 Found 14278 hits with Last Name = 'orr' and Initial = 'mj'
Found 14278 hits with Last Name = 'orr' and Initial = 'mj' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM21281

((11R)-2-methyl-11-(morpholin-4-ylmethyl)-3-(naphth...)Show SMILES Cc1c(C(=O)c2cccc3ccccc23)c2cccc3OC[C@@H](CN4CCOCC4)n1c23 |r| Show InChI InChI=1S/C27H26N2O3/c1-18-25(27(30)22-9-4-7-19-6-2-3-8-21(19)22)23-10-5-11-24-26(23)29(18)20(17-32-24)16-28-12-14-31-15-13-28/h2-11,20H,12-17H2,1H3/t20-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pontificia Universidad Cat£lica de Chile
Curated by ChEMBL
| Assay Description
Agonist activity at CB2 receptor (unknown origin) |
Eur J Med Chem 124: 17-35 (2016)
Article DOI: 10.1016/j.ejmech.2016.08.005
BindingDB Entry DOI: 10.7270/Q2Q2426V |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Hydroxycarboxylic acid receptor 2
(Homo sapiens (Human)) | BDBM50313984
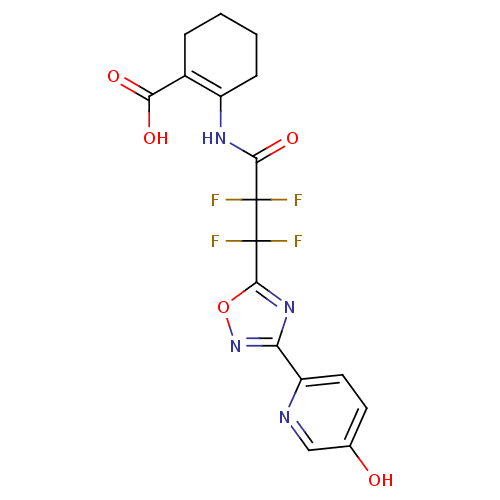
(2-(2,2,3,3-tetrafluoro-3-(3-(5-hydroxypyridin-2-yl...)Show SMILES OC(=O)C1=C(CCCC1)NC(=O)C(F)(F)C(F)(F)c1nc(no1)-c1ccc(O)cn1 |t:3| Show InChI InChI=1S/C17H14F4N4O5/c18-16(19,14(29)23-10-4-2-1-3-9(10)13(27)28)17(20,21)15-24-12(25-30-15)11-6-5-8(26)7-22-11/h5-7,26H,1-4H2,(H,23,29)(H,27,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]NA from cloned human GPR109A receptor expressed in CHO-K1 cells by spectrophotometry |
J Med Chem 53: 2666-70 (2010)
Article DOI: 10.1021/jm100022r
BindingDB Entry DOI: 10.7270/Q2NS0V2P |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM21281

((11R)-2-methyl-11-(morpholin-4-ylmethyl)-3-(naphth...)Show SMILES Cc1c(C(=O)c2cccc3ccccc23)c2cccc3OC[C@@H](CN4CCOCC4)n1c23 |r| Show InChI InChI=1S/C27H26N2O3/c1-18-25(27(30)22-9-4-7-19-6-2-3-8-21(19)22)23-10-5-11-24-26(23)29(18)20(17-32-24)16-28-12-14-31-15-13-28/h2-11,20H,12-17H2,1H3/t20-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pontificia Universidad Cat£lica de Chile
Curated by ChEMBL
| Assay Description
Agonist activity at CB1 receptor (unknown origin) |
Eur J Med Chem 124: 17-35 (2016)
Article DOI: 10.1016/j.ejmech.2016.08.005
BindingDB Entry DOI: 10.7270/Q2Q2426V |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 7
(Homo sapiens (Human)) | BDBM50596945
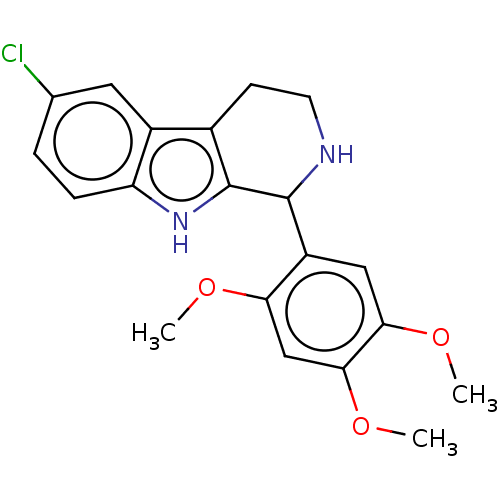
(CHEMBL5207529)Show SMILES Cl.COc1cc(OC)c(cc1OC)C1NCCc2c1[nH]c1ccc(Cl)cc21 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00694
BindingDB Entry DOI: 10.7270/Q2ZP4B4N |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2B
(Homo sapiens (Human)) | BDBM50596945

(CHEMBL5207529)Show SMILES Cl.COc1cc(OC)c(cc1OC)C1NCCc2c1[nH]c1ccc(Cl)cc21 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00694
BindingDB Entry DOI: 10.7270/Q2ZP4B4N |
More data for this
Ligand-Target Pair | |
Hydroxycarboxylic acid receptor 2
(Homo sapiens (Human)) | BDBM50313977
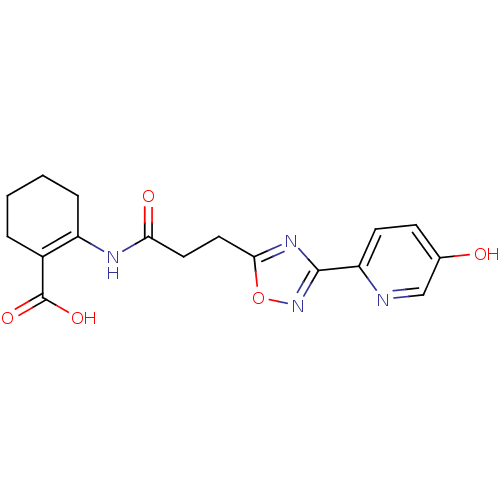
(2-(3-(3-(5-hydroxypyridin-2-yl)-1,2,4-oxadiazol-5-...)Show SMILES OC(=O)C1=C(CCCC1)NC(=O)CCc1nc(no1)-c1ccc(O)cn1 |t:3| Show InChI InChI=1S/C17H18N4O5/c22-10-5-6-13(18-9-10)16-20-15(26-21-16)8-7-14(23)19-12-4-2-1-3-11(12)17(24)25/h5-6,9,22H,1-4,7-8H2,(H,19,23)(H,24,25) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]NA from cloned human GPR109A receptor expressed in CHO-K1 cells by spectrophotometry |
J Med Chem 53: 2666-70 (2010)
Article DOI: 10.1021/jm100022r
BindingDB Entry DOI: 10.7270/Q2NS0V2P |
More data for this
Ligand-Target Pair | |
Hydroxycarboxylic acid receptor 2
(Homo sapiens (Human)) | BDBM23533
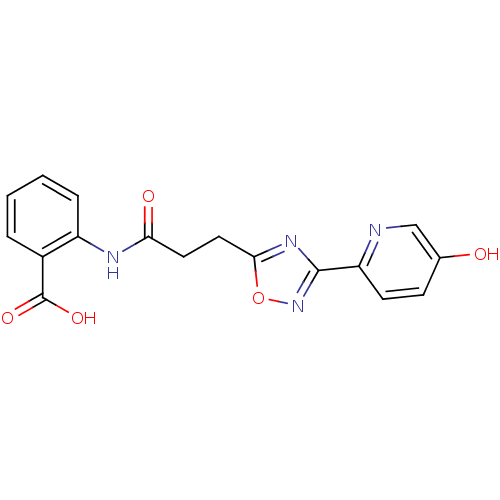
(2-{3-[3-(5-hydroxypyridin-2-yl)-1,2,4-oxadiazol-5-...)Show SMILES OC(=O)c1ccccc1NC(=O)CCc1nc(no1)-c1ccc(O)cn1 Show InChI InChI=1S/C17H14N4O5/c22-10-5-6-13(18-9-10)16-20-15(26-21-16)8-7-14(23)19-12-4-2-1-3-11(12)17(24)25/h1-6,9,22H,7-8H2,(H,19,23)(H,24,25) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]NA from cloned human GPR109A receptor expressed in CHO-K1 cells by spectrophotometry |
J Med Chem 53: 2666-70 (2010)
Article DOI: 10.1021/jm100022r
BindingDB Entry DOI: 10.7270/Q2NS0V2P |
More data for this
Ligand-Target Pair | |
Hydroxycarboxylic acid receptor 2
(Homo sapiens (Human)) | BDBM50313976
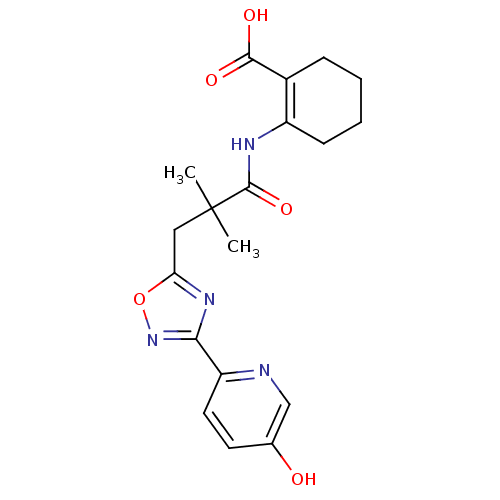
(2-({3-[3-(5-hydroxypyridin-2-yl)-1,2,4-oxadiazol-5...)Show SMILES CC(C)(Cc1nc(no1)-c1ccc(O)cn1)C(=O)NC1=C(CCCC1)C(O)=O |t:21| Show InChI InChI=1S/C19H22N4O5/c1-19(2,18(27)21-13-6-4-3-5-12(13)17(25)26)9-15-22-16(23-28-15)14-8-7-11(24)10-20-14/h7-8,10,24H,3-6,9H2,1-2H3,(H,21,27)(H,25,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]NA from cloned human GPR109A receptor expressed in CHO-K1 cells by spectrophotometry |
J Med Chem 53: 2666-70 (2010)
Article DOI: 10.1021/jm100022r
BindingDB Entry DOI: 10.7270/Q2NS0V2P |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
5-hydroxytryptamine receptor 2B
(Homo sapiens (Human)) | BDBM50596937
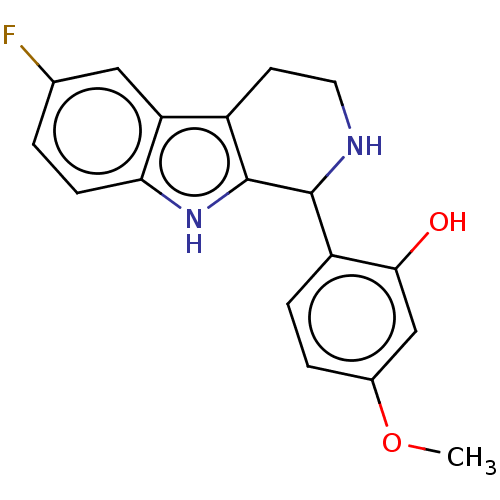
(CHEMBL5204071) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 4.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00694
BindingDB Entry DOI: 10.7270/Q2ZP4B4N |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2B
(Homo sapiens (Human)) | BDBM50596936
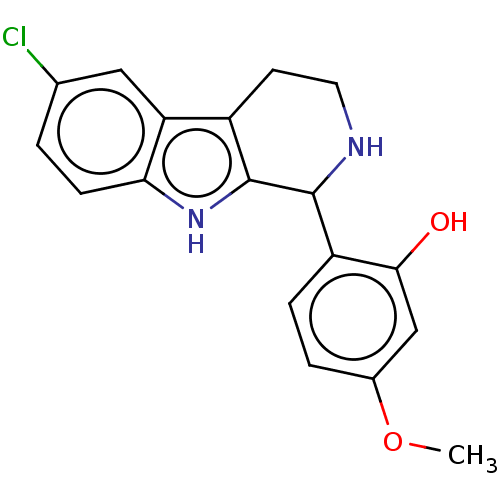
(CHEMBL5170784) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 4.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00694
BindingDB Entry DOI: 10.7270/Q2ZP4B4N |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP2 subtype
(Homo sapiens (Human)) | BDBM17638

(2-{1-[(4-chlorophenyl)carbonyl]-5-methoxy-2-methyl...)Show SMILES COc1ccc2n(C(=O)c3ccc(Cl)cc3)c(C)c(CC(O)=O)c2c1 Show InChI InChI=1S/C19H16ClNO4/c1-11-15(10-18(22)23)16-9-14(25-2)7-8-17(16)21(11)19(24)12-3-5-13(20)6-4-12/h3-9H,10H2,1-2H3,(H,22,23) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 290: 551-60 (1999)
BindingDB Entry DOI: 10.7270/Q2S18110 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 7
(Homo sapiens (Human)) | BDBM50596936

(CHEMBL5170784) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00694
BindingDB Entry DOI: 10.7270/Q2ZP4B4N |
More data for this
Ligand-Target Pair | |
Hydroxycarboxylic acid receptor 2
(Homo sapiens (Human)) | BDBM50313978
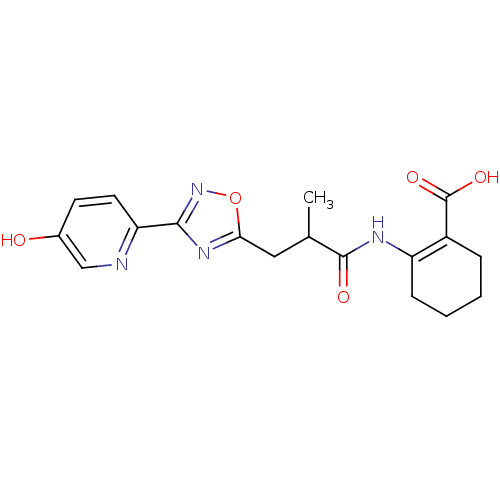
(2-(3-(3-(5-hydroxypyridin-2-yl)-1,2,4-oxadiazol-5-...)Show SMILES CC(Cc1nc(no1)-c1ccc(O)cn1)C(=O)NC1=C(CCCC1)C(O)=O |t:20| Show InChI InChI=1S/C18H20N4O5/c1-10(17(24)20-13-5-3-2-4-12(13)18(25)26)8-15-21-16(22-27-15)14-7-6-11(23)9-19-14/h6-7,9-10,23H,2-5,8H2,1H3,(H,20,24)(H,25,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]NA from cloned human GPR109A receptor expressed in CHO-K1 cells by spectrophotometry |
J Med Chem 53: 2666-70 (2010)
Article DOI: 10.1021/jm100022r
BindingDB Entry DOI: 10.7270/Q2NS0V2P |
More data for this
Ligand-Target Pair | |
Hydroxycarboxylic acid receptor 2
(Homo sapiens (Human)) | BDBM50313978

(2-(3-(3-(5-hydroxypyridin-2-yl)-1,2,4-oxadiazol-5-...)Show SMILES CC(Cc1nc(no1)-c1ccc(O)cn1)C(=O)NC1=C(CCCC1)C(O)=O |t:20| Show InChI InChI=1S/C18H20N4O5/c1-10(17(24)20-13-5-3-2-4-12(13)18(25)26)8-15-21-16(22-27-15)14-7-6-11(23)9-19-14/h6-7,9-10,23H,2-5,8H2,1H3,(H,20,24)(H,25,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]NA from cloned human GPR109A receptor expressed in CHO-K1 cells by spectrophotometry |
J Med Chem 53: 2666-70 (2010)
Article DOI: 10.1021/jm100022r
BindingDB Entry DOI: 10.7270/Q2NS0V2P |
More data for this
Ligand-Target Pair | |
Hydroxycarboxylic acid receptor 2
(Homo sapiens (Human)) | BDBM50313979
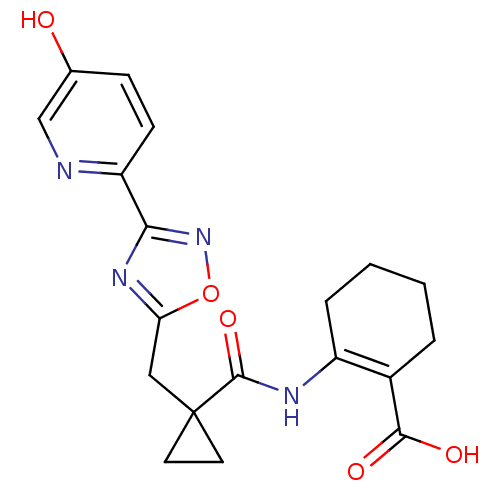
(2-(1-((3-(5-hydroxypyridin-2-yl)-1,2,4-oxadiazol-5...)Show SMILES OC(=O)C1=C(CCCC1)NC(=O)C1(Cc2nc(no2)-c2ccc(O)cn2)CC1 |t:3| Show InChI InChI=1S/C19H20N4O5/c24-11-5-6-14(20-10-11)16-22-15(28-23-16)9-19(7-8-19)18(27)21-13-4-2-1-3-12(13)17(25)26/h5-6,10,24H,1-4,7-9H2,(H,21,27)(H,25,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]NA from cloned human GPR109A receptor expressed in CHO-K1 cells by spectrophotometry |
J Med Chem 53: 2666-70 (2010)
Article DOI: 10.1021/jm100022r
BindingDB Entry DOI: 10.7270/Q2NS0V2P |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Homo sapiens (Human)) | BDBM50132563
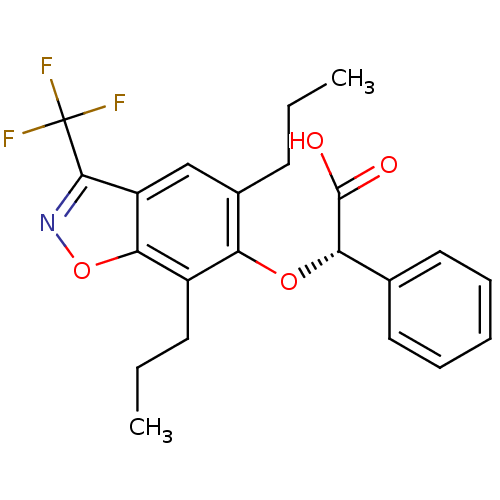
((S)-(5,7-Dipropyl-3-trifluoromethyl-benzo[d]isoxaz...)Show SMILES CCCc1cc2c(noc2c(CCC)c1O[C@H](C(O)=O)c1ccccc1)C(F)(F)F Show InChI InChI=1S/C22H22F3NO4/c1-3-8-14-12-16-19(30-26-20(16)22(23,24)25)15(9-4-2)17(14)29-18(21(27)28)13-10-6-5-7-11-13/h5-7,10-12,18H,3-4,8-9H2,1-2H3,(H,27,28)/t18-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity towards peroxisome proliferator activated receptor alpha (PPAR alpha) |
Bioorg Med Chem Lett 13: 3185-90 (2003)
BindingDB Entry DOI: 10.7270/Q2TQ6229 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM28681
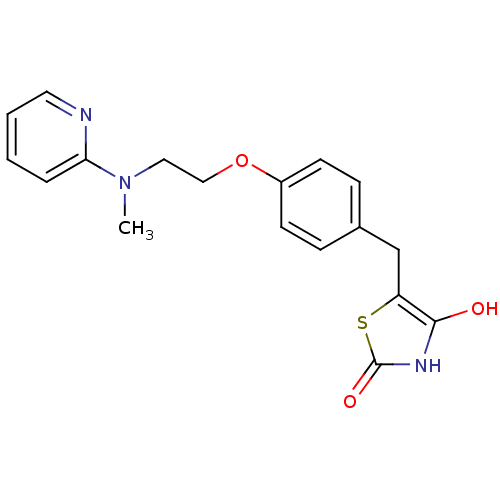
(5-[(4-{2-[methyl(pyridin-2-yl)amino]ethoxy}phenyl)...)Show InChI InChI=1S/C18H19N3O3S/c1-21(16-4-2-3-9-19-16)10-11-24-14-7-5-13(6-8-14)12-15-17(22)20-18(23)25-15/h2-9,22H,10-12H2,1H3,(H,20,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
PubMed
| 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity towards human peroxisome proliferator activated receptor gamma (PPAR gamma) was determined by HTRF assay |
Bioorg Med Chem Lett 13: 3185-90 (2003)
BindingDB Entry DOI: 10.7270/Q2TQ6229 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Peroxisome proliferator-activated receptor alpha
(Homo sapiens (Human)) | BDBM50132574
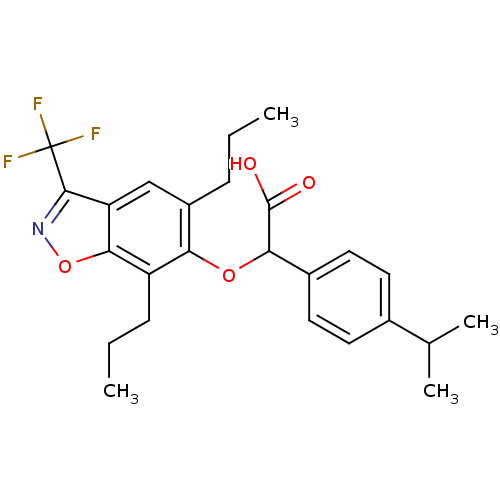
((5,7-Dipropyl-3-trifluoromethyl-benzo[d]isoxazol-6...)Show SMILES CCCc1cc2c(noc2c(CCC)c1OC(C(O)=O)c1ccc(cc1)C(C)C)C(F)(F)F Show InChI InChI=1S/C25H28F3NO4/c1-5-7-17-13-19-22(33-29-23(19)25(26,27)28)18(8-6-2)20(17)32-21(24(30)31)16-11-9-15(10-12-16)14(3)4/h9-14,21H,5-8H2,1-4H3,(H,30,31) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity towards human peroxisome proliferator activated receptor alpha (PPAR alpha) was determined by HTRF assay |
Bioorg Med Chem Lett 13: 3185-90 (2003)
BindingDB Entry DOI: 10.7270/Q2TQ6229 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Homo sapiens (Human)) | BDBM50132567
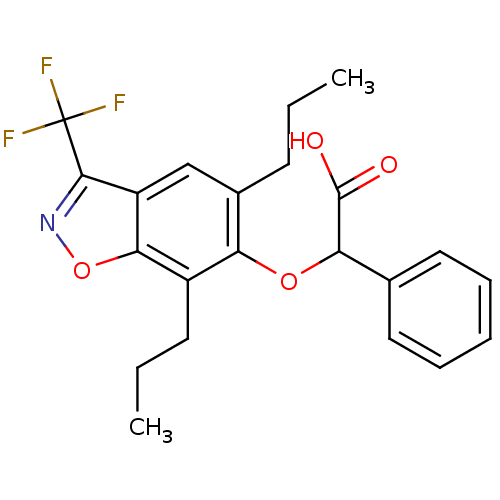
((5,7-Dipropyl-3-trifluoromethyl-benzo[d]isoxazol-6...)Show SMILES CCCc1cc2c(noc2c(CCC)c1OC(C(O)=O)c1ccccc1)C(F)(F)F Show InChI InChI=1S/C22H22F3NO4/c1-3-8-14-12-16-19(30-26-20(16)22(23,24)25)15(9-4-2)17(14)29-18(21(27)28)13-10-6-5-7-11-13/h5-7,10-12,18H,3-4,8-9H2,1-2H3,(H,27,28) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity towards human peroxisome proliferator activated receptor alpha (PPAR alpha) was determined by HTRF assay |
Bioorg Med Chem Lett 13: 3185-90 (2003)
BindingDB Entry DOI: 10.7270/Q2TQ6229 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Homo sapiens (Human)) | BDBM50132564
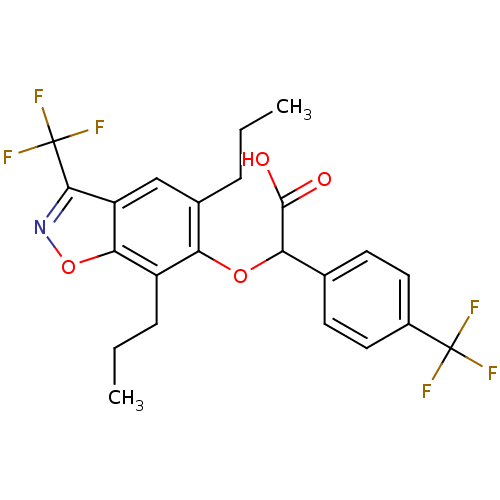
((5,7-Dipropyl-3-trifluoromethyl-benzo[d]isoxazol-6...)Show SMILES CCCc1cc2c(noc2c(CCC)c1OC(C(O)=O)c1ccc(cc1)C(F)(F)F)C(F)(F)F Show InChI InChI=1S/C23H21F6NO4/c1-3-5-13-11-16-19(34-30-20(16)23(27,28)29)15(6-4-2)17(13)33-18(21(31)32)12-7-9-14(10-8-12)22(24,25)26/h7-11,18H,3-6H2,1-2H3,(H,31,32) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity towards peroxisome proliferator activated receptor alpha (PPAR alpha) |
Bioorg Med Chem Lett 13: 3185-90 (2003)
BindingDB Entry DOI: 10.7270/Q2TQ6229 |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(RAT) | BDBM17638

(2-{1-[(4-chlorophenyl)carbonyl]-5-methoxy-2-methyl...)Show SMILES COc1ccc2n(C(=O)c3ccc(Cl)cc3)c(C)c(CC(O)=O)c2c1 Show InChI InChI=1S/C19H16ClNO4/c1-11-15(10-18(22)23)16-9-14(25-2)7-8-17(16)21(11)19(24)12-3-5-13(20)6-4-12/h3-9H,10H2,1-2H3,(H,22,23) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 290: 551-60 (1999)
BindingDB Entry DOI: 10.7270/Q2S18110 |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP2 subtype
(Homo sapiens (Human)) | BDBM17638

(2-{1-[(4-chlorophenyl)carbonyl]-5-methoxy-2-methyl...)Show SMILES COc1ccc2n(C(=O)c3ccc(Cl)cc3)c(C)c(CC(O)=O)c2c1 Show InChI InChI=1S/C19H16ClNO4/c1-11-15(10-18(22)23)16-9-14(25-2)7-8-17(16)21(11)19(24)12-3-5-13(20)6-4-12/h3-9H,10H2,1-2H3,(H,22,23) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 290: 551-60 (1999)
BindingDB Entry DOI: 10.7270/Q2S18110 |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP2 subtype
(Homo sapiens (Human)) | BDBM22369

(4-(4-methanesulfonylphenyl)-3-phenyl-2,5-dihydrofu...)Show SMILES CS(=O)(=O)c1ccc(cc1)C1=C(C(=O)OC1)c1ccccc1 |t:11| Show InChI InChI=1S/C17H14O4S/c1-22(19,20)14-9-7-12(8-10-14)15-11-21-17(18)16(15)13-5-3-2-4-6-13/h2-10H,11H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 290: 551-60 (1999)
BindingDB Entry DOI: 10.7270/Q2S18110 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Homo sapiens (Human)) | BDBM50132561

((5,7-Dipropyl-3-trifluoromethyl-benzo[d]isoxazol-6...)Show SMILES CCCc1cc2c(noc2c(CCC)c1OC(C(O)=O)c1ccc(OCc2ccccn2)cc1)C(F)(F)F Show InChI InChI=1S/C28H27F3N2O5/c1-3-7-18-15-22-25(38-33-26(22)28(29,30)31)21(8-4-2)23(18)37-24(27(34)35)17-10-12-20(13-11-17)36-16-19-9-5-6-14-32-19/h5-6,9-15,24H,3-4,7-8,16H2,1-2H3,(H,34,35) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity towards human peroxisome proliferator activated receptor alpha (PPAR alpha) was determined by HTRF assay |
Bioorg Med Chem Lett 13: 3185-90 (2003)
BindingDB Entry DOI: 10.7270/Q2TQ6229 |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP2 subtype
(Homo sapiens (Human)) | BDBM17638

(2-{1-[(4-chlorophenyl)carbonyl]-5-methoxy-2-methyl...)Show SMILES COc1ccc2n(C(=O)c3ccc(Cl)cc3)c(C)c(CC(O)=O)c2c1 Show InChI InChI=1S/C19H16ClNO4/c1-11-15(10-18(22)23)16-9-14(25-2)7-8-17(16)21(11)19(24)12-3-5-13(20)6-4-12/h3-9H,10H2,1-2H3,(H,22,23) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 19.8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 290: 551-60 (1999)
BindingDB Entry DOI: 10.7270/Q2S18110 |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP2 subtype
(Homo sapiens (Human)) | BDBM17638

(2-{1-[(4-chlorophenyl)carbonyl]-5-methoxy-2-methyl...)Show SMILES COc1ccc2n(C(=O)c3ccc(Cl)cc3)c(C)c(CC(O)=O)c2c1 Show InChI InChI=1S/C19H16ClNO4/c1-11-15(10-18(22)23)16-9-14(25-2)7-8-17(16)21(11)19(24)12-3-5-13(20)6-4-12/h3-9H,10H2,1-2H3,(H,22,23) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 290: 551-60 (1999)
BindingDB Entry DOI: 10.7270/Q2S18110 |
More data for this
Ligand-Target Pair | |
Hydroxycarboxylic acid receptor 2
(Homo sapiens (Human)) | BDBM50313981
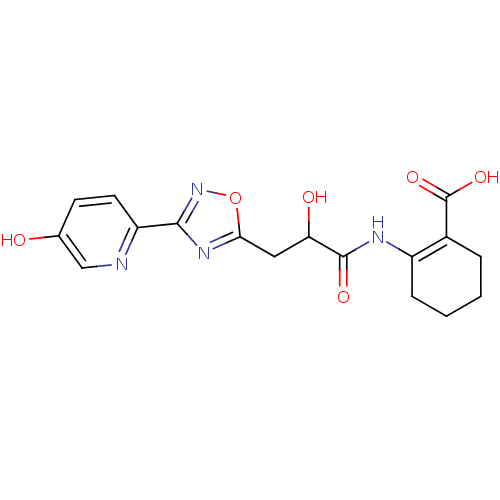
(CHEMBL1088213 | rac-2-(2-hydroxy-3-(3-(5-hydroxypy...)Show SMILES OC(Cc1nc(no1)-c1ccc(O)cn1)C(=O)NC1=C(CCCC1)C(O)=O |t:20| Show InChI InChI=1S/C17H18N4O6/c22-9-5-6-12(18-8-9)15-20-14(27-21-15)7-13(23)16(24)19-11-4-2-1-3-10(11)17(25)26/h5-6,8,13,22-23H,1-4,7H2,(H,19,24)(H,25,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]NA from cloned human GPR109A receptor expressed in CHO-K1 cells by spectrophotometry |
J Med Chem 53: 2666-70 (2010)
Article DOI: 10.1021/jm100022r
BindingDB Entry DOI: 10.7270/Q2NS0V2P |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50596945

(CHEMBL5207529)Show SMILES Cl.COc1cc(OC)c(cc1OC)C1NCCc2c1[nH]c1ccc(Cl)cc21 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00694
BindingDB Entry DOI: 10.7270/Q2ZP4B4N |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Homo sapiens (Human)) | BDBM50132564

((5,7-Dipropyl-3-trifluoromethyl-benzo[d]isoxazol-6...)Show SMILES CCCc1cc2c(noc2c(CCC)c1OC(C(O)=O)c1ccc(cc1)C(F)(F)F)C(F)(F)F Show InChI InChI=1S/C23H21F6NO4/c1-3-5-13-11-16-19(34-30-20(16)23(27,28)29)15(6-4-2)17(13)33-18(21(31)32)12-7-9-14(10-8-12)22(24,25)26/h7-11,18H,3-6H2,1-2H3,(H,31,32) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity towards human peroxisome proliferator activated receptor gamma (PPAR gamma) was determined by HTRF assay |
Bioorg Med Chem Lett 13: 3185-90 (2003)
BindingDB Entry DOI: 10.7270/Q2TQ6229 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 7
(Homo sapiens (Human)) | BDBM50596937

(CHEMBL5204071) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00694
BindingDB Entry DOI: 10.7270/Q2ZP4B4N |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Homo sapiens (Human)) | BDBM50596945

(CHEMBL5207529)Show SMILES Cl.COc1cc(OC)c(cc1OC)C1NCCc2c1[nH]c1ccc(Cl)cc21 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00694
BindingDB Entry DOI: 10.7270/Q2ZP4B4N |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP2 subtype
(Homo sapiens (Human)) | BDBM22369

(4-(4-methanesulfonylphenyl)-3-phenyl-2,5-dihydrofu...)Show SMILES CS(=O)(=O)c1ccc(cc1)C1=C(C(=O)OC1)c1ccccc1 |t:11| Show InChI InChI=1S/C17H14O4S/c1-22(19,20)14-9-7-12(8-10-14)15-11-21-17(18)16(15)13-5-3-2-4-6-13/h2-10H,11H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 290: 551-60 (1999)
BindingDB Entry DOI: 10.7270/Q2S18110 |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP2 subtype
(Homo sapiens (Human)) | BDBM17638

(2-{1-[(4-chlorophenyl)carbonyl]-5-methoxy-2-methyl...)Show SMILES COc1ccc2n(C(=O)c3ccc(Cl)cc3)c(C)c(CC(O)=O)c2c1 Show InChI InChI=1S/C19H16ClNO4/c1-11-15(10-18(22)23)16-9-14(25-2)7-8-17(16)21(11)19(24)12-3-5-13(20)6-4-12/h3-9H,10H2,1-2H3,(H,22,23) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 290: 551-60 (1999)
BindingDB Entry DOI: 10.7270/Q2S18110 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Homo sapiens (Human)) | BDBM50132576
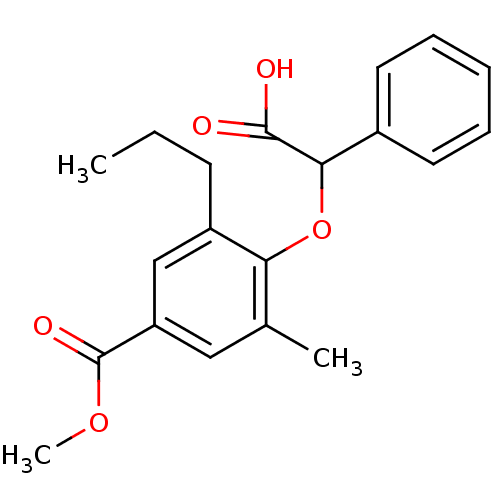
(4-(Carboxy-phenyl-methoxy)-3-methyl-5-propyl-benzo...)Show InChI InChI=1S/C20H22O5/c1-4-8-15-12-16(20(23)24-3)11-13(2)17(15)25-18(19(21)22)14-9-6-5-7-10-14/h5-7,9-12,18H,4,8H2,1-3H3,(H,21,22) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 28 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity towards peroxisome proliferator activated receptor alpha (PPAR alpha) |
Bioorg Med Chem Lett 13: 3185-90 (2003)
BindingDB Entry DOI: 10.7270/Q2TQ6229 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Homo sapiens (Human)) | BDBM50132565
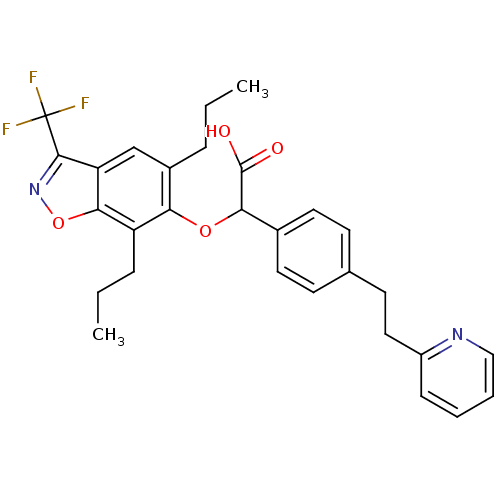
((5,7-Dipropyl-3-trifluoromethyl-benzo[d]isoxazol-6...)Show SMILES CCCc1cc2c(noc2c(CCC)c1OC(C(O)=O)c1ccc(CCc2ccccn2)cc1)C(F)(F)F Show InChI InChI=1S/C29H29F3N2O4/c1-3-7-20-17-23-26(38-34-27(23)29(30,31)32)22(8-4-2)24(20)37-25(28(35)36)19-13-10-18(11-14-19)12-15-21-9-5-6-16-33-21/h5-6,9-11,13-14,16-17,25H,3-4,7-8,12,15H2,1-2H3,(H,35,36) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 28 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity towards human peroxisome proliferator activated receptor alpha (PPAR alpha) was determined by HTRF assay |
Bioorg Med Chem Lett 13: 3185-90 (2003)
BindingDB Entry DOI: 10.7270/Q2TQ6229 |
More data for this
Ligand-Target Pair | |
Hydroxycarboxylic acid receptor 2
(Homo sapiens (Human)) | BDBM50313982
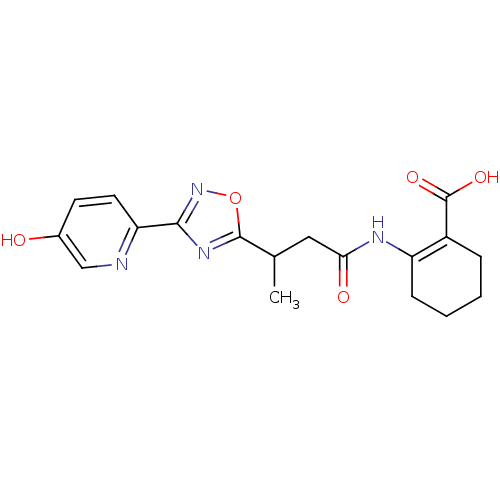
(CHEMBL1084393 | rac-2-(3-(3-(5-hydroxypyridin-2-yl...)Show SMILES CC(CC(=O)NC1=C(CCCC1)C(O)=O)c1nc(no1)-c1ccc(O)cn1 |t:6| Show InChI InChI=1S/C18H20N4O5/c1-10(8-15(24)20-13-5-3-2-4-12(13)18(25)26)17-21-16(22-27-17)14-7-6-11(23)9-19-14/h6-7,9-10,23H,2-5,8H2,1H3,(H,20,24)(H,25,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 29 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]NA from cloned human GPR109A receptor expressed in CHO-K1 cells by spectrophotometry |
J Med Chem 53: 2666-70 (2010)
Article DOI: 10.1021/jm100022r
BindingDB Entry DOI: 10.7270/Q2NS0V2P |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50596945

(CHEMBL5207529)Show SMILES Cl.COc1cc(OC)c(cc1OC)C1NCCc2c1[nH]c1ccc(Cl)cc21 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 29 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00694
BindingDB Entry DOI: 10.7270/Q2ZP4B4N |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50160434
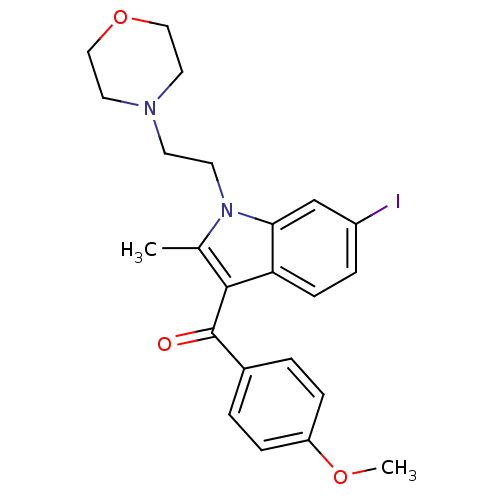
((6-iodo-2-methyl-1-(2-morpholinoethyl)-1H-indol-3-...)Show SMILES COc1ccc(cc1)C(=O)c1c(C)n(CCN2CCOCC2)c2cc(I)ccc12 Show InChI InChI=1S/C23H25IN2O3/c1-16-22(23(27)17-3-6-19(28-2)7-4-17)20-8-5-18(24)15-21(20)26(16)10-9-25-11-13-29-14-12-25/h3-8,15H,9-14H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 31 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pontificia Universidad Cat£lica de Chile
Curated by ChEMBL
| Assay Description
Inverse agonist activity at CB2 receptor (unknown origin) |
Eur J Med Chem 124: 17-35 (2016)
Article DOI: 10.1016/j.ejmech.2016.08.005
BindingDB Entry DOI: 10.7270/Q2Q2426V |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1D
(Homo sapiens (Human)) | BDBM50596936

(CHEMBL5170784) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 32 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00694
BindingDB Entry DOI: 10.7270/Q2ZP4B4N |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Homo sapiens (Human)) | BDBM50596936

(CHEMBL5170784) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 36 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00694
BindingDB Entry DOI: 10.7270/Q2ZP4B4N |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Homo sapiens (Human)) | BDBM50132574

((5,7-Dipropyl-3-trifluoromethyl-benzo[d]isoxazol-6...)Show SMILES CCCc1cc2c(noc2c(CCC)c1OC(C(O)=O)c1ccc(cc1)C(C)C)C(F)(F)F Show InChI InChI=1S/C25H28F3NO4/c1-5-7-17-13-19-22(33-29-23(19)25(26,27)28)18(8-6-2)20(17)32-21(24(30)31)16-11-9-15(10-12-16)14(3)4/h9-14,21H,5-8H2,1-4H3,(H,30,31) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 36 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity towards peroxisome proliferator activated receptor alpha (PPAR alpha) |
Bioorg Med Chem Lett 13: 3185-90 (2003)
BindingDB Entry DOI: 10.7270/Q2TQ6229 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Homo sapiens (Human)) | BDBM50132567

((5,7-Dipropyl-3-trifluoromethyl-benzo[d]isoxazol-6...)Show SMILES CCCc1cc2c(noc2c(CCC)c1OC(C(O)=O)c1ccccc1)C(F)(F)F Show InChI InChI=1S/C22H22F3NO4/c1-3-8-14-12-16-19(30-26-20(16)22(23,24)25)15(9-4-2)17(14)29-18(21(27)28)13-10-6-5-7-11-13/h5-7,10-12,18H,3-4,8-9H2,1-2H3,(H,27,28) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 37 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity towards peroxisome proliferator activated receptor alpha (PPAR alpha) |
Bioorg Med Chem Lett 13: 3185-90 (2003)
BindingDB Entry DOI: 10.7270/Q2TQ6229 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Homo sapiens (Human)) | BDBM50132568
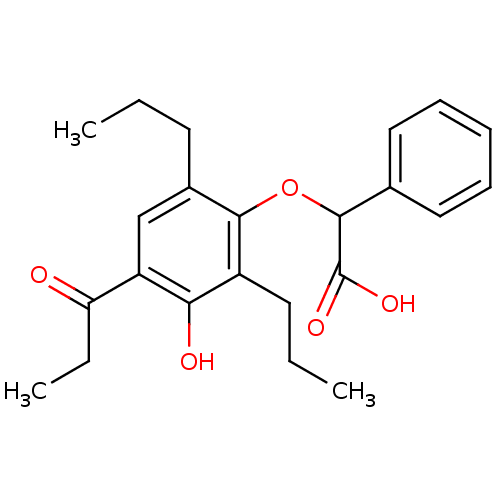
((3-Hydroxy-4-propionyl-2,6-dipropyl-phenoxy)-pheny...)Show SMILES CCCc1cc(C(=O)CC)c(O)c(CCC)c1OC(C(O)=O)c1ccccc1 Show InChI InChI=1S/C23H28O5/c1-4-10-16-14-18(19(24)6-3)20(25)17(11-5-2)21(16)28-22(23(26)27)15-12-8-7-9-13-15/h7-9,12-14,22,25H,4-6,10-11H2,1-3H3,(H,26,27) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 37 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity towards human peroxisome proliferator activated receptor alpha (PPAR alpha) was determined by HTRF assay |
Bioorg Med Chem Lett 13: 3185-90 (2003)
BindingDB Entry DOI: 10.7270/Q2TQ6229 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Homo sapiens (Human)) | BDBM50132568

((3-Hydroxy-4-propionyl-2,6-dipropyl-phenoxy)-pheny...)Show SMILES CCCc1cc(C(=O)CC)c(O)c(CCC)c1OC(C(O)=O)c1ccccc1 Show InChI InChI=1S/C23H28O5/c1-4-10-16-14-18(19(24)6-3)20(25)17(11-5-2)21(16)28-22(23(26)27)15-12-8-7-9-13-15/h7-9,12-14,22,25H,4-6,10-11H2,1-3H3,(H,26,27) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 37 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity towards human peroxisome proliferator activated receptor alpha (PPAR alpha) was determined by HTRF assay |
Bioorg Med Chem Lett 13: 3185-90 (2003)
BindingDB Entry DOI: 10.7270/Q2TQ6229 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50132574

((5,7-Dipropyl-3-trifluoromethyl-benzo[d]isoxazol-6...)Show SMILES CCCc1cc2c(noc2c(CCC)c1OC(C(O)=O)c1ccc(cc1)C(C)C)C(F)(F)F Show InChI InChI=1S/C25H28F3NO4/c1-5-7-17-13-19-22(33-29-23(19)25(26,27)28)18(8-6-2)20(17)32-21(24(30)31)16-11-9-15(10-12-16)14(3)4/h9-14,21H,5-8H2,1-4H3,(H,30,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 38 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity towards human peroxisome proliferator activated receptor gamma (PPAR gamma) was determined by HTRF assay |
Bioorg Med Chem Lett 13: 3185-90 (2003)
BindingDB Entry DOI: 10.7270/Q2TQ6229 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Homo sapiens (Human)) | BDBM50132571
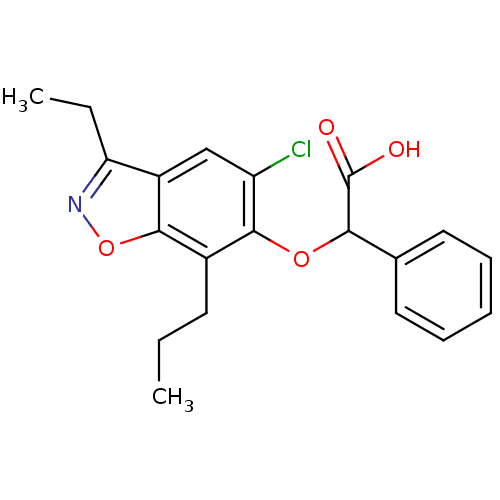
((5-Chloro-3-ethyl-7-propyl-benzo[d]isoxazol-6-ylox...)Show SMILES CCCc1c(OC(C(O)=O)c2ccccc2)c(Cl)cc2c(CC)noc12 Show InChI InChI=1S/C20H20ClNO4/c1-3-8-13-18-14(16(4-2)22-26-18)11-15(21)19(13)25-17(20(23)24)12-9-6-5-7-10-12/h5-7,9-11,17H,3-4,8H2,1-2H3,(H,23,24) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 38 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity towards peroxisome proliferator activated receptor alpha (PPAR alpha) |
Bioorg Med Chem Lett 13: 3185-90 (2003)
BindingDB Entry DOI: 10.7270/Q2TQ6229 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1D
(Homo sapiens (Human)) | BDBM50596945

(CHEMBL5207529)Show SMILES Cl.COc1cc(OC)c(cc1OC)C1NCCc2c1[nH]c1ccc(Cl)cc21 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 39 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00694
BindingDB Entry DOI: 10.7270/Q2ZP4B4N |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM60994
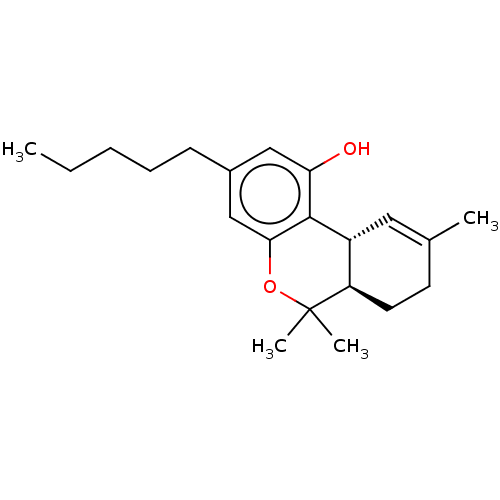
((10R,10aR)-6,6,9-Trimethyl-3-pentyl-6a,7,8,10a-tet...)Show SMILES CCCCCc1cc(O)c2[C@@H]3C=C(C)CC[C@H]3C(C)(C)Oc2c1 |r,t:11| Show InChI InChI=1S/C21H30O2/c1-5-6-7-8-15-12-18(22)20-16-11-14(2)9-10-17(16)21(3,4)23-19(20)13-15/h11-13,16-17,22H,5-10H2,1-4H3/t16-,17-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| DrugBank
Article
PubMed
| 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pontificia Universidad Cat£lica de Chile
Curated by ChEMBL
| Assay Description
Agonist activity at CB2 receptor (unknown origin) |
Eur J Med Chem 124: 17-35 (2016)
Article DOI: 10.1016/j.ejmech.2016.08.005
BindingDB Entry DOI: 10.7270/Q2Q2426V |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM60994

((10R,10aR)-6,6,9-Trimethyl-3-pentyl-6a,7,8,10a-tet...)Show SMILES CCCCCc1cc(O)c2[C@@H]3C=C(C)CC[C@H]3C(C)(C)Oc2c1 |r,t:11| Show InChI InChI=1S/C21H30O2/c1-5-6-7-8-15-12-18(22)20-16-11-14(2)9-10-17(16)21(3,4)23-19(20)13-15/h11-13,16-17,22H,5-10H2,1-4H3/t16-,17-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| DrugBank
Article
PubMed
| 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pontificia Universidad Cat£lica de Chile
Curated by ChEMBL
| Assay Description
Agonist activity at CB1 receptor (unknown origin) |
Eur J Med Chem 124: 17-35 (2016)
Article DOI: 10.1016/j.ejmech.2016.08.005
BindingDB Entry DOI: 10.7270/Q2Q2426V |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(RAT) | BDBM22369

(4-(4-methanesulfonylphenyl)-3-phenyl-2,5-dihydrofu...)Show SMILES CS(=O)(=O)c1ccc(cc1)C1=C(C(=O)OC1)c1ccccc1 |t:11| Show InChI InChI=1S/C17H14O4S/c1-22(19,20)14-9-7-12(8-10-14)15-11-21-17(18)16(15)13-5-3-2-4-6-13/h2-10H,11H2,1H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 41 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 290: 551-60 (1999)
BindingDB Entry DOI: 10.7270/Q2S18110 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data