

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cathepsin K (Homo sapiens (Human)) | BDBM50098576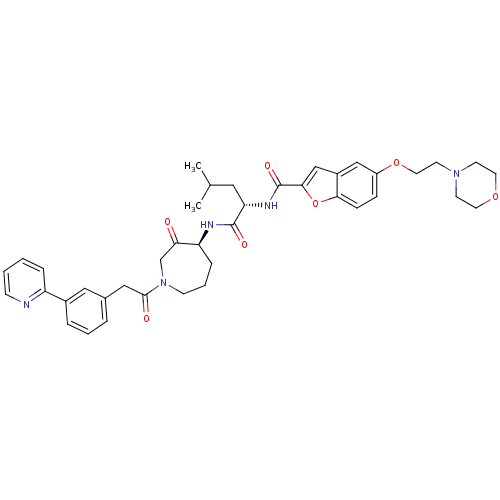 (5-(2-MORPHOLIN-4-YLETHOXY)BENZOFURAN-2-CARBOXYLIC ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB PubMed | 0.00480 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of Human cathepsin K | J Med Chem 44: 1380-95 (2001) BindingDB Entry DOI: 10.7270/Q2QR4WDC | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dimer of Gag-Pol polyprotein [501-599,Q508K,L534I,L564I,C568A,C596A] (Human immunodeficiency virus type 1) | BDBM9236 ((3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yl N-[(2S...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 0.0150 | -61.8 | n/a | n/a | n/a | n/a | n/a | 6.4 | 25 |
University of Illinois at Chicago | Assay Description The Ki values were determined using fluorogenic substrate, 2-(aminobenzoyl)-Thr-Ile-Nle-Phe(p-NO2)-Gln-ArgNH2. A standard curve relating changes in f... | Bioorg Med Chem Lett 12: 1993-6 (2002) Article DOI: 10.1016/s0960-894x(02)00300-1 BindingDB Entry DOI: 10.7270/Q2SJ1HS5 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Serine/threonine-protein kinase PAK 1 (Homo sapiens (Human)) | BDBM50148931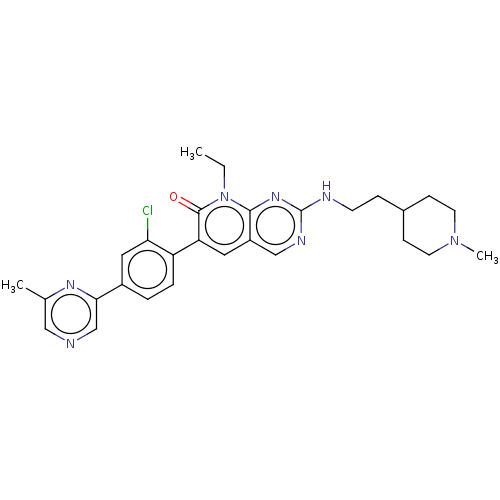 (CHEMBL3770186) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.0230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of full length recombinant human N-terminal GST/His6-tagged PAK1 expressed in sf9 insect cells using tetra LRRWSLG as substrate preincubat... | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112517 BindingDB Entry DOI: 10.7270/Q2Q243W7 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50576898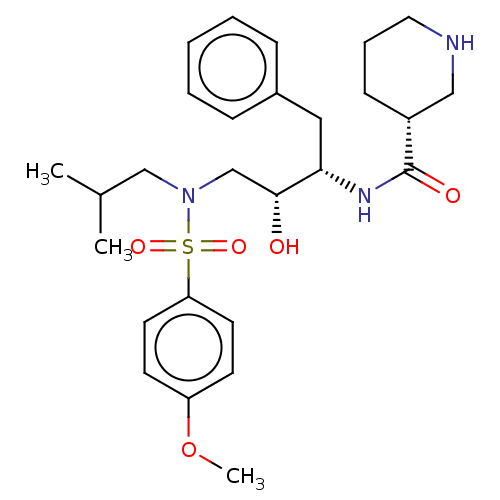 (CHEMBL4877646) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of wild type HIV1 protease using Arg-Glu (EDANS)-Ser-Gln-Asn-Tyr-Pro-Ile-Val-Gln-Lys(DABCYL)-Arg as substrate preincubated for 20 to 30 mi... | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113450 BindingDB Entry DOI: 10.7270/Q2571GTH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50576910 (CHEMBL4862758) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of wild type HIV1 protease using Arg-Glu (EDANS)-Ser-Gln-Asn-Tyr-Pro-Ile-Val-Gln-Lys(DABCYL)-Arg as substrate preincubated for 20 to 30 mi... | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113450 BindingDB Entry DOI: 10.7270/Q2571GTH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50212159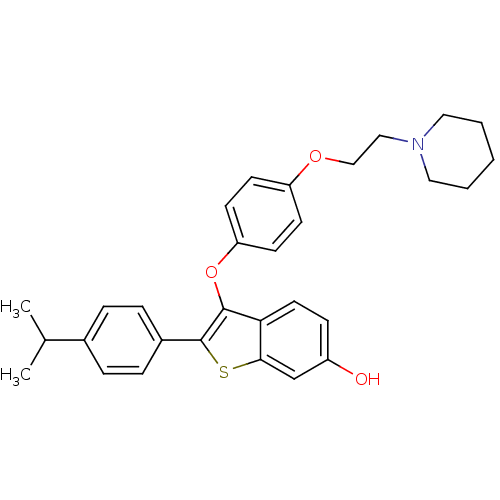 (2-(4-isopropylphenyl)-3-(4-(2-(piperidin-1-yl)etho...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0910 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]estradiol from human recombinant ERalpha | Bioorg Med Chem Lett 17: 3544-9 (2007) Article DOI: 10.1016/j.bmcl.2007.04.044 BindingDB Entry DOI: 10.7270/Q2ZS2W6F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50576913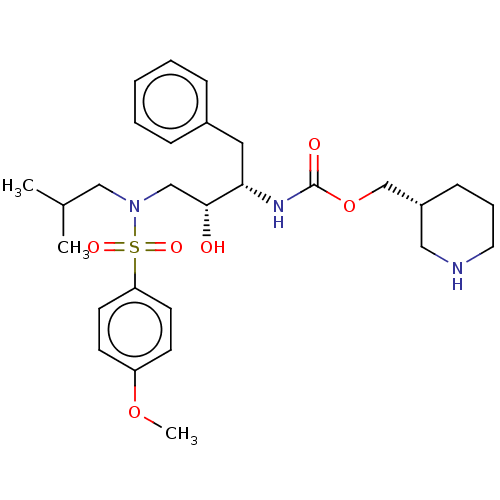 (CHEMBL4868812) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of wild type HIV1 protease using Arg-Glu (EDANS)-Ser-Gln-Asn-Tyr-Pro-Ile-Val-Gln-Lys(DABCYL)-Arg as substrate preincubated for 20 to 30 mi... | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113450 BindingDB Entry DOI: 10.7270/Q2571GTH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50576900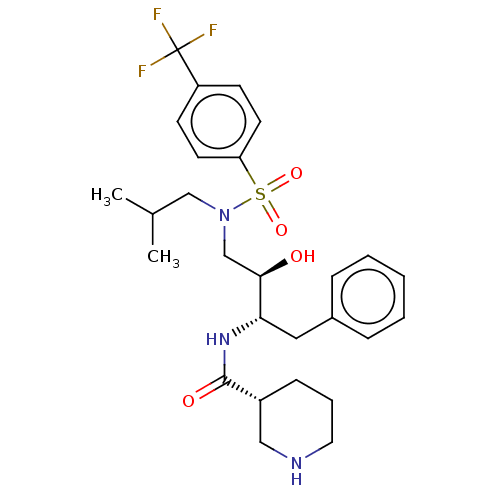 (CHEMBL4852688) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of wild type HIV1 protease using Arg-Glu (EDANS)-Ser-Gln-Asn-Tyr-Pro-Ile-Val-Gln-Lys(DABCYL)-Arg as substrate preincubated for 20 to 30 mi... | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113450 BindingDB Entry DOI: 10.7270/Q2571GTH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50604026 (CHEMBL5192384) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01670 BindingDB Entry DOI: 10.7270/Q2VH5SX2 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50604023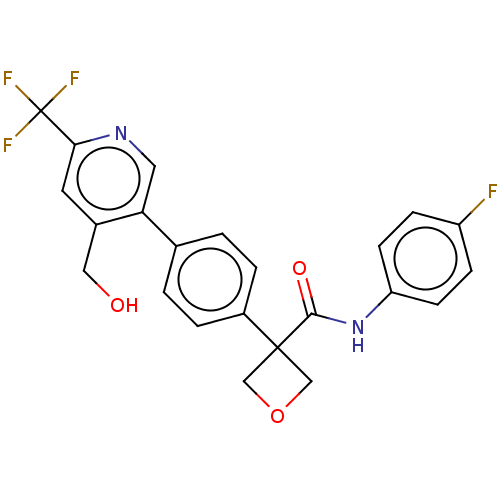 (CHEMBL5192977) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB Article PubMed | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01670 BindingDB Entry DOI: 10.7270/Q2VH5SX2 | ||||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM19769 ((2S)-2-(1-benzofuran-2-ylformamido)-4-methyl-N-[(4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB PubMed | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of Human cathepsin K | J Med Chem 44: 1380-95 (2001) BindingDB Entry DOI: 10.7270/Q2QR4WDC | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM19769 ((2S)-2-(1-benzofuran-2-ylformamido)-4-methyl-N-[(4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Apparent inhibitory constant against human cathepsin K | J Med Chem 48: 6870-8 (2005) Article DOI: 10.1021/jm0502079 BindingDB Entry DOI: 10.7270/Q2WM1CZC | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM50212148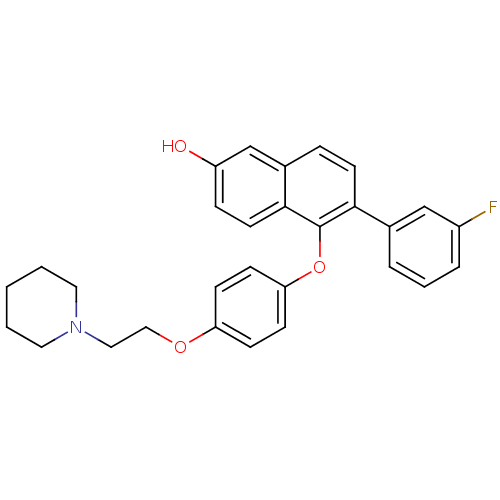 (6-(3-fluorophenyl)-5-(4-(2-(piperidin-1-yl)ethoxy)...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]estradiol from human recombinant ERbeta | Bioorg Med Chem Lett 17: 3544-9 (2007) Article DOI: 10.1016/j.bmcl.2007.04.044 BindingDB Entry DOI: 10.7270/Q2ZS2W6F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50212148 (6-(3-fluorophenyl)-5-(4-(2-(piperidin-1-yl)ethoxy)...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]estradiol from human recombinant ERalpha | Bioorg Med Chem Lett 17: 3544-9 (2007) Article DOI: 10.1016/j.bmcl.2007.04.044 BindingDB Entry DOI: 10.7270/Q2ZS2W6F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM8125 ((3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yl N-[(2S...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of wild type HIV1 protease using Arg-Glu (EDANS)-Ser-Gln-Asn-Tyr-Pro-Ile-Val-Gln-Lys(DABCYL)-Arg as substrate preincubated for 20 to 30 mi... | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113450 BindingDB Entry DOI: 10.7270/Q2571GTH | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50576912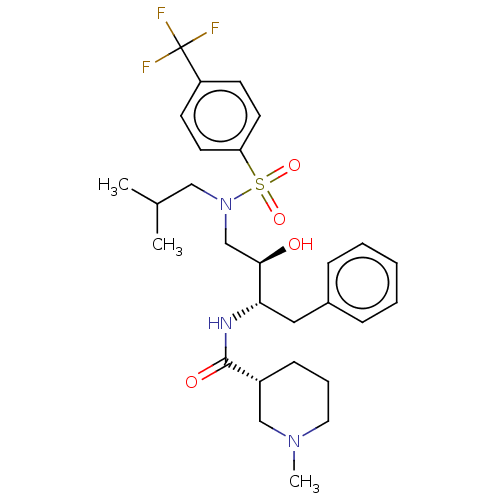 (CHEMBL4861507) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of wild type HIV1 protease using Arg-Glu (EDANS)-Ser-Gln-Asn-Tyr-Pro-Ile-Val-Gln-Lys(DABCYL)-Arg as substrate preincubated for 20 to 30 mi... | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113450 BindingDB Entry DOI: 10.7270/Q2571GTH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50072775 (2-((1R,2R,5R)-5-hydroxy-2-(3-hydroxypropyl)cyclohe...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Mississippi Curated by ChEMBL | Assay Description Agonist activity at recombinant human full length CB2 receptor expressed in HEK293 cell membranes after 2 hrs by [35S]GTP-gammaS binding assay | Eur J Med Chem 143: 983-996 (2018) Article DOI: 10.1016/j.ejmech.2017.11.043 BindingDB Entry DOI: 10.7270/Q2D50QN8 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM19964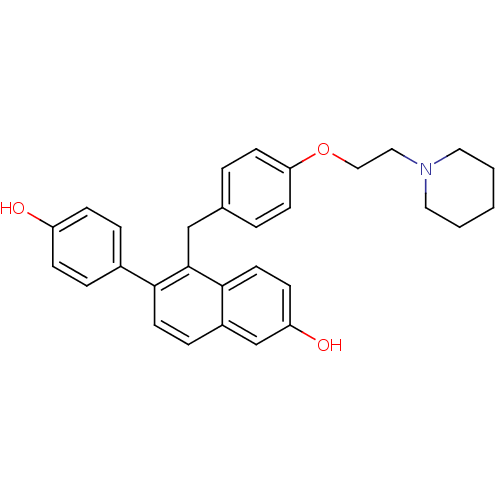 (6-(4-hydroxyphenyl)-5-({4-[2-(piperidin-1-yl)ethox...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents | Article PubMed | 0.200 | -54.8 | n/a | n/a | 1.14 | n/a | n/a | 7.5 | 22 |
Lilly Research Laboratories | Assay Description The binding affinities were determined by a competitive radiometric binding assay using [3H]estradiol as tracer. The Kd for 3H-estradiol was determin... | J Med Chem 48: 6772-5 (2005) Article DOI: 10.1021/jm050723z BindingDB Entry DOI: 10.7270/Q2RR1WHW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50212157 (6-(3,4-difluorophenyl)-5-(4-(2-(piperidin-1-yl)eth...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]estradiol from human recombinant ERalpha | Bioorg Med Chem Lett 17: 3544-9 (2007) Article DOI: 10.1016/j.bmcl.2007.04.044 BindingDB Entry DOI: 10.7270/Q2ZS2W6F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50212149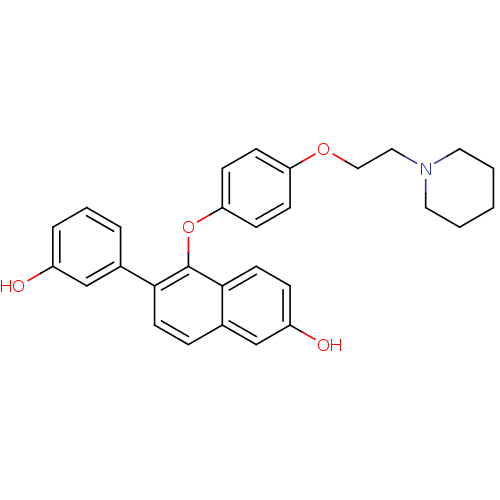 (6-(3-hydroxyphenyl)-5-(4-(2-(piperidin-1-yl)ethoxy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]estradiol from human recombinant ERalpha | Bioorg Med Chem Lett 17: 3544-9 (2007) Article DOI: 10.1016/j.bmcl.2007.04.044 BindingDB Entry DOI: 10.7270/Q2ZS2W6F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50346209 (5-(3-cyclobutyl-2,3,4,5-tetrahydro-1H-benzo[d]azep...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 0.210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from human histamine H3 receptor expressed in SK-N-MC cells | Eur J Med Chem 44: 4413-25 (2009) Article DOI: 10.1016/j.ejmech.2009.06.007 BindingDB Entry DOI: 10.7270/Q28S4Q7C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50212160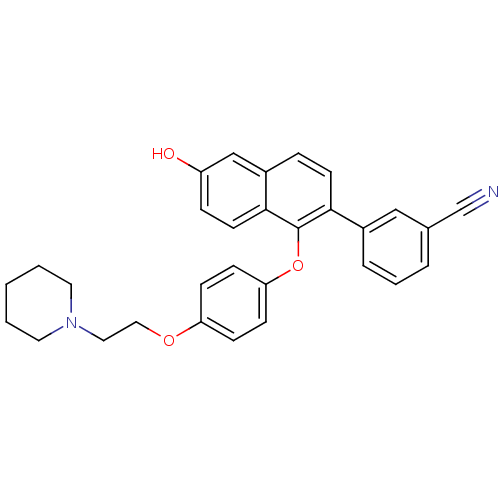 (3-(6-hydroxy-1-(4-(2-(piperidin-1-yl)ethoxy)phenox...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]estradiol from human recombinant ERalpha | Bioorg Med Chem Lett 17: 3544-9 (2007) Article DOI: 10.1016/j.bmcl.2007.04.044 BindingDB Entry DOI: 10.7270/Q2ZS2W6F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM19967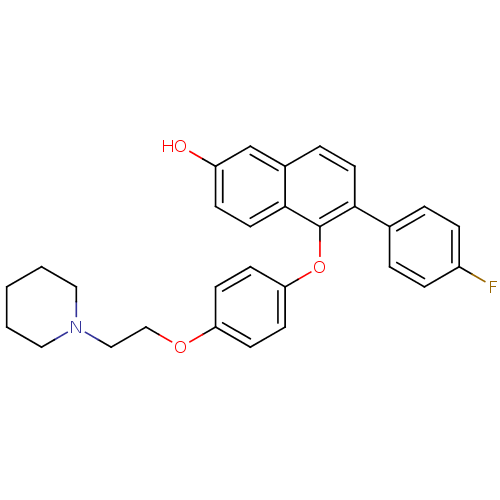 (6-(4-fluorophenyl)-5-{4-[2-(piperidin-1-yl)ethoxy]...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]estradiol from human recombinant ERalpha | Bioorg Med Chem Lett 17: 3544-9 (2007) Article DOI: 10.1016/j.bmcl.2007.04.044 BindingDB Entry DOI: 10.7270/Q2ZS2W6F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM19967 (6-(4-fluorophenyl)-5-{4-[2-(piperidin-1-yl)ethoxy]...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.25 | -54.3 | n/a | n/a | 3.18 | n/a | n/a | 7.5 | 22 |
Lilly Research Laboratories | Assay Description The binding affinities were determined by a competitive radiometric binding assay using [3H]estradiol as tracer. The Kd for 3H-estradiol was determin... | J Med Chem 48: 6772-5 (2005) Article DOI: 10.1021/jm050723z BindingDB Entry DOI: 10.7270/Q2RR1WHW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50084650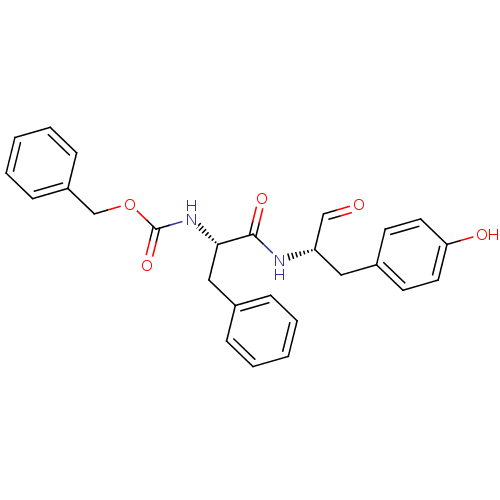 (CHEMBL177914 | {(S)-1-[(S)-1-Formyl-2-(4-hydroxy-p...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 0.260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Apparent inhibitory constant against human cathepsin S | J Med Chem 48: 6870-8 (2005) Article DOI: 10.1021/jm0502079 BindingDB Entry DOI: 10.7270/Q2WM1CZC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50576899 (CHEMBL4852584) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of wild type HIV1 protease using Arg-Glu (EDANS)-Ser-Gln-Asn-Tyr-Pro-Ile-Val-Gln-Lys(DABCYL)-Arg as substrate preincubated for 20 to 30 mi... | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113450 BindingDB Entry DOI: 10.7270/Q2571GTH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50576914 (CHEMBL4866330) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of wild type HIV1 protease using Arg-Glu (EDANS)-Ser-Gln-Asn-Tyr-Pro-Ile-Val-Gln-Lys(DABCYL)-Arg as substrate preincubated for 20 to 30 mi... | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113450 BindingDB Entry DOI: 10.7270/Q2571GTH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM19967 (6-(4-fluorophenyl)-5-{4-[2-(piperidin-1-yl)ethoxy]...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]estradiol from human recombinant ERbeta | Bioorg Med Chem Lett 17: 3544-9 (2007) Article DOI: 10.1016/j.bmcl.2007.04.044 BindingDB Entry DOI: 10.7270/Q2ZS2W6F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM50212157 (6-(3,4-difluorophenyl)-5-(4-(2-(piperidin-1-yl)eth...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]estradiol from human recombinant ERbeta | Bioorg Med Chem Lett 17: 3544-9 (2007) Article DOI: 10.1016/j.bmcl.2007.04.044 BindingDB Entry DOI: 10.7270/Q2ZS2W6F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM19967 (6-(4-fluorophenyl)-5-{4-[2-(piperidin-1-yl)ethoxy]...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.300 | -53.8 | n/a | n/a | 3.18 | n/a | n/a | 7.5 | 22 |
Lilly Research Laboratories | Assay Description The binding affinities were determined by a competitive radiometric binding assay using [3H]estradiol as tracer. The Kd for 3H-estradiol was determin... | J Med Chem 48: 6772-5 (2005) Article DOI: 10.1021/jm050723z BindingDB Entry DOI: 10.7270/Q2RR1WHW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50576916 (CHEMBL4875282) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of wild type HIV1 protease using Arg-Glu (EDANS)-Ser-Gln-Asn-Tyr-Pro-Ile-Val-Gln-Lys(DABCYL)-Arg as substrate preincubated for 20 to 30 mi... | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113450 BindingDB Entry DOI: 10.7270/Q2571GTH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50576911 (CHEMBL4863180) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of wild type HIV1 protease using Arg-Glu (EDANS)-Ser-Gln-Asn-Tyr-Pro-Ile-Val-Gln-Lys(DABCYL)-Arg as substrate preincubated for 20 to 30 mi... | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113450 BindingDB Entry DOI: 10.7270/Q2571GTH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM21190 (4-(2-{[5-amino-2-(furan-2-yl)-[1,2,4]triazolo[1,5-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.302 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nankai University Curated by ChEMBL | Assay Description Antagonist activity at A2AR (unknown origin) | Eur J Med Chem 187: (2020) Article DOI: 10.1016/j.ejmech.2019.111936 BindingDB Entry DOI: 10.7270/Q2S75KSD | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM21190 (4-(2-{[5-amino-2-(furan-2-yl)-[1,2,4]triazolo[1,5-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.302 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nankai University Curated by ChEMBL | Assay Description Antagonist activity at A2AR (unknown origin) | Eur J Med Chem 187: (2020) Article DOI: 10.1016/j.ejmech.2019.111936 BindingDB Entry DOI: 10.7270/Q2S75KSD | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50212147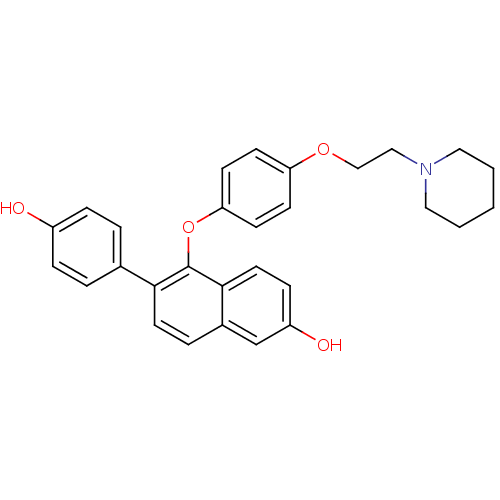 (6-(4-hydroxyphenyl)-5-(4-(2-(piperidin-1-yl)ethoxy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]estradiol from human recombinant ERalpha | Bioorg Med Chem Lett 17: 3544-9 (2007) Article DOI: 10.1016/j.bmcl.2007.04.044 BindingDB Entry DOI: 10.7270/Q2ZS2W6F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50576904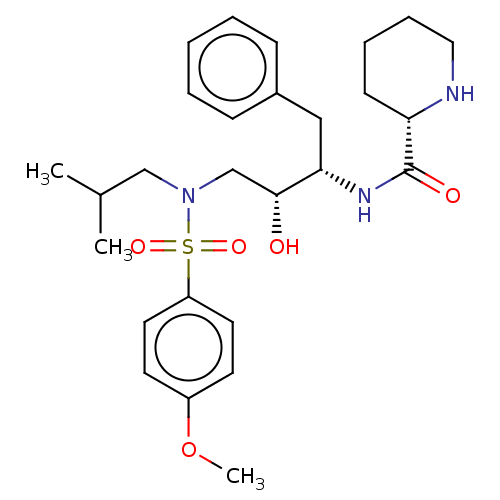 (CHEMBL4860352) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of wild type HIV1 protease using Arg-Glu (EDANS)-Ser-Gln-Asn-Tyr-Pro-Ile-Val-Gln-Lys(DABCYL)-Arg as substrate preincubated for 20 to 30 mi... | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113450 BindingDB Entry DOI: 10.7270/Q2571GTH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM50212160 (3-(6-hydroxy-1-(4-(2-(piperidin-1-yl)ethoxy)phenox...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]estradiol from human recombinant ERbeta | Bioorg Med Chem Lett 17: 3544-9 (2007) Article DOI: 10.1016/j.bmcl.2007.04.044 BindingDB Entry DOI: 10.7270/Q2ZS2W6F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM50212149 (6-(3-hydroxyphenyl)-5-(4-(2-(piperidin-1-yl)ethoxy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]estradiol from human recombinant ERbeta | Bioorg Med Chem Lett 17: 3544-9 (2007) Article DOI: 10.1016/j.bmcl.2007.04.044 BindingDB Entry DOI: 10.7270/Q2ZS2W6F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM19441 (2-(4-hydroxyphenyl)-3-({4-[2-(piperidin-1-yl)ethox...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.370 | -53.3 | n/a | n/a | 4.32 | n/a | n/a | 7.5 | 22 |
Lilly Research Laboratories | Assay Description The binding affinities were determined by a competitive radiometric binding assay using [3H]estradiol as tracer. The Kd for 3H-estradiol was determin... | J Med Chem 48: 6772-5 (2005) Article DOI: 10.1021/jm050723z BindingDB Entry DOI: 10.7270/Q2RR1WHW | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50159110 (1-(3-(4-(piperidin-1-ylmethyl)phenoxy)propyl)piper...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Displacement of N-[3H]methylhistamine from human histamine H3 receptor expressed in SK-N-MC cells | Bioorg Med Chem Lett 16: 897-900 (2006) Article DOI: 10.1016/j.bmcl.2005.11.003 BindingDB Entry DOI: 10.7270/Q2H131M7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50212153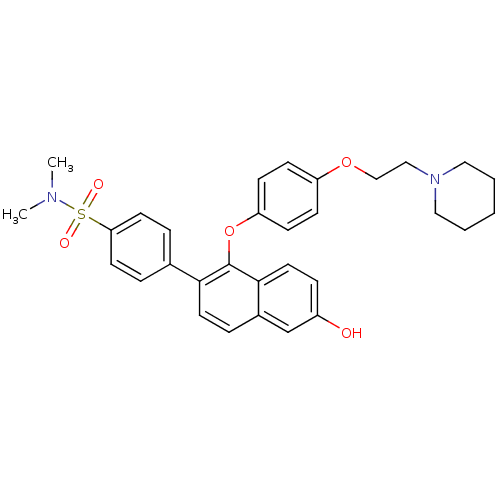 (4-(6-hydroxy-1-(4-(2-(piperidin-1-yl)ethoxy)phenox...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]estradiol from human recombinant ERalpha | Bioorg Med Chem Lett 17: 3544-9 (2007) Article DOI: 10.1016/j.bmcl.2007.04.044 BindingDB Entry DOI: 10.7270/Q2ZS2W6F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50065585 (5-(4-Chloro-3-methyl-phenyl)-9-fluoro-2,2,4-trimet...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Binding affinity for human progesterone receptor isoform A expressed in CV-1 cells | J Med Chem 41: 2779-85 (1998) Article DOI: 10.1021/jm980190c BindingDB Entry DOI: 10.7270/Q2CV4JFM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50065585 (5-(4-Chloro-3-methyl-phenyl)-9-fluoro-2,2,4-trimet...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Binding affinity for human progesterone receptor isoform A expressed in CV-1 cells | J Med Chem 41: 2779-85 (1998) Article DOI: 10.1021/jm980190c BindingDB Entry DOI: 10.7270/Q2CV4JFM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphodiesterase (Macaca mulatta (Rhesus macaque)) | BDBM355246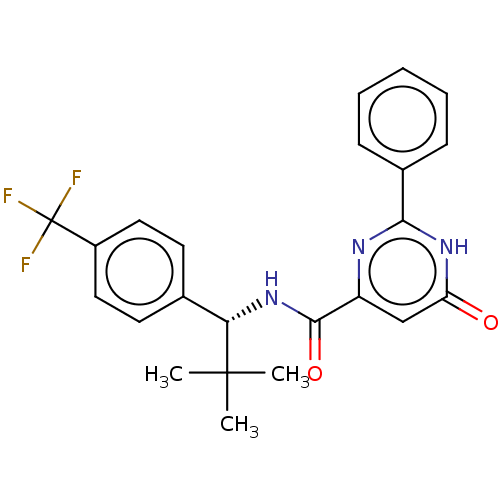 (US9815796, Example 337) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp.; MSD R & D (China) Co. LTD. US Patent | Assay Description In a typical experiment the PDE2 inhibitory activity of the compounds of the present invention was determined in accordance with the following experi... | US Patent US9815796 (2017) BindingDB Entry DOI: 10.7270/Q2V69MQQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM50002402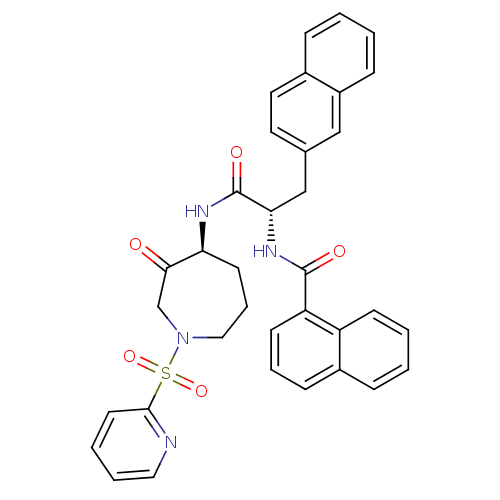 (CHEMBL194643) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Apparent inhibitory constant against human cathepsin L | J Med Chem 48: 6870-8 (2005) Article DOI: 10.1021/jm0502079 BindingDB Entry DOI: 10.7270/Q2WM1CZC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50139391 ((R)-4-(2-(2-(2-methylpyrrolidin-1-yl)ethyl)benzofu...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from human histamine H3 receptor expressed in SK-N-MC cells | Eur J Med Chem 44: 4413-25 (2009) Article DOI: 10.1016/j.ejmech.2009.06.007 BindingDB Entry DOI: 10.7270/Q28S4Q7C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-dependent 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM354912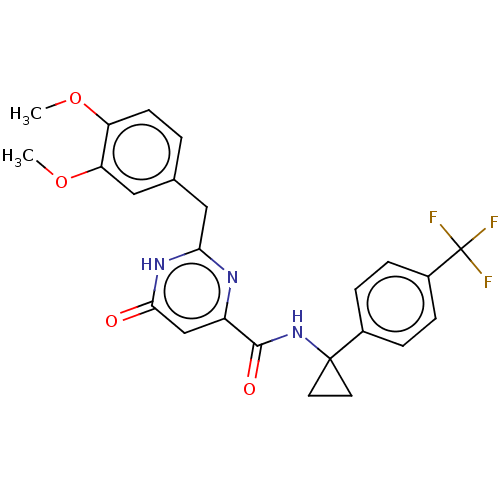 (1-Methylcyclopropanamine Oxalate | US9815796, Exam...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp.; MSD R & D (China) Co. LTD. US Patent | Assay Description In a typical experiment the PDE2 inhibitory activity of the compounds of the present invention was determined in accordance with the following experi... | US Patent US9815796 (2017) BindingDB Entry DOI: 10.7270/Q2V69MQQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM19966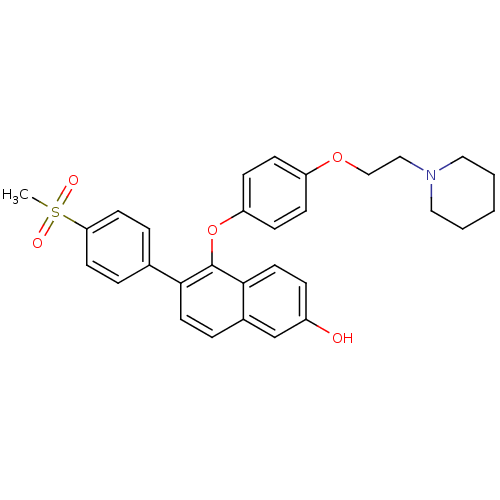 (6-(4-methanesulfonylphenyl)-5-{4-[2-(piperidin-1-y...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.470 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]estradiol from human recombinant ERalpha | Bioorg Med Chem Lett 17: 3544-9 (2007) Article DOI: 10.1016/j.bmcl.2007.04.044 BindingDB Entry DOI: 10.7270/Q2ZS2W6F | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM50084650 (CHEMBL177914 | {(S)-1-[(S)-1-Formyl-2-(4-hydroxy-p...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 0.470 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Apparent inhibitory constant against human cathepsin L | J Med Chem 48: 6870-8 (2005) Article DOI: 10.1021/jm0502079 BindingDB Entry DOI: 10.7270/Q2WM1CZC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM50212147 (6-(4-hydroxyphenyl)-5-(4-(2-(piperidin-1-yl)ethoxy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.480 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]estradiol from human recombinant ERbeta | Bioorg Med Chem Lett 17: 3544-9 (2007) Article DOI: 10.1016/j.bmcl.2007.04.044 BindingDB Entry DOI: 10.7270/Q2ZS2W6F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 31266 total ) | Next | Last >> |