 Found 973 hits with Last Name = 'miyata' and Initial = 'n'
Found 973 hits with Last Name = 'miyata' and Initial = 'n' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Lysine-specific histone demethylase 1A
(Homo sapiens (Human)) | BDBM50346873
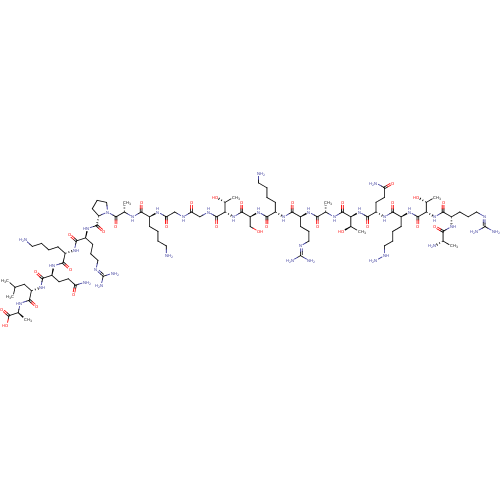
(CHEMBL1797652)Show SMILES [#6]-[#6](-[#6])-[#6]-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6](-[#7])=O)-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#7]-[#6](=O)-[#6@@H]-1-[#6]-[#6]-[#6]-[#7]-1-[#6](=O)-[#6@H](-[#6])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#8])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#7]-[#6](=O)-[#6@H](-[#6])-[#7]-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6](-[#7])=O)-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]-[#6]-[#7]-[#7])-[#7]-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#7]-[#6](=O)-[#6@H](-[#6])-[#7])-[#6@@H](-[#6])-[#8])-[#6@@H](-[#6])-[#8])-[#6@@H](-[#6])-[#8])-[#6](=O)-[#7]-[#6@@H](-[#6])-[#6](-[#8])=O |r| Show InChI InChI=1S/C94H173N37O28/c1-46(2)42-63(84(151)116-50(6)91(158)159)126-81(148)61(30-32-66(99)136)122-76(143)55(23-11-15-35-96)120-79(146)59(27-19-38-109-93(103)104)124-86(153)65-29-21-41-131(65)90(157)49(5)115-75(142)54(22-10-14-34-95)117-69(139)44-111-68(138)43-112-87(154)70(51(7)133)128-85(152)64(45-132)127-80(147)56(24-12-16-36-97)121-78(145)58(26-18-37-108-92(101)102)119-74(141)48(4)114-88(155)71(52(8)134)129-83(150)62(31-33-67(100)137)123-77(144)57(25-13-17-40-113-107)125-89(156)72(53(9)135)130-82(149)60(118-73(140)47(3)98)28-20-39-110-94(105)106/h46-65,70-72,113,132-135H,10-45,95-98,107H2,1-9H3,(H2,99,136)(H2,100,137)(H,111,138)(H,112,154)(H,114,155)(H,115,142)(H,116,151)(H,117,139)(H,118,140)(H,119,141)(H,120,146)(H,121,145)(H,122,143)(H,123,144)(H,124,153)(H,125,156)(H,126,148)(H,127,147)(H,128,152)(H,129,150)(H,130,149)(H,158,159)(H4,101,102,108)(H4,103,104,109)(H4,105,106,110)/t47-,48-,49-,50-,51+,52+,53+,54-,55-,56-,57-,58-,59-,60-,61-,62-,63-,64-,65-,70-,71-,72-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Prefectural University of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of LSD1 |
J Med Chem 54: 8236-50 (2011)
Article DOI: 10.1021/jm201048w
BindingDB Entry DOI: 10.7270/Q2D50NFR |
More data for this
Ligand-Target Pair | |
Lysine-specific histone demethylase 1A
(Homo sapiens (Human)) | BDBM50361478
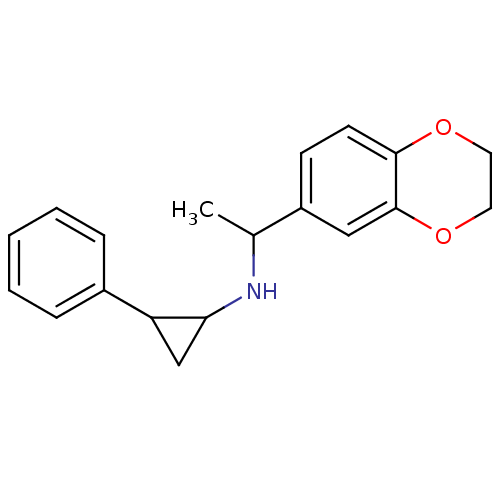
(CHEMBL1938897)Show InChI InChI=1S/C19H21NO2/c1-13(15-7-8-18-19(11-15)22-10-9-21-18)20-17-12-16(17)14-5-3-2-4-6-14/h2-8,11,13,16-17,20H,9-10,12H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Prefectural University of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of LSD1 |
J Med Chem 54: 8236-50 (2011)
Article DOI: 10.1021/jm201048w
BindingDB Entry DOI: 10.7270/Q2D50NFR |
More data for this
Ligand-Target Pair | |
Lysine-specific histone demethylase 1A
(Homo sapiens (Human)) | BDBM50361481
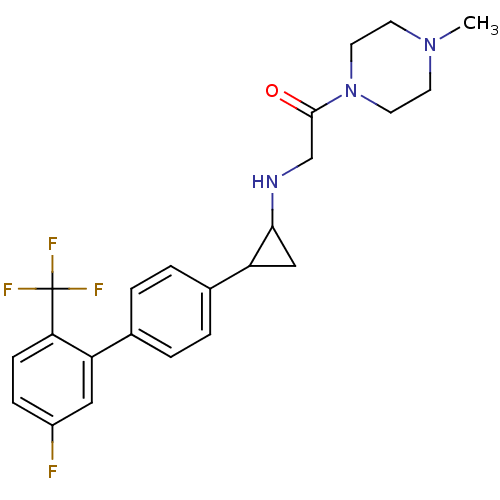
(CHEMBL1938898)Show SMILES CN1CCN(CC1)C(=O)CNC1CC1c1ccc(cc1)-c1cc(F)ccc1C(F)(F)F Show InChI InChI=1S/C23H25F4N3O/c1-29-8-10-30(11-9-29)22(31)14-28-21-13-19(21)16-4-2-15(3-5-16)18-12-17(24)6-7-20(18)23(25,26)27/h2-7,12,19,21,28H,8-11,13-14H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Prefectural University of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of LSD1 |
J Med Chem 54: 8236-50 (2011)
Article DOI: 10.1021/jm201048w
BindingDB Entry DOI: 10.7270/Q2D50NFR |
More data for this
Ligand-Target Pair | |
Lysine-specific histone demethylase 1A
(Homo sapiens (Human)) | BDBM50346875
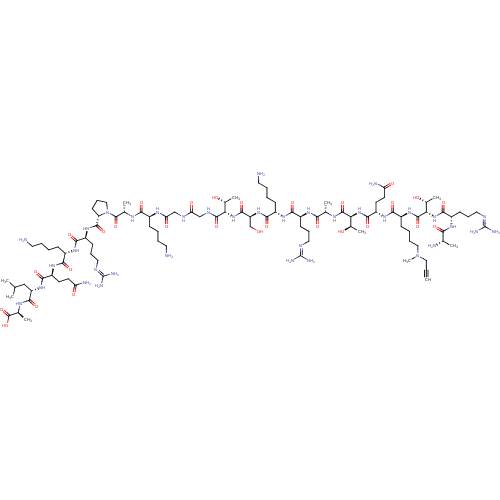
(CHEMBL1797648)Show SMILES [#6]-[#6](-[#6])-[#6]-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6](-[#7])=O)-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#7]-[#6](=O)-[#6@@H]-1-[#6]-[#6]-[#6]-[#7]-1-[#6](=O)-[#6@H](-[#6])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#8])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#7]-[#6](=O)-[#6@H](-[#6])-[#7]-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6](-[#7])=O)-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]-[#6]-[#7](-[#6])-[#6]C#C)-[#7]-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#7]-[#6](=O)-[#6@H](-[#6])-[#7])-[#6@@H](-[#6])-[#8])-[#6@@H](-[#6])-[#8])-[#6@@H](-[#6])-[#8])-[#6](=O)-[#7]-[#6@@H](-[#6])-[#6](-[#8])=O |r| Show InChI InChI=1S/C98H176N36O28/c1-12-43-133(11)44-20-16-28-61(127-93(159)76(57(10)138)132-86(152)64(120-77(143)51(4)102)31-23-42-113-98(109)110)81(147)125-66(34-36-71(104)140)87(153)131-75(56(9)137)92(158)116-52(5)78(144)121-62(29-21-40-111-96(105)106)82(148)123-60(27-15-19-39-101)84(150)129-68(49-135)89(155)130-74(55(8)136)91(157)115-47-72(141)114-48-73(142)119-58(25-13-17-37-99)79(145)117-53(6)94(160)134-45-24-32-69(134)90(156)126-63(30-22-41-112-97(107)108)83(149)122-59(26-14-18-38-100)80(146)124-65(33-35-70(103)139)85(151)128-67(46-50(2)3)88(154)118-54(7)95(161)162/h1,50-69,74-76,135-138H,13-49,99-102H2,2-11H3,(H2,103,139)(H2,104,140)(H,114,141)(H,115,157)(H,116,158)(H,117,145)(H,118,154)(H,119,142)(H,120,143)(H,121,144)(H,122,149)(H,123,148)(H,124,146)(H,125,147)(H,126,156)(H,127,159)(H,128,151)(H,129,150)(H,130,155)(H,131,153)(H,132,152)(H,161,162)(H4,105,106,111)(H4,107,108,112)(H4,109,110,113)/t51-,52-,53-,54-,55+,56+,57+,58-,59-,60-,61-,62-,63-,64-,65-,66-,67-,68-,69-,74-,75-,76-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 107 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Prefectural University of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of LSD1 |
J Med Chem 54: 8236-50 (2011)
Article DOI: 10.1021/jm201048w
BindingDB Entry DOI: 10.7270/Q2D50NFR |
More data for this
Ligand-Target Pair | |
Lysine-specific histone demethylase 1A
(Homo sapiens (Human)) | BDBM50346585
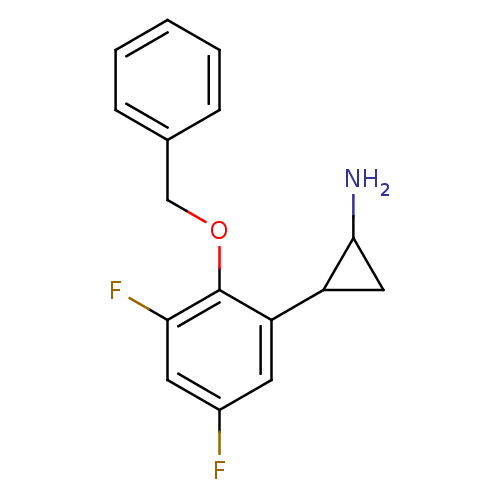
(CHEMBL1795980 | S2101)Show InChI InChI=1S/C16H15F2NO/c17-11-6-13(12-8-15(12)19)16(14(18)7-11)20-9-10-4-2-1-3-5-10/h1-7,12,15H,8-9,19H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 610 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Prefectural University of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of LSD1 |
J Med Chem 54: 8236-50 (2011)
Article DOI: 10.1021/jm201048w
BindingDB Entry DOI: 10.7270/Q2D50NFR |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase NIMA-interacting 1
(Homo sapiens (Human)) | BDBM50518736

(CHEMBL4526940)Show SMILES CC(C)(C)c1ccc(cc1)S(=O)(=O)\N=C1/C=C(SCC(O)=O)C(=O)c2ccccc12 |t:16| Show InChI InChI=1S/C22H21NO5S2/c1-22(2,3)14-8-10-15(11-9-14)30(27,28)23-18-12-19(29-13-20(24)25)21(26)17-7-5-4-6-16(17)18/h4-12H,13H2,1-3H3,(H,24,25)/b23-18+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 625 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nagoya Citi University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant Pin1 (unknown origin) using Suc-AEPF-MCA as substrate preincubated followed by substrate addition and measured after 60 to ... |
Bioorg Med Chem Lett 29: 353-356 (2019)
Article DOI: 10.1016/j.bmcl.2018.12.044
BindingDB Entry DOI: 10.7270/Q27D2ZH0 |
More data for this
Ligand-Target Pair | |
Amine oxidase [flavin-containing] A
(Homo sapiens (Human)) | BDBM50346864
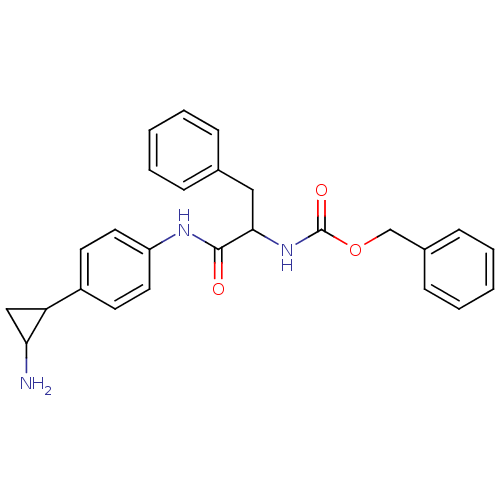
(CHEMBL1797641 | CHEMBL3104337 | US8765820, 8)Show SMILES NC1CC1c1ccc(NC(=O)C(Cc2ccccc2)NC(=O)OCc2ccccc2)cc1 Show InChI InChI=1S/C26H27N3O3/c27-23-16-22(23)20-11-13-21(14-12-20)28-25(30)24(15-18-7-3-1-4-8-18)29-26(31)32-17-19-9-5-2-6-10-19/h1-14,22-24H,15-17,27H2,(H,28,30)(H,29,31) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Prefectural University of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of MAO A |
J Med Chem 54: 8236-50 (2011)
Article DOI: 10.1021/jm201048w
BindingDB Entry DOI: 10.7270/Q2D50NFR |
More data for this
Ligand-Target Pair | |
Lysine-specific histone demethylase 1A
(Homo sapiens (Human)) | BDBM50346864

(CHEMBL1797641 | CHEMBL3104337 | US8765820, 8)Show SMILES NC1CC1c1ccc(NC(=O)C(Cc2ccccc2)NC(=O)OCc2ccccc2)cc1 Show InChI InChI=1S/C26H27N3O3/c27-23-16-22(23)20-11-13-21(14-12-20)28-25(30)24(15-18-7-3-1-4-8-18)29-26(31)32-17-19-9-5-2-6-10-19/h1-14,22-24H,15-17,27H2,(H,28,30)(H,29,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Prefectural University of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of LSD1 |
J Med Chem 54: 8236-50 (2011)
Article DOI: 10.1021/jm201048w
BindingDB Entry DOI: 10.7270/Q2D50NFR |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase NIMA-interacting 1
(Homo sapiens (Human)) | BDBM50518734
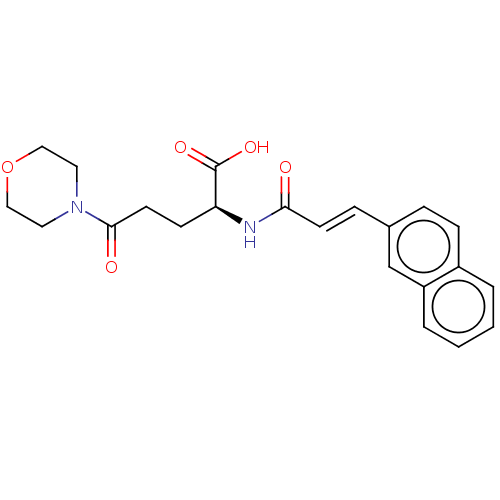
(CHEMBL4467081)Show SMILES OC(=O)[C@H](CCC(=O)N1CCOCC1)NC(=O)\C=C\c1ccc2ccccc2c1 |r| Show InChI InChI=1S/C22H24N2O5/c25-20(9-6-16-5-7-17-3-1-2-4-18(17)15-16)23-19(22(27)28)8-10-21(26)24-11-13-29-14-12-24/h1-7,9,15,19H,8,10-14H2,(H,23,25)(H,27,28)/b9-6+/t19-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.37E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nagoya Citi University
Curated by ChEMBL
| Assay Description
Irreversible inhibition of Pin1 (unknown origin) using suc-Ala-Glu-Pro-Phe-pNA as substrate preincubated for 10 mins followed by substrate addition a... |
Bioorg Med Chem Lett 29: 353-356 (2019)
Article DOI: 10.1016/j.bmcl.2018.12.044
BindingDB Entry DOI: 10.7270/Q27D2ZH0 |
More data for this
Ligand-Target Pair | |
Lysine-specific histone demethylase 1A
(Homo sapiens (Human)) | BDBM50346862
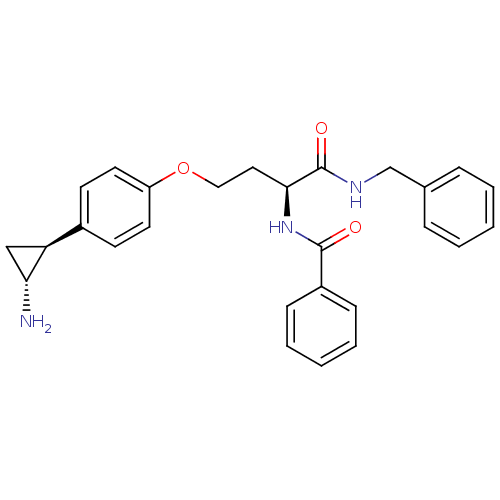
(CHEMBL1215658)Show SMILES N[C@@H]1C[C@H]1c1ccc(OCC[C@H](NC(=O)c2ccccc2)C(=O)NCc2ccccc2)cc1 |r| Show InChI InChI=1S/C27H29N3O3/c28-24-17-23(24)20-11-13-22(14-12-20)33-16-15-25(30-26(31)21-9-5-2-6-10-21)27(32)29-18-19-7-3-1-4-8-19/h1-14,23-25H,15-18,28H2,(H,29,32)(H,30,31)/t23-,24+,25-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Prefectural University of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of LSD1 |
J Med Chem 54: 8236-50 (2011)
Article DOI: 10.1021/jm201048w
BindingDB Entry DOI: 10.7270/Q2D50NFR |
More data for this
Ligand-Target Pair | |
Lysine-specific histone demethylase 1A
(Homo sapiens (Human)) | BDBM50346589
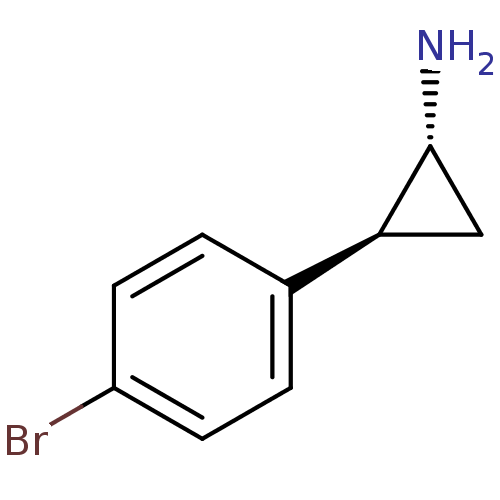
(CHEMBL1795974 | CHEMBL255520)Show InChI InChI=1S/C9H10BrN/c10-7-3-1-6(2-4-7)8-5-9(8)11/h1-4,8-9H,5,11H2/t8-,9+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Prefectural University of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of LSD1 |
J Med Chem 54: 8236-50 (2011)
Article DOI: 10.1021/jm201048w
BindingDB Entry DOI: 10.7270/Q2D50NFR |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase NIMA-interacting 1
(Homo sapiens (Human)) | BDBM50518732

(CHEMBL4473488)Show SMILES OC(=O)[C@@H](CCC(=O)N1CCOCC1)NC(=O)\C=C\c1ccc2ccccc2c1 |r| Show InChI InChI=1S/C22H24N2O5/c25-20(9-6-16-5-7-17-3-1-2-4-18(17)15-16)23-19(22(27)28)8-10-21(26)24-11-13-29-14-12-24/h1-7,9,15,19H,8,10-14H2,(H,23,25)(H,27,28)/b9-6+/t19-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 5.47E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nagoya Citi University
Curated by ChEMBL
| Assay Description
Irreversible inhibition of Pin1 (unknown origin) using suc-Ala-Glu-Pro-Phe-pNA as substrate preincubated for 10 mins followed by substrate addition a... |
Bioorg Med Chem Lett 29: 353-356 (2019)
Article DOI: 10.1016/j.bmcl.2018.12.044
BindingDB Entry DOI: 10.7270/Q27D2ZH0 |
More data for this
Ligand-Target Pair | |
Lysine-specific histone demethylase 1A
(Homo sapiens (Human)) | BDBM50346534
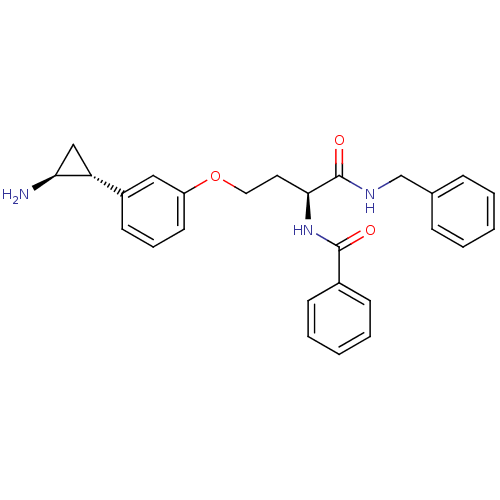
(CHEMBL1797705)Show SMILES N[C@H]1C[C@@H]1c1cccc(OCC[C@H](NC(=O)c2ccccc2)C(=O)NCc2ccccc2)c1 |r| Show InChI InChI=1S/C27H29N3O3/c28-24-17-23(24)21-12-7-13-22(16-21)33-15-14-25(30-26(31)20-10-5-2-6-11-20)27(32)29-18-19-8-3-1-4-9-19/h1-13,16,23-25H,14-15,17-18,28H2,(H,29,32)(H,30,31)/t23-,24+,25+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Prefectural University of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of LSD1 |
J Med Chem 54: 8236-50 (2011)
Article DOI: 10.1021/jm201048w
BindingDB Entry DOI: 10.7270/Q2D50NFR |
More data for this
Ligand-Target Pair | |
Amine oxidase [flavin-containing] B
(Homo sapiens (Human)) | BDBM50361478

(CHEMBL1938897)Show InChI InChI=1S/C19H21NO2/c1-13(15-7-8-18-19(11-15)22-10-9-21-18)20-17-12-16(17)14-5-3-2-4-6-14/h2-8,11,13,16-17,20H,9-10,12H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Prefectural University of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of MAO B |
J Med Chem 54: 8236-50 (2011)
Article DOI: 10.1021/jm201048w
BindingDB Entry DOI: 10.7270/Q2D50NFR |
More data for this
Ligand-Target Pair | |
Solute carrier family 22 member 2
(RAT) | BDBM86694

(N'-(3-Chloro-4-morpholinophenyl)-N-hydroxyform...)Show InChI InChI=1S/C11H14ClN3O2/c12-10-7-9(13-8-14-16)1-2-11(10)15-3-5-17-6-4-15/h1-2,7-8,16H,3-6H2,(H,13,14) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co., Ltd
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 314: 77-85 (2005)
Article DOI: 10.1124/jpet.105.083964
BindingDB Entry DOI: 10.7270/Q2MW2FQQ |
More data for this
Ligand-Target Pair | |
B2 bradykinin receptor
(Homo sapiens (Human)) | BDBM86694

(N'-(3-Chloro-4-morpholinophenyl)-N-hydroxyform...)Show InChI InChI=1S/C11H14ClN3O2/c12-10-7-9(13-8-14-16)1-2-11(10)15-3-5-17-6-4-15/h1-2,7-8,16H,3-6H2,(H,13,14) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co., Ltd
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 314: 77-85 (2005)
Article DOI: 10.1124/jpet.105.083964
BindingDB Entry DOI: 10.7270/Q2MW2FQQ |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(RAT) | BDBM86694

(N'-(3-Chloro-4-morpholinophenyl)-N-hydroxyform...)Show InChI InChI=1S/C11H14ClN3O2/c12-10-7-9(13-8-14-16)1-2-11(10)15-3-5-17-6-4-15/h1-2,7-8,16H,3-6H2,(H,13,14) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co., Ltd
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 314: 77-85 (2005)
Article DOI: 10.1124/jpet.105.083964
BindingDB Entry DOI: 10.7270/Q2MW2FQQ |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(MOUSE) | BDBM86694

(N'-(3-Chloro-4-morpholinophenyl)-N-hydroxyform...)Show InChI InChI=1S/C11H14ClN3O2/c12-10-7-9(13-8-14-16)1-2-11(10)15-3-5-17-6-4-15/h1-2,7-8,16H,3-6H2,(H,13,14) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co., Ltd
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 314: 77-85 (2005)
Article DOI: 10.1124/jpet.105.083964
BindingDB Entry DOI: 10.7270/Q2MW2FQQ |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-9
(RAT) | BDBM86694

(N'-(3-Chloro-4-morpholinophenyl)-N-hydroxyform...)Show InChI InChI=1S/C11H14ClN3O2/c12-10-7-9(13-8-14-16)1-2-11(10)15-3-5-17-6-4-15/h1-2,7-8,16H,3-6H2,(H,13,14) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co., Ltd
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 314: 77-85 (2005)
Article DOI: 10.1124/jpet.105.083964
BindingDB Entry DOI: 10.7270/Q2MW2FQQ |
More data for this
Ligand-Target Pair | |
Dual specificity calcium/calmodulin-dependent 3',5'-cyclic nucleotide phosphodiesterase 1A
(BOVINE) | BDBM86694

(N'-(3-Chloro-4-morpholinophenyl)-N-hydroxyform...)Show InChI InChI=1S/C11H14ClN3O2/c12-10-7-9(13-8-14-16)1-2-11(10)15-3-5-17-6-4-15/h1-2,7-8,16H,3-6H2,(H,13,14) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co., Ltd
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 314: 77-85 (2005)
Article DOI: 10.1124/jpet.105.083964
BindingDB Entry DOI: 10.7270/Q2MW2FQQ |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1B
(Rattus norvegicus (Rat)) | BDBM86694

(N'-(3-Chloro-4-morpholinophenyl)-N-hydroxyform...)Show InChI InChI=1S/C11H14ClN3O2/c12-10-7-9(13-8-14-16)1-2-11(10)15-3-5-17-6-4-15/h1-2,7-8,16H,3-6H2,(H,13,14) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co., Ltd
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 314: 77-85 (2005)
Article DOI: 10.1124/jpet.105.083964
BindingDB Entry DOI: 10.7270/Q2MW2FQQ |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Rattus norvegicus (rat)) | BDBM86694

(N'-(3-Chloro-4-morpholinophenyl)-N-hydroxyform...)Show InChI InChI=1S/C11H14ClN3O2/c12-10-7-9(13-8-14-16)1-2-11(10)15-3-5-17-6-4-15/h1-2,7-8,16H,3-6H2,(H,13,14) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co., Ltd
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 314: 77-85 (2005)
Article DOI: 10.1124/jpet.105.083964
BindingDB Entry DOI: 10.7270/Q2MW2FQQ |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Rattus norvegicus (rat)) | BDBM86694

(N'-(3-Chloro-4-morpholinophenyl)-N-hydroxyform...)Show InChI InChI=1S/C11H14ClN3O2/c12-10-7-9(13-8-14-16)1-2-11(10)15-3-5-17-6-4-15/h1-2,7-8,16H,3-6H2,(H,13,14) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co., Ltd
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 314: 77-85 (2005)
Article DOI: 10.1124/jpet.105.083964
BindingDB Entry DOI: 10.7270/Q2MW2FQQ |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Rattus norvegicus (rat)) | BDBM86694

(N'-(3-Chloro-4-morpholinophenyl)-N-hydroxyform...)Show InChI InChI=1S/C11H14ClN3O2/c12-10-7-9(13-8-14-16)1-2-11(10)15-3-5-17-6-4-15/h1-2,7-8,16H,3-6H2,(H,13,14) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co., Ltd
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 314: 77-85 (2005)
Article DOI: 10.1124/jpet.105.083964
BindingDB Entry DOI: 10.7270/Q2MW2FQQ |
More data for this
Ligand-Target Pair | |
Solute carrier family 22 member 1
(RAT) | BDBM86694

(N'-(3-Chloro-4-morpholinophenyl)-N-hydroxyform...)Show InChI InChI=1S/C11H14ClN3O2/c12-10-7-9(13-8-14-16)1-2-11(10)15-3-5-17-6-4-15/h1-2,7-8,16H,3-6H2,(H,13,14) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co., Ltd
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 314: 77-85 (2005)
Article DOI: 10.1124/jpet.105.083964
BindingDB Entry DOI: 10.7270/Q2MW2FQQ |
More data for this
Ligand-Target Pair | |
Beta-1 adrenergic receptor
(Rattus norvegicus (Rat)) | BDBM86694

(N'-(3-Chloro-4-morpholinophenyl)-N-hydroxyform...)Show InChI InChI=1S/C11H14ClN3O2/c12-10-7-9(13-8-14-16)1-2-11(10)15-3-5-17-6-4-15/h1-2,7-8,16H,3-6H2,(H,13,14) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co., Ltd
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 314: 77-85 (2005)
Article DOI: 10.1124/jpet.105.083964
BindingDB Entry DOI: 10.7270/Q2MW2FQQ |
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member B1 [K65Q]
(Bos taurus (Cattle)) | BDBM86694

(N'-(3-Chloro-4-morpholinophenyl)-N-hydroxyform...)Show InChI InChI=1S/C11H14ClN3O2/c12-10-7-9(13-8-14-16)1-2-11(10)15-3-5-17-6-4-15/h1-2,7-8,16H,3-6H2,(H,13,14) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co., Ltd
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 314: 77-85 (2005)
Article DOI: 10.1124/jpet.105.083964
BindingDB Entry DOI: 10.7270/Q2MW2FQQ |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A
(RAT) | BDBM86694

(N'-(3-Chloro-4-morpholinophenyl)-N-hydroxyform...)Show InChI InChI=1S/C11H14ClN3O2/c12-10-7-9(13-8-14-16)1-2-11(10)15-3-5-17-6-4-15/h1-2,7-8,16H,3-6H2,(H,13,14) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co., Ltd
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 314: 77-85 (2005)
Article DOI: 10.1124/jpet.105.083964
BindingDB Entry DOI: 10.7270/Q2MW2FQQ |
More data for this
Ligand-Target Pair | |
Type-2 angiotensin II receptor
(BOVINE) | BDBM86694

(N'-(3-Chloro-4-morpholinophenyl)-N-hydroxyform...)Show InChI InChI=1S/C11H14ClN3O2/c12-10-7-9(13-8-14-16)1-2-11(10)15-3-5-17-6-4-15/h1-2,7-8,16H,3-6H2,(H,13,14) | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co., Ltd
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 314: 77-85 (2005)
Article DOI: 10.1124/jpet.105.083964
BindingDB Entry DOI: 10.7270/Q2MW2FQQ |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM86694

(N'-(3-Chloro-4-morpholinophenyl)-N-hydroxyform...)Show InChI InChI=1S/C11H14ClN3O2/c12-10-7-9(13-8-14-16)1-2-11(10)15-3-5-17-6-4-15/h1-2,7-8,16H,3-6H2,(H,13,14) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co., Ltd
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 314: 77-85 (2005)
Article DOI: 10.1124/jpet.105.083964
BindingDB Entry DOI: 10.7270/Q2MW2FQQ |
More data for this
Ligand-Target Pair | |
Natriuretic peptides A
(GUINEA PIG) | BDBM86694

(N'-(3-Chloro-4-morpholinophenyl)-N-hydroxyform...)Show InChI InChI=1S/C11H14ClN3O2/c12-10-7-9(13-8-14-16)1-2-11(10)15-3-5-17-6-4-15/h1-2,7-8,16H,3-6H2,(H,13,14) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co., Ltd
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 314: 77-85 (2005)
Article DOI: 10.1124/jpet.105.083964
BindingDB Entry DOI: 10.7270/Q2MW2FQQ |
More data for this
Ligand-Target Pair | |
Gamma-aminobutyric acid receptor subunit alpha-1
(Rattus norvegicus (Rat)) | BDBM86694

(N'-(3-Chloro-4-morpholinophenyl)-N-hydroxyform...)Show InChI InChI=1S/C11H14ClN3O2/c12-10-7-9(13-8-14-16)1-2-11(10)15-3-5-17-6-4-15/h1-2,7-8,16H,3-6H2,(H,13,14) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co., Ltd
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 314: 77-85 (2005)
Article DOI: 10.1124/jpet.105.083964
BindingDB Entry DOI: 10.7270/Q2MW2FQQ |
More data for this
Ligand-Target Pair | |
Voltage-dependent T-type calcium channel subunit alpha-1H
(Homo sapiens (Human)) | BDBM86694

(N'-(3-Chloro-4-morpholinophenyl)-N-hydroxyform...)Show InChI InChI=1S/C11H14ClN3O2/c12-10-7-9(13-8-14-16)1-2-11(10)15-3-5-17-6-4-15/h1-2,7-8,16H,3-6H2,(H,13,14) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co., Ltd
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 314: 77-85 (2005)
Article DOI: 10.1124/jpet.105.083964
BindingDB Entry DOI: 10.7270/Q2MW2FQQ |
More data for this
Ligand-Target Pair | |
Caspase-3
(Homo sapiens (Human)) | BDBM86694

(N'-(3-Chloro-4-morpholinophenyl)-N-hydroxyform...)Show InChI InChI=1S/C11H14ClN3O2/c12-10-7-9(13-8-14-16)1-2-11(10)15-3-5-17-6-4-15/h1-2,7-8,16H,3-6H2,(H,13,14) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co., Ltd
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 314: 77-85 (2005)
Article DOI: 10.1124/jpet.105.083964
BindingDB Entry DOI: 10.7270/Q2MW2FQQ |
More data for this
Ligand-Target Pair | |
Cholecystokinin
(Homo sapiens (Human)) | BDBM86694

(N'-(3-Chloro-4-morpholinophenyl)-N-hydroxyform...)Show InChI InChI=1S/C11H14ClN3O2/c12-10-7-9(13-8-14-16)1-2-11(10)15-3-5-17-6-4-15/h1-2,7-8,16H,3-6H2,(H,13,14) | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co., Ltd
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 314: 77-85 (2005)
Article DOI: 10.1124/jpet.105.083964
BindingDB Entry DOI: 10.7270/Q2MW2FQQ |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(RAT) | BDBM86694

(N'-(3-Chloro-4-morpholinophenyl)-N-hydroxyform...)Show InChI InChI=1S/C11H14ClN3O2/c12-10-7-9(13-8-14-16)1-2-11(10)15-3-5-17-6-4-15/h1-2,7-8,16H,3-6H2,(H,13,14) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co., Ltd
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 314: 77-85 (2005)
Article DOI: 10.1124/jpet.105.083964
BindingDB Entry DOI: 10.7270/Q2MW2FQQ |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Ovis aries (Sheep)) | BDBM86694

(N'-(3-Chloro-4-morpholinophenyl)-N-hydroxyform...)Show InChI InChI=1S/C11H14ClN3O2/c12-10-7-9(13-8-14-16)1-2-11(10)15-3-5-17-6-4-15/h1-2,7-8,16H,3-6H2,(H,13,14) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co., Ltd
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 314: 77-85 (2005)
Article DOI: 10.1124/jpet.105.083964
BindingDB Entry DOI: 10.7270/Q2MW2FQQ |
More data for this
Ligand-Target Pair | |
Corticotropin-releasing factor receptor 1
(Rattus norvegicus (rat)) | BDBM86694

(N'-(3-Chloro-4-morpholinophenyl)-N-hydroxyform...)Show InChI InChI=1S/C11H14ClN3O2/c12-10-7-9(13-8-14-16)1-2-11(10)15-3-5-17-6-4-15/h1-2,7-8,16H,3-6H2,(H,13,14) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co., Ltd
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 314: 77-85 (2005)
Article DOI: 10.1124/jpet.105.083964
BindingDB Entry DOI: 10.7270/Q2MW2FQQ |
More data for this
Ligand-Target Pair | |
D(1A) dopamine receptor
(RAT) | BDBM86694

(N'-(3-Chloro-4-morpholinophenyl)-N-hydroxyform...)Show InChI InChI=1S/C11H14ClN3O2/c12-10-7-9(13-8-14-16)1-2-11(10)15-3-5-17-6-4-15/h1-2,7-8,16H,3-6H2,(H,13,14) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co., Ltd
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 314: 77-85 (2005)
Article DOI: 10.1124/jpet.105.083964
BindingDB Entry DOI: 10.7270/Q2MW2FQQ |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Rattus norvegicus (rat)) | BDBM86694

(N'-(3-Chloro-4-morpholinophenyl)-N-hydroxyform...)Show InChI InChI=1S/C11H14ClN3O2/c12-10-7-9(13-8-14-16)1-2-11(10)15-3-5-17-6-4-15/h1-2,7-8,16H,3-6H2,(H,13,14) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co., Ltd
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 314: 77-85 (2005)
Article DOI: 10.1124/jpet.105.083964
BindingDB Entry DOI: 10.7270/Q2MW2FQQ |
More data for this
Ligand-Target Pair | |
Endothelin-1 receptor
(Homo sapiens (Human)) | BDBM86694

(N'-(3-Chloro-4-morpholinophenyl)-N-hydroxyform...)Show InChI InChI=1S/C11H14ClN3O2/c12-10-7-9(13-8-14-16)1-2-11(10)15-3-5-17-6-4-15/h1-2,7-8,16H,3-6H2,(H,13,14) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co., Ltd
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 314: 77-85 (2005)
Article DOI: 10.1124/jpet.105.083964
BindingDB Entry DOI: 10.7270/Q2MW2FQQ |
More data for this
Ligand-Target Pair | |
Gamma-aminobutyric acid type B receptor subunit 1
(Rattus norvegicus (Rat)) | BDBM86694

(N'-(3-Chloro-4-morpholinophenyl)-N-hydroxyform...)Show InChI InChI=1S/C11H14ClN3O2/c12-10-7-9(13-8-14-16)1-2-11(10)15-3-5-17-6-4-15/h1-2,7-8,16H,3-6H2,(H,13,14) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co., Ltd
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 314: 77-85 (2005)
Article DOI: 10.1124/jpet.105.083964
BindingDB Entry DOI: 10.7270/Q2MW2FQQ |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Cavia porcellus (domestic guinea pig)) | BDBM86694

(N'-(3-Chloro-4-morpholinophenyl)-N-hydroxyform...)Show InChI InChI=1S/C11H14ClN3O2/c12-10-7-9(13-8-14-16)1-2-11(10)15-3-5-17-6-4-15/h1-2,7-8,16H,3-6H2,(H,13,14) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co., Ltd
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 314: 77-85 (2005)
Article DOI: 10.1124/jpet.105.083964
BindingDB Entry DOI: 10.7270/Q2MW2FQQ |
More data for this
Ligand-Target Pair | |
Insulin receptor substrate 1
(RAT) | BDBM86694

(N'-(3-Chloro-4-morpholinophenyl)-N-hydroxyform...)Show InChI InChI=1S/C11H14ClN3O2/c12-10-7-9(13-8-14-16)1-2-11(10)15-3-5-17-6-4-15/h1-2,7-8,16H,3-6H2,(H,13,14) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co., Ltd
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 314: 77-85 (2005)
Article DOI: 10.1124/jpet.105.083964
BindingDB Entry DOI: 10.7270/Q2MW2FQQ |
More data for this
Ligand-Target Pair | |
Interleukin-1 beta
(Homo sapiens (Human)) | BDBM86694

(N'-(3-Chloro-4-morpholinophenyl)-N-hydroxyform...)Show InChI InChI=1S/C11H14ClN3O2/c12-10-7-9(13-8-14-16)1-2-11(10)15-3-5-17-6-4-15/h1-2,7-8,16H,3-6H2,(H,13,14) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co., Ltd
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 314: 77-85 (2005)
Article DOI: 10.1124/jpet.105.083964
BindingDB Entry DOI: 10.7270/Q2MW2FQQ |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM86694

(N'-(3-Chloro-4-morpholinophenyl)-N-hydroxyform...)Show InChI InChI=1S/C11H14ClN3O2/c12-10-7-9(13-8-14-16)1-2-11(10)15-3-5-17-6-4-15/h1-2,7-8,16H,3-6H2,(H,13,14) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co., Ltd
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 314: 77-85 (2005)
Article DOI: 10.1124/jpet.105.083964
BindingDB Entry DOI: 10.7270/Q2MW2FQQ |
More data for this
Ligand-Target Pair | |
Pro-neuropeptide Y
(RAT) | BDBM86694

(N'-(3-Chloro-4-morpholinophenyl)-N-hydroxyform...)Show InChI InChI=1S/C11H14ClN3O2/c12-10-7-9(13-8-14-16)1-2-11(10)15-3-5-17-6-4-15/h1-2,7-8,16H,3-6H2,(H,13,14) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co., Ltd
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 314: 77-85 (2005)
Article DOI: 10.1124/jpet.105.083964
BindingDB Entry DOI: 10.7270/Q2MW2FQQ |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, endothelial
(Homo sapiens (Human)) | BDBM86694

(N'-(3-Chloro-4-morpholinophenyl)-N-hydroxyform...)Show InChI InChI=1S/C11H14ClN3O2/c12-10-7-9(13-8-14-16)1-2-11(10)15-3-5-17-6-4-15/h1-2,7-8,16H,3-6H2,(H,13,14) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co., Ltd
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 314: 77-85 (2005)
Article DOI: 10.1124/jpet.105.083964
BindingDB Entry DOI: 10.7270/Q2MW2FQQ |
More data for this
Ligand-Target Pair | |
Nanos homolog 2
(Mus musculus (Mouse)) | BDBM86694

(N'-(3-Chloro-4-morpholinophenyl)-N-hydroxyform...)Show InChI InChI=1S/C11H14ClN3O2/c12-10-7-9(13-8-14-16)1-2-11(10)15-3-5-17-6-4-15/h1-2,7-8,16H,3-6H2,(H,13,14) | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co., Ltd
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 314: 77-85 (2005)
Article DOI: 10.1124/jpet.105.083964
BindingDB Entry DOI: 10.7270/Q2MW2FQQ |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 1
(RAT) | BDBM86694

(N'-(3-Chloro-4-morpholinophenyl)-N-hydroxyform...)Show InChI InChI=1S/C11H14ClN3O2/c12-10-7-9(13-8-14-16)1-2-11(10)15-3-5-17-6-4-15/h1-2,7-8,16H,3-6H2,(H,13,14) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co., Ltd
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 314: 77-85 (2005)
Article DOI: 10.1124/jpet.105.083964
BindingDB Entry DOI: 10.7270/Q2MW2FQQ |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data