

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Alpha-2A adrenergic receptor [16-465] (Rattus norvegicus (rat)) | BDBM50085677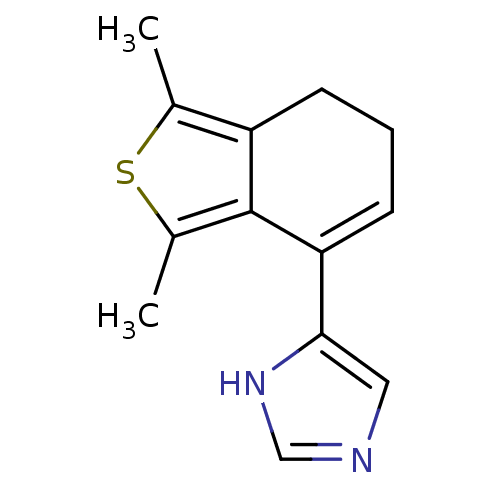 (4-(1,3-Dimethyl-6,7-dihydro-benzo[c]thiophen-4-yl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents | PubMed | 0.00860 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The R. W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro rat alpha-2D adrenergic receptor binding using p-aminoclonidine | J Med Chem 43: 1423-6 (2001) BindingDB Entry DOI: 10.7270/Q2C828HK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-2A adrenergic receptor [16-465] (Rattus norvegicus (rat)) | BDBM50085677 (4-(1,3-Dimethyl-6,7-dihydro-benzo[c]thiophen-4-yl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents | PubMed | 0.00860 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The R. W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity towards alpha-2D adrenergic receptor | J Med Chem 43: 765-8 (2000) BindingDB Entry DOI: 10.7270/Q2251HDX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-2A adrenergic receptor [16-465] (Rattus norvegicus (rat)) | BDBM50085683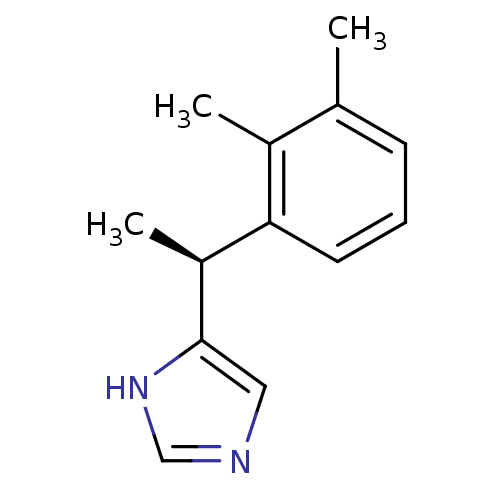 ((+)-4-((S)-alpha,2,3-trimethylbenzyl)imidazole | 4...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 0.0150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The R. W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro rat alpha-2D adrenergic receptor binding using p-aminoclonidine | J Med Chem 43: 1423-6 (2001) BindingDB Entry DOI: 10.7270/Q2C828HK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-2A adrenergic receptor [16-465] (Rattus norvegicus (rat)) | BDBM50085683 ((+)-4-((S)-alpha,2,3-trimethylbenzyl)imidazole | 4...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 0.0150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The R. W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity towards alpha-2D adrenergic receptor | J Med Chem 43: 765-8 (2000) BindingDB Entry DOI: 10.7270/Q2251HDX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM50147083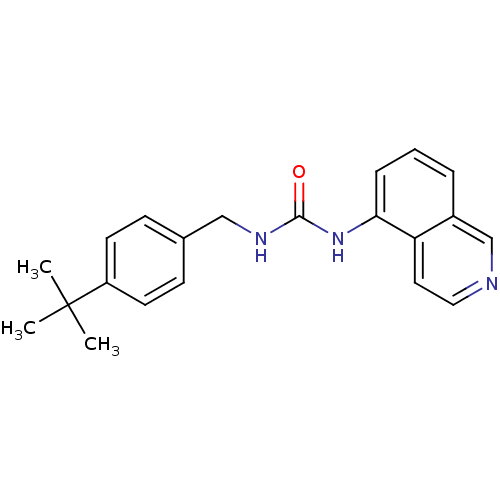 (1-(4-tert-Butyl-benzyl)-3-isoquinolin-5-yl-urea | ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Binding affinity towards human vanilloid receptor subtype 1 expressed in HEK293 cell membrane using [3H]-RTX as radioligand. | Bioorg Med Chem Lett 14: 3053-6 (2004) Article DOI: 10.1016/j.bmcl.2004.04.038 BindingDB Entry DOI: 10.7270/Q2FB52D6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-2A adrenergic receptor [16-465] (Rattus norvegicus (rat)) | BDBM50085679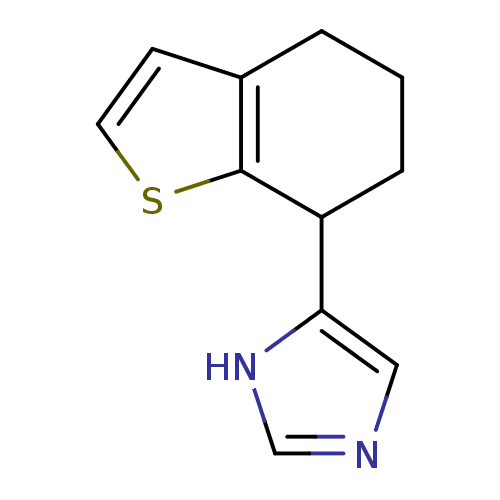 (4-(4,5,6,7-Tetrahydro-benzo[b]thiophen-7-yl)-1H-im...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | 0.350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The R. W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity towards alpha-2D adrenergic receptor | J Med Chem 43: 765-8 (2000) BindingDB Entry DOI: 10.7270/Q2251HDX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-2A adrenergic receptor [16-465] (Rattus norvegicus (rat)) | BDBM50085679 (4-(4,5,6,7-Tetrahydro-benzo[b]thiophen-7-yl)-1H-im...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | 0.350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The R. W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro rat alpha-2D adrenergic receptor binding using p-aminoclonidine | J Med Chem 43: 1423-6 (2001) BindingDB Entry DOI: 10.7270/Q2C828HK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-2A adrenergic receptor [16-465] (Rattus norvegicus (rat)) | BDBM50016897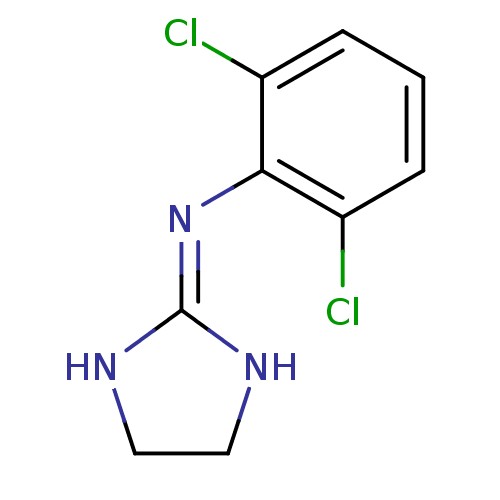 (2-(2,6-dichloroanilino)-1,3-diazacyclopentene-(2) ...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid PDB UniChem Patents Similars | PubMed | 0.390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The R. W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity towards alpha-2D adrenergic receptor | J Med Chem 43: 765-8 (2000) BindingDB Entry DOI: 10.7270/Q2251HDX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-2A adrenergic receptor [16-465] (Rattus norvegicus (rat)) | BDBM50016897 (2-(2,6-dichloroanilino)-1,3-diazacyclopentene-(2) ...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid PDB UniChem Patents Similars | PubMed | 0.390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The R. W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro rat alpha-2D adrenergic receptor binding using p-aminoclonidine | J Med Chem 43: 1423-6 (2001) BindingDB Entry DOI: 10.7270/Q2C828HK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-2A adrenergic receptor [16-465] (Rattus norvegicus (rat)) | BDBM50085678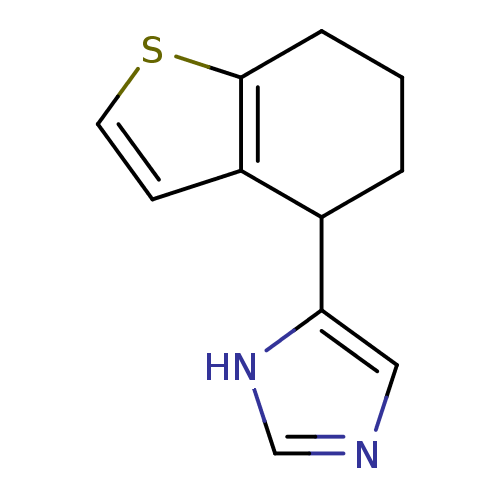 (4-(4,5,6,7-Tetrahydro-benzo[b]thiophen-4-yl)-1H-im...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.560 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The R. W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro rat alpha-2D adrenergic receptor binding using p-aminoclonidine | J Med Chem 43: 1423-6 (2001) BindingDB Entry DOI: 10.7270/Q2C828HK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-2A adrenergic receptor [16-465] (Rattus norvegicus (rat)) | BDBM50085678 (4-(4,5,6,7-Tetrahydro-benzo[b]thiophen-4-yl)-1H-im...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.560 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The R. W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity towards alpha-2D adrenergic receptor | J Med Chem 43: 765-8 (2000) BindingDB Entry DOI: 10.7270/Q2251HDX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM50223336 (1-(1-(cyclopropylmethyl)-6-fluoro-1,2,3,4-tetrahyd...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]RTX from human TRPV1 expressed in HEK293 cells | Bioorg Med Chem Lett 17: 6160-3 (2007) Article DOI: 10.1016/j.bmcl.2007.09.036 BindingDB Entry DOI: 10.7270/Q28G8KFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM50147069 (1-(4-Chloro-3-trifluoromethyl-benzyl)-3-isoquinoli...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Binding affinity towards human vanilloid receptor subtype 1 expressed in HEK293 cell membrane using [3H]-RTX as radioligand. | Bioorg Med Chem Lett 14: 3053-6 (2004) Article DOI: 10.1016/j.bmcl.2004.04.038 BindingDB Entry DOI: 10.7270/Q2FB52D6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM50223313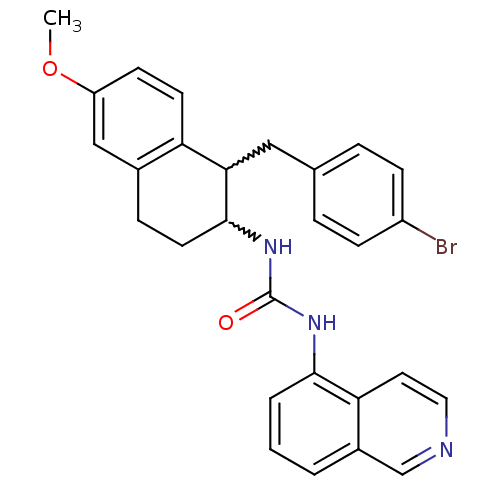 (1-(1-(4-bromobenzyl)-6-methoxy-1,2,3,4-tetrahydron...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]RTX from human TRPV1 expressed in HEK293 cells | Bioorg Med Chem Lett 17: 6160-3 (2007) Article DOI: 10.1016/j.bmcl.2007.09.036 BindingDB Entry DOI: 10.7270/Q28G8KFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM50223314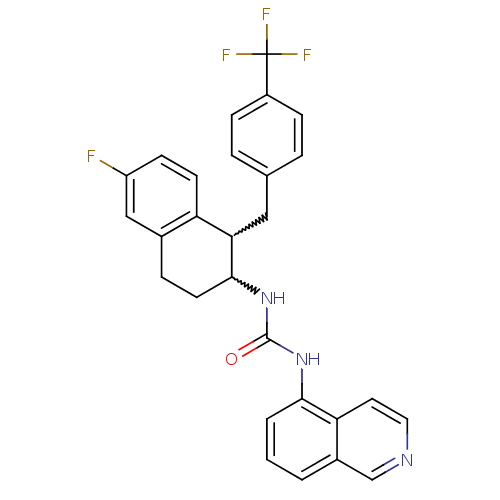 (1-(1-(4-(trifluoromethyl)benzyl)-6-fluoro-1,2,3,4-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]RTX from human TRPV1 expressed in HEK293 cells | Bioorg Med Chem Lett 17: 6160-3 (2007) Article DOI: 10.1016/j.bmcl.2007.09.036 BindingDB Entry DOI: 10.7270/Q28G8KFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM50147080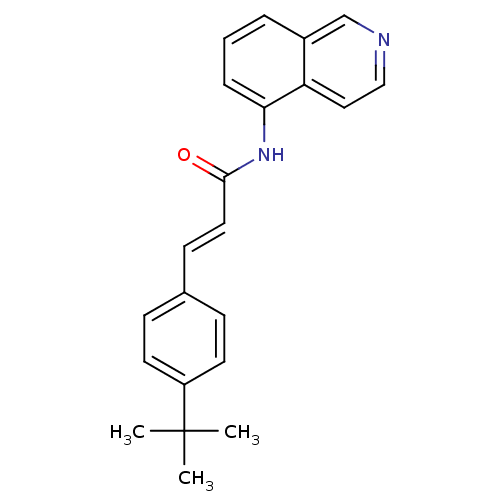 (3-(4-tert-Butyl-phenyl)-N-isoquinolin-5-yl-acrylam...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Antagonistic activity towards human vanilloid receptor subtype 1 expressed in HEK293 cell membrane, as inhibition of agonist-induced intracellular [C... | Bioorg Med Chem Lett 14: 3053-6 (2004) Article DOI: 10.1016/j.bmcl.2004.04.038 BindingDB Entry DOI: 10.7270/Q2FB52D6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM50147067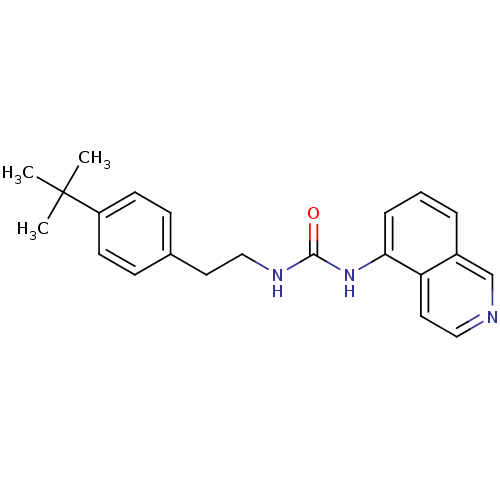 (1-[2-(4-tert-Butyl-phenyl)-ethyl]-3-isoquinolin-5-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Binding affinity towards human vanilloid receptor subtype 1 expressed in HEK293 cell membrane using [3H]-RTX as radioligand. | Bioorg Med Chem Lett 14: 3053-6 (2004) Article DOI: 10.1016/j.bmcl.2004.04.038 BindingDB Entry DOI: 10.7270/Q2FB52D6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM50147063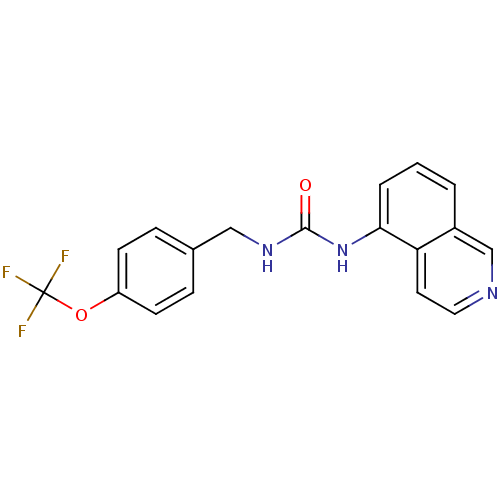 (1-Isoquinolin-5-yl-3-(4-trifluoromethoxy-benzyl)-u...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Binding affinity towards human vanilloid receptor subtype 1 expressed in HEK293 cell membrane using [3H]-RTX as radioligand. | Bioorg Med Chem Lett 14: 3053-6 (2004) Article DOI: 10.1016/j.bmcl.2004.04.038 BindingDB Entry DOI: 10.7270/Q2FB52D6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM50147077 (1-(3,5-Bis-trifluoromethyl-phenyl)-3-isoquinolin-5...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Antagonistic activity towards human vanilloid receptor subtype 1 expressed in HEK293 cell membrane, as inhibition of agonist-induced intracellular [C... | Bioorg Med Chem Lett 14: 3053-6 (2004) Article DOI: 10.1016/j.bmcl.2004.04.038 BindingDB Entry DOI: 10.7270/Q2FB52D6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-2A adrenergic receptor [16-465] (Rattus norvegicus (rat)) | BDBM50085680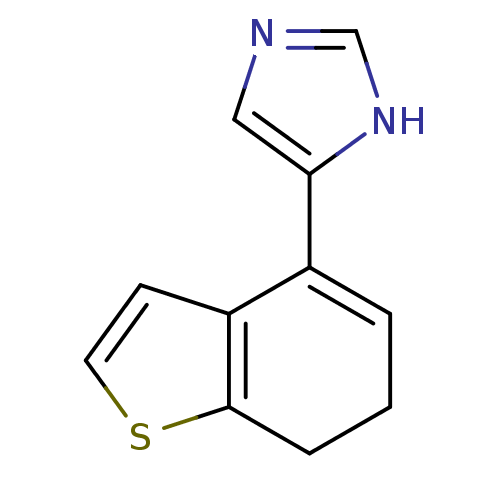 (4-(6,7-Dihydro-benzo[b]thiophen-4-yl)-1H-imidazole...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem | PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The R. W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro rat alpha-2D adrenergic receptor binding using p-aminoclonidine | J Med Chem 43: 1423-6 (2001) BindingDB Entry DOI: 10.7270/Q2C828HK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-2A adrenergic receptor [16-465] (Rattus norvegicus (rat)) | BDBM50085680 (4-(6,7-Dihydro-benzo[b]thiophen-4-yl)-1H-imidazole...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem | PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The R. W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity towards alpha-2D adrenergic receptor | J Med Chem 43: 765-8 (2000) BindingDB Entry DOI: 10.7270/Q2251HDX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM50223330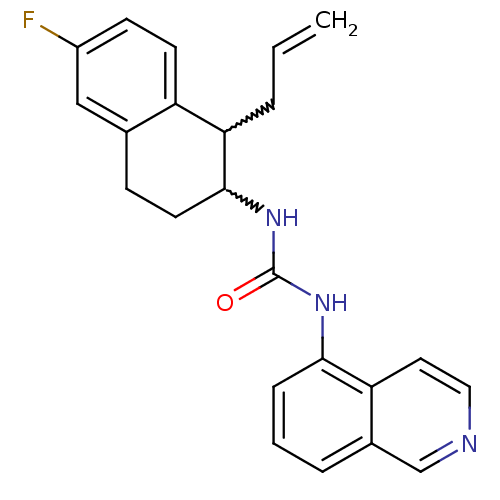 (1-(1-allyl-6-fluoro-1,2,3,4-tetrahydronaphthalen-2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]RTX from human TRPV1 expressed in HEK293 cells | Bioorg Med Chem Lett 17: 6160-3 (2007) Article DOI: 10.1016/j.bmcl.2007.09.036 BindingDB Entry DOI: 10.7270/Q28G8KFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM50223331 (1-(1-benzyl-6-bromo-1,2,3,4-tetrahydronaphthalen-2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]RTX from human TRPV1 expressed in HEK293 cells | Bioorg Med Chem Lett 17: 6160-3 (2007) Article DOI: 10.1016/j.bmcl.2007.09.036 BindingDB Entry DOI: 10.7270/Q28G8KFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM50223328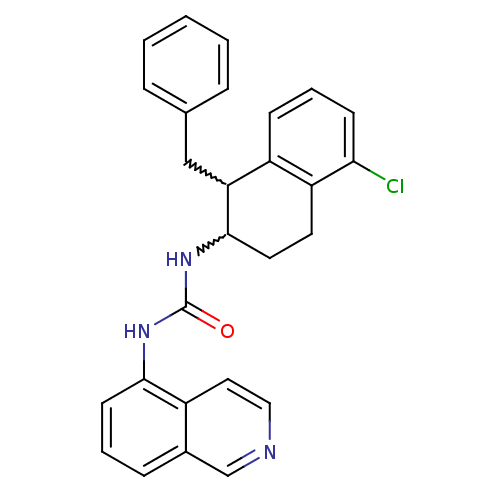 (1-(1-benzyl-5-chloro-1,2,3,4-tetrahydronaphthalen-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]RTX from human TRPV1 expressed in HEK293 cells | Bioorg Med Chem Lett 17: 6160-3 (2007) Article DOI: 10.1016/j.bmcl.2007.09.036 BindingDB Entry DOI: 10.7270/Q28G8KFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM50223338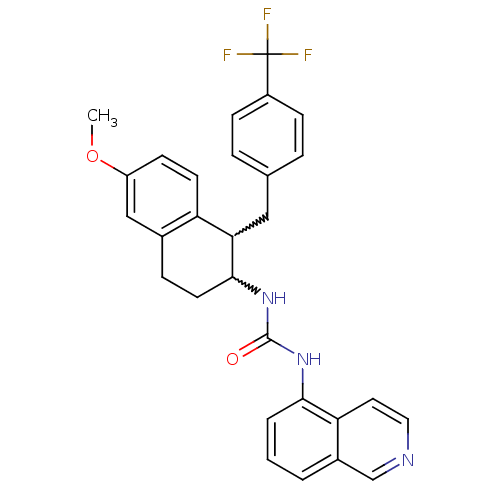 (1-(1-(4-(trifluoromethyl)benzyl)-6-methoxy-1,2,3,4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]RTX from human TRPV1 expressed in HEK293 cells | Bioorg Med Chem Lett 17: 6160-3 (2007) Article DOI: 10.1016/j.bmcl.2007.09.036 BindingDB Entry DOI: 10.7270/Q28G8KFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM50223321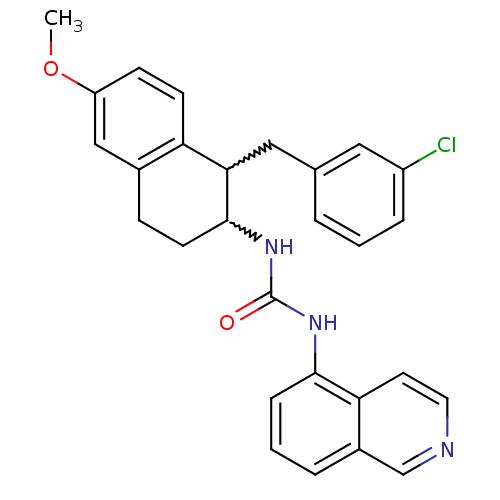 (1-(1-(3-chlorobenzyl)-6-methoxy-1,2,3,4-tetrahydro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]RTX from human TRPV1 expressed in HEK293 cells | Bioorg Med Chem Lett 17: 6160-3 (2007) Article DOI: 10.1016/j.bmcl.2007.09.036 BindingDB Entry DOI: 10.7270/Q28G8KFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-2A adrenergic receptor [16-465] (Rattus norvegicus (rat)) | BDBM50085681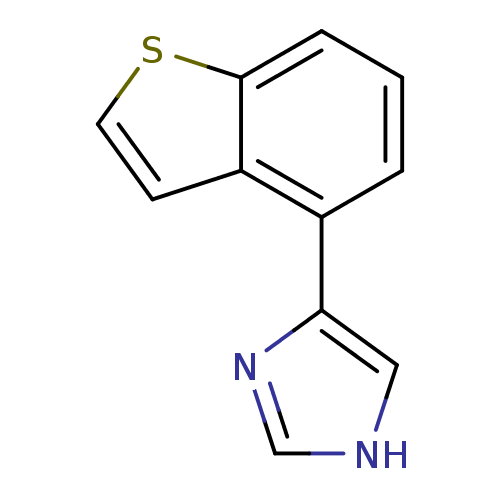 (4-Benzo[b]thiophen-4-yl-1H-imidazole | CHEMBL30534) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The R. W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro rat alpha-2D adrenergic receptor binding using p-aminoclonidine | J Med Chem 43: 1423-6 (2001) BindingDB Entry DOI: 10.7270/Q2C828HK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-2A adrenergic receptor [16-465] (Rattus norvegicus (rat)) | BDBM50085681 (4-Benzo[b]thiophen-4-yl-1H-imidazole | CHEMBL30534) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The R. W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity towards alpha-2D adrenergic receptor | J Med Chem 43: 765-8 (2000) BindingDB Entry DOI: 10.7270/Q2251HDX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-2A adrenergic receptor [16-465] (Rattus norvegicus (rat)) | BDBM50085676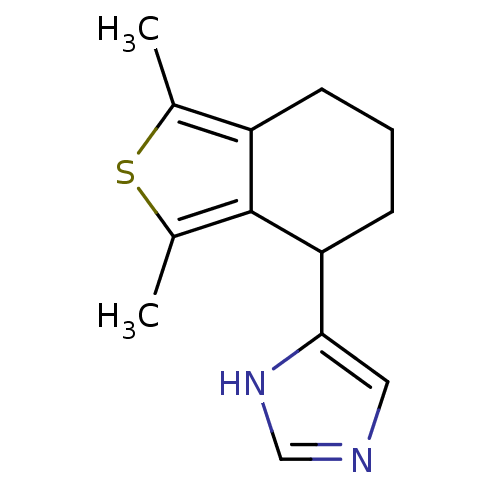 (4-(1,3-Dimethyl-4,5,6,7-tetrahydro-benzo[c]thiophe...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The R. W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity towards alpha-2D adrenergic receptor | J Med Chem 43: 765-8 (2000) BindingDB Entry DOI: 10.7270/Q2251HDX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-2A adrenergic receptor [16-465] (Rattus norvegicus (rat)) | BDBM50085676 (4-(1,3-Dimethyl-4,5,6,7-tetrahydro-benzo[c]thiophe...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The R. W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro rat alpha-2D adrenergic receptor binding using p-aminoclonidine | J Med Chem 43: 1423-6 (2001) BindingDB Entry DOI: 10.7270/Q2C828HK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM50223333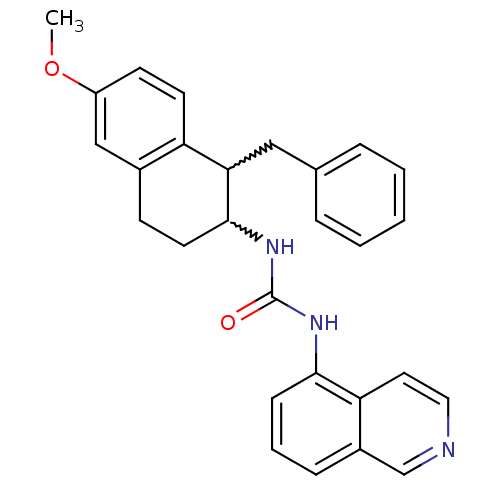 (1-(1-benzyl-6-methoxy-1,2,3,4-tetrahydronaphthalen...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]RTX from human TRPV1 expressed in HEK293 cells | Bioorg Med Chem Lett 17: 6160-3 (2007) Article DOI: 10.1016/j.bmcl.2007.09.036 BindingDB Entry DOI: 10.7270/Q28G8KFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-2A adrenergic receptor [16-465] (Rattus norvegicus (rat)) | BDBM50085682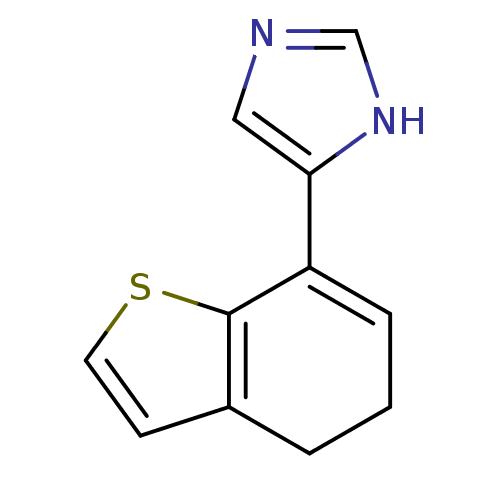 (4-(4,5-Dihydro-benzo[b]thiophen-7-yl)-1H-imidazole...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The R. W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity towards alpha-2D adrenergic receptor | J Med Chem 43: 765-8 (2000) BindingDB Entry DOI: 10.7270/Q2251HDX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-2A adrenergic receptor [16-465] (Rattus norvegicus (rat)) | BDBM50085682 (4-(4,5-Dihydro-benzo[b]thiophen-7-yl)-1H-imidazole...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The R. W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro rat alpha-2D adrenergic receptor binding using p-aminoclonidine | J Med Chem 43: 1423-6 (2001) BindingDB Entry DOI: 10.7270/Q2C828HK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B2 bradykinin receptor (Homo sapiens (Human)) | BDBM50126226 (3-[5-(4-Cyano-benzoyl)-1-methyl-1H-pyrrol-2-yl]-N-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Binding affinity towards human bradykinin receptor B2 | Bioorg Med Chem Lett 13: 1341-4 (2003) BindingDB Entry DOI: 10.7270/Q2HM57T1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM50147060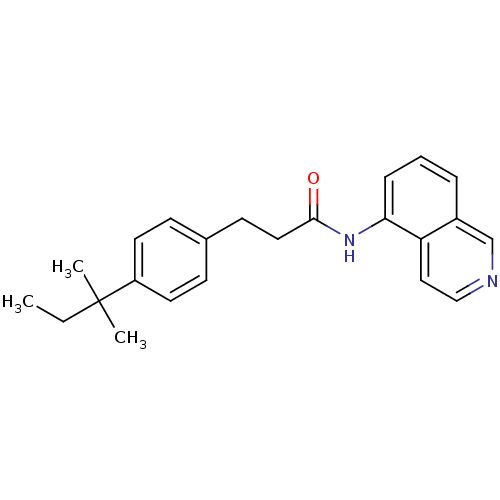 (3-[4-(1,1-Dimethyl-propyl)-phenyl]-N-isoquinolin-5...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Binding affinity towards human vanilloid receptor subtype 1 expressed in HEK293 cell membrane using [3H]-RTX as radioligand. | Bioorg Med Chem Lett 14: 3053-6 (2004) Article DOI: 10.1016/j.bmcl.2004.04.038 BindingDB Entry DOI: 10.7270/Q2FB52D6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM50223335 (1-(1-benzyl-1,2,3,4-tetrahydronaphthalen-2-yl)-3-(...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]RTX from human TRPV1 expressed in HEK293 cells | Bioorg Med Chem Lett 17: 6160-3 (2007) Article DOI: 10.1016/j.bmcl.2007.09.036 BindingDB Entry DOI: 10.7270/Q28G8KFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM50223340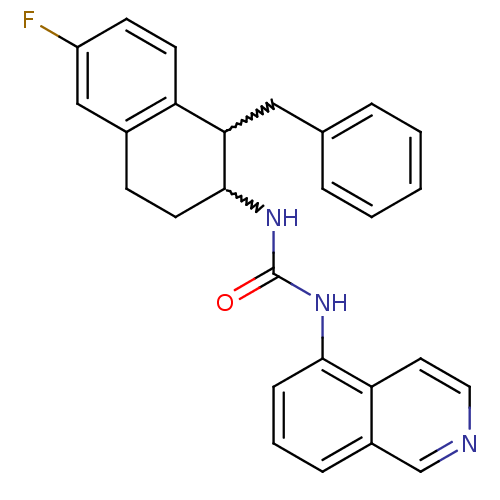 (1-(1-benzyl-6-fluoro-1,2,3,4-tetrahydronaphthalen-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]RTX from human TRPV1 expressed in HEK293 cells | Bioorg Med Chem Lett 17: 6160-3 (2007) Article DOI: 10.1016/j.bmcl.2007.09.036 BindingDB Entry DOI: 10.7270/Q28G8KFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM50223323 (1-(isoquinolin-5-yl)-3-(3-phenyl-2-(4-(trifluorome...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]RTX from human TRPV1 expressed in HEK293 cells | Bioorg Med Chem Lett 17: 6160-3 (2007) Article DOI: 10.1016/j.bmcl.2007.09.036 BindingDB Entry DOI: 10.7270/Q28G8KFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM50223315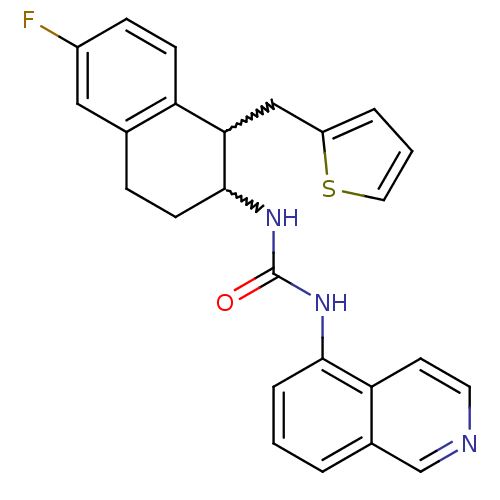 (1-(6-fluoro-1-(thiophen-2-ylmethyl)-1,2,3,4-tetrah...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]RTX from human TRPV1 expressed in HEK293 cells | Bioorg Med Chem Lett 17: 6160-3 (2007) Article DOI: 10.1016/j.bmcl.2007.09.036 BindingDB Entry DOI: 10.7270/Q28G8KFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-2A adrenergic receptor [16-465] (Rattus norvegicus (rat)) | BDBM50085683 ((+)-4-((S)-alpha,2,3-trimethylbenzyl)imidazole | 4...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The R. W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description in vitro alpha-2A adrenergic receptor binding assay from rats ,using RX 821002 as the displaceable ligand | J Med Chem 43: 1423-6 (2001) BindingDB Entry DOI: 10.7270/Q2C828HK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM20334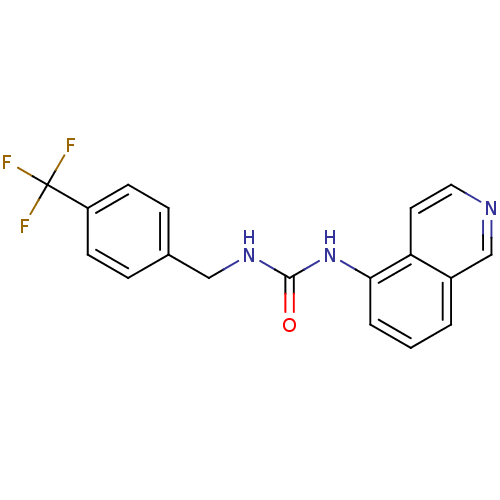 (1-Isoquinolin-5-yl-3-(4-trifluoromethyl-benzyl)-ur...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Binding affinity towards human vanilloid receptor subtype 1 expressed in HEK293 cell membrane using [3H]-RTX as radioligand. | Bioorg Med Chem Lett 14: 3053-6 (2004) Article DOI: 10.1016/j.bmcl.2004.04.038 BindingDB Entry DOI: 10.7270/Q2FB52D6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A/Alpha-1B/Alpha-1D adrenergic receptor (Rattus norvegicus (rat)-Rattus norvegicus (Rat)) | BDBM50085683 ((+)-4-((S)-alpha,2,3-trimethylbenzyl)imidazole | 4...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The R. W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Tested for Binding affinity towards alpha-1 adrenergic receptor | J Med Chem 43: 765-8 (2000) BindingDB Entry DOI: 10.7270/Q2251HDX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM50223316 (1-(1-benzyl-7-chloro-1,2,3,4-tetrahydronaphthalen-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]RTX from human TRPV1 expressed in HEK293 cells | Bioorg Med Chem Lett 17: 6160-3 (2007) Article DOI: 10.1016/j.bmcl.2007.09.036 BindingDB Entry DOI: 10.7270/Q28G8KFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM50147081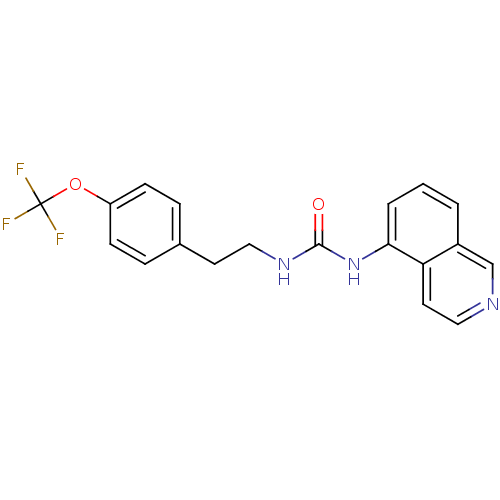 (1-Isoquinolin-5-yl-3-[2-(4-trifluoromethoxy-phenyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Binding affinity towards human vanilloid receptor subtype 1 expressed in HEK293 cell membrane using [3H]-RTX as radioligand. | Bioorg Med Chem Lett 14: 3053-6 (2004) Article DOI: 10.1016/j.bmcl.2004.04.038 BindingDB Entry DOI: 10.7270/Q2FB52D6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM50223337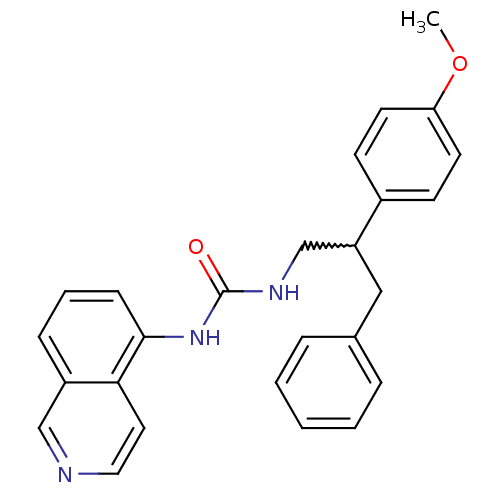 (1-(isoquinolin-5-yl)-3-(2-(4-methoxyphenyl)-3-phen...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]RTX from human TRPV1 expressed in HEK293 cells | Bioorg Med Chem Lett 17: 6160-3 (2007) Article DOI: 10.1016/j.bmcl.2007.09.036 BindingDB Entry DOI: 10.7270/Q28G8KFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM50223334 (1-(1-(4-methoxybenzyl)-6-methoxy-1,2,3,4-tetrahydr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]RTX from human TRPV1 expressed in HEK293 cells | Bioorg Med Chem Lett 17: 6160-3 (2007) Article DOI: 10.1016/j.bmcl.2007.09.036 BindingDB Entry DOI: 10.7270/Q28G8KFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM50223339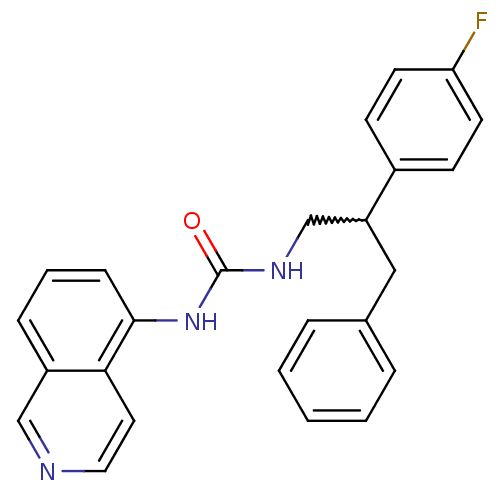 (1-(2-(4-fluorophenyl)-3-phenylpropyl)-3-(isoquinol...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]RTX from human TRPV1 expressed in HEK293 cells | Bioorg Med Chem Lett 17: 6160-3 (2007) Article DOI: 10.1016/j.bmcl.2007.09.036 BindingDB Entry DOI: 10.7270/Q28G8KFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM50376255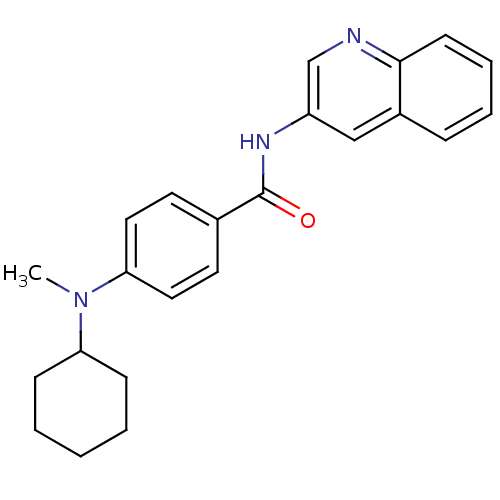 (CHEMBL408401) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]RTX from human TRPV1 receptor expressed in HEK293 cells | Bioorg Med Chem Lett 18: 2730-4 (2008) Article DOI: 10.1016/j.bmcl.2008.02.075 BindingDB Entry DOI: 10.7270/Q27H1KF6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM50147082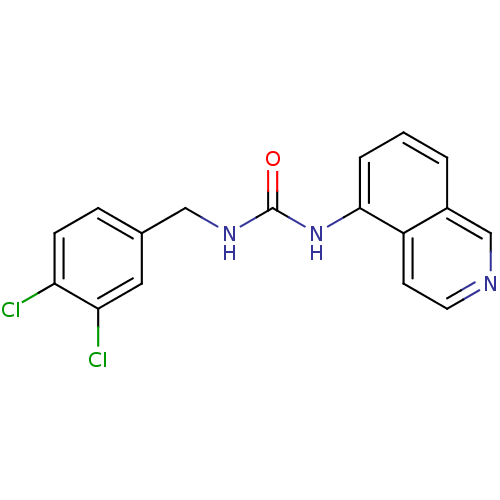 (1-(3,4-Dichloro-benzyl)-3-isoquinolin-5-yl-urea | ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Antagonistic activity towards human vanilloid receptor subtype 1 expressed in HEK293 cell membrane, as inhibition of agonist-induced intracellular [C... | Bioorg Med Chem Lett 14: 3053-6 (2004) Article DOI: 10.1016/j.bmcl.2004.04.038 BindingDB Entry DOI: 10.7270/Q2FB52D6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM50223329 (1-(1-benzyl-6-methoxy-1,2,3,4-tetrahydronaphthalen...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]RTX from human TRPV1 expressed in HEK293 cells | Bioorg Med Chem Lett 17: 6160-3 (2007) Article DOI: 10.1016/j.bmcl.2007.09.036 BindingDB Entry DOI: 10.7270/Q28G8KFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 995 total ) | Next | Last >> |