

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mannose-6-phosphate isomerase (Homo sapiens (Human)) | BDBM50071516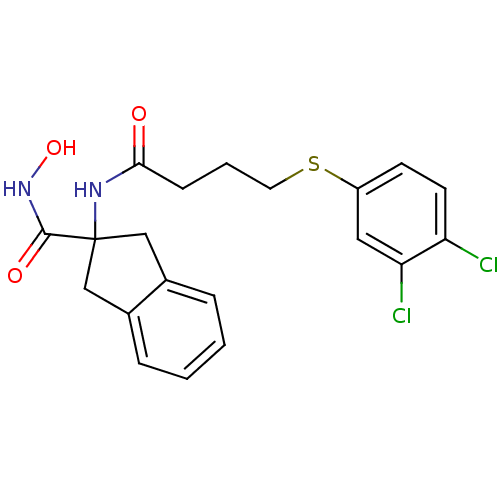 (2-[4-(3,4-Dichloro-phenylsulfanyl)-butyrylamino]-i...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Affymax Research Institute Curated by ChEMBL | Assay Description Compound was tested for inhibition of human phosphomannose isomerase (PMI) | Bioorg Med Chem Lett 8: 2303-8 (1999) BindingDB Entry DOI: 10.7270/Q2JW8D2F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mannose-6-phosphate isomerase (Homo sapiens (Human)) | BDBM50071531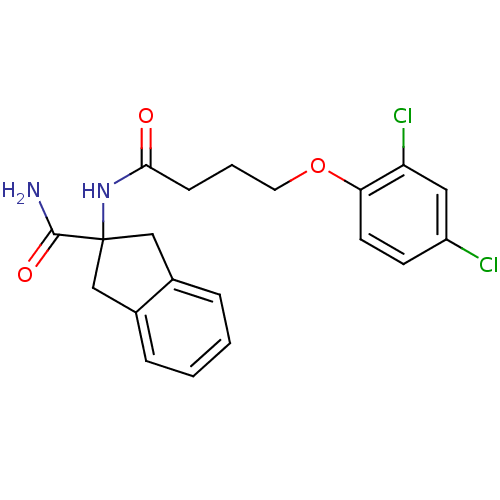 (2-[4-(2,4-Dichloro-phenoxy)-butyrylamino]-indan-2-...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Affymax Research Institute Curated by ChEMBL | Assay Description Compound was tested for inhibition of yeast phosphomannose isomerase (PMI) | Bioorg Med Chem Lett 8: 2303-8 (1999) BindingDB Entry DOI: 10.7270/Q2JW8D2F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mannose-6-phosphate isomerase (Homo sapiens (Human)) | BDBM50071516 (2-[4-(3,4-Dichloro-phenylsulfanyl)-butyrylamino]-i...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Affymax Research Institute Curated by ChEMBL | Assay Description Compound was evaluated for the inhibition constant against phosphomannose isomerase enzyme of Candida albicans (CaPMI) | Bioorg Med Chem Lett 8: 2303-8 (1999) BindingDB Entry DOI: 10.7270/Q2JW8D2F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium/bile acid cotransporter (Homo sapiens (Human)) | BDBM50157077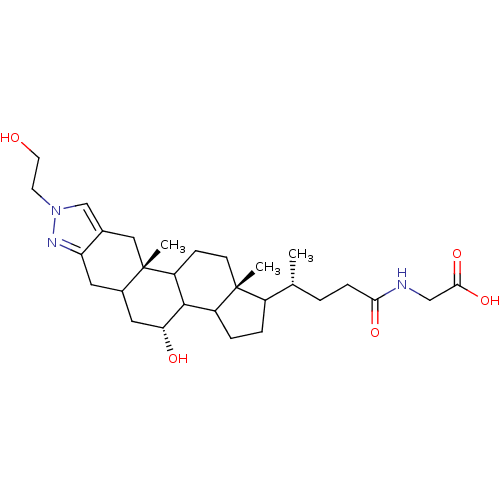 (CHEMBL184982 | {(R)-4-[(4R,10aS,12aR)-4-Hydroxy-8-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
XenoPort Inc. Curated by ChEMBL | Assay Description In vitro inhibition of taurocholate binding to liver bile acid transporter expressed in CHO cells | Bioorg Med Chem Lett 15: 85-7 (2004) Article DOI: 10.1016/j.bmcl.2004.10.027 BindingDB Entry DOI: 10.7270/Q2VT1RKR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium/bile acid cotransporter (Homo sapiens (Human)) | BDBM50157075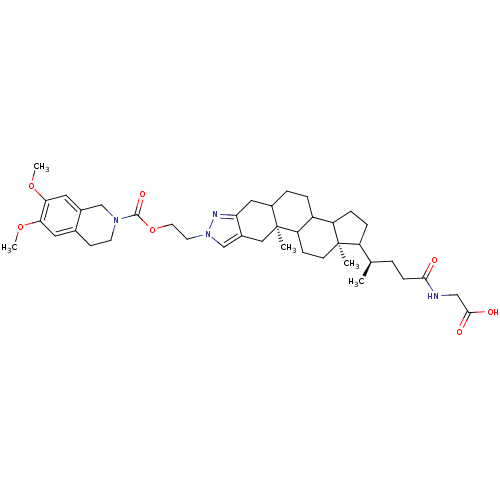 (2-[(4R)-4-[(2S,18R)-6-{2-[(6,7-dimethoxy-1,2,3,4-t...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 220 | n/a | n/a | n/a | n/a | n/a | n/a |
XenoPort Inc. Curated by ChEMBL | Assay Description In vitro inhibition of taurocholate binding to liver bile acid transporter expressed in CHO cells | Bioorg Med Chem Lett 15: 85-7 (2004) Article DOI: 10.1016/j.bmcl.2004.10.027 BindingDB Entry DOI: 10.7270/Q2VT1RKR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium/bile acid cotransporter (Homo sapiens (Human)) | BDBM50157079 (CHEMBL182301 | [(R)-4-((4R,10aS,12aR)-4-Hydroxy-10...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
XenoPort Inc. Curated by ChEMBL | Assay Description In vitro inhibition of taurocholate binding to liver bile acid transporter expressed in CHO cells | Bioorg Med Chem Lett 15: 85-7 (2004) Article DOI: 10.1016/j.bmcl.2004.10.027 BindingDB Entry DOI: 10.7270/Q2VT1RKR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium/bile acid cotransporter (Homo sapiens (Human)) | BDBM50157072 (CHEMBL367757 | {(R)-4-[(10aS,12aR)-8-(3-Hydroxy-be...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
XenoPort Inc. Curated by ChEMBL | Assay Description In vitro inhibition of taurocholate binding to liver bile acid transporter expressed in CHO cells | Bioorg Med Chem Lett 15: 85-7 (2004) Article DOI: 10.1016/j.bmcl.2004.10.027 BindingDB Entry DOI: 10.7270/Q2VT1RKR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium/bile acid cotransporter (Homo sapiens (Human)) | BDBM50157074 (CHEMBL182671 | {(R)-4-[(4R,10aS,12aR)-4-Hydroxy-8-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
XenoPort Inc. Curated by ChEMBL | Assay Description In vitro inhibition of taurocholate binding to liver bile acid transporter expressed in CHO cells | Bioorg Med Chem Lett 15: 85-7 (2004) Article DOI: 10.1016/j.bmcl.2004.10.027 BindingDB Entry DOI: 10.7270/Q2VT1RKR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium/bile acid cotransporter (Homo sapiens (Human)) | BDBM50157076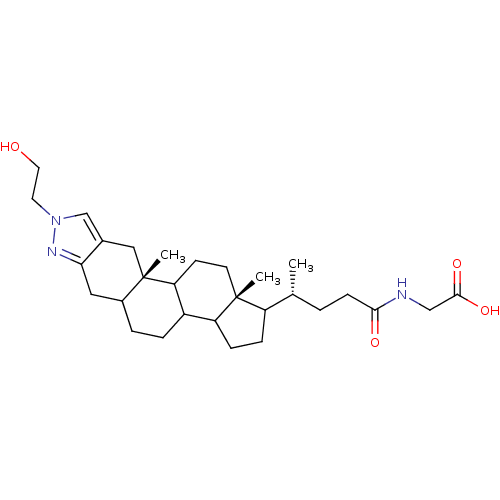 (CHEMBL185033 | {(R)-4-[(10aS,12aR)-8-(2-Hydroxy-et...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a | n/a |
XenoPort Inc. Curated by ChEMBL | Assay Description In vitro inhibition of taurocholate binding to liver bile acid transporter expressed in CHO cells | Bioorg Med Chem Lett 15: 85-7 (2004) Article DOI: 10.1016/j.bmcl.2004.10.027 BindingDB Entry DOI: 10.7270/Q2VT1RKR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ileal sodium/bile acid cotransporter (Homo sapiens (Human)) | BDBM50157072 (CHEMBL367757 | {(R)-4-[(10aS,12aR)-8-(3-Hydroxy-be...) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 900 | n/a | n/a | n/a | n/a | n/a | n/a |
XenoPort Inc. Curated by ChEMBL | Assay Description In vitro inhibition of taurocholate binding to human ileal bile acid transporter expressed in CHO cells | Bioorg Med Chem Lett 15: 85-7 (2004) Article DOI: 10.1016/j.bmcl.2004.10.027 BindingDB Entry DOI: 10.7270/Q2VT1RKR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1/2 (Homo sapiens (Human)) | BDBM50470133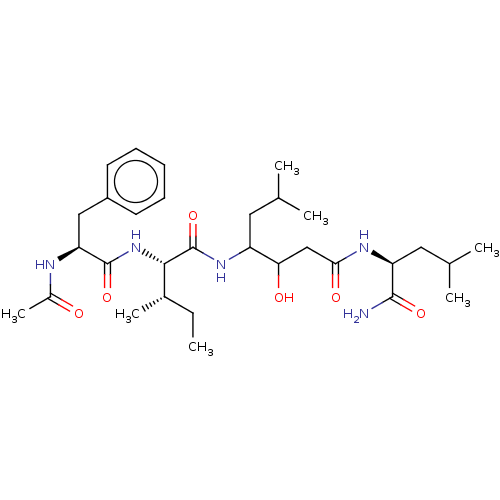 (CHEMBL277046) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Affymax Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against aspartyl protease | J Med Chem 37: 1233-51 (1994) Article DOI: 10.1021/jm00035a001 BindingDB Entry DOI: 10.7270/Q2GT5QWG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ileal sodium/bile acid cotransporter (Homo sapiens (Human)) | BDBM50157074 (CHEMBL182671 | {(R)-4-[(4R,10aS,12aR)-4-Hydroxy-8-...) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
XenoPort Inc. Curated by ChEMBL | Assay Description In vitro inhibition of taurocholate binding to human ileal bile acid transporter expressed in CHO cells | Bioorg Med Chem Lett 15: 85-7 (2004) Article DOI: 10.1016/j.bmcl.2004.10.027 BindingDB Entry DOI: 10.7270/Q2VT1RKR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ileal sodium/bile acid cotransporter (Homo sapiens (Human)) | BDBM50157079 (CHEMBL182301 | [(R)-4-((4R,10aS,12aR)-4-Hydroxy-10...) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
XenoPort Inc. Curated by ChEMBL | Assay Description In vitro inhibition of taurocholate binding to human ileal bile acid transporter expressed in CHO cells | Bioorg Med Chem Lett 15: 85-7 (2004) Article DOI: 10.1016/j.bmcl.2004.10.027 BindingDB Entry DOI: 10.7270/Q2VT1RKR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium/bile acid cotransporter (Homo sapiens (Human)) | BDBM50157078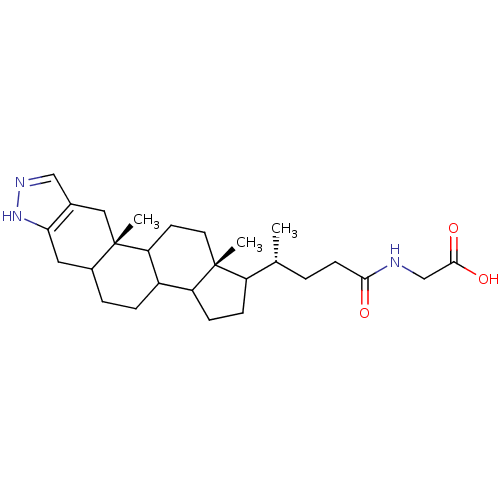 (CHEMBL185179 | [(R)-4-((10aS,12aR)-10a,12a-Dimethy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
XenoPort Inc. Curated by ChEMBL | Assay Description In vitro inhibition of taurocholate binding to liver bile acid transporter expressed in CHO cells | Bioorg Med Chem Lett 15: 85-7 (2004) Article DOI: 10.1016/j.bmcl.2004.10.027 BindingDB Entry DOI: 10.7270/Q2VT1RKR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Homo sapiens (Human)) | BDBM50289023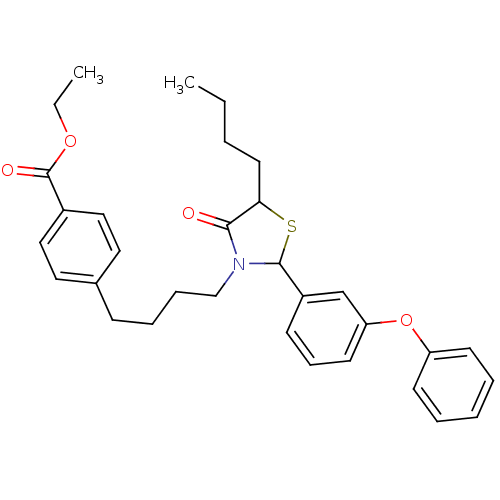 (4-{4-[5-Butyl-4-oxo-2-(3-phenoxy-phenyl)-thiazolid...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | 2.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for inhibition of Prostaglandin G/H synthase 1 | Bioorg Med Chem Lett 6: 707-712 (1996) Article DOI: 10.1016/0960-894X(96)00097-2 BindingDB Entry DOI: 10.7270/Q2BV7H40 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Homo sapiens (Human)) | BDBM50022309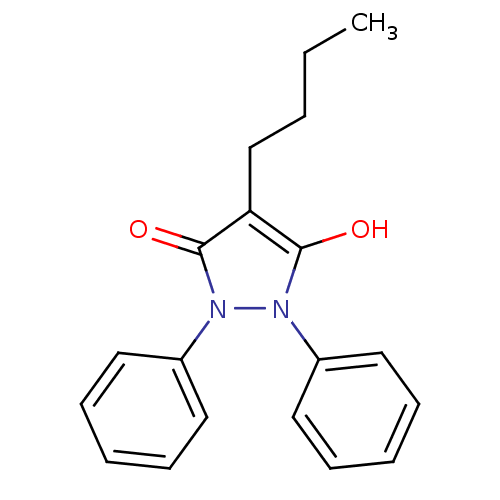 (3,5-Dioxo-1,2-diphenyl-4-n-butylpyrazolidine | 4-b...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | Article | n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for inhibition of Prostaglandin G/H synthase 1 | Bioorg Med Chem Lett 6: 707-712 (1996) Article DOI: 10.1016/0960-894X(96)00097-2 BindingDB Entry DOI: 10.7270/Q2BV7H40 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium/bile acid cotransporter (Homo sapiens (Human)) | BDBM50157080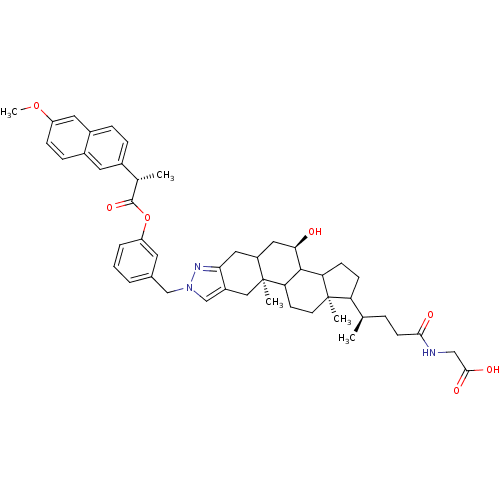 (2-[(4R)-4-[(2S,12R,18R)-12-hydroxy-6-[(3-{[(2S)-2-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
XenoPort Inc. Curated by ChEMBL | Assay Description In vitro inhibition of taurocholate binding to liver bile acid transporter expressed in CHO cells | Bioorg Med Chem Lett 15: 85-7 (2004) Article DOI: 10.1016/j.bmcl.2004.10.027 BindingDB Entry DOI: 10.7270/Q2VT1RKR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Homo sapiens (Human)) | BDBM50289020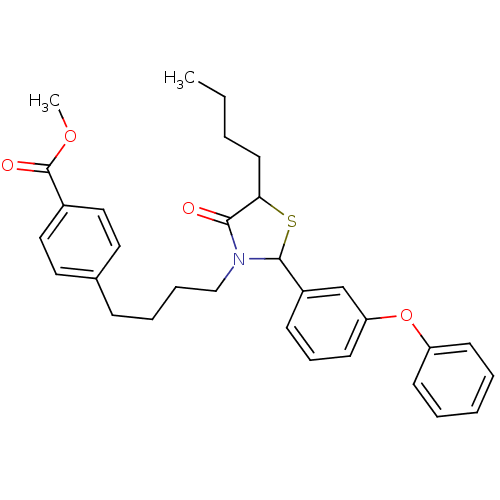 (4-{4-[5-Butyl-4-oxo-2-(3-phenoxy-phenyl)-thiazolid...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 3.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for inhibition of Prostaglandin G/H synthase 1 | Bioorg Med Chem Lett 6: 707-712 (1996) Article DOI: 10.1016/0960-894X(96)00097-2 BindingDB Entry DOI: 10.7270/Q2BV7H40 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mannose-6-phosphate isomerase (Homo sapiens (Human)) | BDBM50071516 (2-[4-(3,4-Dichloro-phenylsulfanyl)-butyrylamino]-i...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Affymax Research Institute Curated by ChEMBL | Assay Description Compound was tested for percentage inhibition of phosphomannose isomerase enzyme of Candida albicans (CaPMI). | Bioorg Med Chem Lett 8: 2303-8 (1999) BindingDB Entry DOI: 10.7270/Q2JW8D2F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mannose-6-phosphate isomerase (Candida albicans) | BDBM50071523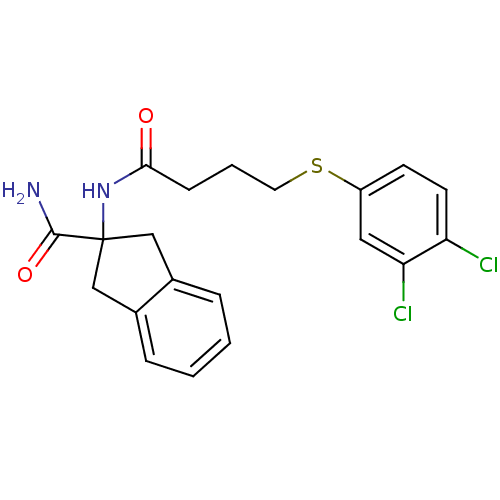 (2-[4-(3,4-Dichloro-phenylsulfanyl)-butyrylamino]-i...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Affymax Research Institute Curated by ChEMBL | Assay Description Inhibition of phosphomannose isomerase enzyme of Candida albicans (CaPMI) | Bioorg Med Chem Lett 8: 2303-8 (1999) BindingDB Entry DOI: 10.7270/Q2JW8D2F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Homo sapiens (Human)) | BDBM50009859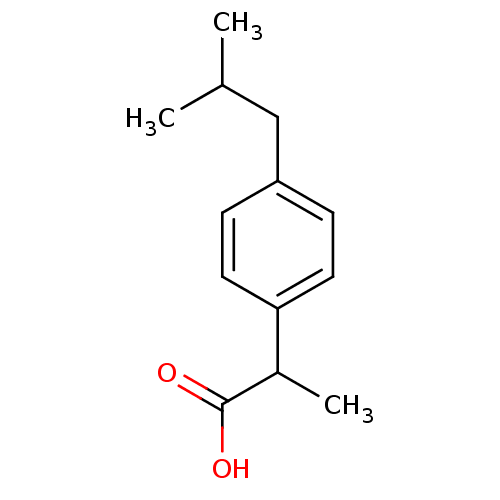 ((+-)-2-(p-isobutylphenyl)propionic acid | (+-)-alp...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PDB Article | n/a | n/a | 6.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for inhibition of Prostaglandin G/H synthase 1 | Bioorg Med Chem Lett 6: 707-712 (1996) Article DOI: 10.1016/0960-894X(96)00097-2 BindingDB Entry DOI: 10.7270/Q2BV7H40 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Sodium/bile acid cotransporter (Homo sapiens (Human)) | BDBM50157081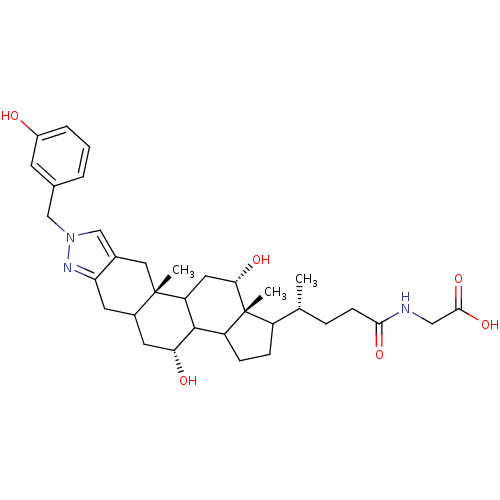 (CHEMBL179911 | {(R)-4-[(4R,10aS,12S,12aR)-4,12-Dih...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
XenoPort Inc. Curated by ChEMBL | Assay Description In vitro inhibition of taurocholate binding to liver bile acid transporter expressed in CHO cells | Bioorg Med Chem Lett 15: 85-7 (2004) Article DOI: 10.1016/j.bmcl.2004.10.027 BindingDB Entry DOI: 10.7270/Q2VT1RKR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Homo sapiens (Human)) | BDBM50289022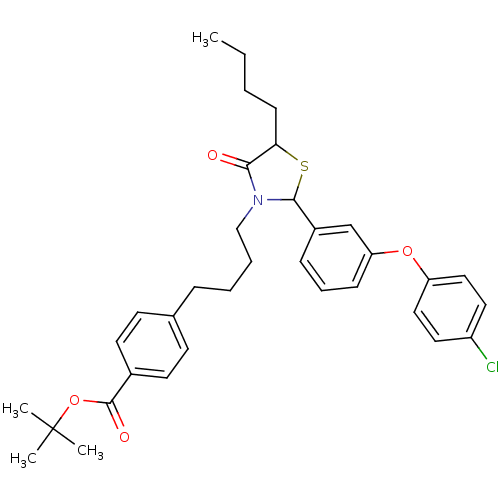 (4-(4-{5-Butyl-2-[3-(4-chloro-phenoxy)-phenyl]-4-ox...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 9.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for inhibition of Prostaglandin G/H synthase 1 | Bioorg Med Chem Lett 6: 707-712 (1996) Article DOI: 10.1016/0960-894X(96)00097-2 BindingDB Entry DOI: 10.7270/Q2BV7H40 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mannose-6-phosphate isomerase (Candida albicans) | BDBM50071519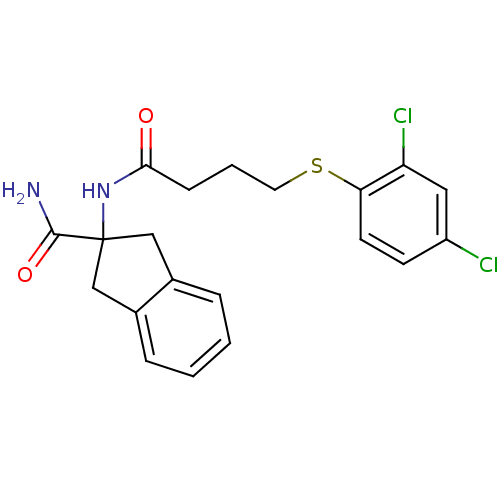 (2-[4-(2,4-Dichloro-phenylsulfanyl)-butyrylamino]-i...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Affymax Research Institute Curated by ChEMBL | Assay Description Inhibition of phosphomannose isomerase enzyme of Candida albicans (CaPMI) | Bioorg Med Chem Lett 8: 2303-8 (1999) BindingDB Entry DOI: 10.7270/Q2JW8D2F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mannose-6-phosphate isomerase (Candida albicans) | BDBM50071539 (2-[4-(3,4-Dichloro-phenoxy)-butyrylamino]-indan-2-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Affymax Research Institute Curated by ChEMBL | Assay Description Inhibition of phosphomannose isomerase enzyme of Candida albicans (CaPMI) | Bioorg Med Chem Lett 8: 2303-8 (1999) BindingDB Entry DOI: 10.7270/Q2JW8D2F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mannose-6-phosphate isomerase (Candida albicans) | BDBM50071535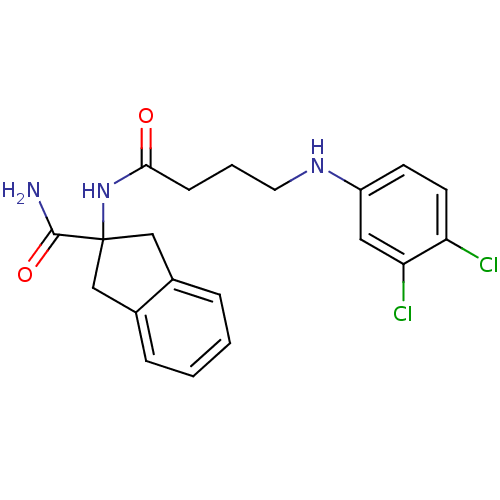 (2-[4-(3,4-Dichloro-phenylamino)-butyrylamino]-inda...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Affymax Research Institute Curated by ChEMBL | Assay Description Inhibition of phosphomannose isomerase enzyme of Candida albicans (CaPMI) | Bioorg Med Chem Lett 8: 2303-8 (1999) BindingDB Entry DOI: 10.7270/Q2JW8D2F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ileal sodium/bile acid cotransporter (Homo sapiens (Human)) | BDBM50157077 (CHEMBL184982 | {(R)-4-[(4R,10aS,12aR)-4-Hydroxy-8-...) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
XenoPort Inc. Curated by ChEMBL | Assay Description In vitro inhibition of taurocholate binding to human ileal bile acid transporter expressed in CHO cells | Bioorg Med Chem Lett 15: 85-7 (2004) Article DOI: 10.1016/j.bmcl.2004.10.027 BindingDB Entry DOI: 10.7270/Q2VT1RKR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mannose-6-phosphate isomerase (Candida albicans) | BDBM50071515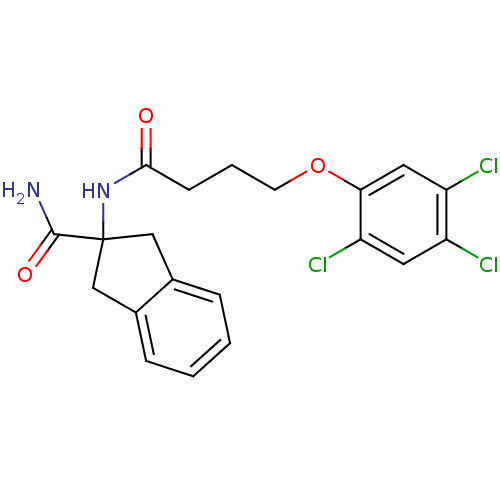 (2-[4-(2,4,5-Trichloro-phenoxy)-butyrylamino]-indan...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Affymax Research Institute Curated by ChEMBL | Assay Description Inhibition of phosphomannose isomerase enzyme of Candida albicans (CaPMI) | Bioorg Med Chem Lett 8: 2303-8 (1999) BindingDB Entry DOI: 10.7270/Q2JW8D2F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ileal sodium/bile acid cotransporter (Homo sapiens (Human)) | BDBM50157075 (2-[(4R)-4-[(2S,18R)-6-{2-[(6,7-dimethoxy-1,2,3,4-t...) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
XenoPort Inc. Curated by ChEMBL | Assay Description In vitro inhibition of taurocholate binding to human ileal bile acid transporter expressed in CHO cells | Bioorg Med Chem Lett 15: 85-7 (2004) Article DOI: 10.1016/j.bmcl.2004.10.027 BindingDB Entry DOI: 10.7270/Q2VT1RKR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mannose-6-phosphate isomerase (Candida albicans) | BDBM50071518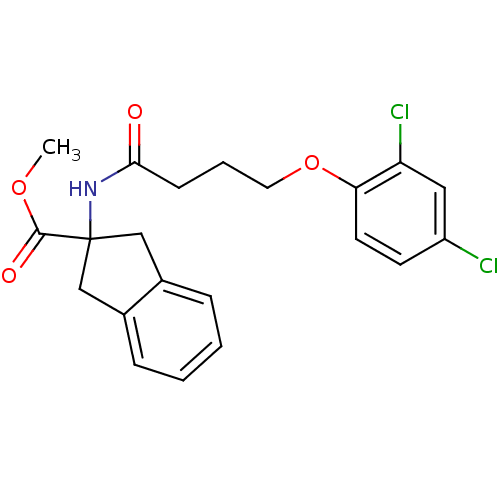 (2-[4-(2,4-Dichloro-phenoxy)-butyrylamino]-indan-2-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Affymax Research Institute Curated by ChEMBL | Assay Description Inhibition of phosphomannose isomerase enzyme of Candida albicans (CaPMI) | Bioorg Med Chem Lett 8: 2303-8 (1999) BindingDB Entry DOI: 10.7270/Q2JW8D2F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mannose-6-phosphate isomerase (Candida albicans) | BDBM50071533 (2-[4-(2,4-Dichloro-phenoxy)-butyrylamino]-indan-2-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Affymax Research Institute Curated by ChEMBL | Assay Description Inhibition of phosphomannose isomerase enzyme of Candida albicans (CaPMI) | Bioorg Med Chem Lett 8: 2303-8 (1999) BindingDB Entry DOI: 10.7270/Q2JW8D2F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Homo sapiens (Human)) | BDBM50289021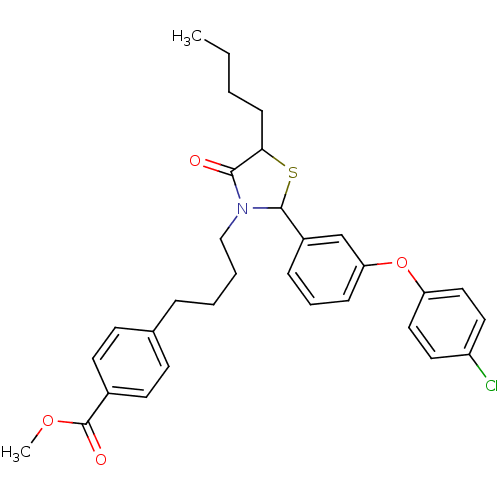 (4-(4-{5-Butyl-2-[3-(4-chloro-phenoxy)-phenyl]-4-ox...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 3.44E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for inhibition of Prostaglandin G/H synthase 1 | Bioorg Med Chem Lett 6: 707-712 (1996) Article DOI: 10.1016/0960-894X(96)00097-2 BindingDB Entry DOI: 10.7270/Q2BV7H40 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mannose-6-phosphate isomerase (Candida albicans) | BDBM50071528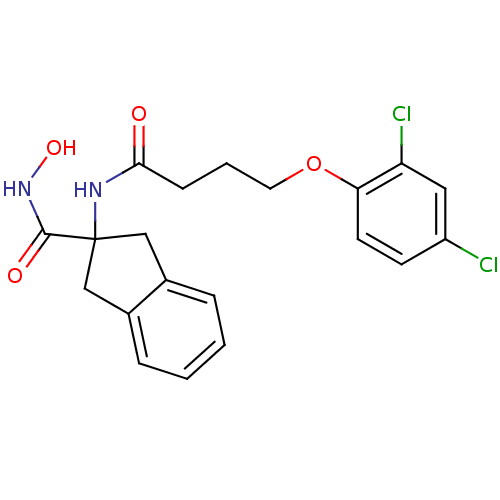 (2-[4-(2,4-Dichloro-phenoxy)-butyrylamino]-indan-2-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Affymax Research Institute Curated by ChEMBL | Assay Description Inhibition of phosphomannose isomerase enzyme of Candida albicans (CaPMI) | Bioorg Med Chem Lett 8: 2303-8 (1999) BindingDB Entry DOI: 10.7270/Q2JW8D2F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mannose-6-phosphate isomerase (Candida albicans) | BDBM50071534 (4-(2,4-Dichloro-phenoxy)-N-(2-hydrazinocarbonyl-in...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Affymax Research Institute Curated by ChEMBL | Assay Description Inhibition of phosphomannose isomerase enzyme of Candida albicans (CaPMI) | Bioorg Med Chem Lett 8: 2303-8 (1999) BindingDB Entry DOI: 10.7270/Q2JW8D2F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mannose-6-phosphate isomerase (Homo sapiens (Human)) | BDBM50071531 (2-[4-(2,4-Dichloro-phenoxy)-butyrylamino]-indan-2-...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Affymax Research Institute Curated by ChEMBL | Assay Description Compound was tested for percentage inhibition of phosphomannose isomerase enzyme of Candida albicans (CaPMI). | Bioorg Med Chem Lett 8: 2303-8 (1999) BindingDB Entry DOI: 10.7270/Q2JW8D2F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mannose-6-phosphate isomerase (Candida albicans) | BDBM50071527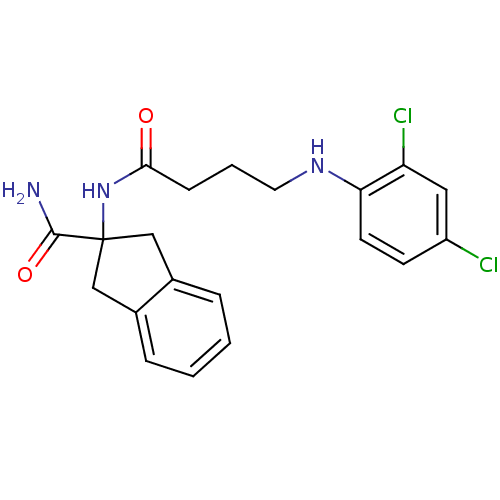 (2-[4-(2,4-Dichloro-phenylamino)-butyrylamino]-inda...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Affymax Research Institute Curated by ChEMBL | Assay Description Inhibition of phosphomannose isomerase enzyme of Candida albicans (CaPMI) | Bioorg Med Chem Lett 8: 2303-8 (1999) BindingDB Entry DOI: 10.7270/Q2JW8D2F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mannose-6-phosphate isomerase (Candida albicans) | BDBM50071517 (2-[4-(4-Chloro-3-methyl-phenoxy)-butyrylamino]-ind...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Affymax Research Institute Curated by ChEMBL | Assay Description Inhibition of phosphomannose isomerase enzyme of Candida albicans (CaPMI) | Bioorg Med Chem Lett 8: 2303-8 (1999) BindingDB Entry DOI: 10.7270/Q2JW8D2F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ileal sodium/bile acid cotransporter (Homo sapiens (Human)) | BDBM50157078 (CHEMBL185179 | [(R)-4-((10aS,12aR)-10a,12a-Dimethy...) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
XenoPort Inc. Curated by ChEMBL | Assay Description In vitro inhibition of taurocholate binding to human ileal bile acid transporter expressed in CHO cells | Bioorg Med Chem Lett 15: 85-7 (2004) Article DOI: 10.1016/j.bmcl.2004.10.027 BindingDB Entry DOI: 10.7270/Q2VT1RKR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium/bile acid cotransporter (Homo sapiens (Human)) | BDBM50157073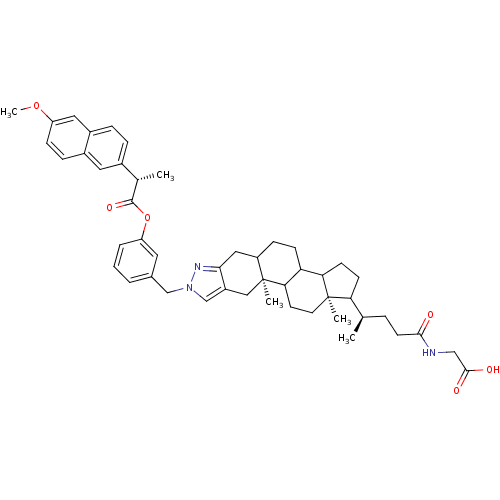 (2-[(4R)-4-[(2S,18R)-6-[(3-{[(2S)-2-(6-methoxynapht...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
XenoPort Inc. Curated by ChEMBL | Assay Description In vitro inhibition of taurocholate binding to liver bile acid transporter expressed in CHO cells | Bioorg Med Chem Lett 15: 85-7 (2004) Article DOI: 10.1016/j.bmcl.2004.10.027 BindingDB Entry DOI: 10.7270/Q2VT1RKR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mannose-6-phosphate isomerase (Candida albicans) | BDBM50071536 (2-[4-(2,4-Dichloro-phenoxy)-butyrylamino]-1,2,3,4-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 9.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Affymax Research Institute Curated by ChEMBL | Assay Description Inhibition of phosphomannose isomerase enzyme of Candida albicans (CaPMI) | Bioorg Med Chem Lett 8: 2303-8 (1999) BindingDB Entry DOI: 10.7270/Q2JW8D2F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mannose-6-phosphate isomerase (Homo sapiens (Human)) | BDBM50071521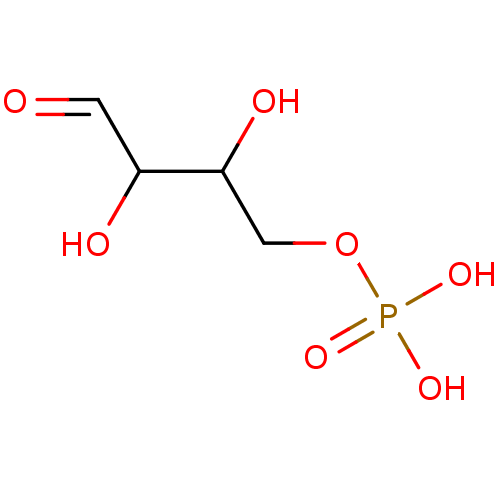 (CHEMBL75612 | Phosphoric acid mono-(2,3-dihydroxy-...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid PDB UniChem Similars | PubMed | n/a | n/a | 1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Affymax Research Institute Curated by ChEMBL | Assay Description Compound was tested for inhibition of human phosphomannose isomerase (PMI) | Bioorg Med Chem Lett 8: 2303-8 (1999) BindingDB Entry DOI: 10.7270/Q2JW8D2F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mannose-6-phosphate isomerase (Homo sapiens (Human)) | BDBM50071520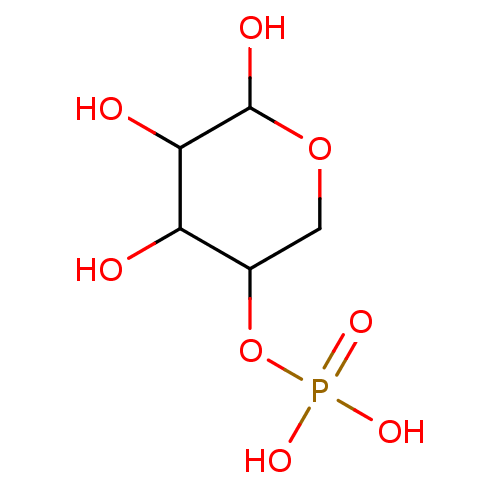 (CHEMBL75583 | Phosphoric acid mono-(4,5,6-trihydro...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Affymax Research Institute Curated by ChEMBL | Assay Description Compound was evaluated for the inhibition constant against phosphomannose isomerase enzyme of Candida albicans (CaPMI) | Bioorg Med Chem Lett 8: 2303-8 (1999) BindingDB Entry DOI: 10.7270/Q2JW8D2F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mannose-6-phosphate isomerase (Homo sapiens (Human)) | BDBM50071521 (CHEMBL75612 | Phosphoric acid mono-(2,3-dihydroxy-...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid PDB UniChem Similars | PubMed | n/a | n/a | 1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Affymax Research Institute Curated by ChEMBL | Assay Description Compound was evaluated for the inhibition constant against human phosphomannose isomerase (huPMI) | Bioorg Med Chem Lett 8: 2303-8 (1999) BindingDB Entry DOI: 10.7270/Q2JW8D2F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mannose-6-phosphate isomerase (Homo sapiens (Human)) | BDBM50071520 (CHEMBL75583 | Phosphoric acid mono-(4,5,6-trihydro...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Affymax Research Institute Curated by ChEMBL | Assay Description Compound was tested for inhibition of yeast phosphomannose isomerase (PMI) | Bioorg Med Chem Lett 8: 2303-8 (1999) BindingDB Entry DOI: 10.7270/Q2JW8D2F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mannose-6-phosphate isomerase (Candida albicans) | BDBM50071532 (4-(2,4-Dichloro-phenoxy)-N-(2-hydroxymethyl-indan-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.40E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Affymax Research Institute Curated by ChEMBL | Assay Description Inhibition of phosphomannose isomerase enzyme of Candida albicans (CaPMI) | Bioorg Med Chem Lett 8: 2303-8 (1999) BindingDB Entry DOI: 10.7270/Q2JW8D2F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mannose-6-phosphate isomerase (Candida albicans) | BDBM50071529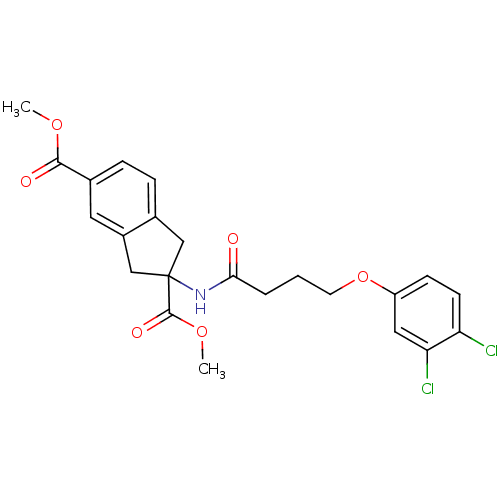 (2-[4-(3,4-Dichloro-phenoxy)-butyrylamino]-indan-2,...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Affymax Research Institute Curated by ChEMBL | Assay Description Inhibition of phosphomannose isomerase enzyme of Candida albicans (CaPMI) | Bioorg Med Chem Lett 8: 2303-8 (1999) BindingDB Entry DOI: 10.7270/Q2JW8D2F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mannose-6-phosphate isomerase (Candida albicans) | BDBM50071525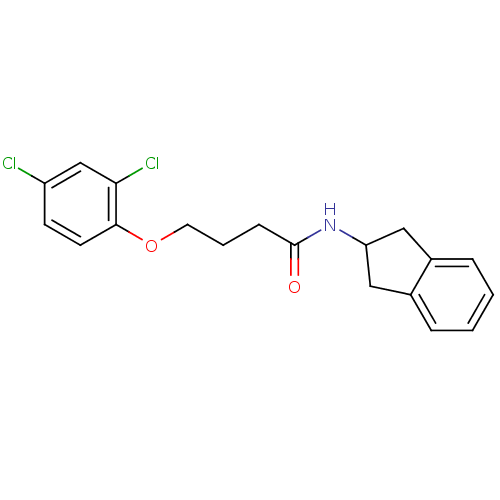 (4-(2,4-Dichloro-phenoxy)-N-indan-2-yl-butyramide |...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Affymax Research Institute Curated by ChEMBL | Assay Description Inhibition of phosphomannose isomerase enzyme of Candida albicans (CaPMI) | Bioorg Med Chem Lett 8: 2303-8 (1999) BindingDB Entry DOI: 10.7270/Q2JW8D2F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mannose-6-phosphate isomerase (Candida albicans) | BDBM50071537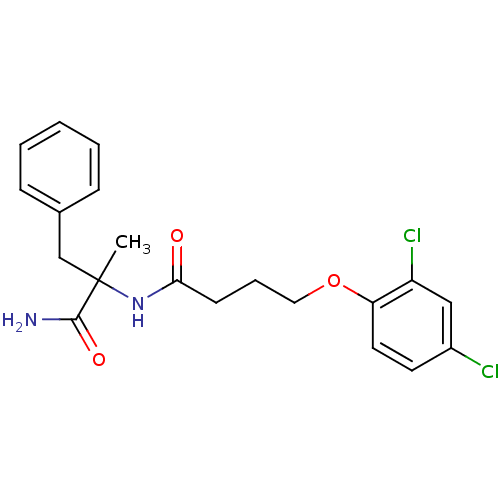 (CHEMBL77470 | N-(1-Carbamoyl-1-methyl-2-phenyl-eth...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Affymax Research Institute Curated by ChEMBL | Assay Description Inhibition of phosphomannose isomerase enzyme of Candida albicans (CaPMI) | Bioorg Med Chem Lett 8: 2303-8 (1999) BindingDB Entry DOI: 10.7270/Q2JW8D2F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mannose-6-phosphate isomerase (Candida albicans) | BDBM50071540 (1-[4-(2,4-Dichloro-phenoxy)-butyrylamino]-cyclopen...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Affymax Research Institute Curated by ChEMBL | Assay Description Inhibition of phosphomannose isomerase enzyme of Candida albicans (CaPMI) | Bioorg Med Chem Lett 8: 2303-8 (1999) BindingDB Entry DOI: 10.7270/Q2JW8D2F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mannose-6-phosphate isomerase (Candida albicans) | BDBM50071522 (1-[4-(2,4-Dichloro-phenoxy)-butyrylamino]-indan-1-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Affymax Research Institute Curated by ChEMBL | Assay Description Inhibition of phosphomannose isomerase enzyme of Candida albicans (CaPMI) | Bioorg Med Chem Lett 8: 2303-8 (1999) BindingDB Entry DOI: 10.7270/Q2JW8D2F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 58 total ) | Next | Last >> |