

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM433537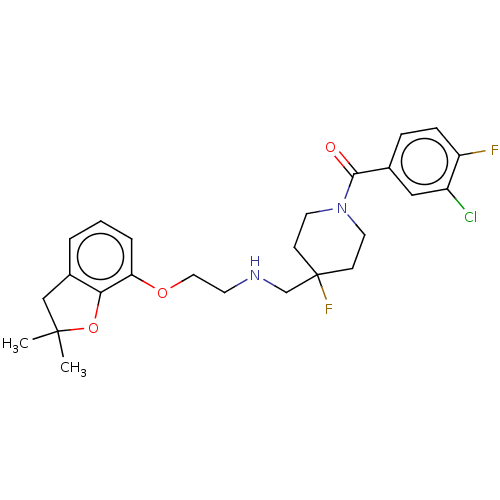 (US10562853, Compound 60) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NEUROLIXIS; UNIVERSITE JAGELLONE US Patent | Assay Description 5-HT1A: Radioligand binding was performed using membranes from CHO-K1 cells stably transfected with the human 5-HT1A receptor. All assays were carrie... | US Patent US10562853 (2020) BindingDB Entry DOI: 10.7270/Q29Z979D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50555494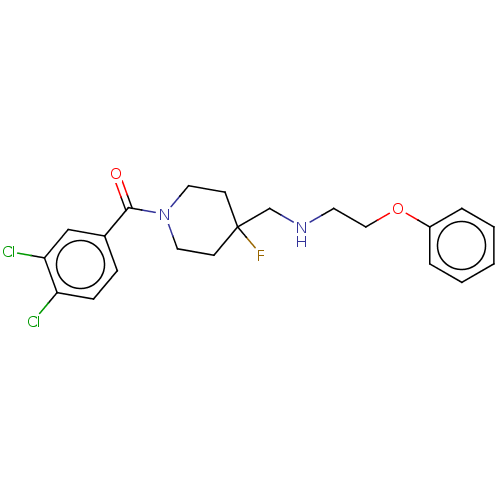 (CHEMBL4742112) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0148 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]8-OH-DPAT from recombinant human 5HT1A receptor stably expressed in CHO-K1 cell membranes measured after 60 mins by microbeta2 sc... | Citation and Details Article DOI: 10.1021/acs.jmedchem.9b00062 BindingDB Entry DOI: 10.7270/Q23F4T9H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM433479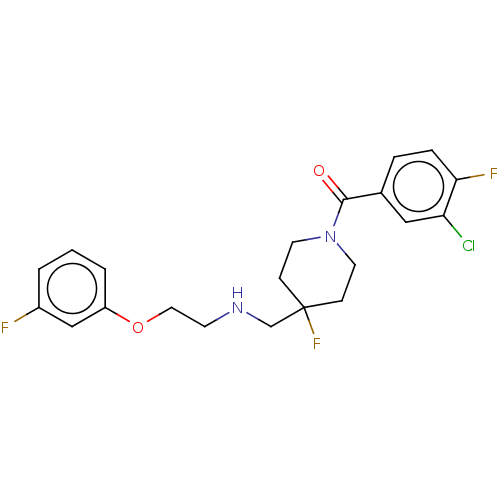 (US10562853, Compound 5) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NEUROLIXIS; UNIVERSITE JAGELLONE US Patent | Assay Description 5-HT1A: Radioligand binding was performed using membranes from CHO-K1 cells stably transfected with the human 5-HT1A receptor. All assays were carrie... | US Patent US10562853 (2020) BindingDB Entry DOI: 10.7270/Q29Z979D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM433491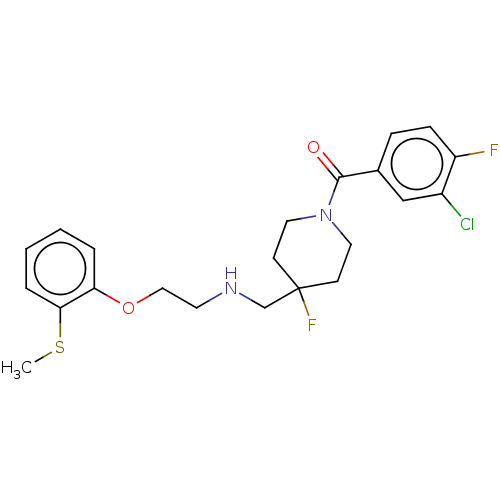 (US10562853, Compound 12) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NEUROLIXIS; UNIVERSITE JAGELLONE US Patent | Assay Description 5-HT1A: Radioligand binding was performed using membranes from CHO-K1 cells stably transfected with the human 5-HT1A receptor. All assays were carrie... | US Patent US10562853 (2020) BindingDB Entry DOI: 10.7270/Q29Z979D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM433554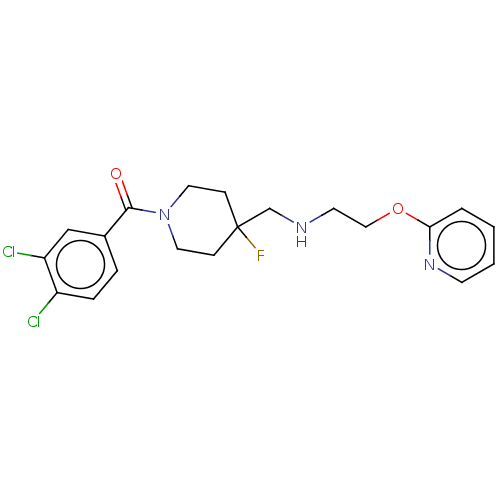 (US10562853, Compound 74) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0339 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]8-OH-DPAT from recombinant human 5HT1A receptor stably expressed in CHO-K1 cell membranes measured after 60 mins by microbeta2 sc... | Citation and Details Article DOI: 10.1021/acs.jmedchem.9b00062 BindingDB Entry DOI: 10.7270/Q23F4T9H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM433480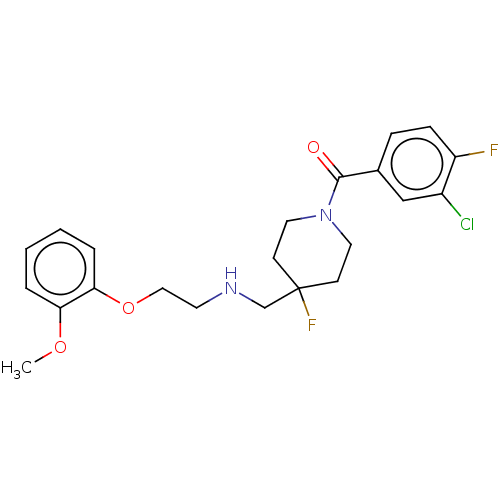 (US10562853, Compound 6) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NEUROLIXIS; UNIVERSITE JAGELLONE US Patent | Assay Description 5-HT1A: Radioligand binding was performed using membranes from CHO-K1 cells stably transfected with the human 5-HT1A receptor. All assays were carrie... | US Patent US10562853 (2020) BindingDB Entry DOI: 10.7270/Q29Z979D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM433478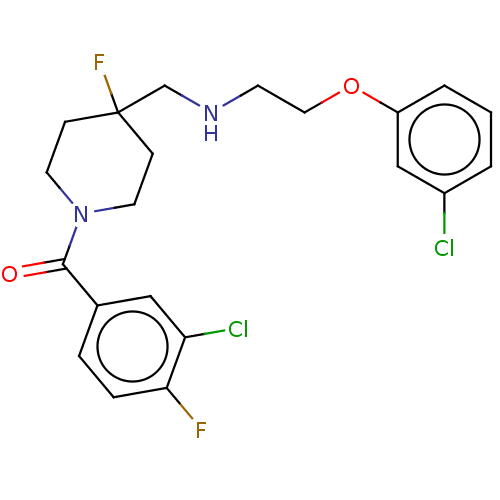 (US10562853, Compound 4) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NEUROLIXIS; UNIVERSITE JAGELLONE US Patent | Assay Description 5-HT1A: Radioligand binding was performed using membranes from CHO-K1 cells stably transfected with the human 5-HT1A receptor. All assays were carrie... | US Patent US10562853 (2020) BindingDB Entry DOI: 10.7270/Q29Z979D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM433549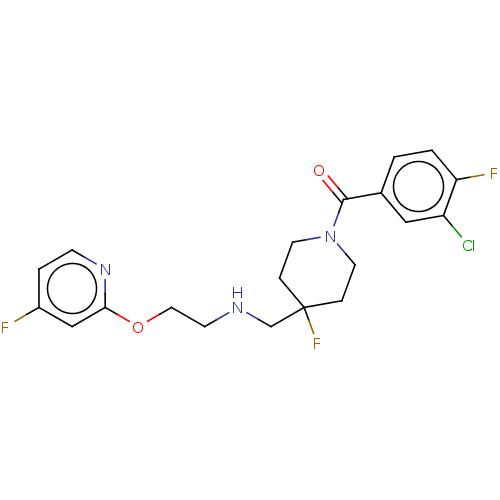 (US10562853, Compound 70) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NEUROLIXIS; UNIVERSITE JAGELLONE US Patent | Assay Description 5-HT1A: Radioligand binding was performed using membranes from CHO-K1 cells stably transfected with the human 5-HT1A receptor. All assays were carrie... | US Patent US10562853 (2020) BindingDB Entry DOI: 10.7270/Q29Z979D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM433483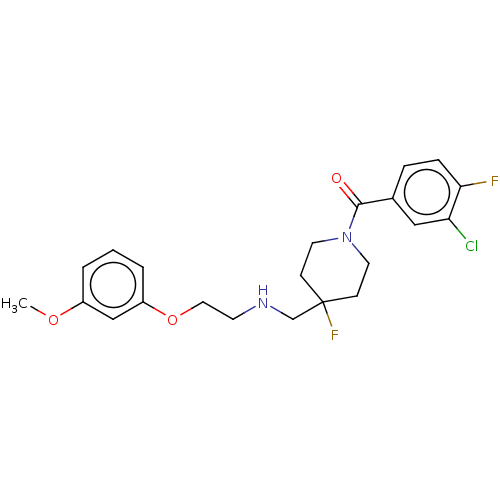 (US10562853, Compound 8) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0520 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NEUROLIXIS; UNIVERSITE JAGELLONE US Patent | Assay Description 5-HT1A: Radioligand binding was performed using membranes from CHO-K1 cells stably transfected with the human 5-HT1A receptor. All assays were carrie... | US Patent US10562853 (2020) BindingDB Entry DOI: 10.7270/Q29Z979D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM433484 (US10562853, Compound 9) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0570 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NEUROLIXIS; UNIVERSITE JAGELLONE US Patent | Assay Description 5-HT1A: Radioligand binding was performed using membranes from CHO-K1 cells stably transfected with the human 5-HT1A receptor. All assays were carrie... | US Patent US10562853 (2020) BindingDB Entry DOI: 10.7270/Q29Z979D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM433474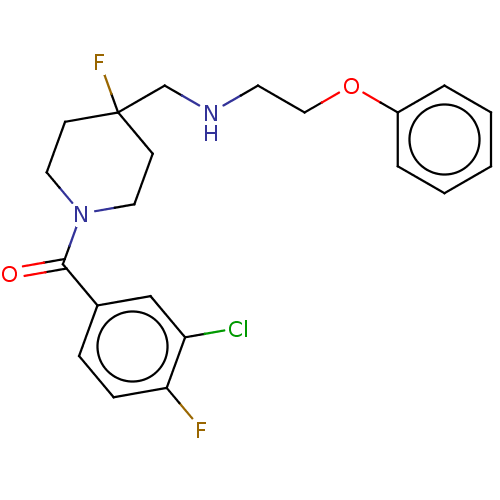 (US10562853, Compound 1) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0610 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NEUROLIXIS; UNIVERSITE JAGELLONE US Patent | Assay Description 5-HT1A: Radioligand binding was performed using membranes from CHO-K1 cells stably transfected with the human 5-HT1A receptor. All assays were carrie... | US Patent US10562853 (2020) BindingDB Entry DOI: 10.7270/Q29Z979D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM433474 (US10562853, Compound 1) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0617 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]8-OH-DPAT from recombinant human 5HT1A receptor stably expressed in CHO-K1 cell membranes measured after 60 mins by microbeta2 sc... | Citation and Details Article DOI: 10.1021/acs.jmedchem.9b00062 BindingDB Entry DOI: 10.7270/Q23F4T9H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM433501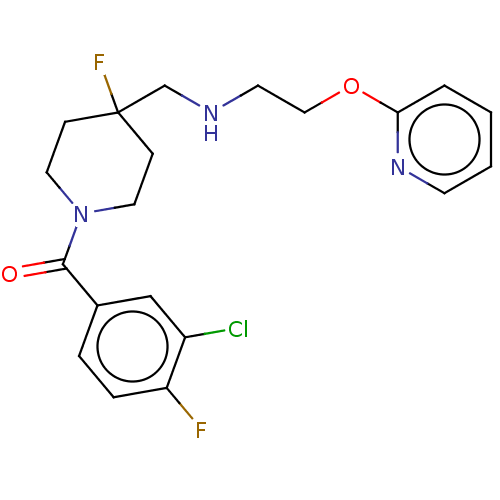 (US10562853, Compound 18) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 0.0646 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]8-OH-DPAT from recombinant human 5HT1A receptor stably expressed in CHO-K1 cell membranes measured after 60 mins by microbeta2 sc... | Citation and Details Article DOI: 10.1021/acs.jmedchem.9b00062 BindingDB Entry DOI: 10.7270/Q23F4T9H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM433501 (US10562853, Compound 18) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | US Patent | 0.0650 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NEUROLIXIS; UNIVERSITE JAGELLONE US Patent | Assay Description 5-HT1A: Radioligand binding was performed using membranes from CHO-K1 cells stably transfected with the human 5-HT1A receptor. All assays were carrie... | US Patent US10562853 (2020) BindingDB Entry DOI: 10.7270/Q29Z979D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM433477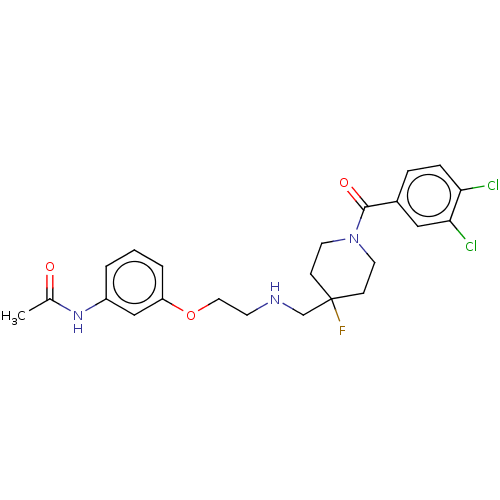 (US10562853, Compound 3) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0730 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NEUROLIXIS; UNIVERSITE JAGELLONE US Patent | Assay Description 5-HT1A: Radioligand binding was performed using membranes from CHO-K1 cells stably transfected with the human 5-HT1A receptor. All assays were carrie... | US Patent US10562853 (2020) BindingDB Entry DOI: 10.7270/Q29Z979D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM433508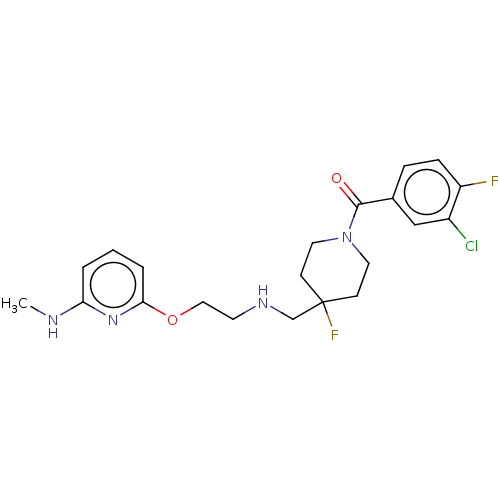 (US10562853, Compound 26) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NEUROLIXIS; UNIVERSITE JAGELLONE US Patent | Assay Description 5-HT1A: Radioligand binding was performed using membranes from CHO-K1 cells stably transfected with the human 5-HT1A receptor. All assays were carrie... | US Patent US10562853 (2020) BindingDB Entry DOI: 10.7270/Q29Z979D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM433509 (US10562853, Compound 27) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NEUROLIXIS; UNIVERSITE JAGELLONE US Patent | Assay Description 5-HT1A: Radioligand binding was performed using membranes from CHO-K1 cells stably transfected with the human 5-HT1A receptor. All assays were carrie... | US Patent US10562853 (2020) BindingDB Entry DOI: 10.7270/Q29Z979D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM433476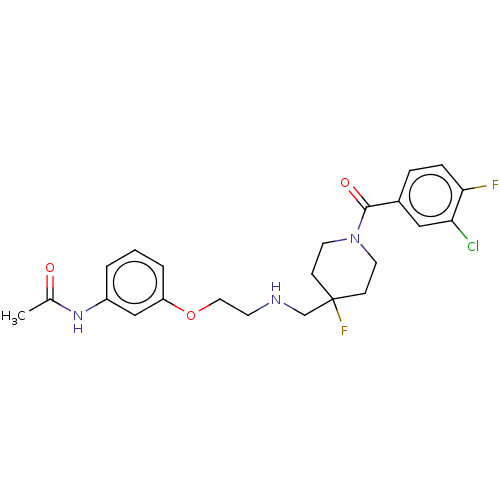 (US10562853, Compound 2) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0830 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NEUROLIXIS; UNIVERSITE JAGELLONE US Patent | Assay Description 5-HT1A: Radioligand binding was performed using membranes from CHO-K1 cells stably transfected with the human 5-HT1A receptor. All assays were carrie... | US Patent US10562853 (2020) BindingDB Entry DOI: 10.7270/Q29Z979D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50041043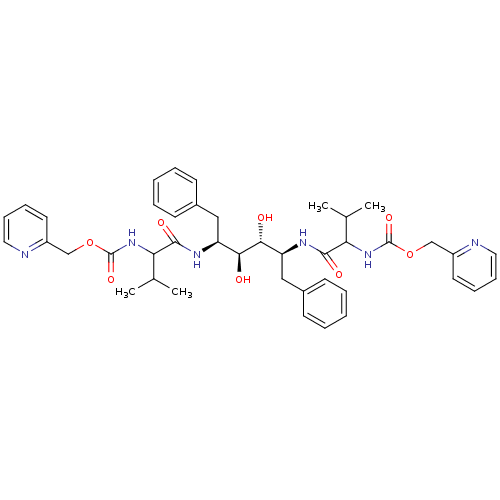 ((1-{(1S,2S,3R,4S)-1-Benzyl-2,3-dihydroxy-4-[3-meth...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for the inhibitory activity against HIV-1 protease | J Med Chem 37: 1035-54 (1994) BindingDB Entry DOI: 10.7270/Q26Q1XWX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM433546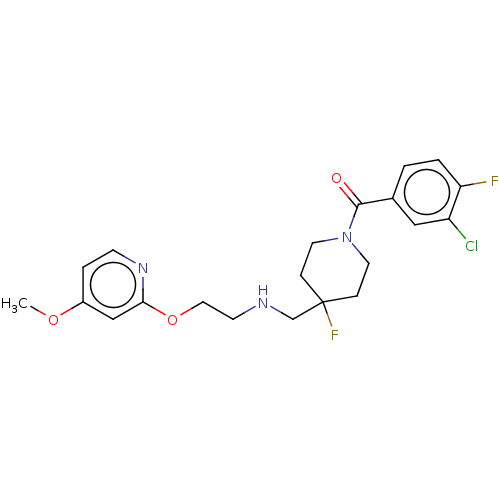 (US10562853, Compound 68) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NEUROLIXIS; UNIVERSITE JAGELLONE US Patent | Assay Description 5-HT1A: Radioligand binding was performed using membranes from CHO-K1 cells stably transfected with the human 5-HT1A receptor. All assays were carrie... | US Patent US10562853 (2020) BindingDB Entry DOI: 10.7270/Q29Z979D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM433523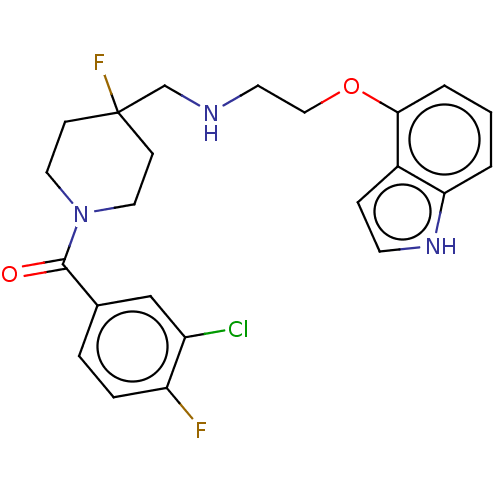 (US10562853, Compound 44) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NEUROLIXIS; UNIVERSITE JAGELLONE US Patent | Assay Description 5-HT1A: Radioligand binding was performed using membranes from CHO-K1 cells stably transfected with the human 5-HT1A receptor. All assays were carrie... | US Patent US10562853 (2020) BindingDB Entry DOI: 10.7270/Q29Z979D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM433526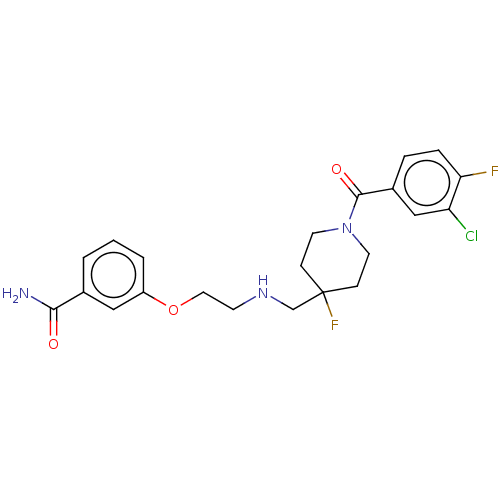 (US10562853, Compound 47) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NEUROLIXIS; UNIVERSITE JAGELLONE US Patent | Assay Description 5-HT1A: Radioligand binding was performed using membranes from CHO-K1 cells stably transfected with the human 5-HT1A receptor. All assays were carrie... | US Patent US10562853 (2020) BindingDB Entry DOI: 10.7270/Q29Z979D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50041045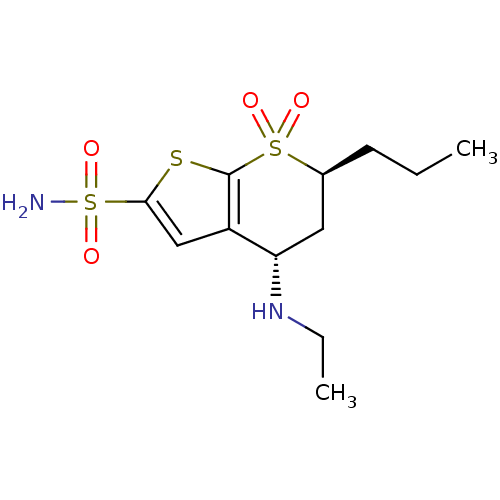 ((4S,6S)-4-Ethylamino-7,7-dioxo-6-propyl-4,5,6,7-te...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for the inhibitory constant towards Human Carbonic anhydrase II (HCA II) | J Med Chem 37: 1035-54 (1994) BindingDB Entry DOI: 10.7270/Q26Q1XWX | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM197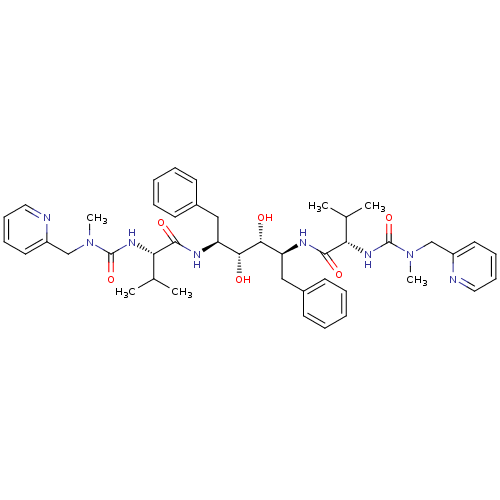 ((2S)-N-[(2S,3R,4R,5S)-3,4-dihydroxy-5-[(2S)-3-meth...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB PubMed | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for the inhibitory activity against HIV-1 protease | J Med Chem 37: 1035-54 (1994) BindingDB Entry DOI: 10.7270/Q26Q1XWX | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM433499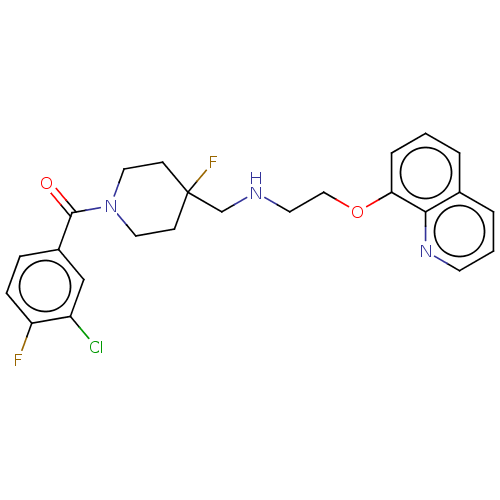 (US10562853, Compound 16) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NEUROLIXIS; UNIVERSITE JAGELLONE US Patent | Assay Description 5-HT1A: Radioligand binding was performed using membranes from CHO-K1 cells stably transfected with the human 5-HT1A receptor. All assays were carrie... | US Patent US10562853 (2020) BindingDB Entry DOI: 10.7270/Q29Z979D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50041023 ((1-{(1S,2S,3S,4S)-1-Benzyl-2,3-dihydroxy-4-[3-meth...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for the inhibitory activity against HIV-1 protease | J Med Chem 37: 1035-54 (1994) BindingDB Entry DOI: 10.7270/Q26Q1XWX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50041037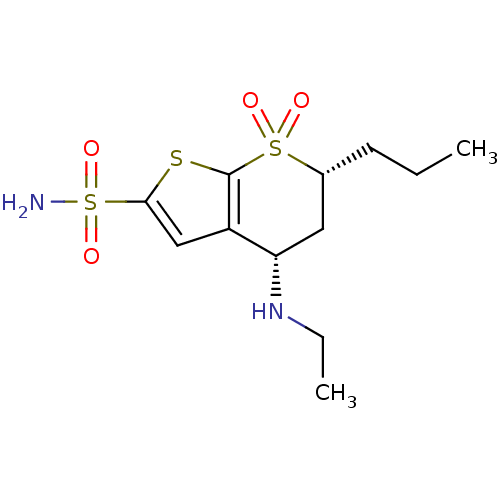 ((4S,6R)-4-Ethylamino-7,7-dioxo-6-propyl-4,5,6,7-te...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for the inhibitory constant towards Human Carbonic anhydrase II (HCA II) | J Med Chem 37: 1035-54 (1994) BindingDB Entry DOI: 10.7270/Q26Q1XWX | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50041035 (CHEMBL269401 | N-{(1S,2R,3R,4S)-1-Benzyl-2,3-dihyd...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for the inhibitory activity against HIV-1 protease | J Med Chem 37: 1035-54 (1994) BindingDB Entry DOI: 10.7270/Q26Q1XWX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM433494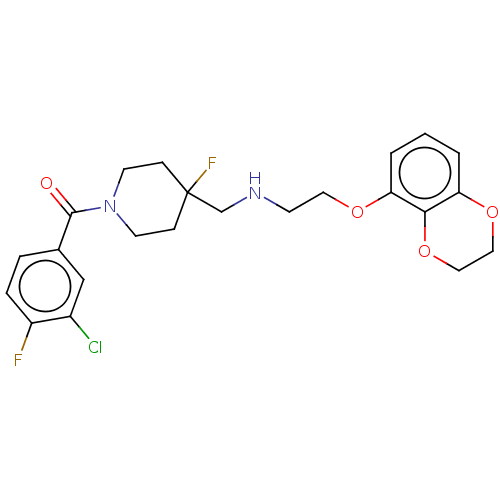 (US10562853, Compound 14) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NEUROLIXIS; UNIVERSITE JAGELLONE US Patent | Assay Description 5-HT1A: Radioligand binding was performed using membranes from CHO-K1 cells stably transfected with the human 5-HT1A receptor. All assays were carrie... | US Patent US10562853 (2020) BindingDB Entry DOI: 10.7270/Q29Z979D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50041032 ((1-{(1S,2R,3R,4S)-1-Benzyl-2,3-dihydroxy-4-[3-meth...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for the inhibitory activity against HIV-1 protease | J Med Chem 37: 1035-54 (1994) BindingDB Entry DOI: 10.7270/Q26Q1XWX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM433552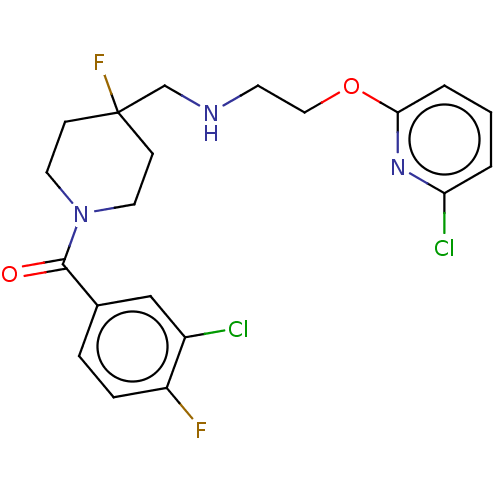 (US10562853, Compound 72) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NEUROLIXIS; UNIVERSITE JAGELLONE US Patent | Assay Description 5-HT1A: Radioligand binding was performed using membranes from CHO-K1 cells stably transfected with the human 5-HT1A receptor. All assays were carrie... | US Patent US10562853 (2020) BindingDB Entry DOI: 10.7270/Q29Z979D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM433495 (US10562853, Compound 15) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NEUROLIXIS; UNIVERSITE JAGELLONE US Patent | Assay Description 5-HT1A: Radioligand binding was performed using membranes from CHO-K1 cells stably transfected with the human 5-HT1A receptor. All assays were carrie... | US Patent US10562853 (2020) BindingDB Entry DOI: 10.7270/Q29Z979D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM433539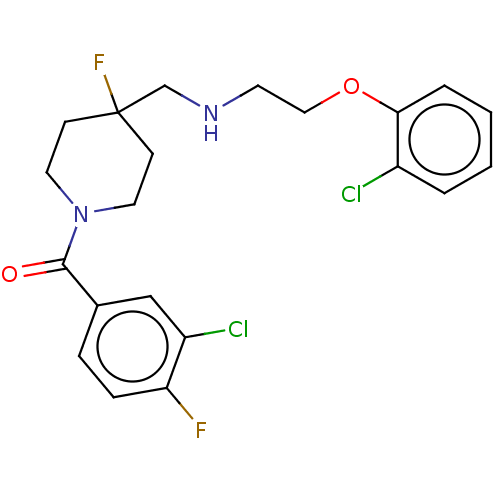 (US10562853, Compound 62) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NEUROLIXIS; UNIVERSITE JAGELLONE US Patent | Assay Description 5-HT1A: Radioligand binding was performed using membranes from CHO-K1 cells stably transfected with the human 5-HT1A receptor. All assays were carrie... | US Patent US10562853 (2020) BindingDB Entry DOI: 10.7270/Q29Z979D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM433527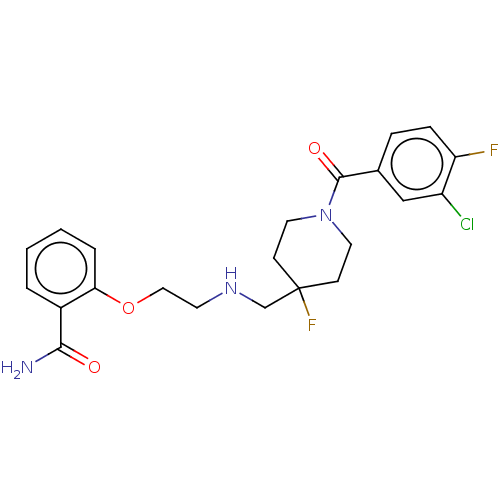 (US10562853, Compound 48) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NEUROLIXIS; UNIVERSITE JAGELLONE US Patent | Assay Description 5-HT1A: Radioligand binding was performed using membranes from CHO-K1 cells stably transfected with the human 5-HT1A receptor. All assays were carrie... | US Patent US10562853 (2020) BindingDB Entry DOI: 10.7270/Q29Z979D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM433554 (US10562853, Compound 74) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NEUROLIXIS; UNIVERSITE JAGELLONE US Patent | Assay Description 5-HT1A: Radioligand binding was performed using membranes from CHO-K1 cells stably transfected with the human 5-HT1A receptor. All assays were carrie... | US Patent US10562853 (2020) BindingDB Entry DOI: 10.7270/Q29Z979D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM433524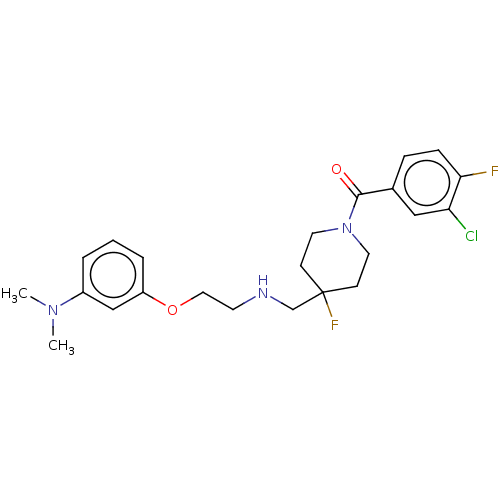 (US10562853, Compound 45) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NEUROLIXIS; UNIVERSITE JAGELLONE US Patent | Assay Description 5-HT1A: Radioligand binding was performed using membranes from CHO-K1 cells stably transfected with the human 5-HT1A receptor. All assays were carrie... | US Patent US10562853 (2020) BindingDB Entry DOI: 10.7270/Q29Z979D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM433531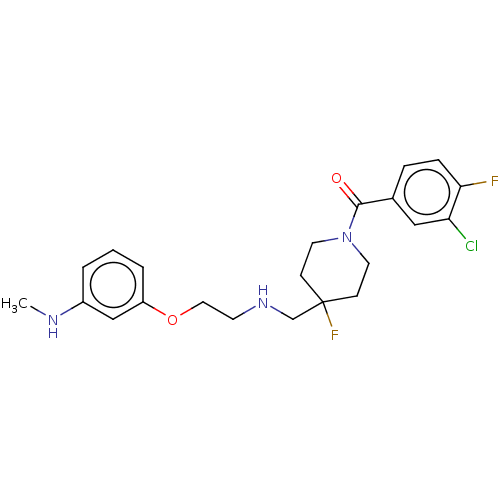 (US10562853, Compound 52) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NEUROLIXIS; UNIVERSITE JAGELLONE US Patent | Assay Description 5-HT1A: Radioligand binding was performed using membranes from CHO-K1 cells stably transfected with the human 5-HT1A receptor. All assays were carrie... | US Patent US10562853 (2020) BindingDB Entry DOI: 10.7270/Q29Z979D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM433548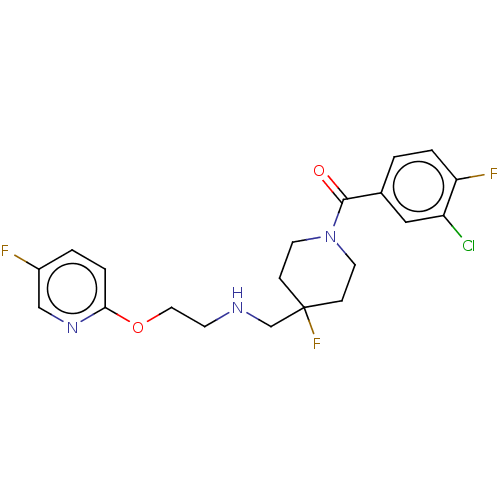 (US10562853, Compound 69) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NEUROLIXIS; UNIVERSITE JAGELLONE US Patent | Assay Description 5-HT1A: Radioligand binding was performed using membranes from CHO-K1 cells stably transfected with the human 5-HT1A receptor. All assays were carrie... | US Patent US10562853 (2020) BindingDB Entry DOI: 10.7270/Q29Z979D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM433507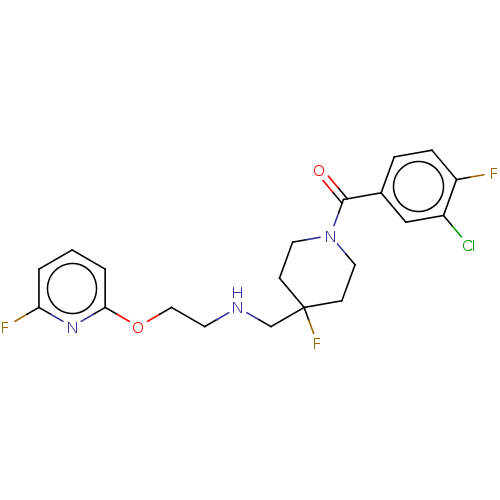 (US10562853, Compound 25) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NEUROLIXIS; UNIVERSITE JAGELLONE US Patent | Assay Description 5-HT1A: Radioligand binding was performed using membranes from CHO-K1 cells stably transfected with the human 5-HT1A receptor. All assays were carrie... | US Patent US10562853 (2020) BindingDB Entry DOI: 10.7270/Q29Z979D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50041027 ((4S,6S)-6-Ethyl-4-ethylamino-7,7-dioxo-4,5,6,7-tet...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for the inhibitory constant towards Human Carbonic anhydrase II (HCA II) | J Med Chem 37: 1035-54 (1994) BindingDB Entry DOI: 10.7270/Q26Q1XWX | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM433525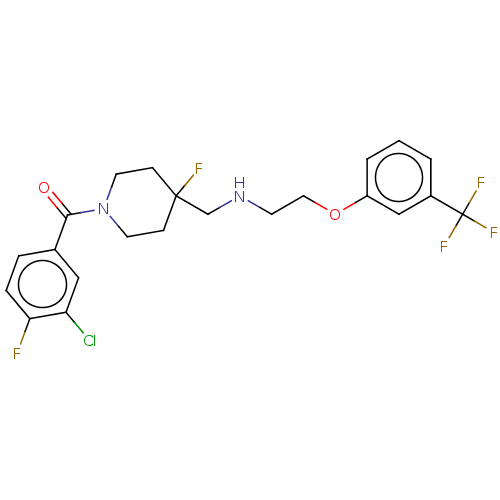 (US10562853, Compound 46) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NEUROLIXIS; UNIVERSITE JAGELLONE US Patent | Assay Description 5-HT1A: Radioligand binding was performed using membranes from CHO-K1 cells stably transfected with the human 5-HT1A receptor. All assays were carrie... | US Patent US10562853 (2020) BindingDB Entry DOI: 10.7270/Q29Z979D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM433538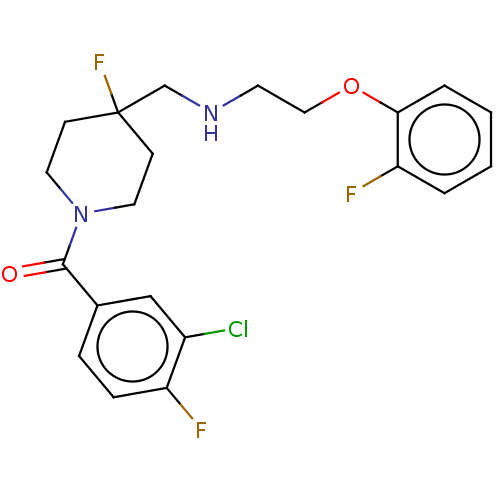 (US10562853, Compound 61) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NEUROLIXIS; UNIVERSITE JAGELLONE US Patent | Assay Description 5-HT1A: Radioligand binding was performed using membranes from CHO-K1 cells stably transfected with the human 5-HT1A receptor. All assays were carrie... | US Patent US10562853 (2020) BindingDB Entry DOI: 10.7270/Q29Z979D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM10884 ((2S,4S)-4-(ethylamino)-2-methyl-1,1-dioxo-2H,3H,4H...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB PubMed | 0.370 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for the inhibitory constant towards Human Carbonic anhydrase II (HCA II) | J Med Chem 37: 1035-54 (1994) BindingDB Entry DOI: 10.7270/Q26Q1XWX | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM433482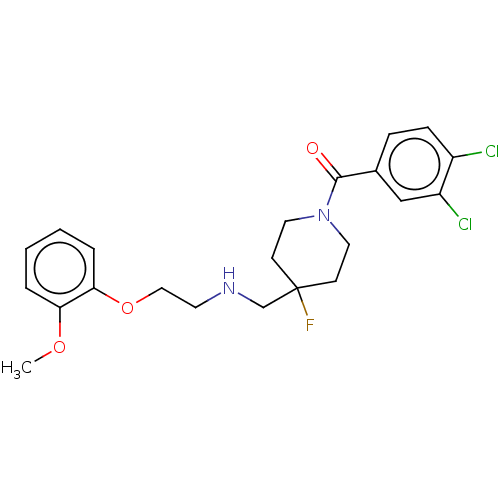 (US10562853, Compound 7) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NEUROLIXIS; UNIVERSITE JAGELLONE US Patent | Assay Description 5-HT1A: Radioligand binding was performed using membranes from CHO-K1 cells stably transfected with the human 5-HT1A receptor. All assays were carrie... | US Patent US10562853 (2020) BindingDB Entry DOI: 10.7270/Q29Z979D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM433528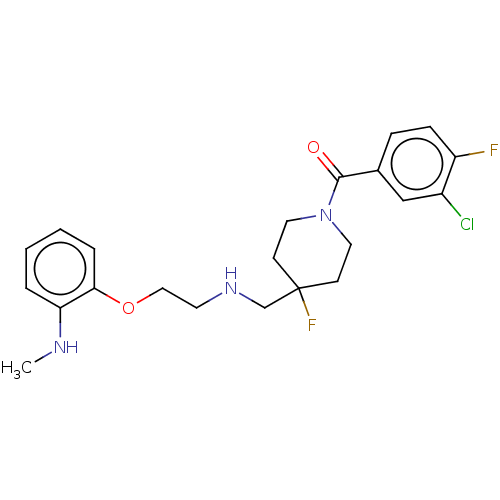 (US10562853, Compound 51) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NEUROLIXIS; UNIVERSITE JAGELLONE US Patent | Assay Description 5-HT1A: Radioligand binding was performed using membranes from CHO-K1 cells stably transfected with the human 5-HT1A receptor. All assays were carrie... | US Patent US10562853 (2020) BindingDB Entry DOI: 10.7270/Q29Z979D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM433505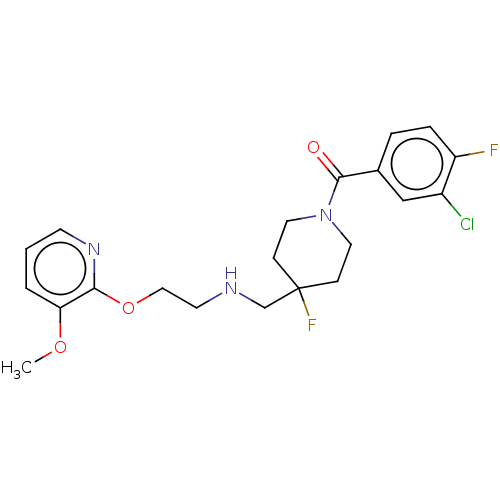 (US10562853, Compound 23) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NEUROLIXIS; UNIVERSITE JAGELLONE US Patent | Assay Description 5-HT1A: Radioligand binding was performed using membranes from CHO-K1 cells stably transfected with the human 5-HT1A receptor. All assays were carrie... | US Patent US10562853 (2020) BindingDB Entry DOI: 10.7270/Q29Z979D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50328639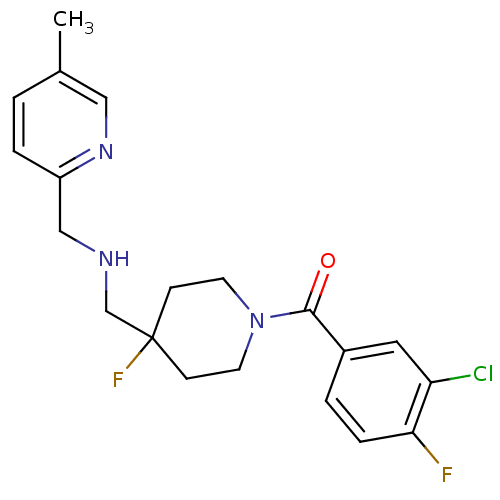 ((3-Chloro-4-fluoro-phenyl)-(4-fluoro-4-{[(5-methyl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | US Patent | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NEUROLIXIS; UNIVERSITE JAGELLONE US Patent | Assay Description 5-HT1A: Radioligand binding was performed using membranes from CHO-K1 cells stably transfected with the human 5-HT1A receptor. All assays were carrie... | US Patent US10562853 (2020) BindingDB Entry DOI: 10.7270/Q29Z979D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM433500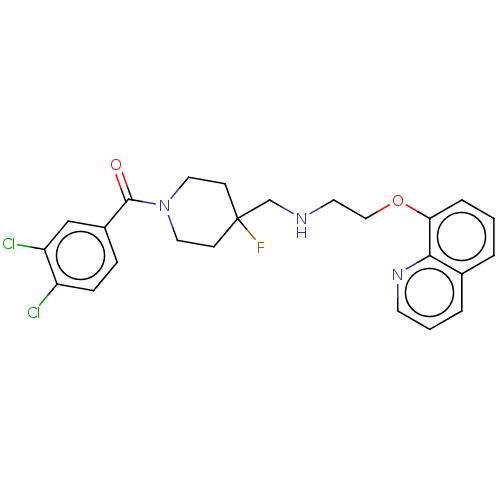 (US10562853, Compound 17) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NEUROLIXIS; UNIVERSITE JAGELLONE US Patent | Assay Description 5-HT1A: Radioligand binding was performed using membranes from CHO-K1 cells stably transfected with the human 5-HT1A receptor. All assays were carrie... | US Patent US10562853 (2020) BindingDB Entry DOI: 10.7270/Q29Z979D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM433536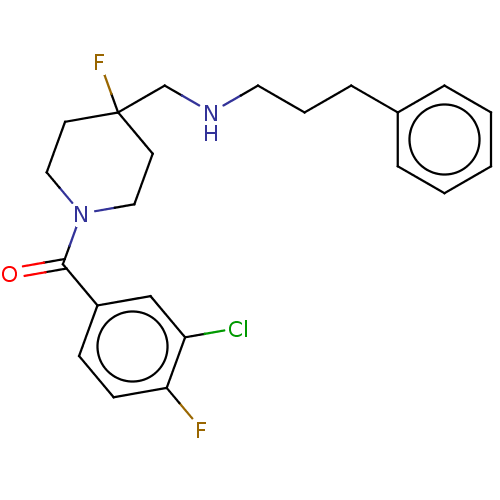 (US10562853, Compound 59) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.479 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]8-OH-DPAT from recombinant human 5HT1A receptor stably expressed in CHO-K1 cell membranes measured after 60 mins by microbeta2 sc... | Citation and Details Article DOI: 10.1021/acs.jmedchem.9b00062 BindingDB Entry DOI: 10.7270/Q23F4T9H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM433492 (US10562853, Compound 13) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NEUROLIXIS; UNIVERSITE JAGELLONE US Patent | Assay Description 5-HT1A: Radioligand binding was performed using membranes from CHO-K1 cells stably transfected with the human 5-HT1A receptor. All assays were carrie... | US Patent US10562853 (2020) BindingDB Entry DOI: 10.7270/Q29Z979D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 2400 total ) | Next | Last >> |