

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50130563 ((3R)-7-Hydroxy-N-[(1S)-1-{[(3R,4R)-4-(3-hydroxyphe...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Science and Technology Curated by ChEMBL | Assay Description Antagonist activity at kappa opioid receptor (unknown origin) assessed as stimulation of [35S]GTPgammaS | Eur J Med Chem 141: 632-647 (2017) Article DOI: 10.1016/j.ejmech.2017.10.012 BindingDB Entry DOI: 10.7270/Q2V98BQ2 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50130563 ((3R)-7-Hydroxy-N-[(1S)-1-{[(3R,4R)-4-(3-hydroxyphe...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Science and Technology Curated by ChEMBL | Assay Description Displacement of [3H]U69,593 from kappa opioid receptor in guinea pig brain membranes after 2 hrs | Eur J Med Chem 141: 632-647 (2017) Article DOI: 10.1016/j.ejmech.2017.10.012 BindingDB Entry DOI: 10.7270/Q2V98BQ2 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50026603 (Buprenorphine | CHEBI:3216) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | DrugBank PC cid PC sid UniChem Patents Similars | DrugBank Article PubMed | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Science and Technology Curated by ChEMBL | Assay Description Antagonist activity at kappa opioid receptor (unknown origin) assessed as stimulation of [35S]GTPgammaS | Eur J Med Chem 141: 632-647 (2017) Article DOI: 10.1016/j.ejmech.2017.10.012 BindingDB Entry DOI: 10.7270/Q2V98BQ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50026614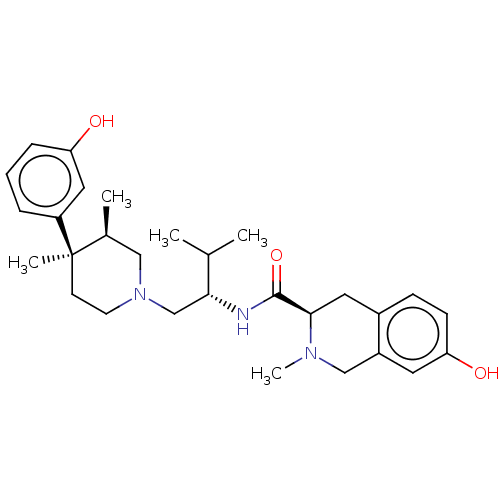 (CHEMBL575508) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0530 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Science and Technology Curated by ChEMBL | Assay Description Displacement of [3H]U69,593 from kappa opioid receptor in guinea pig brain membranes after 2 hrs | Eur J Med Chem 141: 632-647 (2017) Article DOI: 10.1016/j.ejmech.2017.10.012 BindingDB Entry DOI: 10.7270/Q2V98BQ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50464462 (CHEMBL4283681) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Science and Technology Curated by ChEMBL | Assay Description Antagonist activity at human kappa opioid receptor expressed in CHO cells assessed as stimulation of [35S]GTPgammaS | Eur J Med Chem 141: 632-647 (2017) Article DOI: 10.1016/j.ejmech.2017.10.012 BindingDB Entry DOI: 10.7270/Q2V98BQ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50130563 ((3R)-7-Hydroxy-N-[(1S)-1-{[(3R,4R)-4-(3-hydroxyphe...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Science and Technology Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from mu-opioid receptor in rat brain membranes after 2 hrs | Eur J Med Chem 141: 632-647 (2017) Article DOI: 10.1016/j.ejmech.2017.10.012 BindingDB Entry DOI: 10.7270/Q2V98BQ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50012152 (CHEMBL3264441) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Science and Technology Curated by ChEMBL | Assay Description Displacement of [3H]-diprenorphine from human kappa opioid receptor expressed in CHO cells after 1 hr | Eur J Med Chem 141: 632-647 (2017) Article DOI: 10.1016/j.ejmech.2017.10.012 BindingDB Entry DOI: 10.7270/Q2V98BQ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50464462 (CHEMBL4283681) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Science and Technology Curated by ChEMBL | Assay Description Antagonist activity at human delta opioid receptor expressed in CHO cells assessed as stimulation of [35S]GTPgammaS | Eur J Med Chem 141: 632-647 (2017) Article DOI: 10.1016/j.ejmech.2017.10.012 BindingDB Entry DOI: 10.7270/Q2V98BQ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50464462 (CHEMBL4283681) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 5.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Science and Technology Curated by ChEMBL | Assay Description Antagonist activity at human mu opioid receptor expressed in CHO cells assessed as stimulation of [35S]GTPgammaS | Eur J Med Chem 141: 632-647 (2017) Article DOI: 10.1016/j.ejmech.2017.10.012 BindingDB Entry DOI: 10.7270/Q2V98BQ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50464461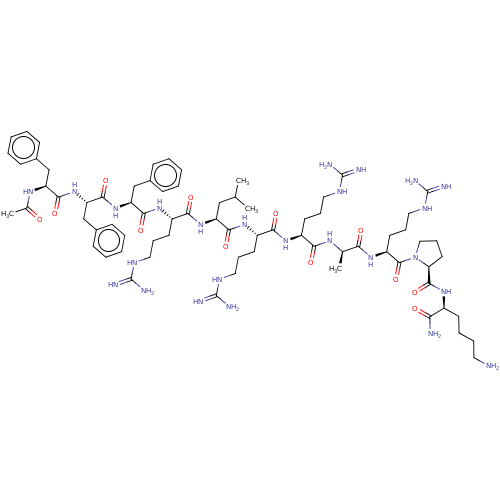 (CHEMBL4290635) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Science and Technology Curated by ChEMBL | Assay Description Antagonist activity at kappa opioid receptor (unknown origin) | Eur J Med Chem 141: 632-647 (2017) Article DOI: 10.1016/j.ejmech.2017.10.012 BindingDB Entry DOI: 10.7270/Q2V98BQ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50027038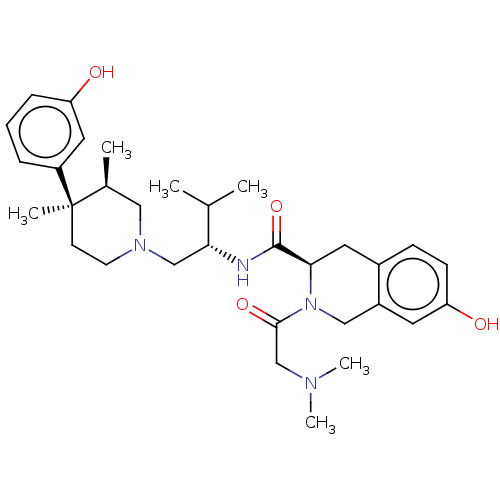 (CHEMBL2112474) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Science and Technology Curated by ChEMBL | Assay Description Displacement of [3H]U69,593 from kappa opioid receptor in guinea pig brain membranes after 2 hrs | Eur J Med Chem 141: 632-647 (2017) Article DOI: 10.1016/j.ejmech.2017.10.012 BindingDB Entry DOI: 10.7270/Q2V98BQ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50464460 (CHEMBL4295159) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Science and Technology Curated by ChEMBL | Assay Description Antagonist activity at kappa opioid receptor (unknown origin) | Eur J Med Chem 141: 632-647 (2017) Article DOI: 10.1016/j.ejmech.2017.10.012 BindingDB Entry DOI: 10.7270/Q2V98BQ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50026614 (CHEMBL575508) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 37 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Science and Technology Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from mu-opioid receptor in rat brain membranes after 2 hrs | Eur J Med Chem 141: 632-647 (2017) Article DOI: 10.1016/j.ejmech.2017.10.012 BindingDB Entry DOI: 10.7270/Q2V98BQ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50012151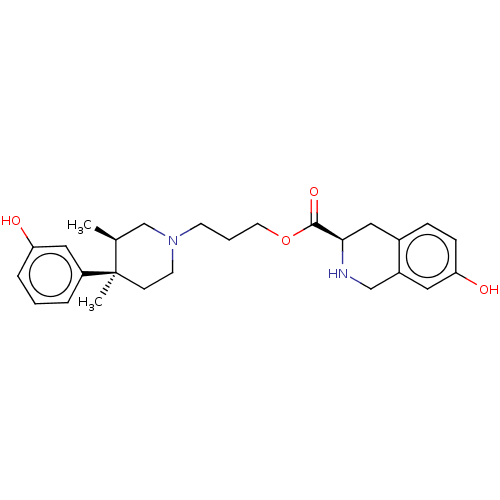 (CHEMBL3264440) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 93 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Science and Technology Curated by ChEMBL | Assay Description Displacement of [3H]-diprenorphine from rat mu-opioid receptor in expressed in rat C6 cell membranes after 1 hr | Eur J Med Chem 141: 632-647 (2017) Article DOI: 10.1016/j.ejmech.2017.10.012 BindingDB Entry DOI: 10.7270/Q2V98BQ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50012150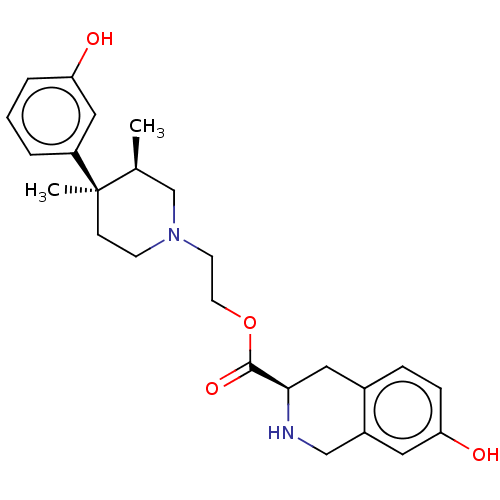 (CHEMBL3264439) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 98 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Science and Technology Curated by ChEMBL | Assay Description Displacement of [3H]-diprenorphine from rat mu-opioid receptor in expressed in rat C6 cell membranes after 1 hr | Eur J Med Chem 141: 632-647 (2017) Article DOI: 10.1016/j.ejmech.2017.10.012 BindingDB Entry DOI: 10.7270/Q2V98BQ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50012150 (CHEMBL3264439) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 135 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Science and Technology Curated by ChEMBL | Assay Description Displacement of [3H]-diprenorphine from human kappa opioid receptor expressed in CHO cells after 1 hr | Eur J Med Chem 141: 632-647 (2017) Article DOI: 10.1016/j.ejmech.2017.10.012 BindingDB Entry DOI: 10.7270/Q2V98BQ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50027038 (CHEMBL2112474) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 164 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Science and Technology Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from mu-opioid receptor in rat brain membranes after 2 hrs | Eur J Med Chem 141: 632-647 (2017) Article DOI: 10.1016/j.ejmech.2017.10.012 BindingDB Entry DOI: 10.7270/Q2V98BQ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50027051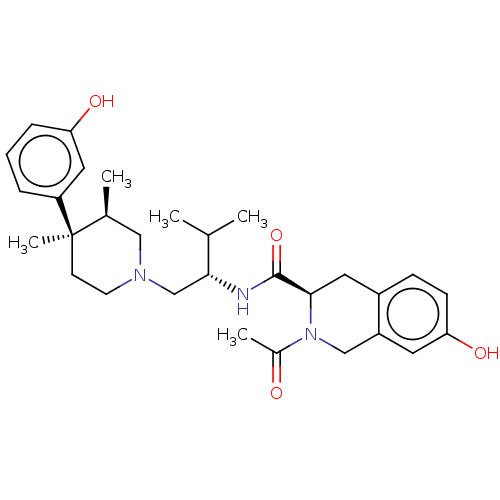 (CHEMBL2112472) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 164 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Science and Technology Curated by ChEMBL | Assay Description Displacement of [3H]U69,593 from kappa opioid receptor in guinea pig brain membranes after 2 hrs | Eur J Med Chem 141: 632-647 (2017) Article DOI: 10.1016/j.ejmech.2017.10.012 BindingDB Entry DOI: 10.7270/Q2V98BQ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50012151 (CHEMBL3264440) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 174 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Science and Technology Curated by ChEMBL | Assay Description Displacement of [3H]-diprenorphine from human kappa opioid receptor expressed in CHO cells after 1 hr | Eur J Med Chem 141: 632-647 (2017) Article DOI: 10.1016/j.ejmech.2017.10.012 BindingDB Entry DOI: 10.7270/Q2V98BQ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50130563 ((3R)-7-Hydroxy-N-[(1S)-1-{[(3R,4R)-4-(3-hydroxyphe...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | >300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Science and Technology Curated by ChEMBL | Assay Description Displacement of [3H]DADLE from delta-opioid receptor in rat brain membranes after 2 hrs | Eur J Med Chem 141: 632-647 (2017) Article DOI: 10.1016/j.ejmech.2017.10.012 BindingDB Entry DOI: 10.7270/Q2V98BQ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50026614 (CHEMBL575508) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 616 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Science and Technology Curated by ChEMBL | Assay Description Displacement of [3H]DADLE from delta-opioid receptor in rat brain membranes after 2 hrs | Eur J Med Chem 141: 632-647 (2017) Article DOI: 10.1016/j.ejmech.2017.10.012 BindingDB Entry DOI: 10.7270/Q2V98BQ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50027051 (CHEMBL2112472) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 764 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Science and Technology Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from mu-opioid receptor in rat brain membranes after 2 hrs | Eur J Med Chem 141: 632-647 (2017) Article DOI: 10.1016/j.ejmech.2017.10.012 BindingDB Entry DOI: 10.7270/Q2V98BQ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50027038 (CHEMBL2112474) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >3.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Science and Technology Curated by ChEMBL | Assay Description Displacement of [3H]DADLE from delta-opioid receptor in rat brain membranes after 2 hrs | Eur J Med Chem 141: 632-647 (2017) Article DOI: 10.1016/j.ejmech.2017.10.012 BindingDB Entry DOI: 10.7270/Q2V98BQ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50027051 (CHEMBL2112472) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >4.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Science and Technology Curated by ChEMBL | Assay Description Displacement of [3H]DADLE from delta-opioid receptor in rat brain membranes after 2 hrs | Eur J Med Chem 141: 632-647 (2017) Article DOI: 10.1016/j.ejmech.2017.10.012 BindingDB Entry DOI: 10.7270/Q2V98BQ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanin-concentrating hormone receptor 1 (Homo sapiens (Human)) | BDBM50169371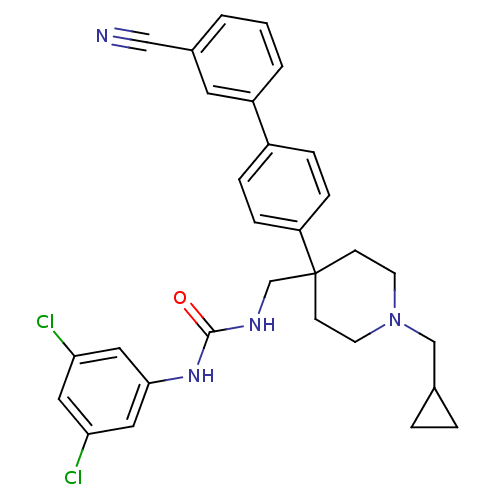 (1-[4-(3'-Cyano-biphenyl-4-yl)-1-cyclopropylmethyl-...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Science and Technology Curated by ChEMBL | Assay Description Antagonist activity at MCHR1 (unknown origin) expressed in CHO cells co-expressing aequorin incubated for 10 mins by luminescence assay | Bioorg Med Chem Lett 29: (2019) Article DOI: 10.1016/j.bmcl.2019.126741 BindingDB Entry DOI: 10.7270/Q2GH9NDS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanin-concentrating hormone receptor 1 (Homo sapiens (Human)) | BDBM50169377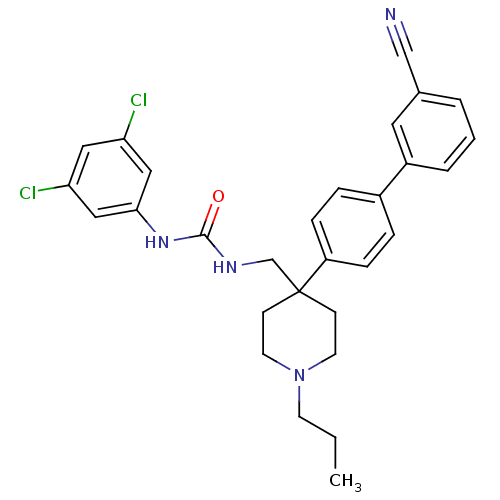 (1-[4-(3'-Cyano-biphenyl-4-yl)-1-propyl-piperidin-4...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.310 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Science and Technology Curated by ChEMBL | Assay Description Antagonist activity at MCHR1 (unknown origin) expressed in CHO cells co-expressing aequorin incubated for 10 mins by luminescence assay | Bioorg Med Chem Lett 29: (2019) Article DOI: 10.1016/j.bmcl.2019.126741 BindingDB Entry DOI: 10.7270/Q2GH9NDS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanin-concentrating hormone receptor 1 (Homo sapiens (Human)) | BDBM50169374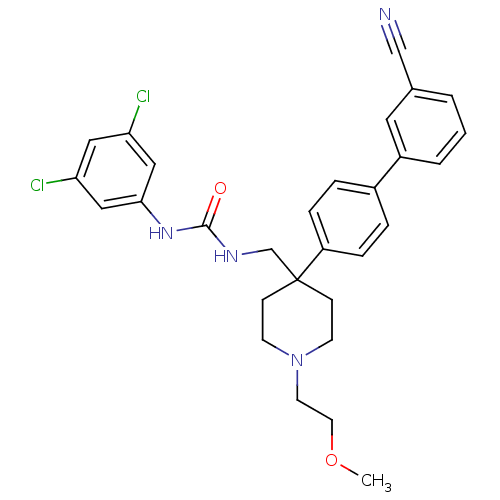 (1-[4-(3'-Cyano-biphenyl-4-yl)-1-(2-methoxy-ethyl)-...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.410 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Science and Technology Curated by ChEMBL | Assay Description Antagonist activity at MCHR1 (unknown origin) expressed in CHO cells co-expressing aequorin incubated for 10 mins by luminescence assay | Bioorg Med Chem Lett 29: (2019) Article DOI: 10.1016/j.bmcl.2019.126741 BindingDB Entry DOI: 10.7270/Q2GH9NDS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase 3 (Plasmodium falciparum (isolate 3D7)) | BDBM50589997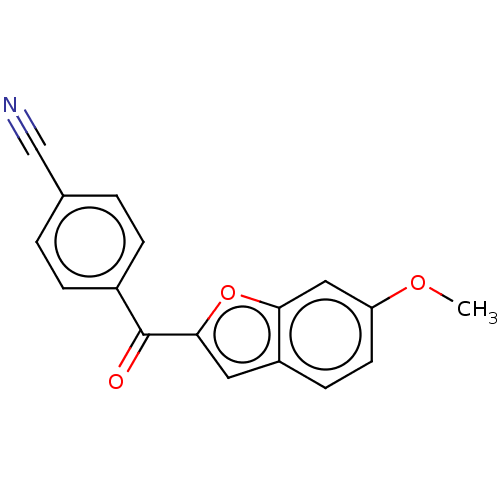 (CHEMBL5208060) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.490 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Bioorg Med Chem Lett 15: 1333-6 (2005) Article DOI: 10.1021/acs.jmedchem.2c00996 BindingDB Entry DOI: 10.7270/Q2445RF2 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase 3 (Plasmodium falciparum (isolate 3D7)) | BDBM50589997 (CHEMBL5208060) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.490 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Bioorg Med Chem Lett 15: 1333-6 (2005) Article DOI: 10.1021/acs.jmedchem.2c00996 BindingDB Entry DOI: 10.7270/Q2445RF2 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanin-concentrating hormone receptor 1 (Homo sapiens (Human)) | BDBM50168812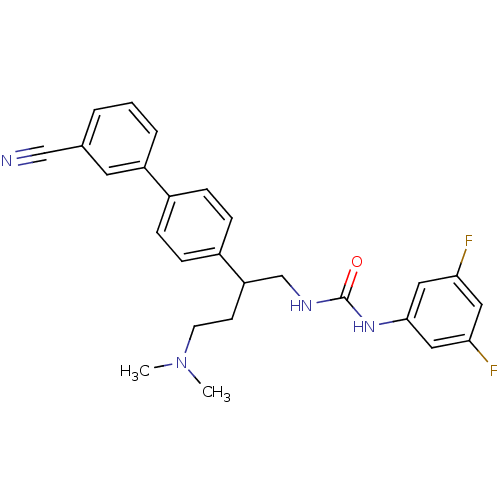 (1-[2-(3'-Cyano-biphenyl-4-yl)-4-dimethylamino-buty...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.880 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Science and Technology Curated by ChEMBL | Assay Description Antagonist activity at MCHR1 (unknown origin) expressed in CHO cells co-expressing aequorin incubated for 10 mins by luminescence assay | Bioorg Med Chem Lett 29: (2019) Article DOI: 10.1016/j.bmcl.2019.126741 BindingDB Entry DOI: 10.7270/Q2GH9NDS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanin-concentrating hormone receptor 1 (Homo sapiens (Human)) | BDBM50168825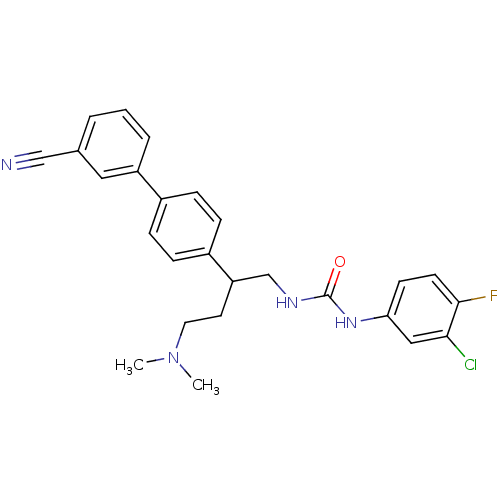 (1-(3-Chloro-4-fluoro-phenyl)-3-[2-(3'-cyano-biphen...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.980 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Science and Technology Curated by ChEMBL | Assay Description Antagonist activity at MCHR1 (unknown origin) expressed in CHO cells co-expressing aequorin incubated for 10 mins by luminescence assay | Bioorg Med Chem Lett 29: (2019) Article DOI: 10.1016/j.bmcl.2019.126741 BindingDB Entry DOI: 10.7270/Q2GH9NDS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanin-concentrating hormone receptor 1 (Homo sapiens (Human)) | BDBM50169382 (1-[4-(3'-Cyano-biphenyl-4-yl)-1-cyclohexyl-piperid...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Science and Technology Curated by ChEMBL | Assay Description Antagonist activity at MCHR1 (unknown origin) expressed in CHO cells co-expressing aequorin incubated for 10 mins by luminescence assay | Bioorg Med Chem Lett 29: (2019) Article DOI: 10.1016/j.bmcl.2019.126741 BindingDB Entry DOI: 10.7270/Q2GH9NDS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanin-concentrating hormone receptor 1 (Homo sapiens (Human)) | BDBM50168800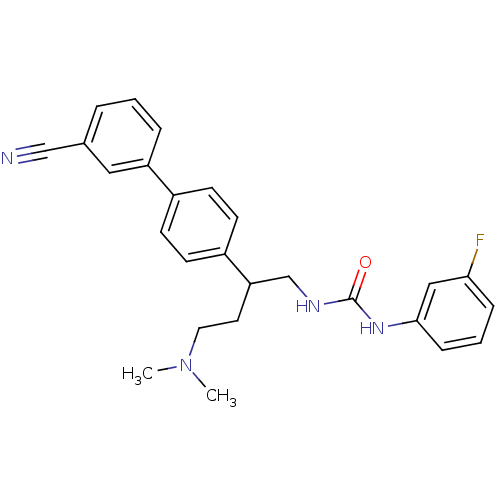 (1-[2-(3'-Cyano-biphenyl-4-yl)-4-dimethylamino-buty...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Science and Technology Curated by ChEMBL | Assay Description Antagonist activity at MCHR1 (unknown origin) expressed in CHO cells co-expressing aequorin incubated for 10 mins by luminescence assay | Bioorg Med Chem Lett 29: (2019) Article DOI: 10.1016/j.bmcl.2019.126741 BindingDB Entry DOI: 10.7270/Q2GH9NDS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Calcium-dependent protein kinase 1 (Plasmodium Falciparum) | BDBM36336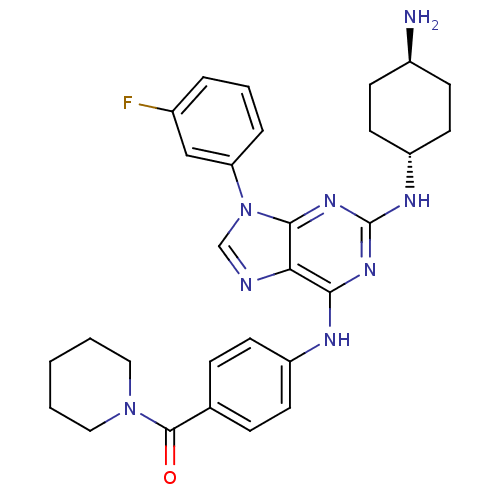 (CID24762166 | Purfalcamine, 1) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Bioorg Med Chem Lett 15: 1333-6 (2005) Article DOI: 10.1021/acs.jmedchem.2c00996 BindingDB Entry DOI: 10.7270/Q2445RF2 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanin-concentrating hormone receptor 1 (Homo sapiens (Human)) | BDBM50169398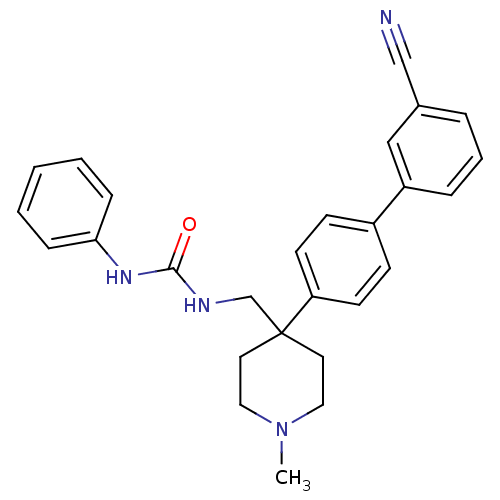 (1-[4-(3'-Cyano-biphenyl-4-yl)-1-methyl-piperidin-4...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Science and Technology Curated by ChEMBL | Assay Description Antagonist activity at MCHR1 (unknown origin) expressed in CHO cells co-expressing aequorin incubated for 10 mins by luminescence assay | Bioorg Med Chem Lett 29: (2019) Article DOI: 10.1016/j.bmcl.2019.126741 BindingDB Entry DOI: 10.7270/Q2GH9NDS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanin-concentrating hormone receptor 1 (Homo sapiens (Human)) | BDBM50192637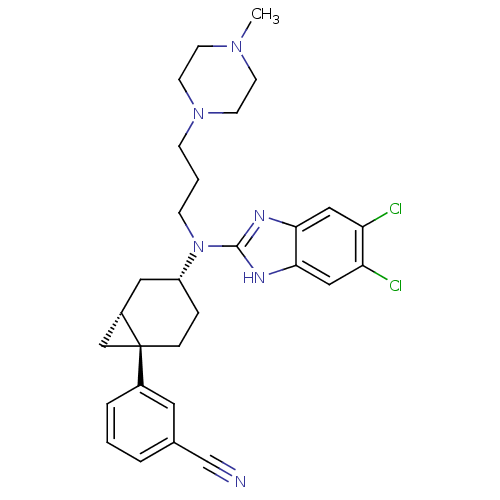 (3-((1R,4R,6R)-4-((5,6-dichloro-1H-benzo[d]imidazol...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Science and Technology Curated by ChEMBL | Assay Description Antagonist activity at MCHR1 (unknown origin) expressed in CHO cells co-expressing aequorin incubated for 10 mins by luminescence assay | Bioorg Med Chem Lett 29: (2019) Article DOI: 10.1016/j.bmcl.2019.126741 BindingDB Entry DOI: 10.7270/Q2GH9NDS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanin-concentrating hormone receptor 1 (Homo sapiens (Human)) | BDBM50168823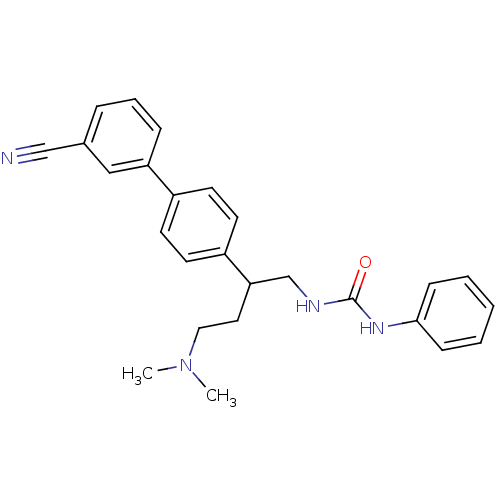 (1-[2-(3'-Cyano-biphenyl-4-yl)-4-dimethylamino-buty...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Science and Technology Curated by ChEMBL | Assay Description Antagonist activity at MCHR1 (unknown origin) expressed in CHO cells co-expressing aequorin incubated for 10 mins by luminescence assay | Bioorg Med Chem Lett 29: (2019) Article DOI: 10.1016/j.bmcl.2019.126741 BindingDB Entry DOI: 10.7270/Q2GH9NDS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanin-concentrating hormone receptor 1 (Homo sapiens (Human)) | BDBM50169391 (1-(3,5-Dichloro-phenyl)-3-[1-methyl-4-(4-pyridin-3...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Science and Technology Curated by ChEMBL | Assay Description Antagonist activity at MCHR1 (unknown origin) expressed in CHO cells co-expressing aequorin incubated for 10 mins by luminescence assay | Bioorg Med Chem Lett 29: (2019) Article DOI: 10.1016/j.bmcl.2019.126741 BindingDB Entry DOI: 10.7270/Q2GH9NDS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanin-concentrating hormone receptor 1 (Homo sapiens (Human)) | BDBM50186837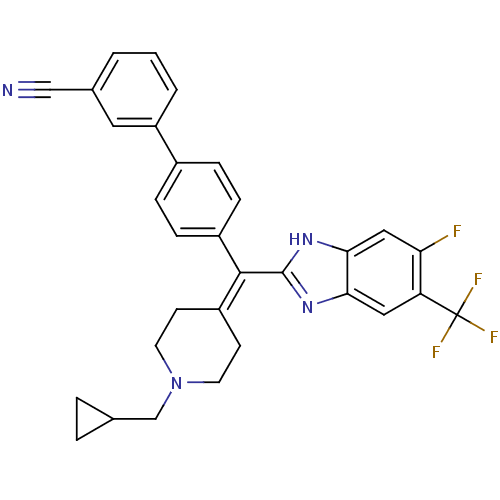 (4'-[(1-cyclopropylmethyl-piperidin-4-ylidene)-(5-f...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Science and Technology Curated by ChEMBL | Assay Description Antagonist activity at MCHR1 (unknown origin) expressed in CHO cells co-expressing aequorin incubated for 10 mins by luminescence assay | Bioorg Med Chem Lett 29: (2019) Article DOI: 10.1016/j.bmcl.2019.126741 BindingDB Entry DOI: 10.7270/Q2GH9NDS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM11639 (4-[5-(4-methylphenyl)-3-(trifluoromethyl)-1H-pyraz...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | n/a | n/a | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Suez Canal University Curated by ChEMBL | Assay Description Inhibition of COX2 (unknown origin) using arachidonic acid as substrate pretreated for 10 mins followed by substrate addition measured after 2 mins b... | Bioorg Med Chem Lett 27: 2377-2383 (2017) Article DOI: 10.1016/j.bmcl.2017.04.020 BindingDB Entry DOI: 10.7270/Q2GM89QB | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Melanin-concentrating hormone receptor 1 (Homo sapiens (Human)) | BDBM50186834 (4'-[6-cyclopropylmethyl-1-(5-fluoro-6-trifluoromet...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Science and Technology Curated by ChEMBL | Assay Description Antagonist activity at MCHR1 (unknown origin) expressed in CHO cells co-expressing aequorin incubated for 10 mins by luminescence assay | Bioorg Med Chem Lett 29: (2019) Article DOI: 10.1016/j.bmcl.2019.126741 BindingDB Entry DOI: 10.7270/Q2GH9NDS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanin-concentrating hormone receptor 1 (Homo sapiens (Human)) | BDBM50529233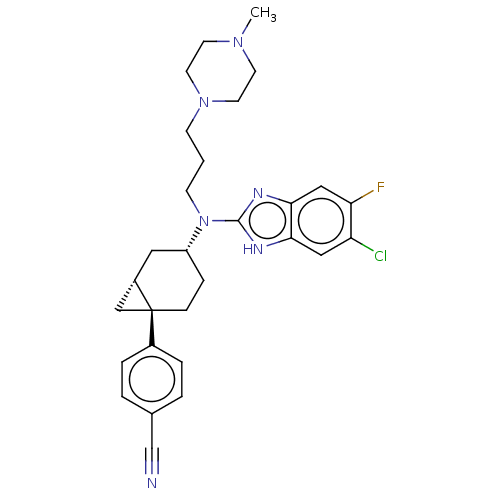 (CHEMBL4465399) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.90 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Science and Technology Curated by ChEMBL | Assay Description Antagonist activity at MCHR1 (unknown origin) expressed in CHO cells co-expressing aequorin incubated for 10 mins by luminescence assay | Bioorg Med Chem Lett 29: (2019) Article DOI: 10.1016/j.bmcl.2019.126741 BindingDB Entry DOI: 10.7270/Q2GH9NDS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanin-concentrating hormone receptor 1 (Homo sapiens (Human)) | BDBM50170613 (1-(3'-Cyano-biphenyl-4-yl)-3-(3-fluoro-4-trifluoro...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Science and Technology Curated by ChEMBL | Assay Description Antagonist activity at MCHR1 (unknown origin) expressed in CHO cells co-expressing aequorin incubated for 10 mins by luminescence assay | Bioorg Med Chem Lett 29: (2019) Article DOI: 10.1016/j.bmcl.2019.126741 BindingDB Entry DOI: 10.7270/Q2GH9NDS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanin-concentrating hormone receptor 1 (Homo sapiens (Human)) | BDBM50529242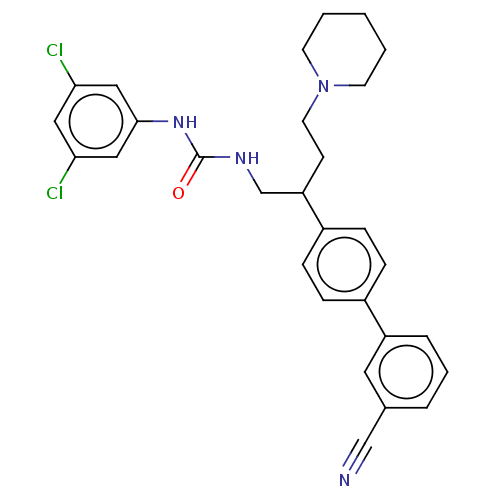 (CHEMBL2112988) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.40 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Science and Technology Curated by ChEMBL | Assay Description Antagonist activity at MCHR1 (unknown origin) expressed in CHO cells co-expressing aequorin incubated for 10 mins by luminescence assay | Bioorg Med Chem Lett 29: (2019) Article DOI: 10.1016/j.bmcl.2019.126741 BindingDB Entry DOI: 10.7270/Q2GH9NDS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanin-concentrating hormone receptor 1 (Homo sapiens (Human)) | BDBM50169372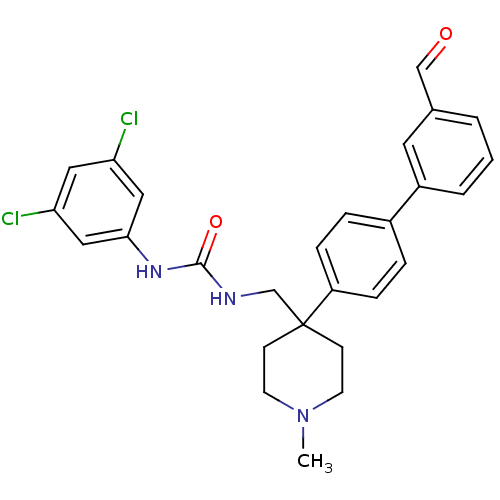 (1-(3,5-Dichloro-phenyl)-3-[4-(3'-formyl-biphenyl-4...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5.5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Science and Technology Curated by ChEMBL | Assay Description Antagonist activity at MCHR1 (unknown origin) expressed in CHO cells co-expressing aequorin incubated for 10 mins by luminescence assay | Bioorg Med Chem Lett 29: (2019) Article DOI: 10.1016/j.bmcl.2019.126741 BindingDB Entry DOI: 10.7270/Q2GH9NDS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanin-concentrating hormone receptor 1 (Homo sapiens (Human)) | BDBM50529239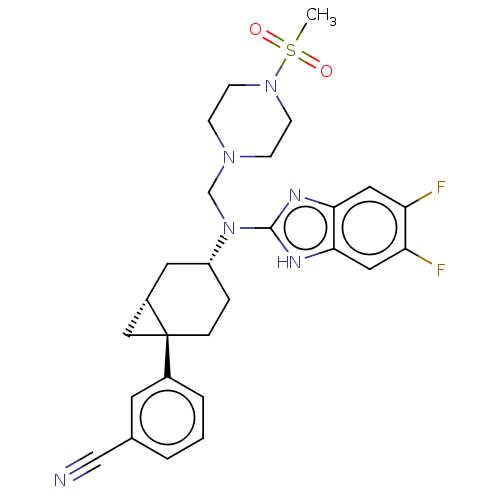 (CHEMBL4444299) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.80 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Science and Technology Curated by ChEMBL | Assay Description Antagonist activity at MCHR1 (unknown origin) expressed in CHO cells co-expressing aequorin incubated for 10 mins by luminescence assay | Bioorg Med Chem Lett 29: (2019) Article DOI: 10.1016/j.bmcl.2019.126741 BindingDB Entry DOI: 10.7270/Q2GH9NDS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanin-concentrating hormone receptor 1 (Homo sapiens (Human)) | BDBM50170191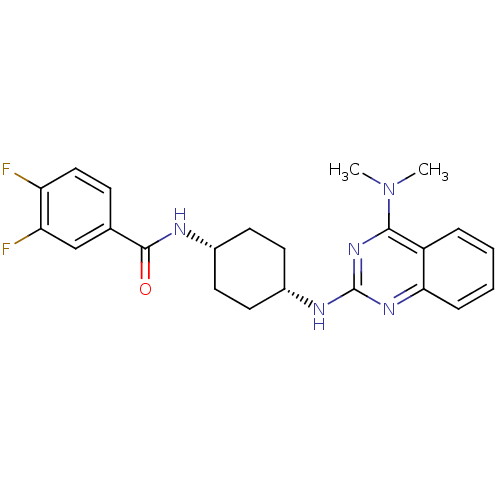 (CHEMBL182150 | N-((cis)-4-(4-(dimethylamino)quinaz...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 8.30 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Science and Technology Curated by ChEMBL | Assay Description Antagonist activity at MCHR1 (unknown origin) expressed in CHO cells co-expressing aequorin incubated for 10 mins by luminescence assay | Bioorg Med Chem Lett 29: (2019) Article DOI: 10.1016/j.bmcl.2019.126741 BindingDB Entry DOI: 10.7270/Q2GH9NDS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanin-concentrating hormone receptor 1 (Homo sapiens (Human)) | BDBM50186826 (4'-[6-benzyl-1-(5-fluoro-6-trifluoromethyl-1H-benz...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Science and Technology Curated by ChEMBL | Assay Description Antagonist activity at MCHR1 (unknown origin) expressed in CHO cells co-expressing aequorin incubated for 10 mins by luminescence assay | Bioorg Med Chem Lett 29: (2019) Article DOI: 10.1016/j.bmcl.2019.126741 BindingDB Entry DOI: 10.7270/Q2GH9NDS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50249139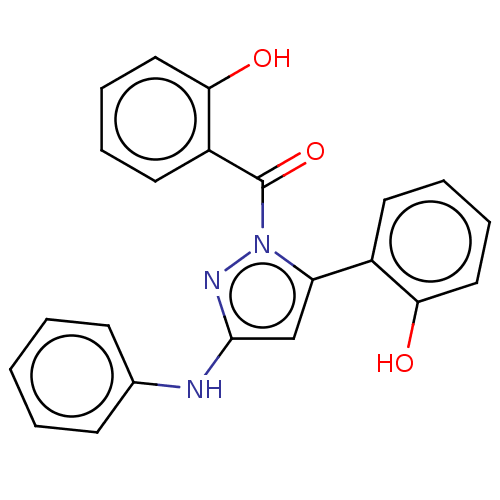 (CHEMBL4065530) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Suez Canal University Curated by ChEMBL | Assay Description Inhibition of COX2 (unknown origin) using arachidonic acid as substrate pretreated for 10 mins followed by substrate addition measured after 2 mins b... | Bioorg Med Chem Lett 27: 2377-2383 (2017) Article DOI: 10.1016/j.bmcl.2017.04.020 BindingDB Entry DOI: 10.7270/Q2GM89QB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50249135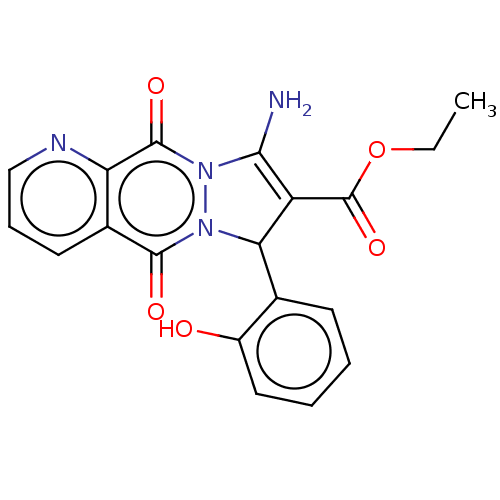 (CHEMBL4071983) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Suez Canal University Curated by ChEMBL | Assay Description Inhibition of COX2 (unknown origin) using arachidonic acid as substrate pretreated for 10 mins followed by substrate addition measured after 2 mins b... | Bioorg Med Chem Lett 27: 2377-2383 (2017) Article DOI: 10.1016/j.bmcl.2017.04.020 BindingDB Entry DOI: 10.7270/Q2GM89QB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 180 total ) | Next | Last >> |