

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50373455 (CHEMBL261107) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.79E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibition of PTP1B by fluorescein diphosphate assay | Bioorg Med Chem Lett 18: 3200-5 (2008) Article DOI: 10.1016/j.bmcl.2008.04.064 BindingDB Entry DOI: 10.7270/Q24M95DX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM13599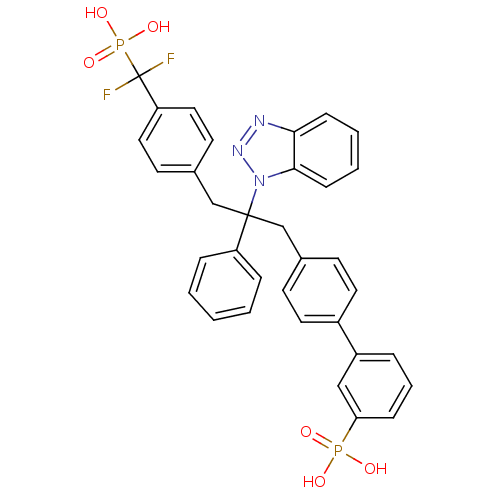 (3-{4-[2-(1H-1,2,3-benzotriazol-1-yl)-3-{4-[difluor...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibitory activity against protein tyrosine phosphatase 1B (PTP1B) was determined in fluorescein diphosphate (FDP) assay | Bioorg Med Chem Lett 14: 1043-8 (2004) Article DOI: 10.1016/j.bmcl.2003.11.076 BindingDB Entry DOI: 10.7270/Q24M9404 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Tyrosine-protein phosphatase non-receptor type 2 (Homo sapiens (Human)) | BDBM13599 (3-{4-[2-(1H-1,2,3-benzotriazol-1-yl)-3-{4-[difluor...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | 6.3 | 22 |
Merck Research Laboratories | Assay Description Hydrolysis of substrate FDP was monitored continuously on a Cytofluor microplate reader with excitation and emission wavelengths set at 440 and 515 ... | Biochemistry 42: 11451-9 (2003) Article DOI: 10.1021/bi035098j BindingDB Entry DOI: 10.7270/Q2HX19XS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 [1-298] (Homo sapiens (Human)) | BDBM13599 (3-{4-[2-(1H-1,2,3-benzotriazol-1-yl)-3-{4-[difluor...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | 6.3 | 22 |
Merck Research Laboratories | Assay Description Hydrolysis of substrate FDP was monitored continuously on a Cytofluor microplate reader with excitation and emission wavelengths set at 440 and 515 ... | Biochemistry 42: 11451-9 (2003) Article DOI: 10.1021/bi035098j BindingDB Entry DOI: 10.7270/Q2HX19XS | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Tyrosine-protein phosphatase non-receptor type 2 (Homo sapiens (Human)) | BDBM13599 (3-{4-[2-(1H-1,2,3-benzotriazol-1-yl)-3-{4-[difluor...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibitory activity against T cell protein tyrosine phosphatase | Bioorg Med Chem Lett 14: 1043-8 (2004) Article DOI: 10.1016/j.bmcl.2003.11.076 BindingDB Entry DOI: 10.7270/Q24M9404 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 2 (Homo sapiens (Human)) | BDBM50142323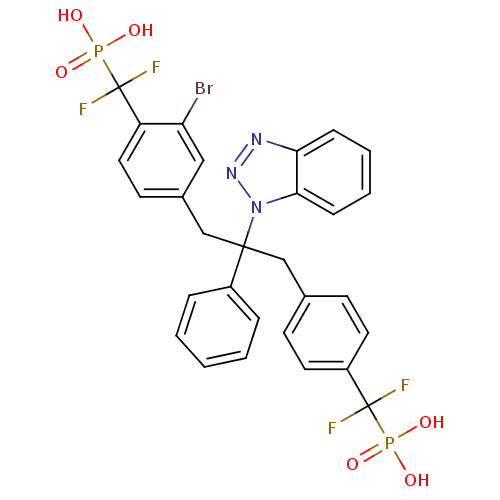 (CHEMBL267488 | [(4-{2-Benzotriazol-1-yl-3-[3-bromo...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibitory activity against T cell protein tyrosine phosphatase | Bioorg Med Chem Lett 14: 1043-8 (2004) Article DOI: 10.1016/j.bmcl.2003.11.076 BindingDB Entry DOI: 10.7270/Q24M9404 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM13604 (6-{4-[2-(1H-1,2,3-benzotriazol-1-yl)-3-{4-[difluor...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibitory activity against protein tyrosine phosphatase 1B (PTP1B) was determined in fluorescein diphosphate (FDP) assay | Bioorg Med Chem Lett 14: 1043-8 (2004) Article DOI: 10.1016/j.bmcl.2003.11.076 BindingDB Entry DOI: 10.7270/Q24M9404 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50142323 (CHEMBL267488 | [(4-{2-Benzotriazol-1-yl-3-[3-bromo...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibitory activity against protein tyrosine phosphatase 1B (PTP1B) was determined in fluorescein diphosphate (FDP) assay | Bioorg Med Chem Lett 14: 1043-8 (2004) Article DOI: 10.1016/j.bmcl.2003.11.076 BindingDB Entry DOI: 10.7270/Q24M9404 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 [1-298] (Homo sapiens (Human)) | BDBM13604 (6-{4-[2-(1H-1,2,3-benzotriazol-1-yl)-3-{4-[difluor...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | 6.3 | 22 |
Merck Research Laboratories | Assay Description Hydrolysis of substrate FDP was monitored continuously on a Cytofluor microplate reader with excitation and emission wavelengths set at 440 and 515 ... | Biochemistry 42: 11451-9 (2003) Article DOI: 10.1021/bi035098j BindingDB Entry DOI: 10.7270/Q2HX19XS | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 [1-298] (Homo sapiens (Human)) | BDBM13603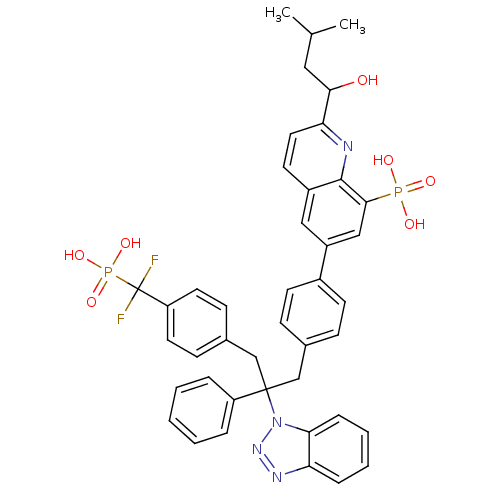 (6-{4-[2-(1H-1,2,3-benzotriazol-1-yl)-3-{4-[difluor...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | 6.3 | 22 |
Merck Research Laboratories | Assay Description Hydrolysis of substrate FDP was monitored continuously on a Cytofluor microplate reader with excitation and emission wavelengths set at 440 and 515 ... | Biochemistry 42: 11451-9 (2003) Article DOI: 10.1021/bi035098j BindingDB Entry DOI: 10.7270/Q2HX19XS | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 [1-298] (Homo sapiens (Human)) | BDBM13601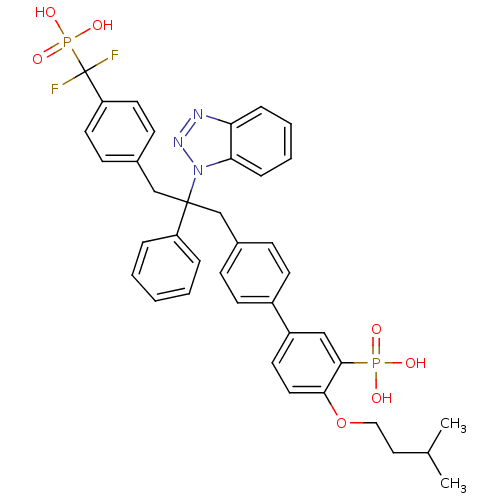 (5-{4-[2-(1H-1,2,3-benzotriazol-1-yl)-3-{4-[difluor...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | 6.3 | 22 |
Merck Research Laboratories | Assay Description Hydrolysis of substrate FDP was monitored continuously on a Cytofluor microplate reader with excitation and emission wavelengths set at 440 and 515 ... | Biochemistry 42: 11451-9 (2003) Article DOI: 10.1021/bi035098j BindingDB Entry DOI: 10.7270/Q2HX19XS | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM13604 (6-{4-[2-(1H-1,2,3-benzotriazol-1-yl)-3-{4-[difluor...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibitory activity against protein tyrosine phosphatase 1B (PTP1B) was determined in fluorescein diphosphate (FDP) assay | Bioorg Med Chem Lett 14: 1043-8 (2004) Article DOI: 10.1016/j.bmcl.2003.11.076 BindingDB Entry DOI: 10.7270/Q24M9404 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50373451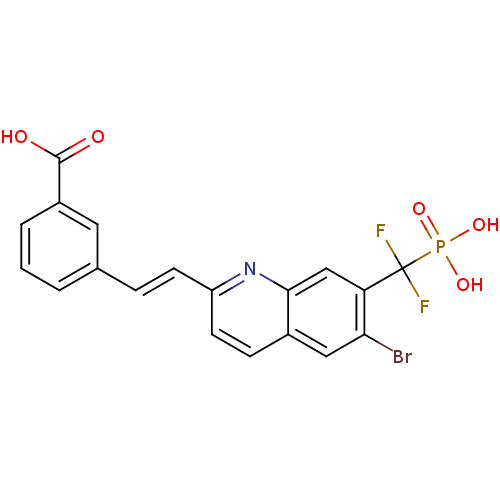 (CHEMBL411295) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibition of PTP1B by fluorescein diphosphate assay | Bioorg Med Chem Lett 18: 3200-5 (2008) Article DOI: 10.1016/j.bmcl.2008.04.064 BindingDB Entry DOI: 10.7270/Q24M95DX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 2 (Homo sapiens (Human)) | BDBM13596 (({4-[2-(1H-1,2,3-benzotriazol-1-yl)-3-{4-[difluoro...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibitory activity against T cell protein tyrosine phosphatase | Bioorg Med Chem Lett 14: 1043-8 (2004) Article DOI: 10.1016/j.bmcl.2003.11.076 BindingDB Entry DOI: 10.7270/Q24M9404 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Rattus norvegicus) | BDBM50000534 (2-Phenethyl-6-[3-(pyridin-4-ylsulfanyl)-propyl]-2,...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre For Therapeutic Research Curated by ChEMBL | Assay Description Tested for its inhibitory activity against arachidonic acid in rat 5-lipoxygenase. | J Med Chem 35: 1299-318 (1992) BindingDB Entry DOI: 10.7270/Q25X27WS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 2 (Homo sapiens (Human)) | BDBM13595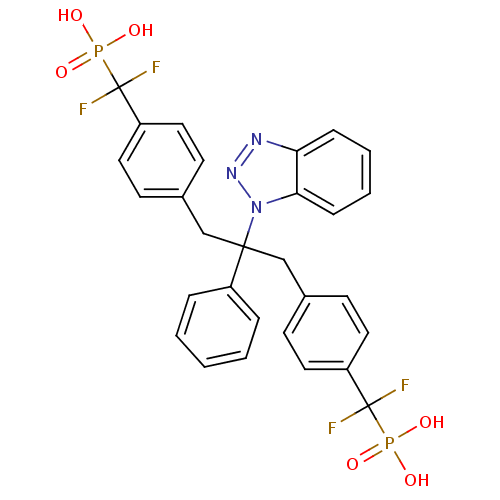 (({4-[2-(1H-1,2,3-benzotriazol-1-yl)-3-{4-[difluoro...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibitory activity against T cell protein tyrosine phosphatase | Bioorg Med Chem Lett 14: 1043-8 (2004) Article DOI: 10.1016/j.bmcl.2003.11.076 BindingDB Entry DOI: 10.7270/Q24M9404 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 [1-298] (Homo sapiens (Human)) | BDBM13600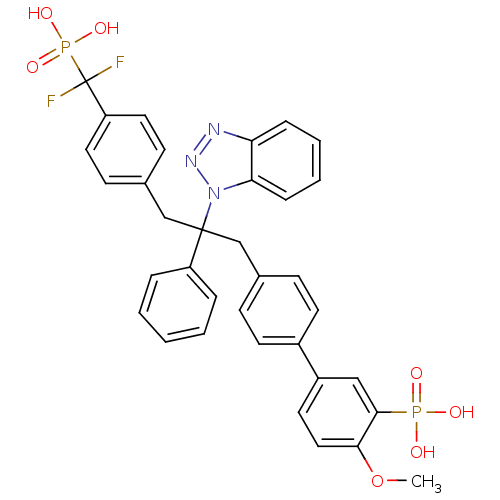 (5-{4-[2-(1H-1,2,3-benzotriazol-1-yl)-3-{4-[difluor...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | 6.3 | 22 |
Merck Research Laboratories | Assay Description Hydrolysis of substrate FDP was monitored continuously on a Cytofluor microplate reader with excitation and emission wavelengths set at 440 and 515 ... | Biochemistry 42: 11451-9 (2003) Article DOI: 10.1021/bi035098j BindingDB Entry DOI: 10.7270/Q2HX19XS | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Tyrosine-protein phosphatase non-receptor type 2 (Homo sapiens (Human)) | BDBM50142335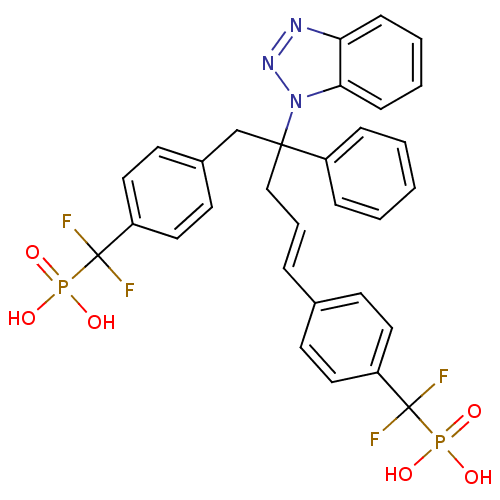 (CHEMBL274435 | [(4-{4-Benzotriazol-1-yl-5-[4-(difl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibitory activity against T cell protein tyrosine phosphatase | Bioorg Med Chem Lett 14: 1043-8 (2004) Article DOI: 10.1016/j.bmcl.2003.11.076 BindingDB Entry DOI: 10.7270/Q24M9404 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 2 (Homo sapiens (Human)) | BDBM13595 (({4-[2-(1H-1,2,3-benzotriazol-1-yl)-3-{4-[difluoro...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | 6.3 | 22 |
Merck Research Laboratories | Assay Description Hydrolysis of substrate FDP was monitored continuously on a Cytofluor microplate reader with excitation and emission wavelengths set at 440 and 515 ... | Biochemistry 42: 11451-9 (2003) Article DOI: 10.1021/bi035098j BindingDB Entry DOI: 10.7270/Q2HX19XS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50142328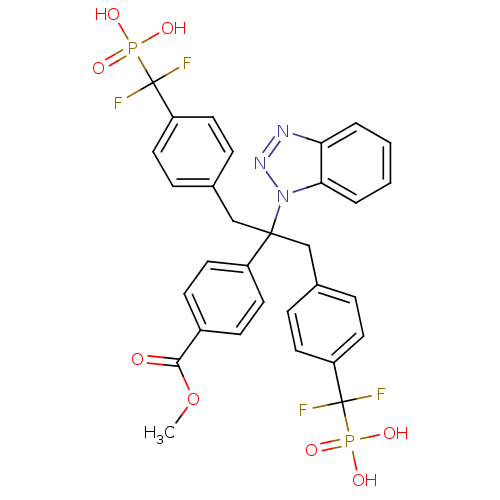 (4-{1-Benzotriazol-1-yl-1-[4-(difluoro-phosphono-me...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibitory activity against Protein tyrosine phosphatase 1B (PTP1B) overexpressed in intact Sf9 cell assay | Bioorg Med Chem Lett 14: 1043-8 (2004) Article DOI: 10.1016/j.bmcl.2003.11.076 BindingDB Entry DOI: 10.7270/Q24M9404 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 [1-298] (Homo sapiens (Human)) | BDBM13605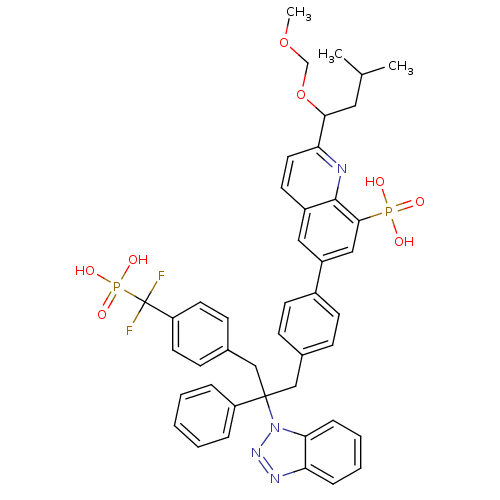 (6-{4-[2-(1H-1,2,3-benzotriazol-1-yl)-3-{4-[difluor...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | 6.3 | 22 |
Merck Research Laboratories | Assay Description Hydrolysis of substrate FDP was monitored continuously on a Cytofluor microplate reader with excitation and emission wavelengths set at 440 and 515 ... | Biochemistry 42: 11451-9 (2003) Article DOI: 10.1021/bi035098j BindingDB Entry DOI: 10.7270/Q2HX19XS | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM13602 (6-{4-[2-(1H-1,2,3-benzotriazol-1-yl)-3-{4-[difluor...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibitory activity against Protein tyrosine phosphatase 1B (PTP1B) overexpressed in intact Sf9 cell assay | Bioorg Med Chem Lett 14: 1043-8 (2004) Article DOI: 10.1016/j.bmcl.2003.11.076 BindingDB Entry DOI: 10.7270/Q24M9404 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 [1-298] (Homo sapiens (Human)) | BDBM13602 (6-{4-[2-(1H-1,2,3-benzotriazol-1-yl)-3-{4-[difluor...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | 6.3 | 22 |
Merck Research Laboratories | Assay Description Hydrolysis of substrate FDP was monitored continuously on a Cytofluor microplate reader with excitation and emission wavelengths set at 440 and 515 ... | Biochemistry 42: 11451-9 (2003) Article DOI: 10.1021/bi035098j BindingDB Entry DOI: 10.7270/Q2HX19XS | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM13596 (({4-[2-(1H-1,2,3-benzotriazol-1-yl)-3-{4-[difluoro...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibitory activity against protein tyrosine phosphatase 1B (PTP1B) was determined in fluorescein diphosphate (FDP) assay | Bioorg Med Chem Lett 14: 1043-8 (2004) Article DOI: 10.1016/j.bmcl.2003.11.076 BindingDB Entry DOI: 10.7270/Q24M9404 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 [1-298] (Homo sapiens (Human)) | BDBM13596 (({4-[2-(1H-1,2,3-benzotriazol-1-yl)-3-{4-[difluoro...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank MMDB PDB Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | 6.3 | 22 |
Merck Research Laboratories | Assay Description Hydrolysis of substrate FDP was monitored continuously on a Cytofluor microplate reader with excitation and emission wavelengths set at 440 and 515 ... | Biochemistry 42: 11451-9 (2003) Article DOI: 10.1021/bi035098j BindingDB Entry DOI: 10.7270/Q2HX19XS | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 [1-298] (Homo sapiens (Human)) | BDBM13595 (({4-[2-(1H-1,2,3-benzotriazol-1-yl)-3-{4-[difluoro...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | 6.3 | 22 |
Merck Research Laboratories | Assay Description Hydrolysis of substrate FDP was monitored continuously on a Cytofluor microplate reader with excitation and emission wavelengths set at 440 and 515 ... | Biochemistry 42: 11451-9 (2003) Article DOI: 10.1021/bi035098j BindingDB Entry DOI: 10.7270/Q2HX19XS | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50142335 (CHEMBL274435 | [(4-{4-Benzotriazol-1-yl-5-[4-(difl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibitory activity against protein tyrosine phosphatase 1B (PTP1B) was determined in fluorescein diphosphate (FDP) assay | Bioorg Med Chem Lett 14: 1043-8 (2004) Article DOI: 10.1016/j.bmcl.2003.11.076 BindingDB Entry DOI: 10.7270/Q24M9404 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Tyrosine-protein phosphatase non-receptor type 2 (Homo sapiens (Human)) | BDBM50142328 (4-{1-Benzotriazol-1-yl-1-[4-(difluoro-phosphono-me...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibitory activity against T cell protein tyrosine phosphatase | Bioorg Med Chem Lett 14: 1043-8 (2004) Article DOI: 10.1016/j.bmcl.2003.11.076 BindingDB Entry DOI: 10.7270/Q24M9404 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50373441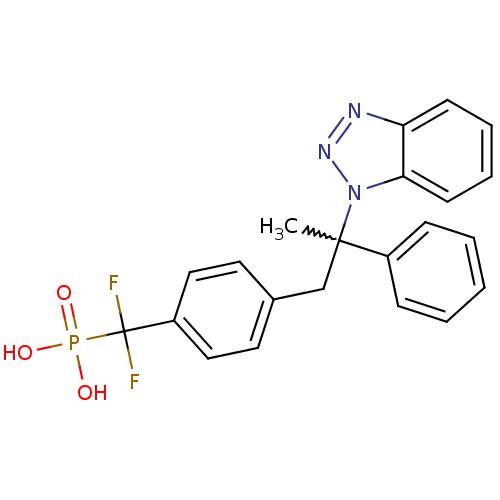 (CHEMBL410646) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibition of PTP1B by fluorescein diphosphate assay | Bioorg Med Chem Lett 18: 3200-5 (2008) Article DOI: 10.1016/j.bmcl.2008.04.064 BindingDB Entry DOI: 10.7270/Q24M95DX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 2 (Homo sapiens (Human)) | BDBM50142324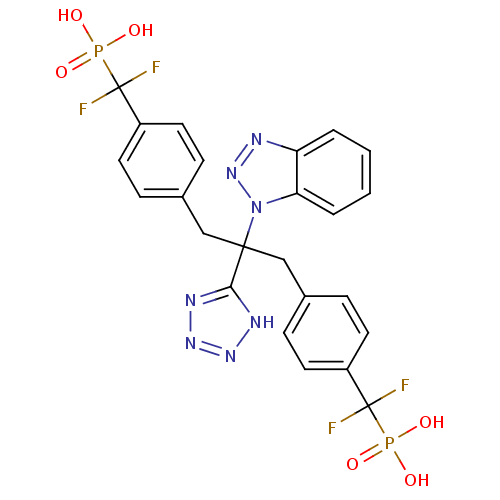 (({4-[2-Benzotriazol-1-yl-3-[4-(difluoro-phosphono-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibitory activity against T cell protein tyrosine phosphatase | Bioorg Med Chem Lett 14: 1043-8 (2004) Article DOI: 10.1016/j.bmcl.2003.11.076 BindingDB Entry DOI: 10.7270/Q24M9404 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM13595 (({4-[2-(1H-1,2,3-benzotriazol-1-yl)-3-{4-[difluoro...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibitory activity against protein tyrosine phosphatase 1B (PTP1B) was determined in fluorescein diphosphate (FDP) assay | Bioorg Med Chem Lett 14: 1043-8 (2004) Article DOI: 10.1016/j.bmcl.2003.11.076 BindingDB Entry DOI: 10.7270/Q24M9404 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Rattus norvegicus) | BDBM50000536 (6-Allyl-2-phenethyl-2,3-dihydro-benzofuran-5-ol | ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre For Therapeutic Research Curated by ChEMBL | Assay Description Tested for its inhibitory activity against arachidonic acid in rat 5-lipoxygenase. | J Med Chem 35: 1299-318 (1992) BindingDB Entry DOI: 10.7270/Q25X27WS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 2 (Homo sapiens (Human)) | BDBM13603 (6-{4-[2-(1H-1,2,3-benzotriazol-1-yl)-3-{4-[difluor...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | 6.3 | 22 |
Merck Research Laboratories | Assay Description Hydrolysis of substrate FDP was monitored continuously on a Cytofluor microplate reader with excitation and emission wavelengths set at 440 and 515 ... | Biochemistry 42: 11451-9 (2003) Article DOI: 10.1021/bi035098j BindingDB Entry DOI: 10.7270/Q2HX19XS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50142330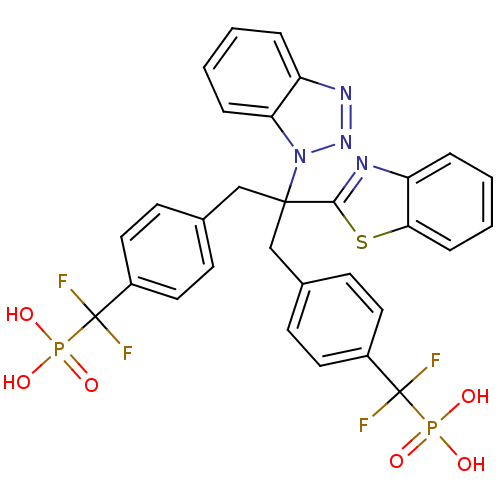 (CHEMBL8662 | [(4-{2-Benzothiazol-2-yl-2-benzotriaz...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibitory activity against protein tyrosine phosphatase 1B (PTP1B) was determined in fluorescein diphosphate (FDP) assay | Bioorg Med Chem Lett 14: 1043-8 (2004) Article DOI: 10.1016/j.bmcl.2003.11.076 BindingDB Entry DOI: 10.7270/Q24M9404 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Tyrosine-protein phosphatase non-receptor type 2 (Homo sapiens (Human)) | BDBM50142329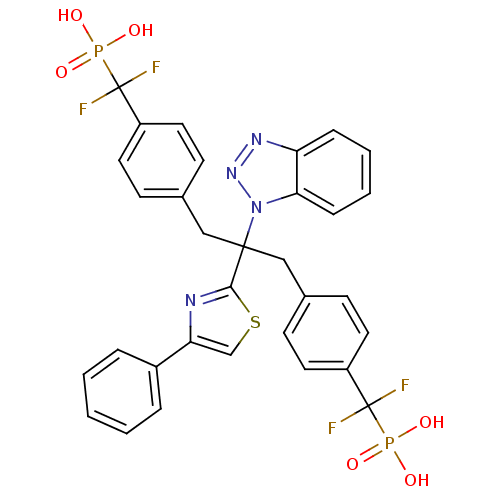 (({4-[2-Benzotriazol-1-yl-3-[4-(difluoro-phosphono-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibitory activity against T cell protein tyrosine phosphatase | Bioorg Med Chem Lett 14: 1043-8 (2004) Article DOI: 10.1016/j.bmcl.2003.11.076 BindingDB Entry DOI: 10.7270/Q24M9404 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50142329 (({4-[2-Benzotriazol-1-yl-3-[4-(difluoro-phosphono-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibitory activity against protein tyrosine phosphatase 1B (PTP1B) was determined in fluorescein diphosphate (FDP) assay | Bioorg Med Chem Lett 14: 1043-8 (2004) Article DOI: 10.1016/j.bmcl.2003.11.076 BindingDB Entry DOI: 10.7270/Q24M9404 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Tyrosine-protein phosphatase non-receptor type 2 (Homo sapiens (Human)) | BDBM13602 (6-{4-[2-(1H-1,2,3-benzotriazol-1-yl)-3-{4-[difluor...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibitory activity against T cell protein tyrosine phosphatase | Bioorg Med Chem Lett 14: 1043-8 (2004) Article DOI: 10.1016/j.bmcl.2003.11.076 BindingDB Entry DOI: 10.7270/Q24M9404 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 2 (Homo sapiens (Human)) | BDBM50142330 (CHEMBL8662 | [(4-{2-Benzothiazol-2-yl-2-benzotriaz...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibitory activity against T cell protein tyrosine phosphatase | Bioorg Med Chem Lett 14: 1043-8 (2004) Article DOI: 10.1016/j.bmcl.2003.11.076 BindingDB Entry DOI: 10.7270/Q24M9404 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50142325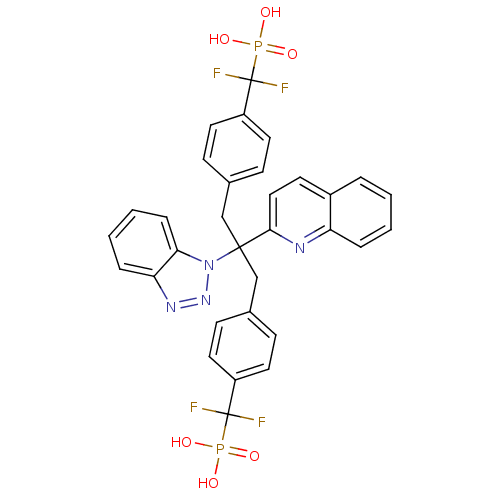 (CHEMBL273474 | [(4-{2-Benzotriazol-1-yl-3-[4-(difl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibitory activity against protein tyrosine phosphatase 1B (PTP1B) was determined in fluorescein diphosphate (FDP) assay | Bioorg Med Chem Lett 14: 1043-8 (2004) Article DOI: 10.1016/j.bmcl.2003.11.076 BindingDB Entry DOI: 10.7270/Q24M9404 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Tyrosine-protein phosphatase non-receptor type 2 (Homo sapiens (Human)) | BDBM13602 (6-{4-[2-(1H-1,2,3-benzotriazol-1-yl)-3-{4-[difluor...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | 6.3 | 22 |
Merck Research Laboratories | Assay Description Hydrolysis of substrate FDP was monitored continuously on a Cytofluor microplate reader with excitation and emission wavelengths set at 440 and 515 ... | Biochemistry 42: 11451-9 (2003) Article DOI: 10.1021/bi035098j BindingDB Entry DOI: 10.7270/Q2HX19XS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 2 (Homo sapiens (Human)) | BDBM13601 (5-{4-[2-(1H-1,2,3-benzotriazol-1-yl)-3-{4-[difluor...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | 6.3 | 22 |
Merck Research Laboratories | Assay Description Hydrolysis of substrate FDP was monitored continuously on a Cytofluor microplate reader with excitation and emission wavelengths set at 440 and 515 ... | Biochemistry 42: 11451-9 (2003) Article DOI: 10.1021/bi035098j BindingDB Entry DOI: 10.7270/Q2HX19XS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 2 (Homo sapiens (Human)) | BDBM13605 (6-{4-[2-(1H-1,2,3-benzotriazol-1-yl)-3-{4-[difluor...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 21 | n/a | n/a | n/a | n/a | 6.3 | 22 |
Merck Research Laboratories | Assay Description Hydrolysis of substrate FDP was monitored continuously on a Cytofluor microplate reader with excitation and emission wavelengths set at 440 and 515 ... | Biochemistry 42: 11451-9 (2003) Article DOI: 10.1021/bi035098j BindingDB Entry DOI: 10.7270/Q2HX19XS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 [1-298] (Homo sapiens (Human)) | BDBM13597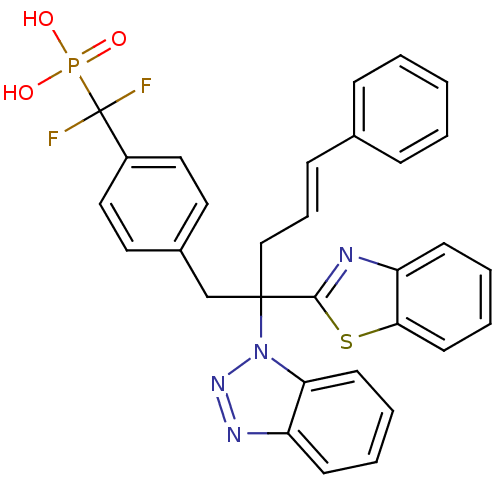 (({4-[(4E)-2-(1,3-benzothiazol-2-yl)-2-(1H-1,2,3-be...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | 6.3 | 22 |
Merck Research Laboratories | Assay Description Hydrolysis of substrate FDP was monitored continuously on a Cytofluor microplate reader with excitation and emission wavelengths set at 440 and 515 ... | Biochemistry 42: 11451-9 (2003) Article DOI: 10.1021/bi035098j BindingDB Entry DOI: 10.7270/Q2HX19XS | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Rattus norvegicus) | BDBM50000526 (2-Phenethyl-6-(3-phenoxy-propyl)-2,3-dihydro-benzo...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre For Therapeutic Research Curated by ChEMBL | Assay Description Tested for its inhibitory activity against arachidonic acid in rat 5-lipoxygenase. | J Med Chem 35: 1299-318 (1992) BindingDB Entry DOI: 10.7270/Q25X27WS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 2 (Homo sapiens (Human)) | BDBM13598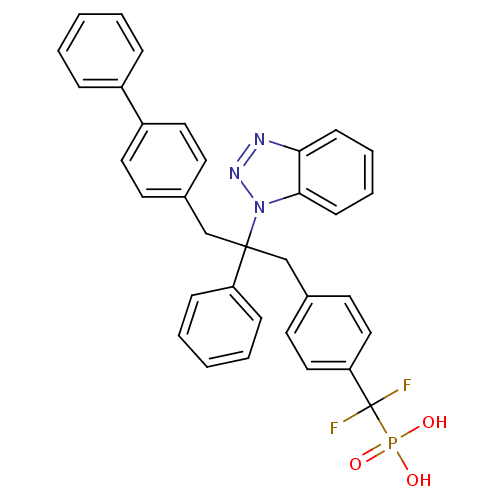 (({4-[2-(1H-1,2,3-benzotriazol-1-yl)-2-phenyl-3-(4-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 24 | n/a | n/a | n/a | n/a | 6.3 | 22 |
Merck Research Laboratories | Assay Description Hydrolysis of substrate FDP was monitored continuously on a Cytofluor microplate reader with excitation and emission wavelengths set at 440 and 515 ... | Biochemistry 42: 11451-9 (2003) Article DOI: 10.1021/bi035098j BindingDB Entry DOI: 10.7270/Q2HX19XS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 2 (Homo sapiens (Human)) | BDBM13598 (({4-[2-(1H-1,2,3-benzotriazol-1-yl)-2-phenyl-3-(4-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibitory activity against T cell protein tyrosine phosphatase | Bioorg Med Chem Lett 14: 1043-8 (2004) Article DOI: 10.1016/j.bmcl.2003.11.076 BindingDB Entry DOI: 10.7270/Q24M9404 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 2 [V121L] (Homo sapiens (Human)) | BDBM13814 (({4-[(4E)-2-(1H-1,2,3-benzotriazol-1-yl)-2-[4-(met...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 24 | n/a | n/a | n/a | n/a | 6.3 | 22 |
Merck Frosst Center for Therapeutic Research | Assay Description Hydrolysis of substrate FDP was monitored continuously on a Cytofluor microplate reader with excitation and emission wavelengths set at 440 and 515 ... | J Biol Chem 281: 8010-5 (2006) Article DOI: 10.1074/jbc.M511827200 BindingDB Entry DOI: 10.7270/Q2C53J3V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Rattus norvegicus) | BDBM50000551 (4-Allyl-2-phenethyl-2,3-dihydro-benzofuran-5-ol | ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre For Therapeutic Research Curated by ChEMBL | Assay Description Tested for its inhibitory activity against arachidonic acid in rat 5-lipoxygenase. | J Med Chem 35: 1299-318 (1992) BindingDB Entry DOI: 10.7270/Q25X27WS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50373449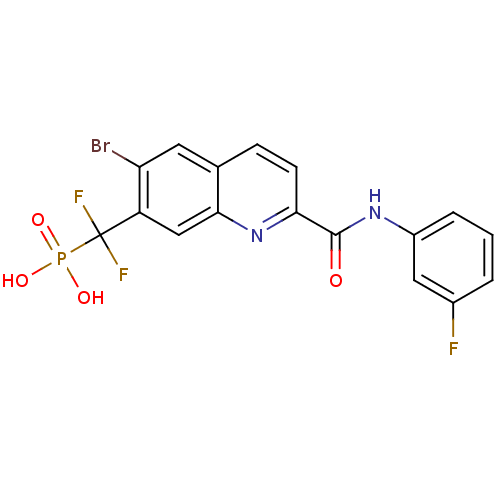 (CHEMBL261362) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibition of PTP1B by fluorescein diphosphate assay | Bioorg Med Chem Lett 18: 3200-5 (2008) Article DOI: 10.1016/j.bmcl.2008.04.064 BindingDB Entry DOI: 10.7270/Q24M95DX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 2 (Homo sapiens (Human)) | BDBM50142325 (CHEMBL273474 | [(4-{2-Benzotriazol-1-yl-3-[4-(difl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibitory activity against T cell protein tyrosine phosphatase | Bioorg Med Chem Lett 14: 1043-8 (2004) Article DOI: 10.1016/j.bmcl.2003.11.076 BindingDB Entry DOI: 10.7270/Q24M9404 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 189 total ) | Next | Last >> |