 Found 1149 hits with Last Name = 'morgan' and Initial = 'n'
Found 1149 hits with Last Name = 'morgan' and Initial = 'n' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM77970
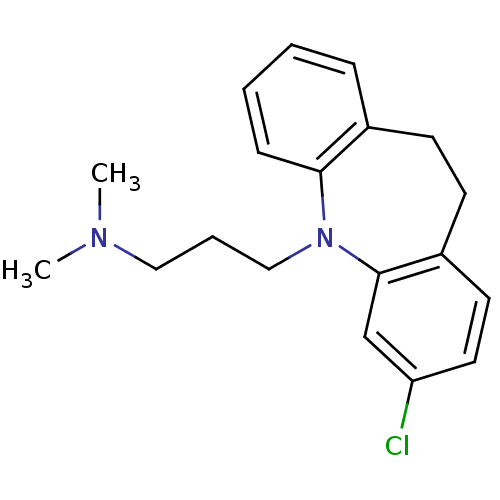
(3-(2-chloranyl-5,6-dihydrobenzo[b][1]benzazepin-11...)Show InChI InChI=1S/C19H23ClN2/c1-21(2)12-5-13-22-18-7-4-3-6-15(18)8-9-16-10-11-17(20)14-19(16)22/h3-4,6-7,10-11,14H,5,8-9,12-13H2,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| DrugBank
PubMed
| 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 283: 1305-22 (1997)
BindingDB Entry DOI: 10.7270/Q25Q4TMX |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Rattus norvegicus (rat)) | BDBM22416

((3S,4R)-3-[(1,3-benzodioxol-5-yloxy)methyl]-4-(4-f...)Show SMILES Fc1ccc(cc1)[C@@H]1CCNC[C@H]1COc1ccc2OCOc2c1 Show InChI InChI=1S/C19H20FNO3/c20-15-3-1-13(2-4-15)17-7-8-21-10-14(17)11-22-16-5-6-18-19(9-16)24-12-23-18/h1-6,9,14,17,21H,7-8,10-12H2/t14-,17-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
PubMed
| 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 283: 1305-22 (1997)
BindingDB Entry DOI: 10.7270/Q25Q4TMX |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM22416

((3S,4R)-3-[(1,3-benzodioxol-5-yloxy)methyl]-4-(4-f...)Show SMILES Fc1ccc(cc1)[C@@H]1CCNC[C@H]1COc1ccc2OCOc2c1 Show InChI InChI=1S/C19H20FNO3/c20-15-3-1-13(2-4-15)17-7-8-21-10-14(17)11-22-16-5-6-18-19(9-16)24-12-23-18/h1-6,9,14,17,21H,7-8,10-12H2/t14-,17-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
PubMed
| 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 283: 1305-22 (1997)
BindingDB Entry DOI: 10.7270/Q25Q4TMX |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Muscarinic acetylcholine receptor M2
(RAT) | BDBM86231

(ATR | ATROPINE | Atropine,(-) | CAS_51-55-8 | CHEM...)Show SMILES CN1C2CCC1CC(C2)OC(=O)C(CO)c1ccccc1 |THB:9:7:1:3.4| Show InChI InChI=1S/C17H23NO3/c1-18-13-7-8-14(18)10-15(9-13)21-17(20)16(11-19)12-5-3-2-4-6-12/h2-6,13-16,19H,7-11H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 283: 1305-22 (1997)
BindingDB Entry DOI: 10.7270/Q25Q4TMX |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Rattus norvegicus (rat)) | BDBM10755

(14C-5-hydroxy tryptamine creatinine disulfate | 2-...)Show InChI InChI=1S/C10H12N2O/c11-4-3-7-6-12-10-2-1-8(13)5-9(7)10/h1-2,5-6,12-13H,3-4,11H2 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| PDB
PubMed
| 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 283: 1305-22 (1997)
BindingDB Entry DOI: 10.7270/Q25Q4TMX |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM82217
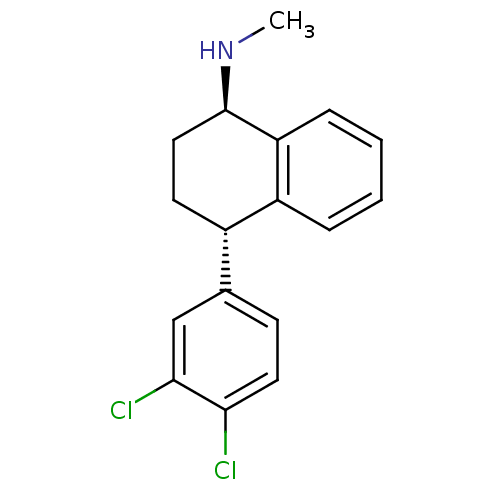
(CHEMBL284994 | CP-52003 | SERTRALINE | [4-(3,4-Dic...)Show SMILES CN[C@@H]1CC[C@@H](c2ccc(Cl)c(Cl)c2)c2ccccc12 Show InChI InChI=1S/C17H17Cl2N/c1-20-17-9-7-12(13-4-2-3-5-14(13)17)11-6-8-15(18)16(19)10-11/h2-6,8,10,12,17,20H,7,9H2,1H3/t12-,17+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 283: 1305-22 (1997)
BindingDB Entry DOI: 10.7270/Q25Q4TMX |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Cavia porcellus (domestic guinea pig)) | BDBM50020712
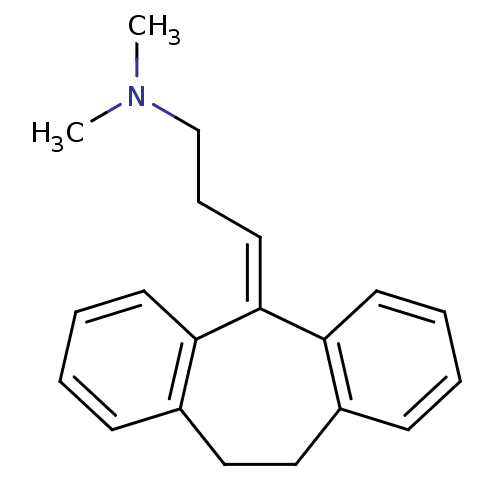
(10,11-dihydro-5-(gamma-dimethylaminopropylidene)-5...)Show SMILES [#6]-[#7](-[#6])-[#6]-[#6]\[#6]=[#6]-1/c2ccccc2-[#6]-[#6]-c2ccccc-12 Show InChI InChI=1S/C20H23N/c1-21(2)15-7-12-20-18-10-5-3-8-16(18)13-14-17-9-4-6-11-19(17)20/h3-6,8-12H,7,13-15H2,1-2H3 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 283: 1305-22 (1997)
BindingDB Entry DOI: 10.7270/Q25Q4TMX |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM19423

(HDAC inhibitor, Compound 1 | N-[2-amino-5-(thiophe...)Show InChI InChI=1S/C19H17N3O2S/c1-12(23)21-15-7-4-13(5-8-15)19(24)22-17-11-14(6-9-16(17)20)18-3-2-10-25-18/h2-11H,20H2,1H3,(H,21,23)(H,22,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| <0.200 | <-55.4 | 1 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Broad Institute of Harvard and MIT
| Assay Description
Purified HDAC1-9 (0.5~5 nM) were incubated with 2 μM carboxyfluorescein (FAM)-labeled acetylated peptide substrate A or B and test compound for ... |
ACS Chem Biol 11: 363-74 (2016)
Article DOI: 10.1021/acschembio.5b00640
BindingDB Entry DOI: 10.7270/Q2BZ64T2 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50356582
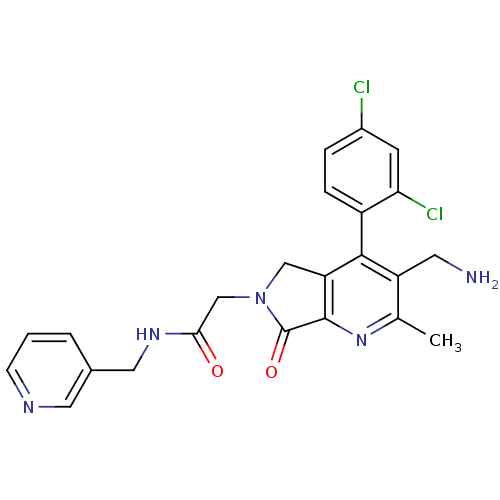
(CHEMBL1910126)Show SMILES Cc1nc2C(=O)N(CC(=O)NCc3cccnc3)Cc2c(c1CN)-c1ccc(Cl)cc1Cl |(14.01,-46.7,;12.68,-47.48,;11.34,-46.71,;10.02,-47.49,;8.55,-47.01,;8.08,-45.55,;7.65,-48.25,;6.11,-48.26,;5.34,-49.59,;6.12,-50.92,;3.8,-49.59,;3.04,-50.93,;1.5,-50.93,;.74,-52.26,;-.8,-52.27,;-1.58,-50.93,;-.81,-49.6,;.73,-49.59,;8.55,-49.5,;10.01,-49.03,;11.35,-49.8,;12.68,-49.03,;14.02,-49.79,;15.35,-49.02,;11.35,-51.33,;10.01,-52.1,;10.01,-53.64,;11.35,-54.41,;11.35,-55.95,;12.69,-53.64,;12.68,-52.1,;14.01,-51.33,)| Show InChI InChI=1S/C23H21Cl2N5O2/c1-13-17(8-26)21(16-5-4-15(24)7-19(16)25)18-11-30(23(32)22(18)29-13)12-20(31)28-10-14-3-2-6-27-9-14/h2-7,9H,8,10-12,26H2,1H3,(H,28,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of DPP4 |
Bioorg Med Chem Lett 21: 6646-51 (2011)
Article DOI: 10.1016/j.bmcl.2011.09.074
BindingDB Entry DOI: 10.7270/Q28W3DQK |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50356591

(CHEMBL1910117)Show SMILES CN(C)C(=O)CN1Cc2c(nc(C)c(CN)c2-c2ccc(Cl)cc2Cl)C1=O |(23.14,-19.79,;23.91,-21.12,;23.14,-22.45,;25.45,-21.12,;26.22,-22.45,;26.22,-19.78,;27.75,-19.78,;28.65,-21.03,;30.12,-20.55,;30.12,-19.01,;31.45,-18.24,;32.78,-19,;34.12,-18.23,;32.79,-20.55,;34.13,-21.32,;35.46,-20.55,;31.45,-21.32,;31.46,-22.86,;30.12,-23.63,;30.12,-25.17,;31.45,-25.94,;31.45,-27.48,;32.79,-25.16,;32.79,-23.62,;34.12,-22.85,;28.66,-18.54,;28.18,-17.07,)| Show InChI InChI=1S/C19H20Cl2N4O2/c1-10-13(7-22)17(12-5-4-11(20)6-15(12)21)14-8-25(9-16(26)24(2)3)19(27)18(14)23-10/h4-6H,7-9,22H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of DPP4 |
Bioorg Med Chem Lett 21: 6646-51 (2011)
Article DOI: 10.1016/j.bmcl.2011.09.074
BindingDB Entry DOI: 10.7270/Q28W3DQK |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Rattus norvegicus (rat)) | BDBM82217

(CHEMBL284994 | CP-52003 | SERTRALINE | [4-(3,4-Dic...)Show SMILES CN[C@@H]1CC[C@@H](c2ccc(Cl)c(Cl)c2)c2ccccc12 Show InChI InChI=1S/C17H17Cl2N/c1-20-17-9-7-12(13-4-2-3-5-14(13)17)11-6-8-15(18)16(19)10-11/h2-6,8,10,12,17,20H,7,9H2,1H3/t12-,17+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 283: 1305-22 (1997)
BindingDB Entry DOI: 10.7270/Q25Q4TMX |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50356589
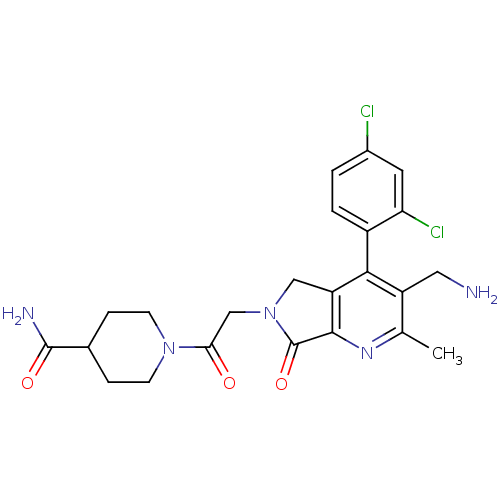
(CHEMBL1910119)Show SMILES Cc1nc2C(=O)N(CC(=O)N3CCC(CC3)C(N)=O)Cc2c(c1CN)-c1ccc(Cl)cc1Cl |(6.41,-30.16,;5.08,-30.93,;3.75,-30.17,;2.42,-30.95,;.95,-30.47,;.48,-29,;.05,-31.71,;-1.49,-31.71,;-2.25,-33.05,;-1.48,-34.38,;-3.79,-33.05,;-4.56,-31.72,;-6.09,-31.72,;-6.87,-33.05,;-6.1,-34.38,;-4.56,-34.39,;-8.41,-33.04,;-9.18,-31.71,;-9.18,-34.38,;.95,-32.96,;2.41,-32.48,;3.75,-33.26,;5.09,-32.48,;6.42,-33.25,;7.75,-32.48,;3.75,-34.79,;2.41,-35.56,;2.41,-37.1,;3.75,-37.87,;3.75,-39.41,;5.09,-37.09,;5.08,-35.56,;6.41,-34.78,)| Show InChI InChI=1S/C23H25Cl2N5O3/c1-12-16(9-26)20(15-3-2-14(24)8-18(15)25)17-10-30(23(33)21(17)28-12)11-19(31)29-6-4-13(5-7-29)22(27)32/h2-3,8,13H,4-7,9-11,26H2,1H3,(H2,27,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of DPP4 |
Bioorg Med Chem Lett 21: 6646-51 (2011)
Article DOI: 10.1016/j.bmcl.2011.09.074
BindingDB Entry DOI: 10.7270/Q28W3DQK |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 9
(Homo sapiens (Human)) | BDBM50442192

(CHEMBL2441845)Show SMILES Cc1[nH]c2cn(CCC(O)=O)c(=O)c2c(c1CN)-c1ccc(Cl)cc1Cl |(11.05,-4.3,;9.72,-5.08,;8.38,-4.32,;7.05,-5.08,;5.58,-4.61,;4.67,-5.87,;3.13,-5.88,;2.36,-4.55,;3.12,-3.21,;2.34,-1.88,;4.66,-3.2,;5.59,-7.12,;5.12,-8.58,;7.06,-6.63,;8.39,-7.4,;9.72,-6.63,;11.06,-7.4,;12.39,-6.62,;8.38,-8.94,;7.05,-9.7,;7.04,-11.24,;8.37,-12.01,;8.37,-13.55,;9.71,-11.24,;9.71,-9.71,;11.05,-8.93,)| Show InChI InChI=1S/C18H17Cl2N3O3/c1-9-12(7-21)16(11-3-2-10(19)6-13(11)20)17-14(22-9)8-23(18(17)26)5-4-15(24)25/h2-3,6,8,22H,4-5,7,21H2,1H3,(H,24,25) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Inhibition of DPP9 (unknown origin) |
J Med Chem 56: 7343-57 (2013)
Article DOI: 10.1021/jm4008906
BindingDB Entry DOI: 10.7270/Q2N01806 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50356585

(CHEMBL1910123)Show SMILES Cc1nc2C(=O)N(CC(=O)N3CCCC3)Cc2c(c1CN)-c1ccc(Cl)cc1Cl |(22.17,-39.13,;20.84,-39.9,;19.5,-39.14,;18.17,-39.91,;16.71,-39.44,;16.24,-37.97,;15.8,-40.68,;14.27,-40.68,;13.5,-42.02,;14.28,-43.35,;11.96,-42.02,;11.05,-40.78,;9.59,-41.26,;9.59,-42.8,;11.06,-43.27,;16.71,-41.93,;18.17,-41.45,;19.51,-42.22,;20.84,-41.45,;22.18,-42.22,;23.51,-41.45,;19.51,-43.76,;18.17,-44.53,;18.17,-46.07,;19.51,-46.84,;19.51,-48.38,;20.84,-46.06,;20.84,-44.52,;22.17,-43.75,)| Show InChI InChI=1S/C21H22Cl2N4O2/c1-12-15(9-24)19(14-5-4-13(22)8-17(14)23)16-10-27(21(29)20(16)25-12)11-18(28)26-6-2-3-7-26/h4-5,8H,2-3,6-7,9-11,24H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of DPP4 |
Bioorg Med Chem Lett 21: 6646-51 (2011)
Article DOI: 10.1016/j.bmcl.2011.09.074
BindingDB Entry DOI: 10.7270/Q28W3DQK |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50356584
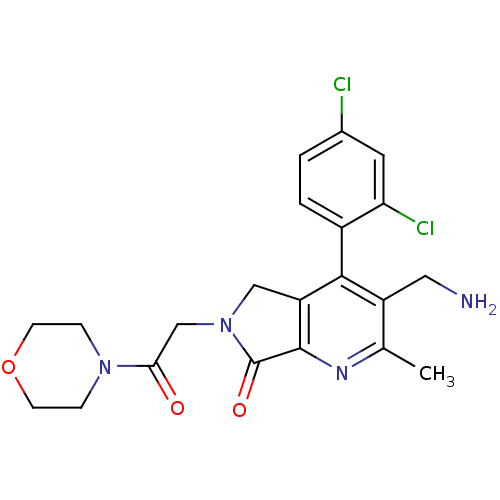
(CHEMBL1910124)Show SMILES Cc1nc2C(=O)N(CC(=O)N3CCOCC3)Cc2c(c1CN)-c1ccc(Cl)cc1Cl |(23.9,-37.72,;22.57,-38.49,;21.24,-37.73,;19.91,-38.51,;18.45,-38.03,;17.97,-36.56,;17.54,-39.27,;16.01,-39.27,;15.24,-40.61,;16.01,-41.94,;13.7,-40.61,;12.93,-39.28,;11.4,-39.28,;10.62,-40.61,;11.39,-41.94,;12.94,-41.95,;18.44,-40.52,;19.91,-40.04,;21.24,-40.82,;22.58,-40.04,;23.91,-40.81,;25.25,-40.04,;21.24,-42.35,;19.91,-43.12,;19.91,-44.66,;21.24,-45.43,;21.24,-46.97,;22.58,-44.65,;22.57,-43.12,;23.9,-42.34,)| Show InChI InChI=1S/C21H22Cl2N4O3/c1-12-15(9-24)19(14-3-2-13(22)8-17(14)23)16-10-27(21(29)20(16)25-12)11-18(28)26-4-6-30-7-5-26/h2-3,8H,4-7,9-11,24H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of DPP4 |
Bioorg Med Chem Lett 21: 6646-51 (2011)
Article DOI: 10.1016/j.bmcl.2011.09.074
BindingDB Entry DOI: 10.7270/Q28W3DQK |
More data for this
Ligand-Target Pair | |
Norepinephrine transporter
(RAT) | BDBM35229

(3-(10,11-dihydro-5H-dibenzo[b,f]azepin-5-yl)-N-met...)Show InChI InChI=1S/C18H22N2/c1-19-13-6-14-20-17-9-4-2-7-15(17)11-12-16-8-3-5-10-18(16)20/h2-5,7-10,19H,6,11-14H2,1H3 | UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 0.310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 283: 1305-22 (1997)
BindingDB Entry DOI: 10.7270/Q25Q4TMX |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50356592

(CHEMBL1910116)Show SMILES CNC(=O)CN1Cc2c(nc(C)c(CN)c2-c2ccc(Cl)cc2Cl)C1=O |(7.18,-19.79,;7.95,-21.12,;9.49,-21.12,;10.26,-22.45,;10.26,-19.78,;11.79,-19.78,;12.69,-21.03,;14.16,-20.55,;14.16,-19.01,;15.49,-18.24,;16.82,-19,;18.16,-18.23,;16.83,-20.55,;18.17,-21.32,;19.5,-20.55,;15.49,-21.32,;15.5,-22.86,;14.16,-23.63,;14.16,-25.17,;15.49,-25.94,;15.49,-27.48,;16.83,-25.16,;16.83,-23.62,;18.16,-22.85,;12.7,-18.54,;12.22,-17.07,)| Show InChI InChI=1S/C18H18Cl2N4O2/c1-9-12(6-21)16(11-4-3-10(19)5-14(11)20)13-7-24(8-15(25)22-2)18(26)17(13)23-9/h3-5H,6-8,21H2,1-2H3,(H,22,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.370 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of DPP4 |
Bioorg Med Chem Lett 21: 6646-51 (2011)
Article DOI: 10.1016/j.bmcl.2011.09.074
BindingDB Entry DOI: 10.7270/Q28W3DQK |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50356583

(CHEMBL1910125)Show SMILES Cc1nc2C(=O)N(CC(=O)Nc3ccnn3C)Cc2c(c1CN)-c1ccc(Cl)cc1Cl |(39.71,-37.55,;38.38,-38.32,;37.04,-37.56,;35.71,-38.33,;34.25,-37.86,;33.78,-36.39,;33.34,-39.1,;31.81,-39.1,;31.04,-40.44,;31.81,-41.77,;29.5,-40.44,;28.73,-41.77,;27.21,-41.94,;26.89,-43.44,;28.22,-44.21,;29.37,-43.18,;30.87,-43.5,;34.25,-40.35,;35.71,-39.87,;37.05,-40.64,;38.38,-39.87,;39.72,-40.64,;41.05,-39.87,;37.05,-42.18,;35.71,-42.95,;35.71,-44.49,;37.04,-45.26,;37.05,-46.8,;38.38,-44.48,;38.38,-42.94,;39.71,-42.17,)| Show InChI InChI=1S/C21H20Cl2N6O2/c1-11-14(8-24)19(13-4-3-12(22)7-16(13)23)15-9-29(21(31)20(15)26-11)10-18(30)27-17-5-6-25-28(17)2/h3-7H,8-10,24H2,1-2H3,(H,27,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of DPP4 |
Bioorg Med Chem Lett 21: 6646-51 (2011)
Article DOI: 10.1016/j.bmcl.2011.09.074
BindingDB Entry DOI: 10.7270/Q28W3DQK |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50356590

(CHEMBL1910118)Show SMILES CCN(CC)C(=O)CN1Cc2c(nc(C)c(CN)c2-c2ccc(Cl)cc2Cl)C1=O |(39.88,-17.76,;39.11,-19.1,;39.89,-20.43,;39.12,-21.76,;39.89,-23.09,;41.43,-20.43,;42.2,-21.76,;42.19,-19.09,;43.73,-19.09,;44.63,-20.33,;46.09,-19.86,;46.1,-18.32,;47.43,-17.55,;48.76,-18.31,;50.09,-17.53,;48.77,-19.86,;50.1,-20.63,;51.43,-19.86,;47.43,-20.63,;47.43,-22.17,;46.09,-22.94,;46.09,-24.47,;47.43,-25.25,;47.43,-26.79,;48.77,-24.47,;48.76,-22.93,;50.09,-22.16,;44.63,-17.84,;44.16,-16.38,)| Show InChI InChI=1S/C21H24Cl2N4O2/c1-4-26(5-2)18(28)11-27-10-16-19(14-7-6-13(22)8-17(14)23)15(9-24)12(3)25-20(16)21(27)29/h6-8H,4-5,9-11,24H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of DPP4 |
Bioorg Med Chem Lett 21: 6646-51 (2011)
Article DOI: 10.1016/j.bmcl.2011.09.074
BindingDB Entry DOI: 10.7270/Q28W3DQK |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Cavia porcellus (domestic guinea pig)) | BDBM35938
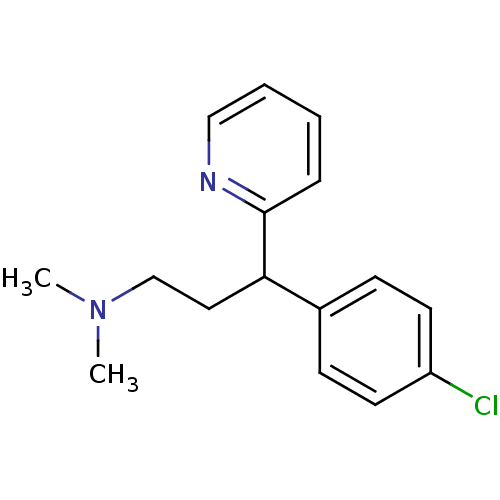
(1-(p-chlorophenyl)-1-(2-pyridyl)-3-N,N-dimethylpro...)Show InChI InChI=1S/C16H19ClN2/c1-19(2)12-10-15(16-5-3-4-11-18-16)13-6-8-14(17)9-7-13/h3-9,11,15H,10,12H2,1-2H3 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.440 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 283: 1305-22 (1997)
BindingDB Entry DOI: 10.7270/Q25Q4TMX |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50356587
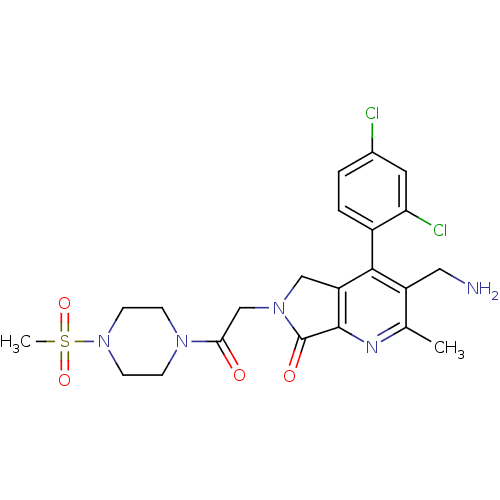
(CHEMBL1910121)Show SMILES Cc1nc2C(=O)N(CC(=O)N3CCN(CC3)S(C)(=O)=O)Cc2c(c1CN)-c1ccc(Cl)cc1Cl |(48.61,-29.07,;47.28,-29.84,;45.95,-29.08,;44.62,-29.86,;43.16,-29.38,;42.68,-27.91,;42.25,-30.62,;40.72,-30.62,;39.95,-31.96,;40.72,-33.29,;38.41,-31.96,;37.64,-30.63,;36.11,-30.63,;35.33,-31.96,;36.1,-33.29,;37.65,-33.3,;33.79,-31.95,;33.03,-30.62,;32.45,-32.72,;33.78,-33.49,;43.15,-31.87,;44.62,-31.4,;45.95,-32.17,;47.29,-31.39,;48.62,-32.16,;49.96,-31.39,;45.95,-33.7,;44.62,-34.47,;44.62,-36.01,;45.95,-36.78,;45.95,-38.32,;47.29,-36,;47.28,-34.47,;48.61,-33.69,)| Show InChI InChI=1S/C22H25Cl2N5O4S/c1-13-16(10-25)20(15-4-3-14(23)9-18(15)24)17-11-28(22(31)21(17)26-13)12-19(30)27-5-7-29(8-6-27)34(2,32)33/h3-4,9H,5-8,10-12,25H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.460 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of DPP4 |
Bioorg Med Chem Lett 21: 6646-51 (2011)
Article DOI: 10.1016/j.bmcl.2011.09.074
BindingDB Entry DOI: 10.7270/Q28W3DQK |
More data for this
Ligand-Target Pair | |
Sodium-dependent noradrenaline transporter
(Homo sapiens (Human)) | BDBM50005536
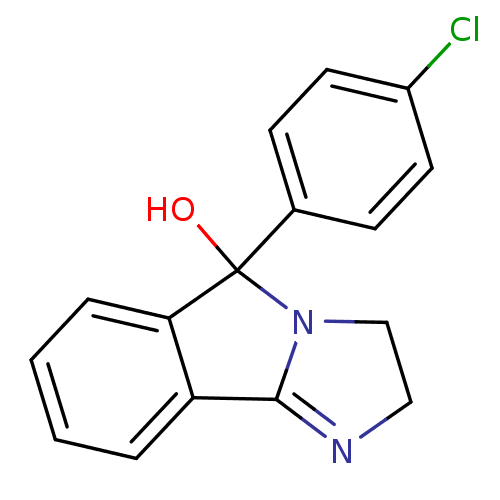
(42-548 | 5-(4-Chloro-phenyl)-2,5-dihydro-3H-imidaz...)Show InChI InChI=1S/C16H13ClN2O/c17-12-7-5-11(6-8-12)16(20)14-4-2-1-3-13(14)15-18-9-10-19(15)16/h1-8,20H,9-10H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PubMed
| 0.460 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 283: 1305-22 (1997)
BindingDB Entry DOI: 10.7270/Q25Q4TMX |
More data for this
Ligand-Target Pair | |
Norepinephrine transporter
(RAT) | BDBM50005536

(42-548 | 5-(4-Chloro-phenyl)-2,5-dihydro-3H-imidaz...)Show InChI InChI=1S/C16H13ClN2O/c17-12-7-5-11(6-8-12)16(20)14-4-2-1-3-13(14)15-18-9-10-19(15)16/h1-8,20H,9-10H2 | UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 0.470 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 283: 1305-22 (1997)
BindingDB Entry DOI: 10.7270/Q25Q4TMX |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Rattus norvegicus (rat)) | BDBM77970

(3-(2-chloranyl-5,6-dihydrobenzo[b][1]benzazepin-11...)Show InChI InChI=1S/C19H23ClN2/c1-21(2)12-5-13-22-18-7-4-3-6-15(18)8-9-16-10-11-17(20)14-19(16)22/h3-4,6-7,10-11,14H,5,8-9,12-13H2,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| PubMed
| 0.470 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 283: 1305-22 (1997)
BindingDB Entry DOI: 10.7270/Q25Q4TMX |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 9
(Homo sapiens (Human)) | BDBM50442198
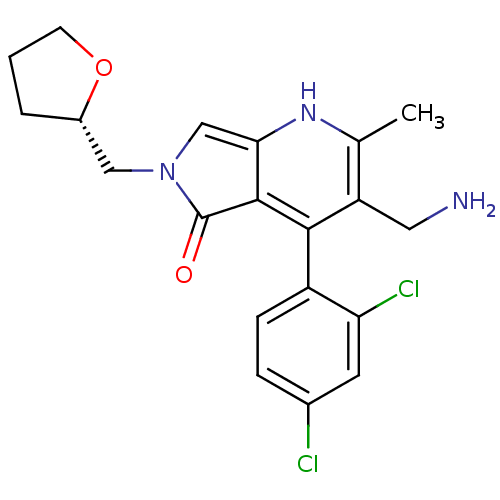
(CHEMBL2441839)Show SMILES Cc1[nH]c2cn(C[C@@H]3CCCO3)c(=O)c2c(c1CN)-c1ccc(Cl)cc1Cl |r,wD:7.6,(24.71,-38.82,;23.38,-39.6,;22.05,-38.83,;20.72,-39.6,;19.24,-39.13,;18.34,-40.39,;16.8,-40.39,;16.02,-39.06,;14.47,-39,;14.06,-37.52,;15.35,-36.67,;16.55,-37.63,;19.25,-41.63,;18.79,-43.1,;20.72,-41.15,;22.05,-41.92,;23.39,-41.15,;24.72,-41.91,;26.05,-41.14,;22.05,-43.46,;20.71,-44.22,;20.71,-45.76,;22.04,-46.53,;22.04,-48.07,;23.38,-45.76,;23.38,-44.22,;24.71,-43.45,)| Show InChI InChI=1S/C20H21Cl2N3O2/c1-11-15(8-23)18(14-5-4-12(21)7-16(14)22)19-17(24-11)10-25(20(19)26)9-13-3-2-6-27-13/h4-5,7,10,13,24H,2-3,6,8-9,23H2,1H3/t13-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Inhibition of DPP9 (unknown origin) |
J Med Chem 56: 7343-57 (2013)
Article DOI: 10.1021/jm4008906
BindingDB Entry DOI: 10.7270/Q2N01806 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50356588

(CHEMBL1910120)Show SMILES CC(=O)N1CCN(CC1)C(=O)CN1Cc2c(nc(C)c(CN)c2-c2ccc(Cl)cc2Cl)C1=O |(11.15,-31.36,;11.92,-32.69,;11.15,-34.03,;13.46,-32.7,;14.24,-31.37,;15.77,-31.37,;16.54,-32.7,;15.78,-34.04,;14.23,-34.03,;18.08,-32.7,;18.85,-34.03,;18.84,-31.36,;20.38,-31.36,;21.28,-32.61,;22.75,-32.13,;22.75,-30.6,;24.08,-29.82,;25.41,-30.58,;26.74,-29.81,;25.42,-32.13,;26.75,-32.9,;28.08,-32.13,;24.08,-32.91,;24.08,-34.44,;22.75,-35.21,;22.74,-36.75,;24.08,-37.52,;24.08,-39.06,;25.42,-36.74,;25.41,-35.21,;26.74,-34.43,;21.28,-30.12,;20.81,-28.65,)| Show InChI InChI=1S/C23H25Cl2N5O3/c1-13-17(10-26)21(16-4-3-15(24)9-19(16)25)18-11-30(23(33)22(18)27-13)12-20(32)29-7-5-28(6-8-29)14(2)31/h3-4,9H,5-8,10-12,26H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.550 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of DPP4 |
Bioorg Med Chem Lett 21: 6646-51 (2011)
Article DOI: 10.1016/j.bmcl.2011.09.074
BindingDB Entry DOI: 10.7270/Q28W3DQK |
More data for this
Ligand-Target Pair | |
Sodium-dependent noradrenaline transporter
(Homo sapiens (Human)) | BDBM35229

(3-(10,11-dihydro-5H-dibenzo[b,f]azepin-5-yl)-N-met...)Show InChI InChI=1S/C18H22N2/c1-19-13-6-14-20-17-9-4-2-7-15(17)11-12-16-8-3-5-10-18(16)20/h2-5,7-10,19H,6,11-14H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PubMed
| 0.630 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 283: 1305-22 (1997)
BindingDB Entry DOI: 10.7270/Q25Q4TMX |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50356586

(CHEMBL1910122)Show SMILES CN1CCN(CC1)C(=O)CN1Cc2c(nc(C)c(CN)c2-c2ccc(Cl)cc2Cl)C1=O |(-10,-42.49,;-8.46,-42.49,;-7.69,-41.16,;-6.16,-41.16,;-5.39,-42.49,;-6.15,-43.83,;-7.69,-43.83,;-3.85,-42.49,;-3.08,-43.82,;-3.08,-41.16,;-1.55,-41.15,;-.64,-42.4,;.82,-41.93,;.82,-40.39,;2.15,-39.61,;3.49,-40.38,;4.82,-39.6,;3.49,-41.93,;4.83,-42.69,;6.16,-41.92,;2.15,-42.7,;2.16,-44.23,;.82,-45,;.82,-46.54,;2.15,-47.31,;2.16,-48.85,;3.49,-46.53,;3.49,-45,;4.82,-44.22,;-.64,-39.91,;-1.11,-38.44,)| Show InChI InChI=1S/C22H25Cl2N5O2/c1-13-16(10-25)20(15-4-3-14(23)9-18(15)24)17-11-29(22(31)21(17)26-13)12-19(30)28-7-5-27(2)6-8-28/h3-4,9H,5-8,10-12,25H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.670 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of DPP4 |
Bioorg Med Chem Lett 21: 6646-51 (2011)
Article DOI: 10.1016/j.bmcl.2011.09.074
BindingDB Entry DOI: 10.7270/Q28W3DQK |
More data for this
Ligand-Target Pair | |
Alpha-2A adrenergic receptor
(Homo sapiens (Human)) | BDBM50013515

((+)-yohimbine | (16alpha,17alpha)-17-hydroxyyohimb...)Show SMILES COC(=O)[C@H]1[C@@H](O)CC[C@H]2CN3CCc4c([nH]c5ccccc45)[C@@H]3C[C@H]12 |r| Show InChI InChI=1S/C21H26N2O3/c1-26-21(25)19-15-10-17-20-14(13-4-2-3-5-16(13)22-20)8-9-23(17)11-12(15)6-7-18(19)24/h2-5,12,15,17-19,22,24H,6-11H2,1H3/t12-,15-,17-,18-,19+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.710 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 283: 1305-22 (1997)
BindingDB Entry DOI: 10.7270/Q25Q4TMX |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Rattus norvegicus (rat)) | BDBM25870
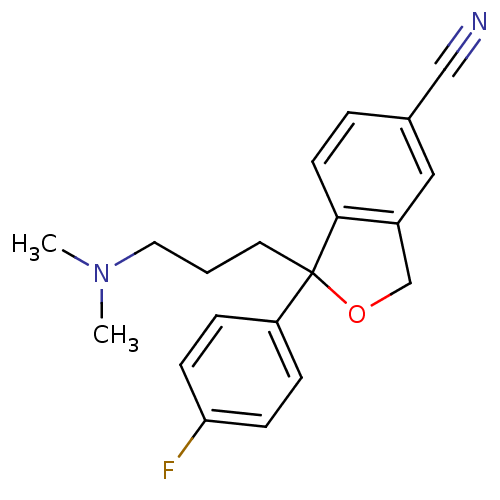
(1-[3-(dimethylamino)propyl]-1-(4-fluorophenyl)-1,3...)Show InChI InChI=1S/C20H21FN2O/c1-23(2)11-3-10-20(17-5-7-18(21)8-6-17)19-9-4-15(13-22)12-16(19)14-24-20/h4-9,12H,3,10-11,14H2,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
PubMed
| 0.75 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 283: 1305-22 (1997)
BindingDB Entry DOI: 10.7270/Q25Q4TMX |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Dipeptidyl peptidase 9
(Homo sapiens (Human)) | BDBM50442183
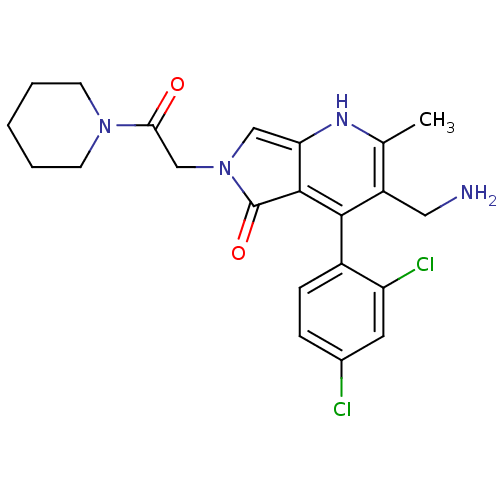
(CHEMBL2441955)Show SMILES Cc1[nH]c2cn(CC(=O)N3CCCCC3)c(=O)c2c(c1CN)-c1ccc(Cl)cc1Cl |(49.34,-35.7,;48.01,-36.47,;46.68,-35.71,;45.35,-36.47,;43.87,-36,;42.97,-37.26,;41.43,-37.27,;40.65,-35.94,;39.11,-35.95,;41.42,-34.6,;42.95,-34.6,;43.71,-33.27,;42.94,-31.94,;41.4,-31.95,;40.63,-33.28,;43.89,-38.51,;43.42,-39.98,;45.36,-38.02,;46.69,-38.79,;48.02,-38.02,;49.35,-38.79,;50.69,-38.01,;46.68,-40.33,;45.35,-41.09,;45.34,-42.63,;46.67,-43.41,;46.67,-44.95,;48.01,-42.63,;48.01,-41.1,;49.34,-40.32,)| Show InChI InChI=1S/C22H24Cl2N4O2/c1-13-16(10-25)20(15-6-5-14(23)9-17(15)24)21-18(26-13)11-28(22(21)30)12-19(29)27-7-3-2-4-8-27/h5-6,9,11,26H,2-4,7-8,10,12,25H2,1H3 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Inhibition of DPP9 (unknown origin) |
J Med Chem 56: 7343-57 (2013)
Article DOI: 10.1021/jm4008906
BindingDB Entry DOI: 10.7270/Q2N01806 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 8
(Homo sapiens (Human)) | BDBM50442191

(CHEMBL2441846)Show SMILES CCN(CC)C(=O)Cn1cc2[nH]c(C)c(CN)c(-c3ccc(Cl)cc3Cl)c2c1=O |(18.85,-1.37,;18.08,-2.7,;16.54,-2.71,;15.77,-1.38,;14.23,-1.39,;15.78,-4.05,;14.24,-4.06,;16.56,-5.38,;18.1,-5.37,;19,-4.11,;20.48,-4.59,;21.8,-3.82,;23.14,-4.58,;24.47,-3.81,;23.14,-6.13,;24.48,-6.9,;25.81,-6.13,;21.81,-6.9,;21.81,-8.44,;20.47,-9.2,;20.47,-10.74,;21.8,-11.52,;21.8,-13.06,;23.14,-10.74,;23.14,-9.21,;24.47,-8.44,;20.48,-6.13,;19.01,-6.62,;18.54,-8.09,)| Show InChI InChI=1S/C21H24Cl2N4O2/c1-4-26(5-2)18(28)11-27-10-17-20(21(27)29)19(15(9-24)12(3)25-17)14-7-6-13(22)8-16(14)23/h6-8,10,25H,4-5,9,11,24H2,1-3H3 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Inhibition of DPP8 (unknown origin) |
J Med Chem 56: 7343-57 (2013)
Article DOI: 10.1021/jm4008906
BindingDB Entry DOI: 10.7270/Q2N01806 |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM22416

((3S,4R)-3-[(1,3-benzodioxol-5-yloxy)methyl]-4-(4-f...)Show SMILES Fc1ccc(cc1)[C@@H]1CCNC[C@H]1COc1ccc2OCOc2c1 Show InChI InChI=1S/C19H20FNO3/c20-15-3-1-13(2-4-15)17-7-8-21-10-14(17)11-22-16-5-6-18-19(9-16)24-12-23-18/h1-6,9,14,17,21H,7-8,10-12H2/t14-,17-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
PubMed
| 0.830 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 283: 1305-22 (1997)
BindingDB Entry DOI: 10.7270/Q25Q4TMX |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM30130

(CHEMBL1201082 | CHEMBL41 | Fluoxetin | Fluoxetine ...)Show InChI InChI=1S/C17H18F3NO/c1-21-12-11-16(13-5-3-2-4-6-13)22-15-9-7-14(8-10-15)17(18,19)20/h2-10,16,21H,11-12H2,1H3 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PubMed
| 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 283: 1305-22 (1997)
BindingDB Entry DOI: 10.7270/Q25Q4TMX |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 9
(Homo sapiens (Human)) | BDBM50442184

(CHEMBL2441954)Show SMILES Cc1[nH]c2cn(CC(=O)N3CCCC3)c(=O)c2c(c1CN)-c1ccc(Cl)cc1Cl |(31.58,-31.64,;30.25,-32.42,;28.91,-31.65,;27.58,-32.42,;26.11,-31.95,;25.21,-33.21,;23.67,-33.22,;22.89,-31.89,;21.35,-31.89,;23.65,-30.55,;25.19,-30.58,;25.7,-29.12,;24.47,-28.19,;23.21,-29.07,;26.12,-34.46,;25.65,-35.92,;27.59,-33.97,;28.92,-34.74,;30.25,-33.97,;31.59,-34.74,;32.92,-33.96,;28.92,-36.28,;27.58,-37.04,;27.58,-38.58,;28.91,-39.35,;28.91,-40.89,;30.25,-38.58,;30.25,-37.04,;31.58,-36.27,)| Show InChI InChI=1S/C21H22Cl2N4O2/c1-12-15(9-24)19(14-5-4-13(22)8-16(14)23)20-17(25-12)10-27(21(20)29)11-18(28)26-6-2-3-7-26/h4-5,8,10,25H,2-3,6-7,9,11,24H2,1H3 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Inhibition of DPP9 (unknown origin) |
J Med Chem 56: 7343-57 (2013)
Article DOI: 10.1021/jm4008906
BindingDB Entry DOI: 10.7270/Q2N01806 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 9
(Homo sapiens (Human)) | BDBM50442193
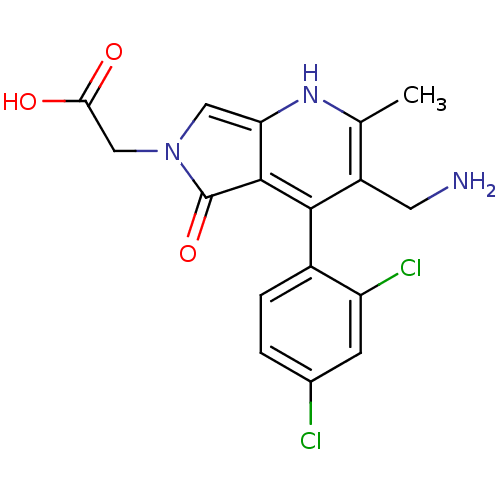
(CHEMBL2441844)Show SMILES Cc1[nH]c2cn(CC(O)=O)c(=O)c2c(c1CN)-c1ccc(Cl)cc1Cl |(23.65,-50.78,;22.32,-51.55,;20.98,-50.79,;19.65,-51.56,;18.18,-51.08,;17.27,-52.34,;15.73,-52.35,;14.96,-51.02,;15.72,-49.68,;13.42,-51.03,;18.19,-53.59,;17.72,-55.06,;19.66,-53.1,;20.99,-53.87,;22.32,-53.1,;23.66,-53.87,;24.99,-53.09,;20.98,-55.41,;19.65,-56.17,;19.64,-57.71,;20.97,-58.49,;20.97,-60.03,;22.31,-57.71,;22.31,-56.18,;23.65,-55.41,)| Show InChI InChI=1S/C17H15Cl2N3O3/c1-8-11(5-20)15(10-3-2-9(18)4-12(10)19)16-13(21-8)6-22(17(16)25)7-14(23)24/h2-4,6,21H,5,7,20H2,1H3,(H,23,24) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Inhibition of DPP9 (unknown origin) |
J Med Chem 56: 7343-57 (2013)
Article DOI: 10.1021/jm4008906
BindingDB Entry DOI: 10.7270/Q2N01806 |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50507371
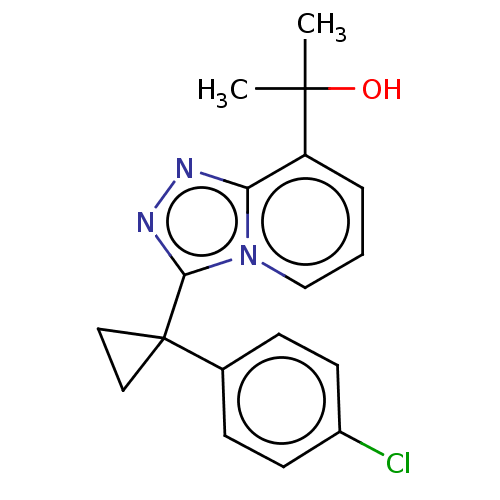
(BMS-823778)Show InChI InChI=1S/C18H18ClN3O/c1-17(2,23)14-4-3-11-22-15(14)20-21-16(22)18(9-10-18)12-5-7-13(19)8-6-12/h3-8,11,23H,9-10H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human 11beta-HSD1 expressed in HEK293 cell microsomes using [3H]cortisone as substrate after 4 hrs by homogeneous immuno-ra... |
ACS Med Chem Lett 9: 1170-1174 (2018)
Article DOI: 10.1021/acsmedchemlett.8b00307
BindingDB Entry DOI: 10.7270/Q20R9SP3 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Norepinephrine transporter
(RAT) | BDBM112777

(NORTRIPTYLINE | US8629135, SW-02)Show SMILES [#6]-[#7]-[#6]-[#6]\[#6]=[#6]-1/c2ccccc2-[#6]-[#6]-c2ccccc-12 Show InChI InChI=1S/C19H21N/c1-20-14-6-11-19-17-9-4-2-7-15(17)12-13-16-8-3-5-10-18(16)19/h2-5,7-11,20H,6,12-14H2,1H3 | UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| PubMed
| 0.990 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 283: 1305-22 (1997)
BindingDB Entry DOI: 10.7270/Q25Q4TMX |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 8
(Homo sapiens (Human)) | BDBM50442183

(CHEMBL2441955)Show SMILES Cc1[nH]c2cn(CC(=O)N3CCCCC3)c(=O)c2c(c1CN)-c1ccc(Cl)cc1Cl |(49.34,-35.7,;48.01,-36.47,;46.68,-35.71,;45.35,-36.47,;43.87,-36,;42.97,-37.26,;41.43,-37.27,;40.65,-35.94,;39.11,-35.95,;41.42,-34.6,;42.95,-34.6,;43.71,-33.27,;42.94,-31.94,;41.4,-31.95,;40.63,-33.28,;43.89,-38.51,;43.42,-39.98,;45.36,-38.02,;46.69,-38.79,;48.02,-38.02,;49.35,-38.79,;50.69,-38.01,;46.68,-40.33,;45.35,-41.09,;45.34,-42.63,;46.67,-43.41,;46.67,-44.95,;48.01,-42.63,;48.01,-41.1,;49.34,-40.32,)| Show InChI InChI=1S/C22H24Cl2N4O2/c1-13-16(10-25)20(15-6-5-14(23)9-17(15)24)21-18(26-13)11-28(22(21)30)12-19(29)27-7-3-2-4-8-27/h5-6,9,11,26H,2-4,7-8,10,12,25H2,1H3 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Inhibition of DPP8 (unknown origin) |
J Med Chem 56: 7343-57 (2013)
Article DOI: 10.1021/jm4008906
BindingDB Entry DOI: 10.7270/Q2N01806 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 8
(Homo sapiens (Human)) | BDBM50442193

(CHEMBL2441844)Show SMILES Cc1[nH]c2cn(CC(O)=O)c(=O)c2c(c1CN)-c1ccc(Cl)cc1Cl |(23.65,-50.78,;22.32,-51.55,;20.98,-50.79,;19.65,-51.56,;18.18,-51.08,;17.27,-52.34,;15.73,-52.35,;14.96,-51.02,;15.72,-49.68,;13.42,-51.03,;18.19,-53.59,;17.72,-55.06,;19.66,-53.1,;20.99,-53.87,;22.32,-53.1,;23.66,-53.87,;24.99,-53.09,;20.98,-55.41,;19.65,-56.17,;19.64,-57.71,;20.97,-58.49,;20.97,-60.03,;22.31,-57.71,;22.31,-56.18,;23.65,-55.41,)| Show InChI InChI=1S/C17H15Cl2N3O3/c1-8-11(5-20)15(10-3-2-9(18)4-12(10)19)16-13(21-8)6-22(17(16)25)7-14(23)24/h2-4,6,21H,5,7,20H2,1H3,(H,23,24) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Inhibition of DPP8 (unknown origin) |
J Med Chem 56: 7343-57 (2013)
Article DOI: 10.1021/jm4008906
BindingDB Entry DOI: 10.7270/Q2N01806 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 8
(Homo sapiens (Human)) | BDBM50442193

(CHEMBL2441844)Show SMILES Cc1[nH]c2cn(CC(O)=O)c(=O)c2c(c1CN)-c1ccc(Cl)cc1Cl |(23.65,-50.78,;22.32,-51.55,;20.98,-50.79,;19.65,-51.56,;18.18,-51.08,;17.27,-52.34,;15.73,-52.35,;14.96,-51.02,;15.72,-49.68,;13.42,-51.03,;18.19,-53.59,;17.72,-55.06,;19.66,-53.1,;20.99,-53.87,;22.32,-53.1,;23.66,-53.87,;24.99,-53.09,;20.98,-55.41,;19.65,-56.17,;19.64,-57.71,;20.97,-58.49,;20.97,-60.03,;22.31,-57.71,;22.31,-56.18,;23.65,-55.41,)| Show InChI InChI=1S/C17H15Cl2N3O3/c1-8-11(5-20)15(10-3-2-9(18)4-12(10)19)16-13(21-8)6-22(17(16)25)7-14(23)24/h2-4,6,21H,5,7,20H2,1H3,(H,23,24) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Inhibition of DPP8 (unknown origin) |
J Med Chem 56: 7343-57 (2013)
Article DOI: 10.1021/jm4008906
BindingDB Entry DOI: 10.7270/Q2N01806 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 9
(Homo sapiens (Human)) | BDBM50442206
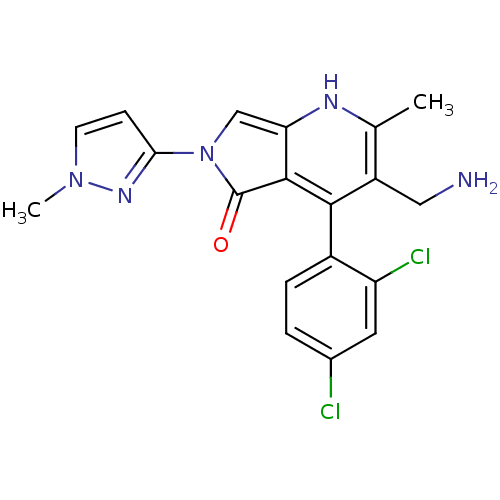
(CHEMBL2441831)Show SMILES Cc1[nH]c2cn(-c3ccn(C)n3)c(=O)c2c(c1CN)-c1ccc(Cl)cc1Cl |(11.24,-14.08,;9.91,-14.86,;8.57,-14.09,;7.25,-14.86,;5.77,-14.39,;4.87,-15.65,;3.33,-15.65,;2.41,-14.41,;.95,-14.9,;.96,-16.44,;-.29,-17.35,;2.42,-16.91,;5.78,-16.89,;5.31,-18.36,;7.25,-16.41,;8.58,-17.18,;9.91,-16.41,;11.25,-17.17,;12.58,-16.4,;8.58,-18.72,;7.24,-19.48,;7.24,-21.02,;8.57,-21.79,;8.57,-23.33,;9.91,-21.02,;9.91,-19.48,;11.24,-18.71,)| Show InChI InChI=1S/C19H17Cl2N5O/c1-10-13(8-22)17(12-4-3-11(20)7-14(12)21)18-15(23-10)9-26(19(18)27)16-5-6-25(2)24-16/h3-7,9,23H,8,22H2,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Inhibition of DPP9 (unknown origin) |
J Med Chem 56: 7343-57 (2013)
Article DOI: 10.1021/jm4008906
BindingDB Entry DOI: 10.7270/Q2N01806 |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50010859
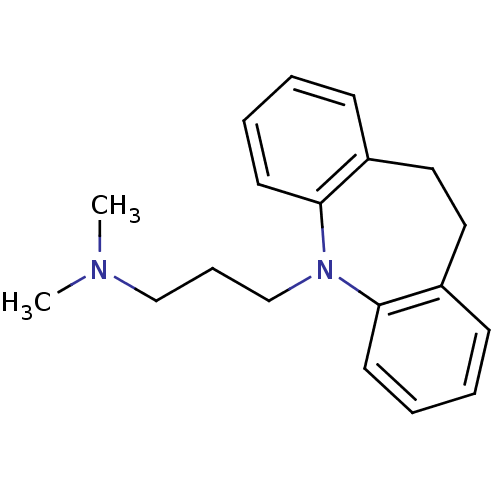
(CHEMBL11 | IMIPRAMINE HYDROCHLORIDE | IMIPRAMINE P...)Show InChI InChI=1S/C19H24N2/c1-20(2)14-7-15-21-18-10-5-3-8-16(18)12-13-17-9-4-6-11-19(17)21/h3-6,8-11H,7,12-15H2,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 283: 1305-22 (1997)
BindingDB Entry DOI: 10.7270/Q25Q4TMX |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Dipeptidyl peptidase 9
(Homo sapiens (Human)) | BDBM50442181
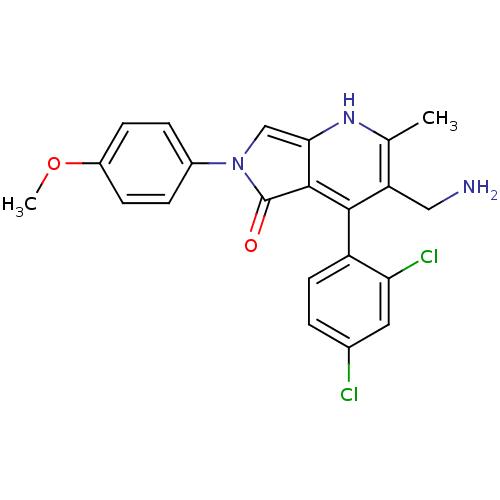
(CHEMBL2441829)Show SMILES COc1ccc(cc1)-n1cc2[nH]c(C)c(CN)c(-c3ccc(Cl)cc3Cl)c2c1=O |(14.54,-2.86,;15.32,-4.18,;16.86,-4.18,;17.62,-2.84,;19.16,-2.83,;19.93,-4.16,;19.18,-5.5,;17.64,-5.51,;21.47,-4.15,;22.38,-2.89,;23.85,-3.36,;25.18,-2.6,;26.52,-3.36,;27.85,-2.59,;26.52,-4.91,;27.86,-5.68,;29.19,-4.9,;25.19,-5.68,;25.18,-7.22,;23.85,-7.98,;23.84,-9.52,;25.17,-10.3,;25.17,-11.84,;26.51,-9.52,;26.51,-7.99,;27.85,-7.22,;23.86,-4.91,;22.39,-5.4,;21.92,-6.87,)| Show InChI InChI=1S/C22H19Cl2N3O2/c1-12-17(10-25)20(16-8-3-13(23)9-18(16)24)21-19(26-12)11-27(22(21)28)14-4-6-15(29-2)7-5-14/h3-9,11,26H,10,25H2,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Inhibition of DPP9 (unknown origin) |
J Med Chem 56: 7343-57 (2013)
Article DOI: 10.1021/jm4008906
BindingDB Entry DOI: 10.7270/Q2N01806 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 9
(Homo sapiens (Human)) | BDBM50442191

(CHEMBL2441846)Show SMILES CCN(CC)C(=O)Cn1cc2[nH]c(C)c(CN)c(-c3ccc(Cl)cc3Cl)c2c1=O |(18.85,-1.37,;18.08,-2.7,;16.54,-2.71,;15.77,-1.38,;14.23,-1.39,;15.78,-4.05,;14.24,-4.06,;16.56,-5.38,;18.1,-5.37,;19,-4.11,;20.48,-4.59,;21.8,-3.82,;23.14,-4.58,;24.47,-3.81,;23.14,-6.13,;24.48,-6.9,;25.81,-6.13,;21.81,-6.9,;21.81,-8.44,;20.47,-9.2,;20.47,-10.74,;21.8,-11.52,;21.8,-13.06,;23.14,-10.74,;23.14,-9.21,;24.47,-8.44,;20.48,-6.13,;19.01,-6.62,;18.54,-8.09,)| Show InChI InChI=1S/C21H24Cl2N4O2/c1-4-26(5-2)18(28)11-27-10-17-20(21(27)29)19(15(9-24)12(3)25-17)14-7-6-13(22)8-16(14)23/h6-8,10,25H,4-5,9,11,24H2,1-3H3 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Inhibition of DPP9 (unknown origin) |
J Med Chem 56: 7343-57 (2013)
Article DOI: 10.1021/jm4008906
BindingDB Entry DOI: 10.7270/Q2N01806 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 8
(Homo sapiens (Human)) | BDBM50442198

(CHEMBL2441839)Show SMILES Cc1[nH]c2cn(C[C@@H]3CCCO3)c(=O)c2c(c1CN)-c1ccc(Cl)cc1Cl |r,wD:7.6,(24.71,-38.82,;23.38,-39.6,;22.05,-38.83,;20.72,-39.6,;19.24,-39.13,;18.34,-40.39,;16.8,-40.39,;16.02,-39.06,;14.47,-39,;14.06,-37.52,;15.35,-36.67,;16.55,-37.63,;19.25,-41.63,;18.79,-43.1,;20.72,-41.15,;22.05,-41.92,;23.39,-41.15,;24.72,-41.91,;26.05,-41.14,;22.05,-43.46,;20.71,-44.22,;20.71,-45.76,;22.04,-46.53,;22.04,-48.07,;23.38,-45.76,;23.38,-44.22,;24.71,-43.45,)| Show InChI InChI=1S/C20H21Cl2N3O2/c1-11-15(8-23)18(14-5-4-12(21)7-16(14)22)19-17(24-11)10-25(20(19)26)9-13-3-2-6-27-13/h4-5,7,10,13,24H,2-3,6,8-9,23H2,1H3/t13-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Inhibition of DPP8 (unknown origin) |
J Med Chem 56: 7343-57 (2013)
Article DOI: 10.1021/jm4008906
BindingDB Entry DOI: 10.7270/Q2N01806 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 8
(Homo sapiens (Human)) | BDBM50442184

(CHEMBL2441954)Show SMILES Cc1[nH]c2cn(CC(=O)N3CCCC3)c(=O)c2c(c1CN)-c1ccc(Cl)cc1Cl |(31.58,-31.64,;30.25,-32.42,;28.91,-31.65,;27.58,-32.42,;26.11,-31.95,;25.21,-33.21,;23.67,-33.22,;22.89,-31.89,;21.35,-31.89,;23.65,-30.55,;25.19,-30.58,;25.7,-29.12,;24.47,-28.19,;23.21,-29.07,;26.12,-34.46,;25.65,-35.92,;27.59,-33.97,;28.92,-34.74,;30.25,-33.97,;31.59,-34.74,;32.92,-33.96,;28.92,-36.28,;27.58,-37.04,;27.58,-38.58,;28.91,-39.35,;28.91,-40.89,;30.25,-38.58,;30.25,-37.04,;31.58,-36.27,)| Show InChI InChI=1S/C21H22Cl2N4O2/c1-12-15(9-24)19(14-5-4-13(22)8-16(14)23)20-17(25-12)10-27(21(20)29)11-18(28)26-6-2-3-7-26/h4-5,8,10,25H,2-3,6-7,9,11,24H2,1H3 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Inhibition of DPP8 (unknown origin) |
J Med Chem 56: 7343-57 (2013)
Article DOI: 10.1021/jm4008906
BindingDB Entry DOI: 10.7270/Q2N01806 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 2
(Homo sapiens (Human)) | BDBM19423

(HDAC inhibitor, Compound 1 | N-[2-amino-5-(thiophe...)Show InChI InChI=1S/C19H17N3O2S/c1-12(23)21-15-7-4-13(5-8-15)19(24)22-17-11-14(6-9-16(17)20)18-3-2-10-25-18/h2-11H,20H2,1H3,(H,21,23)(H,22,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 1.5 | -50.4 | 13 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Broad Institute of Harvard and MIT
| Assay Description
Purified HDAC1-9 (0.5~5 nM) were incubated with 2 μM carboxyfluorescein (FAM)-labeled acetylated peptide substrate A or B and test compound for ... |
ACS Chem Biol 11: 363-74 (2016)
Article DOI: 10.1021/acschembio.5b00640
BindingDB Entry DOI: 10.7270/Q2BZ64T2 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM25870

(1-[3-(dimethylamino)propyl]-1-(4-fluorophenyl)-1,3...)Show InChI InChI=1S/C20H21FN2O/c1-23(2)11-3-10-20(17-5-7-18(21)8-6-17)19-9-4-15(13-22)12-16(19)14-24-20/h4-9,12H,3,10-11,14H2,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
PubMed
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 283: 1305-22 (1997)
BindingDB Entry DOI: 10.7270/Q25Q4TMX |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Sodium-dependent serotonin transporter
(Rattus norvegicus (rat)) | BDBM50028091
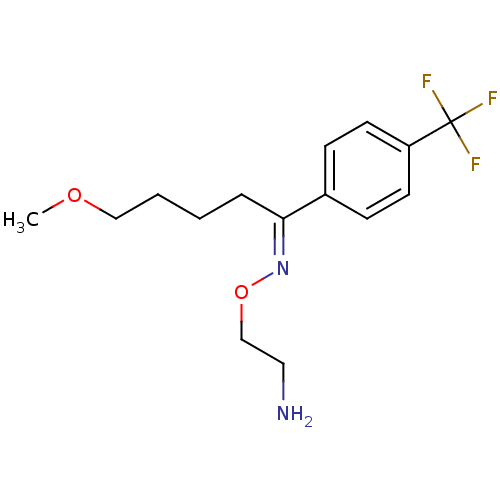
((1E)-5-methoxy-1-[4-(trifluoromethyl)phenyl]pentan...)Show InChI InChI=1S/C15H21F3N2O2/c1-21-10-3-2-4-14(20-22-11-9-19)12-5-7-13(8-6-12)15(16,17)18/h5-8H,2-4,9-11,19H2,1H3/b20-14+ | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
PubMed
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 283: 1305-22 (1997)
BindingDB Entry DOI: 10.7270/Q25Q4TMX |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data