

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Endothelin-converting enzyme 1 (Homo sapiens (Human)) | BDBM50035236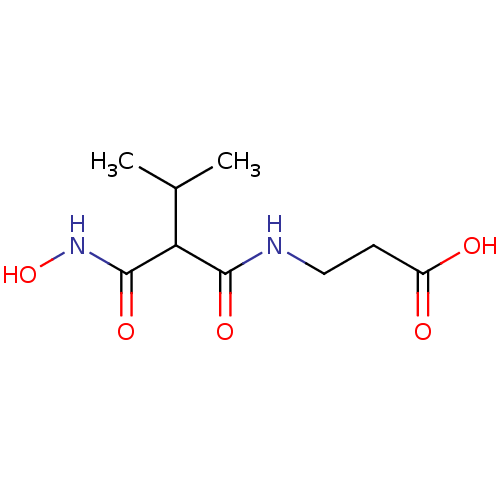 (3-(2-Hydroxycarbamoyl-3-methyl-butyrylamino)-propi...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against human bronchiolar smooth muscle Endothelin-converting enzyme 1 | J Med Chem 38: 2119-29 (1995) BindingDB Entry DOI: 10.7270/Q2D79C2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Oryctolagus cuniculus (rabbit)) | BDBM50035247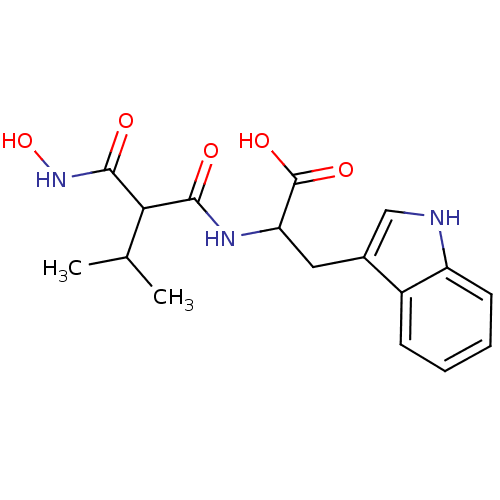 (2-(2-Hydroxycarbamoyl-3-methyl-butyrylamino)-3-(1H...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against big ET-1 of Neutral endopeptidase | J Med Chem 38: 2119-29 (1995) BindingDB Entry DOI: 10.7270/Q2D79C2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Oryctolagus cuniculus (rabbit)) | BDBM50035246 (2-(2-Hydroxycarbamoyl-4-methyl-pentanoylamino)-3-(...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against Leu-enkeph of Neutral endopeptidase | J Med Chem 38: 2119-29 (1995) BindingDB Entry DOI: 10.7270/Q2D79C2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Heat shock protein HSP 90-alpha (Homo sapiens (Human)) | BDBM81917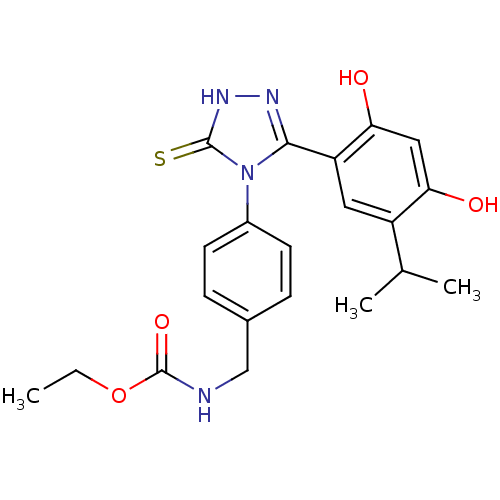 (BX-2819) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0400 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Bayer Healthcare, | Assay Description To assess the affinity of compounds binding to Hsp90, we measured their ability to compete with the binding of a fluorescent analog of GA (GM-Bodipy)... | Chem Biol Drug Des 74: 43-50 (2009) Article DOI: 10.1111/j.1747-0285.2009.00833.x BindingDB Entry DOI: 10.7270/Q2KW5DJW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Oryctolagus cuniculus (rabbit)) | BDBM50035246 (2-(2-Hydroxycarbamoyl-4-methyl-pentanoylamino)-3-(...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against big ET-1 of Neutral endopeptidase | J Med Chem 38: 2119-29 (1995) BindingDB Entry DOI: 10.7270/Q2D79C2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Heat shock protein HSP 90-alpha (Homo sapiens (Human)) | BDBM81916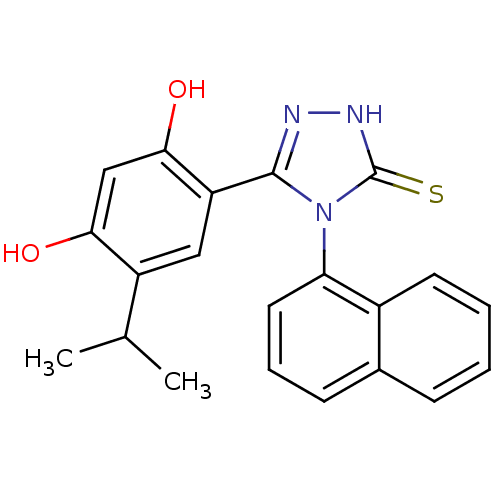 (lspropyl analog, 5) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Bayer Healthcare, | Assay Description To assess the affinity of compounds binding to Hsp90, we measured their ability to compete with the binding of a fluorescent analog of GA (GM-Bodipy)... | Chem Biol Drug Des 74: 43-50 (2009) Article DOI: 10.1111/j.1747-0285.2009.00833.x BindingDB Entry DOI: 10.7270/Q2KW5DJW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Heat shock protein HSP 90-alpha (Homo sapiens (Human)) | BDBM81914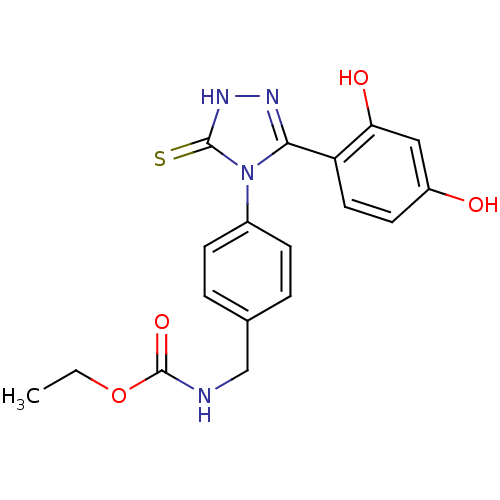 (Ethyl carbamate analog, 3) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Bayer Healthcare, | Assay Description To assess the affinity of compounds binding to Hsp90, we measured their ability to compete with the binding of a fluorescent analog of GA (GM-Bodipy)... | Chem Biol Drug Des 74: 43-50 (2009) Article DOI: 10.1111/j.1747-0285.2009.00833.x BindingDB Entry DOI: 10.7270/Q2KW5DJW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Heat shock protein HSP 90-alpha (Homo sapiens (Human)) | BDBM81915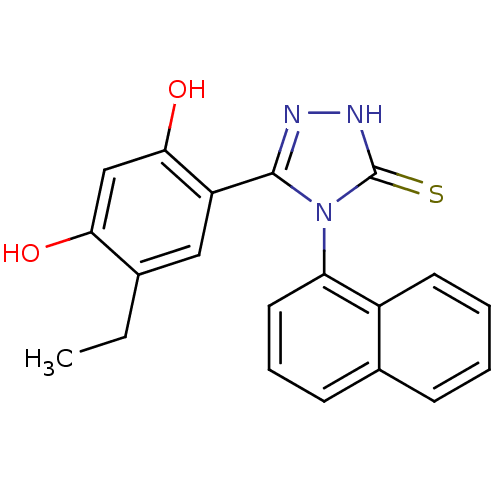 (Ethyl analog, 4) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Bayer Healthcare, | Assay Description To assess the affinity of compounds binding to Hsp90, we measured their ability to compete with the binding of a fluorescent analog of GA (GM-Bodipy)... | Chem Biol Drug Des 74: 43-50 (2009) Article DOI: 10.1111/j.1747-0285.2009.00833.x BindingDB Entry DOI: 10.7270/Q2KW5DJW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-converting enzyme 1 (Homo sapiens (Human)) | BDBM50035247 (2-(2-Hydroxycarbamoyl-3-methyl-butyrylamino)-3-(1H...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against human bronchiolar smooth muscle Endothelin-converting enzyme 1 | J Med Chem 38: 2119-29 (1995) BindingDB Entry DOI: 10.7270/Q2D79C2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-converting enzyme 1 (Homo sapiens (Human)) | BDBM50035246 (2-(2-Hydroxycarbamoyl-4-methyl-pentanoylamino)-3-(...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against human bronchiolar smooth muscle Endothelin-converting enzyme 1 | J Med Chem 38: 2119-29 (1995) BindingDB Entry DOI: 10.7270/Q2D79C2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Heat shock protein HSP 90-alpha (Homo sapiens (Human)) | BDBM81912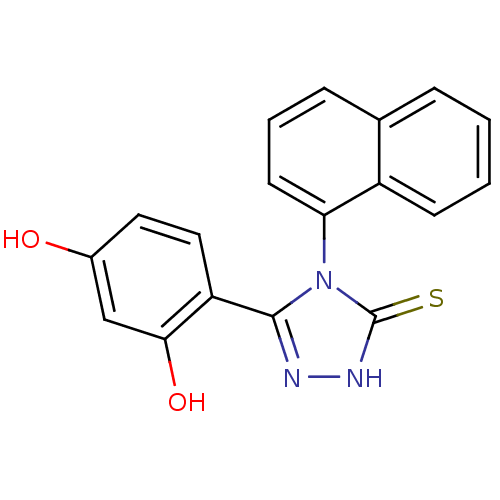 (DC23 | Resorcinol analog, 1) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Bayer Healthcare, | Assay Description To assess the affinity of compounds binding to Hsp90, we measured their ability to compete with the binding of a fluorescent analog of GA (GM-Bodipy)... | Chem Biol Drug Des 74: 43-50 (2009) Article DOI: 10.1111/j.1747-0285.2009.00833.x BindingDB Entry DOI: 10.7270/Q2KW5DJW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Oryctolagus cuniculus (rabbit)) | BDBM50035247 (2-(2-Hydroxycarbamoyl-3-methyl-butyrylamino)-3-(1H...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against Leu-enkeph of Neutral endopeptidase | J Med Chem 38: 2119-29 (1995) BindingDB Entry DOI: 10.7270/Q2D79C2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Heat shock protein HSP 90-alpha (Homo sapiens (Human)) | BDBM81913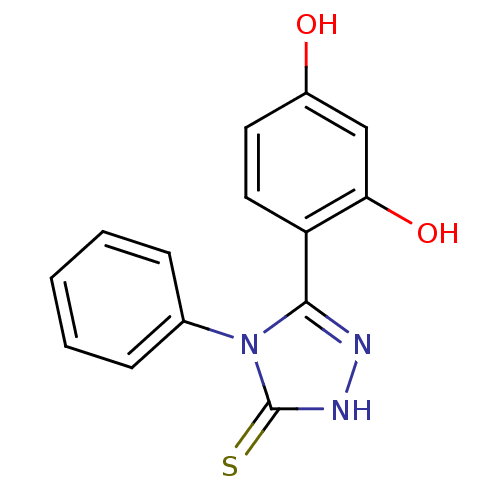 (Unsubstituted phenyl ring analog, 2) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Bayer Healthcare, | Assay Description To assess the affinity of compounds binding to Hsp90, we measured their ability to compete with the binding of a fluorescent analog of GA (GM-Bodipy)... | Chem Biol Drug Des 74: 43-50 (2009) Article DOI: 10.1111/j.1747-0285.2009.00833.x BindingDB Entry DOI: 10.7270/Q2KW5DJW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-converting enzyme 1 (Homo sapiens (Human)) | BDBM50251742 ((3R,4R,5R,6S)-3,4,5-trihydroxy-6-methyl-tetrahydro...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against human bronchiolar smooth muscle Endothelin-converting enzyme 1 | J Med Chem 38: 2119-29 (1995) BindingDB Entry DOI: 10.7270/Q2D79C2M | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Neprilysin (Oryctolagus cuniculus (rabbit)) | BDBM50035251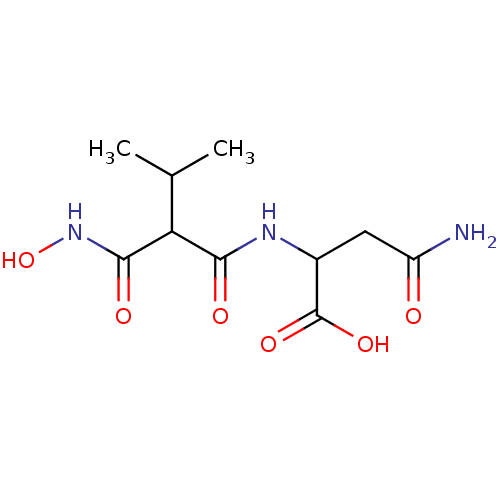 (2-(2-Hydroxycarbamoyl-3-methyl-butyrylamino)-succi...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against Leu-enkeph of Neutral endopeptidase | J Med Chem 38: 2119-29 (1995) BindingDB Entry DOI: 10.7270/Q2D79C2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Oryctolagus cuniculus (rabbit)) | BDBM50105264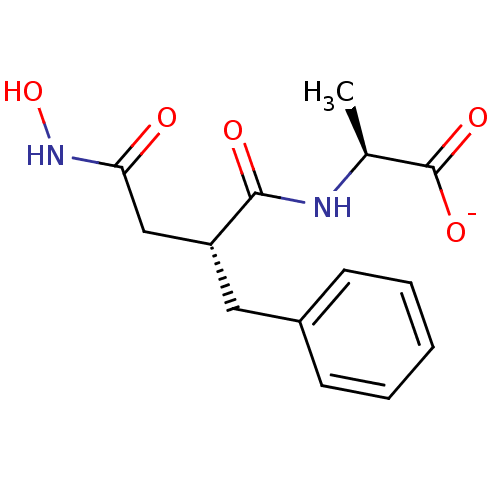 (2-(2-Benzyl-3-hydroxycarbamoyl-propionylamino)-pro...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against Leu-enkeph of Neutral endopeptidase | J Med Chem 38: 2119-29 (1995) BindingDB Entry DOI: 10.7270/Q2D79C2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Oryctolagus cuniculus (rabbit)) | BDBM50035236 (3-(2-Hydroxycarbamoyl-3-methyl-butyrylamino)-propi...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against big ET-1 of Neutral endopeptidase | J Med Chem 38: 2119-29 (1995) BindingDB Entry DOI: 10.7270/Q2D79C2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-converting enzyme 1 (Homo sapiens (Human)) | BDBM50035239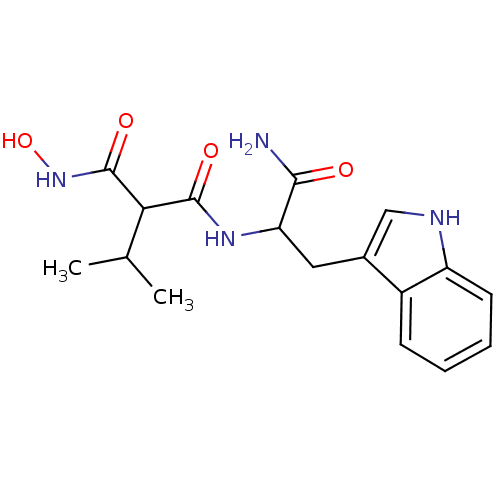 (CHEMBL63317 | N-[1-Carbamoyl-2-(1H-indol-3-yl)-eth...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against human bronchiolar smooth muscle Endothelin-converting enzyme 1 | J Med Chem 38: 2119-29 (1995) BindingDB Entry DOI: 10.7270/Q2D79C2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Oryctolagus cuniculus (rabbit)) | BDBM50035235 (CHEMBL2372437 | N-[1-(Carbamoylmethyl-carbamoyl)-e...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against Leu-enkeph of Neutral endopeptidase | J Med Chem 38: 2119-29 (1995) BindingDB Entry DOI: 10.7270/Q2D79C2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Oryctolagus cuniculus (rabbit)) | BDBM50035236 (3-(2-Hydroxycarbamoyl-3-methyl-butyrylamino)-propi...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against Leu-enkeph of Neutral endopeptidase | J Med Chem 38: 2119-29 (1995) BindingDB Entry DOI: 10.7270/Q2D79C2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Oryctolagus cuniculus (rabbit)) | BDBM50035239 (CHEMBL63317 | N-[1-Carbamoyl-2-(1H-indol-3-yl)-eth...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against Leu-enkeph of Neutral endopeptidase | J Med Chem 38: 2119-29 (1995) BindingDB Entry DOI: 10.7270/Q2D79C2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Oryctolagus cuniculus (rabbit)) | BDBM50035249 (CHEMBL303892 | N-[1-Carbamoyl-2-(1H-indol-3-yl)-et...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against Leu-enkeph of Neutral endopeptidase | J Med Chem 38: 2119-29 (1995) BindingDB Entry DOI: 10.7270/Q2D79C2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Oryctolagus cuniculus (rabbit)) | BDBM50251742 ((3R,4R,5R,6S)-3,4,5-trihydroxy-6-methyl-tetrahydro...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against big ET-1 of Neutral endopeptidase | J Med Chem 38: 2119-29 (1995) BindingDB Entry DOI: 10.7270/Q2D79C2M | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Thermolysin (Bacillus thermoproteolyticus) | BDBM50035249 (CHEMBL303892 | N-[1-Carbamoyl-2-(1H-indol-3-yl)-et...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against thermolysin with 0.5 uM [Leu5]-enkephalin (NEN) | J Med Chem 38: 2119-29 (1995) BindingDB Entry DOI: 10.7270/Q2D79C2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-phosphoinositide-dependent protein kinase 1 (Homo sapiens (Human)) | BDBM17003 (Indolinone based compound, 7l | [(3Z)-3-({4-[3-(am...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences | Assay Description The coupled assay can detect inhibitors of AKT2 activation, as well as direct inhibitors of PDK1 or AKT2. Inactive AKT2 is activated in situ by incub... | Bioorg Med Chem Lett 17: 3819-25 (2007) Article DOI: 10.1016/j.bmcl.2007.05.060 BindingDB Entry DOI: 10.7270/Q29G5K22 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thermolysin (Bacillus thermoproteolyticus) | BDBM50105264 (2-(2-Benzyl-3-hydroxycarbamoyl-propionylamino)-pro...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against thermolysin with 0.5 uM [Leu5]-enkephalin (NEN) | J Med Chem 38: 2119-29 (1995) BindingDB Entry DOI: 10.7270/Q2D79C2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-phosphoinositide-dependent protein kinase 1 (Homo sapiens (Human)) | BDBM17048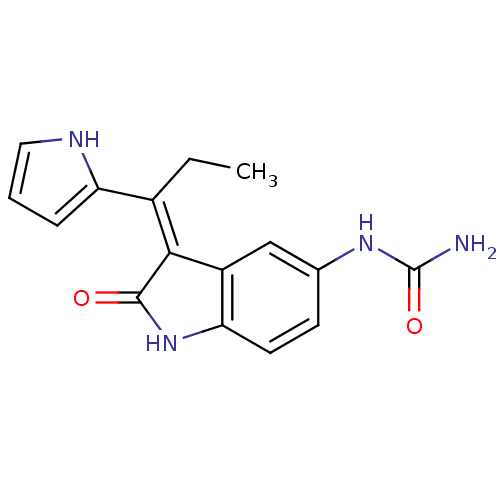 (Indolinone based inhibitor, 4j | [(3Z)-2-oxo-3-[1-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences | Assay Description The coupled assay can detect inhibitors of AKT2 activation, as well as direct inhibitors of PDK1 or AKT2. Inactive AKT2 is activated in situ by incub... | Bioorg Med Chem Lett 17: 3814-8 (2007) Article DOI: 10.1016/j.bmcl.2007.04.071 BindingDB Entry DOI: 10.7270/Q25Q4TBB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-phosphoinositide-dependent protein kinase 1 (Homo sapiens (Human)) | BDBM17014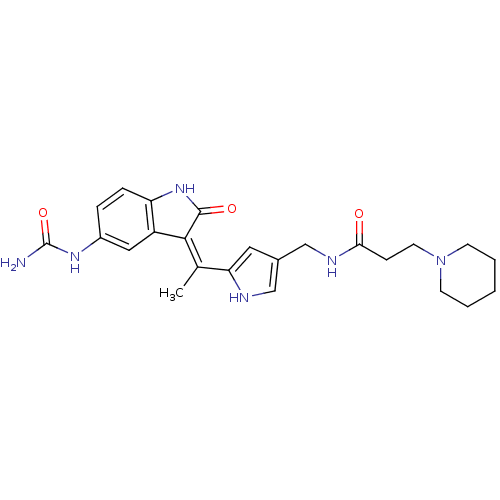 (Indolinone based compound, 22 | N-[(5-{1-[(3Z)-5-(...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences | Assay Description The coupled assay can detect inhibitors of AKT2 activation, as well as direct inhibitors of PDK1 or AKT2. Inactive AKT2 is activated in situ by incub... | Bioorg Med Chem Lett 17: 3819-25 (2007) Article DOI: 10.1016/j.bmcl.2007.05.060 BindingDB Entry DOI: 10.7270/Q29G5K22 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Oryctolagus cuniculus (rabbit)) | BDBM50035240 ((2-Hydroxycarbamoyl-3-methyl-butyrylamino)-acetic ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against Leu-enkeph of Neutral endopeptidase | J Med Chem 38: 2119-29 (1995) BindingDB Entry DOI: 10.7270/Q2D79C2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-converting enzyme 1 (Homo sapiens (Human)) | BDBM50035251 (2-(2-Hydroxycarbamoyl-3-methyl-butyrylamino)-succi...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against human bronchiolar smooth muscle Endothelin-converting enzyme 1 | J Med Chem 38: 2119-29 (1995) BindingDB Entry DOI: 10.7270/Q2D79C2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Oryctolagus cuniculus (rabbit)) | BDBM50035239 (CHEMBL63317 | N-[1-Carbamoyl-2-(1H-indol-3-yl)-eth...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against big ET-1 of Neutral endopeptidase | J Med Chem 38: 2119-29 (1995) BindingDB Entry DOI: 10.7270/Q2D79C2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-phosphoinositide-dependent protein kinase 1 (Homo sapiens (Human)) | BDBM16995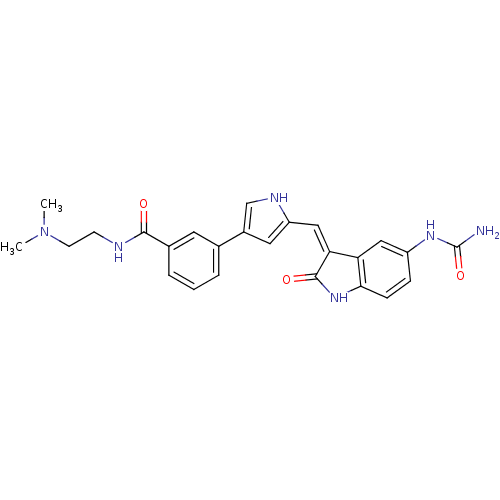 (3-(5-{[(3Z)-5-(carbamoylamino)-2-oxo-2,3-dihydro-1...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | 7.2 | 22 |
Berlex Biosciences | Assay Description The coupled assay can detect inhibitors of AKT2 activation, as well as direct inhibitors of PDK1 or AKT2. Inactive AKT2 is activated in situ by incub... | Bioorg Med Chem Lett 17: 3819-25 (2007) Article DOI: 10.1016/j.bmcl.2007.05.060 BindingDB Entry DOI: 10.7270/Q29G5K22 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-phosphoinositide-dependent protein kinase 1 (Homo sapiens (Human)) | BDBM17010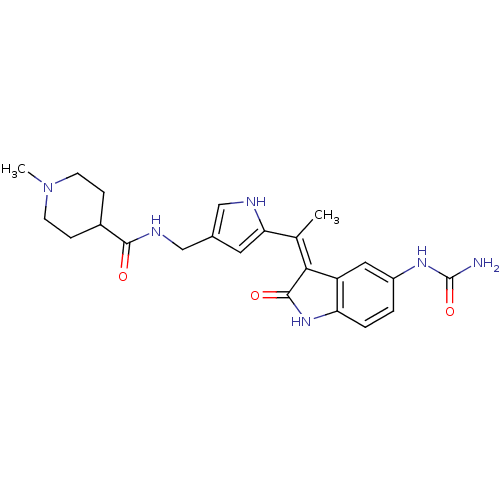 (Indolinone based compound, 18 | N-[(5-{1-[(3Z)-5-(...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences | Assay Description The coupled assay can detect inhibitors of AKT2 activation, as well as direct inhibitors of PDK1 or AKT2. Inactive AKT2 is activated in situ by incub... | Bioorg Med Chem Lett 17: 3819-25 (2007) Article DOI: 10.1016/j.bmcl.2007.05.060 BindingDB Entry DOI: 10.7270/Q29G5K22 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-phosphoinositide-dependent protein kinase 1 (Homo sapiens (Human)) | BDBM16996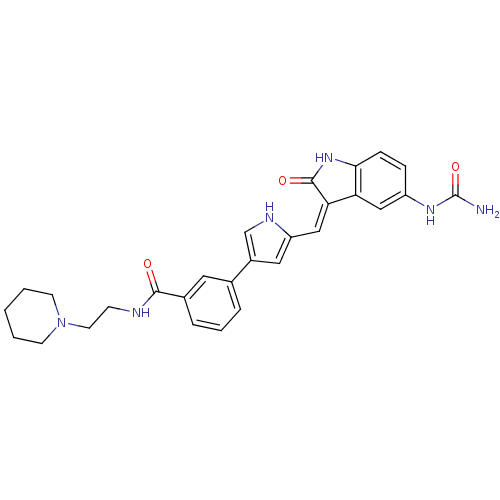 (3-(5-{[(3Z)-5-(carbamoylamino)-2-oxo-2,3-dihydro-1...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | 7.2 | 22 |
Berlex Biosciences | Assay Description The coupled assay can detect inhibitors of AKT2 activation, as well as direct inhibitors of PDK1 or AKT2. Inactive AKT2 is activated in situ by incub... | Bioorg Med Chem Lett 17: 3819-25 (2007) Article DOI: 10.1016/j.bmcl.2007.05.060 BindingDB Entry DOI: 10.7270/Q29G5K22 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Endothelin-converting enzyme 1 (Homo sapiens (Human)) | BDBM50035249 (CHEMBL303892 | N-[1-Carbamoyl-2-(1H-indol-3-yl)-et...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against human bronchiolar smooth muscle Endothelin-converting enzyme 1 | J Med Chem 38: 2119-29 (1995) BindingDB Entry DOI: 10.7270/Q2D79C2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-phosphoinositide-dependent protein kinase 1 (Homo sapiens (Human)) | BDBM17004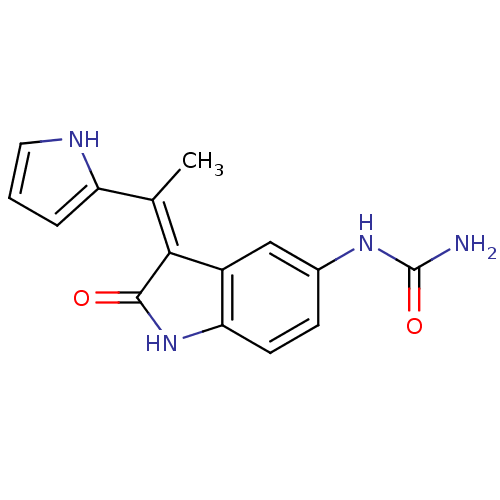 (BX-517 | Indolinone based inhibitor, 4i | [(3Z)-2-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences | Assay Description The coupled assay can detect inhibitors of AKT2 activation, as well as direct inhibitors of PDK1 or AKT2. Inactive AKT2 is activated in situ by incub... | Bioorg Med Chem Lett 17: 3814-8 (2007) Article DOI: 10.1016/j.bmcl.2007.04.071 BindingDB Entry DOI: 10.7270/Q25Q4TBB | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| 3-phosphoinositide-dependent protein kinase 1 (Homo sapiens (Human)) | BDBM17012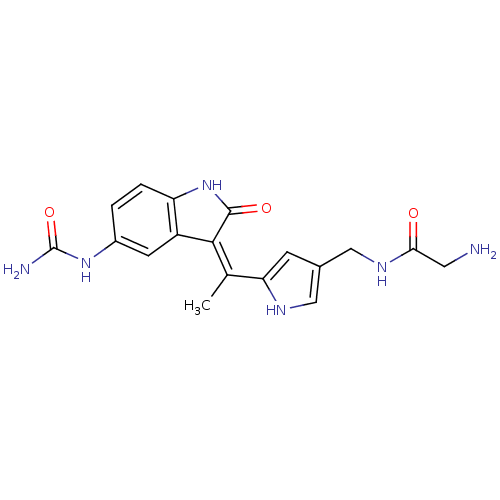 (2-amino-N-[(5-{1-[(3Z)-5-(carbamoylamino)-2-oxo-2,...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences | Assay Description The coupled assay can detect inhibitors of AKT2 activation, as well as direct inhibitors of PDK1 or AKT2. Inactive AKT2 is activated in situ by incub... | Bioorg Med Chem Lett 17: 3819-25 (2007) Article DOI: 10.1016/j.bmcl.2007.05.060 BindingDB Entry DOI: 10.7270/Q29G5K22 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-phosphoinositide-dependent protein kinase 1 (Homo sapiens (Human)) | BDBM17011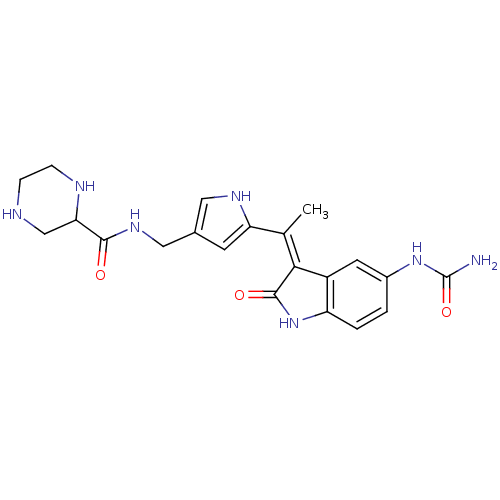 (Indolinone based compound, 19 | N-[(5-{1-[(3Z)-5-(...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences | Assay Description The coupled assay can detect inhibitors of AKT2 activation, as well as direct inhibitors of PDK1 or AKT2. Inactive AKT2 is activated in situ by incub... | Bioorg Med Chem Lett 17: 3819-25 (2007) Article DOI: 10.1016/j.bmcl.2007.05.060 BindingDB Entry DOI: 10.7270/Q29G5K22 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-phosphoinositide-dependent protein kinase 1 (Homo sapiens (Human)) | BDBM17007 (Indolinone based compound, 15 | N-[(5-{1-[(3Z)-5-(...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences | Assay Description The coupled assay can detect inhibitors of AKT2 activation, as well as direct inhibitors of PDK1 or AKT2. Inactive AKT2 is activated in situ by incub... | Bioorg Med Chem Lett 17: 3819-25 (2007) Article DOI: 10.1016/j.bmcl.2007.05.060 BindingDB Entry DOI: 10.7270/Q29G5K22 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thermolysin (Bacillus thermoproteolyticus) | BDBM50035246 (2-(2-Hydroxycarbamoyl-4-methyl-pentanoylamino)-3-(...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against thermolysin with 0.5 uM [Leu5]-enkephalin (NEN) | J Med Chem 38: 2119-29 (1995) BindingDB Entry DOI: 10.7270/Q2D79C2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-phosphoinositide-dependent protein kinase 1 (Homo sapiens (Human)) | BDBM17004 (BX-517 | Indolinone based inhibitor, 4i | [(3Z)-2-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences | Assay Description The coupled assay can detect inhibitors of AKT2 activation, as well as direct inhibitors of PDK1 or AKT2. Inactive AKT2 is activated in situ by incub... | Bioorg Med Chem Lett 17: 3819-25 (2007) Article DOI: 10.1016/j.bmcl.2007.05.060 BindingDB Entry DOI: 10.7270/Q29G5K22 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| 3-phosphoinositide-dependent protein kinase 1 (Homo sapiens (Human)) | BDBM17001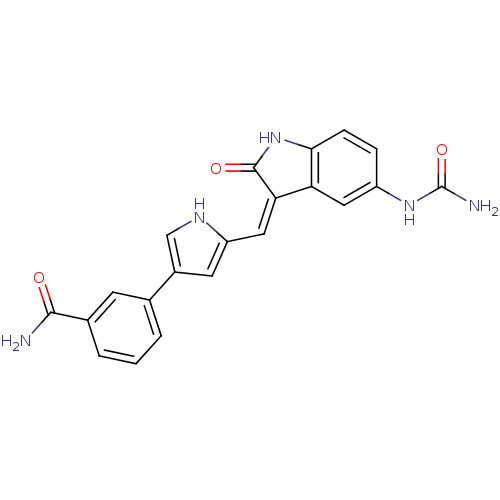 (3-(5-{[(3Z)-5-(carbamoylamino)-2-oxo-2,3-dihydro-1...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences | Assay Description The coupled assay can detect inhibitors of AKT2 activation, as well as direct inhibitors of PDK1 or AKT2. Inactive AKT2 is activated in situ by incub... | Bioorg Med Chem Lett 17: 3819-25 (2007) Article DOI: 10.1016/j.bmcl.2007.05.060 BindingDB Entry DOI: 10.7270/Q29G5K22 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thermolysin (Bacillus thermoproteolyticus) | BDBM50035247 (2-(2-Hydroxycarbamoyl-3-methyl-butyrylamino)-3-(1H...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against thermolysin with 0.5 uM [Leu5]-enkephalin (NEN) | J Med Chem 38: 2119-29 (1995) BindingDB Entry DOI: 10.7270/Q2D79C2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Oryctolagus cuniculus (rabbit)) | BDBM21641 (2-(2-benzyl-3-sulfanylpropanamido)acetic acid | CH...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against Leu-enkeph of Neutral endopeptidase | J Med Chem 38: 2119-29 (1995) BindingDB Entry DOI: 10.7270/Q2D79C2M | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Neprilysin (Oryctolagus cuniculus (rabbit)) | BDBM50035237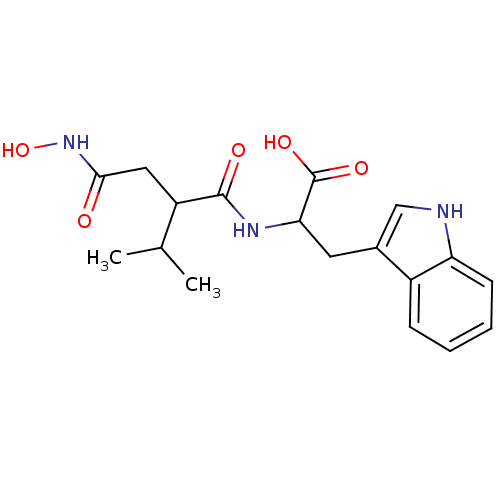 (2-(2-Hydroxycarbamoylmethyl-3-methyl-butyrylamino)...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against Leu-enkeph of Neutral endopeptidase | J Med Chem 38: 2119-29 (1995) BindingDB Entry DOI: 10.7270/Q2D79C2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-phosphoinositide-dependent protein kinase 1 (Homo sapiens (Human)) | BDBM17004 (BX-517 | Indolinone based inhibitor, 4i | [(3Z)-2-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences | Assay Description PDK1 inhibitory activity is measured directly using PDK1, a peptide substrate, 33PATP, and compound followed by capture on P81 phospho-cellulose pape... | Bioorg Med Chem Lett 17: 3814-8 (2007) Article DOI: 10.1016/j.bmcl.2007.04.071 BindingDB Entry DOI: 10.7270/Q25Q4TBB | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| 3-phosphoinositide-dependent protein kinase 1 (Homo sapiens (Human)) | BDBM17009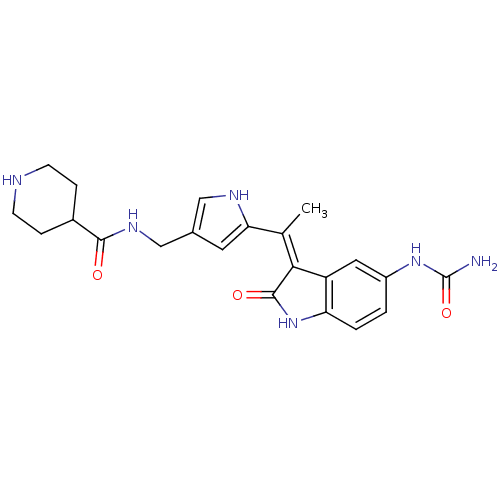 (Indolinone based compound, 17 | N-[(5-{1-[(3Z)-5-(...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences | Assay Description The coupled assay can detect inhibitors of AKT2 activation, as well as direct inhibitors of PDK1 or AKT2. Inactive AKT2 is activated in situ by incub... | Bioorg Med Chem Lett 17: 3819-25 (2007) Article DOI: 10.1016/j.bmcl.2007.05.060 BindingDB Entry DOI: 10.7270/Q29G5K22 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-phosphoinositide-dependent protein kinase 1 (Homo sapiens (Human)) | BDBM17051 (BX-795 | BX-795, 3 | N-(3-{[5-iodo-4-({3-[(thiophe...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | 7.2 | 22 |
Berlex Biosciences | Assay Description The coupled assay can detect inhibitors of AKT2 activation, as well as direct inhibitors of PDK1 or AKT2. Inactive AKT2 is activated in situ by incub... | J Biol Chem 280: 19867-74 (2005) Article DOI: 10.1074/jbc.M501367200 BindingDB Entry DOI: 10.7270/Q21Z42PW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-phosphoinositide-dependent protein kinase 1 (Homo sapiens (Human)) | BDBM16997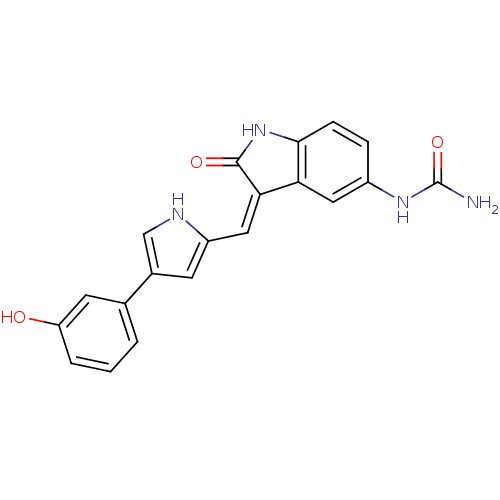 (Indolinone based compound, 7f | [(3Z)-3-{[4-(3-hyd...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | 7.2 | 22 |
Berlex Biosciences | Assay Description The coupled assay can detect inhibitors of AKT2 activation, as well as direct inhibitors of PDK1 or AKT2. Inactive AKT2 is activated in situ by incub... | Bioorg Med Chem Lett 17: 3819-25 (2007) Article DOI: 10.1016/j.bmcl.2007.05.060 BindingDB Entry DOI: 10.7270/Q29G5K22 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Oryctolagus cuniculus (rabbit)) | BDBM50251742 ((3R,4R,5R,6S)-3,4,5-trihydroxy-6-methyl-tetrahydro...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against Leu-enkeph of Neutral endopeptidase | J Med Chem 38: 2119-29 (1995) BindingDB Entry DOI: 10.7270/Q2D79C2M | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Displayed 1 to 50 (of 148 total ) | Next | Last >> |