM50530438

(Mosapramine | Mosapramine hydrochloride)Show SMILES Clc1ccc2CCc3ccccc3N(CCCN3CCC4(CC3)N3CCCCC3NC4=O)c2c1 Show InChI InChI=1S/C28H35ClN4O/c29-23-12-11-22-10-9-21-6-1-2-7-24(21)32(25(22)20-23)16-5-15-31-18-13-28(14-19-31)27(34)30-26-8-3-4-17-33(26)28/h1-2,6-7,11-12,20,26H,3-5,8-10,13-19H2,(H,30,34) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Palack£ University
Curated by ChEMBL
| Assay Description
Agonist activity at dopamine D4 receptor (unknown origin) |
Eur J Med Chem 181: (2019)
Article DOI: 10.1016/j.ejmech.2019.111569
BindingDB Entry DOI: 10.7270/Q2H41VXG |
More data for this
Ligand-Target Pair | |
D(4) dopamine receptor
(Homo sapiens (Human)) | BDBM50530438

(Mosapramine | Mosapramine hydrochloride)Show SMILES Clc1ccc2CCc3ccccc3N(CCCN3CCC4(CC3)N3CCCCC3NC4=O)c2c1 Show InChI InChI=1S/C28H35ClN4O/c29-23-12-11-22-10-9-21-6-1-2-7-24(21)32(25(22)20-23)16-5-15-31-18-13-28(14-19-31)27(34)30-26-8-3-4-17-33(26)28/h1-2,6-7,11-12,20,26H,3-5,8-10,13-19H2,(H,30,34) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Palack£ University
Curated by ChEMBL
| Assay Description
Agonist activity at dopamine D4 receptor (unknown origin) |
Eur J Med Chem 181: (2019)
Article DOI: 10.1016/j.ejmech.2019.111569
BindingDB Entry DOI: 10.7270/Q2H41VXG |
More data for this
Ligand-Target Pair | |
Translocator protein
(Homo sapiens (Human)) | BDBM50274351
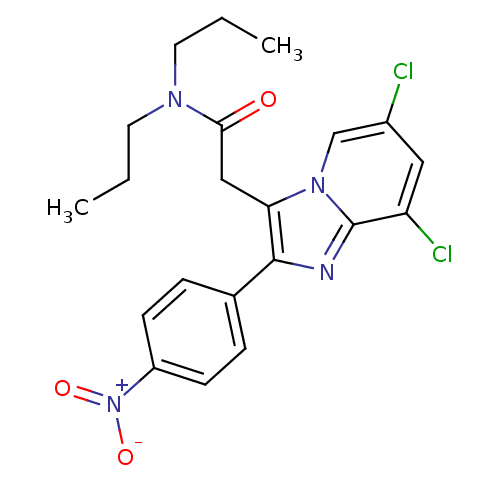
(2-(6,8-Dichloro-2-(4-nitrophenyl)imidazo[1,2-a]pyr...)Show SMILES CCCN(CCC)C(=O)Cc1c(nc2c(Cl)cc(Cl)cn12)-c1ccc(cc1)[N+]([O-])=O Show InChI InChI=1S/C21H22Cl2N4O3/c1-3-9-25(10-4-2)19(28)12-18-20(14-5-7-16(8-6-14)27(29)30)24-21-17(23)11-15(22)13-26(18)21/h5-8,11,13H,3-4,9-10,12H2,1-2H3 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Palack£ University
Curated by ChEMBL
| Assay Description
Inhibition of TSPO (unknown origin) |
Eur J Med Chem 181: (2019)
Article DOI: 10.1016/j.ejmech.2019.111569
BindingDB Entry DOI: 10.7270/Q2H41VXG |
More data for this
Ligand-Target Pair | |
Translocator protein
(Homo sapiens (Human)) | BDBM50274351

(2-(6,8-Dichloro-2-(4-nitrophenyl)imidazo[1,2-a]pyr...)Show SMILES CCCN(CCC)C(=O)Cc1c(nc2c(Cl)cc(Cl)cn12)-c1ccc(cc1)[N+]([O-])=O Show InChI InChI=1S/C21H22Cl2N4O3/c1-3-9-25(10-4-2)19(28)12-18-20(14-5-7-16(8-6-14)27(29)30)24-21-17(23)11-15(22)13-26(18)21/h5-8,11,13H,3-4,9-10,12H2,1-2H3 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Palack£ University
Curated by ChEMBL
| Assay Description
Inhibition of TSPO (unknown origin) |
Eur J Med Chem 181: (2019)
Article DOI: 10.1016/j.ejmech.2019.111569
BindingDB Entry DOI: 10.7270/Q2H41VXG |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(GUINEA PIG) | BDBM50247053

(1-(3-(3-(4-chlorophenyl)propoxy)propyl)piperidine ...)Show InChI InChI=1S/C17H26ClNO/c18-17-9-7-16(8-10-17)6-4-14-20-15-5-13-19-11-2-1-3-12-19/h7-10H,1-6,11-15H2 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Palack£ University
Curated by ChEMBL
| Assay Description
Inhibition of Guinea Pig Ileum H3R |
Eur J Med Chem 181: (2019)
Article DOI: 10.1016/j.ejmech.2019.111569
BindingDB Entry DOI: 10.7270/Q2H41VXG |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(GUINEA PIG) | BDBM50247053

(1-(3-(3-(4-chlorophenyl)propoxy)propyl)piperidine ...)Show InChI InChI=1S/C17H26ClNO/c18-17-9-7-16(8-10-17)6-4-14-20-15-5-13-19-11-2-1-3-12-19/h7-10H,1-6,11-15H2 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Palack£ University
Curated by ChEMBL
| Assay Description
Inhibition of Guinea Pig Ileum H3R |
Eur J Med Chem 181: (2019)
Article DOI: 10.1016/j.ejmech.2019.111569
BindingDB Entry DOI: 10.7270/Q2H41VXG |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50291284
(CHEMBL4170220)Show SMILES CN([C@@H]1CCCc2cc(ccc12)S(=O)(=O)c1cccc(F)c1)C(N)=O |r| Show InChI InChI=1S/C18H19FN2O3S/c1-21(18(20)22)17-7-2-4-12-10-15(8-9-16(12)17)25(23,24)14-6-3-5-13(19)11-14/h3,5-6,8-11,17H,2,4,7H2,1H3,(H2,20,22)/t17-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Palack£ University
Curated by ChEMBL
| Assay Description
Binding affinity to 5HT6R (unknown origin) |
Eur J Med Chem 144: 716-729 (2018)
Article DOI: 10.1016/j.ejmech.2017.12.053
BindingDB Entry DOI: 10.7270/Q2ZK5K6S |
More data for this
Ligand-Target Pair | |
D(4) dopamine receptor
(Homo sapiens (Human)) | BDBM50189854

(6-bromo-2-[4-(2-methoxyphenyl)piperazin-1-ylmethyl...)Show InChI InChI=1S/C19H21BrN4O/c1-25-18-5-3-2-4-17(18)23-10-8-22(9-11-23)13-16-14-24-12-15(20)6-7-19(24)21-16/h2-7,12,14H,8-11,13H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Palack£ University
Curated by ChEMBL
| Assay Description
Agonist activity at dopamine D4 receptor (unknown origin) |
Eur J Med Chem 181: (2019)
Article DOI: 10.1016/j.ejmech.2019.111569
BindingDB Entry DOI: 10.7270/Q2H41VXG |
More data for this
Ligand-Target Pair | |
D(4) dopamine receptor
(Homo sapiens (Human)) | BDBM50189854

(6-bromo-2-[4-(2-methoxyphenyl)piperazin-1-ylmethyl...)Show InChI InChI=1S/C19H21BrN4O/c1-25-18-5-3-2-4-17(18)23-10-8-22(9-11-23)13-16-14-24-12-15(20)6-7-19(24)21-16/h2-7,12,14H,8-11,13H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Palack£ University
Curated by ChEMBL
| Assay Description
Agonist activity at dopamine D4 receptor (unknown origin) |
Eur J Med Chem 181: (2019)
Article DOI: 10.1016/j.ejmech.2019.111569
BindingDB Entry DOI: 10.7270/Q2H41VXG |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50318633

(3-benzenesulfonyl-8-piperazin-1-ylquinoline | CHEM...)Show InChI InChI=1S/C19H19N3O2S/c23-25(24,16-6-2-1-3-7-16)17-13-15-5-4-8-18(19(15)21-14-17)22-11-9-20-10-12-22/h1-8,13-14,20H,9-12H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Palack£ University
Curated by ChEMBL
| Assay Description
Binding affinity to 5HT6R (unknown origin) |
Eur J Med Chem 144: 716-729 (2018)
Article DOI: 10.1016/j.ejmech.2017.12.053
BindingDB Entry DOI: 10.7270/Q2ZK5K6S |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50291286
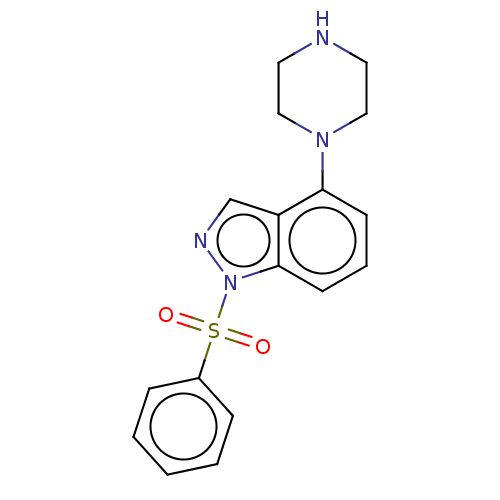
(CHEMBL4169827)Show InChI InChI=1S/C17H18N4O2S/c22-24(23,14-5-2-1-3-6-14)21-17-8-4-7-16(15(17)13-19-21)20-11-9-18-10-12-20/h1-8,13,18H,9-12H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Palack£ University
Curated by ChEMBL
| Assay Description
Binding affinity to 5HT6R (unknown origin) |
Eur J Med Chem 144: 716-729 (2018)
Article DOI: 10.1016/j.ejmech.2017.12.053
BindingDB Entry DOI: 10.7270/Q2ZK5K6S |
More data for this
Ligand-Target Pair | |
D(4) dopamine receptor
(Homo sapiens (Human)) | BDBM50189841
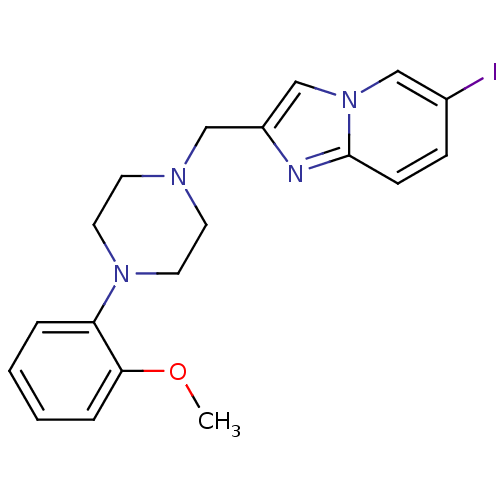
(6-iodo-2-[4-(2-methoxyphenyl)piperazin-1-ylmethyl]...)Show InChI InChI=1S/C19H21IN4O/c1-25-18-5-3-2-4-17(18)23-10-8-22(9-11-23)13-16-14-24-12-15(20)6-7-19(24)21-16/h2-7,12,14H,8-11,13H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Palack£ University
Curated by ChEMBL
| Assay Description
Agonist activity at dopamine D4 receptor (unknown origin) |
Eur J Med Chem 181: (2019)
Article DOI: 10.1016/j.ejmech.2019.111569
BindingDB Entry DOI: 10.7270/Q2H41VXG |
More data for this
Ligand-Target Pair | |
D(4) dopamine receptor
(Homo sapiens (Human)) | BDBM50189841

(6-iodo-2-[4-(2-methoxyphenyl)piperazin-1-ylmethyl]...)Show InChI InChI=1S/C19H21IN4O/c1-25-18-5-3-2-4-17(18)23-10-8-22(9-11-23)13-16-14-24-12-15(20)6-7-19(24)21-16/h2-7,12,14H,8-11,13H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Palack£ University
Curated by ChEMBL
| Assay Description
Agonist activity at dopamine D4 receptor (unknown origin) |
Eur J Med Chem 181: (2019)
Article DOI: 10.1016/j.ejmech.2019.111569
BindingDB Entry DOI: 10.7270/Q2H41VXG |
More data for this
Ligand-Target Pair | |
Translocator protein
(Homo sapiens (Human)) | BDBM50060095

(2-[6,8-Dichloro-2-(4-chloro-phenyl)-imidazo[1,2-a]...)Show SMILES CCCN(CCC)C(=O)Cc1c(nc2c(Cl)cc(Cl)cn12)-c1ccc(Cl)cc1 Show InChI InChI=1S/C21H22Cl3N3O/c1-3-9-26(10-4-2)19(28)12-18-20(14-5-7-15(22)8-6-14)25-21-17(24)11-16(23)13-27(18)21/h5-8,11,13H,3-4,9-10,12H2,1-2H3 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.410 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Palack£ University
Curated by ChEMBL
| Assay Description
Inhibition of TSPO (unknown origin) |
Eur J Med Chem 181: (2019)
Article DOI: 10.1016/j.ejmech.2019.111569
BindingDB Entry DOI: 10.7270/Q2H41VXG |
More data for this
Ligand-Target Pair | |
Translocator protein
(Homo sapiens (Human)) | BDBM50060095

(2-[6,8-Dichloro-2-(4-chloro-phenyl)-imidazo[1,2-a]...)Show SMILES CCCN(CCC)C(=O)Cc1c(nc2c(Cl)cc(Cl)cn12)-c1ccc(Cl)cc1 Show InChI InChI=1S/C21H22Cl3N3O/c1-3-9-26(10-4-2)19(28)12-18-20(14-5-7-15(22)8-6-14)25-21-17(24)11-16(23)13-27(18)21/h5-8,11,13H,3-4,9-10,12H2,1-2H3 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.410 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Palack£ University
Curated by ChEMBL
| Assay Description
Inhibition of TSPO (unknown origin) |
Eur J Med Chem 181: (2019)
Article DOI: 10.1016/j.ejmech.2019.111569
BindingDB Entry DOI: 10.7270/Q2H41VXG |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50044615

(CHEMBL3329435)Show InChI InChI=1S/C18H20N4O2S/c1-14-20-18-16(21-12-10-19-11-13-21)8-5-9-17(18)22(14)25(23,24)15-6-3-2-4-7-15/h2-9,19H,10-13H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to 5HT6R (unknown origin) |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c02009
BindingDB Entry DOI: 10.7270/Q2JQ14RQ |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50530421
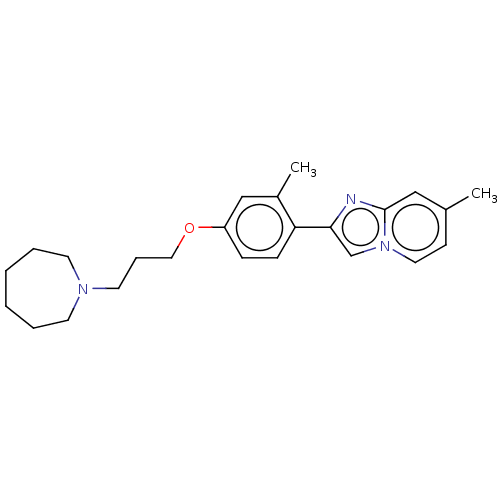
(CHEMBL4585201)Show InChI InChI=1S/C24H31N3O/c1-19-10-14-27-18-23(25-24(27)16-19)22-9-8-21(17-20(22)2)28-15-7-13-26-11-5-3-4-6-12-26/h8-10,14,16-18H,3-7,11-13,15H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Palack£ University
Curated by ChEMBL
| Assay Description
Inhibition of H3R (unknown origin) |
Eur J Med Chem 181: (2019)
Article DOI: 10.1016/j.ejmech.2019.111569
BindingDB Entry DOI: 10.7270/Q2H41VXG |
More data for this
Ligand-Target Pair | |
Translocator protein
(Homo sapiens (Human)) | BDBM22041
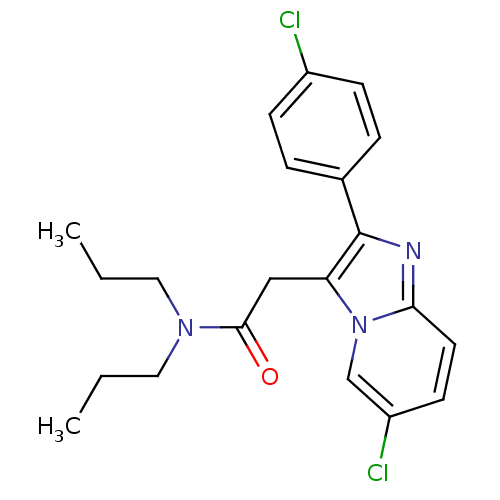
(2-[6-chloro-2-(4-chlorophenyl)imidazo[1,2-a]pyridi...)Show SMILES CCCN(CCC)C(=O)Cc1c(nc2ccc(Cl)cn12)-c1ccc(Cl)cc1 Show InChI InChI=1S/C21H23Cl2N3O/c1-3-11-25(12-4-2)20(27)13-18-21(15-5-7-16(22)8-6-15)24-19-10-9-17(23)14-26(18)19/h5-10,14H,3-4,11-13H2,1-2H3 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Palack£ University
Curated by ChEMBL
| Assay Description
Inhibition of TSPO (unknown origin) |
Eur J Med Chem 181: (2019)
Article DOI: 10.1016/j.ejmech.2019.111569
BindingDB Entry DOI: 10.7270/Q2H41VXG |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50530421

(CHEMBL4585201)Show InChI InChI=1S/C24H31N3O/c1-19-10-14-27-18-23(25-24(27)16-19)22-9-8-21(17-20(22)2)28-15-7-13-26-11-5-3-4-6-12-26/h8-10,14,16-18H,3-7,11-13,15H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Palack£ University
Curated by ChEMBL
| Assay Description
Inhibition of H3R (unknown origin) |
Eur J Med Chem 181: (2019)
Article DOI: 10.1016/j.ejmech.2019.111569
BindingDB Entry DOI: 10.7270/Q2H41VXG |
More data for this
Ligand-Target Pair | |
Translocator protein
(Homo sapiens (Human)) | BDBM22041

(2-[6-chloro-2-(4-chlorophenyl)imidazo[1,2-a]pyridi...)Show SMILES CCCN(CCC)C(=O)Cc1c(nc2ccc(Cl)cn12)-c1ccc(Cl)cc1 Show InChI InChI=1S/C21H23Cl2N3O/c1-3-11-25(12-4-2)20(27)13-18-21(15-5-7-16(22)8-6-15)24-19-10-9-17(23)14-26(18)19/h5-10,14H,3-4,11-13H2,1-2H3 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Palack£ University
Curated by ChEMBL
| Assay Description
Inhibition of TSPO (unknown origin) |
Eur J Med Chem 181: (2019)
Article DOI: 10.1016/j.ejmech.2019.111569
BindingDB Entry DOI: 10.7270/Q2H41VXG |
More data for this
Ligand-Target Pair | |
Translocator protein
(Homo sapiens (Human)) | BDBM50274258
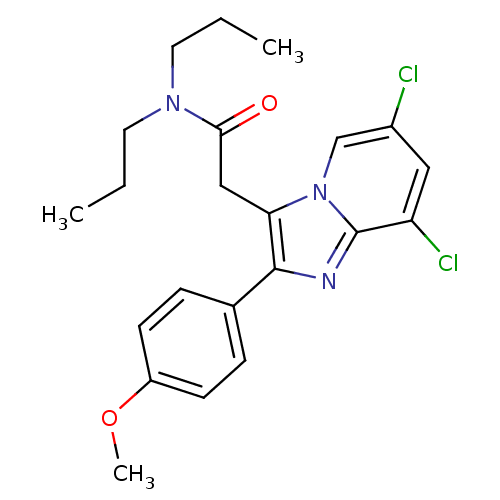
(2-(6,8-Dichloro-2-(4-methoxyphenyl)imidazo[1,2-a]p...)Show SMILES CCCN(CCC)C(=O)Cc1c(nc2c(Cl)cc(Cl)cn12)-c1ccc(OC)cc1 Show InChI InChI=1S/C22H25Cl2N3O2/c1-4-10-26(11-5-2)20(28)13-19-21(15-6-8-17(29-3)9-7-15)25-22-18(24)12-16(23)14-27(19)22/h6-9,12,14H,4-5,10-11,13H2,1-3H3 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.537 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Palack£ University
Curated by ChEMBL
| Assay Description
Inhibition of TSPO (unknown origin) |
Eur J Med Chem 181: (2019)
Article DOI: 10.1016/j.ejmech.2019.111569
BindingDB Entry DOI: 10.7270/Q2H41VXG |
More data for this
Ligand-Target Pair | |
Translocator protein
(Homo sapiens (Human)) | BDBM50274258

(2-(6,8-Dichloro-2-(4-methoxyphenyl)imidazo[1,2-a]p...)Show SMILES CCCN(CCC)C(=O)Cc1c(nc2c(Cl)cc(Cl)cn12)-c1ccc(OC)cc1 Show InChI InChI=1S/C22H25Cl2N3O2/c1-4-10-26(11-5-2)20(28)13-19-21(15-6-8-17(29-3)9-7-15)25-22-18(24)12-16(23)14-27(19)22/h6-9,12,14H,4-5,10-11,13H2,1-3H3 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.537 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Palack£ University
Curated by ChEMBL
| Assay Description
Inhibition of TSPO (unknown origin) |
Eur J Med Chem 181: (2019)
Article DOI: 10.1016/j.ejmech.2019.111569
BindingDB Entry DOI: 10.7270/Q2H41VXG |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50530440
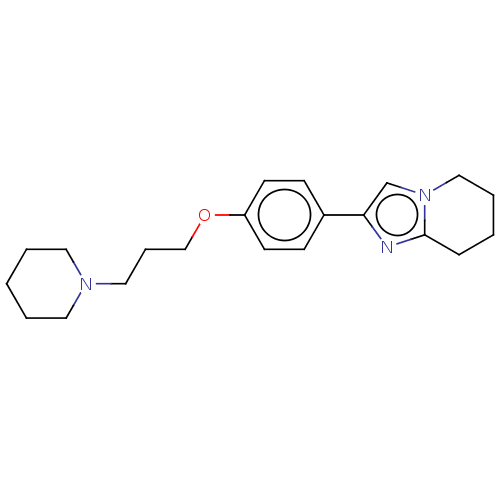
(CHEMBL4548796)Show InChI InChI=1S/C21H29N3O/c1-3-12-23(13-4-1)14-6-16-25-19-10-8-18(9-11-19)20-17-24-15-5-2-7-21(24)22-20/h8-11,17H,1-7,12-16H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.75 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Palack£ University
Curated by ChEMBL
| Assay Description
Inhibition of H3R (unknown origin) |
Eur J Med Chem 181: (2019)
Article DOI: 10.1016/j.ejmech.2019.111569
BindingDB Entry DOI: 10.7270/Q2H41VXG |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50530440

(CHEMBL4548796)Show InChI InChI=1S/C21H29N3O/c1-3-12-23(13-4-1)14-6-16-25-19-10-8-18(9-11-19)20-17-24-15-5-2-7-21(24)22-20/h8-11,17H,1-7,12-16H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.75 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Palack£ University
Curated by ChEMBL
| Assay Description
Inhibition of H3R (unknown origin) |
Eur J Med Chem 181: (2019)
Article DOI: 10.1016/j.ejmech.2019.111569
BindingDB Entry DOI: 10.7270/Q2H41VXG |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50019754
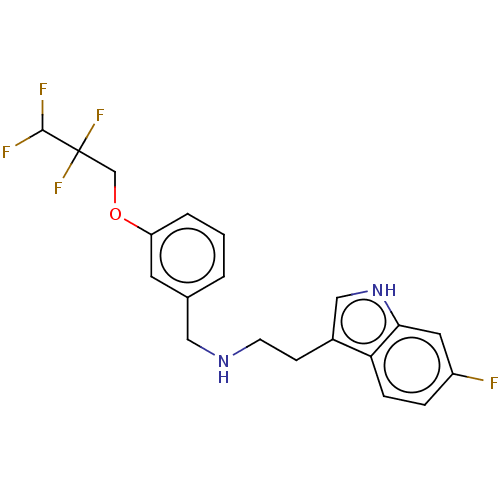
(IDALOPIRDINE | LU-AE58054)Show SMILES FC(F)C(F)(F)COc1cccc(CNCCc2c[nH]c3cc(F)ccc23)c1 Show InChI InChI=1S/C20H19F5N2O/c21-15-4-5-17-14(11-27-18(17)9-15)6-7-26-10-13-2-1-3-16(8-13)28-12-20(24,25)19(22)23/h1-5,8-9,11,19,26-27H,6-7,10,12H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.830 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Palack£ University
Curated by ChEMBL
| Assay Description
Binding affinity to 5HT6R (unknown origin) |
Eur J Med Chem 144: 716-729 (2018)
Article DOI: 10.1016/j.ejmech.2017.12.053
BindingDB Entry DOI: 10.7270/Q2ZK5K6S |
More data for this
Ligand-Target Pair | |
Translocator protein
(Homo sapiens (Human)) | BDBM50274561

(4-(2-(4-Chlorophenyl)-3-(2-(dipropylamino)-2-oxoet...)Show SMILES CCCN(CCC)C(=O)Cc1c(nc2c(NC(=O)CCC(=O)OCC)cccn12)-c1ccc(Cl)cc1 Show InChI InChI=1S/C27H33ClN4O4/c1-4-15-31(16-5-2)24(34)18-22-26(19-9-11-20(28)12-10-19)30-27-21(8-7-17-32(22)27)29-23(33)13-14-25(35)36-6-3/h7-12,17H,4-6,13-16,18H2,1-3H3,(H,29,33) | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.860 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Palack£ University
Curated by ChEMBL
| Assay Description
Inhibition of TSPO (unknown origin) |
Eur J Med Chem 181: (2019)
Article DOI: 10.1016/j.ejmech.2019.111569
BindingDB Entry DOI: 10.7270/Q2H41VXG |
More data for this
Ligand-Target Pair | |
Translocator protein
(Homo sapiens (Human)) | BDBM50274561

(4-(2-(4-Chlorophenyl)-3-(2-(dipropylamino)-2-oxoet...)Show SMILES CCCN(CCC)C(=O)Cc1c(nc2c(NC(=O)CCC(=O)OCC)cccn12)-c1ccc(Cl)cc1 Show InChI InChI=1S/C27H33ClN4O4/c1-4-15-31(16-5-2)24(34)18-22-26(19-9-11-20(28)12-10-19)30-27-21(8-7-17-32(22)27)29-23(33)13-14-25(35)36-6-3/h7-12,17H,4-6,13-16,18H2,1-3H3,(H,29,33) | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.860 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Palack£ University
Curated by ChEMBL
| Assay Description
Inhibition of TSPO (unknown origin) |
Eur J Med Chem 181: (2019)
Article DOI: 10.1016/j.ejmech.2019.111569
BindingDB Entry DOI: 10.7270/Q2H41VXG |
More data for this
Ligand-Target Pair | |
Translocator protein
(Homo sapiens (Human)) | BDBM50274259
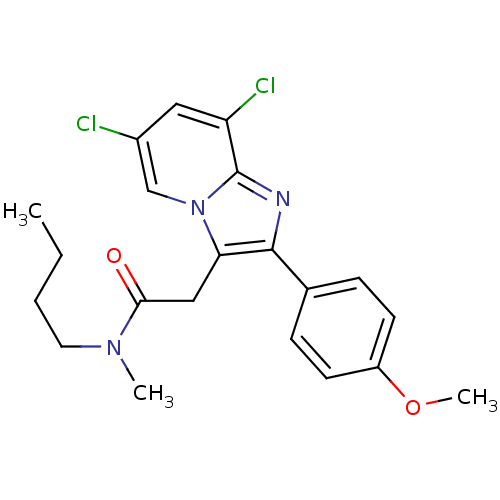
(CHEMBL521186 | N-Butyl-2-(6,8-dichloro-2-(4-methox...)Show SMILES CCCCN(C)C(=O)Cc1c(nc2c(Cl)cc(Cl)cn12)-c1ccc(OC)cc1 Show InChI InChI=1S/C21H23Cl2N3O2/c1-4-5-10-25(2)19(27)12-18-20(14-6-8-16(28-3)9-7-14)24-21-17(23)11-15(22)13-26(18)21/h6-9,11,13H,4-5,10,12H2,1-3H3 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.882 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Palack£ University
Curated by ChEMBL
| Assay Description
Inhibition of TSPO (unknown origin) |
Eur J Med Chem 181: (2019)
Article DOI: 10.1016/j.ejmech.2019.111569
BindingDB Entry DOI: 10.7270/Q2H41VXG |
More data for this
Ligand-Target Pair | |
Translocator protein
(Homo sapiens (Human)) | BDBM50274259

(CHEMBL521186 | N-Butyl-2-(6,8-dichloro-2-(4-methox...)Show SMILES CCCCN(C)C(=O)Cc1c(nc2c(Cl)cc(Cl)cn12)-c1ccc(OC)cc1 Show InChI InChI=1S/C21H23Cl2N3O2/c1-4-5-10-25(2)19(27)12-18-20(14-6-8-16(28-3)9-7-14)24-21-17(23)11-15(22)13-26(18)21/h6-9,11,13H,4-5,10,12H2,1-3H3 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.882 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Palack£ University
Curated by ChEMBL
| Assay Description
Inhibition of TSPO (unknown origin) |
Eur J Med Chem 181: (2019)
Article DOI: 10.1016/j.ejmech.2019.111569
BindingDB Entry DOI: 10.7270/Q2H41VXG |
More data for this
Ligand-Target Pair | |
D(4) dopamine receptor
(Homo sapiens (Human)) | BDBM50189848

(2-[4-(3,4-dichlorophenyl)piperazin-1-ylmethyl]imid...)Show InChI InChI=1S/C18H18Cl2N4/c19-16-5-4-15(11-17(16)20)23-9-7-22(8-10-23)12-14-13-24-6-2-1-3-18(24)21-14/h1-6,11,13H,7-10,12H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.990 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Palack£ University
Curated by ChEMBL
| Assay Description
Agonist activity at dopamine D4 receptor (unknown origin) |
Eur J Med Chem 181: (2019)
Article DOI: 10.1016/j.ejmech.2019.111569
BindingDB Entry DOI: 10.7270/Q2H41VXG |
More data for this
Ligand-Target Pair | |
D(4) dopamine receptor
(Homo sapiens (Human)) | BDBM50189848

(2-[4-(3,4-dichlorophenyl)piperazin-1-ylmethyl]imid...)Show InChI InChI=1S/C18H18Cl2N4/c19-16-5-4-15(11-17(16)20)23-9-7-22(8-10-23)12-14-13-24-6-2-1-3-18(24)21-14/h1-6,11,13H,7-10,12H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.990 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Palack£ University
Curated by ChEMBL
| Assay Description
Agonist activity at dopamine D4 receptor (unknown origin) |
Eur J Med Chem 181: (2019)
Article DOI: 10.1016/j.ejmech.2019.111569
BindingDB Entry DOI: 10.7270/Q2H41VXG |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50174269

(1-(phenylsulfonyl)-4-(piperazin-1-yl)-1H-indole | ...)Show InChI InChI=1S/C18H19N3O2S/c22-24(23,15-5-2-1-3-6-15)21-12-9-16-17(7-4-8-18(16)21)20-13-10-19-11-14-20/h1-9,12,19H,10-11,13-14H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Palack£ University
Curated by ChEMBL
| Assay Description
Binding affinity to 5HT6R (unknown origin) |
Eur J Med Chem 144: 716-729 (2018)
Article DOI: 10.1016/j.ejmech.2017.12.053
BindingDB Entry DOI: 10.7270/Q2ZK5K6S |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50562747

(CHEMBL4756814)Show SMILES CCc1nc2c(nccc2n1S(=O)(=O)c1cccc(Cl)c1)N1CCNCC1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]-LSD from human 5HT6R expressed in HEK293 cell membranes incubated for 1 hr by micro-beta plate reader based analysis |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c02009
BindingDB Entry DOI: 10.7270/Q2JQ14RQ |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50562748
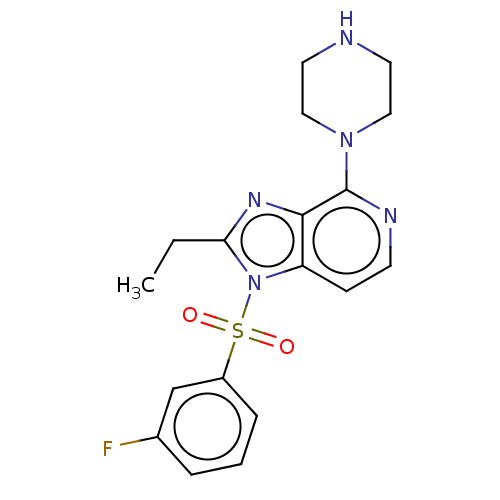
(CHEMBL4760105)Show SMILES CCc1nc2c(nccc2n1S(=O)(=O)c1cccc(F)c1)N1CCNCC1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]-LSD from human 5HT6R expressed in HEK293 cell membranes incubated for 1 hr by micro-beta plate reader based analysis |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c02009
BindingDB Entry DOI: 10.7270/Q2JQ14RQ |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50562751
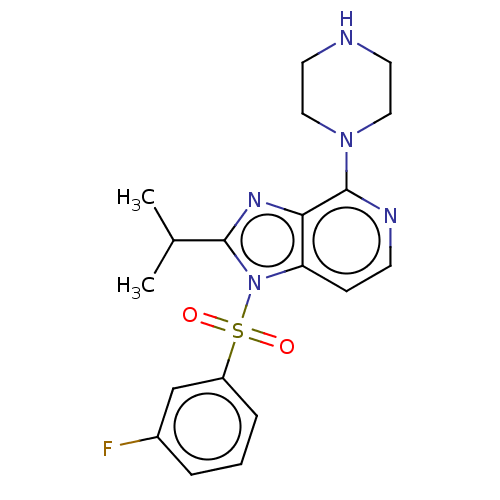
(CHEMBL4760545)Show SMILES CC(C)c1nc2c(nccc2n1S(=O)(=O)c1cccc(F)c1)N1CCNCC1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]-LSD from human 5HT6R expressed in HEK293 cell membranes incubated for 1 hr by micro-beta plate reader based analysis |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c02009
BindingDB Entry DOI: 10.7270/Q2JQ14RQ |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50318633

(3-benzenesulfonyl-8-piperazin-1-ylquinoline | CHEM...)Show InChI InChI=1S/C19H19N3O2S/c23-25(24,16-6-2-1-3-7-16)17-13-15-5-4-8-18(19(15)21-14-17)22-11-9-20-10-12-22/h1-8,13-14,20H,9-12H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Palack£ University
Curated by ChEMBL
| Assay Description
Displacement of [3H]-LSD from human 5HT6R expressed in HEK293 cell membranes after 1 hr by microbeta counting method |
Eur J Med Chem 144: 716-729 (2018)
Article DOI: 10.1016/j.ejmech.2017.12.053
BindingDB Entry DOI: 10.7270/Q2ZK5K6S |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50318633

(3-benzenesulfonyl-8-piperazin-1-ylquinoline | CHEM...)Show InChI InChI=1S/C19H19N3O2S/c23-25(24,16-6-2-1-3-7-16)17-13-15-5-4-8-18(19(15)21-14-17)22-11-9-20-10-12-22/h1-8,13-14,20H,9-12H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]-LSD from human 5HT6R expressed in HEK293 cell membranes incubated for 1 hr by micro-beta plate reader based analysis |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c02009
BindingDB Entry DOI: 10.7270/Q2JQ14RQ |
More data for this
Ligand-Target Pair | |
Translocator protein
(Homo sapiens (Human)) | BDBM50408728

(CHEMBL339816)Show SMILES CCCN(CCC)C(=O)Cc1c(nc2c(N)cccn12)-c1ccc(Cl)cc1 Show InChI InChI=1S/C21H25ClN4O/c1-3-11-25(12-4-2)19(27)14-18-20(15-7-9-16(22)10-8-15)24-21-17(23)6-5-13-26(18)21/h5-10,13H,3-4,11-12,14,23H2,1-2H3 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Palack£ University
Curated by ChEMBL
| Assay Description
Inhibition of TSPO (unknown origin) |
Eur J Med Chem 181: (2019)
Article DOI: 10.1016/j.ejmech.2019.111569
BindingDB Entry DOI: 10.7270/Q2H41VXG |
More data for this
Ligand-Target Pair | |
Translocator protein
(Homo sapiens (Human)) | BDBM50408728

(CHEMBL339816)Show SMILES CCCN(CCC)C(=O)Cc1c(nc2c(N)cccn12)-c1ccc(Cl)cc1 Show InChI InChI=1S/C21H25ClN4O/c1-3-11-25(12-4-2)19(27)14-18-20(15-7-9-16(22)10-8-15)24-21-17(23)6-5-13-26(18)21/h5-10,13H,3-4,11-12,14,23H2,1-2H3 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Palack£ University
Curated by ChEMBL
| Assay Description
Inhibition of TSPO (unknown origin) |
Eur J Med Chem 181: (2019)
Article DOI: 10.1016/j.ejmech.2019.111569
BindingDB Entry DOI: 10.7270/Q2H41VXG |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50562749

(CHEMBL4756098)Show SMILES CC(C)c1nc2c(nccc2n1S(=O)(=O)c1ccccc1)N1CCNCC1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]-LSD from human 5HT6R expressed in HEK293 cell membranes incubated for 1 hr by micro-beta plate reader based analysis |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c02009
BindingDB Entry DOI: 10.7270/Q2JQ14RQ |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50120543
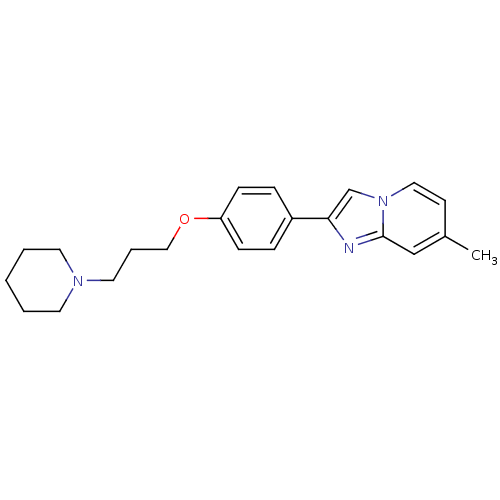
(7-Methyl-2-[4-(3-piperidin-1-yl-propoxy)-phenyl]-i...)Show InChI InChI=1S/C22H27N3O/c1-18-10-14-25-17-21(23-22(25)16-18)19-6-8-20(9-7-19)26-15-5-13-24-11-3-2-4-12-24/h6-10,14,16-17H,2-5,11-13,15H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Palack£ University
Curated by ChEMBL
| Assay Description
Inhibition of H3R (unknown origin) |
Eur J Med Chem 181: (2019)
Article DOI: 10.1016/j.ejmech.2019.111569
BindingDB Entry DOI: 10.7270/Q2H41VXG |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50120543

(7-Methyl-2-[4-(3-piperidin-1-yl-propoxy)-phenyl]-i...)Show InChI InChI=1S/C22H27N3O/c1-18-10-14-25-17-21(23-22(25)16-18)19-6-8-20(9-7-19)26-15-5-13-24-11-3-2-4-12-24/h6-10,14,16-17H,2-5,11-13,15H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Palack£ University
Curated by ChEMBL
| Assay Description
Inhibition of H3R (unknown origin) |
Eur J Med Chem 181: (2019)
Article DOI: 10.1016/j.ejmech.2019.111569
BindingDB Entry DOI: 10.7270/Q2H41VXG |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50562744
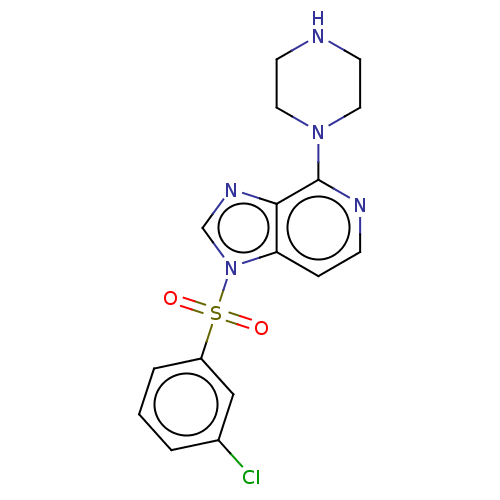
(CHEMBL4777550)Show SMILES Clc1cccc(c1)S(=O)(=O)n1cnc2c(nccc12)N1CCNCC1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]-LSD from human 5HT6R expressed in HEK293 cell membranes incubated for 1 hr by micro-beta plate reader based analysis |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c02009
BindingDB Entry DOI: 10.7270/Q2JQ14RQ |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM50530442
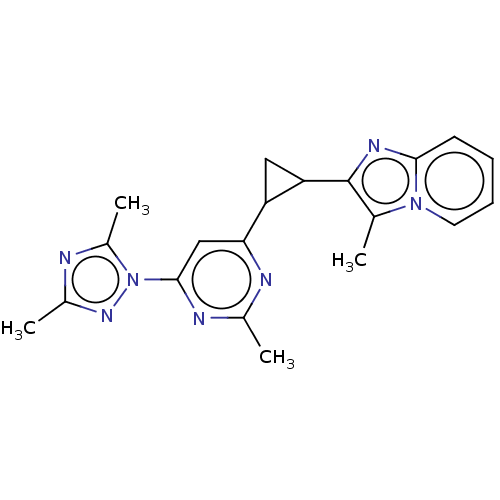
(CHEMBL4461114)Show SMILES Cc1nc(C)n(n1)-c1cc(nc(C)n1)C1CC1c1nc2ccccn2c1C Show InChI InChI=1S/C20H21N7/c1-11-20(24-18-7-5-6-8-26(11)18)16-9-15(16)17-10-19(23-12(2)22-17)27-14(4)21-13(3)25-27/h5-8,10,15-16H,9H2,1-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Palack£ University
Curated by ChEMBL
| Assay Description
Inhibition of human PDE10 |
Eur J Med Chem 181: (2019)
Article DOI: 10.1016/j.ejmech.2019.111569
BindingDB Entry DOI: 10.7270/Q2H41VXG |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM50530442

(CHEMBL4461114)Show SMILES Cc1nc(C)n(n1)-c1cc(nc(C)n1)C1CC1c1nc2ccccn2c1C Show InChI InChI=1S/C20H21N7/c1-11-20(24-18-7-5-6-8-26(11)18)16-9-15(16)17-10-19(23-12(2)22-17)27-14(4)21-13(3)25-27/h5-8,10,15-16H,9H2,1-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Palack£ University
Curated by ChEMBL
| Assay Description
Inhibition of human PDE10 |
Eur J Med Chem 181: (2019)
Article DOI: 10.1016/j.ejmech.2019.111569
BindingDB Entry DOI: 10.7270/Q2H41VXG |
More data for this
Ligand-Target Pair | |
D(4) dopamine receptor
(Homo sapiens (Human)) | BDBM50189845
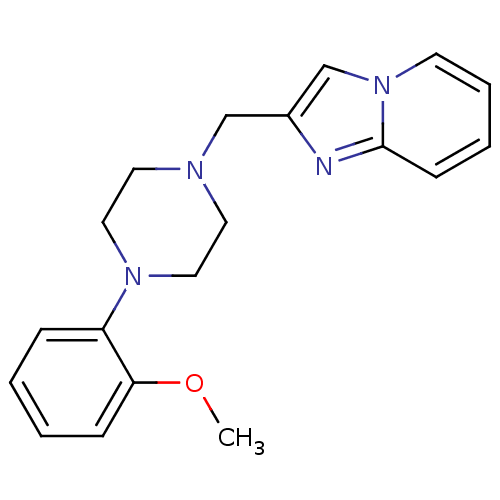
(2-[4-(2-methoxyphenyl)piperazin-1-ylmethyl]imidazo...)Show InChI InChI=1S/C19H22N4O/c1-24-18-7-3-2-6-17(18)22-12-10-21(11-13-22)14-16-15-23-9-5-4-8-19(23)20-16/h2-9,15H,10-14H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Palack£ University
Curated by ChEMBL
| Assay Description
Agonist activity at dopamine D4 receptor (unknown origin) |
Eur J Med Chem 181: (2019)
Article DOI: 10.1016/j.ejmech.2019.111569
BindingDB Entry DOI: 10.7270/Q2H41VXG |
More data for this
Ligand-Target Pair | |
D(4) dopamine receptor
(Homo sapiens (Human)) | BDBM50189845

(2-[4-(2-methoxyphenyl)piperazin-1-ylmethyl]imidazo...)Show InChI InChI=1S/C19H22N4O/c1-24-18-7-3-2-6-17(18)22-12-10-21(11-13-22)14-16-15-23-9-5-4-8-19(23)20-16/h2-9,15H,10-14H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Palack£ University
Curated by ChEMBL
| Assay Description
Agonist activity at dopamine D4 receptor (unknown origin) |
Eur J Med Chem 181: (2019)
Article DOI: 10.1016/j.ejmech.2019.111569
BindingDB Entry DOI: 10.7270/Q2H41VXG |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50562745
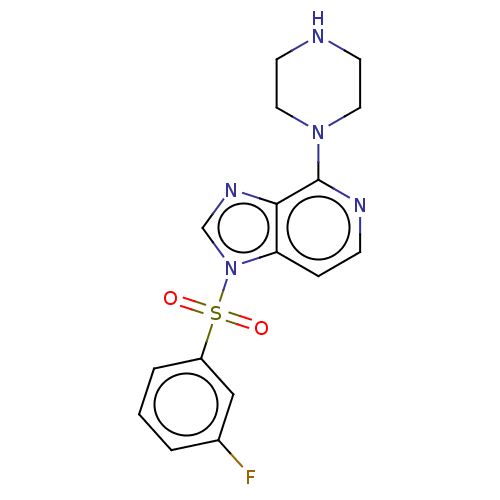
(CHEMBL4743983)Show SMILES Fc1cccc(c1)S(=O)(=O)n1cnc2c(nccc12)N1CCNCC1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]-LSD from human 5HT6R expressed in HEK293 cell membranes incubated for 1 hr by micro-beta plate reader based analysis |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c02009
BindingDB Entry DOI: 10.7270/Q2JQ14RQ |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50562750
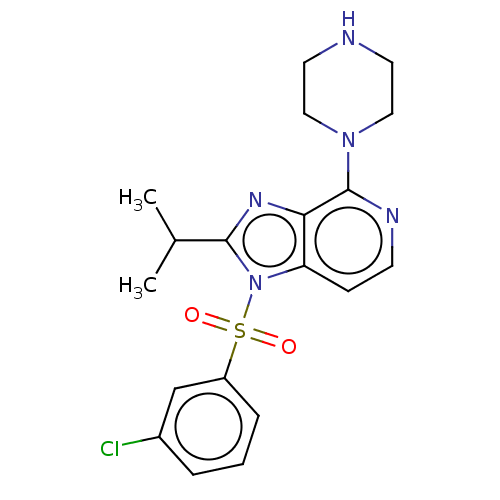
(CHEMBL4742804)Show SMILES CC(C)c1nc2c(nccc2n1S(=O)(=O)c1cccc(Cl)c1)N1CCNCC1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]-LSD from human 5HT6R expressed in HEK293 cell membranes incubated for 1 hr by micro-beta plate reader based analysis |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c02009
BindingDB Entry DOI: 10.7270/Q2JQ14RQ |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50562743
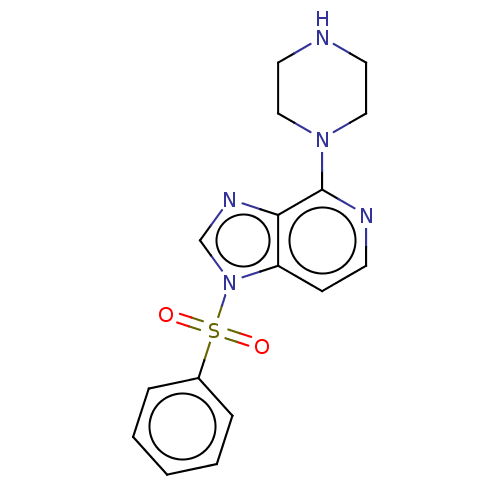
(CHEMBL4759927) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]-LSD from human 5HT6R expressed in HEK293 cell membranes incubated for 1 hr by micro-beta plate reader based analysis |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c02009
BindingDB Entry DOI: 10.7270/Q2JQ14RQ |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data